ABSTRACT.
Prostatic abscess is a common manifestation of melioidosis in men, but the characteristics of female genitourinary melioidosis are incompletely defined. There were 453 cases of melioidosis in Far North Queensland, tropical Australia, between January 1998 and April 2023; genitourinary involvement was less common in women than in men (13/140 [9%] versus 76/313 [24%], odds ratio [95% confidence interval]: 0.32 [0.17–0.60], P = 0.0004). In 11 of these 13 (85%) women, other organs were also affected. The two women with disease involving only the genitourinary tract had underlying anatomical abnormalities: one had an ovarian malignancy, the only case to involve the female reproductive system in the cohort, while the other had a urethral diverticulum. In 3 of 13 (23%) women, genitourinary involvement was identified only with computed tomography, emphasizing the importance of early imaging of patients with melioidosis to identify unexpected foci of disease and to inform the optimal duration of antibiotic therapy.
Melioidosis is an opportunistic infection caused by the environmental, gram-negative bacterium Burkholderia pseudomallei. The organism is endemic to southeast Asia and northern Australia, although it is encountered throughout the tropics.1 Melioidosis most frequently presents as pneumonia, with or without bacteremia, but almost any organ can be involved.2 Genitourinary involvement occurs in approximately 12% of cases of melioidosis, with prostatic abscesses present in 15% to 21% of men with the disease.3–5 As men are more likely to have melioidosis, male genitourinary manifestations, particularly prostatic abscesses, are well described.4,5 However infections of the female genitourinary system are incompletely characterized.6 This study was therefore performed to describe the clinical features of female genitourinary melioidosis in an area of tropical Australia where melioidosis is endemic.
Cairns Hospital is a 531-bed tertiary-referral public hospital located in Far North Queensland, a region of 380,000 km2 in tropical Australia. The hospital serves a population of approximately 290,000 people, 17% of whom identify as Aboriginal or Torres Strait Islander Australians. The hospital’s laboratory is the sole public clinical microbiology provider for the region.
We reviewed all cases of culture-confirmed melioidosis identified at Cairns Hospital between January 1, 1998 and April 30, 2023; since October 2016, these data have been collected prospectively. Patient demographics, comorbidities, and their clinical courses were reviewed and defined as previously described.7 Women were said to have genitourinary involvement if they had infection of the urinary tract (kidney, ureter, bladder, or urethra) and/or female reproductive organs (cervix, vagina, uterus, or adnexa). Genitourinary infection was defined as growth of B. pseudomallei in urine or from another site (blood, sputum, or other body fluid) with concomitant radiological imaging suggesting genitourinary involvement.
Data were deidentified, entered into an electronic database (Microsoft Excel), and analyzed using statistical software (Stata version 14.2). Groups were compared using Fisher’s exact and the χ2 test and logistic regression as appropriate. The Far North Queensland Human Research Ethics Committee provided ethical approval for the study (HREC/15/QCH/46-977).
There were 453 cases of melioidosis during the study period, of which 140 (31%) occurred in women (Table 1). Of these 140 women, 112 (80%) had accessible computed tomography (CT) or ultrasound imaging of the genitourinary tract (104 had CT imaging and eight had ultrasound alone); 117 (84%) had accessible urine culture reports. Only 10/140 (7%) had neither imaging of the genitourinary tract nor urinary culture results available. The individuals missing these data were more likely to have presented before data collection was prospective (7/50 [14%] versus 3/90 [3%], P = 0.04).
Table 1.
Comparison of the characteristics of the women with melioidosis with and without genitourinary involvement
| Variable | With data* | Genitourinary tract involved (n = 13) | Genitourinary tract not involved (n = 127) | P |
|---|---|---|---|---|
| Age (years) | 140 | 53 (45–67) | 52 (41–63) | 0.61 |
| First Nations Australian | 140 | 7 (54) | 73 (57) | 1.0 |
| Acute presentation† | 140 | 10 (77) | 117 (92) | 0.10 |
| Wet season presentation | 140 | 7 (54) | 100 (79) | 0.08 |
| Imaging of the genitourinary tract | 112 | 13/112 (12) | 99/127 (88) | 0.07 |
| Urine collected for culture | 117 | 13/117 (11) | 104/127 (89) | 0.13 |
| Diabetes | 140 | 10 (77) | 62 (49) | 0.08 |
| Glycosylated hemoglobin (%) | 67 | 9.8 (9.6–12.6) | 10 (7.5–12.1) | 0.45 |
| Smoker | 128 | 8/13 (62) | 45/115 (39) | 0.14 |
| Hazardous alcohol consumption‡ | 130 | 3/13 (23) | 23/117 (20) | 0.72 |
| Chronic kidney disease | 133 | 2/13 (15) | 14/120 (12) | 0.66 |
| Chronic lung disease | 130 | 4/13 (31) | 24/117 (21) | 0.48 |
| Malignancy | 129 | 1/13 (8) | 11/116 (9) | 1.0 |
| Immunosuppression | 101 | 2/10 (20) | 16/91 (18) | 1.0 |
| No traditional risk factors for the disease§ | 140 | 1 (8) | 22 (17) | 0.69 |
| Bacteremic | 140 | 6 (46) | 78 (61) | 0.38 |
| Pulmonary involvement | 135 | 10/13 (77) | 77/122 (63) | 0.38 |
| Central nervous system involvement | 132 | 1 (8) | 4/119 (3) | 0.41 |
| Skin or soft tissue infection | 132 | 2 (15) | 24/119 (20) | 1.0 |
| Musculoskeletal | 132 | 5/13 (38) | 12/119 (10) | 0.01 |
| Any abscess | 121 | 7/12 (58) | 44/109 (40) | 0.36 |
| Septic shock | 125 | 2/12 (17) | 27/113 (24) | 0.73 |
| Intensive care unit admission | 140 | 4 (31) | 32 (25) | 0.74 |
| Died | 140 | 1 (8) | 16 (13) | 1.0 |
Numbers are the median (interquartile range) or absolute numbers (%).
Data were collected retrospectively prior to October 2016, resulting in incomplete data prior to this date.
Presentation within 2 months of symptom onset.3
As defined in Australian national guidelines.7
Traditional risk factors: diabetes mellitus, hazardous alcohol consumption, chronic lung disease, chronic kidney disease, immunosuppression, and active malignancy. Note that retrospective data collection of the 49 cases prior to October 2016 may have underestimated the prevalence of these risk factors prior to that date.
Across the entire cohort, women were less likely than men to have genitourinary involvement (13/140 [9%] versus 76/313 [24%], odds ratio [95% confidence interval]: 0.32 [0.17–0.60], P = 0.0004). The proportions of women with genitourinary involvement were similar before and after data were collected prospectively (5/50 [10%] versus 8/90 [9%], P = 1.0). There was nothing in the medical records or discharge summaries of the 10 women without genitourinary tract imaging or urine culture to suggest that they had genitourinary melioidosis.
Most women (11/13, 85%) with genitourinary melioidosis had other organs involved, most commonly the lungs (Table 2). The only two women with genitourinary melioidosis without other organ involvement had a predisposing anatomical abnormality, an ovarian malignancy in one and a urethral diverticulum in the other (Figure 1C and 1D). Diabetes mellitus was more common in women with genitourinary involvement, although the difference failed to reach statistical significance (10/13 [77%] versus 62/127 [49%], P = 0.08).
Table 2.
Detailed findings of the 13 women who had genitourinary melioidosis
| Case | Age (years) | Manifestation | Comorbidities | Presentation | Urinary symptoms | Bacteremia | Pyuria | Bacteriuria | Other organ involvement | ICU | Died | Impact on duration of therapy* |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 46 | Left pyelonephritis | Diabetes, chronic lung disease, hazardous alcohol use | Acute | Dysuria | Yes | > 500 leukocytes | No | Pulmonary, spleen, liver | Yes | Yes | No |
| 2 | 43 | Right kidney abscess | Diabetes, hazardous alcohol use | Acute | Dysuria | No | 50 leukocytes | No | Pulmonary, liver | No | No | No |
| 3 | 58 | Cystitis | Diabetes | Chronic | Dysuria, frequency | No | > 500 leukocytes | Yes | Pulmonary, spleen, subphrenic collection, septic arthritis | Yes | No | No |
| 4 | 51 | Cystitis | Diabetes | Acute | No | No | 60 leukocytes | Yes | Pulmonary, buttock abscess | No | No | No |
| 5 | 64 | Bilateral adnexal mass | Malignancy | Acute | N/A | Yes | 300 leukocytes | No | Nil | No | Yes† | Yes |
| 6 | 46 | Bilateral pyelonephritis | Diabetes | Acute | No | No | < 10 leukocytes‡ | No | Pulmonary | No | No | Yes |
| 7 | 69 | Bilateral pyelonephritis | Diabetes, chronic lung disease, chronic kidney disease | Acute | No† | No | N/A§ | No§ | Pulmonary | No | No | Yes |
| 8 | 57 | Left pyelonephritis | Diabetes, chronic lung disease | Chronic | No | No | 30 leukocytes | Yes | Pulmonary | No | No | Yes |
| 9 | 76 | Periurethral abscess | Nil | Chronic | Dysuria, pelvic pain, frequency | No | > 500 leukocytes | Yes | Nil | No | No | Yes |
| 10 | 52 | Cystitis | Diabetes, chronic kidney disease | Acute | No | Yes | 300 leukocytes | Yes | Septic arthritis | No | No | No |
| 11 | 29 | Pyelonephritis | Diabetes, hazardous alcohol use | Chronic | No | No | 40 leukocytes | Yes | Pulmonary, septic arthritis, splenic and liver abscesses | Yes | No | No |
| 12 | 39 | Right kidney abscess | Diabetes | Acute | No | Yes | > 200 leukocytes | Yes | Septic arthritis, liver, spleen, pulmonary, pancreas, plantar fascii | No | No | No |
| 13 | 75 | Cystitis | Immunosuppression | Chronic | No | No | > 500 leukocytes | Yes | Pulmonary, retroperitoneal abscess, mycotic aneurysm | No | No | No |
ICU = intensive care unit.
As recommended in Australian therapeutic guidelines.23
Discontinued melioidosis treatment at request of patient.
Urine specimen collected 72 hours prior to computed tomography imaging that demonstrated bilateral pyelonephritis.
Patient receiving renal replacement therapy and anuric.
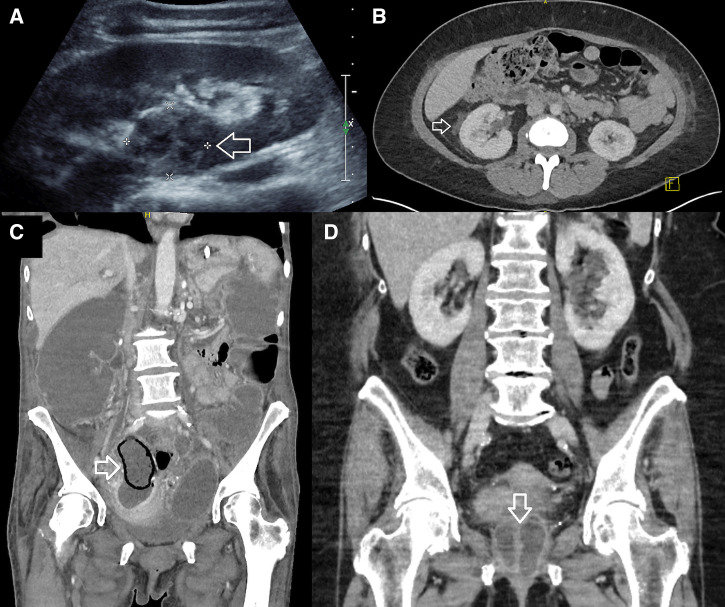
Figure 1.
(A) Case 2: renal ultrasound demonstrating an ill-defined, heterogeneous area within the cortex of the right kidney, consistent with an abscess (arrow). (B) Case 6: axial computerized tomography (CT) demonstrating fat stranding surrounding the right kidney (arrow). (C) Case 5: bilateral adnexal collections consistent with tubo-ovarian abscesses with associated bowel obstruction. Right abscess shows gas in part of the wall (arrow). (D) Case 9: coronal planes on CT of abdomen showing a multiloculated collection (arrow) surrounding the urethra, proximal vagina, and cervix complicating a urethral diverticulum.
Of the 13 cases with genitourinary involvement, 12 (92%) involved the urinary tract and seven (54%) involved the kidney (Table 2, Figure 1A and 1B). Only 4/12 (33%) women with urinary tract involvement had symptoms consistent with a urinary tract infection, but 8/12 (67%) had B. pseudomallei cultured in the urine.
Only one case in the study period appeared to involve the female reproductive organs: a 64-year-old woman with a history of ovarian malignancy who presented with B. pseudomallei bacteremia and a rapidly expanding adnexal lesion surrounded by gas (Figure 1C). The attending infectious diseases specialist thought that this was likely to represent an infected ovarian tumor, although no confirmatory aspirate of the adnexal mass was obtained as the patient opted for a palliative approach.
In 5/13 (38%) cases, the duration of intensive intravenous therapy recommended in the Australian therapeutic guidelines was extended by the presence of urinary involvement. In three of these five cases, the patients had neither urinary symptoms nor urinary growth of B. pseudomallei and the diagnosis of genitourinary involvement was made only with abdominal imaging.
There was only one death among the women with genitourinary involvement that was directly attributable to melioidosis: a 46-year-old woman with significant comorbidity who presented initially with melioidosis pneumonia and improved after 6 weeks of intravenous meropenem. However, she relapsed 26 weeks later after poor adherence to oral trimethoprim-sulfamethoxazole eradication therapy, presenting with pyelonephritis and hepatic and splenic abscesses.
Genitourinary involvement in cases of melioidosis is well described, but as the infection predominantly affects men, and the incidence of prostatic abscess is so striking, there has been very limited discussion about the genitourinary manifestations of the disease in women. In this Australian cohort, 9% of women with culture-confirmed melioidosis had genitourinary involvement, although this was most commonly in the setting of multiorgan involvement. Indeed, it was striking how few women with melioidosis had isolated genitourinary disease, and the two patients that did had underlying anatomical abnormalities predisposing them to infection (Figure 1C and 1D).
One patient had a urethral diverticulum, although it was notable that no other woman in the series had any abnormality of the urinary tract, such as prosthetic material or nephrolithiasis, that might increase the risk of infection.8 It was even more striking that in over 25 years, there was only a single case that was believed to involve the reproductive tract. The limited involvement of the female reproductive organs in this cohort contrasts starkly with tuberculosis, which may have a presentation similar to that of B. pseudomallei in melioidosis-endemic regions.9 Indeed, melioidosis of the female reproductive system is rarely reported in the literature, beyond occasional case reports.10
Although B. pseudomallei clearly has a tropism for prostatic tissue, it is unclear why some women develop genitourinary complications and others do not. Although B. pseudomallei has virulence factors that influence disease manifestations,11 the route of transmission, duration of symptoms, and host factors, especially comorbidity, are likely to have a greater impact on clinical presentation.12 Melioidosis is an opportunistic infection, and diabetes mellitus is the most common predisposing factor globally.3,13,14 In our series, 77% of women with genitourinary involvement had diabetes mellitus compared with 49% of the cases without genitourinary involvement, although in this small cohort, this difference did not reach statistical significance. An association between diabetes mellitus and genitourinary involvement in melioidosis is biologically plausible, as diabetes increases the risk of urinary tract infections from other organisms, a result of the glycosuria and incomplete bladder emptying that can complicate the disease.15 The risk of urinary tract infections with B. pseudomallei may be further increased by the neutrophil dysfunction seen in patients with diabetes that is believed to play an important role in the pathogenesis of melioidosis.16
The predominant mode of transmission of B. pseudomallei is believed to be percutaneous inoculation, during exposure to soil or contaminated water or after inhalation during severe weather events.17,18 Genitourinary involvement, in both men and women, appears to develop after hematogenous dissemination from the initial site of infection. Sexual transmission of B. pseudomallei has been suggested as a mode of infection but would appear to occur extremely rarely, if it occurs at all.19 There was one case report that proposed that a United States serviceman transmitted B. pseudomallei to his wife sexually, but her asymptomatic infection was diagnosed only with serology; vaginal and cervical cultures for B. pseudomallei were negative.20 In our cohort, there was no evidence of any sexual transmission, nor indeed any person-to-person transmission, of B. pseudomallei.
It was notable that over two-thirds of patients with genitourinary disease had no symptoms referrable to the genitourinary tract. The study therefore highlights the importance of judicious imaging to facilitate the diagnosis of unexpected intra-abdominal involvement, not only genitourinary involvement but also hepatic and splenic disease.21 Australian clinicians use CT of the chest, abdomen, and pelvis early in the management of almost all patients with melioidosis to identify unexpected foci of disease.2,4 A combination of plain imaging and ultrasound may be used in areas with limited access to CT, although this approach may be less sensitive.21,22 Earlier diagnosis of unexpected foci of disease expedites source control, when necessary, and allows the selection of an appropriate duration of intensive therapy that reduces the risk of recrudescence and relapse.5 In Australia, genitourinary melioidosis is usually treated with a minimum of 4 weeks of intravenous therapy, compared with 2 weeks of therapy for uncomplicated pneumonia, unless another focus is found that warrants a longer course.23 In this series, almost a quarter of the women with genitourinary involvement had the duration of their intensive therapy extended on the basis of imaging. This may have contributed to the very low relapse and case fatality rate seen in these women.
The study has many limitations. The fact that 7% of the women in the cohort had neither genitourinary tract imaging nor urine culture means that the incidence of genitourinary involvement may have been underestimated. However, the proportion of women diagnosed with genitourinary involvement was no higher later in the study period, when there were fewer missing data, greater access to CT imaging, and a larger number of cases.24 The small number of cases of female genitourinary involvement limited the power of the study and increased the risk of type II statistical errors. However, as the database contains 453 cases of culture-confirmed melioidosis, it does suggest that melioidosis of the female genitourinary tract is relatively uncommon. Although we argue that imaging identified unexpected genitourinary foci in several patients, leading to longer courses of intensive therapy and resulting in low rates of relapse, these patients may have had similarly positive outcomes with shorter durations of therapy. Indeed, in most patients, the knowledge of genitourinary involvement did not have a substantial impact on the duration or nature of the patients’ therapy.
In this series from tropical Australia, genitourinary involvement occurred in 9% of women with melioidosis, although this was usually in the setting of multiorgan involvement. Only one case appeared to involve the reproductive system. In several cases, genitourinary involvement was established only with abdominal imaging, emphasizing the importance of early imaging in patients with melioidosis to identify unexpected foci of disease, to expedite source control (if appropriate), and to inform the optimal duration of therapy.
REFERENCES
- 1. Currie BJ, 2022. Melioidosis and Burkholderia pseudomallei: progress in epidemiology, diagnosis, treatment and vaccination. Curr Opin Infect Dis 35: 517–523. [DOI] [PubMed] [Google Scholar]
- 2. Smith S, Hanson J, Currie BJ, 2018. Melioidosis: an Australian perspective. Trop Med Infect Dis 3: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Currie BJ. et al. , 2021. The Darwin Prospective Melioidosis Study: a 30-year prospective, observational investigation. Lancet Infect Dis 21: 1737–1746. [DOI] [PubMed] [Google Scholar]
- 4. Morse LP, Moller CC, Harvey E, Ward L, Cheng AC, Carson PJ, Currie BJ, 2009. Prostatic abscess due to Burkholderia pseudomallei: 81 cases from a 19-year prospective melioidosis study. J Urol 182: 542–547. [DOI] [PubMed] [Google Scholar]
- 5. Kozlowska J, Smith S, Roberts J, Pridgeon S, Hanson J, 2018. Prostatic abscess due to Burkholderia pseudomallei: facilitating diagnosis to optimize management. Am J Trop Med Hyg 98: 227–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chong Vh VH, Sharif F, Bickle I, 2014. Urogenital melioidosis: a review of clinical presentations, characteristic and outcomes. Med J Malaysia 69: 257–260. [PubMed] [Google Scholar]
- 7. Stewart JD, Smith S, Binotto E, McBride WJ, Currie BJ, Hanson J, 2017. The epidemiology and clinical features of melioidosis in Far North Queensland: implications for patient management. PLoS Negl Trop Dis 11: e0005411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Storme O, Tiran Saucedo J, Garcia-Mora A, Dehesa-Davila M, Naber KG, 2019. Risk factors and predisposing conditions for urinary tract infection. Ther Adv Urol 11: 1756287218814382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sharma JB, Sharma E, Sharma S, Dharmendra S, 2018. Female genital tuberculosis: revisited. Indian J Med Res 148: S71–S83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Abbink FC, Orendi JM, de Beaufort AJ, 2001. Mother-to-child transmission of Burkholderia pseudomallei. N Engl J Med 344: 1171–1172. [DOI] [PubMed] [Google Scholar]
- 11. Gora H, Hasan T, Smith S, Wilson I, Mayo M, Woerle C, Webb JR, Currie BJ, Hanson J, Meumann EM, 2022. Melioidosis of the central nervous system; impact of the bimABm allele on patient presentation and outcome. Clin Infect Dis 2022: ciac111. [DOI] [PubMed] [Google Scholar]
- 12. Cheng AC, Day NPJ, Mayo MJ, Gal D, Currie BJ, 2005. Burkholderia pseudomallei strain type, based on pulsed-field gel electrophoresis, does not determine disease presentation in melioidosis. Microbes Infect 7: 104–109. [DOI] [PubMed] [Google Scholar]
- 13. Chantratita N. et al. , 2023. Characteristics and one year outcomes of melioidosis patients in Northeastern Thailand: a prospective, multicenter cohort study. Lancet Reg Health Southeast Asia 9: 100118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hanson J, Smith S, Stewart J, Horne P, Ramsamy N, 2021. Melioidosis – a disease of socioeconomic disadvantage. PLoS Negl Trop Dis 15: e0009544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nitzan O, Elias M, Chazan B, Saliba W, 2015. Urinary tract infections in patients with type 2 diabetes mellitus: review of prevalence, diagnosis, and management. Diabetes Metab Syndr Obes 8: 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kronsteiner B. et al. , 2019. Diabetes alters immune response patterns to acute melioidosis in humans. Eur J Immunol 49: 1092–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Currie BJ, Jacups SP, 2003. Intensity of rainfall and severity of melioidosis, Australia. Emerg Infect Dis 9: 1538–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fairhead LJ, Smith S, Sim BZ, Stewart AGA, Stewart JD, Binotto E, Law M, Hanson J, 2022. The seasonality of infections in tropical Far North Queensland, Australia: a 21-year retrospective evaluation of the seasonal patterns of six endemic pathogens. PLOS Glob Public Health 2: e0000506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chang CY, Lau NLJ, Currie BJ, Podin Y, 2020. Disseminated melioidosis in early pregnancy – an unproven cause of foetal loss. BMC Infect Dis 20: 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McCormick JB, Sexton DJ, McMurray JG, Carey E, Hayes P, Feldman RA, 1975. Human-to-human transmission of Pseudomonas pseudomallei . Ann Intern Med 83: 512–513. [DOI] [PubMed] [Google Scholar]
- 21. Maude RR. et al. , 2012. Prospective observational study of the frequency and features of intra-abdominal abscesses in patients with melioidosis in northeast Thailand. Trans R Soc Trop Med Hyg 106: 629–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Expert Panel on Urological Imaging , Smith AD et al. 2022. ACR Appropriateness Criteria® acute pyelonephritis: 2022 update. J Am Coll Radiol 19: S224–S239. [DOI] [PubMed] [Google Scholar]
- 23. Therapeutic Guidelines , 2021. Melioidosis. Available at: https://www.tg.org.au. Accessed May 16, 2023.
- 24. Smith S, Horne P, Rubenach S, Gair R, Stewart J, Fairhead L, Hanson J, 2021. Increased incidence of melioidosis in Far North Queensland, Queensland, Australia, 1998–2019. Emerg Infect Dis 27: 3119–3123. [DOI] [PMC free article] [PubMed] [Google Scholar]