Abstract
Purpose
The objective is to create a comprehensive model that integrates clinical, semantic, and radiomics features to forecast the 5-year progression-free survival (PFS) of individuals diagnosed with non-distant metastatic Nasopharyngeal Carcinoma (NPC).
Methods
In a retrospective analysis, we included clinical and MRI data from 313 patients diagnosed with primary NPC. Patient classification into progressive and non-progressive categories relied on the occurrence of recurrence or distant metastasis within a 5-year timeframe. Initial screening comprised clinical features and statistically significant image semantic features. Subsequently, MRI radiomics features were extracted from all patients, and optimal features were selected to formulate the Rad-Score.Combining Rad-Score, image semantic features, and clinical features to establish a combined model Evaluation of predictive efficacy was conducted using ROC curves and nomogram specific to NPC progression. Lastly, employing the optimal ROC cutoff value from the combined model, patients were dichotomized into high-risk and low-risk groups, facilitating a comparison of 10-year overall survival (OS) between the groups.
Results
The combined model showcased superior predictive performance for NPC progression, reflected by AUC values of 0.84, an accuracy rate of 81.60%, sensitivity at 0.77, and specificity at 0.81 within the training group. In the test set, the AUC value reached 0.81, with an accuracy of 74.6%, sensitivity at 0.82, and specificity at 0.66.
Conclusion
The amalgamation of Rad-Score, clinical, and imaging semantic features from multi-parameter MRI exhibited significant promise in prognosticating 5-year PFS for non-distant metastatic NPC patients. The combined model provided quantifiable data for informed and personalized diagnosis and treatment planning.
Keywords: Nasopharyngeal carcinoma, MRI, Machine learning, Nomogram, Long-term
Graphical Abstract
1. Introduction
Nasopharyngeal Carcinoma (NPC) manifests as a heterogeneous malignant tumour originating from the nasopharyngeal mucosal epithelium, demonstrating notable sensitivity to radiotherapy [1]. In the year 2020, the Global Cancer Centre reported 133,354 newly confirmed cases of NPC, with over 70% occurring in Southern China and Southeast Asia [2]. Given its heightened responsiveness to radiotherapy and the anatomical constraints of the nasopharynx, radiotherapy remains the primary treatment modality for individuals afflicted with non-distant metastatic NPC [3].
Amidst the progressions in medical equipment, the continual evolution of radiotherapy technology, and the widespread integration of artificial intelligence, particularly the prevalent adoption of Intensity-Modulated Radiotherapy (IMRT) in NPC [4], [5], patients have entered an era of prolonged survival. However, the incidence of distant metastasis remains notably high at 20%∼25% [5], with some advanced patients facing a 5-year survival rate of approximately 60%∼85% [6]. Consequently, recurrence and distant metastasis persist as primary contributors to therapeutic failures [7], [8]. As NPC advances, it presents a multitude of clinical challenges. Chiefly, the clinical attributes of recurrent and metastatic NPC are distinct, with around 22% of locally recurrent patients and 33% of those with distant metastases showing no clinical symptoms [9], [10]. Furthermore, over 70% of local recurrences are in advanced stages, rendering rescue interventions challenging [11]. Secondly, different medical institutions adhere to varying monitoring intervals, often leading to suboptimal utilization of medical resources or delayed diagnosis of recurrences and distant metastases for certain patients [10]. Presently, while NCCN guidelines emphasize pre-treatment imaging methods for detecting recurrences and metastases, the ESMO guidelines lack detailed recommendations. Therefore, early prognostication of recurrence and distant metastasis in NPC patients, coupled with the enhancement of patient stratification and the formulation of personalised and precise treatment strategies based on risk factors and failure patterns, stands as pivotal for improving therapeutic efficacy and extending the survival duration of NPC patients, thereby occupying a central role in clinical patient management.
The TNM staging system serves as the definitive metric for assessing the prognosis of NPC. However, current research indicates that reliance solely on anatomy-based TNM staging falls short in providing precise clinical guidance [12], [13], [14]. Notably, the invasion of the carotid artery (CAI) has emerged as a significant prognostic indicator for specific malignancies in the head and neck region [15], [16]. In the context of NPC, the impact of the primary tumour extending to the carotid sheath or the invasion of the carotid sheath by metastatic lymph nodes has been unequivocally substantiated [17], [18].
In recent years, radiomics has gained traction as a method to investigate tumour heterogeneity. It extracts textural attributes from medical images, screens these attributes, and constructs models to assess tumour heterogeneity and biological characteristics. This yields quantitative parameters for clinical use, enhancing diagnosis, distinguishing between different tumour types, and predicting individual outcomes, thereby providing more personalised treatment options for cancer patients. Based on MRI radiomics, it has demonstrated higher value in various studies related to the diagnosis and differential diagnosis of head and neck tumours [19], risk stratification [20], prognosis prediction [21], and survival application [22]. The nomogram serves as a reflection of tumour space heterogeneity and assumes the role of a novel biomarker [23], [24], [25].
This study sought to leverage clinical and multi-parameter MRI images of non-metastatic NPC to construct 5-year PFS models encompassing clinical-image semantic features, radiomics, and their combination, with the aim of exploring their predictive potential. Patients were categorised based on the optimal model, and the 10-year OS rates among different risk groups were juxtaposed, thereby providing a quantitative foundation for the clinical treatment strategies of NPC.
2. Materials and methods
2.1. Materials for clinical cases
This study has obtained approval from the local research ethics committee (approval number: NO.20220722/08/01/002). Given its retrospective nature, individual informed consent is not obligatory. The study protocol adheres to the Helsinki Declaration and is executed in accordance with relevant guidelines and regulations. A total of 517 patients diagnosed with stage I-IVa Nasopharyngeal Carcinoma (NPC) were compiled from the affiliated Oncology Hospital of the Chinese Academy of Sciences between June 2012 and December 2016. The inclusion criteria comprised: 1) patients with histopathologically confirmed NPC; 2) patients classified as stage I-IVa according to the 2017 AJCC eighth edition; 3) age not less than 18 years, with a follow-up duration of at least 60 months, who received complete Intensity-Modulated Radiotherapy (IMRT) treatment; 4) patients with bulky NPC who underwent pre-treatment MRI scans, including transverse T1-weighted imaging (T1WI), fat-suppressed T2-weighted imaging (FS T2WI), and fat-suppressed contrast-enhanced T1-weighted imaging (FS CE-T1WI), with no pronounced artefacts. Patients who continued to progress after treatment were not encompassed in our investigation.
According to the new eighth edition of the AJCC staging system, all patients were reclassified into new stages. The process of patient selection and categorisation is illustrated in Fig. 1.
Fig. 1.
The case screening process for the progressive group and the non-progressive group involved the screening of 203 and 110 eligible cases, respectively.
Demographic information, including sex, age, pathological type, T stage, N stage, induction chemotherapy (IC) regimen, treatment duration, and synchronous chemotherapy course, was meticulously collected.
2.1.1. Treatment and follow-up
All enrolled patients underwent 0–3 cycles of induction chemotherapy, comprising either the docetaxel + cisplatin + 5-fluorouracil (TPF) or cisplatin + 5-fluorouracil (PF) regimens. Subsequently, 0–2 cycles of concurrent chemotherapy were administered three to four weeks after the completion of induction chemotherapy. All patients underwent intensity-modulated radiotherapy (IMRT). The prescribed radiation dose for the nasopharyngeal primary lesion planning target (PGTVnx) ranged from 63.0 to 70.8 Gy, and for the lymph node planning target (PGTVnd) it ranged from 60.0 to 72.1 Gy. Recurrence and metastasis were defined as new pathologically confirmed NPC tumours appearing six months post-radiotherapy. In cases where pathological evidence was unavailable, recurrence and metastasis were identified through MRI, SPECT, CT, or ultrasound examination, in conjunction with clinical multidisciplinary consultation evaluation.
Following treatment completion, patients underwent follow-up evaluations every 1–3 months during the initial two years, biannually in the subsequent 2–3 years, and annually thereafter. The primary endpoint was the 5-year progression-free survival (PFS) rate for all patients, while the secondary endpoint was the 10-year OS rate. Endpoint calculation commenced from the treatment initiation date to the occurrence of the first defined event or the latest follow-up visit, which was conducted until February 23, 2023.
2.2. MR inspection square method
MR images were obtained using two distinct MR scanners, specifically Siemens Magnetom Symphony 1.5 T and Siemens Skyra 3.0 T. Cross-sectional T1-weighted images (T1WI) and fast spin-echo T2-weighted images (fs T2WI) were initially acquired. Subsequently, axial fast spin-echo contrast-enhanced T1-weighted images (fs CE-T1WI) were obtained following the administration of a gadolinium-based contrast agent at a dosage of 0.01 mmol/kg. While recommendations for neck MRI slightly varied, the protocol primarily comprised the following parameters: 1) axial T1WI: TR/TE 1700–1800 ms/910 ms, flip angle of 90°, matrix of 256 × 168, slice thickness of 5.00 mm, and slice spacing of 1.00 mm; 2) axial fs T2WI: TR/TE 5700–6360 ms/4995 ms, flip angle of 90°, matrix of 256 × 168, slice thickness of 5.00 mm, and slice spacing of 1.00 mm; 3) axial fs CE-T1WI: TR/TE 450–605 ms/8.89 ms, flip angle of 90°, matrix of 256 × 168, slice thickness of 5.00 mm, and slice spacing of 1.00 mm.
2.3. MRI semantic features
All MR images underwent independent review by two experienced head and neck radiation physicians with 8 and 15 years of experience, respectively. Relevant clinical information, such as cranial nerve palsy and lymph node size, was considered during assessment. Tumour invasion of the internal carotid artery, external carotid artery, and common carotid artery, as well as cervical lymph nodes, was evaluated based on radiological criteria from established sources [18], [26]. To assess inter-rater reliability for cervical vessel sheath involvement by tumours and lymph nodes, after a three-month interval, one of the radiologists (XYZ) re-evaluated the cases, and Cohen's kappa coefficient was calculated for evaluation.
2.4. Tumour segmentation, radiomics feature extraction, and selection
MRI Preprocessing and Image Segmentation: ① Acquisition of DICOM images for pre-treatment nasopharyngeal MR scans was conducted from the Picture Archiving and Communication System (PACS) of the hospital for each patient. ② Manual layer-by-layer segmentation of primary nasopharyngeal carcinoma (NPC) tumours on T1-weighted imaging (T1WI), fast spin-echo T2-weighted imaging (fs T2WI), and contrast-enhanced fast spin-echo T1-weighted imaging (fs T1WI+C) was performed using ITK-SNAP software (version 3.8.0). The resulting delineations were stored as regions of interest (ROIs). This segmentation task was carried out by two senior radiation department therapists, each possessing 8 and 15 years of diagnostic experience in head and neck radiology. Inter- and intra-observer agreement was evaluated using intra-class correlation coefficients (ICCs).
Image Preprocessing, radiomics Feature Extraction, and Selection: The original images, along with their corresponding delineated ROIs, were imported into the uAI Research Portal software. Initially, the original images underwent normalization, followed by the extraction of radiomics features.Radiomics features were derived from distinct groups, encompassing First Order, Shape, Gray Level Co-occurrence Matrix (GLCM), Gray Level Dependence Matrix (GLDM), Gray Level Size Zone Matrix (GLSZM), Gray Level Run Length Matrix (GLRLM), and Neighbourhood Gray Tone Difference Matrix (NGTDM). Various filters, including Original, Laplacian of Gaussian (LoG), wavelet, Additive Gaussian Noise, Box Mean, Binomial Blur Image, Box Sigma Image, Normalize, Laplacian Sharpening, Discrete Gaussian, Mean, Speckle Noise, Recursive Gaussian, and Shot Noise, were applied, resulting in the extraction of 2286 features from each sequence. Subsequently, the dataset was partitioned into a training cohort (TC) and a test cohort (VC) in an 8:2 ratio through random assignment. Employing the maximum relevance minimum redundancy (mRMR) algorithm, 20 features were retained based on their maximum correlation and minimum redundancy. Following this, the least absolute shrinkage and selection operator (LASSO) regression was applied to further reduce the dimensionality of each feature, ultimately yielding the statistically significant radiomics signature referred to as Rad-Score. The formulae for feature extraction and model development are detailed in https://pyradiomics.readthedocs.io/en/latest/features.html#. The predictive performance of the models was assessed by calculating the Area Under the Curve (AUC), accuracy, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) separately for the training set and test set.
2.5. Model establishment, verification, and analysis
Statistical analysis was performed using version and version MedCalc software 19.1 of MedCalc and 4.2.1 of R software statistical software were employed for the analyses. Measurement data adhering to a normal distribution were expressed as mean ± standard deviation (" ± s"), and inter-group comparisons were executed using the two independent samples t-test. In instances where measurement data deviated from a normal distribution, presentation took the form of median (interquartile range) [M (P25 and P75)], and inter-group comparisons were conducted using the Mann-Whitney U test. The χ2 test was utilised for the comparison of categorical data between groups. The radiomics model was constructed based on the Rad-score. To discern independent risk factors for the progression of NPC patients, multivariate logistic regression analyses were undertaken on potential predictors, encompassing sex, age, pathological type, T stage, N stage, induction chemotherapy regimen and duration, simultaneous chemotherapy course, and invasion of the carotid sheath by the tumour and lymph nodes. The identified independent risk factors, along with the Rad-Score, were amalgamated to formulate a comprehensive model. Model goodness-of-fit was assessed through the Hosmer-Lemeshow test. Receiver Operating Characteristic (ROC) curves were crafted, and a nomogram was developed to prognosticate NPC progression. The efficacy of each model was evaluated using the Area Under the Curve (AUC), with AUC values compared among different models through the Delong test. The optimal cut-off value was determined based on the AUC of the combined model. Subsequently, patients were categorised into high and low-risk groups, and 10-year overall survival (OS) curves were generated for all patients.
3. Results
3.1. Clinical characteristics selection
Based on the occurrence of recurrence or distant metastasis within a 5-year timeframe among NPC patients, they were categorised into two distinct groups: the progressive group (n = 110 cases) and the non-progressive group (n = 203 cases). The Kappa test yielded values of 0.920 and 0.879 for cervical artery tumour invasion and cervical artery lymph node invasion, respectively. Similarly, the Kappa test recorded values of 0.914 and 0.901 for cervical artery lymph node invasion. Notably, a statistically significant disparity in cervical artery sheath involvement was observed between the two groups based on N stage and tumour invasion, as presented in Table 1. These identified risk factors were subsequently integrated into the construction of a clinical-semantic feature pre-detection model for NPC progression type.
Table 1.
Comparison of clinical and imaging semantic features between the two groups.
| Clinical data and semantic features | Non-progress(203) | Progress(110) | P value | 95% CI | |
|---|---|---|---|---|---|
| Sex | male | 142 | 87 | 0.072 | -0.008–0.191 |
| female | 61 | 23 | |||
| Age (year) | 50.31 ± 9.88 | 51.92 ± 9.18 | 0.168 | -3.859–0.633 | |
| Pathological type | Type 1 | 24 | 17 | 0.914 | -0.363–0.262 |
| Type 2 | 106 | 52 | |||
| Type3 | 26 | 14 | |||
| Type 4 | 1 | 2 | |||
| Type 5 | 46 | 25 | |||
| Induction chemotherapy regimen | NO | 25 | 14 | 0.195 | -0.064–0.252 |
| PF | 86 | 56 | |||
| TPF | 92 | 40 | |||
| Course of induction chemotherapy | 0 | 25 | 14 | 0.886 | -0.084–0.056 |
| 1 | 2 | 1 | |||
| 2 | 5 | 3 | |||
| 3 | 171 | 92 | |||
| Synchronous course of chemotherapy | 0 | 12 | 4 | 0.861 | -0.141–0.087 |
| 1 | 11 | 8 | |||
| 2 | 180 | 98 | |||
| T status | 1 | 5 | 3 | 0.330 | -0.217–0.094 |
| 2 | 12 | 6 | |||
| 3 | 123 | 60 | |||
| 4 | 63 | 40 | |||
| N status | 0 | 3 | 2 | 0.005 | -0.416∼−0.084 |
| 1 | 84 | 34 | |||
| 2 | 97 | 47 | |||
| 3 | 19 | 27 | |||
| Tumor invasion | NO | 158 | 70 | 0.020 | -0.235∼−0.021 |
| Yes | 46 | 39 | |||
| Lymph node invasion | NO | 117 | 66 | 0.686 | -0.092–0.139 |
| Yes | 86 | 44 | |||
The classification is as follows: Type 1 – Squamous carcinoma, Type 2 – Non-keratinizing undifferentiated carcinoma, Type 3 – Non-keratinizing differentiated carcinoma, Type 4 – Non-keratinizing mixed cell carcinoma, Type 5 – Basal cell-like carcinoma.
3.2. Extraction and selection of radiomics features
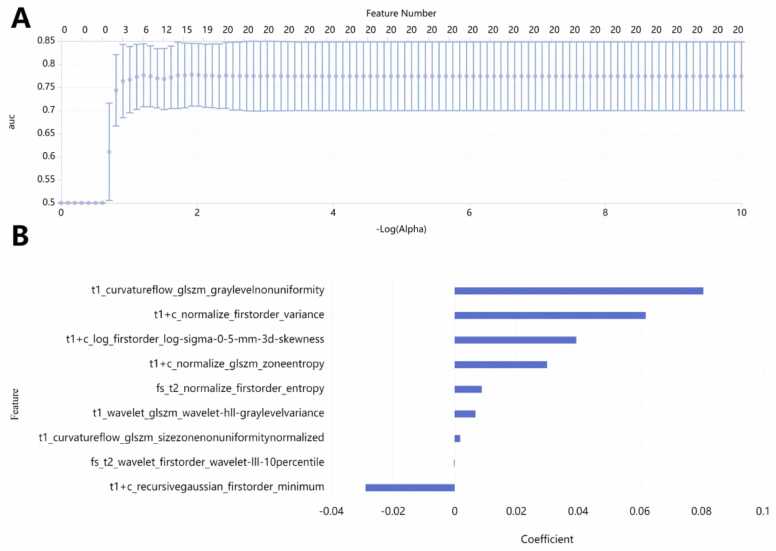
Initially, the mRMR square method was employed to select 20 texture features from a pool of over 6000 features extracted from T1-weighted imaging (T1WI), fast spin-echo T2-weighted imaging (FS T2WI), and contrast-enhanced fast spin-echo T1-weighted imaging (FS CE-T1WI) images (Fig. 2 A). Subsequently, the LASSO algorithm was applied for further refinement. To mitigate the risk of overfitting, a 10-fold cross-validation was utilised for selecting the tuning parameter λ. Ultimately, 9 optimal subsets were retained (Fig. 2B). The Rad-Score was computed by calculating the weighted sum of these features based on their correlation coefficients, as outlined in Table 2. The Rad-Score box-plot-scatter diagram, illustrating both the training and testing sets, is presented in Fig. 3.
Fig. 2.
(A) The mRMR square method was utilised to select 20 texture features. (B) Subsequently, nine key imaging features were chosen along with their corresponding correlation coefficients, distinguishing between the progressive group and the non-progressive group.
Table 2.
The nine optimal radiomics features have been selected, each accompanied by its corresponding weighted coefficients.
| Sequence | MRI radiomics features | Weighting coefficient |
|---|---|---|
| T1WI | curvatureflow_glszm_graylevelnonuniformity | 0.0806 |
| FS T1 + C | normalize_firstorder_variance | 0.0619 |
| FS T1 + C | log_firstorder_log-sigma-0-5-mm-3d-skewness | 0.0394 |
| FS T1 + C | normalize_glszm_zoneentropy | 0.0299 |
| FS T2WI | normalize_firstorder_entropy | 0.0088 |
| T1WI | wavelet_glszm_wavelet-hll-graylevelvariance | 0.0068 |
| T1WI | curvatureflow_glszm_sizezonenonuniformitynormalized | 0.0017 |
| FS T2WI | wavelet_firstorder_wavelet-III-10percentile | -0.0002 |
| FS T1 + C | recursivegaussian_firstorder_minimum | -0.0289 |
The weight values are automatically generated by the software during the feature selection process, as illustrated in Fig. 2 of the radiomics feature selection method.
Fig. 3.
Box-plot-scatter diagram of Rad-Scores for the training and testing sets (P < 0.05).
3.3. Construction and verification of the combined model
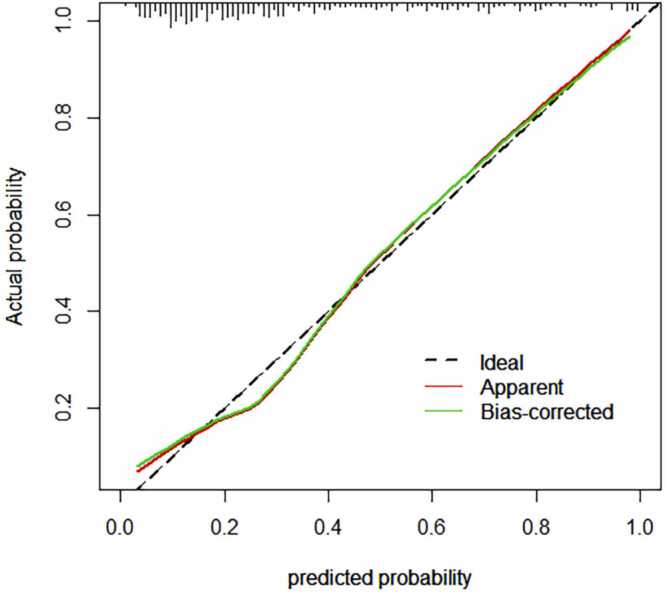
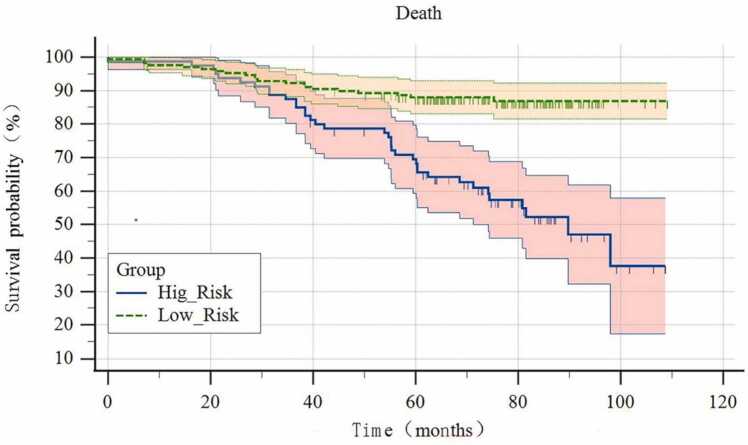
The risk of NPC progression was evaluated utilising the Rad-Score, N-stages, and tumour invasion of the carotid sheath as pivotal risk factors. The Area Under the Curve (AUC) values (Fig. 4), accuracy, sensitivity, specificity, Positive Predictive Value (PPV), and Negative Predictive Value (NPV) under the Receiver Operating Characteristic (ROC) curve were computed for both the training set and test set of the combined model. The specific values are presented in Table 3. Notably, the combined model exhibited the highest predictive value, as illustrated in Table 4, Fig. 5, Fig. 6, depicting the line diagram and fitting curve. Referring to the optimal cut-off value from the ROC curve of the combined model, the patients were categorised into high and low-risk groups, and the 10-year OS curve comparing the two groups is portrayed in Fig. 7.
Fig. 4.
ROC curves were generated for the Radomics model, clinical-semantic feature model, and combined model using the training and test sets of the two groups.
Table 3.
Evaluation indicators of multiple models of training and test sets.
| Model | AUC | 95% CI | accuracy | sensitivity | specificity | NPV | PPV | |
|---|---|---|---|---|---|---|---|---|
| TS | Radomics | 0.807 | 0.753–0.854 | 78.80 | 69.32 | 85.19 | 55.68 | 91.36 |
| Clinical-semantic | 0.615 | 0.551–0.675 | 68.40 | 42.05 | 81.68 | 90.74 | 27.27 | |
| Combined | 0.840 | 0.788–0.883 | 81.60 | 77.27 | 81.48 | 90.74 | 64.77 | |
| VS | Radomics | 0.786 | 0.665–0.879 | 74.60 | 54.55 | 92.68 | 85.37 | 54.55 |
| Clinical-semantic | 0.683 | 0.554–0.795 | 71.43 | 50.00 | 80.49 | 90.24 | 36.36 | |
| Combined | 0.809 | 0.690–0897 | 74.60 | 81.82 | 65.85 | 85.37 | 54.55 |
TS:training set, VS: test set
Table 4.
Delong test of AUC values of training set between different models.
| Radomics VS.Clinical-semantic | Radomics VS. Combined | Clinical-semantic vs. Combined | ||
|---|---|---|---|---|
| TS | Z | 3.831 | 2.020 | 5.555 |
| P | <0.001 | 0.043 | <0.001 |
Note: There are statistical differences among the three models, and the combined model has the highest predictive value. TS:training set
Fig. 5.
Diagram illustrating the training set for the progressive and non-progressive groups. CSI: Carotid Sheath Invasion, denoting the extent of tumor infiltration.
Fig. 6.
Fitting curve of progressive group and non-progressive training set.
Fig. 7.
There is a significant difference in 10-year OS between high and low risk groups. ( p < 0.001).
4. Discussion
In this investigation, we developed a combined clinical-semantic-radiomics model to predict the risk of 5-year PFS for NPC. The findings revealed that N staging and tumour invasion into the carotid sheath were significant factors influencing the 5-year PFS risk in NPC patients. Pre-treatment multi-parameter MRI radiomics emerged as a potent tool for anticipating NPC progression, with the combined model demonstrating the most robust predictive efficacy. Moreover, the nomogram developed from the combined model serves as a valuable quantitative tool for early-stage risk assessment, stratifying patients into high and low-risk categories based on the joint model's Area Under the Curve (AUC) value. Notably, a significant discrepancy in 10-year OS between the two groups is evident.
The intrusion of tumours into blood vessels is recognised as an unfavourable predictor of survival outcomes [27], [28]. Notably, the invasion of the carotid sheath by tumours, which houses an abundant vascular network, acts as a conduit for distant transmission. Our study discerns that the infiltration of the carotid sheath by tumours holds predictive value for 5-year PFS in non-metastatic NPC patients, potentially because such tumours bear an elevated tumour load [29]. Intriguingly, our research reveals that metastatic lymph nodes invading the carotid sheath exhibit no significant statistical variance in NPC progression. This observation could stem from the potential of induction chemotherapy to alleviate the tumour burden, eradicating early-stage micrometastases while demonstrating enhanced tolerance [30]. A notable portion of the patients in our dataset underwent induction chemotherapy. Furthermore, our study underscores that the combined model also possesses substantial predictive worth in forecasting 10-year OS in NPC patients.
The most prominent MRI radiomics features distinguishing the progressive and non-progressive groups primarily centre on tumour heterogeneity: GLSZM-gln measures the diversity of grey intensity values within images, with lower values signifying greater uniformity in intensity; First-order variance calculates the average squared distance between each intensity value and the mean value, serving as a mean distribution measure. First-order 3-D skewness quantifies the "peak" of the image ROI's mean value distribution, where higher skewness signifies concentration towards the distribution tail. Greater uncertainty or randomness in GLSZM-zoneentropy measurement area size and grey level distribution corresponds to higher values, indicative of heightened texture pattern heterogeneity. These traits underscore that elevated unevenness in NPC's grey intensity value distribution and heightened heterogeneity correlate with an elevated likelihood of NPC patients experiencing recurrence or distant metastasis.
MRI represents a conventional and pivotal tool in the preliminary stages of treatment for NPC patients and in determining appropriate therapeutic interventions. Moreover, radiomic features derived from multi-parameter MRI underscore the significance of tumour heterogeneity during the initial phases of tumour progression. The Delong test establishes that the composite model, which integrates clinical-MRI semantic features and radiomics studies, exhibits the most favourable predictive efficacy for NPC progression. NPC patients at an elevated risk present with pronounced tumour heterogeneity and a more substantial tumour load, thereby further heightening the susceptibility to disease progression. Early pre-testing and prompt intervention constitute the foundation for refining and advancing clinical treatments for NPC.
The current investigation is not without limitations. Firstly, our study adopts a retrospective analysis, potentially introducing selective bias. Secondly, the data originates from a single centre, and our future endeavours involve acquiring data from multiple centres to enhance the generalizability of the augmented model. Thirdly, NPC patients often experience prolonged lifespans, resulting in a limited dataset within both deceased and non-deceased groups. In response to this, we intend to expand our follow-up efforts and accumulate additional data over time to bolster the robustness and reliability of our model.
5. Conclusion
In summary, the amalgamated model, rooted in pre-treatment multi-parameter MRI radiomics and complemented by clinical and MRI semantic features, possesses the capability to prognosticate the 5-year PFS in non-metastatic NPC patients at an early stage. Through the comprehensive risk classification across all NPC patients, substantial variations in the 10-year OS become evident among distinct risk groups within the NPC population. Consequently, this model facilitates an accurate prognosis and stratification of progression risk in NPC patients, guided by high-risk factors associated with recurrence and distant metastasis. This provides a non-invasive, high-throughput quantitative foundation for timely intervention and optimization of treatment, offering a means to balance survival advantages against medical costs.
Ethic statement
Based on the obtainable data, the original data supporting the conclusions of this article will be presented by the authors without undue retention. The research involving human participants conducted by members of the 903 Medical Hospital of the Joint Logistics Protection Department of the Chinese People's Liberation Army and Lun Li Sheng Ming has been scrutinized and authorized (Approval No. 20220722/08/01/002).
Funding
This research is supported by Natural Science Foundation of Zhejiang Province (Y22H185692), Medical Science and Technology Project of Zhejiang Province (NO. 2023KY209, NO. 2021RC108), and Hangzhou Science and Technology Commission (NO. B20220177).
CRediT authorship contribution statement
Jiang Feng: Writing – review & editing, Supervision, Resources, Funding acquisition. Liu Miao: Writing – review & editing, Supervision. Han Jing: Software, Methodology. Chen Shiyu: Investigation. Ding Zhongxiang: Writing – review & editing, Supervision, Funding acquisition, Conceptualization. Wang Mengze: Resources. Dong Hao: Writing – original draft, Methodology. Xi Yuzhen: Writing – original draft, Software, Methodology, Investigation, Funding acquisition, Data curation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Miao Liu, Email: xhqqbliumiao@163.com.
Feng Jiang, Email: jiangfeng@zjcc.org.cn.
Zhongxiang Ding, Email: hangzhoudzx73@126.com.
References
- 1.Martel, de C., Georges D., Bray F., et al. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob. Health. 2020;8(2):e180–e190. doi: 10.1016/S2214-109X(19)30488-7. [DOI] [PubMed] [Google Scholar]
- 2.Chen Y.P., Chan A.T.C., Le Q.T., et al. Nasopharyngeal carcinoma. Lancet. 2019;394(10192):64–80. doi: 10.1016/S0140-6736(19)30956-0. [DOI] [PubMed] [Google Scholar]
- 3.Sung H., Ferlay J., Siegel R.L., et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 4.Au K.H., Ngan R.K.C., Ng A.W.Y., et al. Treatment outcomes of nasopharyngeal carcinoma in modern era after intensity modulated radiotherapy (IMRT) in Hong Kong: a report of 3328 patients (HKNPCSG 1301 study) Oral. Oncol. 2018;77:16–21. doi: 10.1016/j.oraloncology.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Tian Y.M., Liu M.Z., Zeng L., et al. Long-term outcome and pattern of failure for patients with nasopharyngeal carcinoma treated with intensity-modulated radiotherapy. Head. Neck. 2019;41(5):1246–1252. doi: 10.1002/hed.25545. [DOI] [PubMed] [Google Scholar]
- 6.Tang X.R., Li Y.Q., Liang S.B., et al. Development and validation of a gene expression-based signature to predict distant metastasis in locoregionally advanced nasopharyngeal carcinoma: a retrospective, multicentre, cohort study. Lancet Oncol. 2018;19(3):382–393. doi: 10.1016/S1470-2045(18)30080-9. [DOI] [PubMed] [Google Scholar]
- 7.Blanchard P., Lee A., Marguet S., et al. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC meta-analysis. Lancet Oncol. 2015;16(6):645–655. doi: 10.1016/S1470-2045(15)70126-9. [DOI] [PubMed] [Google Scholar]
- 8.Lee N., Harris J., Garden A.S., et al. Intensity-modulated radiation therapy with or without chemotherapy for nasopharyngeal carcinoma: radiation therapy oncology group phase II trial 0225. J. Clin. Oncol. 2009;27(22):3684–3690. doi: 10.1200/JCO.2008.19.9109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tian Y., Huang W.Z., Zeng L., et al. The Failure patterns of nasopharygeal carcinoma after intensity-modulated radiotherapy and implications for surveillance. Cancer Manag. Res. 2022;14:2813–2823. doi: 10.2147/CMAR.S347864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou G.Q., Lv J.W., Tang L.L., et al. Evaluation of the national comprehensive cancer network and european society for medical oncology nasopharyngeal carcinoma surveillance guidelines. Front. Oncol. 2020;10 doi: 10.3389/fonc.2020.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang L., Guo Y., Xu J., et al. Clinical analysis of recurrence patterns in patients with nasopharyngeal carcinoma treated with intensity-modulated radiotherapy. Ann. Otol. Rhinol. Laryngol. 2017;126(12):789–797. doi: 10.1177/0003489417734229. [DOI] [PubMed] [Google Scholar]
- 12.Ren Y., Qiu H., Yuan Y., et al. Evaluation of 7th edition of AJCC staging system for nasopharyngeal carcinoma. J. Cancer. 2017;8(9):1665–1672. doi: 10.7150/jca.19197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang Y.T., Chen K.H., Yang J., et al. Establishment of a prognostic nomogram for patients with locoregionally advanced nasopharyngeal carcinoma incorporating TNM stage, post-induction chemotherapy tumor volume and epstein-barr virus DNA load. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.683475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang L., Dong D., Li H., et al. Development and validation of a magnetic resonance imaging-based model for the prediction of distant metastasis before initial treatment of nasopharyngeal carcinoma: a retrospective cohort study. EBioMedicine. 2019;40:327–335. doi: 10.1016/j.ebiom.2019.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui C., Li H., Ma H., et al. Staging of T2 and T3 nasopharyngeal carcinoma: proposed modifications for improving the current AJCC staging system. Cancer Med. 2020;9(20):7572–7579. doi: 10.1002/cam4.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang W., Quan T., Zhao Q., et al. MRI of nasopharyngeal carcinoma: parapharyngeal subspace involvement has prognostic value and influences T-staging in the IMRT era. Eur. Radio. 2022;32(1):262–271. doi: 10.1007/s00330-021-08113-3. [DOI] [PubMed] [Google Scholar]
- 17.Chan J.Y.W., Wong S.T.S., Wei W.I. Stage II recurrent nasopharyngeal carcinoma: Prognostic significance of retropharyngeal nodal metastasis, parapharyngeal invasion, and carotid encasement. Head. Neck. 2018;40(1):103–110. doi: 10.1002/hed.24976. [DOI] [PubMed] [Google Scholar]
- 18.Qiu W., Zhong X., Jiang J., et al. Prognostic significance of cervical radiologic carotid artery invasion by lymph node on magnetic resonance imaging in nasopharyngeal carcinoma. Cancer Imaging. 2023;23(1) doi: 10.1186/s40644-023-00544-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.M G., L F., S A., et al. Can magnetic resonance radiomics analysis discriminate parotid gland tumors? A pilot study. Diagnostics (Basel) 2020;3:900. doi: 10.3390/diagnostics10110900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qiang M., Li C., Sun Y., et al. A prognostic predictive system based on deep learning for locoregionally advanced nasopharyngeal carcinoma. J. Natl. Cancer Inst. 2021;113(5):606–615. doi: 10.1093/jnci/djaa149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xi Y., Ge X., Ji H., et al. Prediction of response to induction chemotherapy plus concurrent chemoradiotherapy for nasopharyngeal carcinoma based on MRI radiomics and delta radiomics: a two-center retrospective study. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.824509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.W D., B X., T T., et al. Radiomics in nasopharyngeal carcinoma. Clin. Med. Insights Oncol. 2022;24 doi: 10.1177/11795549221079186. 11795549221079186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang Y.T., Chen K.H., Liang Z.G., et al. A nomogram to predict survival and guide individualized induction chemotherapy in T3-4N1M0 nasopharyngeal carcinoma. Curr. Probl. Cancer. 2022;46(6) doi: 10.1016/j.currproblcancer.2022.100897. [DOI] [PubMed] [Google Scholar]
- 24.Luo J., Hu X., Ge X. Conditional survival nomogram for monitoring real-time survival of young non-metastatic nasopharyngeal cancer survivors. J. Cancer Res. Clin. Oncol. 2023;149(12):10181–10188. doi: 10.1007/s00432-023-04952-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu C., Huang H., Liu X., et al. a clinical-radiomics nomogram based on computed tomography for predicting risk of local recurrence after radiotherapy in nasopharyngeal carcinoma. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.637687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quan T., Guan W., Huang W., et al. Carotid space involvement is a prognostic factor and marker for induction chemotherapy in patients with nasopharyngeal carcinoma. Oral. Oncol. 2022;135 doi: 10.1016/j.oraloncology.2022.106230. [DOI] [PubMed] [Google Scholar]
- 27.Dias, Rodrigues D., Breda E., Sousa F., et al. Nasopharyngeal carcinoma in a non-endemic country-Validation of the new NPC staging system. Acta Otorrinolaringol. Esp. (Engl. Ed. ) 2023;74(1):39–49. doi: 10.1016/j.otoeng.2022.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Wong F.C., Ng A.W., Lee V.H., et al. Whole-field simultaneous integrated-boost intensity-modulated radiotherapy for patients with nasopharyngeal carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2010;76(1):138–145. doi: 10.1016/j.ijrobp.2009.01.084. [DOI] [PubMed] [Google Scholar]
- 29.Toumi N., Ennouri S., Charfeddine I., et al. Local and lymph node relapse of nasopharyngeal carcinoma: a single-center experience. Ear. Nose. Throat J. 2021;100(5_suppl):795S–800S. doi: 10.1177/0145561320908955. [DOI] [PubMed] [Google Scholar]
- 30.Chen S., Yang D., Liao X., et al. Failure patterns of recurrence and metastasis after intensity-modulated radiotherapy in patients with nasopharyngeal carcinoma: results of a multicentric clinical study. Front. Oncol. 2022;11 doi: 10.3389/fonc.2021.693199. [DOI] [PMC free article] [PubMed] [Google Scholar]