Highlights
-
•
The RSV-F Fc fusion proteins, native construct and structural stabilized construct, were successfully produced in plant.
-
•
The plant produced RSV-F Fc fusion proteins were recognized by the monoclonal antibody Motavizumab specific against RSV-F protein.
-
•
The plant produced RSV-F Fc fusion proteins showed immunogenicity in mice.
Keywords: Subunit vaccine, Respiratory syncytial virus, plant-produced recombinant protein, Nicotianabenthamiana
Abstract
Respiratory syncytial virus (RSV) is a highly infectious respiratory virus that causes serious illness, particularly in young children, elderly people, and those with immunocompromised individuals. RSV infection is the leading cause of infant hospitalization and can lead to serious complications such as pneumonia and bronchiolitis. Currently, there is an RSV vaccine approved exclusively for the elderly population, but no approved vaccine specifically designed for infants or any other age groups. Therefore, it is crucial to continue the development of an RSV vaccine specifically tailored for these populations. In this study, the immunogenicity of the two plant-produced RSV-F Fc fusion proteins (Native construct and structural stabilized construct) were examined to assess them as potential vaccine candidates for RSV. The RSV-F Fc fusion proteins were transiently expressed in Nicotiana benthamiana and purified using protein A affinity column chromatography. The recombinant RSV-F Fc fusion protein was recognized by the monoclonal antibody Motavizumab specific against RSV-F protein. Moreover, the immunogenicity of the two purified RSV-F Fc proteins were evaluated in mice by formulating with different adjuvants. According to our results, the plant-produced RSV-F Fc fusion protein is immunogenic in mice. These preliminary findings, demonstrate the immunogenicity of plant-based RSV-F Fc fusion protein, however, further preclinical studies such as antigen dose and adjuvant optimization, safety, toxicity, and challenge studies in animal models are necessary in order to prove the vaccine efficacy.
1. Introduction
Respiratory Syncytial Virus (RSV) is a common virus that is responsible for infections of the lungs and respiratory tract. It has been reported that RSV cause more than 33.1 million cases, 3.2 million hospitalization, and 118,200–149,400 deaths in the USA in 2018 [1]. While, in Thailand, RSV is responsible for about 19,000 cases from 2015 to 2020 [2], Among the affected individuals, 70 % experienced pneumonia, while approximately 15 % suffered from both bronchiolitis and bronchitis [2]. Even though the data on the RSV mortality rate in Thailand is limited, according to the Ministry of Public Health, there have been 2 deaths reported from 2017 to 2020 in Chaiyaphum province and Nakhon Ratchasima province [3]. Furthermore, RSV is responsible for the illness globally, causing almost 29 % of all cases of pediatric pneumonia [4]. The infection in infants and young children may result in severe bronchiolitis, which can be fatal. Recurrent upper respiratory tract infections may also range from asymptomatic illness to symptomatic upper respiratory tract disease. RSV is also increasingly emerging as a significant pathogen among elderly, and infection increases hospitalization rates among those aged 65 and older as well as mortality rates among the elderly [5]. Adults with underlying chronic lung disease, congenital heart disease, and immunodeficiency are more likely to experience severe disease [6,7]. RSV infection has been linked with high mortality rates in patients who have undergone lung or bone marrow transplants [8]. Therefore, developing a preventative measure through vaccination is quite important. Since the 1960s, after the first use of a formalin-inactivated virus (FI-RSV) vaccination in infants caused severe vaccine-associated increased respiratory illness following their first natural infection with RSV, therefore, an effort to find an effective vaccine has been delayed for many years [9,10].
Recently the United States Food and Drug Administration (FDA) approved the first RSV vaccine developed by GSK, called Arexvy™. This vaccine is effective for individuals aged 60 years and older, and it has shown an impressive efficacy rate of up to 83 % in preventing RSV-related lower tract respiratory infection (LTRI). The vaccine works equally well against both RSV A and B subtypes, offering robust protection. For RSV-related LTRI, the vaccine demonstrated efficacy rates of 85 % and 81 % against RSV A and B subtypes, respectively. In the case of RSV-related acute respiratory infection, it showed efficacy rates of 72 % and 71 % against the same subtypes, respectively. These findings clearly emphasize the Arexvy™vaccine's effectiveness against RSV subtypes thereby reducing the burden of RSV-related illnesses [11].
RSV virion contains fusion glycoprotein (F protein) and attachment glycoprotein (G protein) [12], which are present on the surface of the virus. These two main RSV components are known to trigger neutralizing antibodies upon infection. The primary function of the F (fusion) and G (attachment) proteins is to facilitate the attachment of the virus to the host cell. The F glycoprotein from RSV clinical isolates of both the A and B subtype shared amino acid identity with 90 % or more [13,14] hence it is considered as a highly conserved region. Thus, the F protein has become an attractive target for developing vaccines [[15], [16], [17], [18]]. The RSV-F protein is composed of 574 amino acids (aa) and belongs to the class I fusion protein family. It was initially produced as a precursor known as F0. This F0 precursor undergoes cleavage by the host furin protease, leading to the release of a 27 kDa protein fragment (F-p27) and generating the prefusion F (Pre-F) protein. Pre-F protein consists of two fragments, F2 (20 kDa) and F1 (50 kDa), linked together by a disulfide bond [12,19]. The F protein easily changes conformation from the pre-fusion form into the post-fusion form resulting in lower induction neutralizing antibodies [20,21]. To retain the structure of RSV-F into perfusion stage, many structural based design has been used such as disulfide bridging and cavity filling have been introduced into the F structure called, DS-Cav1 [22] or mutation in their structure to reduce repulsion in the structure [23]. Recently, a study conducted by Lee et al. found that the F-p27 peptide is highly expressed on the surface of virion of RSV-infected cells and lung samples from RSV-infected mice [24]. Also, serum and nasal washes from RSV-infected patients contain a high number of antibodies against F-p27 [25]. These results imply that the immunological response mediated by F-p27 may be involved in the replication of the virus and F-p27 should be considered as inclusion in an RSV vaccine development. Moreover, the previous RSV vaccine candidate based on a pre-fusion form containing F-p27 peptide elicited high neutralizing antibodies and protected animals against RSV challenge in mice compared to the pre-fusion form without F-p27 [26].
A vaccine for RSV was previously produced in transgenic tomatoes as an oral vaccine. Oral immunization of mice with the transgenic tomato resulted in the induction of RSV-F specific antibodies, both of serum and mucosal. Additionally, the immunized mice showed an increased titer of serum antibodies when exposed to inactivated RSV antigen [27]. RSV-F protein also has been previously expressed in a mammalian cell as a protein subunit vaccine, and it has been reported to elicit strong immunogenicity and neutralizing antibody activity in mice [13,23]. Therefore, in this study, a protein subunit vaccine was developed by using F native protein (including F-p27, F native construct) and structural stabilized F protein (SC-TM construct) by fusing with Fc region of human IgG1 and expressed in Nicotiana benthamiana. Utilizing plant expression platforms can provide many advantages compared to conventional expression platforms [28,29]. For instance, plant presents a lower production cost, only 0.1–1 % of the cost of recombinant protein production compared to the mammalian cell culture platform [30], also, a plant transient expression system can produce functional protein in typically a few days, which is much faster than other expression systems, such as mammalian cells or transgenic plants. Furthermore, the plant expression platforms are easy to scale up and plants can perform post-translational modifications, such as glycosylation [31,32], which is important for the function and stability of proteins [31,33]. Plant system was used for producing various vaccines, monoclonal antibodies, diagnostic reagents, and other biopharmaceuticals [[34], [35], [36]]. Hence an attempt was made to assess the immunogenicity and neutralizing activity of the plant-produced RSV vaccine by combining the recombinant antigens with either 3M-052 alum or 3M-SE adjuvants.
2. Materials and methods
2.1. Gene construction
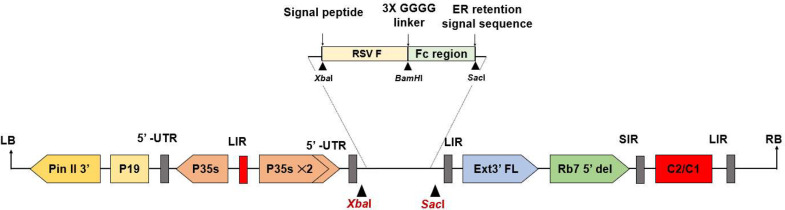
The native sequence encoding RSV fusion protein strain A was obtained from GenBank (Accession number: ACO83301.1) amino acid 26-529. The SC-TM (structural stabilized) protein sequence was obtained from the Protein data bank (PDB: accession number: 5C6B_F) amino acid 26-517. These sequences were codon optimized for expression in Nicotiana benthamiana, XbaI and BamHI restriction sites were added for cloning. Both constructs were fused with the Fc tag which derived from the Fc region of human immunoglobulin G1 (IgG1) (GenBank accession number: 4CDH_A) using peptide linker 3XGGGG at C-terminal with BamHI and SacI restriction site. The barley alpha-amylase signal peptide was added to the N terminus, and SEKDEL was added to the C-terminus of both the constructs before cloning into the geminiviral plant expression vector (pBYR2eK2Md; pBYR2e) as shown in Fig. 1. The recombinant vectors were then transformed into Agrobacterium tumefaciens GV3101 via electroporation.
Fig. 1.
Schematic representation of the T-DNA region of the pBYR2e RSV-F Fc fusion plant expression vector. The T-DNA region plays a crucial role in facilitating the transfer of the gene of interest into plant cells. It includes the left and right borders, known as RB and LB, which serve as the boundaries for gene transfer. The Pin II 3′ sequence derived from potato proteinase inhibitor II acts as a border element. The vector also incorporates several important components, such as the Tomato Bushy Stunt Virus (TBSV) RNA silencing suppressor, P19; the Cauliflower Mosaic Virus (CaMV) 35s promoter, P35s; the CaMV enhancer, P35s × 2; the tobacco extension gene region, Ext3′ FL, 3′; the tobacco RB7 promoter, Rb7 5′ del; the Bean Yellow Dwarf Virus (BeYDV) short intergenic region, SIR; the BeYDV long intergenic region, LIR; and the BeYDV replication initiation proteins, Rep and RepA, along with C2/C1.
2.2. Transient expression of recombinant protein in N. benthamiana
Agrobacterium tumefaciens GV3101 harboring an expression vector was cultured in Luria–Bertani (LB) medium supplemented with 50 µg/ml rifampicin, gentamicin, and kanamycin. The culture was grown overnight at 28 °C with continuous shaking at 200 rpm. The Agrobacterium culture was then diluted in infiltration buffer [10 mM MES and 10 mM MgSO4, pH 5.5] to yield a final optical density (OD600) of 0.2. The diluted Agrobacterium suspension was infiltrated into the 6- to 8-week-old wild-type plants (N. benthamiana) by vacuum infiltration. In this procedure, entire plants were immersed in an Agrobacterium suspension, and a vacuum pump was used to generate negative atmospheric pressure (approximately 200 mbar for 2 min). Subsequently, the vacuum is released, and the plants are allowed to grow further. Infiltrated leaves were harvested on day 4 after infiltration.
2.3. Protein extraction and purification
Infiltrated leaves were homogenized with 1x PBS (phosphate-buffered saline, pH 7.4) and centrifuged at 18,000 g at 4 °C for 30 min. The supernatant was then filtered through a 0.45 µm membrane filter (Merck, Rahway, New Jersey, USA) before loading into a protein A column which was packed with MabSelectSURE protein A bead (Cytiva, Buckinghamshire, UK). After 10 column volumes of washing with 1x PBS pH 7.4, the recombinant protein was eluted with 0.1 M glycine, pH 2.7, and neutralized with 1.5 M Tris pH 8.0. The neutralized recombinant protein was dialyzed against 1x PBS pH 7.4 overnight with SnakeSkin Dialysis Tubing (10 K MWCO) (ThermoFisher Scientific, MA, USA).
2.4. Protein quantification
Protein quantification was performed by using the Pierce BCA™ kit (ThermoFisher Scientific, MA, USA). Two microliters of protein sample were diluted with deionized water to a final volume of 50 µL and added into 96 wells plate (Corning, NY, USA). Then, a BCA working solution which comprise 49 parts of Reagent A, and 1 part of Reagent B was added into wells and incubated at 25 °C for 30 min. Optical density at 560 nm (OD560) was measured with a multimode reader (Perkin Elmer, MA, USA). The standard curve was plotted by using bovine serum albumin at the concentration of 100 µg/ml to 1500 µg/ml (ThermoFisher Scientific, MA, USA).
2.5. SDS-PAGE and western blot
Two micrograms of purified proteins were denatured by heating at 95 °C for 5 min with SDS loading buffer and separated on 4–12 % sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS–PAGE) under reducing and non-reducing conditions. Protein bands are visualized with Instant blue staining (Abcam, Cambridge, UK). Precision plus protein all blue was used as standard (Bio-rad, Hercules, USA) for SDS-PAGE and PageRuler™ Plus prestained protein ladder was used as standard in western blot (ThermoFisher Scientific, MA, USA). For western blot analysis, protein bands were electro-transferred to a 0.45 µm nitrocellulose membrane (Bio-rad, Hercules, USA) and blocked with 5 % skim milk in PBS (Difco, NJ, USA). The membrane was then probed with HRP-conjugated goat anti-human IgG gamma heavy chain (Southern Biotech, Birmingham, AL, USA) at 1:4000 dilution in 3 % skim milk in PBS. After washing three times with PBS + 0.05 % Tween 20 (PBS-T), the signal was developed by using West Pico ECL reagent (ThermoFisher Scientific, MA, USA) and visualized by ImageQuantTM LAS500 (GE Healthcare, Uppsala, Sweden).
2.6. Binding of plant-produced RSV-F fusion protein to plant-produced Motavizumab
96-well plate (Corning, NY, USA) was coated with 2 µg/ml of plant-produced F-native protein, plant-produced SC-TM protein, and plant-produced SARS-CoV-2 Receptor binding domain protein [36] (as a negative control) in phosphate buffer (20 mM, pH 7.4), 25 µl per well, overnight at 4 °C. After washing three times with PBS-T, the plate was blocked with 200 µL of 5 % skim milk in PBS at 37 °C for 1 h. Then, the plate was washed three times with PBS-T and incubated with serially diluted plant-produced Motavizumab and incubated at 37 °C for another 2 h. After sample incubation, the solution was removed and washed three times with PBS-T. After washing, the plate was incubated with 1:2500 HRP-conjugated goat anti-human IgG kappa light chain (Southern Biotech, Birmingham, AL, USA), and incubation was continued at 37 °C for another hour. After secondary antibody incubation, the wells were emptied and washed. The TMB one substrate solution (Promega, Madison, WI, USA) was added and incubated for 10 min, the reaction was stopped by adding 1 M sulfuric acid and the absorbance was measured using a multimode reader (Perkin Elmer, MA, USA) at 450 nm.
2.7. Mice immunization with the RSV-F vaccine
The protocol for mice immunization was subjected to review and approval by the Institutional Animal Care and Use Committee, Faculty of Medicine, Chulalongkorn University (Protocol no. 007/2566). The selection of the dose utilized in this study was based on a preliminary investigation conducted by Krarup et al. [23]. Therefore, recombinant F protein was formulated with 20 µg/ml 3M-052 and squalene-based oil-in-water emulsion in PBS (3M-SE) or 20 µg/ml 3M-052 and alum in PBS (3M-Alum). Four-week-old female ICR mice were divided into 6 groups (n = 5 per group) as follows: control group: Placebo (3M-SE); 5 µg of F-Native + 3M-SE; 5 µg of SC-TM + 3M-SE; control group: Placebo (3M-Alum); 5 µg of F-Native + 3M-Alum; 5 µg of SC-TM + 3M-Alum. On days 0 and 21, mice were intramuscularly (IM) injected with the RSV-F vaccine or placebo. Sera were collected at day 0 (pre-immune serum) and day 35 (14 days after the second immunization).
2.8. Evaluation of RSV specific antibody titer by ELISA
96-well plate (Corning, NY, USA) was coated with 2 µg/ml of RSV-F Protein (Extracellular domain, His Tag) (Sino biological, Cat#11049-V08B, Beijing, China) in phosphate buffer (20 mM, pH 7.4), 25 µl per well, overnight at 4 °C. After washing three times with PBS-T, the plate was blocked with 200 µL of 5 % skim milk in PBS at 37 °C for 1 h. Then, the plate was washed three times and subsequently, the mouse sera were two-fold serially diluted with 1x PBS starting at 1:100 was incubated on the wells for 2 h at 37 °C and PBS was used as a negative control. After sera binding, the plate was washed and 1:2000 goat anti-mouse IgG HRP conjugated antibody in PBS (Southern Biotech, Birmingham, AL, USA) were added into the wells and the plate was incubated for another 1 h at 37 °C. After secondary antibody incubation, the wells were emptied and washed. A TMB one substrate solution (Promega, Madison, WI, USA) was added and incubated for 10 min, the reaction was stopped by adding 1 M sulfuric acid and the absorbance was measured using a multimode reader (Perkin Elmer, MA, USA) at 450 nm. The endpoint titers were determined as the highest dilution of sera, which had A450 more than the cut-off value. The cutoff value was calculated from A450 of pre-immunized sera in the dilution of 1:100 [37]. All samples were performed in duplicates. All data in each group were compared by using nonparametric test in GraphPad Prism software version 9.3 and the values of P< 0.05 were considered statistically significant.
2.9. Cells and virus cultivation
RSV strain A2 (VR-1540) was obtained from the American Type Culture Collection (ATCC, Rockville, MD) and propagated in HEp-2 cells (ATCC CCL-23) in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 2 % FBS, 100 U/mL of penicillin, and 100 µg/mL of streptomycin. Vero cells (ATCC CCL-81) were used to perform the microneutralization assay. Vero cells were cultured in Minimum Essential Medium (MEM) supplemented with 10 % FBS, 2 mM L-glutamine, non-essential amino acids, and 100 U/mL of penicillin/streptomycin. All culture media reagents and supplements were obtained from Gibco® (ThermoFisher Scientific, Detroit, MI, USA).
2.10. Microneutralization assay (MNA)
The MNA protocol was modified from Boukhvalova et al. [38]. In brief, the mouse sera were heat inactivated at 56 °C for 30 min. The sera then were diluted four-fold in triplicate, starting from 1:10. The MEM supplemented with 2 % FBS, 100 U/mL of penicillin, and 100 µg/mL of streptomycin was used as the dilution buffer throughout the assay. The RSV strain A2 was diluted in dilution buffer to achieve an infectious dose of 100 TCID50 (50 % tissue culture infectious dose) in the final volume of the assay. Equal volumes of 100 TCID50 RSV strain A2 were added to the serial dilution sera in 96-well U-bottom plates, which were then incubated at 37 °C with 5 % CO2 for 1 h. After 1 h. of incubation, one hundred microliters of the serum-virus mixture were transferred to a 96-well flat bottom plate, then mixed with 50 µL of freshly trypsinized Vero cells in dilution buffer (20,000 cells/50 µL). The serum-virus-cell mixture was incubated for three days at 37 °C with 5 % CO2. The virus control, cell control, and virus back-titration were all present on each plate. The convalescent serum from an RSV-recovered patient, or intravenous immunoglobulin (IVIG) serves as the positive control while H4 (a monoclonal antibody against SARS-CoV-2) and Nivolumab (a monoclonal antibody against PD-1) were served as a negative control in this assay (supplementary data).
After three days of incubation, the media was discarded and washed once with Dulbecco's Phosphate Buffer Saline (D-PBS). The cells were fixed with ice-cold 80 % acetone/20 % D-PBS for 20 min at 4 °C. The plates were washed three times with 1xPBS-T before being blocked for 1 h with a blocking buffer (4 % BSA and 0.01 % Tween-20 in 1xPBS). Human RSV fusion glycoprotein was detected with rabbit anti RSV-F mAb (Sino Biological, Cat#11049-R302, Beijing, China) diluted 1:3000 in 1x PBS containing 0.5 % BSA and 0.01 % Tween-20 added to each well and incubated for 1 h at 37 °C. The detection antibody was removed by washing the plate three times, then 1:2000 HRP-conjugated goat anti-rabbit polyclonal antibody (Dako, Glostrup, Denmark A/S) was added, and the plate was incubated at 37 °C for 1 h. Plates were washed three times, and then TMB substrate was added (KPL, MA, USA) for 10 min. The reaction was stopped with 1 N HCl. Absorbance was measured at 450 and 620 nm (reference wavelength) with an ELISA plate reader (Tecan Sunrise®, Männedorf, Switzerland). The average A450/620 values of cell control and virus control were used to normalize 0 % and 100 % of infection, respectively. The inhibitory concentration (IC50) was calculated using a normalized response with a variable slope in GraphPad Prism software version 9.3. Sera with nondetectable titers were assigned with a value of LOD (Log2 IC50 = 4.9). All data in each group were compared by using nonparametric test in GraphPad Prism software version 9.3 and the values of P < 0.05 were considered statistically significant.
2.11. Statistical analysis
The statistical analyses were analyzed using GraphPad Prism 9.3. To calculate the results of RSV specific IgG and neutralizing titer, a nonparametric test (one-way analysis of variance (ANOVA)) was used. All P values < 0.05 were defined as statistically significant.
3. Results
3.1. Expression and purification of RSV-F Fc fusion protein from N. benthamiana
The recombinant F native protein (native construct) was constructed based on a native sequence without introducing any mutation into the sequence whereas the SC-TM construct was made by deleting the F-p27 protein, mutation on the furin cleavage site, and three-point mutation (N67I, S215P and E487Q) into the sequence was introduced to retain the structure into pre-fusion form. The coding sequence of the F protein was linked with the Fc tag, codon-optimized to enhance expression in plants, and cloned into an expression vector, pBYR2e (Fig. 1). The recombinant vector was transformed into Agrobacterium and infiltrated into the tobacco plants. The recombinant protein was then extracted and purified using protein A chromatography.
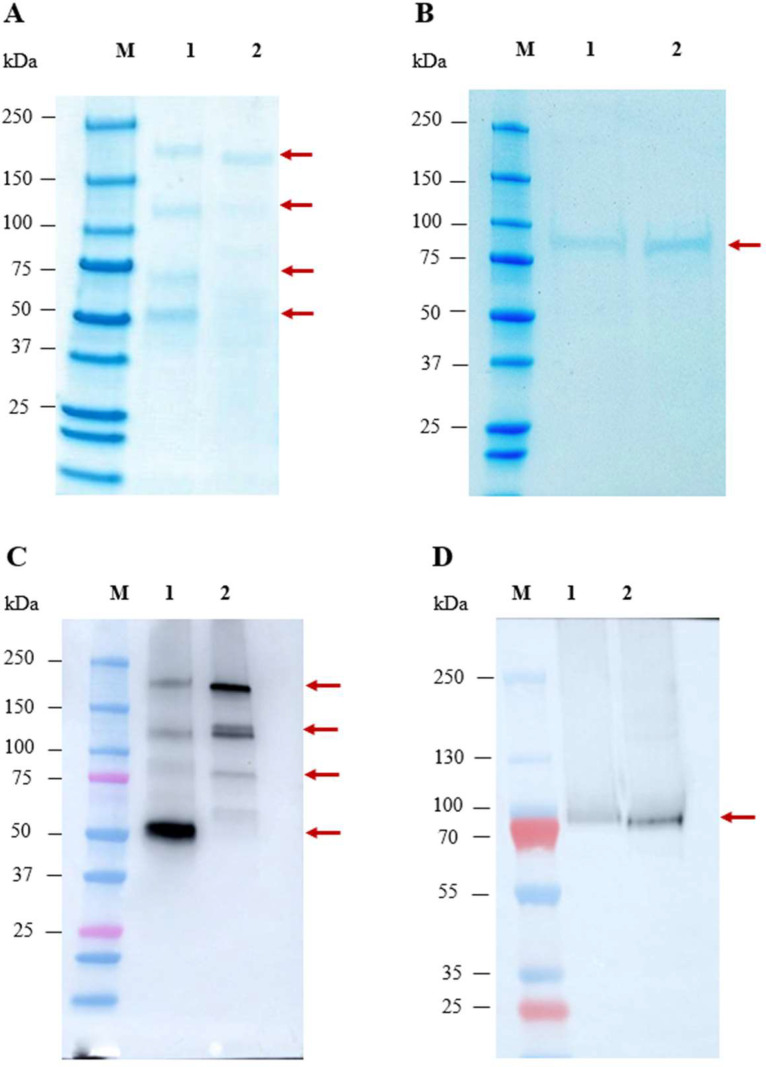
The purified recombinant proteins was analyzed by SDS-PAGE and western blot. For RSV-F native, four major bands were observed (indicated by an arrow) in the range of approximately 50–160 kDa, with one prominent band approximately 50 kDa. In the case of SC-TM, non-reducing conditions showed one major band at approximately 160 kDa and faint bands spanning from 50–160 kDa (Fig. 2A). The multiple bands observed under non-reducing conditions likely correspond to different protein forms, including the monomer (~ 70 kDa), dimer (~ 140 kDa), trimer (~ 200 kDa), and some protein at approximately 50 kDa. The presence of the 50 kDa protein may be attributed to incomplete denaturation during gel electrophoresis, leading to varying migration rates of the protein components. Importantly, we did not observe any degraded product below 50 kDa on SDS-PAGE gel, and western blot analysis using anti-human IgG Fc HRP showed a positive signal, indicating the presence of our target protein. Therefore, it is highly probable that one of these bands represents our target protein. Under reducing conditions, only a major band of approximately 80 kDa was observed, as expected (Fig. 2B). This is because the plant lacks the furin protease [37] and, therefore, could not cleave the native sequence, resulting in a single chain that can be observed on the reduced gel. The identity of the proteins in both non-reducing and reducing conditions was confirmed through western blot analysis using an anti-human gamma (Fc tag) antibody (Fig. 2C and D). The yield of plant-produced RSV-F native and SC-TM proteins were determined to be 3.60 and 6.25 mg per kilogram fresh leaf weight respectively.
Fig. 2.
SDS-PAGE and western blot analysis of plant-produced RSV-F Fc fusion protein. SDS-PAGE stained with Instant Blue™ under non-reducing (a) and reducing condition (b) whereas, lane M, Protein ladder; lane 1, purified RSV F-Native Fc fusion protein; lane 2, purified SC-TM Fc fusion protein. Western blot analysis under non-reducing conditions (c) and reducing condition (d), the membrane was probed with anti-human IgG Fc HRP. The arrowhead indicates the major band.
3.2. Antigen recognition by mAb motavizumab specific to RSV-F protein
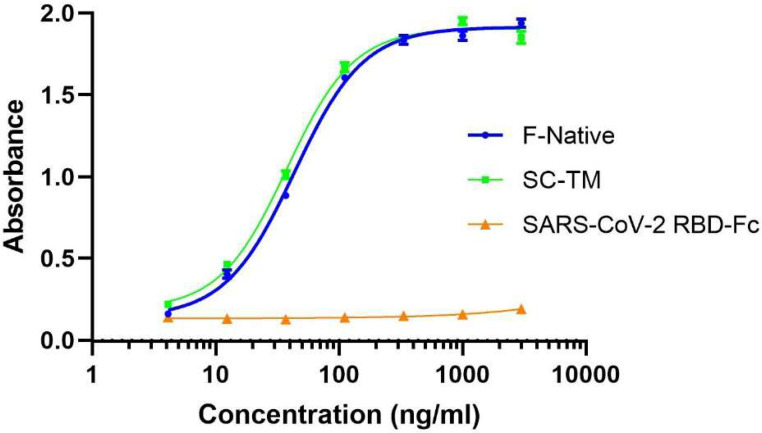
We also evaluated the binding of recombinant F protein with RSV-specific antibodies by ELISA. The result showed that both F native (with F-p27) and SC-TM proteins have similar binding activities with motavizumab, a monoclonal antibody specific against RSV-F protein. In contrast, the plant-produced RBD-Fc (negative control) did not show binding efficiency to motavizumab as expected (Fig. 3).
Fig. 3.
The binding activity of the plant-produced RSV-F Fc fusion protein to Motavizumab (anti-RSV mAb) was analyzed by ELISA. The plant-produced RBD-Fc was used as a negative control. Data are presented as mean ± SD of triplicates.
3.3. Immunogenicity and neutralizing activity of plant-produced RSV-F Fc fusion protein in mice
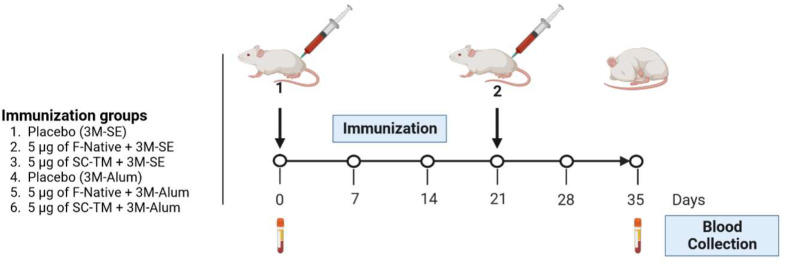
The immunogenicity of RSV-F Fc produced in plants was evaluated. The immunogenicity of the purified protein was investigated in mice using a two-dose prime-boost strategy. Intramuscular immunization of ICR mice was performed on day 0 and 21, with 5 µg of protein formulated with 3M-052 adjuvant and alum or squalene emulsion. Blood samples were collected on day 0 (pre-immune serum) and 35 to assess the overall immune response (Fig. 4).
Fig. 4.
Schematic illustration of immunization protocol and blood collection. Groups of mice (five mice per group) were intramuscularly immunized with 5 µg of RSV-F Fc fusion protein with 3M-SE or 3M-Alum adjuvant. A booster dose was administered 21 days after the first immunization. Mice sera were collected on day 0 (pre-immune sera) and day 35 post-immunization.
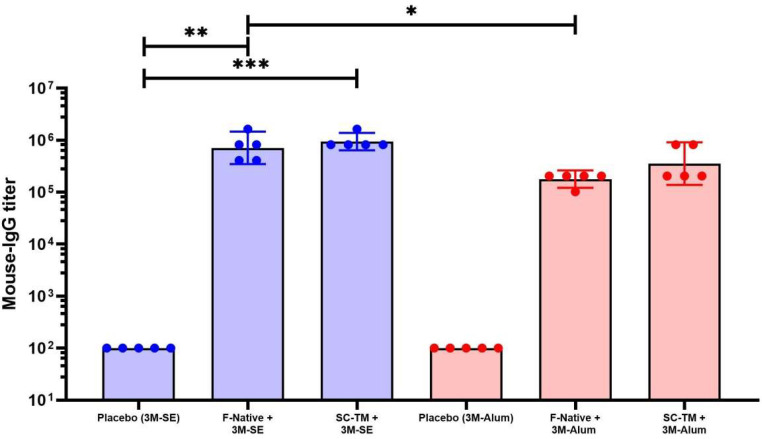
The RSV-F specific ELISA showed that both F native and SC-TM protein formulated in squalene emulsion showed significantly higher IgG titer than the control group with P < 0.01 and P < 0.001, respectively. However, no significant difference was observed in the alum group.
The effect of adjuvant on the immunogenicity of different proteins were evaluated. The F native protein formulated with squalene emulsion showed significantly higher titer than the F native protein with alum with P < 0.05. However, no significant difference was observed in the SC-TM construct group, with P value of 0.0679 (Fig. 5).
Fig. 5.
Immunogenicity of RSV-F Fc fusion protein in mice. The mice sera was collected on day 35 and RSV specific titer was analyzed by ELISA using RSV-F his protein as the capture antigen. Data were presented as GMT ± 95 % CI of the endpoint titer (n = 5). A nonparametric test was used to compare each group. (∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001).
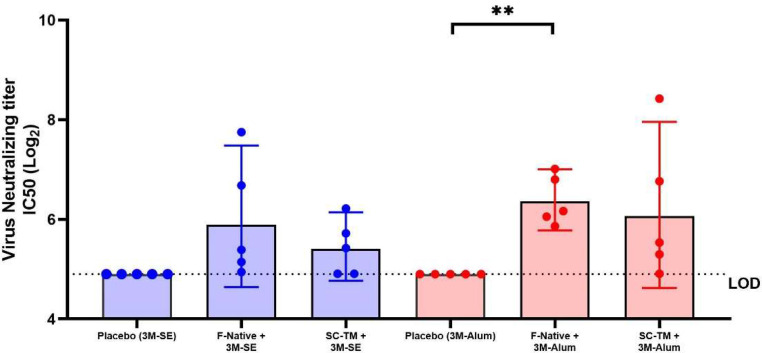
In order to evaluate the neutralizing activity, microneutralization assay was performed. The results showed that both F native and SC-TM proteins formulated in squalene emulsion did not induce significant neutralizing antibodies (Nab) compared to the control group. Whereas the vaccine formulated in alum, the F native protein but not the SC-TM protein significantly induce Nab compared to the control group with P < 0.01.
The F native protein formulated in squalene emulsion did not show significant Nab titer compared with alum formulation. Also, SC-TM protein with squalene emulsion did not show significant Nab titer compared with alum formulation. These results suggested that the mice induced RSV-specific antibodies but failed to neutralize the RSV significantly (Fig. 6).
Fig. 6.
Neutralizing antibody against RSV strain A2 in immunized sera. The mice sera were collected on day 35 and the neutralizing titer was evaluated by microneutralization. Values below the limit of detection (LOD) are represented as LOD in the plot. Data presented as GMT ± 95 % CI (n = 5). A nonparametric test was used to compare each group. (∗∗, P < 0.01).
4. Discussion
RSV-related illness is the most frequent cause of pediatric and neonatal hospitalizations among elderly and high-risk adults. In newborns and young children, severe RSV infections are linked to chronic and recurrent asthmatic episodes and resulting in fatality in some severe cases. According to the CDC, up to 300 children in the USA, under the age of five die every year, and up to ten thousand deaths among 65 and older adults due to RSV illness [38]. Thus, the development of an effective and safe vaccination is urgently needed. Since the emergence of RSV, many research groups are focusing on developing RSV vaccine and RSV-specific antibodies for prophylaxis. To date, two monoclonal antibodies, palivizumab and nirsevimab, have received the FDA approval for RSV treatment. Immunotherapy has the potential to significantly reduce the risk of severe RSV infections while also protecting vulnerable populations.
Generally, the native trimeric RSV-F (without F-p27) is known to be metastable whereas lower energy post-fusion structures are known to aggregate [39,40] and induce less or no neutralizing antibodies. A recent study suggests that the F-p27 peptide is abundantly expressed on the surface of the virion of RSV-infected cells and lung samples from RSV-infected mice. Additionally, individuals with RSV infection have significant levels of antibodies against F-p27 in their blood and nasal washes. This finding suggests that F-p27 could play a significant role in the immunogenicity of RSV-F antigen. In this study, we presented the immunogenicity of plant-produced F native containing F-p27 peptide fused with Fc protein and the structural stabilized SC-TM Fc fusion protein. The RSV-F Fc fusion proteins were transiently expressed and purified from the plant crude extracts by protein A affinity chromatography. The purified RSV-F Fc fusion protein was recognized by monoclonal antibody against RSV-F, motavizumab. The results indicated that the RSV-F Fc protein can be produced in plants The two RSV-F Fc proteins were then formulated with 3M-052, a synthetic TLR-7/8 agonist [41] with different adjuvants (Squalene emulsions and Alum) and investigated on the ability to stimulate an immune response in animals. 3M-052 is a synthetic small molecule adjuvant that acts as a toll-like receptor (TLR) 7/8 agonist, activating specific receptors in the immune system and triggering and enhancing the immune response. These adjuvants have been selected based on their safety profile, ability to enhance the immune response to the desired level, and compatibility with the various vaccine antigens [[41], [42], [43], [44]]. In mice, RSV-F-Fc fusion protein induced RSV-specific titer after intramuscular injection with a 2-dose prime–boost strategy. The microneutralization assay clearly demonstrated that the native protein, when formulated in 3M-Alum, elicited a significant increase in neutralizing antibodies compared to the control group. However, it is importantto note that a high titer alone does not guarantee a corresponding high level of neutralizing activity. Although a high titer indicates a substantial concentration of antibodies, it does not directly translate to the efficacy of those antibodies in neutralizing the virus. Several critical factors, including antibody quality, specificity, and affinity, significantly influence the neutralizing activity exhibited by the antibodies [45].
RSV F protein was previously expressed in tomato and reported to induce both serum and mucosal RSV-F specific antibodies when administered in mice orally [27]. Many RSV vaccine prototypes are produced based on F-stabilized protein without F-p27 [23,46] and expressed in mammalian cells. A previous report suggests that RSV-F Fc fusion protein produced in mammalian cells tends to form in hexamer [47], while the plant-produced RSV F Fc fusion protein form dimer.
The use of Fc as a fusion partner in therapeutics has been widely used due to its ability to extend the half-life of the target protein, enhance its immunogenicity, enhance the recombinant protein expression, as well as increase its solubility and stability [[48], [49], [50]]. Previous studies suggested that the Fc portion can sometimes alter the quaternary structure of the protein, which results in far lower induction of neutralizing antibodies compared to antigens without the Fc portion [51]. In addition, the Fc fusion tag also increases the size of the antigen, which could make it more difficult for immune cells to access the important neutralizing epitopes. These factors can substantially alter antigenicity of the target protein.
The limitation of this study is the lack of a suitable comparator, such as mammalian-produced DS-CAV variants, which are known to stimulate strong neutralizing titers [20,22]. Including a well-established comparator would have provided valuable insights about the observed neutralization patterns and allowed for comprehensive analysis of our plant-produced vaccine candidates. Addressing this limitation by including a suitable comparator could have enhanced the significance and interpretation of our findings.
5. Conclusion
In conclusion, the development of an RSV vaccine would have significant public health benefits and would help to reduce the disease burden. Here, we demonstrated the quick and efficient production of RSV-F Fc fusion protein in plant expression platform. The plant-produced RSV subunit vaccine induced an immunogenic response in mice. However, this study warrants further studies on dose optimization, adjuvant selection for eliciting neutralizing antibodies and efficacy studies in animal models.
Institutional review board statement
The animal protocol usage in this study was reviewed and approved by the committee from faculty of medicine, Chulalongkorn university.
CRediT authorship contribution statement
Nuttapat Pisuttinusart: Conceptualization, Investigation, Writing – original draft, Writing – review & editing, Methodology. Balamurugan Shanmugaraj: Investigation, Writing – original draft, Writing – review & editing. Chanya Srisaowakarn: Investigation, Methodology. Chutitorn Ketloy: Methodology. Eakachai Prompetchara: Methodology, Writing – original draft. Arunee Thitithanyanont: Conceptualization, Investigation, Methodology. Waranyoo Phoolcharoen: Conceptualization, Funding acquisition, Investigation, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
WP from Chulalongkorn University is a founder/shareholder of Baiya Phytopharm Co., Ltd., Thailand.
Acknowledgments
Acknowledgments
We appreciate the technical assistance provided by the technicians and staff during the experimental study. The author would like to thank the 72nd anniversary of His Majesty King Bhumibol Adulyadej for the graduate school doctoral fellowship and Overseas Research Experience Scholarship (ORES) for Graduate Students from both the Graduate School and the Faculty of Pharmaceutical Science, Chulalongkorn University.
Funding
This research is funded by Thailand Science research and Innovation Fund Chulalongkorn University.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.btre.2023.e00826.
Appendix. Supplementary materials
Data availability
No data was used for the research described in the article.
References
- 1.Mazur N.I., Higgins D., Nunes M.C., Melero J.A., Langedijk A.C., Horsley N., et al. The respiratory syncytial virus vaccine landscape: lessons from the graveyard and promising candidates. Lancet Infect. Dis. 2018;18:e295–e311. doi: 10.1016/s1473-3099(18)30292-5. [DOI] [PubMed] [Google Scholar]
- 2.Sitthikarnkha P., Uppala R., Niamsanit S., Sutra S., Thepsuthammarat K., Techasatian L., et al. Burden of respiratory syncytial virus related acute lower respiratory tract infection in hospitalized thai children: a 6-year national data analysis. Children. 2022;9:1990. doi: 10.3390/children9121990. (Basel) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tangprasert P., Bangkok Post, Korat on alert for RSV respiratory virus after boy's death. Available from: https://www.bangkokpost.com/thailand/general/2391635/korat-on-alert-for-rsv-respiratory-virus-after-boys-death, 2022 (accessed 09.02.2023).
- 4.Rudan I., O'Brien K.L., Nair H., Liu L., Theodoratou E., Qazi S., et al. Epidemiology and etiology of childhood pneumonia in 2010: estimates of incidence, severe morbidity, mortality, underlying risk factors and causative pathogens for 192 countries. J. Glob. Health. 2013;3 doi: 10.7189/jogh.03.010401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chuaychoo B., Rattanasaengloet K., Banlengchit R., Horthongkham N., Athipanyasilp N., Totanarungroj K., et al. Characteristics, complications, and mortality of respiratory syncytial virus compared with influenza infections in hospitalized adult patients in Thailand. Int. J. Infect. Dis. 2021;110:237–246. doi: 10.1016/j.ijid.2021.07.045. [DOI] [PubMed] [Google Scholar]
- 6.Sommer C., Resch B., Simões E.A. Risk factors for severe respiratory syncytial virus lower respiratory tract infection. Open Microbiol. J. 2011;5:144–154. doi: 10.2174/1874285801105010144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi T., Balsells E., Wastnedge E., Singleton R., Rasmussen Z.A., Zar H.J., et al. Risk factors for respiratory syncytial virus associated with acute lower respiratory infection in children under five years: systematic review and meta-analysis. J. Glob. Health. 2015;5 doi: 10.7189/jogh.05.020416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu C., Ho D.Y., Boeckh M. In: Principles and Practice of Transplant Infectious Diseases. Safdar A., editor. Springer; New York, NY: 2019. Respiratory Viral Infections in Transplant Recipients. [DOI] [Google Scholar]
- 9.Graham B.S. Biological challenges and technological opportunities for respiratory syncytial virus vaccine development. Immunol. Rev. 2011;239:149–166. doi: 10.1111/j.1600-065X.2010.00972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graham B.S. The journey to RSV vaccines — heralding an era of structure-based design. N. Engl. J. Med. 2023;388:579–581. doi: 10.1056/NEJMp2216358. [DOI] [PubMed] [Google Scholar]
- 11.Papi A., Ison M.G., Langley J.M., Lee D.-G., Leroux-Roels I., Martinon-Torres F., et al. Respiratory syncytial virus prefusion F protein vaccine in older adults. N. Engl. J. Med. 2023;388:595–608. doi: 10.1056/NEJMoa2209604. [DOI] [PubMed] [Google Scholar]
- 12.Dudas R.A., Karron R.A. Respiratory syncytial virus vaccines. Clin. Microbiol. Rev. 1998;11:430–439. doi: 10.1128/cmr.11.3.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel N., Tian J.H., Flores R., Jacobson K., Walker M., Portnoff A., et al. Flexible RSV prefusogenic fusion glycoprotein exposes multiple neutralizing epitopes that may collectively contribute to protective immunity. Vaccines. 2020;8 doi: 10.3390/vaccines8040607. (Basel) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McLellan J.S., Ray W.C., Peeples M.E. Structure and function of respiratory syncytial virus surface glycoproteins. Curr. Top. Microbiol. Immunol. 2013;372:83–104. doi: 10.1007/978-3-642-38919-1_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simões E.A.F., Center K.J., Tita A.T.N., Swanson K.A., Radley D., Houghton J., et al. Prefusion F protein–based respiratory syncytial virus immunization in pregnancy. N. Engl. J. Med. 2022;386:1615–1626. doi: 10.1056/NEJMoa2106062. [DOI] [PubMed] [Google Scholar]
- 16.Gilbert B.E., Patel N., Lu H., Liu Y., Guebre-Xabier M., Piedra P.A., et al. Respiratory syncytial virus fusion nanoparticle vaccine immune responses target multiple neutralizing epitopes that contribute to protection against wild-type and palivizumab-resistant mutant virus challenge. Vaccine. 2018;36:8069–8078. doi: 10.1016/j.vaccine.2018.10.073. [DOI] [PubMed] [Google Scholar]
- 17.Aliprantis A.O., Shaw C.A., Griffin P., Farinola N., Railkar R.A., Cao X., et al. A phase 1, randomized, placebo-controlled study to evaluate the safety and immunogenicity of an mRNA-based RSV prefusion F protein vaccine in healthy younger and older adults. Hum. Vaccin. Immunother. 2021;17:1248–1261. doi: 10.1080/21645515.2020.1829899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walsh E.E., Falsey A.R., Scott D.A., Gurtman A., Zareba A.M., Jansen K.U., et al. A randomized phase 1/2 study of a respiratory syncytial virus prefusion F vaccine. J. Infect. Dis. 2022;225:1357–1366. doi: 10.1093/infdis/jiab612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.González-Reyes L., Ruiz-Argüello M., García-Barreno B., Calder L., López J., Albar J., et al. Cleavage of the human respiratory syncytial virus fusion protein at two distinct sites is required for activation of membrane fusion. Proc. Natl. Acad. Sci. U.S.A. 2001;98:9859–9864. doi: 10.1073/pnas.151098198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steff A.-M., Monroe J., Friedrich K., Chandramouli S., Nguyen T.L.-A., Tian S., et al. Pre-fusion RSV F strongly boosts pre-fusion specific neutralizing responses in cattle pre-exposed to bovine RSV. Nat. Commun. 2017;8:1085. doi: 10.1038/s41467-017-01092-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Killikelly A.M., Kanekiyo M., Graham B.S. Pre-fusion F is absent on the surface of formalin-inactivated respiratory syncytial virus. Sci. Rep. 2016;6:34108. doi: 10.1038/srep34108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joyce M.G., Bao A., Chen M., Georgiev I.S., Ou L., Bylund T., et al. Crystal structure and immunogenicity of the DS-Cav1-stabilized fusion glycoprotein from respiratory syncytial virus subtype B. Pathog. Immun. 2019;4:294–323. doi: 10.20411/pai.v4i2.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krarup A., Truan D., Furmanova-Hollenstein P., Bogaert L., Bouchier P., Bisschop I.J.M., et al. A highly stable prefusion RSV F vaccine derived from structural analysis of the fusion mechanism. Nat. Commun. 2015;6:8143. doi: 10.1038/ncomms9143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee J., Lee Y., Klenow L., Coyle E.M., Tang J., Ravichandran S., et al. Protective antigenic sites identified in respiratory syncytial virus fusion protein reveals importance of p27 domain. EMBO Mol. Med. 2022;14:e13847. doi: 10.15252/emmm.202013847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ye X., Cabral de Rezende W., Iwuchukwu O.P., Avadhanula V., Ferlic-Stark L.L., Patel K.D., et al. Antibody response to the furin cleavable twenty-seven amino acid peptide (p27) of the fusion protein in respiratory syncytial virus (RSV) infected adult hematopoietic cell transplant (HCT) recipients. Vaccines. 2020;8 doi: 10.3390/vaccines8020192. (Basel) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel N., Massare M.J., Tian J.-H., Guebre-Xabier M., Lu H., Zhou H., et al. Respiratory syncytial virus prefusogenic fusion (F) protein nanoparticle vaccine: structure, antigenic profile, immunogenicity, and protection. Vaccine. 2019;37:6112–6124. doi: 10.1016/j.vaccine.2019.07.089. [DOI] [PubMed] [Google Scholar]
- 27.Sandhu J.S., Krasnyanski S.F., Domier L.L., Korban S.S., Osadjan M.D., Buetow D.E. Oral immunization of mice with transgenic tomato fruit expressing respiratory syncytial virus-F protein induces a systemic immune response. Transgenic Res. 2000;9:127–135. doi: 10.1023/A:1008979525909. [DOI] [PubMed] [Google Scholar]
- 28.He W., Baysal C., Lobato Gómez M., Huang X., Alvarez D., Zhu C., et al. Contributions of the international plant science community to the fight against infectious diseases in humans—part 2: Affordable drugs in edible plants for endemic and re-emerging diseases. Plant Biotechnol. J. 2021;19:1921–1936. doi: 10.1111/pbi.13658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lobato Gómez M, Huang X, Alvarez D, He W, Baysal C, Zhu C, et al. Contributions of the international plant science community to the fight against human infectious diseases – part 1: epidemic and pandemic diseases. Plant Biotechnol. J. 2021;19:20–1901. doi: 10.1111/pbi.13657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen M., Liu X., Wang Z., Song J., Qi Q., Wang P.G. Modification of plant N-glycans processing: the future of producing therapeutic protein by transgenic plants. Med. Res. Rev. 2005;25:343–360. doi: 10.1002/med.20022. [DOI] [PubMed] [Google Scholar]
- 31.Xu J., Ge X., Dolan M.C. Towards high-yield production of pharmaceutical proteins with plant cell suspension cultures. Biotechnol. Adv. 2011;29:278–299. doi: 10.1016/j.biotechadv.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Shanmugaraj B, I. Bulaon C.J., Phoolcharoen W. Plant molecular farming: a viable platform for recombinant biopharmaceutical production. Plants. 2020;9 doi: 10.3390/plants9070842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pogue G.P., Vojdani F., Palmer K.E., Hiatt E., Hume S., Phelps J., et al. Production of pharmaceutical-grade recombinant aprotinin and a monoclonal antibody product using plant-based transient expression systems. Plant Biotechnol. J. 2010;8:638–654. doi: 10.1111/j.1467-7652.2009.00495.x. [DOI] [PubMed] [Google Scholar]
- 34.Shanmugaraj B., Bulaon C.J.I., Malla A., Phoolcharoen W. Biotechnological insights on the expression and production of antimicrobial peptides in plants. Molecules. 2021;26:4032. doi: 10.3390/molecules26134032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rattanapisit K., Yusakul G., Shanmugaraj B., Kittirotruji K., Suwatsrisakul P., Prompetchara E., et al. Plant-produced recombinant SARS-CoV-2 receptor-binding domain; an economical, scalable biomaterial source for COVID-19 diagnosis. Biomater. Transl. 2021;2:43–49. doi: 10.3877/cma.j.issn.2096-112X.2021.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shanmugaraj B., Khorattanakulchai N., Panapitakkul C., Malla A., Im-erbsin R., Inthawong M., et al. Preclinical evaluation of a plant-derived SARS-CoV-2 subunit vaccine: protective efficacy, immunogenicity, safety, and toxicity. Vaccine. 2022;40:4440–4452. doi: 10.1016/j.vaccine.2022.05.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mamedov T., Musayeva I., Acsora R., Gun N., Gulec B., Mammadova G., et al. Engineering, and production of functionally active human Furin in N. benthamiana plant: in vivo post-translational processing of target proteins by furin in plants. PLOS One. 2019;14 doi: 10.1371/journal.pone.0213438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hansen C.L., Chaves S.S., Demont C., Viboud C. Mortality associated with influenza and respiratory syncytial virus in the US, 1999-2018. JAMA Netw. Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.0527. -e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Begoña Ruiz-Argüello M., González-Reyes L., Calder L.J., Palomo C., Martín D., Saíz M.J., et al. Effect of proteolytic processing at two distinct sites on shape and aggregation of an anchorless fusion protein of human respiratory syncytial virus and fate of the intervening segment. Virology. 2002;298:317–326. doi: 10.1006/viro.2002.1497. [DOI] [PubMed] [Google Scholar]
- 40.Smith G., Raghunandan R., Wu Y., Liu Y., Massare M., Nathan M., et al. Respiratory syncytial virus fusion glycoprotein expressed in insect cells form protein nanoparticles that induce protective immunity in cotton rats. PLOS One. 2012;7:e50852. doi: 10.1371/journal.pone.0050852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kasturi S.P., Rasheed M.A.U., Havenar-Daughton C., Pham M., Legere T., Sher Z.J., et al. 3M-052, a synthetic TLR-7/8 agonist, induces durable HIV-1 envelope-specific plasma cells and humoral immunity in nonhuman primates. Sci. Immunol. 2020;5 doi: 10.1126/sciimmunol.abb1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Phoolcharoen W., Shanmugaraj B., Khorattanakulchai N., Sunyakumthorn P., Pichyangkul S., Taepavarapruk P., et al. Preclinical evaluation of immunogenicity, efficacy and safety of a recombinant plant-based SARS-CoV-2 RBD vaccine formulated with 3M-052-Alum adjuvant. Vaccine. 2023;41:2781–2792. doi: 10.1016/j.vaccine.2023.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smirnov D., Schmidt J.J., Capecchi J.T., Wightman P.D. Vaccine adjuvant activity of 3M-052: an imidazoquinoline designed for local activity without systemic cytokine induction. Vaccine. 2011;29:5434–5442. doi: 10.1016/j.vaccine.2011.05.061. [DOI] [PubMed] [Google Scholar]
- 44.Van Hoeven N., Fox C.B., Granger B., Evers T., Joshi S.W., Nana G.I., et al. A formulated TLR7/8 agonist is a flexible, highly potent and effective adjuvant for pandemic influenza vaccines. Sci. Rep. 2017;7:46426. doi: 10.1038/srep46426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.CHMP CfMPfHU . European Medicines Agency; 2017. Guideline on Immunogenicity Assessment of Therapeutic Proteins; p. 24. 01-12-2017 ed. [Google Scholar]
- 46.Swanson K.A., Rainho-Tomko J.N., Williams Z.P., Lanza L., Peredelchuk M., Kishko M., et al. A respiratory syncytial virus (RSV) F protein nanoparticle vaccine focuses antibody responses to a conserved neutralization domain. Sci. Immunol. 2020;5:eaba6466. doi: 10.1126/sciimmunol.aba6466. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y., Zhou Z., Zhu S.-L., Zu X., Wang Z., Zhang L.-K., et al. A novel RSV F-Fc fusion protein vaccine reduces lung injury induced by respiratory syncytial virus infection. Antiviral Res. 2019;165:11–22. doi: 10.1016/j.antiviral.2019.02.017. [DOI] [PubMed] [Google Scholar]
- 48.Huang C. Receptor-Fc fusion therapeutics, traps, and MIMETIBODY technology. Curr. Opin. Biotechnol. 2009;20:692–699. doi: 10.1016/j.copbio.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 49.Czajkowsky D.M., Hu J., Shao Z., Pleass R.J. Fc-fusion proteins: new developments and future perspectives. EMBO Mol. Med. 2012;4:1015–1028. doi: 10.1002/emmm.201201379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang C., Gao X., Gong R. Engineering of Fc fragments with optimized physicochemical properties implying improvement of clinical potentials for Fc-based therapeutics. Front. Immunol. 2017;8:1860. doi: 10.3389/fimmu.2017.01860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jungmin C., et al. Effect of Fc fusion on folding and immunogenicity of middle east respiratory syndrome coronavirus spike protein. J. Microbiol. Biotechnol. 2019;29:813–819. doi: 10.4014/jmb.1903.03043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.