Abstract
A newly identified intraerythrocytic Babesia-like organism, WA1, and its relatives were recently shown to be infectious for humans in the western United States. The purpose of the present study was to determine the susceptibilities of selected mouse genotypes to WA1 infection in an attempt to develop a murine model of the human disease. Several mouse strains were inoculated intraperitoneally with various passages of WA1-parasitized erythrocytes. Parasitemia was evaluated by blood smears and by PCR with blood samples collected at various intervals after inoculation. Hematologic parameters were monitored in blood samples at all intervals. C57BL/6 and C57BL/10 mice exhibited mortality rates of <10%. BALB/cJ, CBAJ, and 129/J mice had higher peak parasitemias than did C57BL mice, with mortality rates of 40, 50, and 50%, respectively. A/J, AKR/N, C3H, and DBA/1J mice also had higher peak parasitemia and mortality rates (>95%). An F1 cross of C57BL/6 (resistant) and C3H.RKK (susceptible) mice had a mortality rate similar to that of the resistant parental strain. Histopathology of BALB/cJ and C3H mice at 9 and 14 days after inoculation revealed erythrophagocytosis and deposition of an iron-negative pigment in multiple organs. Morbidly ill C3H mice at 14 days had severe pulmonary edema, hemoglobinuria, and glomerulonephritis.
Organisms of the genus Babesia represent a nearly ubiquitous family of tick-transmitted intraerythrocytic protozoa with a worldwide distribution in wild and domestic animals. More than 100 species of babesial parasites have been identified to date (family Piroplasmorida), each of which is capable of infecting one or more vertebrate hosts. These parasites, together with members of the genus Theileria, are referred to as piroplasms because of their pear-shaped intraerythrocytic stages (21). Until early in this decade, Babesia microti (United States) and B. divergens (Europe) were recognized as the only human pathogens among them. In 1991, a zoonotic Babesia-like piroplasm (WA1) was identified in a human case from the state of Washington. WA1 was later found to be genetically and antigenically distinct from B. microti and B. divergens (10) and related to B. gibsoni, a well-known canine pathogen. Additional relatives of WA1 have previously been recognized as human pathogens in California (10, 13). WA1 and its relatives have previously been associated with flu-like symptoms in humans. In some cases, disseminated intravascular coagulation, pulmonary edema, and renal insufficiency have been observed (10, 13). However, previous seroprevalence studies have suggested that some cases may be asymptomatic.
Babesiosis in humans is characterized by anorexia, fatigue, fever, sweating, and generalized myalgia (2, 17, 21). Several studies of the pathobiology of experimental babesial infection in hamsters (Mesocricetus auratus), laboratory mice, and primates have been published over the past few decades (1, 4, 8, 16), with such infections demonstrating various resemblances to the human disease. These studies have dealt mostly with B. microti, the etiologic agent of human babesiosis in the northeastern and midwestern United States. In hamsters, infection with B. microti is patent for 3 to 5 weeks, with severe anemia, marked parasitemia, and a low mortality rate. This is followed by a chronic carrier state, during which no parasites are detectable on blood smears but parasitemia can be reactivated by splenectomy. In mice, B. microti also produces transient parasitemia, followed by an asymptomatic carrier state, which is the most common outcome of infection (16).
In contrast to B. microti, WA1 has previously been found to be unusually virulent in experimentally infected Syrian hamsters, with acute disease and a high mortality rate occurring within 10 days of inoculation (5, 22). The purpose of the present study was to develop a murine model of WA1 infection, since laboratory mice are more amenable to immunologic and genetic manipulation.
MATERIALS AND METHODS
Mice.
Random-sex, 3- to 4-week-old inbred mice were used in all studies. A/J, AKR/N, BALB/cJ, CBA/J, C3H.RKK, C3H/HeJ, C3H.SW, C3H.NB/Sn, C3H.HTG/Sn, C57BL/6, C57BL/10, C57BL/10K, C57BL/10M, C57BL/10Q, DBA/1J, and 129/J mice were either bred in our colony or purchased from Jackson Laboratory, Bar Harbor, Maine.
Parasite strains.
The WA1 isolate used in this study was originally isolated from a blood sample of a human patient in the state of Washington (10), maintained by hamster inoculation, and cryopreserved in liquid nitrogen. When a mouse inoculation experiment was performed, cryopreserved blood was first inoculated intraperitoneally (i.p.) into hamsters and the percentage of parasitized erythrocytes (PRBC) was determined for subsequent inoculation in mice. Hamster blood samples were collected when WA1 parasitemia reached 80 to 90%; this level of parasitemia usually occurred between 10 and 12 days after inoculation. With the exception of one experiment, all mice were inoculated within 24 h of collection of infected-hamster blood samples in order to avoid the experimental variability associated with the loss of parasite viability. Cryopreserved hamster blood samples, 100 μl/animal, were utilized for a single initial experiment with C3H.RKK and BALB/cJ mice.
For infectious dosing experiments, different concentrations of PRBC, ranging from 105 to 108 PRBC/100 μl/animal were used for BALB/cJ, C3H.RKK, C57BL/6, and C57BL/10 mice. In subsequent experiments, we utilized 107 PRBC/100 μl/animal.
Hematology.
The percentage of PRBC was estimated by counting the percentage of PRBC among a total of 300 RBC examined on Giemsa-stained blood smears. Parasitemia was monitored initially at 2-day intervals during the first 2 weeks and subsequently every 7 days for up to 2 months. Leukocyte (WBC) counts, RBC counts, hematocrits, and platelet counts were also evaluated at the same time intervals by utilizing a Coulter Counter STKS (Coulter Electronic).
PCR.
Whole-blood specimens were collected in EDTA anticoagulant and stored at 4°C prior to further analysis. DNA in 100 to 200 μl of blood was extracted with an IsoQuick nucleic acid extraction kit (ORCA Research, Inc.). The final pellet was resuspended in 25 μl of 0.5× TE buffer (5 mM Tris [pH 8], 5 mM EDTA). Five microliters of template DNA was used in a 50-μl PCR mixture containing 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, 200 μM (each) deoxyribonucleotide triphosphates, 0.001% bovine serum albumin, 10% glycerol, 0.5% Tween 20, 50 μg of isopsoralen compound 10 (HRI) per ml, 0.25 U of AmpliTaq polymerase, 25 pmol (each) of primers (11, 12) Bab 1.3 (5′ CTA AGT ATA AGC TTT TAT ACA GCG 3′) and Bab 1.3W (5′ CTA AGT ATA AAC TTT TAT ATG GTG 3′), and 50 pmol of primer Bab 4.1 (5′ AGC TGA TAG GTC AGA AAC TTG AAT GA 3′). Amplification was performed by 5 min of denaturation at 94°C, followed by 50 cycles of 1-min denaturation at 94°C and 1-min annealing at 55°C, ending with a 5-min extension at 72°C. The reaction products were electrophoretically separated, and the amplification product was visualized after being stained with ethidium bromide.
Histology.
The brain, liver, kidney, heart, spleen, trachea, lungs, stomach, small intestine, mesenteric lymph node, pancreas, salivary glands, eyes, adrenals, calvarium, spinal column, and rear legs were sampled at 9 and 14 days after inoculation of BALB/cJ and C3H mice. Tissues were immersion fixed in 10% buffered formalin (pH 7.2), embedded in paraffin, and processed by standard techniques. Sections were stained with hematoxylin and eosin or Perl’s iron stain and evaluated microscopically for lesions.
RESULTS
Dose effect.
The median lethal doses (LD50s) for C57BL/6 (H-2b), C57BL/10 (H-2b), C3H.RKK (H-2k), and BALB/cJ (H-2b) mice were established by the method of Reed and Muench (14). Groups of 10 or more mice were inoculated i.p. with 105, 106, 107, and 108 PRBC and then monitored for up to 4 weeks. Moribund mice were euthanized. LD50s appeared to be strain dependent, with C57BL/6 and C57BL/10 mice exhibiting high levels of resistance (LD50, 108), BALB/cJ mice showing intermediate levels of susceptibility (LD50, 2 × 107), and C3H mice demonstrating high levels of susceptibility (LD50, 6 × 104). Most deaths occurred between days 10 and 12 after inoculation.
Strain distribution of resistance and susceptibility to WA1.
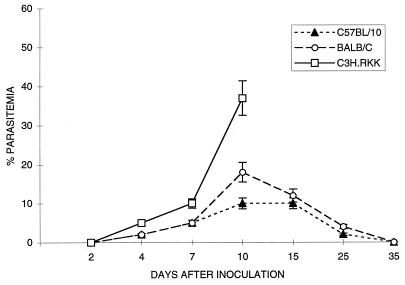
The susceptibilities of 10 mouse strains to WA1 infection were determined by using single doses (107 PRBC) of inoculum for a minimum of 20 animals in each strain cohort. Infection was monitored at the same intervals as used in the LD50 experiment. During the first 4 days after inoculation, no parasites were detected in blood smears from any of the inoculated animals. From days 4 to 7, a low parasitemia rate (5 to 7%) was observed consistently in C3H mice and less consistently in other strains. After this initial incubation period, substantial parasitemia developed, peaking between days 10 and 12 after inoculation; it affected up to 40% of RBC of A/J AKR/N, DBA/1J, and C3H mice. The results for a C3H strain are used as an example in Fig. 1. Mice of susceptible strains developed increased respiratory rates, unsteady gaits, lethargy, and rough coats and subsequently exhibited high rates of mortality (Table 1). Lower peak parasitemia and mortality rates were observed for BALB/cJ, C57BL/6, C57BL/10 (Fig. 1), CBA/J, and 129/J mice on days 10 to 12, with decreases in parasitemia after day 12. Intermediately susceptible strains (BALB/cJ, CBA/J, and 129/J) had moderate mortality rates for the same time intervals. PCR testing of blood samples from all of these strains was positive as early as 2 days after inoculation and persisted for up to 8 weeks (end of experiment). In Table 1, the mouse strains examined are classified as resistant, intermediate, and susceptible, based on the percent mortality after 15 days (no deaths occurred after this interval).
FIG. 1.
WA1 parasitemia in the indicated strains of mice. Each datum point is the mean for three animals. No parasitemia data are shown for C3H.RKK mice after 10 days due to the high mortality rate (>95%). Significant differences (P < 0.05) were observed between parasitemia rates only 10 days after inoculation. Error bars indicate standard errors.
TABLE 1.
Mouse strain susceptibilities
| Susceptibility and mouse strain | Haplotype | Mortality (%) |
|---|---|---|
| Resistant | ||
| C57BL/6 | H-2b | <10 |
| C57BL/10 | H-2b | <10 |
| (C57BL/6 × C3H.RKK)F1 | <10 | |
| Intermediate | ||
| 129/J | H-2b | 50 |
| BALB/cJ | H-2d | 40 |
| CBA/J | H-2k | 50 |
| Susceptible | ||
| A/J | H-2a | >95 |
| AKR/N | H-2k | >95 |
| DBA/1J | H-2q | >95 |
| C3H.RKK | H-2k | >95 |
| C3H/HeJ | H-2k | >95 |
C57BL/6 (resistant) mice were crossed with C3H.RKK (susceptible) mice, and F1 hybrids were tested by inoculation as described above. All of the F1 hybrids (30 mice) showed susceptibility to infection which was equivalent to that of the C57BL/6 parent strain (Table 1).
Resistance and susceptibility of H-2 congenic strains.
We next investigated the role of the major histocompatibility complex (MHC) in relative resistance or susceptibility to WA1 by evaluating a series of H-2 haplotypes (Table 2). The effects of H-2 on two mouse genetic backgrounds, C57BL/10 and C3H, were studied. These strains are congenic at the H-2 complex. The following five haplotypes were examined: H-2b, H-2k, H-2m, H-2p, H-2q. All C57BL/10 congenic mice were relatively disease resistant, independent of H-2 haplotype. In contrast, C3H mice were universally susceptible, regardless of H-2 haplotype. We concluded from this analysis that the H-2 complex plays little or no role in resistance to WA1.
TABLE 2.
MHC influence
| Susceptibility and mouse strain | Haplotype | Mortality (%) |
|---|---|---|
| Resistant | ||
| C57BL/10 | H-2b | <10 |
| C57BL/10K | H-2k | <10 |
| C57BL/10M | H-2m | <10 |
| C57BL/10Q | H-2q | <10 |
| Susceptible | ||
| C3H.RKK | H-2k | >95 |
| C3H/HeJ | H-2k | >95 |
| C3H.SW | H-2b | >90 |
| C3H.NB/Sn | H-2p | >90 |
| C3H.HTG/Sn | H-2q | >90 |
Hematologic evaluation.
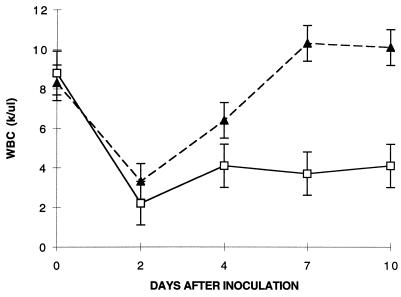
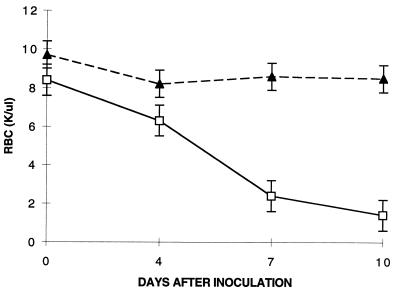
Most of the parasitic forms observed in all of the mouse strains tested were ring form merozoites. Multiple infection with from six to eight ring forms per RBC was often observed, especially in highly parasitemic animals. A small percentage of RBC (<5%) contained pyriform elements in pairs or tetrads. Hematologic parameters showed progressive leukopenia for susceptible C3H mice, which persisted up to the time of death. In contrast, C57BL/10 mice demonstrated transient leukopenia, followed by a return to normal levels within 4 days of inoculation (Fig. 2). Susceptible C3H mice developed progressively severe anemia, whereas resistant C57BL/10 mice developed only a minor decline in RBC count within the first 10 days (Fig. 3). Surviving BALB/cJ mice had RBC and WBC counts similar to those of C57BL/10 mice; in contrast, the BALB/cJ mice that died had values similar to those of C3H mice (data not shown). Thrombocytopenia was also observed in susceptible animals (data not shown).
FIG. 2.
WBC counts (in thousands [k] per microliter) and WA1 infection in the indicated strains of mice. Each datum point is the mean for three animals. Error bars indicate standard errors. ▴, C57BL/10; □, C3H.RKK.
FIG. 3.
RBC counts (in thousands [K] per microliter) and WA1 infection in the indicated strains of mice. Each datum point is the mean for three animals. Error bars indicate standard errors. ▴, C57BL/10; □, C3H.RKK.
Pathologic assessment of BALB and C3H mice.
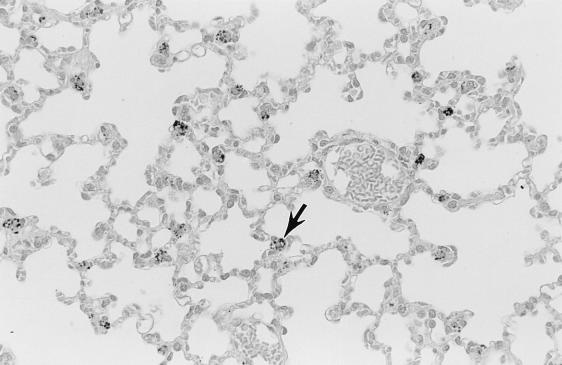
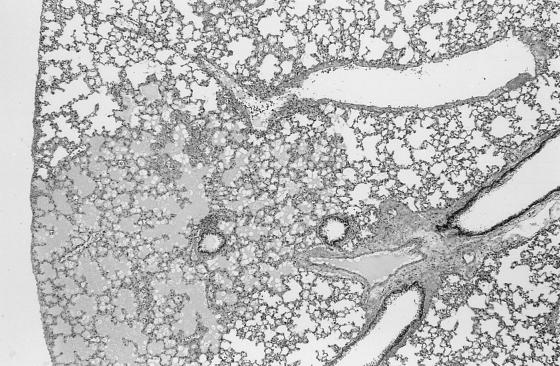
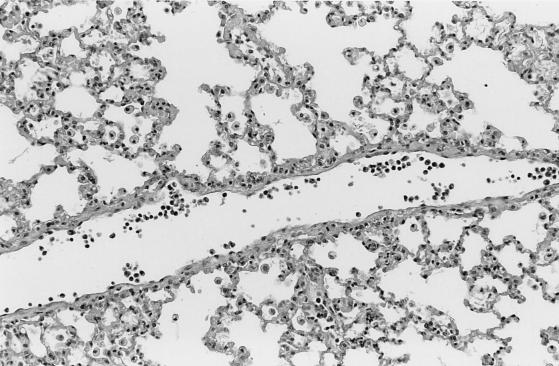
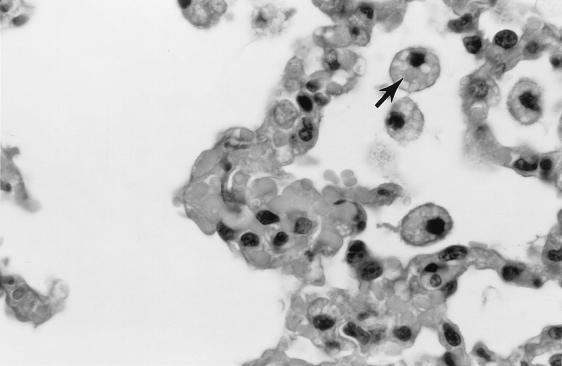
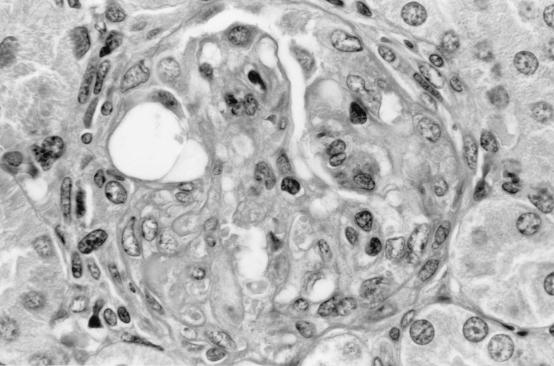
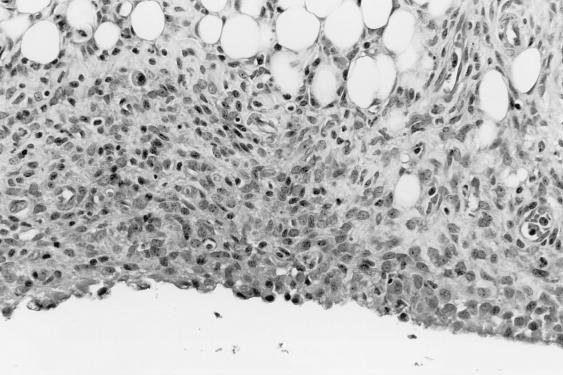
Pathology was assessed in BALB/cJ and C3H mice on days 9 and 14 after i.p. inoculation with cryopreserved infected hamster blood. Four BALB/cJ mice were examined on day 9, five BALB/cJ mice were examined on day 14, and four C3H mice were examined on days 9 and 14. On day 9, all mice of both genotypes had splenomegaly, with marked extramedullary hematopoiesis (predominantly erythropoiesis) and reactive lymphoid hyperplasia. In addition, all mice had pulmonary alveolar macrophage erythrophagocytosis, with accumulation of prominent large golden granules of iron-negative pigment within the cytoplasm of alveolar septal cells (Fig. 4). Erythrophagocytosis and pigmentation were also present in hepatic Kupffer cells and splenic sinusoidal lining cells. Rare microgranulomata and foci of extramedullary hematopoiesis were present in hepatic sinusoids. On day 14, C3H mice were morbidly ill, with gross pulmonary consolidation. Alveoli were flooded with proteinaceous fluid (Fig. 5) and extravasated RBC, with interstitial hypercellularity due to mixed WBC infiltration, prominent pigment-laden interstitial macrophages, and margination of WBCs along endothelial surfaces of vessels (Fig. 6 and 7). BALB/cJ mice had similar lesions, but they were less severe. Three of the four C3H mice, but none of the five BALB/cJ mice, had marked proliferative glomerulonephritis, with proteinaceous casts in tubular lumina (Fig. 8). Necropsies of additional moribund C3H mice revealed similar pulmonary and renal lesions. BALB/cJ (but not C3H) mice also had diffuse hepatic oval cell hyperplasia, and two of the five BALB/cJ mice (but no C3H mice) examined on day 14 had bilateral arthritis in their knee joints, characterized by nonsuppurative synovial proliferation (Fig. 9).
FIG. 4.
Iron-negative pigment within phagocytes (typified by cell at tip of arrow) of pulmonary alveolar septa of a C3H mouse at 9 days after WA1 inoculation. Such pigment is not present in the lungs of healthy mice. Section was stained with hematoxylin. Magnification, ×110.
FIG. 5.
Pulmonary edema, with flooding of alveoli with proteinaceous fluid (affected lung in lower left, surrounded by normal, empty alveolar spaces) in a C3H mouse at 14 days after WA1 inoculation. Section was stained with hematoxylin and eosin. Magnification, ×28.
FIG. 6.
Interstitial pneumonitis, with margination of WBCs along vascular lumen (vessel horizontally transverses section), infiltration of alveolar septa with mixed WBCs (note hypercellularity of alveolar walls), accumulation of macrophages in alveolar spaces, and infiltration of alveolar septa with mixed WBCs in the lung of a C3H mouse at 14 days after WA1 inoculation. Section was stained with hematoxylin and eosin. Magnification, ×110.
FIG. 7.
Erythrophagocytosis by alveolar macrophages in the lung of a C3H mouse at 14 days after WA1 inoculation. Note the engulphed RBC in the cytoplasm of an alveolar macrophage (arrow). Section was stained with hematoxylin and eosin. Magnification, ×550.
FIG. 8.
Proliferative glomerulonephritis in the kidney of a C3H mouse at 14 days after WA1 inoculation. There is diffuse adhesion of the glomerular tuft to Bowman’s capsule, obliterating Bowman’s space. The glomerular tuft is hypoperfused and hypercellular. Section was stained with hematoxylin and eosin. Magnification, ×550.
FIG. 9.
Proliferative synovitis in the areolar synovium of the knee joint of a BALB/cJ mouse at 14 days after WA1 inoculation. The synovial membrane, which is normal and approximately one to two cells thick, is distorted by marked proliferation of fusiform cells, with sparse WBC infiltration. Section was stained with hematoxylin and eosin. Magnification, ×220.
DISCUSSION
Experimental infections of mice with the Babesia-like WA1 organism resulted in rather striking differences in mouse strain susceptibility, with severe disease and high mortality rates in A/J, AKR/N, DBA/1J, and C3H mice but almost complete resistance in C57BL/6 and C57BL/10 mice. Other strains (BALB/cJ, CBA/J, and 129/J) exhibited intermediate susceptibilities. These differences did not appear to be influenced by MHC haplotypes, since mouse strains with identical MHC haplotypes but differing genetic backgrounds showed differences that correlated with the genetic background and since different H-2 haplotypes on the same genetic background had little (if any) discernible influence on mortality rates.
The pathology in WA1-infected C3H mice resembled some of the observations reported for WA1-infected hamsters, in which high parasitemia, splenomegaly, and pulmonary edema were seen, but we did not observe multifocal necrosis in multiple organs in susceptible mice. Lesions in hamster lungs, spleens, and vessels were also dominated by macrophages (5, 22), which was not a feature in infected mice. Glomerular hypercellularity was noted in infected hamsters, but hamsters did not develop the severe proliferative glomerulonephritis noted in C3H mice. Joint lesions in two of the five BALB/cJ mice examined on day 14 were unique, but an analysis of larger samples is needed to assess the relevance to WA1 infection.
C57BL mice are intrinsically resistant to many intracellular pathogens, such as Listeria monocytogenes and Plasmodium chabaudi chabaudi (6, 18). The mechanism of this resistance has not been fully elucidated, but it may be related to induction of a predominant and early cytotoxic T-cell response associated with Th1 differentiation. However, innate immune responses may also play an important role. In the mouse model of L. monocytogenes infection, the control of infection appears to be dependent on innate mechanisms. Recent studies have identified a pathway where cytokines, such as interleukin-12 (IL-12) and tumor necrosis factor alpha, are produced by macrophages infected with intracellular pathogens and function to stimulate gamma interferon (IFN-γ) production by NK cells (6, 7, 9, 18, 19). These cytokines play multiple roles in the innate immune response, including activation of macrophages and increased expression of adhesion molecules that are required for neutrophil extravasation (15). Perhaps the innate differences that make C57BL mice resistant to infection by a variety of intracellular pathogens are also responsible for the resistance to WA1. Conversely, it is also possible that high-level susceptibility to WA1 in C3H mice is a function of an overly vigorous cytotoxic T-lymphocyte response, resulting in toxicity, a mechanism apparently underlying the pathogenesis of malarial infections (3). Further analysis of inflamatory mediators in these mice may shed light on these possibilities.
T-helper-cell responses may be biphasic, especially during different stages of infection. In infections by the rodent malarial parasite P. chabaudi chabaudi, a Th1 response predominates during the early or acute phase, followed by the appearance of a Th2 response (20). Splenocytes from resistant mouse strains (such as C57BL/6) produce high levels of IFN-γ within the first week, followed by production of IL-4, IL-5, and IL-10 (Th2 cytokines) at 2 to 4 weeks postinfection. In contrast, susceptible mice, such as C3H, produce low levels of IFN-γ and high levels of IL-4, IL-5, and IL-10 within the first week postinfection. However, since both Th1 and Th2 responses are associated with disease resolution, the relative inflexibility of host responses (i.e., commitment to ongoing Th1 responses when humoral immune responses are necessary for resolution) could result in increased disease susceptibility. The innate predisposition of host immune responses may account for susceptibilities to several types of pathogens. The absence of an identified arthropod vector for WA1 makes it impossible to stimulate all stages of parasite development in order to address this question in the setting of natural transmission. However, even under the conditions used, this is the first known demonstration of heightened susceptibility of C3H mice to infection by any type of intracellular blood-stage pathogen resulting in a such high rate of mortality. The rapid, usually lethal outcome of infection in these mice may become useful for identifying critical immunogenetic determinants by evaluation of recombinant inbred strains.
Infections with WA1 and its relatives in humans probably range in severity from an asymptomatic carrier state (10, 13) to a severe, life-threatening illness, which is consistent with the range of severity observed in mice. The one fatal case of infection by WA1 was associated with pulmonary edema and multiorgan failure, including renal failure. Renal failure was also observed in a second, nonfatal case (13). To date, most of the clinically apparent cases of WA1 infection have been in asplenic hosts, thus predisposing them to infection by intraerythrocytic and other pathogens. Other host genetic factors, perhaps analogous to those in C3H mice, may play additional roles in modulating the severity of disease.
In summary, we have identified an unusual pattern of mouse strain susceptibility to infection by the WA1 piroplasm, a recently recognized human pathogen. Resistance to infection appears to be a dominant trait that is independent of the host H-2 genotype. Further studies of the basis for the observed differences in strains are under way.
REFERENCES
- 1.Brandt F, Healy G R, Welch M. Human babesiosis: the isolation of Babesia microti in golden hamsters. J Parasitol. 1977;63:934–937. [PubMed] [Google Scholar]
- 2.Bredt A B, Weinstein W M, Cohen S. Treatment of babesiosis in asplenic patients. JAMA. 1981;245:1938–1939. [PubMed] [Google Scholar]
- 3.Clark I A, Chaudhri G, Cowden W B. Roles of tumor necrosis factor in the illness and pathology of malaria. Trans R Soc Trop Med Hyg. 1989;83:436–440. doi: 10.1016/0035-9203(89)90240-x. [DOI] [PubMed] [Google Scholar]
- 4.Cullen J M, Levine J F. Pathology of experimental Babesia microti infection in the Syrian hamster. Lab Anim Sci. 1987;37:640–643. [PubMed] [Google Scholar]
- 5.Dao A H, Eberhard H L. Pathology of acute fatal babesiosis in hamsters experimentally infected with the WA-1 strain of Babesia. Lab Invest. 1996;74:853–859. [PubMed] [Google Scholar]
- 6.Harty J H, Lenz L L, Bevan M J. Primary and secondary immune response to Listeria monocytogenes. Curr Opin Immunol. 1996;8:526–530. doi: 10.1016/s0952-7915(96)80041-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harty J H, Bevan M J. Specific immunity to Listeria monocytogenes in the absence of IFN γ. Immunity. 1995;3:109–117. doi: 10.1016/1074-7613(95)90163-9. [DOI] [PubMed] [Google Scholar]
- 8.Lykins J D, Ristic M, Weisiger R M. Babesia microti: pathogenesis of parasite of human origin in the hamster. Exp Parasitol. 1975;37:388–397. doi: 10.1016/0014-4894(75)90008-9. [DOI] [PubMed] [Google Scholar]
- 9.Mossman T R, Sad S. The expanding universe of T cell subsets: Th1, Th2 and more. Immunol Today. 1996;3:143–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 10.Persing D H, Herwaldt B L, Glasser C, Lane R S, Thomford J W, Mathiesen D, Krause P J, Phillip D F, Conrad P A. Infection with a Babesia-like organism in northern California. N Engl J Med. 1995;332:298–303. doi: 10.1056/NEJM199502023320504. [DOI] [PubMed] [Google Scholar]
- 11.Persing D H, Mathiesen D, Marshall W F, et al. Detection of Babesia microti by polymerase chain reaction. J Clin Microbiol. 1992;30:2097–2103. doi: 10.1128/jcm.30.8.2097-2103.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Persing D H. PCR detection of Babesia microti. In: Persing D H, Smith T F, Tenover F C, White T J, editors. Diagnostic molecular microbiology: principles and applications. Washington, D.C: American Society for Microbiology; 1993. pp. 475–479. [Google Scholar]
- 13.Quick R E, Herwaldt B L, Thomford J T, Garnett M E, Eberhard M L, Wilson M, Spach D H, Dickerson J W, Telford III S R, Steingart K R, Pollock R, Persing D H, Kobayashi J M, Juranek D D, Conrad P A. Babesiosis in Washington state: a new species of Babesia? Ann Intern Med. 1993;119:284–290. doi: 10.7326/0003-4819-119-4-199308150-00006. [DOI] [PubMed] [Google Scholar]
- 14.Reed L J, Muench H. A simple method for estimating fifty percent end points. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 15.Rogers H W, Unanue E R. Neutrophils are involved in acute, nonspecific resistance to Listeria monocytogenes in mice. Infect Immun. 1993;61:5090–5096. doi: 10.1128/iai.61.12.5090-5096.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruebush M J, Hanson W L. Susceptibility of five strains of mice to Babesia microti of human origin. J Parasitol. 1979;65:430–433. [PubMed] [Google Scholar]
- 17.Scholtens R G, Braff E H, Healy G R, Gleason N. A case of babesiosis in man in the United States. Am J Trop Med Hyg. 1968;17:810–813. doi: 10.4269/ajtmh.1968.17.810. [DOI] [PubMed] [Google Scholar]
- 18.Stevenson M M, Huang D Y, Podoba J E, Nowotarski M E. Macrophage activation during Plasmodium chabaudi infection in resistant C57BL/6 and susceptible A/J mice. Infect Immun. 1992;60:1193–1201. doi: 10.1128/iai.60.3.1193-1201.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stevenson M M, Tam M F, Wolf S F, Sher A. IL-12 induced protection against blood-stage Plasmodium chabaudi requires IFN γ and TNF α and occurs via a nitric oxide-dependent mechanism. J Immunol. 1995;155:2545–2556. [PubMed] [Google Scholar]
- 20.Taylor-Robinson A W, Phillips R S, Severn A, Moncada S, Liew F Y. The role of Th1 and Th2 cells in a rodent malaria. Science. 1993;25:1931–1934. doi: 10.1126/science.8100366. [DOI] [PubMed] [Google Scholar]
- 21.Telford S R, III, Gorenflot A, Brasseur P, Spielman A. Babesial infections in human hosts. In: Kreier J P, editor. Parasitic protozoa. Vol. 5. New York, N.Y: Academic Press; 1993. pp. 1–27. [Google Scholar]
- 22.Wozniak E J, Lowenstine L, Hemmer R, Robinson T, Conrad P. Comparative pathogenesis of human WA1 and Babesia microti isolates in a Syrian hamster model. Lab Anim Sci. 1996;46:507–515. [PubMed] [Google Scholar]