Abstract
Advanced stages of mycobacterial diseases such as leprosy and tuberculosis are characterized by a loss of T-cell function. The basis of this T-cell dysfunction is not well understood. The present report demonstrates major alterations in the expression of signal transduction molecules in T cells of leprosy patients. These alterations were most frequently observed in lepromatous leprosy (LL) patients. Of 29 LL patients, 69% had decreased T-cell receptor ζ-chain expression, 48% had decreased p56lck tyrosine kinase, and 63% had a loss of nuclear transcription factor NF-κB p65. An electrophoretic mobility shift assay with the gamma interferon core promoter region revealed a loss of the Th1 DNA-binding pattern in LL patients. In contrast, tuberculoid leprosy patients had only minor signal transduction alterations. These novel findings might improve our understanding of the T-cell dysfunction observed in leprosy and other infectious diseases and consequently might lead to better immunologic evaluation of patients.
Leprosy is caused by an obligate intracellular pathogen, Mycobacterium leprae, and is characterized by a clinical spectrum with two polar forms and several intermediate stages, all closely related to the immune response of the patient to the mycobacteria. In patients with tuberculoid leprosy (TL), growth of the mycobacteria is restricted; such patients display strong delayed-type hypersensitivity (DTH) responses to M. leprae antigens. In contrast, patients with lepromatous leprosy (LL) have high numbers of mycobacteria, multiple lesions, and no DTH (14, 22). Recent studies suggest that immune suppression in LL patients can be explained in part by the fact that LL lesions are infiltrated by T cells that produce interleukin-4 (IL-4) and IL-10, a Th2 response, while TL is characterized by infiltration by T lymphocytes producing gamma interferon (IFN-γ) and IL-2, a Th1 response (10, 12, 24, 28, 30).
Suppression of the T-cell response is also observed in diseases such as cancer. Recent work with murine models and cancer patients has demonstrated alterations in the molecules mediating signal transduction and the activation of T lymphocytes. These alterations include decreased expression of the T-cell receptor ζ-chain (TCRζ), p56lck tyrosine kinase, and nuclear transcription factor NF-κB p65 (3, 5, 11, 15, 32). The alterations are accompanied by a diminished ability to mobilize Ca2+ in response to activation signals, decreased cytotoxic function, and decreased production of IFN-γ. Similar changes, at least in NF-κB, have been described for T cells made tolerant to bacterial lipopolysaccharide (33). T cells from leprosy patients demonstrated similar alterations in signal transduction molecules. These changes were most frequently found in T cells of patients with LL, an advanced form of the disease characterized by a loss of cellular response and a decrease in Th1 cytokine production.
MATERIALS AND METHODS
Patients.
Forty-three leprosy patients monitored at the leprosy clinic of the Universidad del Valle (Cali, Colombia) and seven clinic workers, used as healthy controls, were included in this study. The patients were classified according to the system of Ridley and Jopling (22), based on clinical presentation, histopathology, and response to lepromin. Lepromin was a kind gift from the World Health Organization (S. K. Nordeen, World Health Organization, Geneva, Switzerland). After providing signed informed consent, each patient was injected subcutaneously with 0.1 ml of lepromin (30 × 106 to 40 × 106 bacteria/ml). Induration reactions were measured 21 days later (Mitsuda reaction; positive, ≥5 mm; negative, <5-mm induration). Of 43 patients, 29 were classified as having LL and 14 as having TL (Table 1). At the time of their entry into the study, patients had already been treated for 1 to 6 months. TL patients received therapy with dapsone and rifampin (6 months), while LL patients received dapsone, rifampin, and clofazimine (24 months), both in accordance with World Health Organization guidelines (29). Patients were carefully evaluated during treatment, and those patients showing complete clinical resolution of their lesions were considered responders, while patients requiring additional treatment (>2 years) were considered nonresponders.
TABLE 1.
Ages and genders of leprosy patients studied
| Disease | No. of patients
|
Mean age (yr)a | ||
|---|---|---|---|---|
| Male | Female | Total | ||
| LL | 15 | 14 | 29 | 38.5 |
| TL | 4 | 10 | 14 | 44 |
| Total | 19 | 24 | 43 | |
Mean age of healthy controls, 37 years.
Cell preparation.
Enriched peripheral blood T cells (PBL-T) were obtained by passing PBL over T-cell enrichment columns (R&D Systems, Minneapolis, Minn.). Briefly, mononuclear cells enriched over Ficoll-Paque (Pharmacia Biotech, Uppsala, Sweden) were resuspended at 108 cells/ml and loaded onto T-cell enrichment columns. After 10 min of incubation, the columns were washed with 15 ml of washing buffer provided by the manufacturer. The cells were collected, counted, and tested for enrichment by flow cytometry. The resulting suspension, for most samples, was >85% CD3+ cells, <5% CD16+ cells, and <2% each of granulocytes, B cells, and monocytes.
Determination of TCRζ and tyrosine kinase expression.
Enriched T cells were tested by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting (32). Briefly, 106 CD3+ cells were electrophoresed in 14 and 8% Tris-glycine gels for TCRζ and tyrosine kinase p56lck, respectively (Novex Experimental Technology, San Diego, Calif.). The membranes were immunoblotted with anti-ζ-chain antiserum (Onco-Zeta 1; Biomira USA [previously Onco Therapeutics Inc.], Cranbury, N.J.) and anti-human CD3-ɛ (DAKO, Carpinteria, Calif.) at 1:1,000 and 1:6,000 dilutions, respectively, or with anti-p56lck (Upstate Biotechnology Inc., Lake Placid, N.Y.) at a 1:1,000 dilution for 1 h. Membranes were washed and incubated with horseradish peroxidase-conjugated anti-rabbit immunoglobulin (Amersham, Buckinghamshire, United Kingdom) for 20 min and developed with an enhanced chemiluminescence kit (Amersham). X-Omat AR film (Eastman Kodak Co., Rochester, N.Y.) was used to obtain the autoradiographs. The cutoff value for defining low expression of the ζ-chain was determined to be 2 standard deviations below the mean optical density value for all of the normal samples tested (approximately 55% of the mean). However, due to the normal experimental variations seen with gels, the bands in each gel were compared only to the normal controls included in such gel by densitometry.
Nuclear extracts for NF-κB and DNA-binding proteins.
Nuclear extracts from T cells were prepared as described previously (5). For the evaluation of NF-κB and c-Rel expression, 10 μg of nuclear extract was electrophoresed in an 8% gel. The immunoblots were performed with the following antibodies: anti-p50 antiserum 1157 (21), rabbit anti-c-Rel antiserum 265 (21), and anti-p65 antiserum 1226 (21). For the electrophoretic mobility shift assay (EMSA), 2 μg of nuclear extract was preincubated in reaction buffer (5) for 10 min at room temperature; and a 32P-labeled oligonucleotide corresponding to the IFN-γ core promoter region (positions −70 to −40) with the sequence 5′ AAAACTGTGAAAATACGTAATCCTCAGGAGA 3′ (16) was then added to the reaction mixture and the mixture was incubated for 20 min. The complexes were separated on a 5% polyacrylamide gel and exposed to autoradiography.
Cytokine testing.
Enriched T cells were stimulated with anti-CD3 (10 ng of Ortho Clone OKT-3 [Ortho Pharmaceutical, Raritan, N.J.] per ml) for 48 h; the supernatants were tested by enzyme-linked immunosorbent assay for IL-1, IL-2, IL-4, IL-6, IL-10, and IFN-γ according to the manufacturer’s instructions (IL-1 from Cistron, Pine Brook, N.J.; IL-2 from Dupont-New England Nuclear, Boston, Mass.; IL-4 from R&D Systems; IL-6 and IL-10 from Biosource, Camarillo, Calif.; and IFN-γ from Medgenix Diagnostics SA, Flevas, Belgium).
Statistical methods.
Fisher exact tests were used to test for homogeneity of proportions between leprosy types and for dichotomous outcomes between immunological competence and signal transduction assays. Wilcoxon ranked-sum tests were used to test for differences in cytokine production between LL and TL patients and healthy controls. Probabilities were calculated by a two-tailed test and were reported in all cases. For some analyses, not all patients were tested due to limited sample availability (not to any other selection criteria).
RESULTS
Purified PBL-T from 43 leprosy patients and 7 healthy clinic workers were tested for the expression of signal transduction molecules and for cytokine production. As shown in Table 1, 29 patients had LL and 14 had TL. None of the patients with LL displayed DTH to lepromin, while 12 of 14 TL patients had DTH to lepromin, with indurations ranging from 5 to 15 mm (mean = 10.9 mm).
TCRζ expression and p56lck tyrosine kinase.
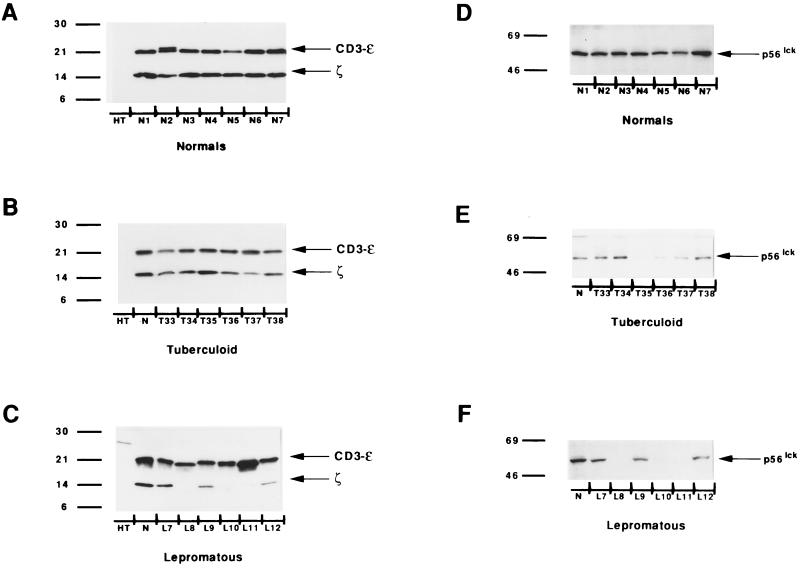
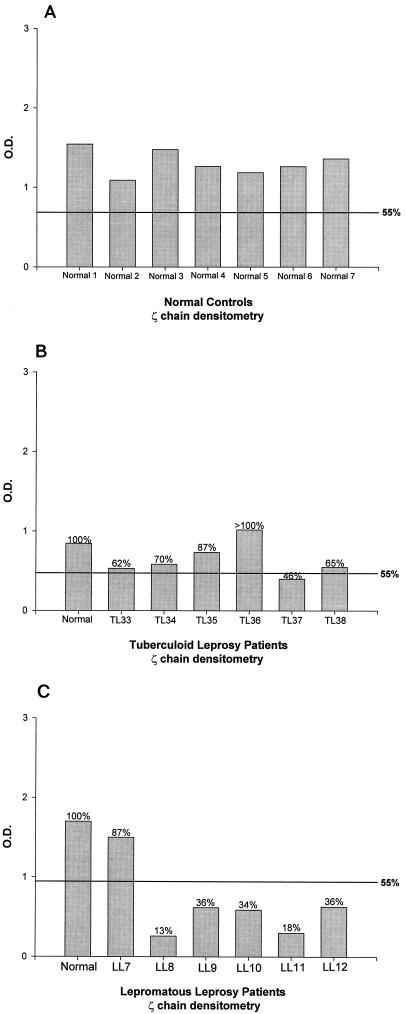
The surface expression of the TCR in patients with leprosy, as tested by immunofluorescence with anti-CD3 (CD3-ɛ chain) and anti-TCRα/β monoclonal antibodies, did not show any difference from that of healthy controls (Table 2). However, the expression of TCRζ in T cells showed a marked variation. Representative Western blots in Fig. 1 show that healthy controls (Fig. 1A) and 12 of 14 (86%) TL patients (Fig. 1B) expressed equivalent levels of TCRζ and the CD3-ɛ-chain. Densitometry confirmed these observations (Fig. 2A and B). The levels of ζ-chain expression in the Colombian controls were comparable to those in the seven healthy U.S. controls (data not shown). In contrast, 20 of 29 (69%) LL patients (Fig. 1C and 2C) had decreased (<55% of normal levels) or undetectable levels of TCRζ (P = 0.0011).
TABLE 2.
Expression of TCR as measured by mean channel immunofluorescence
| Disease | No. of subjects | Level of immunofluorescence (mean ± SD)
|
|
|---|---|---|---|
| TCRα/β | CD3-ɛ | ||
| LL | 11 | 24.06 ± 0.77 | 34.59 ± 1.34 |
| TL | 9 | 24.31 ± 0.87 | 34.51 ± 1.07 |
| None | 4 | 24.36 ± 1.36 | 34.22 ± 0.74 |
FIG. 1.
Reduced expression of TCRζ and p56lck in LL patients. Representative Western blots of TCRζ and CD3-ɛ (A to C) and p56lck tyrosine kinase (D to F). HT, B-cell line used as a negative control; N, normal human T cells. Numbers on the left are molecular masses in kilodaltons.
FIG. 2.
Densitometry values for the ζ-chain from Fig. 1A to C are presented in panels A to C, respectively. TCRζ expression levels that were ≤55% of the mean levels for the normal controls on each gel were considered to be decreased (see Materials and Methods). O.D., optical density.
Changes were also seen with p56lck tyrosine kinase expression. Fourteen of 29 (48%) LL patients had decreased p56lck expression, as seen in patients L8, L10, and L11 (Fig. 1F), while only 5 of 14 (36%) TL patients (Fig. 1E) showed changes. The differences in p56lck expression between groups of patients were not statistically significant. All of the healthy controls showed equivalent levels of p56lck (Fig. 1D).
Expression of nuclear NF-κB.
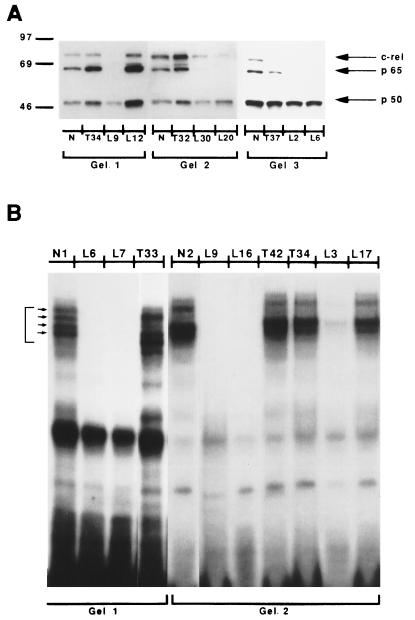
Nuclear transcription factors such as NF-κB and c-Rel family proteins play a major role in the activation or repression of cytokine genes. The translocation of the NF-κB p65-p50 heterodimer to the nucleus is associated with increased production of some cytokines, while the presence of the NF-κB p50-p50 homodimer in the nucleus is associated with the inhibition of their production (6). Figure 3A shows the results of three experiments testing the presence of nuclear NF-κB family members in activated T cells of three TL and six LL patients. A total of 10 of 16 (63%) LL patients tested lacked nuclear p65 and/or c-Rel but had normal levels of p50. In contrast, 7 of 8 TL patients tested expressed normal nuclear levels of all three NF-κB family members, (P = 0.0064). Only one TL patient (T37) showed an absence of nuclear c-Rel but normal expression of p65 (Fig. 3A).
FIG. 3.
Decreased expression of nuclear NF-κB and absence of Th1 DNA-binding pattern in LL patients. (A) Western blotting for NF-κB and c-Rel, p65, and p50 was done with nuclear extracts from purified T cells taken from normal (healthy) controls (N) and tuberculoid (T) and lepromatous (L) patients and previously activated for 2 h with 1 μg of anti-CD3 per ml. Numbers on the left are molecular masses in kilodaltons. (B) EMSA for Th1 with an IFN-γ core promoter region. The four bands shown by the arrows are consistently seen in cells from healthy controls and Th1 cells.
DNA-binding pattern and cytokine production.
Using an IFN-γ core promoter region in an EMSA, Young et al. (31) demonstrated a distinct DNA-binding pattern for Th1 and Th2 clones. PBL-T from healthy controls show a Th1 DNA-binding pattern. Figure 3B shows data for PBL-T from three TL and six LL patients. T cells from healthy controls (subjects N1 and N2) and from nine of nine TL patients (data for patients T33, T42, and T34 only are shown in Fig. 3B) presented the four bands characteristic of Th1 cells. In contrast, 10 of 17 LL patients tested showed an absence of the Th1 DNA-binding pattern (patients L6, L7, L9, L16, and L3). These changes were not due to sample degradation, since other nuclear binding proteins such as NF-κB p50 and octamer binding protein were expressed at normal levels (data not shown).
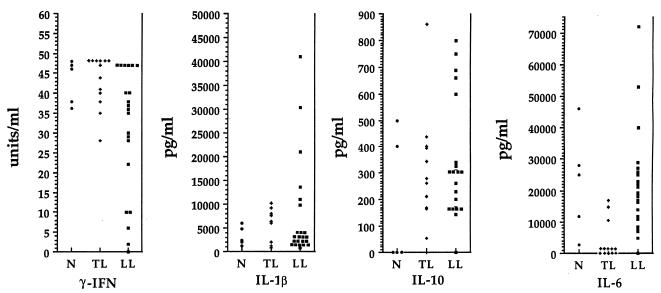
Cytokine production by T cells was measured after in vitro stimulation with anti-CD3 (Fig. 4). The nonparametric Wilcoxon test indicated that LL patients produced significantly lower levels of IFN-γ and higher levels of IL-6 than TL patients did (P = 0.016 and P = 0.0001, respectively). Healthy controls and TL patients did not differ in the production of IFN-γ, but TL patients produced lower levels of IL-6 than the healthy controls (P = 0.005). Overall levels of IL-1β and IL-10 were slightly higher but not significantly different in LL patients. Therefore, there was not an absolute correlation between the type of leprosy and the production of Th1 or Th2 cytokines by PBL-T, suggesting that not all patients, even those within the same clinical group, have the same biological or clinical behavior. However, two distinct subsets of patients could be identified within the LL group (Table 3): those producing high and those producing low levels of IL-10 (levels above and below the mean, respectively; P = 0.0001). These differences also correlated with signal transduction changes. As shown in Table 3, those patients producing high levels of IL-10 displayed TCRζ decrease and loss of the Th1 binding pattern more often than those producing low levels of IL-10. No significant differences were observed between the groups with regard to IFN-γ production. IL-2 and IL-4 were not detected in patients or healthy controls at the time points tested. It is possible that costimulation with anti-CD28 might provide a better signal for carefully evaluating the difference in cytokine production between these groups, as has been recently shown for patients with Hodgkin’s disease (20).
FIG. 4.
Predominant Th2 cytokine patterns in LL patients. Levels of cytokine production (IFN-γ, IL-1β, IL-10, and IL-6) in normal (healthy) controls (N) and TL and LL patients were determined after stimulation of PBL-T with soluble anti-CD3 for 48 h.
TABLE 3.
Alterations in Th1 EMSA patterns and TCRζ expression in LL patients
| Group | Mean level of:
|
No. of patients with decreased TCRζ expression/no. testedb | No. of patients with loss of Th1/no. tested | |
|---|---|---|---|---|
| IL-10a (pg/ml) | IFN-γ (U/ml) | |||
| Normal controls | 180 | 43.00 | 0/5 | 0/5 |
| LL patients | ||||
| Low IL-10 | 222 | 32.96 | 8/15 | 3/7 |
| High IL-10 | 731 | 26.44 | 7/8 | 6/7 |
P = 0.0001 for high-IL-10 versus low-IL-10 patients. P = 0.0039 for healthy controls versus high-IL-10 patients. P = 0.6297 for healthy controls versus low-IL-10 patients.
Decreased TCRζ expression was defined as values ≤55% of those for healthy controls, as measured by densitometry.
DISCUSSION
The clinical presentation of leprosy is closely related to the patient’s immune response to M. leprae. LL patients lose their protective T-cell response, while patients with TL maintain T-cell immunity despite having developed the disease. Initial reports suggested the presence of suppressor cells in patients with LL as an explanation for the decreased T-cell response in these individuals (13, 18, 23). More recently, a new model has been proposed in which the clinical presentations of infectious diseases caused by intracellular microorganisms, such as leprosy (30), leishmaniasis (7, 25), and AIDS (1), are closely correlated with the preferential response of one of the T helper cell subsets, namely, Th1 or Th2 (2). A selective decrease in Th1 function and the predominance of a Th2 response lead to the loss of cellular immunity and enhanced antibody production. The results presented here provide new insights into the previously described loss of the Th1 response (16) by demonstrating certain alterations in nuclear transcription factors and an absence of the Th1 DNA-binding pattern. The loss of nuclear NF-κB p65 might provide some clues to explain this shift. The presence of the NF-κB p65-p50 heterodimer is associated with increased production of IFN-γ, while the p50-p50 homodimer acts as a repressor of the gene for this cytokine (26). In addition, c-Rel also binds to the intronic region of the IFN-γ gene, apparently acting in a way similar to that of NF-κB p65. Therefore, the absence of nuclear NF-κB p65 and c-Rel and the presence of p50 alone in T cells from LL patients could explain in part the decreased IFN-γ production. These data are similar to those of recent reports showing that in vitro tolerization by lipopolysaccharide involves the nuclear translocation of p50 homodimers of NF-κB (33).
However, the decreased Th1 function alone does not appear to be the only cause of the anergy seen in these patients. The decreased expression of TCRζ and tyrosine kinase p56lck could further impair the function of T cells in LL patients. Moreover, the loss of TCRζ expression is seen more frequently in LL patients who have an absent Th1 DNA-binding pattern and increased production of IL-10. The essential role of the ζ-chain in T-cell activation has been shown by the absence of response to antigenic stimulation in T-cell lines lacking the ζ-chain (27). Similarly, knockout mice lacking the ζ-chain have few T cells and consequently are severely immunodeficient (9). In this study, LL patients with decreased ζ-chain expression frequently had alterations in other signal transduction molecules and diminished production of Th1 cytokines. The impact of decreased ζ-chain expression on the clonal response to specific antigens is currently being studied. Lai and colleagues (8) have shown that T cells from cancer patients with low expression of the ζ-chain also show alterations in tyrosine phosphorylation patterns and in their responses to antigens. It is interesting that the two TL patients who had decreased expression of TCRζ and p56lck also had no DTH to lepromin. Reactivity to other antigens as measured by DTH was not studied prior to initiation of treatment in this group of patients; therefore, the impact of these changes on the response to other antigens is unknown.
These observations are similar to those described recently for cancer patients by members of our laboratory and others (8, 15, 32). Patients with metastatic melanoma who had decreased TCRζ expression had low production of IFN-γ and shorter survival times (32). Interestingly, despite major signal transduction changes in T cells, neither cancer nor leprosy patients have generalized immunosuppression. This suggests that signal transduction changes may lead to the inability of T cells to respond to tumors or mycobacteria but that other elements of host defense, namely, granulocytes, macrophages, and B cells, must still be capable of a protective response. Alternatively, a strong antigenic signal leading to activation might restore normal signal transduction proteins, as seen from the incubation with anti-CD3 and anti-CD28 (20).
The mechanisms inducing these alterations are still unclear. Genetic defects in the structure of the TCR are not frequent. Regueiro et al. (19) described one patient with a congenital absence of the CD3-ɛ chain and a severe state of immunosuppression. However, it is unlikely that the patients discussed here had congenital TCRζ defects, especially since none of them had a clinical history of immunodeficiency. These alterations probably represent a quantitative change in the expression of the ζ-chain induced by the disease process. Preliminary work has failed to demonstrate the induction of signal transduction alterations in normal T cells by incubation with cytokines such as transforming growth factor β, IL-4, or IL-10. Alternatively, in vitro anergy models suggest that chronic TCR stimulation in the absence of adequate costimulatory signals can induce some signal transduction alterations (4, 17). It is therefore possible that soluble factors produced by macrophages infected with M. leprae and the presence of a chronic antigenic stimulus from the increasing numbers of mycobacteria in LL patients could lead to the signal transduction defects in T cells described here. Studies of cancer patients suggest that these abnormalities could be related to increased turnover of signal transduction proteins rather than to abnormal transcription (data not shown).
Because of the prolonged duration of treatment (2 years for LL patients), samples from only five LL patients were tested after completion of therapy. Preliminary data show that patients responding to treatment also correct the signal transduction defects (data not shown). These preliminary data could suggest a possible correlation between the reexpression of these signal transduction molecules and an improvement in clinical status. However, further testing of patients that complete treatment will be necessary to assess this possibility and evaluate the clinical significance of the changes in signal transduction molecules and of their reexpression with effective treatment.
In summary, alterations in the expression of signal transduction molecules of T cells are found in leprosy patients, especially LL patients. These findings provide new insights into the immunopathology of this disease and possibly into the mechanisms leading to anergy. It is also possible that the patients with signal transduction alterations represent a subset of individuals within the major clinical classification whose disease has different biological and/or clinical behavior and who might benefit from careful monitoring with signal transduction assays. Additional research will be needed to confirm this possibility and to further explore whether novel therapeutic approaches that correct these signal transduction alterations result in the recovery of a protective immune response.
ACKNOWLEDGMENTS
This work was supported by NCI contract NO1-CO-56000 with SAIC and by Cooperative Research and Development Agreement CACR-0109 with Biomira Inc. Work in Colombia was supported by NIAID-TMRC grant P50-A1 and by Biomira Inc.
We thank Brendan Curti, Larry Kwak, Igor Espinoza-Delgado, and Claudio Dansky-Ullmann for their critical review of the manuscript and their constructive ideas on the presentation of the material. We also thank Louise Finch and William Kopp for their help with flow cytometry, Helen Rager for cytokine testing, and Mircea Popescu and Richard Robb from Biomira USA for the anti-TCRζ antibody. Finally, we thank the nursing staff and patients of the leprosy clinic in Cali, Colombia, for their collaboration in this study.
REFERENCES
- 1.Clerici M, Shearer G M. A Th1-Th2 switch is a critical step in the etiology of HIV infection. Immunol Today. 1993;14:107–111. doi: 10.1016/0167-5699(93)90208-3. [DOI] [PubMed] [Google Scholar]
- 2.Del-Prete G, Romagnani S. The role of Th1 and Th2 subsets in human infectious diseases. Trends Microbiol. 1994;2:4–6. doi: 10.1016/0966-842x(94)90336-0. [DOI] [PubMed] [Google Scholar]
- 3.Finke J H, Zea A H, Stanley J, Longo D L, Mizoguchi H, Tubbs R R, Wiltrout R H, O’Shea J J, Kudoh S, Klein E, Bukowski R M, Ochoa A C. Loss of T cell receptor ζ-chain and p56lck in T cells infiltrating human renal cell carcinoma. Cancer Res. 1993;53:5613–5616. [PubMed] [Google Scholar]
- 4.Gajewski T, Qian D, Fields P, Fitch F. Anergic T-lymphocyte clones have altered inositol phosphate, calcium and tyrosine kinase signaling pathways. Proc Natl Acad Sci USA. 1994;91:38–42. doi: 10.1073/pnas.91.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghosh P, Sica A, Young H A, Franco J L, Wiltrout R H, Longo D L, Rice N R, Komschlies K L. Alterations in NFκB/Rel family proteins in splenic T cells from tumor-bearing mice and reversal following therapy. Cancer Res. 1994;54:2969–2972. [PubMed] [Google Scholar]
- 6.Grilli M, Chiu J J, Lenardo M J. NFκB and Rel: participants in a multiform transcriptional regulatory system. Int Rev Cytol. 1990;143:1–62. doi: 10.1016/s0074-7696(08)61873-2. [DOI] [PubMed] [Google Scholar]
- 7.Heinzel F P, Sadick M D, Holaday B J, Coffman R L, Locksley R M. Reciprocal expression of interferon-gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. J Exp Med. 1989;169:59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lai P, Rabinovich H, Crowley-Nowick P A, Cell N C, Mantovani G, Whiteside T L. Alterations in expression and function of signal-transducing proteins in tumor-associated T and natural killer cells in patients with ovarian carcinoma. Clin Cancer Res. 1996;2:161–173. [PubMed] [Google Scholar]
- 9.Love P E, Shores E W, Lee E J, Grinberg A, Munitz T I, Westphal H, Singer A. Differential effects of ζ and ɛ transgenes on early α/β T cell development. J Exp Med. 1994;179:1485–1494. doi: 10.1084/jem.179.5.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Misra N, Murtaza A, Walker B, Narayan P S, Misra R S, Ramesh V, Singh S, Colston M J, Nath I. Cytokine profile of circulating T cells of leprosy patients reflects both indiscriminate and polarized T-helper subsets: T-helper phenotype is stable and influenced by related antigens of Mycobacterium leprae. Immunology. 1995;86:97–103. [PMC free article] [PubMed] [Google Scholar]
- 11.Mizoguchi H, O’Shea J J, Longo D L, Loeffler C M, McVicar D, Ochoa A C. Alterations in signal transduction molecules in lymphocytes from tumor-bearing mice. Science. 1992;258:1795–1798. doi: 10.1126/science.1465616. [DOI] [PubMed] [Google Scholar]
- 12.Modlin R L. Th1-Th2 paradigm: insights from leprosy. J Investig Dermatol. 1994;102:828–832. doi: 10.1111/1523-1747.ep12381958. [DOI] [PubMed] [Google Scholar]
- 13.Modlin R L, Kato H, Mehra V, Nelson E E, Xue-Dong F, Rea T H, Pattengale P K, Bloom B R. Genetically restricted suppressor T cell clones derived from lepromatous leprosy lesions. Nature. 1986;322:459–461. doi: 10.1038/322459a0. [DOI] [PubMed] [Google Scholar]
- 14.Modlin R L, Melancon-Kaplan J S M, Young S M, Pirmez C, Kino H, Convit J, Rea T H, Bloom B R. Learning from lesions: patterns of tissue inflammation in leprosy. Proc Natl Acad Sci USA. 1988;85:1213–1217. doi: 10.1073/pnas.85.4.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakagomi H, Petterson M, Magnusson I, Juhlin C, Matzuda M, Mellsted H, Taupin J L, Vivier E, Anderson P, Kiessling R. Decreased expression of the signal-transducing ζ-chains in tumor infiltrating T cells and NK cells of patients with colorectal carcinoma. Cancer Res. 1993;53:5610–5612. [PubMed] [Google Scholar]
- 16.Ochoa M T, Valderrama L, Ochoa A C, Zea A H, Escobar C E, Moreno L H, Falabella R. Lepromatous and tuberculoid leprosy: clinical presentation and cytokine responses. Int J Dermatol. 1996;35:786–790. doi: 10.1111/j.1365-4362.1996.tb02974.x. [DOI] [PubMed] [Google Scholar]
- 17.Quill H, Riley M, Cho E, Casnellie J, Reed J, Torigoe T. Anergic Th1 cells express altered levels of the protein tyrosine kinases p56lck and p59fyn. J Immunol. 1992;149:2887–2893. [PubMed] [Google Scholar]
- 18.Rea T H. Suppressor cell activity and phenotypes in the blood or tissues of patients with leprosy. Clin Exp Immunol. 1983;54:298–304. [PMC free article] [PubMed] [Google Scholar]
- 19.Regueiro J R, Arnaiz-Villena A, Ortiz de Landazuri M, Martin-Villa J M, Vicario J L, Pascual-Ruiz V, Guerra-Garcia F, Alcami J, Lopez-Botet M, Manzanarez J. Familial defect of CD3 (T3) expression by T cells associated with rare gut epithelial cell autoantibodies. Lancet. 1986;i:1274–1275. doi: 10.1016/s0140-6736(86)91413-3. [DOI] [PubMed] [Google Scholar]
- 20.Renner C, Ohnesorge S, Gerhard H, Bauer S, Jung W, Pfitzenmeier P-P, Pfreundschuh M. T cells from patients with Hodgkin’s disease have a defective T-cell receptor ζ chain expression that is reversible by T-cell stimulation with CD3 and CD28. Blood. 1996;88:236–241. [PubMed] [Google Scholar]
- 21.Rice N, Mackichan M L, Israel A. The precursor of NFκB p50 has IκB-like functions. Cell. 1992;71:243–253. doi: 10.1016/0092-8674(92)90353-e. [DOI] [PubMed] [Google Scholar]
- 22.Ridley D S, Jopling W H. Classification of leprosy according to immunity. A five group system. Int J Lepr. 1966;34:255–273. [PubMed] [Google Scholar]
- 23.Salgame P, Modlin R L, Bloom B R. On the mechanism of human T cell suppression. Int Immunol. 1989;1:121–129. doi: 10.1093/intimm/1.2.121. [DOI] [PubMed] [Google Scholar]
- 24.Scollard D M. Inside the skin: the local immune and inflammatory milieu in leprosy. Am J Trop Med Hyg. 1991;44:17–23. [PubMed] [Google Scholar]
- 25.Scott P, Natovitz P, Coffman R L, Pearce E, Sher A. Immunoregulation of cutaneous leishmaniasis. T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigens. J Exp Med. 1988;168:1675–1684. doi: 10.1084/jem.168.5.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sica A, Tan T H, Rice N, Kretzchmar M, Ghosh P, Young H A. The c-rel protooncogene product c-Rel but not NFκB binds to the intronic region of the human interferon-γ gene at a site related to an interferon-stimulable response element. Proc Natl Acad Sci USA. 1992;89:1740–1744. doi: 10.1073/pnas.89.5.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sussman J J, Bonifacino J S, Lippincott-Schwart J, Weissman A M, Saito T, Klausner R D, Ashwell J D. Failure to synthesize the T cell CD3 ζ-chain: structure and function of a partial T cell receptor complex. Cell. 1988;52:85–95. doi: 10.1016/0092-8674(88)90533-8. [DOI] [PubMed] [Google Scholar]
- 28.Van-Voorhis W C, Kaplan G, Sarno E N, Horwitz M A, Steiman R M, Lewis W R, Nogueira N, Hair L S, Gattass C R, Arrick B A, Cohn Z A. The cutaneous infiltrates of leprosy: cellular characteristics and the predominant T cell phenotypes. N Engl J Med. 1982;307:1593–1597. doi: 10.1056/NEJM198212233072601. [DOI] [PubMed] [Google Scholar]
- 29.WHO Expert Committee on Leprosy. 1988. WHO Expert Committee on Leprosy: sixth report. WHO Tech. Rep. Ser. 768.
- 30.Yamamura M, Uyemura K, Deans R J, Weimberg K, Rea T H, Bloom B R, Modlin R L. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991;254:277–282. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]
- 31.Young H A, Ghosh P, Ye J, Lederer J, Lichtman A, Gerard J R, Penix L, Wilson C B, Melvin A J, McGurn M E, Lewis D B, Taub D D. Differentiation of the T helper phenotypes by analysis of the methylation state of the interferon-γ gene. J Immunol. 1994;153:3603–3610. [PubMed] [Google Scholar]
- 32.Zea A H, Curti B D, Longo D L, Alvord W G, Strobl S L, Mizoguchi H, Creekmore S P, O’Shea J J, Powers G C, Urba W J, Ochoa A C. Alterations in T cell receptor and signal transduction molecules in melanoma patients. Clin Cancer Res. 1995;1:1327–1335. [PubMed] [Google Scholar]
- 33.Ziegler-Heitbrock H W, Wedel A, Schraut W, Ströbel M, Wendelgass P, Sternsdof T, Bäuerle P A, Haas J G, Riethmüller G. Tolerance to lipopolysaccharide involves mobilization of nuclear factor κB with predominance of p50 homodimers. J Biol Chem. 1994;269:17001–17004. [PubMed] [Google Scholar]