ABSTRACT.
Malaria remains a leading cause of childhood morbidity and mortality in sub-Saharan Africa, particularly among children under 5 years of age. To help address this challenge, the WHO recommends chemoprevention for certain populations. For children and infants, the WHO recommends seasonal malaria chemoprevention (SMC), perennial malaria chemoprevention (PMC; formerly intermittent preventive treatment in infants [IPTi]), and, more recently, intermittent preventive treatment in school children (IPTsc). This review describes the contextual factors, including feasibility, acceptability, health equity, financial considerations, and values and preferences, that impact implementation of these strategies. A systematic search was conducted on July 5, 2022, and repeated April 13, 2023, to identify relevant literature. Two reviewers independently screened titles for eligibility, extracted data from eligible articles, and identified and summarized themes. Of 6,295 unique titles identified, 65 were included. The most frequently evaluated strategy was SMC (n = 40), followed by IPTi (n = 18) and then IPTsc (n = 6). Overall, these strategies were highly acceptable, although with IPTsc, there were community concerns with providing drugs to girls of reproductive age and the use of nonmedical staff for drug distribution. For SMC, door-to-door delivery resulted in higher coverage, improved caregiver acceptance, and reduced cost. Lower adherence was noted when caregivers were charged with giving doses 2 and 3 unsupervised. For SMC and IPTi, travel distances and inclement weather limited accessibility. Sensitization and caregiver education efforts, retention of high-quality drug distributors, and improved transportation were key to improving coverage. Additional research is needed to understand the role of community values and preferences in chemoprevention implementation.
INTRODUCTION
Substantial progress has been made in reducing the burden of malaria; the 2022 World Malaria Report noted a case reduction from 368 to 222 per 1,000 population at risk between 2000 and 2019.1 This progress can be attributed to a variety of malaria interventions, including vector control, chemoprevention, and effective treatment with artemisinin-based combination therapies. However, malaria remains a leading cause of childhood morbidity and mortality. The last few years have seen a leveling off in the gains against malaria; globally, countries are off track by 48% to achieve the targets set by the most recent WHO Global Technical Strategy for malaria control.2 To accelerate progress and meet the 2030 WHO targets, additional investments are needed to improve uptake of existing interventions and to implement new tools.
In June 2022, the WHO Global Malaria Program released updated guidance on malaria chemoprevention,3 informed by reviews of available evidence. These guidelines expanded recommendations in favor of broader implementation of seasonal malaria chemoprevention (SMC) and perennial malaria chemoprevention (PMC; formerly known as intermittent preventive treatment in infants [IPTi]) in terms of ages targeted, drugs used, and number of cycles. These factors should be based on analysis of local epidemiological and seasonal patterns. The new guidance also included recommendations for the use of intermittent preventive treatment in school-aged children (IPTsc) for the first time. Although the WHO has changed the terminology from IPTi to PMC in recognition of the fact that delivery now extends beyond the period of infancy, this review will refer to IPTi, as all literature to date uses this older terminology.
Seasonal malaria chemoprevention has been implemented much more extensively than IPTi or IPTsc. Since being recommended by the WHO in 2012, SMC has been widely implemented in West Africa. Scale-up of SMC across the Sahel region was facilitated by the ACCESS-SMC pilot project, implemented in 2015 to 2017. To date, only one country, Sierra Leone, has implemented IPTi routinely outside of operations research since the WHO’s initial recommendation in 2010. Intermittent preventive treatment in infants is a desirable intervention in Sierra Leone, as SMC is not feasible in the absence of a significant rainy season. Despite the high acceptability and efficacy reported in Sierra Leone, the success of IPTi has not translated into implementation in other countries. Concerns related to sulfadoxine-pyrimethamine (SP) resistance, unconvincing efficacy results, gaps in coverage, and anticipated logistical challenges with integrating IPTi with the Expanded Program on Immunizations (EPI) has slowed expansion beyond implementation studies and pilot programs. Recently, there has been renewed interest in IPTi (now renamed PMC), with a large pilot implementation under way in four countries (Benin, Cameroon [ClinicalTrials.gov ID NCT05889052], Côte d’Ivoire [ClinicalTrials.gov ID NCT05856357], and Mozambique), while IPTsc has been conducted only in the context of research (Table 1).
Table 1.
Chemoprevention strategies
| Strategy | Description |
|---|---|
| SMC | The administration of a full treatment of antimalarial medicine during the malaria season to prevent illness. Historically, SMC has been administered to children 3 to 59 months of age in areas with highly seasonal malaria transmission, with SP+AQ given monthly during the transmission season for a maximum of four monthly doses. The current WHO recommendation does not specify strict ages, transmission intensity thresholds, number of cycles, or specific drugs.3 |
| IPTi/PMC | Previous WHO recommendations for IPTi included three doses of SP at 2, 3, and 9 months of age concomitantly with vaccinations given through the EPI in areas where SP resistance was not high. The 2023 WHO recommendation for PMC removes the specifications on number of doses and targeted ages for these doses, and expands the eligible age group beyond 1 year where severe disease burden is high. |
| IPTsc | In settings with moderate to high perennial or seasonal malaria transmission, the WHO recommends that school-aged children (5 to 15 years of age) receive antimalarials at regular intervals, facilitated through schools. |
EPI = Expanded Program on Immunization; IPTi = intermittent preventive treatment during infancy; IPTsc = intermittent preventive treatment in school-aged children; PMC = perennial malaria chemoprevention; SMC = seasonal malaria chemoprevention; SP+AQ = sulfadoxine-pyrimethamine plus amodiaquine.
The evidence profiles for SMC, IPTi, and IPTsc presented in the 2023 WHO guidelines support the efficacy and safety of all three chemoprevention strategies but with differing degrees of confidence.3 The SMC profile reported expansive and high-quality evidence that SMC is a tool to reduce clinical malaria, parasite prevalence, and anemia in children under 5 years of age in both low- and high-transmission settings.4–7 The IPTi profile summarizing the available literature concluded that IPTi probably reduces the incidence of clinical malaria and anemia.8–10 The evidence for IPTsc was limited and considered low quality because of serious risk of bias and inconsistency; however, based on available data, IPTsc may decrease clinical malaria, anemia, and parasite prevalence.11
Given recommendations to consider expanded use of these malaria chemoprevention interventions, it is relevant to assess not only their continued effectiveness but how to optimize implementation using key contextual factors recognized by the WHO. We conducted a systematic review of the literature, presenting themes and lessons learned concerning five contextual factors related to chemoprevention strategies that target children (SMC, IPTi, and IPTsc) based on the new WHO-INTEGRATE (INTEGRATe Evidence) framework.12 These contextual factors cover community-specific and health system considerations that may impact the success of a chemoprevention program. Two factors consider community members’ interest in supporting and participating in chemoprevention programs: 1) values and preferences and 2) acceptability. Three factors highlight the logistical considerations for the successful implementation and accessibility of these programs: health equity and nondiscrimination, feasibility and health system considerations, and financial and economic considerations.
MATERIALS AND METHODS
Source selection.
PubMed was searched on July 5, 2022, and again on April 13, 2023, using the following terms [(malaria AND (prevent* OR prophylax*) AND (value OR knowledg* OR Accept* OR Feasib* OR equit* OR cost OR resource)] NOT (pregnan*). Additional titles were identified by reviewing reference lists of included sources and based on input from other subject matter experts. All titles were uploaded to Covidence (Veritas Health Innovation Ltd., Melbourne, Australia), a systematic review software. Sources that pertained to SMC, IPTi, or IPTsc were included. Sources describing interventions not focused on children, such as those for pregnant women or the general population, were excluded. Duplicates, efficacy studies with no data on contextual factors, meta-analyses, and studies where data were collected prior to 2000 were removed. All titles and abstracts were independently screened by two reviewers (P. G. and J. L.), and any discordances were discussed until an agreement regarding inclusion was reached.
Data extraction.
Full-text review and data extraction were completed by the same two reviewers using a form designed in Covidence (Supplemental Appendix A). The form captured study identifiers (title, lead author, publication year), intervention details [target county, study aim, study design, study start and end dates, population description, number of participants, drug(s) evaluated and dosing schedule], and contextual factors (acceptability, health equity, feasibility and health system considerations, financial and economic considerations, values and preferences). All data extractions were compared, and disagreements between reviewers were discussed until an agreement was reached.
Identification of themes.
Each contextual factor was reviewed separately for common themes. The extraction results were exported from Covidence into a comma-separated values (CSV) file, and data for each contextual factor were summarized.
The domains were analyzed within the following definitions based on the new WHO-INTEGRATE (INTEGRATe Evidence) framework12 with the exception of values and preferences, which is not part of the INTEGRATe framework but which had been requested by the WHO as part of initial reviews.
Feasibility and health system considerations.
“Feasibility and health system considerations recognize that the most appropriate and feasible interventions may vary significantly across different contexts, both across countries and across jurisdictions within countries. Legislation and governance, the structure of the health system and existing programs, as well as human resources and infrastructure, should be taken into account.”
Acceptability.
“The extent to which those benefiting from an intervention as well as other relevant stakeholder groups consider the intervention to be appropriate, based on anticipated or experienced cognitive and emotional responses to the intervention.”
Health equity, equality, and nondiscrimination.
“The extent to which the intervention benefits all populations, does not discriminate against anyone on the basis of sex, age, ethnicity, culture, language, sexual orientation or gender identity, disability status, education, socioeconomic status, residence or any other characteristic.”
Financial and economic considerations.
“Financial and economic considerations acknowledge that available financial (budgetary) resources are constrained and take into account the economic impact of an intervention on the health system, government or society as a whole.”
Values and preferences.
Information on “the relative importance assigned to health outcomes by those affected by them; how such importance varies within and across populations; and whether this importance or variability is surrounded by uncertainty.”
RESULTS
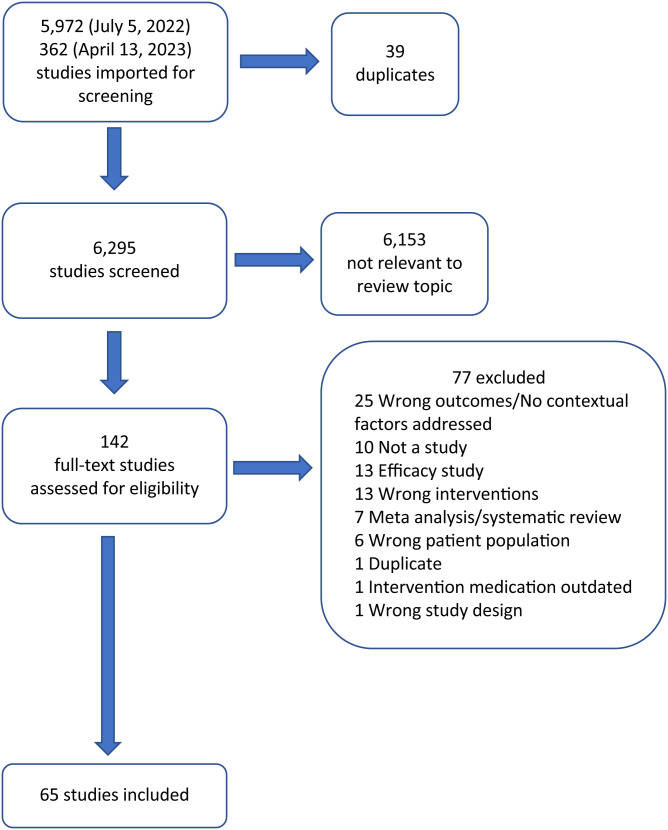
Of the 6,295 unique titles screened, 142 were included for full-text review. The majority of sources were excluded during initial title and abstract screening because they did not discuss malaria or chemoprevention. Of the 142 full texts reviewed, 65 were extracted (Figure 1). Of the extracted articles, 40 discussed SMC, 18 discussed IPTi, six discussed IPTsc, and two described chemoprevention for children that could not be categorized as SMC, IPTi, or IPTsc. For SMC, 16 studies were completed between 2006 and 2014, 13 between 2015 and 2017 (during the same period as the ACCESS-SMC project), 10 between 2018 and 2022, and for one study the year was not specified. Individual studies could describe multiple interventions, countries, or contextual factors. The majority of articles were conducted in Ghana (n = 15), Tanzania (n = 10), and Nigeria (n = 10) (Supplemental Appendix B). The most frequently discussed contextual factors were feasibility and acceptability, with 52 and 38 articles discussing these factors, respectively. Financial considerations and health equity were less frequently described, with 23 and 18 titles describing these factors, respectively. Values and preferences were discussed minimally and appeared in only four sources.
Figure 1.
Flow chart of literature search.
Feasibility and health system considerations.
Challenges to chemoprevention implementation largely fit into four categories: training and retaining staff, identifying and accessing eligible children, ensuring supply availability, and correct dosing.
Staffing.
Chemoprevention studies described a variety of community-based staff involved in drug distribution. Most frequently, these individuals were from the local community, received training specific to chemoprevention, and may have received incentive pay during the implementation period. For simplicity, all community-based staff involved in chemoprevention distribution are referred to as community health workers (CHWs). Clinic-based staff were associated with a facility, most frequently received a regular salary, and incorporated chemoprevention into broader work duties. These included health workers, nurses, clinic health workers, and traveling clinic care teams and are referred to as clinic staff in this review.
Training of staff was achieved through both formal training and cascade training, whereby formally trained staff provided on-the-job training or organized lessons for additional staff.
For SMC, training was widely reported as successful, with CHWs quickly understanding their responsibilities and the role of SMC.13–16 Refresher trainings were needed to build staff skills and confidence, with staff retained for multiple seasons showing improved skills and capacity.17,18 Several studies noted that additional supervision was necessary to promote continued adherence to intervention protocols15,19–21 although one study did note that little follow-up supervision was necessary for continued success.11 The most common protocol violation was not directly observing the first dose of SMC at all or not observing the child for a full 30 minutes after administering the drugs.16,22 In one study, 56% of caretakers reported that CHWs did not directly observe the first dose of SMC;23 in another, 44% of CHWs did not follow protocol.24 Studies reported that the number of supervisory visits was limited by resource constraints and supervisors not wanting to complete supervision tasks.21,22
As literacy was required for completing SMC documentation, in communities with lower literacy rates, programs partnered literate and illiterate CHWs as working pairs.18,22 One study noted that low literacy rates slowed initial training,25 and another reported challenges with recruiting a sufficient number of literate CHWs.10 Several studies noted general staffing shortages.26–29
Retention of previously trained CHWs was a key concern for SMC programs, as recruiting and training new staff each year was both costly and time-consuming.15,18,30 Although existing CHWs reported being proud of their work and viewing it as important,18,19 many reported financial challenges associated with the role, which reduced retention. Across studies, CHWs reported delays in payments, having to borrow money or pay out of pocket for transportation, and having to temporarily forgo their own farm work to participate in SMC campaigns.16,17,19,21,30–32
Similar to SMC training, most IPTi training was well regarded, and clinic staff quickly understood how to incorporate chemoprevention into EPI visits,33,34 though one study noted that 62% of staff believed they needed more training.35 One study noted that only limited supervision was needed for trained staff,36 whereas a different study emphasized the importance of continued supervision.37
Challenges with retention and payment complaints were less frequently reported in IPTi research, potentially because IPTi tasks were often completed by clinic staff used by the health facility. However, some staff felt they should be compensated for offering the drugs, as it took time away from other tasks.34,35,37 A time and motion study estimated that the time required to integrate IPTi into routine vaccination activities was 12.4 minutes per nurse per day and did not require adjustments to work schedules.37 Another study estimated that IPTi represented ∼11% of the clinic staff member’s time.38 In instances where only select clinic staff were trained for IPTi, staff who did not receive the formal training were reluctant to administer IPTi.35,37 Researchers suggested that untrained staff envied the staff who had received formal training. One study found that clinic staff gave pills to caretakers to administer at home to save time despite explicit instructions requiring directly observed therapy.27
The need for repeat training due to turnover was also seen with IPTsc.39 One study of IPTsc had success with a “train the trainer” approach whereby a subset of teachers received formal IPTsc training and then relayed the information to other school staff. During this same study, the number of teachers implementing ITPsc was scaled up based on school enrollment to allow for efficient drug distribution, resulting in minimal curriculum disruptions.40
Accessing eligible children.
Both door-to-door distribution and facility-based fixed-point distribution, which leverages existing clinic staff and health facility infrastructure, have been studied for SMC delivery. Door-to-door distribution of SMC was found to reach more children and have higher coverage than fixed-point distribution strategies.41–44 One study reported that coverage with door-to-door distribution reached 76% of eligible children whereas facility-based distribution reached only 62%.45 Even when facility-based teams traveled to increase accessibility, CHW teams reached 74% of eligible children compared with 48% reached by a clinic trekking team. This difference in coverage was attributed to CHWs remaining accessible to communities throughout the month, while trekking teams visited villages only on select days.46 Another study reported that CHWs were able to reach children in more remote areas than facility-based teams, with CHWs traveling 190 km each month compared with 137 km traveled by facility-based teams.42 In many studies, caregivers preferred the convenience of door-to-door delivery, which did not take time away from daily tasks.17,42 Current SMC programs use the door-to-door approach.
Regardless of distribution strategy, SMC programs had difficulties reaching children and infants living far from central distribution sites or health centers and during rainy seasons when road conditions were poor.16,22,29 Absenteeism, i.e., children or caregivers not being home during a visit, was also a challenge for SMC.13,32,47–49 In communities where rainy season and farming season coincided, these issues were compounded and dramatically reduced coverage during later SMC cycles.44,49 To address this challenge, one study delivered SMC on weekends, when parents were less likely to be working.50 In many reports, bicycles, motorbikes, and drivers were viewed as essential for reaching eligible children in future campaigns.22,30,46,48
Difficulties related to travel and caregiver absenteeism were also a challenge for IPTi programs.20,26,36 One study in Ghana estimated that 25% of the eligible population was not reached by EPI clinics during the rainy season.20 Unlike with SMC, which often delivers chemoprevention to children’s homes, IPTi is distributed largely through fixed-point distribution at health facilities, putting the burden of travel on caregivers. For some caregivers, time spent traveling to the clinic, long wait times, and overcrowded waiting rooms discouraged clinic attendance.35,51 However, some mothers viewed clinic visits as important social opportunities, which motivated attendance.27,35 Co-delivery of IPTi with EPI leveraged the established vaccination visit for chemoprevention delivery; however, missed opportunities, where infants received vaccinations but not IPTi, were noted.26,33 One study estimated that 30% of eligible children receiving a vaccine did not receive IPTi.52 Reasons for these missed opportunities were unclear, but possibilities include vaccination outreach campaigns that did not include IPTi, unavailability of SP (stock-out), or parental acceptance of IPTi.
Supply availability.
While SP plus amodiaquine (AQ) and SP alone were the most common chemoprevention drug regimens, several other drug regimens were used (Supplemental Appendix B).
Estimating supply needs prior to implementation was a challenge for both SMC and IPTi programs, particularly in areas with outdated census information or nomadic populations.18,33 One SMC study noted that CHWs distributing door-to-door did not have reliable access to gloves or soap and water,22 resulting in sanitation concerns. Drug stockouts were a commonly identified problem for both SMC17–19,31 and IPTi programs,27,37 with reports of 10%37 and 24%52 of facilities experiencing IPTi drug stockouts. IPTi studies also noted that the limited access to clean water also made dosing smaller children, who required pills to be crushed in water, difficult,26,33 and two IPTi studies reported that caregivers viewed medication administration using shared cups or spoons as unhygienic.27,35 ITPsc studies were limited and did not describe supply challenges.
Correct dosing.
Eligibility for SMC is based on the child’s age, with most programs limiting eligibility to children 3 to 59 months old. Determining which children were eligible was difficult when caregivers were unsure of a child’s age.16,22 This uncertainty led to dosing ineligible children.24,44 CHWs had an advantage over clinic staff, as they often knew the ages of the children in their community.18 Among eligible children, drugs were frequently administered by age, not by weight,20,41 potentially resulting in over- or underdosing of children. Notably, operationalizing weight-based dosing would require additional equipment and training for community staff and was not described outside of the study setting.
Directly observed therapy (DOT) was common for the first dose of an SMC drug course; however, DOT was not typically feasible for all three doses,41 and the second and third doses were left at home to be administered by the caregivers. During DOT, if a child vomited the initial dose, drugs were readministered. Caregivers, however, did not have surplus drugs to readminister if the child vomited after the second or third doses.41 Other children did not receive their subsequent doses because parents forgot, did not have time, or were not home or the doses were lost or intentionally saved in case of future illness.23,41 CHWs estimated that based on the number of tablets remaining during household visits on the third day of each monthly SMC dose, 10% to 20% of caregivers did not give the second and third doses.48 In one study, caregivers self-reported giving subsequent doses only 35% of the time despite 93.6% reporting that CHWs left subsequent doses and 86.8% reporting that they knew how to give SMC correctly.24
Similar adherence challenges were seen with IPTi in studies where SP was given with amodiaquine or artesunate.27,35 A pharmacological study estimated that three-dose coverage for IPTi was 39% to 50%,53 and in another study, 15% of mothers reported not giving the subsequent doses because they forgot or thought it was a fever reducer to be given as needed.54
Acceptability.
Overall, acceptability of chemoprevention interventions was high. Caregivers and community members primarily viewed SMC as an effective and safe way to protect their children from malaria.26,32,41,55 Caregivers reported that clinic staff and CHWs were knowledgeable about SMC and could be trusted.17,19,56 For delivery of SMC, caregivers preferred CHWs over clinic staff.28,42,57 This preference was attributed to the trust established by existing relationships with the community members, shared customs and beliefs, and the proximity of CHWs in comparison with that of clinic staff.17,21,42,57
Barriers to SMC acceptability included concerns about side effects,25,30,41 fears that drugs were expired or dangerous,8 and general apprehension about accepting free drugs.17,49 These challenges were mediated by CHWs, who clearly explained the function of the medicine and described common side effects.13,22,25,49 Sensitization campaigns, education during drug distribution, and endorsements from community leaders were key strategies for promoting SMC acceptability.28,32,41,49 These strategies were developed in partnership with community members, as local contexts influenced their success. For example, one study in Ghana noted that political opinions resulted in unwillingness to participate in community activities that were endorsed by opposition leadership.14 Beyond the caregivers’ initial acceptance of SMC, several early studies noted children spitting out doses due to the bitter taste of AQ, which inconvenienced drug distributors and impacted caregivers’ willingness to give second and third doses.25,49,58 Caregivers and staff also noted that crushing and dissolving pills took a significant amount of time.26,30,34,35,38,47 A sweetened dispersible formulation was approved by the Global Fund Expert Review Panel for Pharmaceutical Products in February 2016. By 2017, all SMC programs used the sweetened, dispersible SP+AQ formulation. One study from Nigeria reported that this formulation was more acceptable, did not require crushing, and was less frequently spat out by children.17
Similar findings were reported for IPTi. Overall, caregivers understood the purpose of IPTi drugs as malaria prevention, which resulted in high acceptance.27,34,35,54,59 Barriers included caregivers’ concerns with accepting free drugs32 and fear of side effects, which reduced maternal interest in IPTi.27 Misunderstandings also reduced uptake. The most common misunderstanding was the belief among caregivers that the drugs were postvaccination fever reducers that did not need to be given to nonfebrile children.27,34,54 Other studies reported community members spreading rumors that blood samples taken during studies were being used for nonstudy purposes.54,59 Researchers emphasized the need for sensitization and educational campaigns to counteract misinformation and promote interest in IPTi. Specific strategies included soliciting endorsements from opinion leaders17 and creating posters33,56 to promote IPTi.
Delivery of IPTi through routine health services resulted in higher acceptability, as the intervention was seen as part of established community norms or parental responsibilities.27,41,56,59 The coadministration of chemoprevention alongside vaccines was also shown to increase vaccination coverage.33,34,60 Researchers suggested that the additional benefit of IPTi further incentivized mothers to return for their routine vaccine visits. Staff acceptance of the interventions was also high, with studies reporting positive perceptions of IPTi, with staff noting benefits including improved compliance with EPI and reduced malaria cases.26,33,34 In several studies, the power dynamic between caregivers and clinic staff and social pressures influenced IPTi uptake. Some caregivers were motivated by fear of disapproval, social consequences, or reduced access to future health facility services should they decline IPTi.27,34,35,56 Two studies reported that healthcare staff had been rude54 or had publicly reprimanded caregivers.35
Unlike SMC, which was an established intervention, and IPTi, which was associated with trusted EPI activities, IPTsc studies reported challenges with parental buy-in with the new program. Opinions about IPTsc varied widely, and although parents appreciated that the intervention reached many children in the community, others were concerned that drugs could be expired or have negative side effects.11,61 Some parents did not believe that teachers should be distributing drugs because they were not health care professionals.11,39 Communication was also a challenge with IPTsc. Many parents missed informational sessions and signed informed consents sent home with students without having a clear understanding of the intervention.11 One study leveraged political leaders to inform the public at community gatherings to improve awareness.40 Another study used extensive screening protocols to ensure understanding, but these efforts reduced IPTsc coverage by excluding students when guardians could not be contacted.61 Such exhaustive measures may not be feasible outside of a study setting.
Health equity, equality, and nondiscrimination.
Eighteen articles discussed health equity, equality, and nondiscrimination. Seasonal malaria chemoprevention has been shown to be equitable with regards to socioeconomic status, with an ACCESS-SMC Partnership study noting that “door-to-door distribution was successful in reaching the poorest in the community.”31 Door-to-door coverage in The Gambia was also found to achieve high coverage.44
Although one study found no association between wealth and child’s receipt of IPTi,33 another study reported that IPTi delivered through the EPI program had the potential to “cluster coverage of interventions among certain groups, while others receive nothing, thereby increasing inequalities.”36 Although the interventions were free, travel distance, weather, family support systems, and farming activities affected accessibility. In some areas, the EPI clinics furthered inequities, with caregivers being given differential treatment both from the clinic staff and from peers based on how they dressed for appointments or perceived compliance with staff recommendations. In one study, clinic visits were an opportunity to dress up and socialize, which improved attendance; however, poorly dressed mothers could face discrimination by clinic staff and peer disapproval, which led to clinic avoidance and exacerbated existing inequities based on income.35 In another, it was reported that mothers feared treatment denial due to lack of up-to-date vaccination records, when clinic staff asked to see children’s health cards prior to treatment to confirm age and weight. This led some mothers to falsify dates on their baby’s health cards to allow them to continue to receive treatment at the clinics.56
Ensuring equitable access to chemoprevention across a community was particularly challenging for IPTsc, as wealth often affected school attendance. Several research studies noted that low school attendance and poor enrollment resulted in high loss to follow-up during the study period.11,61 One study addressed absenteeism by staffing two CHWs whose main role was to find and dose children who missed school on the day of administration, thus reducing the equity gap.40 A second limitation of IPTsc was establishing eligibility criteria. Because many antimalarial drugs are not recommended in the first trimester of pregnancy, girls post-menarche were excluded from studies.61 Although it was operationally easier to exclude all girls of reproductive age, it denied them the benefits of IPTsc and unintentionally “raised suspicion in the community regarding the reasons for excluding older girls.”61
Financial and economic considerations.
Initial costs for chemoprevention programs were high, regardless of the intervention. Once programs were established, drug costs and the mode of delivery were critical factors in the cost analysis.
Seasonal malaria chemoprevention costs varied based on whether CHWs or nurses were responsible for drug delivery and whether door-to-door or fixed-point delivery was used. Supervision costs for SMC were lower with fixed-point delivery using clinic staff, but estimated drug administration costs were higher based on the average hourly nursing wage and opportunity costs, as nurses spent time away from other responsibilities to dispense SMC.42 The cumulative cost for SMC delivered door-to-door by CHWs or village health workers was estimated to be lower.42 Furthermore, expanding SMC to include broader age groups resulted in cost reduction per dose distributed in both Senegal and Ghana.50,62,63 Modeling studies have demonstrated similar cost benefits.62
When individual-level costs are evaluated, SMC studies reported frequent challenges with the economic burden put on CHWs. Although CHWs were compensated for implementing SMC during research studies,50 compensation was not typical outside of the research context. Delays in payments, lack of incentives, rigid reimbursement processes,26,32,48 and CHWs forgoing personal responsibilities, like farming, to distribute SMC added to the financial burden of SMC programs on individual CHWs.
Unlike SMC, which most frequently requires additional staff for door-to-door delivery or additional clinic visits for administration, IPTi leverages existing touchpoints with infants, which can reduce costs. A pooled analysis by Conteh et al. reviewing IPTi delivered via EPI reported health system cost savings or no increase in health system costs associated with integrating IPTi with EPI.62 Other studies that evaluated IPTi labor costs noted that after initial training, implementation of IPTi did not add additional labor expenses, as IPTi was incorporated into the existing nurses’ schedules without incurring overtime.35,60 One study assessing IPTi implementation separated the costs for developing a health system that included ministry buy-in and policy change from the costs for implementation, including behavior change, sensitization within the community, training, and drug procurement, and found that training and drug expenses drove up implementation costs.64
With IPTsc, human resources were the leading drivers of cost. Although there were initial training and labor costs with all interventions, cost savings were assumed in subsequent years, notably if the same teachers continued to distribute drugs, as they did not require extended retrainings.65 Teachers were paid per diem for delivering chemoprevention during the study period.59
For all chemoprevention programs, intervention costs varied substantially based on the treatment regimen selected. Although data are available regarding drug costs, the wide variability in treatment options and courses across the eligible studies did not allow for general synthesis of data for financial and economic considerations. Other country-specific costs associated with drug acquisition that should be considered include customs, clearance, warehousing, and distribution costs.66
One study looked at costs associated with implementing digital data collection platforms with SMC. Although initial implementation of a digital platform was costly, implementing countries felt that cost savings could be achieved over time.25 Costs associated with launching a digital platform differed based on the availability and cost of internet and the decision to purchase devices or to ask workers to download an application on personal devices. No studies in this review discussed costs for digital data collection for IPTi or IPTsc.
Values and preferences.
There are limited data on values and preferences among all chemoprevention interventions. A total of four articles discussed values and preferences. One qualitative study of SMC conducted in Burkina Faso and Mali13 noted the importance of child health within the community. Seasonal malaria chemoprevention was easily adopted into their existing health care practices. Another article discussed values in Niger that influence caregivers’ perceptions of SMC, including competing priorities with the caregivers’ “life philosophy, their personal effectiveness/perceived capacity, and their history of understanding malaria’s risk.”23 Similarly, a study looking at the community response to IPTi integration with the EPI noted that IPTi was accepted, as it aligned with existing practices of enhancing the child’s health.35 A study looking at IPTsc done in the Democratic Republic of Congo noted that parents preferred to use medications for clinical treatment than for prevention.11
DISCUSSION
Understanding contextual factors is vital to the successful implementation of malaria chemoprevention strategies. Most available studies with information on these factors focused on SMC, followed by IPTi, with very little on IPTsc; however, lessons learned irrespective of the target group may be helpful in informing the expansion and optimization of existing initiatives or establishment of new programs.
The feasibility of SMC was affected primarily by staffing, mode of delivery, and drug availability. Community health workers were generally preferred by caregivers over health facility staff, as they often had established relationships with community members and were conveniently located within the community.42,57 Although these CHWs found their work satisfying during studies when they received timely compensation, they often reported challenges during routine campaigns when experiencing payment delays, having to fund their own transportation, and forgoing their farm work, which they found demoralizing.16,19,26,30 The call for sustainable funding for CHWs is not new, and commitments to stable and reliable financial support for CHW-staffed interventions are needed to ensure the continued success of chemoprevention programs.67,68
Stockouts were a common challenge during SMC implementation and were attributed to inaccurate census data, population migration, and incorrect assessment of children’s ages.18,22,33 Solutions to address stockouts include strengthening supply chain communication and procuring adequate stock for SMC implementation. For example, a study in Kenya piloted a mobile phone text messaging program that used a Web-based tool to report real-time stock data,69 whereas Guinea enhanced routine collection of malaria commodities using a monthly routine malaria information system.67 Both methods were successful in reducing stockouts.
SMC studies also reported challenges with determining appropriate dosing for children, particularly when caregivers were unsure of the child’s age.16,22 Utilizing CHWs who lived in the community and knew the children18 helped address this issue. Unfortunately, using age instead of weight for dosing20,41 can lead to over- or underdosing children. Consideration for using a measuring stick or “dose pole” designed for malaria, similar to those used to determine drug dosages for neglected tropical diseases70 may mitigate this issue in the future.
Adherence, especially caregivers’ adherence to the second and third doses of three-dose drug regimens, was low in multiple studies.27,35,41,53,54 A systematic review by Bruxvoort et al. identified factors associated with nonadherence to antimalarials prescribed for treatment that translate in chemoprevention, including language barriers between caregivers and pharmacists, caregiver education level, potential side effects, and factors related to the consultation (not understanding the instructions, being counseled on what to do in case of side effects, choice of medication).71 Although articles identified challenges with adherence and some factors associated with low adherence, few strategies beyond improved education during consultations were presented to address this challenge. No strategies were systematically evaluated. Additional research is needed to develop and assess strategies to promote caregiver compliance with administration of subsequent doses at home.
Studies of SMC reported staffing challenges, caregiver absenteeism, and low compliance with administration of second and third doses as key factors impacting SMC feasibility. Although some studies reported that nurses complained that IPTi duties took time away from regular work tasks,34 or felt they should not be responsible for administering IPTi when not trained to do so,35,37 other studies reported that nurses successfully incorporated IPTi duties into their existing workloads without incurring overtime or requiring additional compensation.37 Standardized training for all nurses on IPTi, either by including it in the nursing school curriculum or as part of onboarding training, could change perceptions of IPTi as a routine EPI task, thereby improving nurses’ willingness to dedicate time to implement IPTi and reducing recurrent training costs. Challenges with absenteeism to the clinics may be addressed by integrating door-to-door delivery strategies into IPTi programs. The high coverage achieved through door-to-door delivery with SMC programs suggests that CHWs can successfully reach a large proportion of children in a community and could similarly reach difficult-to-reach infants who are not able to use the EPI platform.45 A hybrid strategy in which CHWs complete follow-up visits with caregivers who miss an IPTi visit, as was done with children who were absent during IPTsc administration, may be a potential solution.40 The downside to this would be the increased workload to the CHWs.
Community acceptability of SMC26,32,55 and IPTi27,34,35,54,59 was high. Barriers to acceptance were largely centered around misinformation and hygiene concerns when drugs were crushed and dissolved for administration. Sensitization campaigns and consultations with trusted health care providers were successful in combating misinformation.22,25,49 In one study, the use of dispersible drugs, which do not require crushing pills and are generally formulated to taste better, enhanced both acceptability and feasibility.17
Fewer studies discussed IPTsc acceptability, but the issues presented in the available literature differed from those seen with SMC and IPTi. With SMC and IPTi, caregivers perceived CHWs and clinic staff to be competent in chemoprevention administration, but for IPTsc, parents had mixed opinions about teachers providing drugs to school children.11,39 Parental awareness of chemoprevention programs and buy-in were also lower with IPTsc, and the negative impact of excluding girls of childbearing age was unique to IPTsc.61 As a newer intervention, additional resources are needed to introduce IPTsc to communities to improve acceptability. Lessons learned from SMC and IPTi programs, including best practices for community announcements, educational campaigns, and community-tailored advertising, can help inform IPTsc sensitization strategies. For all interventions, stakeholder involvement at the initial stages of program implementation ensured community engagement and provided an opportunity to address misinformation early on, increasing intervention acceptance. Given the importance of men as household decision makers,13,72 community engagement should reach both men and women. Power dynamics were noted with IPTi, where caregivers complied with the interventions for fear of repercussions from health care workers,27,34,35,54,56 so efforts should be placed towards enhancing caregiver-provider relationships.
Regarding equity, SMC with door-to-door coverage was found to be the most equitable, as it reached children in rural and remote areas.29,44 Although facility- and school-based IPTi and IPTsc interventions are reliable, and efficiently leverage existing touchpoints, a limitation of these delivery systems is that they depend on caregivers visiting health facilities and students attending school, which may be difficult, particularly for remote or lower-resource populations.36 With IPTsc, absenteeism, dropouts, and low enrollment in some populations could result in missing children who might be at greatest risk for malaria and poor outcomes, thus most likely to benefit from chemoprevention.11,61 An inequity specific to IPTsc is the exclusion of girls of reproductive age if pregnancy cannot be ruled out, because most antimalarials are not recommended for chemoprevention in pregnancy,21 particularly in the first trimester. This inequity could be addressed through further research into drugs that are safe and effective for chemoprevention including in the first trimester.40
The major financial drivers for all interventions were drug and delivery costs and human resources. Data on drug costs could not be summarized because of the variability in treatment options and courses in the studies reviewed. In one study, door-to-door delivery of SMC by CHWs had the lowest cost in comparison to delivery at outpatient departments or EPI outreach clinics in Ghana. The main cost driver for delivery by CHWs was supervision, which was impacted by the cost of traveling to sites. Supervision costs were lower with facility-based distribution, but coverage was lower, resulting in a higher per-dose cost.42 In another study, additional per-dose cost savings were realized when eligibility was expanded to include older children.63 One study evaluated costs for digital data collection with SMC and noted that although the initial implementation of a digital platform is costly, cost savings could be achieved over time.25 At initial IPTi implementation, the cost drivers were largely from community sensitization, policy change, training, and drug acquisition61 but once IPTi was integrated with EPI, there was no reported increase in health system costs.62 Intervention costs of IPTsc varied based on the treatment regimen selected. A recent systematic literature review on cost and cost effectiveness of malaria control interventions completed by Conteh et al. had similar findings, where the largest costs for SMC were reported as training, supervision, and distribution costs and those for IPTi were drug costs, training, and education/communication activities.73 The review did not report on IPTsc.
In conclusion, this review shows that contextual factors have a significant impact on chemoprevention sustainability and should be taken into consideration during implementation and to optimize existing programs. The findings of this review can also be used to inform IPTsc implementation and expansion. Although the review looked primarily at chemoprevention, the contextual factors can also be considered during implementation of other malaria interventions.
Limitations.
Although there is extensive research on SMC efficacy, assessing contextual factors with the aim of improving implementation is a less frequent research objective. Literature describing contextual factors is predominantly from six countries, namely, Ghana, Tanzania, Nigeria, Senegal, Burkina Faso, and Mali, limiting its generalizability and applicability elsewhere. As IPTsc is not widely implemented, there were no operational evaluations available for review. No publications in this review discussed IPTsc outside a study context. Similarly, although IPTi has been implemented in Sierra Leone, the available literature largely discusses the intervention within the context of research studies. Implementation of IPTsc and IPTi outside of a study context may differ significantly. For all interventions, studies discussing the values and preferences of the communities were limited. Where data is available, social desirability bias may have influenced participants’ responses. Cost estimates were also difficult to compare or summarize across different countries and different programs because of what is assumed or included in the cost. Finally, although efforts were made to capture all literature, including gray literature, unpublished research or titles not captured in initial searches may have been missed.
Supplemental files
Note: Supplemental material appears at www.ajtmh.org.
REFERENCES
- 1. WHO , 2022. World Malaria Report 2022. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 2. WHO , 2021. Global Technical Strategy for Malaria 2016–2030, 2021 Update. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 3. WHO , 2023. WHO Guidelines for Malaria, March 14, 2023. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 4. Cairns M. et al. , 2021. Effectiveness of seasonal malaria chemoprevention (SMC) treatments when SMC is implemented at scale: case-control studies in 5 countries. PLoS Med 18: e1003727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bakai TA, Thomas A, Iwaz J, Atcha-Oubou T, Tchadjobo T, Nagham K, Rabilloud M, Voirin N, 2022. Effectiveness of seasonal malaria chemoprevention in three regions of Togo: a population-based longitudinal study from 2013 to 2020. Malar J 21: 400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Manga IA. et al. , 2022. Effectiveness of seasonal malaria chemoprevention administered in a mass campaign in the Kedougou region of Senegal in 2016: a case-control study. Wellcome Open Res 7: 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thwing J, Williamson J, Cavros I, Bhattar A, Gutman J, 2022. Seasonal Malaria Chemoprevention for Malaria in Children in Areas with Seasonal Malaria. Available at: https://zenodo.org/record/6535577. Accessed May 22, 2023.
- 8. Esu EB, Oringanje C, Meremikwu MM, 2021. Intermittent preventive treatment for malaria in infants. Cochrane Database Syst Rev 7: CD011525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cairns M, Carneiro I, Milligan P, Owusu-Agyei S, Awine T, Gosling R, Greenwood B, Chandramohan D, 2008. Duration of protection against malaria and anaemia provided by intermittent preventive treatment in infants in Navrongo, Ghana. PLoS One 3: e2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aponte JJ. et al. , 2009. Efficacy and safety of intermittent preventive treatment with sulfadoxine-pyrimethamine for malaria in African infants: a pooled analysis of six randomised, placebo-controlled trials. Lancet 374: 1533–1542. [DOI] [PubMed] [Google Scholar]
- 11. Matangila JR, Fraeyman J, Mbula Kambulu ML, Mpanya A, Inocêncio da Luz R, Lutumba P, Van Geertruyden JP, Bastiaens H, 2017. The perception of parents and teachers about intermittent preventive treatment for malaria in school children in a semi-rural area of Kinshasa, in the Democratic Republic of Congo. Malar J 16: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rehfuess EA, Stratil JM, Scheel IB, Portela A, Norris SL, Baltussen R, 2019. The WHO-INTEGRATE evidence to decision framework version 1.0: integrating WHO norms and values and a complexity perspective. BMJ Glob Health 4 (Suppl 1): e000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pitt C. et al. , 2012. Intermittent preventive treatment of malaria in children: a qualitative study of community perceptions and recommendations in Burkina Faso and Mali. PLoS One 7: e32900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ahorlu CK, Koram KA, Seake-Kwawu A, Weiss MG, 2011. Two-year evaluation of intermittent preventive treatment for children (IPTc) combined with timely home treatment for malaria control in Ghana. Malar J 10: 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bicaba A, Serme L, Chetaille G, Kombate G, Bila A, Haddad S, 2020. Longitudinal analysis of the capacities of community health workers mobilized for seasonal malaria chemoprevention in Burkina Faso. Malar J 19: 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Compaoré R, Yameogo MWE, Millogo T, Tougri H, Kouanda S, 2017. Evaluation of the implementation fidelity of the seasonal malaria chemoprevention intervention in Kaya health district, Burkina Faso. PLoS One 12: e0187460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ogbulafor N. et al. , 2023. Facilitators and barriers to seasonal malaria chemoprevention (SMC) uptake in Nigeria: a qualitative approach. Malar J 22: 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moukénet A, Donovan L, Honoré B, Baker K, Smith H, Richardson S, Ward C, 2022. Extending delivery of seasonal malaria chemoprevention to children aged 5–10 years in Chad: a mixed-methods study. Glob Health Sci Pract 10: e2100161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tine RCK. et al. , 2013. Acceptability by community health workers in Senegal of combining community case management of malaria and seasonal malaria chemoprevention. Malar J 12: 467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kweku M, Webster J, Adjuik M, Abudey S, Greenwood B, Chandramohan D, 2009. Options for the delivery of intermittent preventive treatment for malaria to children: a community randomised trial. PLoS One 4: e7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Okereke E. et al. , 2023. Optimizing the role of ‘lead mothers’ in seasonal malaria chemoprevention (SMC) campaigns: formative research in Kano State, northern Nigeria. Malar J 22: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kombate G, Guiella G, Baya B, Serme L, Bila A, Haddad S, Bicaba A, 2019. Analysis of the quality of seasonal malaria chemoprevention provided by community health workers in Boulsa health district, Burkina Faso. BMC Health Serv Res 19: 472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Koko DC, Maazou A, Jackou H, Eddis C, 2022. Analysis of attitudes and practices influencing adherence to seasonal malaria chemoprevention in children under 5 years of age in the Dosso Region of Niger. Malar J 21: 375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Oresanya OB, Ahmadu A, Adesoro O, Maranda L, Morosso D, Maxwell K, 2019. An assessment of quality of delivery of seasonal malaria chemoprevention using low literate community health workers in Nigeria. Am J Trop Med Hyg 101 ( Suppl ): 119–120. [Google Scholar]
- 25. Balla K. et al. , 2022. Introducing field digital data collection systems into seasonal malaria chemoprevention campaigns: opportunities for robust evidence development and national e-health strategies. BMJ Glob Health 7: e007899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Audibert C, Tchouatieu AM, 2021. Perception of malaria chemoprevention interventions in infants and children in eight sub-Saharan African countries: an end user perspective study. Trop Med Infect Dis 6: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pool R, Mushi A, Schellenberg JA, Mrisho M, Alonso P, Montgomery C, Tanner M, Mshinda H, Schellenberg D, 2008. The acceptability of intermittent preventive treatment of malaria in infants (IPTi) delivered through the expanded programme of immunization in southern Tanzania. Malar J 7: 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Strachan CE. et al. , 2016. The use of formative research to inform the design of a seasonal malaria chemoprevention intervention in northern Nigeria. Malar J 15: 474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bonkoungou M. et al. , 2018. Seasonal malaria chemoprevention, an effective intervention for reducing malaria morbidity and mortality. Am J Trop Med Hyg 99 ( Suppl ): 663. [Google Scholar]
- 30. Traore A. et al. , 2022. Extending seasonal malaria chemoprevention to five cycles: a pilot study of feasibility and acceptability in Mangodara district, Burkina Faso. BMC Public Health 22: 442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. ACCESS-SMC Partnership , 2020. Effectiveness of seasonal malaria chemoprevention at scale in west and central Africa: an observational study. Lancet 396: 1829–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Oresanya O. et al. , 2022. Co-implementing vitamin A supplementation with seasonal malaria chemoprevention in Sokoto State, Nigeria: a feasibility and acceptability study. BMC Health Serv Res 22: 871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lahuerta M. et al. , 2021. Evaluation of health system readiness and coverage of intermittent preventive treatment of malaria in infants (IPTi) in Kambia district to inform national scale-up in Sierra Leone. Malar J 20: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. de Sousa A. et al. , 2012. Acceptability of coupling intermittent preventive treatment in infants with the expanded programme on immunization in three francophone countries in Africa. Trop Med Int Health TM IH 17: 308–315. [DOI] [PubMed] [Google Scholar]
- 35. Gysels M. et al. , 2009. Community response to intermittent preventive treatment of malaria in infants (IPTi) delivered through the expanded programme of immunization in five African settings. Malar J 8: 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chandramohan D, Webster J, Smith L, Awine T, Owusu-Agyei S, Carneiro I, 2007. Is the Expanded Programme on Immunisation the most appropriate delivery system for intermittent preventive treatment of malaria in West Africa? Trop Med Int Health TM IH 12: 743–750. [DOI] [PubMed] [Google Scholar]
- 37. Manzi F. et al. , 2009. Intermittent preventive treatment for malaria and anaemia control in Tanzanian infants; the development and implementation of a public health strategy. Trans R Soc Trop Med Hyg 103: 79–86. [DOI] [PubMed] [Google Scholar]
- 38. de Sousa A, Salama P, Chopra M, 2010. Implementing intermittent preventive treatment in infants. Lancet 375: 121. [DOI] [PubMed] [Google Scholar]
- 39. Temperley M, Mueller DH, Njagi JK, Akhwale W, Clarke SE, Jukes MCH, Estambale BBA, Brooker S, 2008. Costs and cost-effectiveness of delivering intermittent preventive treatment through schools in western Kenya. Malar J 7: 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Makenga G. et al. , 2023. Implementation research of a cluster randomized trial evaluating the implementation and effectiveness of intermittent preventive treatment for malaria using dihydroartemisinin-piperaquine on reducing malaria burden in school-aged children in Tanzania: methodology, challenges, and mitigation. Malar J 22: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bâ E-H. et al. , 2018. Implementation, coverage and equity of large-scale door-to-door delivery of seasonal malaria chemoprevention (SMC) to children under 10 in Senegal. Sci Rep 8: 5489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Patouillard E, Conteh L, Webster J, Kweku M, Chandramohan D, Greenwood B, 2011. Coverage, adherence and costs of intermittent preventive treatment of malaria in children employing different delivery strategies in Jasikan, Ghana. PLoS One 6: e24871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Barry A. et al. , 2018. Optimal mode for delivery of seasonal malaria chemoprevention in Ouelessebougou, Mali: a cluster randomized trial. PLoS One 13: e0193296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ceesay S. et al. , 2016. Implementation of seasonal malaria chemoprevention in the Gambia. Am J Trop Med Hyg 95 ( Suppl ): 576. [Google Scholar]
- 45. Issiaka D. et al. , 2015. Determining the optimal mode for delivery of seasonal malaria chemoprevention in Mali. Am J Trop Med Hyg 93 ( Suppl ): 496. [Google Scholar]
- 46. Bojang KA, Akor F, Conteh L, Webb E, Bittaye O, Conway DJ, Jasseh M, Wiseman V, Milligan PJ, Greenwood B, 2011. Two strategies for the delivery of IPTc in an area of seasonal malaria transmission in The Gambia: a randomised controlled trial. PLoS Med 8: e1000409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Druetz T, Corneau-Tremblay N, Millogo T, Kouanda S, Ly A, Bicaba A, Haddad S, 2018. Impact evaluation of seasonal malaria chemoprevention under routine program implementation: a quasi-experimental study in Burkina Faso. Am J Trop Med Hyg 98: 524–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Antwi CD, Bates LA, King R, Mahama PR, Tagbor H, Cairns M, Newell JN, 2016. Facilitators and barriers to uptake of an extended seasonal malaria chemoprevention programme in Ghana: a qualitative study of caregivers and community health workers. PLoS One 11: e0166951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chatio S, Ansah NA, Awuni DA, Oduro A, Ansah PO, 2019. Community acceptability of seasonal malaria chemoprevention of morbidity and mortality in young children: a qualitative study in the Upper West Region of Ghana. PLoS One 14: e0216486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pitt C, Ndiaye M, Conteh L, Sy O, Hadj Ba E, Cissé B, Gomis JF, Gaye O, Ndiaye JL, Milligan PJ, 2017. Large-scale delivery of seasonal malaria chemoprevention to children under 10 in Senegal: an economic analysis. Health Policy Plan 32: 1256–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Konaté D. et al. , 2021. Effectiveness and community acceptance of extending seasonal malaria chemoprevention to children 5 to 14 years of age in Dangassa, Mali. Am J Trop Med Hyg 106: 648–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Armstrong Schellenberg JRM, Shirima K, Maokola W, Manzi F, Mrisho M, Mushi A, Mshinda H, Alonso P, Tanner M, Schellenberg DM, 2010. Community effectiveness of intermittent preventive treatment for infants (IPTi) in rural southern Tanzania. Am J Trop Med Hyg 82: 772–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sottas O, Guidi M, Thieffry B, Schneider M, De’costerd L, Mueller I, Genton B, Csajka C, Senn N, 2019. Adherence to intermittent preventive treatment for malaria in Papua New Guinean infants: a pharmacological study alongside the randomized controlled trial. PLoS One 14: e0210789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pell C, Straus L, Phuanukoonnon S, Lupiwa S, Mueller I, Senn N, Siba P, Gysels M, Pool R, 2010. Community response to intermittent preventive treatment of malaria in infants (IPTi) in Papua New Guinea. Malar J 9: 369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ansah NA, Malm K, Chatio ST, Ansah P, Tampuulo S, Awuni D, Oduro AR, 2016. Evaluation of the impact of implementation of seasonal malaria chemoprevention on morbidity and mortality in young children: a qualitative study in Northern Ghana. Am J Trop Med Hyg 95 ( Suppl ): 131. [Google Scholar]
- 56. Mushi AK. et al. , 2008. Development of behaviour change communication strategy for a vaccination-linked malaria control tool in southern Tanzania. Malar J 7: 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kpormegbe SK, Ahorlu CK, 2014. The role of community participation in intermittent preventive treatment of childhood malaria in southeastern Ghana. Ghana Med J 48: 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ndiaye JLA. et al. , 2019. Seasonal malaria chemoprevention combined with community case management of malaria in children under 10 years of age, over 5 months, in south-east Senegal: a cluster-randomised trial. PLoS Med 16: e1002762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pool R, Munguambe K, Macete E, Aide P, Juma G, Alonso P, Menendez C, 2006. Community response to intermittent preventive treatment delivered to infants (IPTi) through the EPI system in Manhiça, Mozambique. Trop Med Int Health 11: 1670–1678. [DOI] [PubMed] [Google Scholar]
- 60. Dicko A. et al. , 2011. Increase in EPI vaccines coverage after implementation of intermittent preventive treatment of malaria in infant with sulfadoxine-pyrimethamine in the district of Kolokani, Mali: results from a cluster randomized control trial. BMC Public Health 11: 573–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Staedke S. et al. , 2018. Assessment of community-level effects of intermittent preventive treatment for malaria in schoolchildren in Jinja, Uganda (START-IPT trial): a cluster-randomised trial. Lancet Glob Health 6: e668–e679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Conteh L. et al. , 2010. The cost-effectiveness of intermittent preventive treatment for malaria in infants in Sub-Saharan Africa. PLoS One 5: e10313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Nonvignon J, Aryeetey GC, Issah S, Ansah P, Malm K, Ofosu W, Tagoe T, Agyemang SA, Aikins M, 2016. Cost-effectiveness of seasonal malaria chemoprevention in upper west region of Ghana. Malar J 15: 367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Manzi F, Hutton G, Schellenberg J, Tanner M, Alonso P, Mshinda H, Schellenberg D, 2008. From strategy development to routine implementation: the cost of intermittent preventive treatment in infants for malaria control. BMC Health Serv Res 8: 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Maccario R. et al. , 2017. Cost analysis of a school-based comprehensive malaria program in primary schools in Sikasso region, Mali. BMC Public Health 17: 572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gilmartin C, Nonvignon J, Cairns M, Milligan P, Bocoum F, Winskill P, 2021. Seasonal malaria chemoprevention in the Sahel subregion of Africa: a cost-effectiveness and cost-savings analysis. Lancet Glob Health 9: e199–e208. [DOI] [PubMed] [Google Scholar]
- 67. Sun Y. et al. , 2018. Evaluating the quality of routinely reported data on malaria commodity stocks in Guinea, 2014–2016. Malar J 17: 461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Saint-Firmin PP, Diakite B, Ward K, Benard M, Stratton S, Ortiz C, Dutta A, Traore S, 2021. Community health worker program sustainability in Africa: evidence from costing, financing, and geospatial analyses in Mali. Glob Health Sci Pract 9 ( Suppl 1 ): S79–S97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Githinji S. et al. , 2013. Reducing stock-outs of life saving malaria commodities using mobile phone text-messaging: SMS for life study in Kenya. PLoS One 8: e54066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. USAID Neglected Tropical Disease Division , 2022. The Simple Tool Used to Determine Dosage when Treating for NTDs. Available at: https://www.neglecteddiseases.gov/the-simple-tool-used-to-determine-dosage-when-treating-for-ntds/. Accessed May 1, 2023.
- 71. Bruxvoort K, Goodman C, Kachur SP, Schellenberg D, 2014. How patients take malaria treatment: a systematic review of the literature on adherence to antimalarial drugs. PLoS One 9: e84555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Strachan CE. et al. , 2016. The use of formative research to inform the design of a seasonal malaria chemoprevention intervention in northern Nigeria. Malar J 15: 474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Conteh L, Shuford K, Agboraw E, Kont M, Kolaczinski J, Patouillard E, 2021. Costs and cost-effectiveness of malaria control interventions: a systematic literature review. Value Health 24: 1213–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.