Abstract
Pre-reading abilities are predictive of later reading ability and can be assessed before reading begins. However, the neural correlates of pre-reading abilities in young children are not fully understood. To address this, we examined 246 datasets collected in an accelerated longitudinal design from 81 children aged 2–6 years (age = 4.6 ± 0.98 years, 47 males). Children completed pre-reading assessments (NEPSY-II Phonological Processing and Speeded Naming) and underwent a diffusion magnetic resonance imaging (MRI) scan to assess white matter connectivity. We defined a core neural network of reading and language regions based on prior literature, and structural connections within this network were assessed using graph theory analysis. Linear mixed models accounting for repeated measures were used to test associations between children’s pre-reading performance and graph theory measures for the whole bilateral reading network and each hemisphere separately. Phonological Processing scores were positively associated with global efficiency, local efficiency, and clustering coefficient in the bilateral and right hemisphere networks, as well as local efficiency and clustering coefficient in the left hemisphere network. Our findings provide further evidence that structural neural correlates of Phonological Processing emerge in early childhood, before and during early reading instruction.
Keywords: DTI, Structural connectivity, Connectome, Pre-reading, Pre-school, Development
1. Background
Reading disorders affect approximately 10% of children and account for almost 80% of known learning disorders (Kohli et al., 2018). These disorders include deficits in comprehension, fluency, and phonological deficits (Elliott and Grigorenko, 2014). Most reading disorders go undiagnosed until children have been struggling in school for years, which can have a substantial impact on their lives. Early recognition and diagnosis are important for early intervention. However, reading develops early, and pre-reading skills begin to emerge early in development and are strong predictors of future reading performance (Gabrieli, 2009). Phonological awareness and rapid automatized naming (RAN) are two key pre-reading skills (Eden and Flowers, 2009, Vander Stappen and Reybroeck, 2018). Phonological awareness includes sound knowledge at the phoneme level and the manipulation of phonemes by segmenting or combining phonemes and words (Chard and Dickson, 1999). Phonological awareness skills in children as young as 3 years old predict future reading performance (Kibby et al., 2014, Lafrance and Gottardo, 2005, Nelson et al., 2012). RAN (also called naming speed) encompasses processing speed and phonological access/retrieval abilities and can be assessed based on the ability to rapidly identify objects, colors, letters, and/or numbers (Vander Stappen and Reybroeck, 2018, Wolf and Bowers, 1999). Individuals with poor reading skills tend to perform worse at RAN than skilled readers (Eden and Flowers, 2009, Elliott and Grigorenko, 2014). Both phonological awareness and RAN are great indicators of future reading ability, however, phonological awareness tends to be a better predictor during the preschool and early school years, whereas RAN emerges as a stronger predictor later in childhood as children become more proficient at reading (Cohen et al., 2018, Kirby et al., 2003, Taibah and Haynes, 2011). This shift likely reflects a transition from relying on phonemic decoding to fluent sight-word recognition as children progress in reading. The relative importance of these predictors may also interact with other factors such as socioeconomic status (Romeo et al., 2022).
Reading involves the recruitment of several brain regions with distinct functions that communicate with each other, thereby forming a brain network (Wilcox et al., 2022). Multiple functional and structural neuroimaging studies have revealed a largely left-lateralized reading network involving the inferior frontal/precentral gyri, superior temporal gyrus/sulcus, temporo-parietal cortex, and occipito-temporal cortex (Wilcox et al., 2022). The inferior frontal gyrus (including Broca’s area) is involved in working memory, phonological processing, and phonemic integration, and the inferior frontal and precentral gyri are involved in motor planning and articulation. The superior temporal gyrus (including the primary auditory cortex (Heschl’s Gyrus) and Wernicke’s area) plays an important role in phonological retrieval and semantic processing. Adjacent temporo-parietal cortex regions (including the angular and supramarginal gyri) are important for integrating the audiovisual inputs of reading for decoding print (Martin et al., 2015, Wilcox et al., 2022). The occitpito-temporal cortex (including fusiform, lingual, and inferior temporal gyri) is associated with the visual component of reading and is important for rapid word recognition, fluency, and orthographic processing. Activation in these regions has been consistently shown to be associated with reading in adults and even young children and emerging readers (Benischek et al., 2020, Houdé et al., 2010, Martin et al., 2015). Moreover, these regions have been shown to be sensitive to changes in response to reading interventions in children with reading disorders (Perdue et al., 2022).
Studying the brain’s structural connections (i.e., white matter) and how they relate to early pre-reading skills in young children has already helped provide an understanding of the processes underlying reading development by highlighting microstructural properties and brain connections that underlie reading, as well as identifying atypical patterns of connectivity between regions that are associated with reading difficulties (Ben-Shachar et al., 2007, Perdue et al., 2022). This line of research complements functional connectivity studies, which have shown how synchronized dynamic activation of brain regions correlates with reading performance (Martin et al., 2015). Prior studies consistently show positive associations between phonological awareness and white matter fractional anisotropy values in language and reading pathways including the arcuate, uncinate, and inferior fronto-occipital fasciculi in children (Keller and Just, 2009, Reynolds et al., 2019, Saygin et al., 2013, Walton et al., 2018), as well as negative associations with mean diffusivity values (Reynolds et al., 2019, Walton et al., 2018). These associations are consistent with findings in adult studies (Vandermosten et al., 2012, Welcome and Joanisse, 2014). Recent studies also suggest widespread relationships between white matter structure and phonological awareness, along with more limited relationships between white matter and reading, thus providing additional support for using pre-reading skills as a target for investigating the brain-behavior relationships that underly reading (Koirala et al., 2021).
In addition to studying white matter microstructure in relation to pre-reading skills, characteristics of structural brain networks can be quantified. Graph theory analysis mathematically represents brain regions and the functional and/or structural connectivity between them as graphs (Bullmore and Sporns, 2009). Characteristics of neural networks can then be delineated from graph theory measures at both the local (i.e., between neighboring regions) and global (i.e., between all network regions) levels. Studying these connectivity measures using graph theory is useful for quantifying network properties and gaining insight into the complexity of brain organization. Additionally, as opposed to studying individual regions or pathways, connectome-based studies can provide additional information by looking at the network as a whole, which may be more sensitive to changes that can’t be seen by studying isolated individual regions and pathways.
Brain networks have provided useful information about the neural correlates of reading in older children and adolescents, but brain networks and pre-reading skills have not been well-studied in younger children. School-aged children with reading difficulties have decreased functional global and local efficiency in the whole brain compared to typical readers (Vourkas et al., 2011). School-aged children with reading difficulties also show abnormally high global efficiency in the right hemisphere and lower clustering in left hemisphere fronto-temporal language regions compared to typical readers, indicating a higher reliance on bilateral networks and reduced efficiency in the typical left-lateralized reading network (Mao et al., 2021). Moreover, a longitudinal study showed that higher correlations between functional connectivity and phonological awareness in young children predicted later reading performance (Yu et al., 2018). A recent study examining functional connectivity in both Chinese adults and school-aged children found that adults had increased functional connectivity in semantic-visual processing regions (I.e., inferior occipital cortex) and lower functional connectivity in phonological awareness processing regions (I.e., inferior frontal and superior temporal gyri) compared to children (Zhou et al., 2021). These findings suggest a shift in the brain from reliance more on phonological processing regions at younger ages to semantic-visual processing regions at older ages (Zhou et al., 2021). In pre-school children (in a sample overlapping the one used here), pre-reading performance was associated with stronger functional connectivity between key reading brain regions including the angular gyrus as well as Broca’s and Wernicke's areas, suggesting that functional brain connectivity correlates of pre-reading skills are apparent even before formal reading instruction begins (Benischek et al., 2020). This evidence linking functional connectivity and pre-reading abilities motivates investigation of the underlying structural networks.
In school-aged children, Lou et al. (2021) found the structural connectivity strength between central and peripheral nodes in the structural connectome of reading regions (e.g., inferior frontal and occipital, middle and superior temporal, supramarginal, precentral, and Heschl’s gyri) was associated with reading efficiency and phonemic decoding, while local efficiency was associated with phonemic decoding. They also found no associations of RAN with any graph theory measures (Lou et al., 2021). Another study in school-aged children found stronger academic performance associated with overall better organization of the white matter connectome (Bathelt et al., 2018). These studies show that structural network properties underlie reading in older children, but the extent to which these network properties are present in early childhood and how they relate to emerging reading skills is unclear.
In this study, we investigated how the brain’s structural reading network properties, modeled with graph theory, were associated with pre-reading abilities (phonological awareness and RAN) using an accelerated longitudinal design in a large cohort of children aged 2-6 years. We hypothesized that stronger structural connectivity in the language network would be associated with better pre-reading skills. Studying these associations in this early period of development addresses current gaps in the literature in this age period and contributes to the existing body of work linking reading and brain structure in older children and adults. Moreover, studying these language measures and brain development at an early stage may lay the foundation for future work examining early interventions for reading deficits.
2. Methods
2.1. Participants and pre-reading assessments
A total of 246 datasets collected using an accelerated longitudinal design were included in the analysis; these were from 81 children aged 3.0–6.9 years (47 males/34 females) were included in the analysis. Participants were recruited from the Alberta Pregnancy Outcomes and Nutrition (APrON) study, and from the Calgary community. The average age at the time of scan was 4.6 ± 0.98 years (Table 1). The dataset included 29 participants with one scan only, 9 with 2 scans, and 43 with ≥ 3 scans (Fig. 1). All participants were born at ≥ 35 weeks’ gestation, spoke English as their primary language, and had no diagnosed genetic disorders, intellectual impairments, motor disorders, developmental disorders, or brain injuries. Participants were within the age range of early pre-reading (3–6 years), however, reading abilities were not assessed for this study, and so children were not necessarily all pre-readers. The study was approved by the University of Calgary’s Conjoint Health Research Ethics Board (REB13–0020). Written consent was obtained from each child’s legal guardian and verbal assent was obtained from the children.
Table 1.
Participants demographics and pre-reading scores.
| Participant demographics | Overall N = 81 |
|---|---|
| Sex | Males (47) |
|
Ethnicity |
White (85.2%) |
| Household Income (per year) | > $100,000 CAD (72.8%) |
| Total dataset | N = 246 |
|---|---|
| Age (years) | |
| Mean (SD) | 4.6 (0.98) |
| Median [Min, Max] | 4.4 [3.0, 6.9] |
| Phonological Processing (PP) Standard Score | |
| Mean (SD) | 11.5 (2.91) |
| Median [Min, Max] | 12.0 [1.0, 18.0] |
| Speeded Naming (SN) Scaled Score | |
| Mean (SD) | 11.9 (2.76) |
| Median [Min, Max] | 12.0 [4.0, 19.0] |
Fig. 1.
Distribution of participant ages at each study visit (n = 246, females are shown in orange and males in purple). Each participant (n = 81) is represented in a row and each circle represents one study visit.
Pre-reading skills were assessed at each visit using the age-normed Phonological Processing (PP) and Speeded Naming (SN) subtests from the Developmental Neuropsychological Assessment-Second Edition (NEPSY-II; Korkman et al., 2007). The PP subtest is used to assess children’s ability to segment phonemes and words; the SN subtest is used to assess children’s ability to rapidly identify pictured shapes and colors. Each assessment took approximately 5–10 min to complete depending on the child’s abilities, and were mostly acquired on the same day as the scan, but always within two weeks.
2.2. Image acquisition and preprocessing
Children underwent a diffusion MRI scan on the same research-dedicated General Electric 3 T MR750w system with a 32-channel head coil at the Alberta Children's Hospital in Calgary, Canada. Diffusion tensor imaging (DTI) data were acquired using single shot spin echo echo-planar imaging sequence of 1.6 × 1.6 × 2.2 mm resolution (resampled to 0.78 × 0.78 × 2.2 mm on scanner), TR = 6750 ms; TE = 79 ms, 30 gradient encoding directions at b = 750 s/mm2, and 5 images at b = 0 s/mm2, scan time 4:03 min (See Reynolds et al., 2020 for further details).
DTI images were preprocessed using ExploreDTI (Leemans, 2009). Raw DICOM images were converted to NIFTI format, and b-matrices were generated. Preprocessing steps included flip/permute images, correction for signal drift, Gibbs ringing correction, head motion correction, and correction for eddy current distortions. Diffusion tensor was calculated to extract fractional anisotropy (FA) values, and whole brain tractography was performed with seedpoint resolution = 2 × 2 × 2 mm3, seed FA threshold = 0.15, fiber length range = 50–500 mm, angle threshold = 30°, step size = 1.
2.3. Graph theory analysis
To create structural DTI-based connectomes, the Automated Anatomical Labeling atlas (AAL; Tzourio-Mazoyer et al., 2002) was used to parcellate the brain into 90 regions, excluding the cerebellum. AAL gray matter regions were dilated by 3 mm to ensure white matter tracts could reach the AAL regions using FSL (Jenkinson et al., 2012), and whole brain tractography was used to generate connectivity matrices of the brain regions of interest in ExploreDTI (Leemans et al., 2009). Each connection was weighted by the average FA values within the connected fiber tracts. The brain regions parcellated from the AAL 90 represents the nodes of the graph while the average FA values of the tracts connecting the nodes represent the edges.
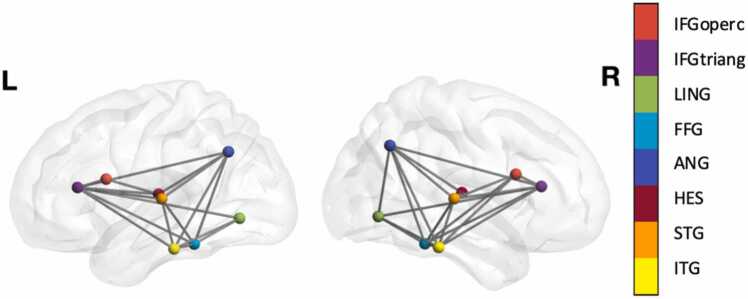
We selected a specific network of brain regions that have been consistently shown to be associated with language and reading (Hertrich et al., 2020, Martin et al., 2015, Turker et al., 2019) for analysis: opercular part of the inferior frontal gyrus, the triangular part of the inferior frontal gyrus, the lingual gyrus, the fusiform gyrus, the angular gyrus, Heschl’s gyrus, the superior temporal gyrus, and the inferior temporal gyrus (Fig. 2). The connectivity matrix of those regions was extracted from the whole brain matrix and the following analyses were conducted.
Fig. 2.
16 Regions of interest (8 from each hemisphere) in the network: opercular part of the inferior frontal gyrus (IFGoperc), the triangular part of the inferior frontal gyrus (IFGtriang), the lingual gyrus (LING), the fusiform gyrus (FFG), the angular gyrus (ANG), Heschl’s gyrus (HES), the superior temporal gyrus (STG), and the inferior temporal gyrus (ITG). Networks were visualized using BrainNet Viewer (Xia et al., 2013).
Graph theory measures of network connectivity (local efficiency, global efficiency, clustering coefficient, and nodal degree) were calculated using the Brain Connectivity Toolbox (Rubinov and Sporns, 2010). These measures were selected given their relationship with reading skills in older children (Mao et al., 2021, Vourkas et al., 2011). Global efficiency measures how efficient the transfer of information is across the whole network. Local efficiency measures how efficient the transfer of information is between local neighboring nodes. Higher efficiency refers to shorter distances (edges) between brain regions (nodes) and therefore higher integration of information and communication across the network. The clustering coefficient is the density of connections between regions and is a measure of functional specialization within regions. Nodal degree is the number of connections (edges) projecting to/from each region. Metrics were calculated from the bilateral networks (16 ×16 matrix), and left and right networks (8 ×8 matrix), given the evidence of bilateral and right hemisphere contributions to reading and language processing early in development (Benischek et al., 2020, Reynolds et al., 2019), and the well-established importance of the left hemisphere for reading throughout life.
2.4. Statistical analysis
Statistical analysis was conducted in R (R Core Team, 2021). To account for varying number of scans by participants, linear mixed-effect models were used to test associations between pre-reading scores and graph theory measures with participant included as a random factor with the equation: pre-reading score ∼ graphic metric + age + sex + graphic metric*age + [1|Subject]. Graph theory metrics, age, sex, and the interaction between age and graph theory measures were fixed effects in the model, and subjects were included as a random effect to account for repeated measures. Maximum likelihood estimation was used. Separate models were used to test each graph theory metric from each network (bilateral, left, and right). False discovery rate correction (FDR) at q < 0.05 was used to correct for 24 multiple comparisons (2 pre-reading assessments x 4 graph theory metrics x 3 brain networks = 24).
3. Results
3.1. Pre-reading assessments
3.1.1. Phonological processing
Mixed effect analysis showed Phonological Processing (PP) scores were significantly associated with average global efficiency (b= 88.6, p = 0.007, q= 0.028), local efficiency (b= 160.27, p = 0.002, q= 0.024), and clustering coefficient (b= 95.06, p = 0.013 q= 0.045), in the bilateral network and right hemisphere network (b= 71.13, p = 0.015, q= 0.045; b= 126.30, p = 0.004, q= 0.028; b= 88.51, p = 0.002, q= 0.024, respectively), as well as average local efficiency (b=136.56, p = 0.005, q= 0.028) and clustering coefficient (b= 95.65, p = 0.006, q= 0.028, respectively) in the left hemisphere network (Table 2). All associations survived FDR correction for multiple comparisons.
Table 2.
Associations between graph theory measures and Phonological Processing.
| Graph Theory Metric | Graph Theory Metric X Age | |||||
|---|---|---|---|---|---|---|
| Bilateral Network | ||||||
| Beta (CI) | t239 | p-value | Beta (CI) | t239 | p-value | |
| Global Efficiency | 88.6 (25.0, 152.2) | 2.74 |
0.007 q= 0.028 |
-20.1 (−33.9, −6.3) | -2.87 | 0.004 |
| Local Efficiency | 160.3 (57.2, 263.4) | 3.06 |
0.002 q= 0.024 |
-34.2 (56.4, −12.0) | -3.04 | 0.003 |
| Clustering Coefficient | 95.1 (20.0, 170.1) | 2.5 |
0.013 q= 0.045 |
-20.7 (−37.2, −4.2) | -2.48 | 0.014 |
| Nodal Degree | 1.5 (−0.3, 3.3) |
1.68 | 0.095 | -0.3 (−0.7, 0.1) | -1.73 | 0.085 |
| Left Network | ||||||
| Beta (CI) | t239 | p-value | Beta (CI) | t239 | p-value | |
| Global Efficiency | 60.1 (−2.75, 122.86) |
1.88 | 0.061 | -13.9 (−27.5, −0.3) | -2.01 | 0.045 |
| Local Efficiency | 136.6 (41.5, 231.7) | 2.83 |
0.005 q= 0.028 |
-32.3 (−52.7, −11.9) | -3.12 | 0.002 |
| Clustering Coefficient | 95.7 (28.2, 163.2) | 2.79 |
0.006 q= 0.028 |
-22.8 (−37.0, −8.7) | -3.17 | 0.002 |
| Nodal Degree | 0.5 (−1.9, 2.9) |
0.43 | 0.666 | -0.1 (−0.7, 0.4) | -0.48 | 0.635 |
| Right Network | ||||||
| Beta (CI) | t239 | p-value | Beta (CI) | t239 | p-value | |
| Global Efficiency | 71.1 (14.1, 128.2) | 2.46 |
0.015 q= 0.045 |
-12.4 (−24.7, −0.2) | -2.00 | 0.046 |
| Local Efficiency | 126.3 (40.9, 211.8) | 2.91 |
0.004 q= 0.028 |
-24.3 (−42.2, −6.5) | -2.69 | 0.008 |
| Clustering Coefficient | 88.5 (33.0, 144.0) | 3.14 |
0.002 q= 0.024 |
-19.4 (−31.4, −7.4) | -3.18 | 0.002 |
| Nodal Degree | 1.7 (−0.7, 4.0) |
1.39 | 0.167 | -0.3 (−0.8, 0.3) | -0.96 | 0.340 |
The age-by-graph theory metric interactions were significant for global efficiency (p = 0.004), local efficiency (p = 0.003), and clustering coefficient (p = 0.014) in the bilateral network as well the left (p = 0.045, p = 0.002, p = 0.002, respectively), and right hemisphere (p = 0.046, p = 0.008, p = 0.002, respectively) networks.
In order to visualize the age-by-graph theory metric interactions found with PP, we split the data by the median age (4.44 y) and observed that younger children displayed a more positive relationship between PP and graph theory metrics than older children (Fig. 3).
Fig. 3.
To visualize the significant age-by-graph theory metric interactions for Phonological Processing, we used a median split at age 4.4 years. This figure shows phonological processing-brain interactions for: global efficiency (top row), local efficiency (middle row), and clustering coefficient (bottom row) in each hemisphere (first and second columns) and bilaterally (third column) by age group. Age groups were split at the median (4.4 years) with older children shown in purple and younger children shown in green. In all cases, the relationship was stronger in younger children than older children.
3.1.2. Speeded naming
No significant associations were found between Speeded Naming (SN) scores and graph theory metrics in any of the networks (all p > .2) (Table 3).
Table 3.
Associations between graph theory measures and Speeded Naming.
| Graph Theory Metric | Graph Theory Metric X Age | |||||
|---|---|---|---|---|---|---|
| Bilateral Network | ||||||
| Beta (CI) | t239 | p-value | Beta (CI) | t239 | p-value | |
| Global Efficiency | -13.5 (−76.3, 49.4) |
-0.42 | 0.674 | 3.6 (−10.3, 17.2) | 0.52 | 0.604 |
| Local Efficiency | 46.4 (−56.0, 148.9) |
0.89 | 0.373 | -8.3 (−30.4, 13.8) |
-0.74 | 0.461 |
| Clustering Coefficient | 33.3 (−40.7, 107.3) |
0.89 | 0.376 | 5.8 (−22.1, 10.5) |
-0.70 | 0.483 |
| Nodal Degree | -0.8 (−2.5, 0.9) |
-0.90 | 0.368 | 0.2 (−0.2, 0.6) |
0.96 | 0.339 |
| Left Network | ||||||
| Beta (CI) | t239 | p-value | Beta (CI) | t239 | p-value | |
| Global Efficiency | 36.0 (−25.5, 97.5) |
1.15 | 0.250 | -4.70 (−18.0, 8.6) | -0.70 | 0.487 |
| Local Efficiency | 60.7 (−33.5, 154.8) |
1.27 | 0.206 | -8.14 (−28.4, 12.1) | -0.79 | 0.429 |
| Clustering Coefficient | 34.7 (−31.6101.0) | 1.03 | 0.303 | -4.9 (−18.9,9.0) | -0.70 | 0.486 |
| Nodal Degree | 0.7 (−1.6, 3.0) |
0.59 | 0.559 | -0.1 (−0.6, 0.4) | -0.27 | 0.790 |
| Right Network | ||||||
| Beta (CI) | t239 | p-value | Beta (CI) | t239 | p-value | |
| Global Efficiency | 15.3 (−41.0, 71.7) |
0.54 | 0.592 | -4.6 (−16.7, 7.4) |
-0.76 | 0.450 |
| Local Efficiency | 36.9 (−47.4, 121.2) |
0.86 | 0.389 | -10.6 (−28.3, 7.1) |
-1.18 | 0.239 |
| Clustering Coefficient | 24.2 (−31.1, 79.4) |
0.86 | 0.389 | -5.2 (−17.1, 6.8) |
-0.85 | 0.398 |
| Nodal Degree | -0.03 (−2.3, 2.3) |
-0.02 | 0.983 | -6.79e-03 (−0.5, 0.5) | -0.03 | 0.979 |
4. Discussion
Here, we show that structural connectome properties of the language network are associated with phonological awareness in children aged 2–6 years. Our findings extend previous studies of brain structure and reading in older children and show that these associations are present even in younger children before and during the early stages of formal reading instructions. This key pre-reading skill is positively associated with structural connectivity at both the global and local levels in young children. Associations between brain metrics and phonological awareness were stronger in younger children, suggesting that these relationships may weaken slightly with age, although these associations were not significant. No significant associations with naming speed were found, though these associations may emerge in older children or in different networks, as the reading becomes more proficient.
Stronger structural connectivity, measured by higher global efficiency, local efficiency, and clustering coefficient in the reading network, was associated with better PP scores in preschool children, supporting our hypothesis. This shows that structural brain connectivity underlies emerging reading skills even before and during the earliest stages of formal reading instruction. Structural connectivity in individual white matter pathways is associated with pre-reading skills in young children (Saygin et al., 2013, Walton et al., 2018, Wang et al., 2021); our findings show that the organization of the reading network is also associated with phonological awareness. Our findings are in good agreement with previous studies in school-aged children showing higher structural network efficiency associated with better reading and academic performance (Bathelt et al., 2018, Lou et al., 2021). This also adds to previous findings in an overlapping sample showing better pre-reading abilities to be associated with stronger functional connectivity (Benischek et al., 2020). Thus, stronger functional and structural connectivity are associated with better phonological awareness performance. Pre-reading skills are predictors of future reading ability (Kibby et al., 2014), and network properties are altered in children with reading disabilities (Vourkas et al., 2011), and in response to reading intervention (Horowitz-Kraus et al., 2015, Richards et al., 2018). Together with our findings, this suggests that these network properties changes are evident even in young children, and altered structural network connectivity may also underlie future reading ability/disability.
PP scores were positively associated with structural connectivity properties in both hemispheres as well as the bilateral network. Language and reading processing in the brain are generally left-lateralized (Schlaggar and McCandliss, 2007), and while macrostructural lateralization is present even in young children, lateralization of microstructural and functional brain properties tends to increase across early childhood (Reynolds et al., 2019). Thus, our findings likely reflect reduced hemispheric specialization in early childhood, where both hemispheres are important for pre-reading skills. These findings are supported by functional studies showing associations in right hemisphere regions in young children (Benischek et al., 2020), as well as an age-related decrease in right hemisphere activation during language tasks across childhood (Holland et al., 2007, Olulade et al., 2020). More longitudinal data examining age-related changes in network connectivity and reading will help further understand the role of the right hemisphere in these skills as children mature.
Our findings from this large longitudinal sample of young children motivate graph theory structural connectivity as a plausible predictor of later reading outcomes, similar to prior findings using other brain metrics in longitudinal studies of early childhood. Longitudinal studies found increased brain activity correlated with reading fluency in school aged children (McNorgan et al., 2011; Ozernov‐Palchik et al., 2023). Additionally, functional connectivity correlates of pre-reading skills (I.e., phonological processing) during the pre-reading period predicted later reading development (Jasińska et al., 2021; Yu et al., 2018). Similar positive trend associations between reading development in school aged children and white matter properties (Moulton et al., 2019, Vanderauwera et al., 2018) were found in longitudinal studies. Thus, accordingly, our findings complement these studies and further demonstrate the emergence of these neural correlates and their prediction of pre-reading skills before and during the early stages of learning. In future research, we will examine if the structural connectome of reading networks predicts future reading ability.
The associations between structural network properties and PP weakened with age; younger children had more positive associations between PP scores and graphic metrics than older children. The decreasing associations with age may be due to changes in reading network connectivity and specialization over the course of development. In general, the brain undergoes pruning and specialization that support cognitive processing, and these maturation/specialization processes have also been seen in brain connectivity (Battista et al., 2018). Our findings are consistent with prior studies showing that connectivity in phonology-related regions decreases with age, while connectivity in semantic and orthography-related regions increases with age, (Liu et al., 2018; Zhou et al., 2021). Preschool aged children rely more on decoding and phonological processing during the early stages of reading instruction and word recognition later (Hogan et al., 2005), which may manifest in these age-brain interactions.
Speeded Naming scores were not associated with structural network properties. This is interesting given that RAN is a predictor of future reading skills (Norton and Wolf, 2012). However, previous studies suggest that phonological awareness is a better predictor of reading ability early, while naming speed becomes a stronger predictor later (Cohen et al., 2018, Kirby et al., 2003, Taibah and Haynes, 2011). This likely reflects the shift from children relying on phonemic decoding of words letter by letter, to rapidly recalling words as they become proficient readers. Moreover, letter/number identification assessments of naming speed tend to be a better predictor of reading than the object identification assessments that our sample have completed (Araújo et al., 2015). Another explanation for these results is that naming speed may involve additional regions not reflected in the network studied here and associations would only be evident in a more widespread network of brain areas.
Other factors that we were unable to assess may play a role in the relationships studied here. We did not directly assess reading skills in the children and thus cannot be certain that they are all “pre-readers”. Moreover, in addition to having high-average scores, most participants had parents with high education and income, which are known to moderate brain-reading associations (Noble et al., 2006, Romeo et al., 2022). Future studies should explore these factors in the context of associations between brain connectivity and pre-reading skills in young children.
5. Conclusion
In conclusion, our large longitudinal dataset of young children shows associations between structural network connectivity and pre-reading skills that emerge before and during early formal reading instruction. This is important given that most reading disorders go undiagnosed until children have been struggling in school for years, which negatively impacts their academic performance and mental health. Thus, focus on early diagnosis and interventions for reading difficulties is necessary.
CRediT authorship contribution statement
Perdue Meaghan: Conceptualization, Methodology, Writing – review & editing. Donnici Claire: Data curation, Writing – review & editing. Long Xiangyu: Methodology, Writing – review & editing. Lebel Catherine: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – review & editing. Dewey Deborah: Conceptualization, Writing – review & editing. Ghasoub Mohammad: Conceptualization, Data curation, Formal analysis, Methodology, Visualization, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the Canadian Institutes of Health Research (IHD-134090, MOP-136797), the Jacobs Foundation, and a grant from the Alberta Children’s Hospital Foundation. MG receives salary support from the Hotchkiss Brain Institute. CL receives salary support from the Canada Research Chairs Program.
Data availability
Neuroimaging and cognitive data used in this study are freely available through the Open Science Framework here: https://osf.io/axz5r/. For more information, see Reynolds, J.E., Long, X., Paniukov, D., Bagshawe, M., Lebel, C., 2020. Calgary Preschool magnetic resonance imaging (MRI) dataset. Data Brief 29: 105224.
References
- Araújo S., Reis A., Petersson K.M., Faísca L. Rapid automatized naming and reading performance: a meta-analysis. J. Educ. Psychol. 2015;107(3):868–883. doi: 10.1037/edu0000006. [DOI] [Google Scholar]
- Bathelt J., Gathercole S.E., Butterfield S., the CALM team, Astle D.E. Children’s academic attainment is linked to the global organization of the white matter connectome. Dev. Sci. 2018;21(5) doi: 10.1111/desc.12662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battista C., Evans T.M., Ngoon T.J., Chen T., Chen L., Kochalka J., Menon V. Mechanisms of interactive specialization and emergence of functional brain circuits supporting cognitive development in children. npj Sci. Learn. 2018;3(1) doi: 10.1038/s41539-017-0017-2. Article 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benischek A., Long X., Rohr C.S., Bray S., Dewey D., Lebel C. Pre-reading language abilities and the brain’s functional reading network in young children. NeuroImage. 2020;217 doi: 10.1016/j.neuroimage.2020.116903. [DOI] [PubMed] [Google Scholar]
- Ben-Shachar M., Dougherty R.F., Wandell B.A. White matter pathways in reading. Curr. Opin. Neurobiol. 2007;17(2):258–270. doi: 10.1016/j.conb.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Bullmore E., Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 2009;10(3):186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Chard D.J., Dickson S.V. Phonological awareness: instructional and assessment guidelines. Interv. Sch. Clin. 1999;34(5):261–270. doi: 10.1177/105345129903400502. [DOI] [Google Scholar]
- Cohen M., Mahé G., Laganaro M., Zesiger P. Does the relation between rapid automatized naming and reading depend on age or on reading level? A behavioral and ERP study. Front. Hum. Neurosci. 2018;12 doi: 10.3389/fnhum.2018.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden G.F., Flowers D.L. Encyclopedia of Neuroscience. Elsevier; 2009. Dyslexia: neurodevelopmental basis; pp. 741–747. [DOI] [Google Scholar]
- Elliott J.G., Grigorenko E.L. The Dyslexia Debate. first ed. Cambridge University Press; 2014. [DOI] [Google Scholar]
- Gabrieli J.D.E. Dyslexia: a new synergy between education and cognitive neuroscience. Science. 2009;325(5938):280–283. doi: 10.1126/science.1171999. [DOI] [PubMed] [Google Scholar]
- Hertrich I., Dietrich S., Ackermann H. The margins of the language network in the brain. Front. Commun. 2020;5 doi: 10.3389/fcomm.2020.519955. [DOI] [Google Scholar]
- Hogan T.P., Catts H.W., Little T.D. The relationship between phonological awareness and reading: implications for the assessment of phonological awareness. Lang. Speech Hear. Serv. Sch. 2005;36(4):285–293. doi: 10.1044/0161-1461(2005/029). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland S.K., Vannest J., Mecoli M., Jacola L.M., Tillema J.-M., Karunanayaka P.R., Schmithorst V.J., Yuan W., Plante E., Byars A.W. Functional MRI of language lateralization during development in children. Int. J. Audiol. 2007;46(9):533–551. doi: 10.1080/14992020701448994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz-Kraus T., Toro-Serey C., DiFrancesco M. Increased resting-state functional connectivity in the cingulo-opercular cognitive-control network after intervention in children with reading difficulties. PLOS ONE. 2015;10(7) doi: 10.1371/journal.pone.0133762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houdé O., Rossi S., Lubin A., Joliot M. Mapping numerical processing, reading, and executive functions in the developing brain: an fMRI meta‐analysis of 52 studies including 842 children. Dev. Sci. 2010;13(6):876–885. doi: 10.1111/j.1467-7687.2009.00938.x. [DOI] [PubMed] [Google Scholar]
- Jasińska K.K., Shuai L., Lau A.N.L., Frost S., Landi N., Pugh K.R. Functional connectivity in the developing language network in 4–year‐old children predicts future reading ability. Dev. Sci. 2021;24(2) doi: 10.1111/desc.13041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Beckmann C.F., Behrens T.E.J., Woolrich M.W., Smith S.M. FSL. NeuroImage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Keller T.A., Just M.A. Altering cortical connectivity: remediation-induced changes in the white matter of poor readers. Neuron. 2009;64(5):624–631. doi: 10.1016/j.neuron.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibby M.Y., Lee S.E., Dyer S.M. Reading performance is predicted by more than phonological processing. Front. Psychol. 2014;5 doi: 10.3389/fpsyg.2014.00960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby J.R., Parrila R.K., Pfeiffer S.L. Naming speed and phonological awareness as predictors of reading development. J. Educ. Psychol. 2003;95(3):453–464. doi: 10.1037/0022-0663.95.3.453. [DOI] [Google Scholar]
- Kohli A., Sharma S., Padhy S.K. Specific learning disabilities: issues that remain unanswered. Indian J. Psychol. Med. 2018;40(5):399–405. doi: 10.4103/IJPSYM.IJPSYM_86_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koirala N., Perdue M.V., Su X., Grigorenko E.L., Landi N. Neurite density and arborization is associated with reading skill and phonological processing in children. NeuroImage. 2021;241 doi: 10.1016/j.neuroimage.2021.118426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkman M., Kirk U., Kemp S. (NEPSY - II) [Database record] APA PsycTests. 2007 doi: 10.1037/t15125-000. [DOI] [Google Scholar]
- Lafrance A., Gottardo A. A longitudinal study of phonological processing skills and reading in bilingual children. Appl. Psycholinguist. 2005;26(4):559–578. doi: 10.1017/S0142716405050307. [DOI] [Google Scholar]
- Leemans A., Jeurissen B., Sijbers J., Jones D.K., 2009. ExploreDTI: a graphical toolbox for processing, analyzing, and visualizing diffusion MR data. In: 17th Annual Meeting of International Society for Magnetic Resonance in Medicine. Hawaii, USA, p. 3537.
- Liu X., Gao Y., Di Q., Hu J., Lu C., Nan Y., Booth J.R., Liu L. Differences between child and adult large‐scale functional brain networks for reading tasks. Hum. Brain Mapp. 2018;39(2):662–679. doi: 10.1002/hbm.23871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou C., Cross A.M., Peters L., Ansari D., Joanisse M.F. Rich-club structure contributes to individual variance of reading skills via feeder connections in children with reading disabilities. Dev. Cogn. Neurosci. 2021;49 doi: 10.1016/j.dcn.2021.100957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J., Liu L., Perkins K., Cao F. Poor reading is characterized by a more connected network with wrong hubs. Brain Lang. 2021;220 doi: 10.1016/j.bandl.2021.104983. [DOI] [PubMed] [Google Scholar]
- Martin A., Schurz M., Kronbichler M., Richlan F. Reading in the brain of children and adults: a meta-analysis of 40 functional magnetic resonance imaging studies: reading in the brain of children and Adults. Hum. Brain Mapp. 2015;36(5):1963–1981. doi: 10.1002/hbm.22749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNorgan C., Alvarez A., Bhullar A., Gayda J., Booth J.R. Prediction of reading skill several years later depends on age and brain region: implications for developmental models of reading. J. Neurosci. 2011;31(26):9641–9648. doi: 10.1523/JNEUROSCI.0334-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulton E., Bouhali F., Monzalvo K., Poupon C., Zhang H., Dehaene S., Dehaene-Lambertz G., Dubois J. Connectivity between the visual word form area and the parietal lobe improves after the first year of reading instruction: a longitudinal MRI study in children. Brain Struct. Funct. 2019 doi: 10.1007/s00429-019-01855-3. [DOI] [PubMed] [Google Scholar]
- Nelson J.M., Lindstrom J.H., Lindstrom W., Denis D. The structure of phonological processing and its relationship to basic reading. Exceptionality. 2012;20(3):179–196. doi: 10.1080/09362835.2012.694612. [DOI] [Google Scholar]
- Noble K.G., Wolmetz M.E., Ochs L.G., Farah M.J., McCandliss B.D. Brain-behavior relationships in reading acquisition are modulated by socioeconomic factors. Dev. Sci. 2006;9(6) doi: 10.1111/j.1467-7687.2006.00542.x. [DOI] [PubMed] [Google Scholar]
- Norton E.S., Wolf M. Rapid automatized naming (RAN) and reading fluency: implications for understanding and treatment of reading disabilities. Annu. Rev. Psychol. 2012;63(1):427–452. doi: 10.1146/annurev-psych-120710-100431. [DOI] [PubMed] [Google Scholar]
- Olulade O.A., Seydell-Greenwald A., Chambers C.E., Turkeltaub P.E., Dromerick A.W., Berl M.M., Gaillard W.D., Newport E.L. The neural basis of language development: changes in lateralization over age. Proc. Natl. Acad. Sci. 2020;117(38):23477–23483. doi: 10.1073/pnas.1905590117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozernov‐Palchik O., Sury D., Turesky T.K., Yu X., Gaab N. Longitudinal changes in brain activation underlying reading fluency. Hum. Brain Mapp. 2023;44(1):18–34. doi: 10.1002/hbm.26048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdue M.V., Mahaffy K., Vlahcevic K., Wolfman E., Erbeli F., Richlan F., Landi N. Reading intervention and neuroplasticity: a systematic review and meta-analysis of brain changes associated with reading intervention. Neurosci. Biobehav. Rev. 2022;132:465–494. doi: 10.1016/j.neubiorev.2021.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . R Foundation for Statistical Computing,; Vienna, Austria: 2021. R: A Language and Environment for Statistical Computing.https://www.R-project.org/ [Google Scholar]
- Reynolds J.E., Long X., Grohs M.N., Dewey D., Lebel C. Structural and functional asymmetry of the language network emerge in early childhood. Dev. Cogn. Neurosci. 2019;39 doi: 10.1016/j.dcn.2019.100682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds J.E., Long X., Paniukov D., Bagshawe M., Lebel C. Calgary Preschool magnetic resonance imaging (MRI) dataset. Data Brief. 2020;29 doi: 10.1016/j.dib.2020.105224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards T.L., Berninger V.W., Yagle K., Abbott R.D., Peterson D. Brain’s functional network clustering coefficient changes in response to instruction (RTI) in students with and without reading disabilities: multi-leveled reading brain’s RTI. Cogent Psychol. 2018;5(1):1424680. doi: 10.1080/23311908.2018.1424680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo R.R., Perrachione T.K., Olson H.A., Halverson K.K., Gabrieli J.D.E., Christodoulou J.A. Socioeconomic dissociations in the neural and cognitive bases of reading disorders. Dev. Cogn. Neurosci. 2022;58 doi: 10.1016/j.dcn.2022.101175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M., Sporns O. Complex network measures of brain connectivity: Uses and interpretations. NeuroImage. 2010;52(3):1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Saygin Z.M., Norton E.S., Osher D.E., Beach S.D., Cyr A.B., Ozernov-Palchik O., Yendiki A., Fischl B., Gaab N., Gabrieli J.D.E. Tracking the roots of reading ability: white matter volume and integrity correlate with phonological awareness in prereading and early-reading kindergarten children. J. Neurosci. 2013;33(33):13251–13258. doi: 10.1523/JNEUROSCI.4383-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaggar B.L., McCandliss B.D. Development of neural systems for reading. Annu. Rev. Neurosci. 2007;30(1):475–503. doi: 10.1146/annurev.neuro.28.061604.135645. [DOI] [PubMed] [Google Scholar]
- Taibah N.J., Haynes C.W. Contributions of phonological processing skills to reading skills in Arabic speaking children. Read. Writ. 2011;24(9):1019–1042. doi: 10.1007/s11145-010-9273-8. [DOI] [Google Scholar]
- Turker S., Reiterer S.M., Schneider P., Seither-Preisler A. Auditory cortex morphology predicts language learning potential in children and teenagers. Front. Neurosci. 2019;13 doi: 10.3389/fnins.2019.00824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Vander Stappen C., Reybroeck M.V. Phonological awareness and rapid automatized naming are independent phonological competencies with specific impacts on word reading and spelling: an intervention study. Front. Psychol. 2018;9 doi: 10.3389/fpsyg.2018.00320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderauwera J., De Vos A., Forkel S.J., Catani M., Wouters J., Vandermosten M., Ghesquière P. Neural organization of ventral white matter tracts parallels the initial steps of reading development: a DTI tractography study. Brain Lang. 2018;183:32–40. doi: 10.1016/j.bandl.2018.05.007. [DOI] [PubMed] [Google Scholar]
- Vandermosten M., Boets B., Poelmans H., Sunaert S., Wouters J., Ghesquiere P. A tractography study in dyslexia: neuroanatomic correlates of orthographic, phonological and speech processing. Brain. 2012;135(3):935–948. doi: 10.1093/brain/awr363. [DOI] [PubMed] [Google Scholar]
- Vourkas M., Micheloyannis S., Simos P.G., Rezaie R., Fletcher J.M., Cirino P.T., Papanicolaou A.C. Dynamic task-specific brain network connectivity in children with severe reading difficulties. Neurosci. Lett. 2011;488(2):123–128. doi: 10.1016/j.neulet.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton M., Dewey D., Lebel C. Brain white matter structure and language ability in preschool-aged children. Brain Lang. 2018;176:19–25. doi: 10.1016/j.bandl.2017.10.008. [DOI] [PubMed] [Google Scholar]
- Wang N.Y.-H., Wang H.-L.S., Liu Y.-C., Chang Y.-P.E., Weng J.-C. Investigating the white matter correlates of reading performance: evidence from Chinese children with reading difficulties. PLOS ONE. 2021;16(3) doi: 10.1371/journal.pone.0248434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welcome S.E., Joanisse M.F. Individual differences in white matter anatomy predict dissociable components of reading skill in adults. NeuroImage. 2014;96:261–275. doi: 10.1016/j.neuroimage.2014.03.069. [DOI] [PubMed] [Google Scholar]
- Wilcox G., MacMaster F.P., Makarenko E. Cognitive Neuroscience Foundations for School Psychologists: Brain-Behavior Relationships in the Classroom. first ed. Routledge; 2022. [DOI] [Google Scholar]
- Wolf M., Bowers P.G. The double-deficit hypothesis for the developmental dyslexias. J. Educ. Psychol. 1999;91(3):415–438. doi: 10.1037/0022-0663.91.3.415. [DOI] [Google Scholar]
- Xia M., Wang J., He Y. BrainNet viewer: a network visualization tool for human brain connectomics. PLoS ONE. 2013;8(7) doi: 10.1371/journal.pone.0068910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Raney T., Perdue M.V., Zuk J., Ozernov-Palchik O., Becker B.L.C., Raschle N.M., Gaab N. Emergence of the neural network underlying phonological processing from the prereading to the emergent reading stage: a longitudinal study. Hum. Brain Mapp. 2018;39(5):2047–2063. doi: 10.1002/hbm.23985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W., Cui X., Shi B., Su M., Cao M. The development of brain functional connectome during text reading. Dev. Cogn. Neurosci. 2021;48 doi: 10.1016/j.dcn.2021.100927. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Neuroimaging and cognitive data used in this study are freely available through the Open Science Framework here: https://osf.io/axz5r/. For more information, see Reynolds, J.E., Long, X., Paniukov, D., Bagshawe, M., Lebel, C., 2020. Calgary Preschool magnetic resonance imaging (MRI) dataset. Data Brief 29: 105224.