Abstract
Recent studies point to the need to incorporate non-falciparum species detection into malaria surveillance activities in sub-Saharan Africa, where 95% of malaria cases occur. Although Plasmodium falciparum infection is typically more severe, diagnosis, treatment, and control for P. malariae, P. ovale spp., and P. vivax may be more challenging. The prevalence of these species throughout sub-Saharan Africa is poorly defined. Tanzania has geographically heterogeneous transmission levels but an overall high malaria burden. In order to estimate the prevalence of malaria species in Mainland Tanzania, 1,428 samples were randomly selected from 6,005 asymptomatic isolates collected in cross-sectional community surveys across four regions and analyzed via qPCR to detect each Plasmodium species. P. falciparum was most prevalent, with P. malariae and P. ovale spp. detected at lower prevalence (<5%) in all four regions. P. vivax was not detected. Malaria elimination efforts in Tanzania will need to account for these non-falciparum species.
Keywords: malaria, Plasmodium malariae, Plasmodium ovale, Plasmodium vivax, non-falciparum species, Tanzania, asymptomatic malaria
Tanzania has one of the highest malaria burdens in the world, accounting for 4.1% of global malaria deaths in 2021[1]. While most malaria cases in Tanzania and elsewhere in sub-Saharan Africa are caused by Plasmodium falciparum, four other Plasmodium species (P. vivax, P. malariae, P. ovale curtisi, and P. ovale wallikeri) are present to varying degrees. There is also data to suggest that these species have higher prevalence than previously known, and may become more prevalent as P. falciparum is controlled and ultimately eliminated[2–6] in line with the WHO goal of a 90% reduction in global malaria burden by 2030[7]. Non-falciparum malaria may require different control measures, due to major differences in biology, including different anopheline vectors with different seasonal peaks[8], relapse and/or chronic infections[9,10], lower parasitemia[11], and higher rates of asymptomatic infection[8].
Previous work in Mainland Tanzania has characterized non-falciparum prevalence in schoolchildren (5–16 years)[6] and non-falciparum positivity rates among symptomatic patients[12]. In schoolchildren, P. ovale spp. prevalence (24%) was similar to that of P. falciparum (22%)[6], while in symptomatic patients, P. falciparum was much more abundant than non-falciparum malaria, although P. ovale spp. positivity rates surpassed 5% in seven of ten regions[12]. In both studies, P. malariae was less common than either P. falciparum or P. ovale spp., and P. vivax was rare[6,12]. In this study, we characterize the prevalence of all malaria species among asymptomatic individuals across all ages in three regions with moderate and high malaria transmission intensity, and in children under five in one region with high transmission.
The study protocol was approved by the Tanzanian Medical Research Coordinating Committee (MRCC) of the National Institute for Medical Research (NIMR) and involved approved standard procedures for informed consent and sample deidentification. Additional details are described elsewhere[13]. Deidentified samples were considered non-human subjects’ research at the University of North Carolina and Brown University.
A random subset of 1,428 dried blood spot (DBS) samples were drawn from a total of 5,860 asymptomatic samples. 694 samples were drawn from a total of 2,647 collected from all age groups during cross-sectional community surveys in Kigoma (n=252/878, high transmission), Ruvuma (n=186/741, high transmission), and Tanga (n=256/1,028, moderate transmission) regions during the Molecular Surveillance of Malaria in Tanzania (MSMT) project in 2021[13]. The random subset was representative of the regional sample distribution (X2 (3,2) = 2.43, p (2 df) = 0.3, Table S1), but not representative of the age group distribution (X2 (3,2) = 10.46, p (2 df) = 0.005, Table S2), or the sex distribution (X2 (2,2) = 43.65, p (1 df) < 0.001, Table S3). An additional 734 samples were drawn from 3,213 collected from children under five during cross-sectional household surveys for the group antenatal care project (GANC)[14,15] in Geita in 2021.
The molecular analyses used to detect Plasmodium spp. in each sample are described in detail elsewhere[12]. Briefly, we performed a separate 18S qPCR assay for each species, which allows for both the detection of each species as well as a semi-quantitative parasitemia estimate. For each region, we calculated prevalence for each species, including both single-species and mixed-species infections. Regional-level maps of prevalence for each species were created using the R package sf (version 1.0–9) based on shape files available from GADM.org and naturalearthdata.com accessed via the R package rnaturalearth (version 0.3.2)[16]. Variation in species-specific prevalence by region and age group (young children <5 years, school-aged children 5–16 years, and adults >16 years, as previously described[12]) was assessed for significance with generalized linear models or ANOVA, as appropriate, in R.
Excluding the Geita participants who were all under five and whose ages were not recorded, the median age of the remaining 694 participants was 20 years (IQR 8–47) with a range of 6 months to 87 years. Including the Geita participants, children (≤16 years old) constituted 74.2% of participants (n=1,060), while adults (>16 years old) constituted the remaining 25.8% (n=368). Young children (<5 years old) comprised 77.9% (n=826) of the child participants, while the remaining 22.1% (n=234) were school-aged (5–16 years old). Sex identifications were available for 694 participants and female-skewed, with 505 female (72.8%) and 189 male participants (27.2%). 21.1% (n=301) of sampled individuals were RDT-positive.
Among all 1,428 samples analyzed, P. falciparum was detected in 34.2% (n=488, 95% CI: 31.7%–36.7%), P. malariae in 1.5% (n=22, 95% CI: 0.99%–2.4%), and P. ovale spp. in 3.4% (n=49, 95% CI: 2.6%–4.5%). P. vivax was not detected. P. malariae infections were nearly evenly split between single-species infections (45.5%, n=10/22) and mixed-species infections with P. falciparum (40.9%, n=9/22), with the remaining three (13.6%) being triple infections with P. falciparum and P. ovale spp. (Table 1). In contrast, most P. ovale spp. infections were mixed with P. falciparum (65.3%, n=32/49), with single-species infections being less common (28.6%, n=14/49) and triple infections comprising the remainder (Table 1). P. malariae had the highest median parasitemia at 164,080 p/μL (IQR 9,942–1,333,100 p/μL), followed by P. falciparum at 55,200 p/μL (2,910–775,000 p/μL) and P. ovale spp. at 11,868 p/μL (1,271–70,840 p/μL). However, there was no significant difference in parasitemia by species (ANOVA p=0.9).
Table 1.
Infection composition proportions for isolates infected with P. malariae and P. ovale spp.
| Infection Type | Count | Proportion |
|---|---|---|
| Pm | 10 | 45.5% |
| Pm/Pf | 9 | 40.9% |
| Pm/Pf/Po | 3 | 13.6% |
| All Pm Infections | 22 | |
| Po | 14 | 28.6% |
| Po/Pf | 32 | 65.3% |
| Pm/Pf/Po | 3 | 6.1% |
| All Po Infections | 49 |
Sum of proportions may not equal 100% due to rounding.
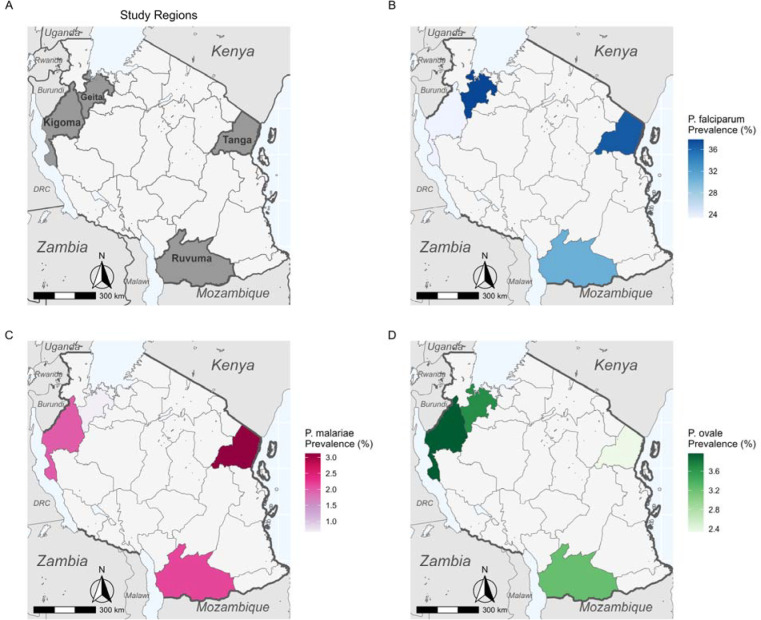
All three species were detected in each region (Figure 1). Geita and Tanga had the highest P. falciparum prevalence (37.8% and 36.7%, n=278/734 and 94/256 respectively, Table S4). P. malariae was relatively rare in all four regions, with the highest prevalence in Tanga (3.1%, n=8/256) and the lowest in Geita (0.7%, n=5/734, Table S4). P. ovale spp. prevalence was slightly higher than P. malariae (3.2%−4.0%, n=6/186–10/252) in all regions except Tanga (2.3%, n=6/256, Table S4). There was significant variation (by ANOVA) in prevalence between regions for P. falciparum (p<0.001) and P. malariae (p=0.04) but not for P. ovale spp.
Figure 1 –
Maps of Tanzania showing A) location of study regions, B) P. falciparum regional prevalence, C) P. malariae regional prevalence, and D) P. ovale spp. regional prevalence. P. vivax was not detected, so is not mapped.
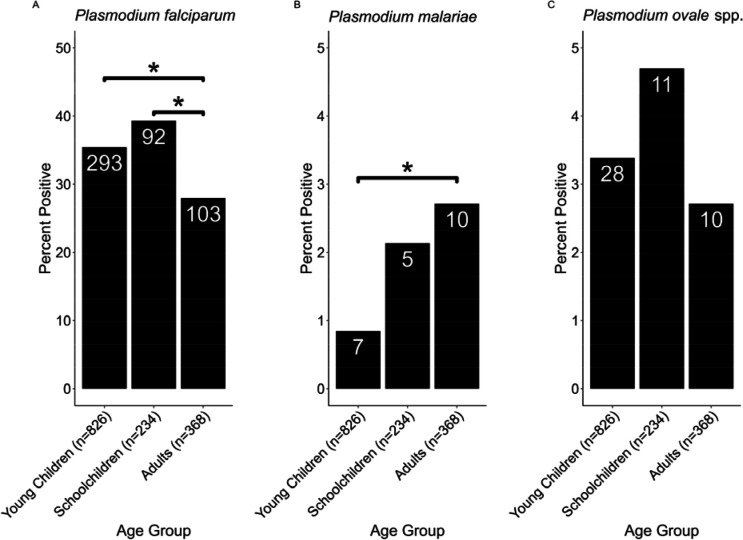
While age was a significant (GLM p<0.001) determinant of P. falciparum infection, there was no significant effect of age for either P. malariae or P. ovale spp. Age group was a significant (ANOVA p<0.05) determinant of infection likelihood for both P. falciparum and P. malariae, but not P. ovale spp. (Figure 2). While children were significantly (p<0.05) more likely than adults to have P. falciparum, adults were more likely to have P. malariae (p<0.05, Figure 2). There was no significant interaction between age group and region for either P. falciparum or P. malariae, but the interaction was nearly significant (p=0.055) for P. ovale spp.
Figure 2 –
Tukey Analysis of Malaria Species Prevalence by Age Group. A total of 826, 234, and 368 were in the Young Children (<5 years), Schoolchildren (5–16 years), and Adult (>16 years) groups, respectively. The total number of samples per group for each species is shown in the X-axis labels while the number of positive samples for each group is shown in the bar labels. Comparisons marked with a * are significant at the p<0.05 level. Panel A shows P. falciparum prevalence by age group. Significant pairwise comparisons are marked, while the other is insignificant. Panel B shows P. malariae prevalence by age group. One significant pairwise comparison is marked, while the others are insignificant. Panel C shows P. ovale spp. prevalence by age group. No pairwise comparisons are significant.
This study builds on previous research with schoolchildren and clinic patients to describe the prevalence of different malaria species within four regions of Mainland Tanzania. Although P. falciparum is the most prevalent species, both P. malariae and P. ovale spp. prevalence surpasses 3% in at least one region, and could increase as P. falciparum is locally eliminated. In contrast to a 2017 study on schoolchildren, which found P. ovale spp. prevalence to be similar to P. falciparum[6], we found P. ovale spp. prevalence to be much lower than that of P. falciparum. In addition, the schoolchildren survey mostly identified P. ovale spp. as single-species infections and P. malariae as mixed with P. falciparum[6], whereas we found similar proportions of single-species and mixed-species P. malariae infections and most of our P. ovale spp. samples were mixed with P. falciparum. However, our sample sizes are small and may not necessarily be representative of the full picture, particularly in Geita where samples were only collected from children under 5 years old. In addition, our study only includes four regions, only one of which (Tanga) overlaps with the previous study, and we include a wider age range.
In Tanga, we may have found lower P. ovale spp. prevalence than the previous study due to the inclusion of adults, who are less likely to test positive for this species, whereas schoolchildren are a major asymptomatic infectious reservoir[17–19], although we did not replicate a significant difference in this study. In addition, the disruptions to malaria control caused by the COVID-19 pandemic, particularly in 2020–2021[20], could have an increase in P. falciparum prevalence. Indeed, there was no difference in the malaria prevalence in the villages of Magoda, Mamboleo, and Mpapayu between 2019 (24.9%) and 2021 (24.5%, unpublished data). However, the prevalence dropped to 6.4% in 2022 (unpublished data), so further longitudinal study may clarify the impact of resumed intense P. falciparum control. However, the malaria prevalence in Tanga dropped from 34.8% to 26.2% between 2020 and 2021 (unpublished data), so P. falciparum control in this region was likely effective during the course of this study.
Unlike in our study of symptomatic patients[12], we did not find schoolchildren to be significantly more likely to test positive for either P. malariae or P. ovale spp. Although not significant, this trend is present in P. ovale spp. prevalences in this study, so the lack of significance in these species is likely an artifact of small sample sizes (Table 1, Figure 2, Figure S1). Our finding that P. malariae was significantly more prevalent among adults likely reflects the presence of chronic infections[10].
Although P. falciparum remains the most prevalent species in these four regions, P. malariae and P. ovale spp. are present in all four, whereas P. vivax is not detected. Achieving malaria elimination in Tanzania will require ongoing surveillance of these species. While standard treatments successfully clear P. falciparum, the 3.4% of patients in this study with P. ovale spp. may relapse. This study serves as a complement to previous studies focusing on schoolchildren and symptomatic patients to paint a full picture of the non-falciparum malaria landscape for communities in Mainland Tanzania. Ongoing analysis of samples collected in 2022 and 2023 will allow us to detect temporal trends in prevalence, and forthcoming genomic analysis of P. malariae and P. ovale spp. isolates from Tanzania will inform our understanding of population structure and diversity in these species.
Supplementary Material
Acknowledgements:
The authors wish to thank participants and parents/guardians of all children who took part in the surveillance. We acknowledge the contribution of the following project staff and other colleagues who participated in data collection and/or laboratory processing of samples: Raymond Kitengeso, Ezekiel Malecela, Muhidin Kassim, Athanas Mhina, August Nyaki, Juma Tupa, Anangisye Malabeja, Emmanuel Kessy, George Gesase, Tumaini Kamna, Grace Kanyankole, Oswald Osca, Richard Makono, Ildephonce Mathias, Godbless Msaki, Rashid Mtumba, Gasper Lugela, Gineson Nkya, Daniel Chale, Richard Malisa, Sawaya Msangi, Ally Idrisa, Francis Chambo, Kusa Mchaina, Neema Barua, Christian Msokame, Rogers Msangi, Salome Simba, Hatibu Athumani, Mwanaidi Mtui, Rehema Mtibusa, Jumaa Akida, Ambele Yatinga, and Tilaus Gustav. We also acknowledge the finance, administrative and logistic support team at NIMR: Christopher Masaka, Millen Meena, Beatrice Mwampeta, Gracia Sanga, Neema Manumbu, Halfan Mwanga, Arison Ekoni, Twalipo Mponzi, Pendael Nasary, Denis Byakuzana, Alfred Sezary, Emmanuel Mnzava, John Samwel, Daud Mjema, Seth Nguhu, Thomas Semdoe, Sadiki Yusuph, Alex Mwakibinga, Rodrick Ulomi and Andrea Kimboi. We are also grateful to the management of the National Institute for Medical Research, National Malaria Control Program and President’s Office-Regional Administration and Local Government (regional administrative secretaries of the 14 regions, and district officials, staff from all 100 HFs and Community Health Workers from the 4 community cross sectional regions). Technical and logistics support from the Bill and Melinda Gates Foundation team is highly appreciated. The following reagents were obtained through BEI Resources, NIAID, NIH: Diagnostic Plasmid Containing the Small Subunit Ribosomal RNA Gene (18S) from Plasmodium falciparum, MRA-177; Plasmodium vivax, MRA-178; Plasmodium malariae, MRA-179; and Plasmodium ovale, MRA-180, contributed by Peter A. Zimmerman. Permission to publish the manuscript was sought and obtained from the Director General of NIMR.
Funding
This work was supported, in part, by the Bill & Melinda Gates Foundation [grant number 002202]. Under the grant conditions of the Foundation, a Creative Commons Attribution 4.0 Generic License has already been assigned to the Author Accepted Manuscript version that might arise from this submission. Data collection in Geita was funded by USAID/PMI through Jhpiego and CDC. JJJ also received funding from NIH K24AI134990.
Footnotes
Competing Interests: We declare no competing interests.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the U.S. Centers for Disease Control and Prevention.
Data availability:
Data is available upon reasonable request to the corresponding author.
References:
- 1.World Health Organization. World malaria report 2022. Geneva; 2022. [Google Scholar]
- 2.Akala HM, Watson OJ, Mitei KK, Juma DW, Verity R, Ingasia LA, et al. Plasmodium interspecies interactions during a period of increasing prevalence of Plasmodium ovale in symptomatic individuals seeking treatment: an observational study. The Lancet Microbe [Internet]. 2021. [cited 2022 Mar 31];2:e141–50. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2666524721000094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Betson M, Clifford S, Stanton M, Kabatereine NB, Stothard JR. Emergence of Nonfalciparum Plasmodium Infection Despite Regular Artemisinin Combination Therapy in an 18-Month Longitudinal Study of Ugandan Children and Their Mothers. J Infect Dis [Internet]. Oxford Academic; 2018. [cited 2022 Mar 31];217:1099–109. Available from: https://academic.oup.com/jid/article/217/7/1099/4791878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yman V, Wandell G, Mutemi DD, Miglar A, Asghar M, Hammar U, et al. Persistent transmission of Plasmodium malariae and Plasmodium ovale species in an area of declining Plasmodium falciparum transmission in eastern Tanzania. Marks F, editor. PLoS Negl Trop Dis [Internet]. Public Library of Science; 2019. [cited 2021 Apr 19];13:e0007414. Available from: 10.1371/journal.pntd.0007414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguiffo-Nguete D, Nongley Nkemngo F, Ndo C, Agbor J-P, Boussougou-Sambe ST, Salako Djogbénou L, et al. Plasmodium malariae contributes to high levels of malaria transmission in a forest–savannah transition area in Cameroon. Parasites Vectors 2023 161 [Internet]. BioMed Central; 2023. [cited 2023 Jan 30];16:1–10. Available from: 10.1186/s13071-022-05635-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sendor R, Mitchell CL, Chacky F, Mohamed A, Mhamilawa LE, Molteni F, et al. Similar Prevalence of Plasmodium falciparum and Non-P. falciparum Malaria Infections among Schoolchildren, Tanzania. Emerg Infect Dis [Internet]. 2023;29:1143–53. Available from: https://wwwnc.cdc.gov/eid/article/29/6/22-1016_article [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. Global Technical Strategy for Malaria 2016–2030 [Internet]. Geneva; 2015. Available from: https://www.who.int/malaria/publications/atoz/9789241564991/en/ [Google Scholar]
- 8.Tarimo BB, Nyasembe VO, Ngasala B, Basham C, Rutagi IJ, Muller M, et al. Seasonality and transmissibility of Plasmodium ovale in Bagamoyo District, Tanzania. Parasit Vectors [Internet]. BioMed Central; 2022. [cited 2022 Feb 16];15:56. Available from: 10.1186/s13071-022-05181-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins WE, Jeffery GM. Plasmodium ovale: Parasite and disease. Clin Microbiol Rev [Internet]. American Society for Microbiology; 2005. [cited 2022 Mar 31];18:570–81. Available from: 10.1128/CMR.18.3.570-581.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oriero EC, Amenga-Etego L, Ishengoma DS, Amambua-Ngwa A. Plasmodium malariae, current knowledge and future research opportunities on a neglected malaria parasite species. Crit Rev Microbiol [Internet]. Taylor & Francis; 2021. [cited 2021 Jan 29];0:1–13. Available from: 10.1080/1040841X.2020.1838440 [DOI] [PubMed] [Google Scholar]
- 11.Roucher C, Rogier C, Sokhna C, Tall A, Trape JF. A 20-Year Longitudinal Study of Plasmodium ovale and Plasmodium malariae Prevalence and Morbidity in a West African Population. PLoS One [Internet]. Public Library of Science; 2014. [cited 2023 Mar 24];9:e87169. Available from: 10.1371/journal.pone.0087169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Popkin-Hall ZR, Seth MD, Madebe RA, Budodo R, Bakari C, Francis F, et al. Malaria species positivity rates among symptomatic individuals across regions of differing transmission intensities in Mainland Tanzania. medRxiv [Internet]. Cold Spring Harbor Laboratory Press; 2023. [cited 2023 Nov 8];2023.09.19.23295562. Available from: 10.1101/2023.09.19.23295562v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rogier E, Battle N, Bakari C, Seth MD, Nace D, Herman C, et al. Plasmodium falciparum pfhrp2 and pfhrp3 gene deletions among patients enrolled at 100 health facilities throughout Tanzania: February to July 2021. medRxiv [Internet]. Cold Spring Harbor Laboratory Press; 2023. [cited 2023 Aug 7];2023.07.29.23293322. Available from: 10.1101/2023.07.29.23293322v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emerson C, Ulimboka S, Lemwayi R, Kinyina A, Nhiga SL, Aaron S, et al. Women attending antenatal care as a sentinel surveillance population for malaria in Geita region, Tanzania: feasibility and acceptability to women and providers. Malar J [Internet]. BioMed Central Ltd; 2023. [cited 2023 Jul 24];22:1–10. Available from: 10.1186/s12936-023-04480-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gutman JR, Mwesigwa JN, Arnett K, Kangale C, Aaron S, Babarinde D, et al. Using antenatal care as a platform for malaria surveillance data collection: study protocol. Malar J [Internet]. BioMed Central Ltd; 2023. [cited 2023 Jul 24];22:1–10. Available from: 10.1186/s12936-023-04521-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Massicotte P, South A. rnaturalearth: World Map Data from Natural Earth [Internet]. 2023. Available from: https://cran.r-project.org/package=rnaturalearth [Google Scholar]
- 17.Abdulraheem MA, Ernest M, Ugwuanyi I, Abkallo HM, Nishikawa S, Adeleke M, et al. High prevalence of Plasmodium malariae and Plasmodium ovale in co-infections with Plasmodium falciparum in asymptomatic malaria parasite carriers in southwestern Nigeria. Int J Parasitol [Internet]. Pergamon; 2022. [cited 2023 May 4];52:23–33. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0020751921002393 [DOI] [PubMed] [Google Scholar]
- 18.Andolina C, Rek JC, Briggs J, Okoth J, Musiime A, Ramjith J, et al. Sources of persistent malaria transmission in a setting with effective malaria control in eastern Uganda: a longitudinal, observational cohort study. Lancet Infect Dis [Internet]. Elsevier; 2021. [cited 2021 Jul 12];21:1568–78. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1473309921000724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walldorf JA, Cohee LM, Coalson JE, Bauleni A, Nkanaunena K, Kapito-Tembo A, et al. School-Age Children Are a Reservoir of Malaria Infection in Malawi. PLoS One [Internet]. Public Library of Science; 2015. [cited 2023 May 4];10:e0134061. Available from: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0134061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Q, Yan W, Qin C, Du M, Liu M, Liu J. Millions of excess cases and thousands of excess deaths of malaria occurred globally in 2020 during the COVID-19 pandemic. J Glob Health [Internet]. 2022;12:05045. Available from: https://jogh.org/2022/jogh-12-05045 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is available upon reasonable request to the corresponding author.