SUMMARY
The cerebral cortex—the brain’s covering and largest region—has increased in size and complexity in humans and supports higher cognitive functions such as language and abstract thinking. There is a growing understanding of the human cerebral cortex, including the diversity and number of cell types that it contains, as well as of the developmental mechanisms that shape cortical structure and organization. In this Review, we discuss recent progress in our understanding of molecular and cellular processes, as well as mechanical forces, that regulate the folding of the cerebral cortex. Advances in human genetics, coupled with experimental modeling in gyrencephalic species, have provided insights into the central role of cortical progenitors in the gyrification and evolutionary expansion of the cerebral cortex. These studies are essential for understanding the emergence of structural and functional organization during cortical development and the pathogenesis of neurodevelopmental disorders associated with cortical malformations.
eTOC:
During brain development, neural stem cells generate many cell types that assemble to build the cerebral cortex, the substrate for higher cognitive functions. In a review, Akula and Exposito-Alonso et al. examine our current understanding of the mechanisms underlying cortical development and folding, and how alterations may cause brain malformations.
INTRODUCTION
The cerebral cortex constitutes the majority of the human brain by mass, and the neocortex—the newest evolutionary addition to the cerebral cortex—has expanded extraordinarily in humans when compared to our phylogenetically closest relatives, the great apes 1,2. Recent progress in describing the human neocortex has revealed unique features relating to the number and diversity of cell types 3–8. However, our understanding of how the neocortex has acquired its shape and organization remains incomplete. Some of the remarkable human cognitive abilities that resulted from the increases in size and complexity of the neocortex are disrupted in patients with cortical malformations, neurodevelopmental disorders characterized by abnormal cortical structure and/or organization, and understanding the process of neocortical formation may give us insights into these disorders 9–12.
The presence of folds in the neocortex is a hallmark in many mammals, including humans. Neocortical folding is characterized by patterns of fissures called sulci and ridges called gyri, hence the name gyrencephalic for describing species with a folded cortex. The thickness of the neocortex varies across the folds: the grey matter (where neuronal cell bodies reside) is thickest at the peaks of gyri and thinnest at the deepest points of sulci. Therefore, areas corresponding to gyri and sulci can be inferred from the variation in thickness 13,14. Cortical folding is thought to provide compaction of the neocortical structure, allowing a larger surface area to fit in a smaller volume. In humans, primary gyri and sulci are the first convolutions to emerge during development, are the largest folds, and display consistent locations across individuals 14. In contrast, secondary and tertiary folds are formed later in development and vary to some degree in their locations and orientations 15–18. Importantly, patterns of cortical folding may have consequences for functional connectivity: for instance, neurons separated by a gyrus are more likely to be functionally connected than those separated by a sulcus 19–21.
Not all mammals have a gyrencephalic neocortex. Common animal models in neuroscience research such as mice and rats are lissencephalic (i.e., their cortices are smooth). Cortical gyrification was first proposed to have emerged from common lissencephalic ancestors as neocortical size increased 22. However, broad comparative analysis of mammalian brain anatomy suggests differently. Cortical gyrification is observed even in Monotremes and Marsupials 23, far removed from the common ancestor of mice and gyrencephalic species, and within Rodentia, members with larger brains such as capybaras show gyrification. Thus, in rodents, and particularly in mice and rats, lissencephaly more likely represents a derived trait, likely selected for through demands for a smaller head or less energetically expensive cortex 24. A gyrencephalic cortex was very likely present in the ancestral animal of all living mammals today, suggesting that cortical folding was a core feature of the early neocortex 25,26. In gyrencephalic species with only primary folds, such as ferrets, the sulcation pattern is essentially invariant between animals 27. Although mice do not develop cortical gyration, they remain useful model organisms to explore aspects of neocortical development, even related to cortical folding, because of the high degree of conservation in mechanisms of neocortical development across all mammals. Recently, understanding cortical folding has benefited from the study of cortical folding disorders in human individuals 28, and the more recent development of genetic manipulation in gyrencephalic models like ferrets have led to a new era of exploring the biology of cortical folding 29–31.
In this Review, we explore and discuss advances in our understanding of the development of cortical folding as a central process underlying the emergence of shape in the human brain. We provide a comprehensive review on the role that cortical progenitors play in the formation of cortical folding, with interdisciplinary consideration of studies of the biophysics of this process. Our attention is also focused on comparative analyses across species that have provided key insights into the cellular mechanisms underlying cortical folding. We review signaling pathways that regulate the dynamics and maintenance of cortical progenitors and concentrate on specific examples for which extensive studies of animal modeling as well as human genetics converge to inform us about their function in cortical gyrification. We discuss recent data establishing a key role for Sonic Hedgehog signaling in cortical folding, based on experiments in ferret models and genetic studies of patients with cortical malformations. Next, we highlight findings of evolutionary mechanisms that illustrate the importance of human-specific genetic changes in regulating the behavior of cortical progenitors in the developing cortex, and how these evolutionary specializations may have impacted cortical size and complexity. Finally, we provide a perspective on recent studies that point towards emerging cellular mechanisms shaping the development of neocortical gyrification.
CORTICAL DEVELOPMENT AND NEURAL PROGENITOR CELLS
As corticogenesis begins, neuroepithelial cells (NECs) give rise to cortical progenitor cells known as radial glial cells (RGC) that are responsible for generating diverse populations of cortical projection neurons (Figure 1). Apical RGCs (aRGCs), whose cell somata reside in the ventricular zone (VZ), initially undergo symmetric divisions to amplify the pool of progenitor cells 32–34. aRGCs extend long processes that span from the ventricular surface apically to the pial surface basally 34,35 (Figure 1). As the neurogenic period of cortical development begins, aRGCs begin also to divide asymmetrically to produce a daughter neuron and another aRGC, which is known as a neurogenic and self-renewal division 32. Furthermore, aRGCs can produce nonpolar, division-limited intermediate progenitors (IPCs), which upon division will generate two daughter neurons, hence amplifying the output of neurons produced during neurogenesis 32,36–38 (Figure 1). The vast majority of IPCs undergo their mitotic division in the subventricular zone (SVZ), although these progenitors can be found across germinal zones (VZ and SVZ), which determines their classification as apical or basal IPCs, respectively 38–42. bIPCs can exhibit proliferative, self-renewal capacities in primates, in contrast to mouse IPCs that generally undergo self-consuming divisions 42. As corticogenesis proceeds, aRGCs generate a second class of radial glial progenitors called basal RGCs (bRGCs; also known as outer radial glial cells) that reside basally within the subventricular zone (SVZ), delaminate from the apical belt of adherens junctions attached to the ventricular surface, and retain their polarity typically by extending a basal process connected to the basal surface of the developing cortex although can display remarkable diversity of morphological subtypes 39,42–44 (Figure 1).
Figure 1. Temporal stages and progenitor types in the human developing neocortex.
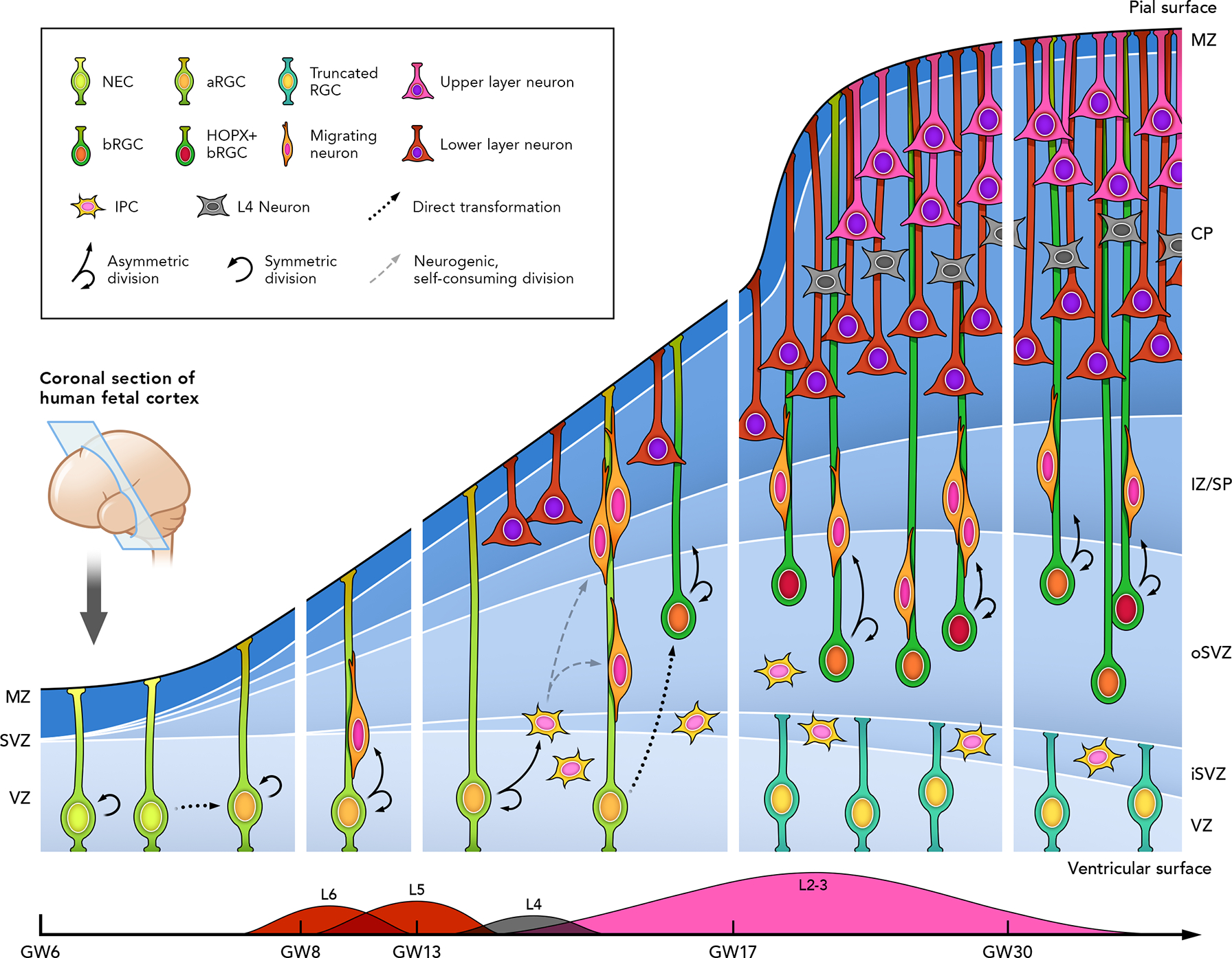
During early human brain development, a layer of neuroepithelial cells (NECs), spanning from the ventricular surface to the pial surface, populates the developing neural tube and undergoes self-renewing divisions to generate more NECs (symmetric divisions) in early developmental stages. They then elongate and differentiate into radial glial cells (RGCs), which also undergo symmetric divisions to expand the population of progenitors. During the neurogenic period, RGCs begin to divide asymmetrically to generate neurons while self-maintaining the progenitor pool, either generating neurons directly or producing neurons indirectly through intermediate progenitor cells (IPCs). Apical radial glial cells (aRGCs) are defined by residing in the ventricular zone (VZ) and by establishing contacts at both the apical and basal surfaces of the developing cortex. Later in development, aRGCs can also give rise to basal radial glial cells (bRGCs) by delamination of the apical belt of adherens junctions attached to the ventricular surface and translocation of their somas to the subventricular zone (SVZ). Based on marker expression, bRGCs have multiple subtypes, including HOPX+ bRGCs. Migrating neurons generated by aRGCs or bRGCs use the RG scaffold of both types of progenitors to migrate through the intermediate zone (IZ) into the developing cortical plate (CP), which contributes to the growth of the CP. By the second trimester of pregnancy (gestational week 17), aRGCs transform into truncated RGCs with their basal process terminating in the border between the inner and outer layers of the SVZ (iSVZ/oSVZ); thus, the RG scaffold becomes truncated at the iSVZ/oSVZ border. Cortical neurons are born in an inside-out fashion, with neurons destined to deeper layers (L6) born first and neurons destined to superficial layers (L2) born last. Density plots shown on the bottom represent the different neurogenic stages that preferentially generate neurons committed to each cortical layer. The extended neurogenic period for superficial layer neurons (L2–3), coincides with the expansion of bRGC proliferation and is considered a hallmark of human brain evolution.
The SVZ can be anatomically subdivided into an inner and an outer layer (iSVZ and oSVZ, respectively): the iSVZ contains randomly organized cells, whereas the oSVZ shows radially organized cells and is the main location of bRGCs 42–45. The inner fiber layer constitutes the anatomical landmark separating the iSVZ and oSVZ and emerges around embryonic day 72 in the macaque developing cortex (roughly corresponding to the beginning of the second trimester of gestation in humans) 45. A growing body of literature has documented that numerous successive rounds of proliferative divisions of bRGCs (in some cases, summing up to 6 division rounds) and to a lower degree of bIPCs, ensure the progressive accumulation of neurons during cortical development. These findings have formed the basis for the view that the high proliferative capacity of bRGCs may underlie the expansion of oSVZ and the increased cortical neuron numbers in primates, achieving its highest levels in humans 42–49.
Nascent, immature neurons in the developing cortex utilize the radial glial processes from RGCs that extend to the basal pial surface—adhering to them through cell adhesion mechanisms—to migrate radially through an intermediate zone (IZ) to finally reach the developing cortical plate (CP), where they will mature and form synaptic connections to begin elaborating developing cortical circuits (Figure 1). Thus, radial glial processes (also known as RG fibers) form cytoarchitectonic scaffolds that are essential for radial migration of neurons. The CP appears by splitting a primordial basal zone that was above the early VZ before neurogenesis, known as the pre-plate (PP), separating it into a cell-rich subplate (SP) of early pioneer neurons below the CP, and the marginal zone (MZ) above the CP; the MZ terminates at the pial surface. Although early models suggested that nascent neurons may migrate in narrow columns called “radial units” 50, analysis of excitatory neuron clones derived from single or few RGCs show a more conical distribution in mouse, widening as daughter neurons migrate further from the VZ. In ferrets, clonal marking and live imaging analyses of cortical slice cultures show even more prominent tangential dispersion, suggesting that migrating neurons use the radial glial scaffold as guides in general, but do not rigidly adhere to specific RG fibers, allowing them to disperse more horizontally in the developing CP of gyrencephalic species 40,51,52. Together with the RG fibers displaying a fanned array in sites of developing folds 40, these data provide key insights into the mechanisms for increased surface area in the gyrencephalic cortex (discussed in the next section).
After they are generated from the early neuroepithelium, RGCs undergo multiple rounds of symmetric division to increase the pool of progenitors and later asymmetric divisions to both generate a daughter neuron and self-renew the progenitor cell 32,53,54. However, as neurogenesis proceeds, individual RGCs will themselves exit the cell cycle to produce terminally differentiated daughter cells. The timing of the switch from symmetric to asymmetric (i.e., expanding the number of progenitors vs. producing neurons), as well as the timing of cell cycle exit for aRGCs and bRGCs, dictates their neurogenic potential. RGCs that proliferate for shorter times produce fewer daughter cells. Thus, regulation of the cell cycle and division mode of different types of RGCs is a determining factor for brain size and cortical thickness 55,56.
While the fundamental components of neurogenesis described above are conserved in all developing mammalian cortices examined 57,58, the relative abundance of progenitor cell types and their behaviors vary with brain size and folding pattern across species, which provides correlative implications about the cellular bases of cortical thickness, size, and gyrification 49,56. Gyrencephalic species like ferrets and humans have thicker and more complex SVZs relative to lissencephalic mice. Beyond increased frequency in the developing cortex, basal progenitors have greater diversity in subtype and neurogenic potential in primates than in mice 38,49,55,56,59. A basal progenitor type reminiscent of human bRGCs, with marked epithelial features, appears to be present only in small numbers in the SVZ of mice, which primarily rely on aRGCs and IPCs during their short neurogenic period 47,60. In contrast, bRGCs, rather than aRGCs, predominantly produce cortical neurons in primates, including humans, and are considered essential in the generation of superficial layer neurons, which have extraordinarily expanded in numbers and diversity in humans 3,49,55,56,61.
MECHANISMS OF CORTICAL FOLDING
The process of cortical folding undergoes several developmental stages—spanning from embryonic stages through postnatal development in humans—and requires a complex interplay of mechanisms 18,49. On the one hand, physical studies of cortical folding suggest that mechanical forces from differential tangential expansion of the CP relative to the germinal layers (VZ and SVZ) are sufficient as an important factor to induce cortical gyration 62. On the other hand, twin studies indicate that gyration patterns are heritable, with patterns of sulci and gyri being significantly more similar between mono-zygotic twins than between control pairs. Higher order gyri and sulci show greater variability across individuals relative to lower order ones 14,15,17. Together, these data suggest that physical models may require additional influences to account for patterns of gyrification, and that genetic factors might regulate the emergence of cortical gyrification, to a certain degree in a deterministic fashion, especially of the primary, deeper sulci 13,49,63. In the next subsections, we will review the mechanical forces and cellular processes underlying cortical folding and discuss how the interactions between these mechanisms form the bases for the current model of cortical folding 22,49,55,56,64,65. This model proposes a central role for RGC dynamics in producing cortical gyration, based on a wealth of studies identifying genetic programs expressed in these progenitors during development that guide the expanding cortex to fold in a controlled manner. These developmental programs produce the necessary conditions for mechanical forces to fold the cortex—such as regions of relative cell production and expansion neighboring regions of slower growth—while maintaining minimal stochasticity for the development of primary gyri; the increased variability in higher order gyri appears to reflect a greater degree of stochasticity in their formation. Studying the interplay between both factors (mechanical forces and genetic and cellular programs) is essential to gain a better understanding of the mechanisms that orchestrate folding in the developing cortex.
Mechanical forces underlying cortical folding
Prior to 2010, numerous models of the physics of cortical folding were debated, including one in which external force from the skull caused folding of the developing cortex 14, or one in which internal tension forces from axonal growth pulled cortical regions into a wrinkled shape 66,67. However, these two hypotheses are not strongly supported by more recent data: the developing skull does not place mechanical constraint because cranial sutures do not ossify until the completion of brain growth, and reducing cranial pressure does not influence gyrification in animal models 14. However, influence in the reverse direction is possible, and pressure from an expanding brain or fluid compartment can increase head size, as seen in cases of macrocephaly, both in mice and humans 14,68,69. The axon tension model is also inconsistent with experimental investigations demonstrating that localized severing of axons in gyrencephalic brains during development does not influence cortical folding 70.
The prevailing physical model of cortical gyration is based on differential tangential growth of the basal portion of the developing cortex (CP and MZ) relative to the underlying germinal zones (VZ and SVZ) 62. The foundations for exploring this model were laid by observations across animals that cortical folding is best correlated with cortical grey matter thickness and surface area rather than with brain size 71, and computational models showing the sufficiency of differential tangential expansion to produce convolutions resembling gyri in structures like the developing cortex 72,73. Building on these analyses, studies with physical models using polymer gels constructed of an inner region that expands less than an outer basal region produced patterns of gyration that are grossly similar in scale and pattern to those seen in the human cortex 74,75. By using simple polymer gel expansion models with initial parameters derived from fetal MRI imaging at 20 weeks of gestation, these physical models yielded striking similarity to human cortical gyration patterns 76. Variations in the stiffness of the inner and outer regions of these compound gel structures impacted the pattern and wavelength of folding, suggesting that the material properties of the tissue play key roles in folding 75,76. These findings are consistent with the notion that mechanical force from differential tangential expansion of the outer layer (CP and MZ) relative to the inner layer (VZ and SVZ) of the developing cortex is sufficient to cause folding in a pattern similar to that of the human cortex.
Even though studies of the physical causes of cortical gyration are compelling, they do not capture all the features of cortical folding seen in vivo. First, while mechanical models of cortical folding can correctly predict how folds arise from specific initial conditions, presumably these initial conditions explain the recurrence of specific folding patterns across individuals; and hence still require mechanistic understanding. Second, mechanical models do not yet encompass the physical properties of the distinct developmental layers and only recently have started to incorporate contributions that influence folding at microscopic rather than macroscopic levels, although it is striking how accurate they are already.. Finally, these models require tuning of initial parameters such as specific thickness of the layers modeled and rate of expansion pulled from biological estimates, and changes in these parameters have dramatic impacts on the outcomes of predicted gyrification 76,77.
Roles of radial glial cells in cortical folding
If differential tangential expansion during cortical development exerts mechanical forces that can induce folding, what is the cellular substrate responsible for the emergence of such forces in the developing cortex? Regional variation in neuronal density is viewed as a determinant factor in the differential growth of the cortical mantle 49,56. Not only can this differential accumulation of neurons can lead to region-specific rates of tangential expansion of the cortical surface area, but also the subsequent maturation of neurons (e.g., dendritic arbor development) has been suggested to further contribute to differential growth in developing gyri and sulci 78. Multiple lines of evidence over the past decade support a major role for the behavior of cortical progenitors (proliferation and neurogenesis) in the differential accumulation of neurons, ultimately influencing cortical folding. Initial anatomical analyses of primate developing cortices showed regional differences in the size of germinal layers, in particular the oSVZ 45. Subsequent studies demonstrated that the distribution of dividing cells is heterogeneous across the cortical mantle in gyrencephalic species, observations consistently reported in ferrets and monkeys 40,79,80, and several studies documented the differential distribution of these basal progenitors in regions that will generate prospective gyri (proto-gyri) and regions destined to become prospective sulci (proto-sulci) 39,40,81 (Figure 2A). The cellular density in the oSVZ relative to other germinal layers during development predicts the overall degree of cortical folding across mammalian species, independently of cortical size, such that the size of the oSVZ in development is not just correlated with brain size but also specifically with the gyrification index 40,41. More recently, experimental manipulations showed that increasing or reducing proliferation of bRGCs in ferrets leads to bidirectional modulation of cortical folding 40,81–83, and amplifying the limited pool of bRGCs in mice results in some degree of cortical folding 84–86 (further discussed in the following sections).
Figure 2. Cellular and molecular mechanisms of radial glial cells promote cortical folding.
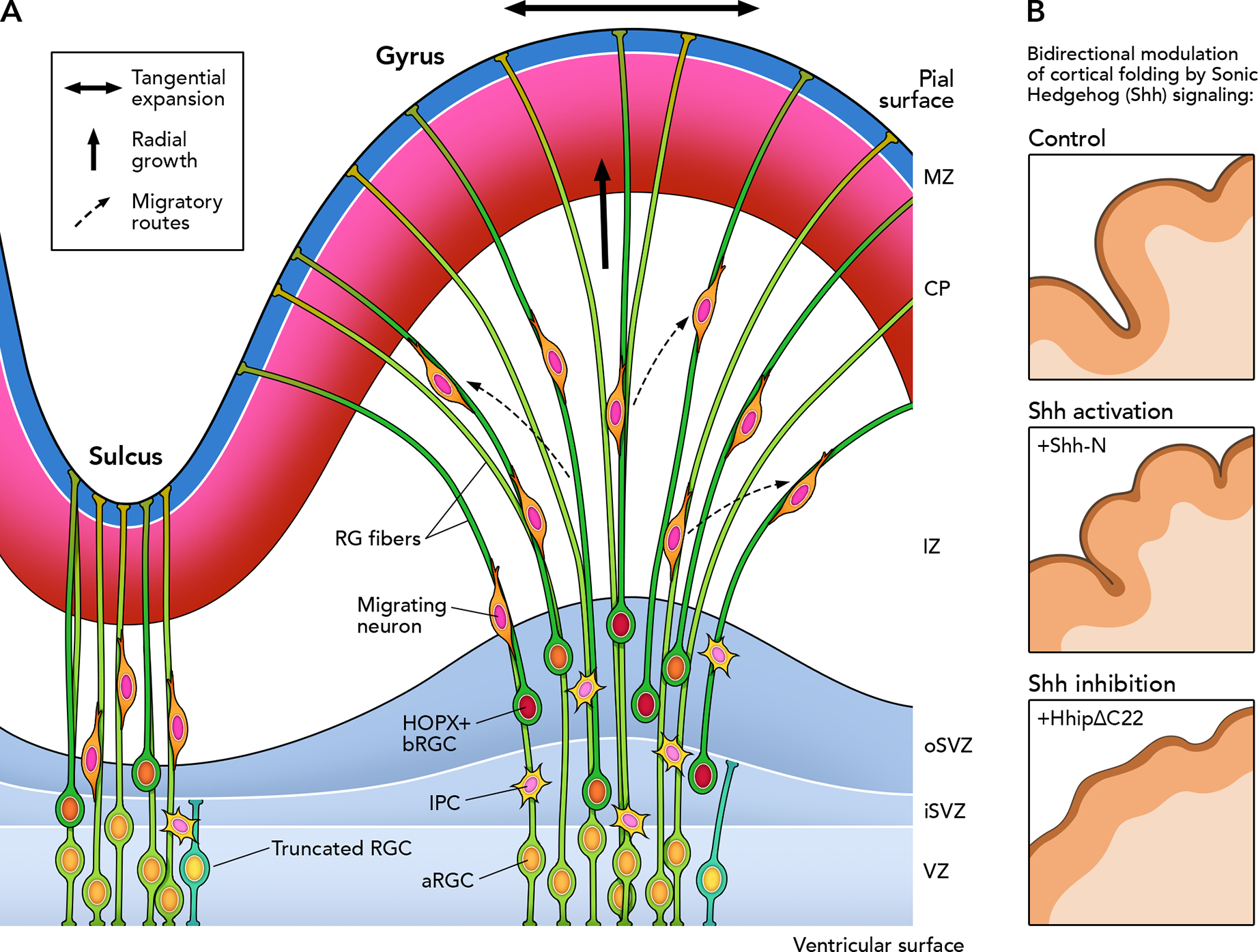
(A) The current model of cortical folding proposes a key role for basal radial glial cells (bRGCs), which are characterized by a highly proliferative capacity and are especially abundant in the outer subventricular zone (oSVZ). Regional differences in neurogenesis are found across the developing cortical mantle in gyrencephalic species (especially noticeable in the oSVZ): regions destined to prospective gyrus (proto-gyri) have greater densities of diving progenitor cells than regions destined to prospective sulci (proto-sulci) 40,80. Consistently, a subclass of bRGCs labeled by the marker HOPX in the developing ferret cortex is found in greater densities in proto-gyri 31. The differential density of basal progenitors results in differential production and accumulation of neurons in the developing cortical plate (CP), which causes a greater degree of tangential expansion (horizontal double arrow) in proto-gyri relative to proto-sulci. Moreover, there is a progressive divergence in the trajectory of radial glial (RG) fibers during gyrus formation due to the highly proliferative population of bRGCs that intercalate their fibers with preexisting ones 40. This fanned array of RG fibers in prospective gyral regions contributes to the tangential spread of migrating neurons guided by this scaffolding. In contrast, radial glial fibers typically show parallel trajectories in proto-sulci, which likely limits the tangential spread of migrating neurons. (B) Schematic illustrating the effects of Sonic Hedgehog (Shh) signaling in cortical folding. HOPX+ bRGCs were shown to respond to activation and inhibition of Shh signaling activity in inverse ways: Shh stimulation (with the amino-terminal fragment of Shh, Shh-N) results in increased densities of HOPX+ bRGCs, whereas Shh suppression (with the competitive inhibitor HhipΔ22) results in a reduced population of HOPX+ bRGCs 31. These effects lead to bidirectional changes in cortical folding: Shh stimulation results in larger gyri, whereas Shh inhibition leads to smaller gyri 31.
These findings formed the bases for a proposed mechanism underlying the emergence of primary gyri and primary sulci: elevated rates of neurogenesis in proto-gyri during corticogenesis will result in a higher accumulation of neurons and rapid tangential expansion of the CP, whereas lower rates of neurogenesis in proto-sulci (neighboring regions to proto-gyri) will lead to a lesser degree of accumulation of neurons and tangential growth, thus the developing cortex will begin to form folds and fissures (Figure 2A). A potential source for differential bRGC distribution across the developing cortex, and perhaps even local bRGC behavior, may be the differential gene expression observed between proto-gyri and proto-sulci in the developing cortex, and such expression patterns (also called protomaps of gene expression) are proposed to reflect the presence of early region-specific genetic programs guiding cortical folding and arealization in a stereotypical manner 13,63,87,88. It remains to be investigated whether the development of tertiary or higher order gyri—characteristic of highly folded cortices such as the human cortex and evidently variable across individuals—follow similar principles than the formation of primary gyri, which are present in ferrets, the animal model used in most experimental investigations into gyrification mechanisms so far.
Neurogenesis from bRGCs, which explains part of the broad mechanism of differential tangential expansion, does not appear to account for the degree of gyrification seen in vivo entirely 62. Aside from proliferation, bRGCs have also been found to contribute to cortical folding through the RG fiber scaffold they provide to nascent neurons, which guides and defines their radial migration and final positioning in the CP. Once bRGCs are initially born from aRGCs by delamination from the ventricular surface (roughly at embryonic day 34 in ferrets, and gestational week 12 in humans), their basal RG fibers begin to intercalate with those from preexisting aRGCs 39–41. The progressive accumulation of basal processes adds complexity to the fiber arrangement, causing fanning outward of the RG scaffold (Figure 2A). This is more prominent in regions with increased bRGC abundance, which correlates with prospective gyri 39,40. These data suggest that bRGCs contribute to differential tangential expansion of the CP not only by increasing the neurogenic potential, but also by promoting tangential dispersion of neurons in the developing cortex by increasing the complexity of the RG fiber network. Additionally, aRGCs appear to transform their basal processes at gestational week 16.5 in humans to a shorter fiber that becomes truncated at the border with the oSVZ, referred to as “truncated” RGCs, with these truncated progenitors presumably producing many late-born neurons 35. These truncated RGCs define a RG scaffold in humans that becomes discontinuous or interrupted between the iSVZ and oSVZ (with the short process of truncated aRGCs spanning the depth of the VZ and iSVZ and the long process of bRGCs extending from the oSVZ up to the CP) in the second trimester of gestation, posing intriguing questions about the migration and dispersion of late-born neurons and how this phenomenon may shape cortical architecture.
In addition to these RGC-driven mechanisms, there is evidence that mechanisms intrinsic to migrating neurons can also influence cortical folding. Migrating neurons associate with numerous fibers of the RG scaffold promiscuously to take more tortuous and divergent paths (by branching their leading process) in developing gyrencephalic cortices than they do in developing lissencephalic cortices, where clonal analyses of migrating neurons show a more limited conical distribution 40,51,52,89,90. Accordingly, the expression of FLRT1 and FLRT3—genes encoding cell adhesion molecules—by migrating neurons in mice appears to restrict their tangential dispersion and their deletion leads to the emergence of cortical sulci; in contrast, the downregulated expression of FLRT1/3 found in ferrets and humans was suggested to be a regulatory mechanism potentially underlying the distinct migration patterns observed in the gyrencephalic neocortex 91,92. Thus, the architecture of the RG fiber scaffold, together with cell-autonomous mechanisms of neuronal migration, critically influences cortical folding by impacting the tangential dispersion of migrating neurons.
MOLECULAR MEDIATORS REGULATING RADIAL GLIAL CELL BEHAVIOR
Experimental studies in both mice and ferrets have provided direct evidence of the role of RGC behavior, and especially of bRGCs, in cortical folding and identified molecular signaling pathways involved in the genetic regulation of RGC function. Several ectopically expressed factors in mouse cortex can induce pseudogyration (folding of the entire developing cortical thickness including ventricular surface, usually due to increased size), but not convincing true ectopic gyration (cortical folding without disruption of the apical membrane shape, usually due to increased tangential dispersion). Thus, parallel experiments in ferrets investigating those same factors have now examined them in an animal model—the ferret—with the cellular substrate to modulate true cortical gyration. For example, local overexpression of fibroblast growth factor 2 (FGF2) or FGF8 via in utero electroporation in mice is sufficient to cause local growth reminiscent of gyration, attributed to increased self-renewal of aRGCs, the inverse of the phenotype described in an Fgf2 knock-out (KO) mouse model 93,94. A similar experiment of FGF8 misexpression in ferrets caused frank polymicrogyria (a cortical malformation characterized by many small gyri creating excessive folding), and histological examination revealed an increased density of bRGCs not seen in the mouse experiments that is potentially driving the change in gyration 95. In a similar pattern, modulating cell cycle of RGCs through overexpression of the cell cycle proteins Cdk4/CyclinD1 via in utero electroporation in mice promoted increased Tbr2+ IPCs in the SVZ, which increases cortical surface area in mice, but did not distinctly lead to local gyri-reminiscent growth 96. Remarkably, the same manipulation in ferrets generates new cortical folds with normal laminar organization 81. These differences in phenotypes between mouse and ferret models may be explained by the distinct cytoarchitecture of the gyrencephalic developing cortex, characterized by expanded basal progenitors in the SVZ, that might be necessary for such phenotype. Alternatively, the underlying molecular programs involved in this phenotype in ferrets may not be shared by the mouse developing cortex 81,96. A unique case of a single gene perturbation in mice causing a phenotype of true cortical gyration is the loss of Trnp1 in the embryonic mouse cortex, loss of which led to an expansion of the small population of bRGC-like progenitors found in mice, thus causing an expanded oSVZ, increased fanning of the local RG scaffolding, and formation of cortical folds 84. Importantly, the cortical folding induced in these mice emerged from radial growth of the germinal zone followed by the tangential expansion of neurons in the cortical plate, recapitulating the endogenous process of gyrification described in the gyrencephalic cortex 84.
Beyond these examples of single gene perturbations in both murine and gyrencephalic models, numerous other genetic factors have been shown to modulate RGC behavior through studies using in utero electroporation, KO models, or drug treatments [excellently reviewed by 65]. These factors, including IGF1 97, INSM1 98, NT3 94, lysophosphatidic acid 99, or modulation of beta-catenin signaling 68, modulate RGC proliferation, and can cause cortical lamination or even cortical shape changes, although none of these induce true gyration in their murine models. Thus far it seems that modulating genetic factors in lissencephalic animal models lacking the key cellular components of the developing cortex required for folding (that is, an abundant population of bRGCs) may not be sufficient to induce true cortical gyration. Likely, given the evolutionary history of mice and the emergence of pseudogyration in most experiments described above, it seems most plausible that mice have shed or suppressed much of the cellular substrate needed to form folds during corticogenesis and, as a result, developed a lissencephalic cortex. Thus, future experiments in gyrencephalic models such as ferret may be able to examine gyrification mechanisms specifically in a developing cortex with more endogenous substrate required for cortical folding.
Molecular cues from the extracellular environment have also been found to play a crucial role in cortical progenitor behavior, hence influencing cortical shape. In this context, the constituents of the extracellular matrix (ECM) are essential for cellular and tissue organization and dynamics in the human developing cortex, and in particular the proliferation of cortical progenitors, which has been thoroughly reviewed elsewhere 100,101. These functions of ECM proteins have been shown to be mediated both by modulating the activity of major signaling pathways (e.g., FGF signaling and Hedgehog signaling) and by direct signaling via ECM receptors (e.g., laminin binding to integrins). A seminal study identified three ECM components (HAPLN1, luminican and collagen I) involved in regulating the morphogenesis of the human developing cortex: treatment of 11–16 gestation week fetal neocortical slices with these ECM proteins as soluble factors induced folding within 24h of culture 102. Importantly, signaling mediated by integrins is required to promote the proliferation of both types of basal progenitors (bRGCs and bIPCs) in ferrets and mice 44,103–105. Beyond ECM-related molecular signaling, other cell-extrinsic factors, such as retinoic acid, have been shown to regulate proliferation of RGCs 106. Taken together, extracellular signals are essential in modulating the behavior of cortical progenitors and, therefore, act as determinant factors in the emergence of shape in the developing cerebral cortex.
SONIC HEDGEHOG SIGNALING AND CORTICAL GYRATION
Recent findings indicate that Sonic hedgehog (Shh) signaling is a key regulator of cortical gyration through its function in bRGCs, further extending the broad spectrum of Shh roles in nervous system development (such as its well described role in the specification of the dorsal-ventral axis of the neural tube and spinal cord as well as cortical patterning). Shh is the best studied of the three hedgehog signaling ligands and its expression is expanded in its breadth and concentration in the developing cortices of gyrencephalic animals relative to lissencephalic ones 31,107. Shh acts as a mitogen in the developing cortex and promotes the proliferation of RGCs, stimulating their amplification over neurogenic divisions 108–110. While Shh might act as a mitogen in aRGCs in some capacity early in neurogenesis, its effects are more apparent on the proliferative capacity and stemness of bRGCs and IPCs in the SVZ and on the generation of bRGCs from aRGCs 42,47,111. Shh function in the SVZ promotes the capacity of bRGCs and IPCs to self-renew through symmetric divisions rather than terminally differentiate, thereby expanding bRGC and IPC populations 31. The role of Shh as a mitogen can be illustrated further by case examples that examined the effects of titrating Shh signaling activity during cortical development: (1) increased Shh signaling by constitutively activating the pathway in RGCs caused macrocephaly and even some cortical folding (pseudogyration) in mice 86; (2) decreased Shh due to defective Shh secretion into the cerebrospinal fluid led to a smaller neocortex and cerebellar hypoplasia due to decreased RGC proliferation in CHMP1A-null mice 112; and (3) prenatal treatment of ferrets with Shh signaling modulators in the critical window for bRGC production fine-tuned the density and dynamics of bRGCs: an Shh inhibitor led to decreased bRGC abundance, and an Shh agonist resulted in augmented bRGC pool size 111. This experiment did not induce noticeable effects on the aRGC population, suggesting a specific role for Shh signaling activity in basal progenitors 111.
Recent work has described greater radial glial diversity in gyrencephalic mammals (ferret, macaque, and human) relative to lissencephalic mice 40,42,59. The specific sensitivity to Shh stimulation by a subclass of bRGCs expressing HOPX (encoding a homeodomain-containing protein) has been recently reported. The HOPX+ bRGC subclass has greatly expanded in gyrencephalic mammals and accumulate in proto-gyri in higher numbers compared to the HOPX− bRGC subclass 31 (Figure 2A). Importantly, Shh activity specifically suppressed differentiation of HOPX+ bRGCs, thus increasing their neurogenic potential through progenitor self-renewal 31. Local suppression of Shh signaling in ferrets using a competitive inhibitor of Shh (HhipΔC22) led to decreased HOPX+ bRGCs and locally decreased cortical folding, demonstrating a necessary and sufficient relationship between Shh signaling, local gyrification, and the sustained local proliferation of bRGCs 31 (Figure 2B). Overall, in agreement with cortical folding theory described above, these studies show the potential of HOPX+ bRGCs to promote local differential tangential expansion through an Shh-mediated mechanism inducing elevated proliferation in proto-gyri. These results extend pioneer studies showing that ectopic constitutive Shh stimulation in the VZ in mice was sufficient to form cortical folds in the otherwise lissencephalic brain 86. Altogether, the function of Shh signaling pathway in HOPX+ bRGCs emerges as a central molecular mediator of cortical folding.
Evidence for the key role of Shh signaling in cortical folding also comes from described cortical malformation syndromes in which cortical gyration is abnormal. Although mutations in SHH have been traditionally associated with forebrain patterning malformations such as holoprosencephaly (a disorder in which the embryonic forebrain fails to form two distinct hemispheres), they have also been associated with microcephaly, polymicrogyria (characterized aberrant number of small folds), and schizencephaly (characterized by abnormal slits or clefts), as part of the phenotypic spectrum associated with dysfunctional Shh signaling 113–115. Mutations in genes associated with the Shh signaling pathway, such as receptors PTCH1 and CDON, have also been associated with cortical malformations 115–117.
In addition, mutations in genes involved in modulating Shh signaling have also been linked to non-holoprosencephaly cortical malformations, providing further support to the notion that disrupted Shh signaling can lead to defects in cortical folding. These include the microcephaly/cerebellar hypoplasia associated with recessive mutations in CHMP1A 112,118, and the recently described polymicrogyria syndrome caused by bi-allelic mutations in TMEM161B 119,120. While disruption of TMEM161B through bi-allelic missense mutations in humans causes cortical gyration abnormalities, loss of Tmem161b function in mice causes several Shh-related malformations such as holoprosencephaly, eye defects, and craniofacial abnormalities, as well as morphologically abnormal primary cilia in the ventricular zone of the developing cortex 119. During cortical development, TMEM161B is expressed preferentially in HOPX+ bRGCs relative to other bRGC populations. Disrupting Tmem161b function in cortical progenitors in ferrets led to decreased gyral size and sulci depth, confirming its role in promoting normal cortical gyration 119. Furthermore, Tmem161b plays also a role in the integrity of the RG fiber scaffold, which as described above is different locally between proto-gyri and proto-sulci 120. Overall, these findings, especially in context of the ferret studies examining Shh stimulation of HOPX+ bRGCs described above, suggest a decreased sensitivity to Shh signaling in a developing gyrencephalic cortex deficient in Tmem161b, which provides important insights into the pathogenic mechanisms underlying the polymicrogyria syndrome described in patients.
More generally than the example of polymicrogyria associated with TMEM161B mutation, cortical malformations associated with dysfunction in the primary cilium (the key cellular organelle necessary for downstream transcriptional activation induced by Shh ligand), frequently include microcephaly and polymicrogyria as part of their phenotypic spectra 121,122. Although primary cilia also are involved in numerous other signaling processes, disruption of normal Shh signaling due to abnormal primary cilia may be in part explanatory for the cortical folding malformations seen in primary ciliopathies such as Joubert or Meckel-Gruber Syndrome 121,122.
EVOLUTIONARY EXPANSION OF RADIAL GLIAL CELLS
The evolution of neocortical circuits is thought to have enabled remarkable computational abilities required for human higher cognitive function 123,124. These evolutionary changes include the expansion of the neocortical cytoarchitecture (i.e., the cellular composition and distribution), and such enlargement is especially prominent in superficial layers 2 and 3 (also known as supragranular layers given their location above the granular layer 4) 125 (Figure 3A). Indeed, comparative transcriptomic analyses recently showed an extraordinary diversification of projection neuronal types within superficial layers of the human neocortex 3 (Figure 3A). The enlargement and diversification of superficial layer neurons may, therefore, provide a biological substrate for increased intracortical, associative connectivity that is essential in higher cognitive abilities, given that superficial layer neurons form cortico-cortical synaptic connections. As discussed above, numerous studies have documented that bRGCs are more numerous and diverse in gyrencephalic species as compared to lissencephalic species and, in particular, the oSVZ is substantially expanded in the primate developing cortex 39,40,42–45. Therefore, it is thought that the expansion of basal progenitors might underlie changes in neocortical cytoarchitecture that occurred over the course of evolution. The last decade has seen a quest for human-specific genomic changes that shaped the development of the cerebral cortex by controlling the behavior of cortical progenitors, thus elucidating molecular mechanisms that played an important role in human cortical evolution.
Figure 3. Evolutionary expansion of the human neocortex and cortical progenitors.
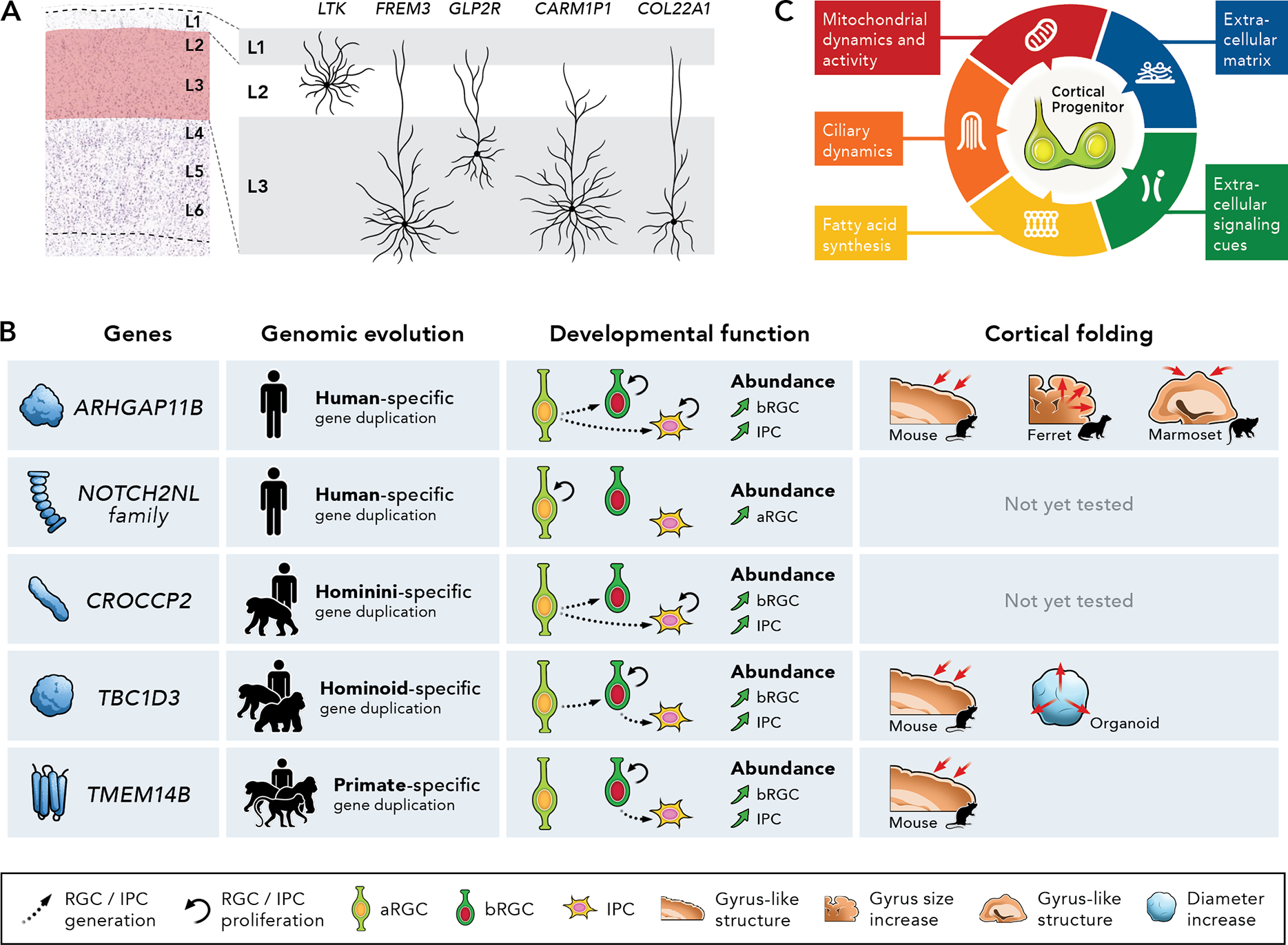
(A) A hallmark of human neocortical evolution is the expansion of superficial layer neurons. Left, image of a coronal section of the human neocortex illustrating the relative thickness of cortical layers (L1-L6). Red shade highlights the expansion of superficial layers, especially L2–3, in the human neocortex. Image adapted from Allen Institute for Brain Science. Image credit: Allen Institute for Brain Science: https://human.brain-map.org/ish/specimen/show/79946224 (specifically, Nissl-stained brain section from the right-hand side panel: Image 7 of 8). Right, drawing illustrating the expanded neuronal diversity found in superficial layers of the human neocortex [as described in 3]; labels indicate the marker genes of specific types of excitatory pyramidal cells. (B) Gene duplications in evolution that regulate the behavior of cortical progenitors. ARHGAP11B arose 5 million years ago by partial duplication of ARHGAP11A. Three human-specific paralogs (NOTCH2NLA, NOTCH2NLB, and NOTCH2NLC) derived from duplication of NOTCH2. CROCCP2 arose through partial duplication of exons 13–21 of CROCC in the Hominini lineage (chimpanzees and humans). TBC1D3 arose in the Hominoid lineage (great apes) from a segmental duplication, with multiple copies found in the human genome and only a single copy found in the chimpanzee genome. TMEM14B arose in the primate lineage, including Old World and New World monkeys and apes. In parallel, experimental manipulations in mice, ferrets, and marmosets, as well as cerebral cortical organoids, have revealed a role for these genes in inducing cortical folding induction and/or cortical size expansion. Symbols represent the animal models in which cortical folding appeared as a result of overexpression experiments. (C) Distinctive features of cortical progenitors during human brain development. The schema compiles 5 cellular features reviewed in this work: extracellular matrix components, responsiveness to extracellular signaling cues, regulation of fatty acid synthesis, mitochondrial dynamics and metabolism, and ciliary dynamics and trafficking.
Gene duplication is a major evolutionary driving force. Many recent studies have documented the essential function that the emergence of novel genes during evolution played in the specialized behavior of human basal progenitors (thoroughly reviewed in 56,126) (Figure 3B). ARGHAP11B, a gene that arose on the human lineage after the divergence from the chimpanzee lineage by partial duplication of the Rho guanosine triphosphatase-activating protein ARHGAP11A and subsequently underwent a splice-site mutation, increases basal progenitor proliferation, as revealed by overexpression experiments in mice, ferrets, and marmosets 82,85,127. CROCCP2, a gene duplicated in the hominin lineage (chimpanzees and humans) that is highly expressed during human corticogenesis, enhances the generation of basal progenitors 128. Three human-specific paralogs derived from duplication of NOTCH2 (NOTCH2NLA, NOTCH2NLB, and NOTCH2NLC) play critical roles in expanding the pool of cortical progenitors 129,130. The overexpression of TBC1D3 (a great ape-specific gene) and of TMEM14B (a primate-specific gene) in mice promote the expansion of basal progenitors 131–133. As a result of the effects in basal progenitors, an increasing body of research in ferret models has revealed roles for many of these human-evolved genes in promoting both cortical neuron production and cortical folding (Figure 3).
Changes in gene regulation constitute another major force driving cortical evolution. A broad range of regulatory mechanisms have been found to be impacted not only by species-specific genomic changes during evolution but also by genetic variants associated with neurodevelopmental disorders. These range from mutations in enhancers and promoters to changes in alternative splicing 134–138, from the emergence of novel microRNAs and to that of novel long non-coding RNAs 139–141. Gene expression changes in the developing cortex can occur at different spatiotemporal scales, including cell types, cortical areas, and developmental windows 59,88,142–145. Such fine-tuned expression patterns likely reflect a positive selection necessary for precise functional organization of the cerebral cortex. Importantly, experimental manipulation in mice has shown a direct link between gene regulation and cortical expansion: the human accelerated region HARE5 upregulates the expression of FZD8, a receptor of the Wnt signaling pathway, and promotes accelerated cell cycle of cortical progenitors resulting in increased cortical size 137. Despite the increasing number of evolutionarily dynamic non-coding regions linked to cortical development, the functional evaluation of these loci in animal models remains limited and, therefore, their impact in regulating cortical shape and folding is still poorly understood.
Notably, several recent studies have revealed specialized molecular and cellular features of human cortical progenitors (Figure 3C). These include elevated expression of extracellular matrix proteins 44,102,146, responsiveness to extracellular signaling cues through innovations in receptor expression 147–149, molecular pathways controlling fatty acid synthesis 83, specialized mitochondrial dynamics and metabolism 150,151, and ciliary dynamics and trafficking 128. These mechanisms have been shown to modulate a variety of developmental processes, including not only the behavior of cortical progenitors but also the cell fate during neurogenesis as well as the protracted timing of neuronal maturation. Therefore, it is foreseeable that research over the next years will continue to decode evolutionary genetic mechanisms underlying these developmental processes, thus shedding new light into the organization of the human cerebral cortex. The recent development of novel comparative genomic strategies will provide new and exciting opportunities to approach these questions 152,153.
CONCLUSIONS AND PERSPECTIVES
In the past decade, a wave of studies have revealed genetic mechanisms that—as discussed in this Review—regulate the highly proliferative capacity of basal radial glial cells and their effect on differential tangential expansion of the developing cortex, thereby establishing a direct link between the behavior of cortical progenitors to the emergence of cerebral cortical folding. Notwithstanding their significance, the study of how the human cerebral cortex acquires its shape during development appears to be at its beginning rather than its end. Advances in comparative genomics have identified a rapidly increasing number of candidate human-specific regulators of cortical development, both in coding and non-coding regions of the genome 130,138,154. Neuroimaging approaches coupled with genome-wide analyses have uncovered many genomic loci significantly associated with various neuroimaging phenotypes related to morphological variables of sulci and gyri 155. Single-cell transcriptomic analyses have found striking differences in gene expression across cortical regions and developmental stages, revealing molecular and cellular patterns for cortical arealization 88,142–144. Furthermore, it is striking to note the many variations in cortical gyrification patterns across mammalian species 156–158, the evolutionary specializations of defined cortical areas (especially within the frontal lobe) 159–162, as well as the effects of environmental perturbations in cortical cytoarchitecture 40,163. Altogether, these data suggest that only a minor fraction of genetic programs involved in shaping the structural and functional organization of the neocortex has been functionally dissected so far, as recently discussed in the context of the evolution of cortical connectivity 124, and many questions remained to be addressed. How do region-specific programs give rise to distinct functional cortical areas? What temporal windows define the specification of these areas? Which evolutionary mechanisms determine species-specific variation in cortical shape and organization? We think that the next years will see intense research on the mechanisms of the emergence of structural and functional organization in the neocortex, and that these developmental insights will have deep implications for our understanding of human cognitive functions.
Recent work is beginning to shed new light on additional cellular processes involved in shaping neocortical architecture. In later stages of development, cortical progenitors acquire new roles and shift their neurogenic fate into a gliogenic one, becoming committed to generate astrocytes as well as oligodendrocytes 34. A recent study found an important role for localized astrogenesis in gyri formation, and this process is regulated by FGF signaling 164. This is consistent with earlier observations in the macaque developing cortex that suggested a contribution of gliogenesis in folding of the cortical surface 165. Since glial cells represent extraordinarily voluminous populations in the neocortex 7,8, further understanding of the role of gliogenesis in cortical folding will complement the current knowledge on neurogenesis-driven mechanisms and lead to more comprehensive models on the emergence of shape during cortical development.
Finally, technological and bioinformatic advances, together with increased sample sizes, have spurred the identification of genetic variants associated with cortical malformations, implicating rapidly increasing numbers of genetic factors in recent years 166. This emphasizes the need to functionally interrogate novel genetic variants to continue dissecting the pathogenesis of these neurodevelopmental disorders. These studies will also be critical in expanding our understanding of molecular and cellular mechanisms involved in the development of cortical shape.
Acknowledgements:
We are grateful to Andrew C. Kay for design consultation and illustration support. S.K.A. is supported by the Harvard Stem Cell Institute, NIH T32 MH020017 and T32 GM007753, and the Paul and Daisy Soros Fellowship for New Americans. D.E-A. is supported by the EMBO Postdoctoral Fellowship (ALTF 336–2022). This work was supported by UM1HG006348. C.A.W. is supported by the NINDS (R01 NS035129 and R01 NS032457) and by the Allen Discovery Center program, a Paul G. Allen Frontiers Group advised program of the Paul G. Allen Family Foundation. C.A.W. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Declaration of Interests:
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Sousa AMM, Meyer KA, Santpere G, Gulden FO, and Sestan N (2017). Evolution of the Human Nervous System Function, Structure, and Development. Cell 170, 226–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaas J (2020). Evolutionary Neuroscience 2nd ed. Kaas J, ed. (Academic Press; ). [Google Scholar]
- 3.Berg J, Sorensen SA, Ting JT, Miller JA, Chartrand T, Buchin A, Bakken TE, Budzillo A, Dee N, Ding S-L, et al. (2021). Human neocortical expansion involves glutamatergic neuron diversification. Nature 598, 151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakken TE, Jorstad NL, Hu Q, Lake BB, Tian W, Kalmbach BE, Crow M, Hodge RD, Krienen FM, Sorensen SA, et al. (2021). Comparative cellular analysis of motor cortex in human, marmoset and mouse. Nature 598, 111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jorstad NL, Song JHT, Exposito-Alonso D, Suresh H, Castro N, Krienen FM, Yanny AM, Close J, Gelfand E, Travaglini KJ, et al. (2022). Comparative transcriptomics reveals human-specific cortical features. bioRxiv, 2022.09.19.508480. 10.1101/2022.09.19.508480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma S, Skarica M, Li Q, Xu C, Risgaard RD, Tebbenkamp ATN, Mato-Blanco X, Kovner R, Krsnik Ž, de Martin X, et al. (2022). Molecular and cellular evolution of the primate dorsolateral prefrontal cortex. Science, eabo7257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herculano-Houzel S (2009). The human brain in numbers: a linearly scaled-up primate brain. Front. Hum. Neurosci. 3, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sherwood CC, Stimpson CD, Raghanti MA, Wildman DE, Uddin M, Grossman LI, Goodman M, Redmond JC, Bonar CJ, Erwin JM, et al. (2006). Evolution of increased glia-neuron ratios in the human frontal cortex. Proc. Natl. Acad. Sci. U. S. A. 103, 13606–13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jayaraman D, Bae B-I, and Walsh CA (2018). The Genetics of Primary Microcephaly. Annu. Rev. Genomics Hum. Genet. 19, 177–200. [DOI] [PubMed] [Google Scholar]
- 10.Faheem M, Naseer MI, Rasool M, Chaudhary AG, Kumosani TA, Ilyas AM, Pushparaj P, Ahmed F, Algahtani HA, Al-Qahtani MH, et al. (2015). Molecular genetics of human primary microcephaly: an overview. BMC Med. Genomics 8 Suppl 1, S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanaka T, and Gleeson JG (2000). Genetics of brain development and malformation syndromes. Curr. Opin. Pediatr. 12, 523–528. [DOI] [PubMed] [Google Scholar]
- 12.Hu WF, Chahrour MH, and Walsh CA (2014). The diverse genetic landscape of neurodevelopmental disorders. Annu. Rev. Genomics Hum. Genet. 15, 195–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Juan Romero C, and Borrell V (2017). Genetic maps and patterns of cerebral cortex folding. Curr. Opin. Cell Biol. 49, 31–37. [DOI] [PubMed] [Google Scholar]
- 14.Welker W (1990). Why Does Cerebral Cortex Fissure and Fold? In Cerebral Cortex: Comparative Structure and Evolution of Cerebral Cortex, Part II, Jones EG and Peters A, eds. (Springer US; ), pp. 3–136. [Google Scholar]
- 15.Lohmann G, von Cramon DY, and Steinmetz H (1999). Sulcal variability of twins. Cereb. Cortex 9, 754–763. [DOI] [PubMed] [Google Scholar]
- 16.Lohmann G, von Cramon DY, and Colchester ACF (2008). Deep sulcal landmarks provide an organizing framework for human cortical folding. Cereb. Cortex 18, 1415–1420. [DOI] [PubMed] [Google Scholar]
- 17.Schmitt JE, Raznahan A, Liu S, and Neale MC (2021). The Heritability of Cortical Folding: Evidence from the Human Connectome Project. Cereb. Cortex 31, 702–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller JA, D’Esposito M, and Weiner KS (2021). Using tertiary sulci to map the “cognitive globe” of prefrontal cortex. J. Cogn. Neurosci. 33, 1698–1715. [DOI] [PubMed] [Google Scholar]
- 19.Cachia A, Borst G, Jardri R, Raznahan A, Murray GK, Mangin J-F, and Plaze M (2021). Towards Deciphering the Fetal Foundation of Normal Cognition and Cognitive Symptoms From Sulcation of the Cortex. Front. Neuroanat. 15, 712862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hilgetag CC, and Barbas H (2006). Role of mechanical factors in the morphology of the primate cerebral cortex. PLoS Comput. Biol. 2, e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klyachko VA, and Stevens CF (2003). Connectivity optimization and the positioning of cortical areas. Proc. Natl. Acad. Sci. U. S. A. 100, 7937–7941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zilles K, Palomero-Gallagher N, and Amunts K (2013). Development of cortical folding during evolution and ontogeny. Trends Neurosci. 36, 275–284. [DOI] [PubMed] [Google Scholar]
- 23.Ashwell KWS, and Hardman CD (2012). Distinct development of the cerebral cortex in platypus and echidna. Brain Behav. Evol. 79, 57–72. [DOI] [PubMed] [Google Scholar]
- 24.Dehay C, Kennedy H, and Kosik KS (2015). The outer subventricular zone and primate-specific cortical complexification. Neuron 85, 683–694. [DOI] [PubMed] [Google Scholar]
- 25.Lewitus E, Kelava I, Kalinka AT, Tomancak P, and Huttner WB (2014). An adaptive threshold in mammalian neocortical evolution. PLoS Biol. 12, e1002000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Leary MA, Bloch JI, Flynn JJ, Gaudin TJ, Giallombardo A, Giannini NP, Goldberg SL, Kraatz BP, Luo Z-X, Meng J, et al. (2013). The placental mammal ancestor and the post-K-Pg radiation of placentals. Science 339, 662–667. [DOI] [PubMed] [Google Scholar]
- 27.Smart IH, and McSherry GM (1986). Gyrus formation in the cerebral cortex in the ferret. I. Description of the external changes. J. Anat. 146, 141–152. [PMC free article] [PubMed] [Google Scholar]
- 28.Akula SK, Chen AY, et al. (2023). Exome sequencing and the identification of new genes and shared mechanisms in polymicrogyria. JAMA Neurol. (accepted). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gilardi C, and Kalebic N (2021). The Ferret as a Model System for Neocortex Development and Evolution. Front Cell Dev Biol 9, 661759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson MB, Sun X, Kodani A, Borges-Monroy R, Girskis KM, Ryu SC, Wang PP, Patel K, Gonzalez DM, Woo YM, et al. (2018). Aspm knockout ferret reveals an evolutionary mechanism governing cerebral cortical size. Nature 556, 370–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsumoto N, Tanaka S, Horiike T, Shinmyo Y, and Kawasaki H (2020). A discrete subtype of neural progenitor crucial for cortical folding in the gyrencephalic mammalian brain. Elife 9. 10.7554/eLife.54873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noctor SC, Martínez-Cerdeño V, Ivic L, and Kriegstein AR (2004). Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat. Neurosci. 7, 136–144. [DOI] [PubMed] [Google Scholar]
- 33.Noctor SC, Martínez-Cerdeño V, and Kriegstein AR (2008). Distinct behaviors of neural stem and progenitor cells underlie cortical neurogenesis. J. Comp. Neurol. 508, 28–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kriegstein A, and Alvarez-Buylla A (2009). The glial nature of embryonic and adult neural stem cells. Annu. Rev. Neurosci. 32, 149–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nowakowski TJ, Pollen AA, Sandoval-Espinosa C, and Kriegstein AR (2016). Transformation of the Radial Glia Scaffold Demarcates Two Stages of Human Cerebral Cortex Development. Neuron 91, 1219–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haubensak W, Attardo A, Denk W, and Huttner WB (2004). Neurons arise in the basal neuroepithelium of the early mammalian telencephalon: a major site of neurogenesis. Proc. Natl. Acad. Sci. U. S. A. 101, 3196–3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miyata T, Kawaguchi A, Saito K, Kawano M, Muto T, and Ogawa M (2004). Asymmetric production of surface-dividing and non-surface-dividing cortical progenitor cells. Development 131, 3133–3145. [DOI] [PubMed] [Google Scholar]
- 38.Pebworth M-P, Ross J, Andrews M, Bhaduri A, and Kriegstein AR (2021). Human intermediate progenitor diversity during cortical development. Proc. Natl. Acad. Sci. U. S. A. 118. 10.1073/pnas.2019415118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reillo I, and Borrell V (2012). Germinal zones in the developing cerebral cortex of ferret: ontogeny, cell cycle kinetics, and diversity of progenitors. Cereb. Cortex 22, 2039–2054. [DOI] [PubMed] [Google Scholar]
- 40.Reillo I, de Juan Romero C, García-Cabezas MÁ, and Borrell V (2011). A role for intermediate radial glia in the tangential expansion of the mammalian cerebral cortex. Cereb. Cortex 21, 1674–1694. [DOI] [PubMed] [Google Scholar]
- 41.Pilz G-A, Shitamukai A, Reillo I, Pacary E, Schwausch J, Stahl R, Ninkovic J, Snippert HJ, Clevers H, Godinho L, et al. (2013). Amplification of progenitors in the mammalian telencephalon includes a new radial glial cell type. Nat. Commun. 4, 2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Betizeau M, Cortay V, Patti D, Pfister S, Gautier E, Bellemin-Ménard A, Afanassieff M, Huissoud C, Douglas RJ, Kennedy H, et al. (2013). Precursor diversity and complexity of lineage relationships in the outer subventricular zone of the primate. Neuron 80, 442–457. [DOI] [PubMed] [Google Scholar]
- 43.Hansen DV, Lui JH, Parker PRL, and Kriegstein AR (2010). Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature 464, 554–561. [DOI] [PubMed] [Google Scholar]
- 44.Fietz SA, Kelava I, Vogt J, Wilsch-Bräuninger M, Stenzel D, Fish JL, Corbeil D, Riehn A, Distler W, Nitsch R, et al. (2010). OSVZ progenitors of human and ferret neocortex are epithelial-like and expand by integrin signaling. Nat. Neurosci. 13, 690–699. [DOI] [PubMed] [Google Scholar]
- 45.Smart IHM, Dehay C, Giroud P, Berland M, and Kennedy H (2002). Unique morphological features of the proliferative zones and postmitotic compartments of the neural epithelium giving rise to striate and extrastriate cortex in the monkey. Cereb. Cortex 12, 37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.LaMonica BE, Lui JH, Hansen DV, and Kriegstein AR (2013). Mitotic spindle orientation predicts outer radial glial cell generation in human neocortex. Nat. Commun. 4, 1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shitamukai A, Konno D, and Matsuzaki F (2011). Oblique radial glial divisions in the developing mouse neocortex induce self-renewing progenitors outside the germinal zone that resemble primate outer subventricular zone progenitors. J. Neurosci. 31, 3683–3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fietz SA, and Huttner WB (2011). Cortical progenitor expansion, self-renewal and neurogenesis-a polarized perspective. Curr. Opin. Neurobiol. 21, 23–35. [DOI] [PubMed] [Google Scholar]
- 49.Llinares-Benadero C, and Borrell V (2019). Deconstructing cortical folding: genetic, cellular and mechanical determinants. Nat. Rev. Neurosci. 20, 161–176. [DOI] [PubMed] [Google Scholar]
- 50.Rakic P (1988). Specification of cerebral cortical areas. Science 241, 170–176. [DOI] [PubMed] [Google Scholar]
- 51.Gertz CC, and Kriegstein AR (2015). Neuronal Migration Dynamics in the Developing Ferret Cortex. J. Neurosci. 35, 14307–14315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ware ML, Tavazoie SF, Reid CB, and Walsh CA (1999). Coexistence of widespread clones and large radial clones in early embryonic ferret cortex. Cereb. Cortex 9, 636–645. [DOI] [PubMed] [Google Scholar]
- 53.Kornack DR, and Rakic P (1995). Radial and horizontal deployment of clonally related cells in the primate neocortex: relationship to distinct mitotic lineages. Neuron 15, 311–321. [DOI] [PubMed] [Google Scholar]
- 54.Noctor SC, Flint AC, Weissman TA, Dammerman RS, and Kriegstein AR (2001). Neurons derived from radial glial cells establish radial units in neocortex. Nature 409, 714–720. [DOI] [PubMed] [Google Scholar]
- 55.Taverna E, Götz M, and Huttner WB (2014). The cell biology of neurogenesis: toward an understanding of the development and evolution of the neocortex. Annu. Rev. Cell Dev. Biol. 30, 465–502. [DOI] [PubMed] [Google Scholar]
- 56.Pinson A, and Huttner WB (2021). Neocortex expansion in development and evolution-from genes to progenitor cell biology. Curr. Opin. Cell Biol. 73, 9–18. [DOI] [PubMed] [Google Scholar]
- 57.Cárdenas A, and Borrell V (2020). Molecular and cellular evolution of corticogenesis in amniotes. Cell. Mol. Life Sci. 77, 1435–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Namba T, and Huttner WB (2017). Neural progenitor cells and their role in the development and evolutionary expansion of the neocortex. Wiley Interdiscip. Rev. Dev. Biol. 6. 10.1002/wdev.256. [DOI] [PubMed] [Google Scholar]
- 59.Pollen AA, Nowakowski TJ, Chen J, Retallack H, Sandoval-Espinosa C, Nicholas CR, Shuga J, Liu SJ, Oldham MC, Diaz A, et al. (2015). Molecular identity of human outer radial glia during cortical development. Cell 163, 55–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang X, Tsai J-W, LaMonica B, and Kriegstein AR (2011). A new subtype of progenitor cell in the mouse embryonic neocortex. Nat. Neurosci. 14, 555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stepien BK, Vaid S, and Huttner WB (2021). Length of the Neurogenic Period-A Key Determinant for the Generation of Upper-Layer Neurons During Neocortex Development and Evolution. Front Cell Dev Biol 9, 676911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kroenke CD, and Bayly PV (2018). How Forces Fold the Cerebral Cortex. J. Neurosci. 38, 767–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.de Juan Romero C, Bruder C, Tomasello U, Sanz-Anquela JM, and Borrell V (2015). Discrete domains of gene expression in germinal layers distinguish the development of gyrencephaly. EMBO J. 34, 1859–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lui JH, Hansen DV, and Kriegstein AR (2011). Development and evolution of the human neocortex. Cell 146, 18–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Del-Valle-Anton L, and Borrell V (2022). Folding brains: from development to disease modeling. Physiol. Rev. 102, 511–550. [DOI] [PubMed] [Google Scholar]
- 66.Bray D (1984). Axonal growth in response to experimentally applied mechanical tension. Dev. Biol. 102, 379–389. [DOI] [PubMed] [Google Scholar]
- 67.Van Essen DC (1997). A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature 385, 313–318. [DOI] [PubMed] [Google Scholar]
- 68.Chenn A, and Walsh CA (2002). Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science 297, 365–369. [DOI] [PubMed] [Google Scholar]
- 69.Jones SG, and Samanta D (2022). Macrocephaly (StatPearls Publishing; ). [PubMed] [Google Scholar]
- 70.Xu G, Knutsen AK, Dikranian K, Kroenke CD, Bayly PV, and Taber LA (2010). Axons pull on the brain, but tension does not drive cortical folding. J. Biomech. Eng. 132, 071013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mota B, and Herculano-Houzel S (2015). Cortical folding scales universally with surface area and thickness, not number of neurons. Science 349, 74–77. [DOI] [PubMed] [Google Scholar]
- 72.Dervaux J, and Ben Amar M (2008). Morphogenesis of growing soft tissues. Phys. Rev. Lett. 101, 068101. [DOI] [PubMed] [Google Scholar]
- 73.Toro R, and Burnod Y (2005). A morphogenetic model for the development of cortical convolutions. Cereb. Cortex 15, 1900–1913. [DOI] [PubMed] [Google Scholar]
- 74.Bayly PV, Okamoto RJ, Xu G, Shi Y, and Taber LA (2013). A cortical folding model incorporating stress-dependent growth explains gyral wavelengths and stress patterns in the developing brain. Phys. Biol. 10, 016005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tallinen T, Chung JY, Biggins JS, and Mahadevan L (2014). Gyrification from constrained cortical expansion. Proc. Natl. Acad. Sci. U. S. A. 111, 12667–12672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tallinen T, Chung JY, Rousseau F, Girard N, Lefèvre J, and Mahadevan L (2016). On the growth and form of cortical convolutions. Nat. Phys. 12, 588–593. [Google Scholar]
- 77.Budday S, Steinmann P, and Kuhl E (2014). The role of mechanics during brain development. J. Mech. Phys. Solids 72, 75–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang X, Studholme C, Grigsby PL, Frias AE, Cuzon Carlson VC, and Kroenke CD (2017). Folding, But Not Surface Area Expansion, Is Associated with Cellular Morphological Maturation in the Fetal Cerebral Cortex. J. Neurosci. 37, 1971–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lukaszewicz A, Savatier P, Cortay V, Giroud P, Huissoud C, Berland M, Kennedy H, and Dehay C (2005). G1 phase regulation, area-specific cell cycle control, and cytoarchitectonics in the primate cortex. Neuron 47, 353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lukaszewicz A, Cortay V, Giroud P, Berland M, Smart I, Kennedy H, and Dehay C (2006). The concerted modulation of proliferation and migration contributes to the specification of the cytoarchitecture and dimensions of cortical areas. Cereb. Cortex 16 Suppl 1, i26–34. [DOI] [PubMed] [Google Scholar]
- 81.Nonaka-Kinoshita M, Reillo I, Artegiani B, Martínez-Martínez MÁ, Nelson M, Borrell V, and Calegari F (2013). Regulation of cerebral cortex size and folding by expansion of basal progenitors. EMBO J. 32, 1817–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kalebic N, Gilardi C, Albert M, Namba T, Long KR, Kostic M, Langen B, and Huttner WB (2018). Human-specific ARHGAP11B induces hallmarks of neocortical expansion in developing ferret neocortex. Elife 7. 10.7554/eLife.41241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pinson A, Xing L, Namba T, Kalebic N, Peters J, Oegema CE, Traikov S, Reppe K, Riesenberg S, Maricic T, et al. (2022). Human TKTL1 implies greater neurogenesis in frontal neocortex of modern humans than Neanderthals. Science 377, eabl6422. [DOI] [PubMed] [Google Scholar]
- 84.Stahl R, Walcher T, De Juan Romero C, Pilz GA, Cappello S, Irmler M, Sanz-Aquela JM, Beckers J, Blum R, Borrell V, et al. (2013). Trnp1 regulates expansion and folding of the mammalian cerebral cortex by control of radial glial fate. Cell 153, 535–549. [DOI] [PubMed] [Google Scholar]
- 85.Florio M, Albert M, Taverna E, Namba T, Brandl H, Lewitus E, Haffner C, Sykes A, Wong FK, Peters J, et al. (2015). Human-specific gene ARHGAP11B promotes basal progenitor amplification and neocortex expansion. Science 347, 1465–1470. [DOI] [PubMed] [Google Scholar]
- 86.Wang L, Hou S, and Han Y-G (2016). Hedgehog signaling promotes basal progenitor expansion and the growth and folding of the neocortex. Nat. Neurosci. 19, 888–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Elsen GE, Hodge RD, Bedogni F, Daza RAM, Nelson BR, Shiba N, Reiner SL, and Hevner RF (2013). The protomap is propagated to cortical plate neurons through an Eomes-dependent intermediate map. Proc. Natl. Acad. Sci. U. S. A. 110, 4081–4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bhaduri A, Sandoval-Espinosa C, Otero-Garcia M, Oh I, Yin R, Eze UC, Nowakowski TJ, and Kriegstein AR (2021). An atlas of cortical arealization identifies dynamic molecular signatures. Nature 598, 200–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Martínez-Martínez MÁ, Ciceri G, Espinós A, Fernández V, Marín O, and Borrell V (2019). Extensive branching of radially-migrating neurons in the mammalian cerebral cortex. J. Comp. Neurol. 527, 1558–1576. [DOI] [PubMed] [Google Scholar]
- 90.Reid CB, Liang I, and Walsh C (1995). Systematic widespread clonal organization in cerebral cortex. Neuron 15, 299–310. [DOI] [PubMed] [Google Scholar]
- 91.del Toro D, Ruff T, Cederfjäll E, Villalba A, Seyit-Bremer G, Borrell V, and Klein R (2017). Regulation of Cerebral Cortex Folding by Controlling Neuronal Migration via FLRT Adhesion Molecules. Cell 169, 621–635.e16. [DOI] [PubMed] [Google Scholar]
- 92.Del Toro D, Carrasquero-Ordaz MA, Chu A, Ruff T, Shahin M, Jackson VA, Chavent M, Berbeira-Santana M, Seyit-Bremer G, Brignani S, et al. (2020). Structural Basis of Teneurin-Latrophilin Interaction in Repulsive Guidance of Migrating Neurons. Cell 180, 323–339.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rash BG, Lim HD, Breunig JJ, and Vaccarino FM (2011). FGF signaling expands embryonic cortical surface area by regulating Notch-dependent neurogenesis. J. Neurosci. 31, 15604–15617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lukaszewicz A, Savatier P, Cortay V, Kennedy H, and Dehay C (2002). Contrasting effects of basic fibroblast growth factor and neurotrophin 3 on cell cycle kinetics of mouse cortical stem cells. J. Neurosci. 22, 6610–6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Masuda K, Toda T, Shinmyo Y, Ebisu H, Hoshiba Y, Wakimoto M, Ichikawa Y, and Kawasaki H (2015). Pathophysiological analyses of cortical malformation using gyrencephalic mammals. Sci. Rep. 5, 15370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lange C, Huttner WB, and Calegari F (2009). Cdk4/cyclinD1 overexpression in neural stem cells shortens G1, delays neurogenesis, and promotes the generation and expansion of basal progenitors. Cell Stem Cell 5, 320–331. [DOI] [PubMed] [Google Scholar]
- 97.Mairet-Coello G, Tury A, and DiCicco-Bloom E (2009). Insulin-like growth factor-1 promotes G(1)/S cell cycle progression through bidirectional regulation of cyclins and cyclin-dependent kinase inhibitors via the phosphatidylinositol 3-kinase/Akt pathway in developing rat cerebral cortex. J. Neurosci. 29, 775–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tavano S, Taverna E, Kalebic N, Haffner C, Namba T, Dahl A, Wilsch-Bräuninger M, Paridaen JTML, and Huttner WB (2018). Insm1 Induces Neural Progenitor Delamination in Developing Neocortex via Downregulation of the Adherens Junction Belt-Specific Protein Plekha7. Neuron 97, 1299–1314.e8. [DOI] [PubMed] [Google Scholar]
- 99.Kingsbury MA, Rehen SK, Contos JJA, Higgins CM, and Chun J (2003). Non-proliferative effects of lysophosphatidic acid enhance cortical growth and folding. Nat. Neurosci. 6, 1292–1299. [DOI] [PubMed] [Google Scholar]
- 100.Long KR, and Huttner WB (2019). How the extracellular matrix shapes neural development. Open Biol. 9, 180216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Amin S, and Borrell V (2020). The Extracellular Matrix in the Evolution of Cortical Development and Folding. Front Cell Dev Biol 8, 604448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Long KR, Newland B, Florio M, Kalebic N, Langen B, Kolterer A, Wimberger P, and Huttner WB (2018). Extracellular Matrix Components HAPLN1, Lumican, and Collagen I Cause Hyaluronic Acid-Dependent Folding of the Developing Human Neocortex. Neuron. 10.1016/j.neuron.2018.07.013. [DOI] [PubMed] [Google Scholar]
- 103.Stenzel D, Wilsch-Bräuninger M, Wong FK, Heuer H, and Huttner WB (2014). Integrin αvβ3 and thyroid hormones promote expansion of progenitors in embryonic neocortex. Development 141, 795–806. [DOI] [PubMed] [Google Scholar]
- 104.Kalebic N, Gilardi C, Stepien B, Wilsch-Bräuninger M, Long KR, Namba T, Florio M, Langen B, Lombardot B, Shevchenko A, et al. (2019). Neocortical Expansion Due to Increased Proliferation of Basal Progenitors Is Linked to Changes in Their Morphology. Cell Stem Cell 24, 535–550.e9. [DOI] [PubMed] [Google Scholar]
- 105.Güven A, Kalebic N, Long KR, Florio M, Vaid S, Brandl H, Stenzel D, and Huttner WB (2020). Extracellular matrix-inducing Sox9 promotes both basal progenitor proliferation and gliogenesis in developing neocortex. Elife 9. 10.7554/eLife.49808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Siegenthaler JA, Ashique AM, Zarbalis K, Patterson KP, Hecht JH, Kane MA, Folias AE, Choe Y, May SR, Kume T, et al. (2009). Retinoic acid from the meninges regulates cortical neuron generation. Cell 139, 597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yabut OR, and Pleasure SJ (2018). Sonic Hedgehog Signaling Rises to the Surface: Emerging Roles in Neocortical Development. Brain Plast 3, 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Komada M (2012). Sonic hedgehog signaling coordinates the proliferation and differentiation of neural stem/progenitor cells by regulating cell cycle kinetics during development of the neocortex. Congenit. Anom. 52, 72–77. [DOI] [PubMed] [Google Scholar]
- 109.Komada M, Saitsu H, Kinboshi M, Miura T, Shiota K, and Ishibashi M (2008). Hedgehog signaling is involved in development of the neocortex. Development 135, 2717–2727. [DOI] [PubMed] [Google Scholar]
- 110.Saade M, Gutiérrez-Vallejo I, Le Dréau G, Rabadán MA, Miguez DG, Buceta J, and Martí E (2013). Sonic hedgehog signaling switches the mode of division in the developing nervous system. Cell Rep. 4, 492–503. [DOI] [PubMed] [Google Scholar]
- 111.Hou S, Ho W-L, Wang L, Kuo B, Park JY, and Han Y-G (2021). Biphasic Roles of Hedgehog Signaling in the Production and Self-Renewal of Outer Radial Glia in the Ferret Cerebral Cortex. Cereb. Cortex 31, 4730–4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Coulter ME, Dorobantu CM, Lodewijk GA, Delalande F, Cianferani S, Ganesh VS, Smith RS, Lim ET, Xu CS, Pang S, et al. (2018). The ESCRT-III Protein CHMP1A Mediates Secretion of Sonic Hedgehog on a Distinctive Subtype of Extracellular Vesicles. Cell Rep. 24, 973–986.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nanni L, Ming JE, Bocian M, Steinhaus K, Bianchi DW, Die-Smulders C, Giannotti A, Imaizumi K, Jones KL, Campo MD, et al. (1999). The mutational spectrum of the sonic hedgehog gene in holoprosencephaly: SHH mutations cause a significant proportion of autosomal dominant holoprosencephaly. Hum. Mol. Genet. 8, 2479–2488. [DOI] [PubMed] [Google Scholar]
- 114.Schell-Apacik CC, Ertl-Wagner B, Panzel A, Klausener K, Rausch G, Muenke M, von Voss H, and Hehr U (2009). Maternally inherited heterozygous sequence change in the sonic hedgehog gene in a male patient with bilateral closed-lip schizencephaly and partial absence of the corpus callosum. Am. J. Med. Genet. A 149A, 1592–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hehr U, Pineda-Alvarez DE, Uyanik G, Hu P, Zhou N, Hehr A, Schell-Apacik C, Altus C, Daumer-Haas C, Meiner A, et al. (2010). Heterozygous mutations in SIX3 and SHH are associated with schizencephaly and further expand the clinical spectrum of holoprosencephaly. Hum. Genet. 127, 555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ming JE, Kaupas ME, Roessler E, Brunner HG, Golabi M, Tekin M, Stratton RF, Sujansky E, Bale SJ, and Muenke M (2002). Mutations in PATCHED-1, the receptor for SONIC HEDGEHOG, are associated with holoprosencephaly. Hum. Genet. 110, 297–301. [DOI] [PubMed] [Google Scholar]
- 117.Bae G-U, Domené S, Roessler E, Schachter K, Kang J-S, Muenke M, and Krauss RS (2011). Mutations in CDON, encoding a hedgehog receptor, result in holoprosencephaly and defective interactions with other hedgehog receptors. Am. J. Hum. Genet. 89, 231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mochida GH, Ganesh VS, de Michelena MI, Dias H, Atabay KD, Kathrein KL, Huang H-T, Hill RS, Felie JM, Rakiec D, et al. (2012). CHMP1A encodes an essential regulator of BMI1-INK4A in cerebellar development. Nat. Genet. 44, 1260–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Akula SK, Marciano JH, Lim Y, Exposito-Alonso D, Hylton NK, Hwang GH, Neil JE, Dominado N, Bunton-Stasyshyn RK, Song JHT, et al. (2023). TMEM161B regulates cerebral cortical gyration, Sonic Hedgehog signaling, and ciliary structure in the developing central nervous system. Proc. Natl. Acad. Sci. U. S. A. 120, e2209964120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang L, Heffner C, loi Vong K, Barrows C, Ha Y-J, Lee S, Lara-Gonzalez P, Jhamb I, Meer DVD, Loughnan R, et al. (2023). TMEM161B modulates radial glial scaffolding in neocortical development. Proc. Natl. Acad. Sci. U. S. A. 120, e2209983120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Reiter JF, and Leroux MR (2017). Genes and molecular pathways underpinning ciliopathies. Nat. Rev. Mol. Cell Biol. 18, 533–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Andreu-Cervera A, Catala M, and Schneider-Maunoury S (2021). Cilia, ciliopathies and hedgehog-related forebrain developmental disorders. Neurobiol. Dis. 150, 105236. [DOI] [PubMed] [Google Scholar]
- 123.Galakhova AA, Hunt S, Wilbers R, Heyer DB, de Kock CPJ, Mansvelder HD, and Goriounova NA (2022). Evolution of cortical neurons supporting human cognition. Trends Cogn. Sci. 26, 909–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vanderhaeghen P, and Polleux F (2023). Developmental mechanisms underlying the evolution of human cortical circuits. Nat. Rev. Neurosci. 10.1038/s41583-023-00675-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hutsler JJ, Lee D-G, and Porter KK (2005). Comparative analysis of cortical layering and supragranular layer enlargement in rodent carnivore and primate species. Brain Res. 1052, 71–81. [DOI] [PubMed] [Google Scholar]
- 126.Mora-Bermúdez F, and Huttner WB (2022). What Are the Human-Specific Aspects of Neocortex Development? Front. Neurosci. 16, 878950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Heide M, Haffner C, Murayama A, Kurotaki Y, Shinohara H, Okano H, Sasaki E, and Huttner WB (2020). Human-specific ARHGAP11B increases size and folding of primate neocortex in the fetal marmoset. Science 369, 546–550. [DOI] [PubMed] [Google Scholar]
- 128.Van Heurck R, Bonnefont J, Wojno M, Suzuki IK, Velez-Bravo FD, Erkol E, Nguyen DT, Herpoel A, Bilheu A, Beckers S, et al. (2022). CROCCP2 acts as a human-specific modifier of cilia dynamics and mTOR signaling to promote expansion of cortical progenitors. Neuron. 10.1016/j.neuron.2022.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fiddes IT, Lodewijk GA, Mooring M, Bosworth CM, Ewing AD, Mantalas GL, Novak AM, van den Bout A, Bishara A, Rosenkrantz JL, et al. (2018). Human-Specific NOTCH2NL Genes Affect Notch Signaling and Cortical Neurogenesis. Cell 173, 1356–1369.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Suzuki IK, Gacquer D, Van Heurck R, Kumar D, Wojno M, Bilheu A, Herpoel A, Lambert N, Cheron J, Polleux F, et al. (2018). Human-Specific NOTCH2NL Genes Expand Cortical Neurogenesis through Delta/Notch Regulation. Cell 173, 1370–1384.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hou Q-Q, Xiao Q, Sun X-Y, Ju X-C, and Luo Z-G (2021). TBC1D3 promotes neural progenitor proliferation by suppressing the histone methyltransferase G9a. Sci Adv 7. 10.1126/sciadv.aba8053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ju X-C, Hou Q-Q, Sheng A-L, Wu K-Y, Zhou Y, Jin Y, Wen T, Yang Z, Wang X, and Luo Z-G (2016). The hominoid-specific gene TBC1D3 promotes generation of basal neural progenitors and induces cortical folding in mice. Elife 5. 10.7554/eLife.18197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liu J, Liu W, Yang L, Wu Q, Zhang H, Fang A, Li L, Xu X, Sun L, Zhang J, et al. (2017). The Primate-Specific Gene TMEM14B Marks Outer Radial Glia Cells and Promotes Cortical Expansion and Folding. Cell Stem Cell 21, 635–649.e8. [DOI] [PubMed] [Google Scholar]
- 134.McLean CY, Reno PL, Pollen AA, Bassan AI, Capellini TD, Guenther C, Indjeian VB, Lim X, Menke DB, Schaar BT, et al. (2011). Human-specific loss of regulatory DNA and the evolution of human-specific traits. Nature 471, 216–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bae B-I, Tietjen I, Atabay KD, Evrony GD, Johnson MB, Asare E, Wang PP, Murayama AY, Im K, Lisgo SN, et al. (2014). Evolutionarily dynamic alternative splicing of GPR56 regulates regional cerebral cortical patterning. Science 343, 764–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Reilly SK, Yin J, Ayoub AE, Emera D, Leng J, Cotney J, Sarro R, Rakic P, and Noonan JP (2015). Evolutionary genomics. Evolutionary changes in promoter and enhancer activity during human corticogenesis. Science 347, 1155–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Boyd JL, Skove SL, Rouanet JP, Pilaz L-J, Bepler T, Gordân R, Wray GA, and Silver DL (2015). Human-chimpanzee differences in a FZD8 enhancer alter cell-cycle dynamics in the developing neocortex. Curr. Biol. 25, 772–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Doan RN, Bae B-I, Cubelos B, Chang C, Hossain AA, Al-Saad S, Mukaddes NM, Oner O, Al-Saffar M, Balkhy S, et al. (2016). Mutations in Human Accelerated Regions Disrupt Cognition and Social Behavior. Cell 167, 341–354.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Arcila ML, Betizeau M, Cambronne XA, Guzman E, Doerflinger N, Bouhallier F, Zhou H, Wu B, Rani N, Bassett DS, et al. (2014). Novel primate miRNAs coevolved with ancient target genes in germinal zone-specific expression patterns. Neuron 81, 1255–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rani N, Nowakowski TJ, Zhou H, Godshalk SE, Lisi V, Kriegstein AR, and Kosik KS (2016). A Primate lncRNA Mediates Notch Signaling during Neuronal Development by Sequestering miRNA. Neuron 90, 1174–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nowakowski TJ, Rani N, Golkaram M, Zhou HR, Alvarado B, Huch K, West JA, Leyrat A, Pollen AA, Kriegstein AR, et al. (2018). Regulation of cell-type-specific transcriptomes by microRNA networks during human brain development. Nat. Neurosci. 21, 1784–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nowakowski TJ, Bhaduri A, Pollen AA, Alvarado B, Mostajo-Radji MA, Di Lullo E, Haeussler M, Sandoval-Espinosa C, Liu SJ, Velmeshev D, et al. (2017). Spatiotemporal gene expression trajectories reveal developmental hierarchies of the human cortex. Science 358, 1318–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhu Y, Sousa AMM, Gao T, Skarica M, Li M, Santpere G, Esteller-Cucala P, Juan D, Ferrández-Peral L, Gulden FO, et al. (2018). Spatiotemporal transcriptomic divergence across human and macaque brain development. Science 362. 10.1126/science.aat8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Li M, Santpere G, Imamura Kawasawa Y, Evgrafov OV, Gulden FO, Pochareddy S, Sunkin SM, Li Z, Shin Y, Zhu Y, et al. (2018). Integrative functional genomic analysis of human brain development and neuropsychiatric risks. Science 362. 10.1126/science.aat7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Eze UC, Bhaduri A, Haeussler M, Nowakowski TJ, and Kriegstein AR (2021). Single-cell atlas of early human brain development highlights heterogeneity of human neuroepithelial cells and early radial glia. Nat. Neurosci. 24, 584–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Fietz SA, Lachmann R, Brandl H, Kircher M, Samusik N, Schröder R, Lakshmanaperumal N, Henry I, Vogt J, Riehn A, et al. (2012). Transcriptomes of germinal zones of human and mouse fetal neocortex suggest a role of extracellular matrix in progenitor self-renewal. Proc. Natl. Acad. Sci. U. S. A. 109, 11836–11841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lui JH, Nowakowski TJ, Pollen AA, Javaherian A, Kriegstein AR, and Oldham MC (2014). Radial glia require PDGFD-PDGFRβ signalling in human but not mouse neocortex. Nature 515, 264–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mayer S, Chen J, Velmeshev D, Mayer A, Eze UC, Bhaduri A, Cunha CE, Jung D, Arjun A, Li E, et al. (2019). Multimodal Single-Cell Analysis Reveals Physiological Maturation in the Developing Human Neocortex. Neuron 102, 143–158.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Xing L, Kalebic N, Namba T, Vaid S, Wimberger P, and Huttner WB (2020). Serotonin Receptor 2A Activation Promotes Evolutionarily Relevant Basal Progenitor Proliferation in the Developing Neocortex. Neuron 108, 1113–1129.e6. [DOI] [PubMed] [Google Scholar]
- 150.Iwata R, Casimir P, and Vanderhaeghen P (2020). Mitochondrial dynamics in postmitotic cells regulate neurogenesis. Science 369, 858–862. [DOI] [PubMed] [Google Scholar]
- 151.Iwata R, Casimir P, Erkol E, Boubakar L, Planque M, Gallego López IM, Ditkowska M, Gaspariunaite V, Beckers S, Remans D, et al. (2023). Mitochondria metabolism sets the species-specific tempo of neuronal development. Science, eabn4705. [DOI] [PubMed] [Google Scholar]
- 152.Song JHT, Grant RL, Behrens VC, Kučka M, Roberts Kingman GA, Soltys V, Chan YF, and Kingsley DM (2021). Genetic studies of human-chimpanzee divergence using stem cell fusions. Proc. Natl. Acad. Sci. U. S. A. 118. 10.1073/pnas.2117557118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Agoglia RM, Sun D, Birey F, Yoon S-J, Miura Y, Sabatini K, Pașca SP, and Fraser HB (2021). Primate cell fusion disentangles gene regulatory divergence in neurodevelopment. Nature 592, 421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mangan RJ, Alsina FC, Mosti F, Sotelo-Fonseca JE, Snellings DA, Au EH, Carvalho J, Sathyan L, Johnson GD, Reddy TE, et al. (2022). Adaptive sequence divergence forged new neurodevelopmental enhancers in humans. Cell 185, 4587–4603.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.van der Meer D, Kaufmann T, Shadrin AA, Makowski C, Frei O, Roelfs D, Monereo-Sánchez J, Linden DEJ, Rokicki J, Alnæs D, et al. (2021). The genetic architecture of human cortical folding. Sci Adv 7, eabj9446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.DeFelipe J (2011). The Evolution of the Brain, the Human Nature of Cortical Circuits, and Intellectual Creativity. Front. Neuroanat. 5. 10.3389/fnana.2011.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Manger PR, Prowse M, Haagensen M, and Hemingway J (2012). Quantitative analysis of neocortical gyrencephaly in African elephants (Loxodonta africana) and six species of cetaceans: comparison with other mammals. J. Comp. Neurol. 520, 2430–2439. [DOI] [PubMed] [Google Scholar]
- 158.Pillay P, and Manger PR (2007). Order-specific quantitative patterns of cortical gyrification. Eur. J. Neurosci. 25, 2705–2712. [DOI] [PubMed] [Google Scholar]
- 159.Schenker NM, Buxhoeveden DP, Blackmon WL, Amunts K, Zilles K, and Semendeferi K (2008). A comparative quantitative analysis of cytoarchitecture and minicolumnar organization in Broca’s area in humans and great apes. J. Comp. Neurol. 510, 117–128. [DOI] [PubMed] [Google Scholar]
- 160.Semendeferi K, Armstrong E, Schleicher A, Zilles K, and Van Hoesen GW (2001). Prefrontal cortex in humans and apes: a comparative study of area 10. Am. J. Phys. Anthropol. 114, 224–241. [DOI] [PubMed] [Google Scholar]
- 161.Bauernfeind AL, de Sousa AA, Avasthi T, Dobson SD, Raghanti MA, Lewandowski AH, Zilles K, Semendeferi K, Allman JM, Craig ADB, et al. (2013). A volumetric comparison of the insular cortex and its subregions in primates. J. Hum. Evol. 64, 263–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Preuss TM, and Wise SP (2022). Evolution of prefrontal cortex. Neuropsychopharmacology 47, 3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dehay C, Giroud P, Berland M, Killackey H, and Kennedy H (1996). Contribution of thalamic input to the specification of cytoarchitectonic cortical fields in the primate: effects of bilateral enucleation in the fetal monkey on the boundaries, dimensions, and gyrification of striate and extrastriate cortex. J. Comp. Neurol. 367, 70–89. [DOI] [PubMed] [Google Scholar]
- 164.Shinmyo Y, Saito K, Hamabe-Horiike T, Kameya N, Ando A, Kawasaki K, Duong TAD, Sakashita M, Roboon J, Hattori T, et al. (2022). Localized astrogenesis regulates gyrification of the cerebral cortex. Sci Adv 8, eabi5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rash BG, Duque A, Morozov YM, Arellano JI, Micali N, and Rakic P (2019). Gliogenesis in the outer subventricular zone promotes enlargement and gyrification of the primate cerebrum. Proc. Natl. Acad. Sci. U. S. A. 116, 7089–7094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chung C, Yang X, Bae T, Vong KI, Mittal S, Donkels C, Westley Phillips H, Li Z, Marsh APL, Breuss MW, et al. (2023). Comprehensive multi-omic profiling of somatic mutations in malformations of cortical development. Nat. Genet, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]


