Abstract
Concerns about the potential neurotoxic effects of anesthetics on developing brain exist. When making clinical decisions, the timing and dosage of anesthetic exposure are critical factors to consider due to their associated risks. In our study, we investigated the impact of repeated anesthetic exposures on the brain development trajectory of a cohort of rhesus monkeys (n = 26) over their first 2 yr of life, utilizing longitudinal magnetic resonance imaging data. We hypothesized that early or high-dose anesthesia exposure could negatively influence structural brain development. By employing the generalized additive mixed model, we traced the longitudinal trajectories of brain volume, cortical thickness, and white matter integrity. The interaction analysis revealed that age and cumulative anesthetic dose were variably linked to white matter integrity but not to morphometric measures. Early high-dose exposure was associated with increased mean, axial, and radial diffusivities across all white matter regions, compared to late-low-dose exposure. Our findings indicate that early or high-dose anesthesia exposure during infancy disrupts structural brain development in rhesus monkeys. Consequently, the timing of elective surgeries and procedures that require anesthesia for children and pregnant women should be strategically planned to account for the cumulative dose of volatile anesthetics, aiming to minimize the potential risks to brain development.
Keywords: neurodevelopment, anesthesia, neurotoxicity, rhesus macaque, magnetic resonance imaging, diffusion tensor imaging
Introduction
Every year, ~3.9 million surgeries are performed on children age 0 to 17 yr in United States (Rabbitts and Groenewald 2020). The potential for lasting neurotoxic effects of agents used to induce general anesthesia and sedation has been a subject of concern and research for the last 20 yr (Andropoulos and Greene 2017). However, the minimal potential neurotoxic dose, frequency, and the age at which the developing brain may be the most vulnerable to anesthesia are unknown. Therefore, it is critical to investigate the effects of different timing and dose of anesthesia on brain and behavioral development.
Animal studies have reported anesthetic and sedative drugs can be neurotoxic to the developing brain. In rodent studies, exposure of combination of commonly used anesthetic agent (i.e. midazolam, nitrous oxide, and isoflurane) resulted in neuronal apoptosis, defects in synaptic function in the hippocampus and cognitive impairments in juveniles and young adults (Jevtovic-Todorovic et al. 2003; Istaphanous and Loepke 2009). Several nonhuman primate (NHP) studies reported single or multiple exposures to anesthetic agents resulted in long-term neurodevelopmental defects: infant rhesus monkeys that were exposed to sevoflurane for 4 h on days of life 6 to 10, followed by subsequent exposures on days of life 14 and 28, demonstrated increase anxiety compared with controls that underwent only a brief period of maternal separation (Raper et al. 2015). Neonatal exposures to sevoflurane in rhesus monkeys altered synaptic ultrastructure 4 yr after three 4-h exposures to sevoflurane during the first five postnatal weeks. Synapse areas were reduced in the largest synapses in the hippocampal CA1 and dorsolateral prefrontal cortex (dlPFC) (Fehr et al. 2022). In another study, infant rhesus macaques that were exposed to isoflurane for 5 h on days of life 6, 9, and 12 demonstrated motor reflex deficits at 1 mo of age and increased anxiety at 12 mo of age (Coleman et al. 2017). Neuronal apoptosis in the fetal and neonatal NHP brain was induced by prolonged exposure (5 to 24 h) to isoflurane (Brambrink et al. 2010; Creeley et al. 2014), ketamine (Slikker Jr et al. 2007; Brambrink et al. 2012b) or propofol (Creeley et al. 2013).
While animal studies have found neurotoxic effects of anesthetic medications, it is unclear whether anesthesia has any long-term neurodevelopmental effects in human. From observational clinical studies, single exposure was not associated with detrimental side effects on academic achievement, intelligence, and cognitive domain (Wilder et al. 2009; Flick et al. 2011; Graham et al. 2016; O'Leary et al. 2016; Glatz et al. 2017; O'Leary et al. 2019). However, repeated exposure to anesthesia and surgery before age of two was a risk factor for later development of learning disabilities (Flick et al. 2011). Another study also reported children receiving equal or more than two anesthetics were at increased risk for learning disabilities and the risk was associated with longer cumulative duration of anesthesia exposure (Wilder et al. 2009). Due to lack of substantial clinical evidence, U.S. Food and Drug Administration (FDA) modified their warning in 2017, stating that medically necessary procedures in pregnant women and children under age of three should not be delayed and that practitioners should follow their usual practice paradigm (FDA 2017). The warning also states that additive high-quality research is needed to investigate the effects of repeated and prolonged anesthesia exposure in children, including vulnerable populations.
Live imaging studies using longitudinal magnetic resonance imaging (MRI) could be useful to investigate the mechanisms of neurotoxicity and prolonged effects of anesthesia on brain development in primates. A recent study evaluated the cumulated effects of exposure to ketamine, telazol, and isoflurane on early brain development by combining the UNC-Wisconsin Neurodevelopmental Rhesus Data (https://data.kitware.com) and Yerkes National Primate Research Center cohort, and reported marked reductions in fractional anisotropy (FA) and increases in axial, radial, and mean diffusivities (AD, RD, and MD), across the brain as a result of repeated anesthesia exposure at ages of 12 or 18 mo (Young et al. 2021). However, this study employed cross-sectional analysis approach focusing on the accumulated anesthesia dose and did not take account of the timing of first anesthesia. Another longitudinal imaging study of 34 rhesus monkeys (23 females, 11 males) analyzed with linear mixed-effect model reported remarkably fast increase in FA and decrease in MD in the first few weeks after birth followed by slow but continuous increase in FA and decrease in MD for the first year of life (Aggarwal et al. 2021). However, our previous results from diffusion MRI dataset of UNC-Wisconsin Neurodevelopmental Rhesus Data reported biphasic patterns of MD from 2 to 22 mo of age (Kim et al. 2020). Here, we speculated that these biphasic changes of MD could be associated with anesthetics used for husbandry and MRI scan or linear mixed models may not properly capture nonlinear changes of microstructural white matter properties during the brain development (Sorensen et al. 2021).
Considering neurotoxic effects of anesthesia, as discussed in our previous study, there is potential for anesthesia to impact brain volume development in primates (Kim et al. 2020). Previous NHP studies have shown that transient reductions in the intracranial volume occur between 5 and 9 mo of age, irrespective of the timing of weaning (after birth vs. 6 to 7 mo), housing conditions (individually housed vs. grouped with peers), size of the housing, and the age of first MRI scan (Malkova et al. 2006; Scott et al. 2016). Intriguingly, this period corresponds to late infancy in human (20 to 36 mo old), a period characterized by ongoing increases in cortical gray matter and white matter volumes (Bethlehem et al. 2022). This suggests that the influences of anesthesia during infancy could lead to more complex and delayed patterns of brain volume development.
In this study, to better characterize the dynamics of structural brain development and address the effects of multiple exposure to anesthesia, we analyzed a longitudinal structural and diffusion MRI dataset of rhesus monkeys (the UNC-Wisconsin Neurodevelopmental Rhesus Data) from late infancy to juvenile stages (months 2 to 24, which approximately corresponds to 8 mo to 8 yr old in the human). We employed the generalized additive mixed effect (GAMM) model to fit the nonlinear changes of MRI markers for brain morphometry and white matter integrity: the smooth functions were constructed as weighted sum of multiple basis functions to the age and anesthesia configuration as covariates. Here, we hypothesized that earlier or higher dose exposure to anesthesia during repeated MRI scans adversely affects structural brain development from late infancy to juvenile stages.
Materials and methods
MRI dataset
For longitudinal analysis, we selected 26 subjects (11 females and 15 males) among 32 rhesus macaques. Six animals were excluded due to following reasons: the timing of the first MRI scan was too late (>16 mo of age) for group comparison, there was severe signal loss of diffusion-weighted images (DWI) in prefrontal area or available time points of longitudinal MRIs were ˂3. Quality controlled longitudinal MRI equal to or more than three time points between 2 and 24 mo of age (equivalent to 8 mo to 8 yr old in the human) were finally selected (Young et al. 2017). Details of longitudinal MRI dataset and imaging parameters were described in our previous study (Kim et al. 2020). Briefly, T1-weighted (T1w), T2-weighted (T2w), and DWI of rhesus macaques were acquired from a publicly available database (UNC-Wisconsin Neurodevelopmental Rhesus Data). All NHPs were scanned on a GE Signa 3 T scanner (General Electric Medical Systems, Milwaukee, WI) in the Waisman Center at University of Wisconsin-Madison. All monkeys were oriented identically within a stereotaxic platform; situated within the human 8-channel brain array coil.
Anesthesia
All study subjects were exposed to anesthesia 3 to 6 times for longitudinal MRI scan from 2 wk to 24 mo. Subjects were given a preanesthetic (ketamine hydrochloride 10 mg/kg I.M.) for transport to the MRI facility. For infants younger than 6 mo of age, immobilization during the scan was achieved with inhaled isoflurane. Subjects older than 7 mo of age were immobilized throughout the scanning procedure by an initial administration of ketamine hydrochloride (10 mg/kg I.M.) followed by dexmedetomidine (0.01 mg/kg I.M.). The effects were reversed at the end of the session by administering atipamezole (0.15 mg/kg I.V.). The plane of anesthesia was monitored with a pulse oximeter to track heart rate and oxygen saturation in both younger and older subjects. In this study, we employed the normalized measure of anesthesia for the analyses as described in the previous study (Total Normalized Exposure [TNE] = Total ketamine/10 + Total isoflurane/2) (Young et al. 2021). A summary of descriptive information about the anesthesia is in Supplemental Table S1. All anesthesia exposures were normalized per prior MRI-related exposure, and then aggregated per subject into a single TNE. This normalization allows us to relate scan sessions with mainly ketamine and those with ketamine and isoflurane and put the exposure in a combined measure. High correlation with TNE (r = 0.73) within the cohort precludes adding dexmedetomidine as covariates into separate models to estimate their effects in the corresponding sample.
As shown in Fig. 1, it differs quite a bit across animals due to differences in husbandry-related exposure as well as length of imaging exposure. From the anesthesia data, we found the early exposure subjects were related to higher accumulated anesthesia dose because scanning was achieved with isoflurane (1.5 vol%) infants younger than 6 mo of age for smooth and rapid induction and to facilitate intubation. Dexmedetomidine exposure was not incorporated as they were quite consistent across all scanning sessions.
Fig. 1.
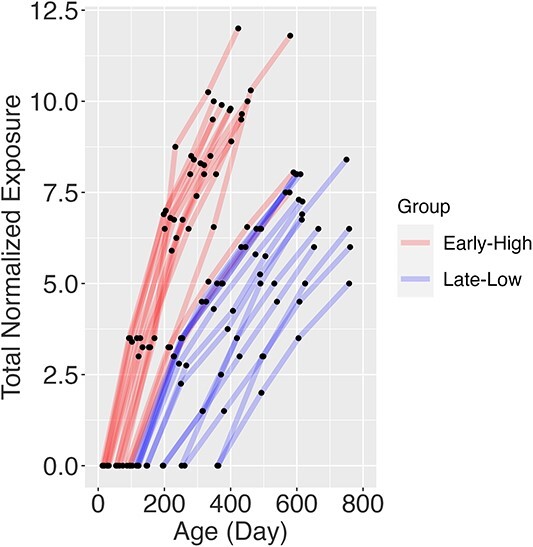
Accumulated normalized anesthesia dose (TNE) relative to the ages during the longitudinal MRI scan. The study subjects can be divided into two groups (early high vs. late-low) based on the slope of the curve.
Image processing
Details of image processing steps were described in the previous study (Kim et al. 2020). Using these T1w, T2w, and quality controlled diffusion-weighted images, we analyzed longitudinal volumetric, cortical thickness, and white matter integrity across the multiple time points. For regional volumetric analyses, the parcellation map defined in the UNC Primate Brain Atlas (Styner et al. 2007) was warped to the population-specific template using deformable registration and manually corrected. The Atlas label includes occipital, temporal auditory, subcortical, frontal, prefrontal, cerebellum, insula, cingulate, parietal, prefrontal, corpus callosum, temporal visual, temporal limbic, and pons and medulla.
The corrected parcellation map of the population-specific template was then warped to each time point using the longitudinal transforms applied in the individual tissue segmentation. Intracranial volumes (ICVs) were measured directly from the brain masks, and region-of-interest (ROI) volumes were measured using the parcellation map warped to the individual brain. By taking advantage of individual segmentation and parcellation maps, mean values of cortical thickness at the gray matter and parcellated regions were estimated using kellyKapowski function of Advanced Normalization Tools (Tustison et al. 2013). We estimated diffusion tensor imaging scalars from the DWIs. Parcellation and parcellation maps on T1w images were warped to the DWI space for ROI analysis using the nonlinear transforms from T1w to FA images within the subject. To correct segmentation errors around the internal and external capsules due to low tissue contrast in T1w and T2w images, we applied the age-adaptive FA threshold to separate WM from GM; all individual FA images were warped to the SST and mean FA values at WM/GM boundary were extracted.
Statistical analysis
For longitudinal regression analysis at the whole brain and ROI levels, we used the Generalized Additive Mixed Model function (gamm4) in the R statistical package (version 4.2: http://r-project.org). The function fits complex brain development patterns with smoothing splines to the age and evaluates the effects of different anesthesia profiles on MRI measures (volume, cortical thickness, and white matter integrity). MRI measurements were analyzed at both whole brain and ROI levels using an interaction model that accounted for age (a within-subject variable) and total anesthesia exposure group (TNE_Group, a between-subject variable), as depicted in Fig. 1. In our model, we chose to exclude variables such as sex, body weight, the lateral side of the brain, and age at first anesthesia exposure from our covariates. This decision stemmed from our preliminary analysis that indicated no significant impact of sex on MRI measurements, a high correlation between body weight and age, and our intent to concentrate on symmetrical brain development, thus streamlining the model and increasing statistical robustness. Moreover, the age at first anesthesia exposure was strongly linked with TNE. Accordingly, our statistical model was structured as MRI measure ~ s (Age, by = TNE_Group) + TNE_Group + (1|Subject), allowing us to discern the interaction effects by permitting the age-related trajectory of MRI measurements to differ among the TNE_Groups. To assess the influence of TNE_Group on structural brain development, we divided the subjects into two groups: an Early High-dose exposure group (n = 13) and a Late-Low-dose exposure group (n = 13), based on the median age of first exposure, which was 100 days (corresponding to 400 days in humans) and slope of age-TNE curve as show in Fig. 1.
ROI volume measures were also normalized by ICV. We excluded data points at <100 and >600 days of age for statistical analyses due to small sample size either in early or late MRI scans to avoid overfitting and biased estimates of high-degree polynomials. Bonferroni correction was applied for multiple comparisons in ROI analyses. The adjusted P-value was estimated from the desired alpha level (0.05) divided by the number of cortical and subcortical regions (13 ROIs) used in the analyses.
Results
We described the interaction among age and TNE_Group on brain tissue volume, cortical thickness, and white matter integrity at the whole brain and ROI levels.
Brain tissue volumes
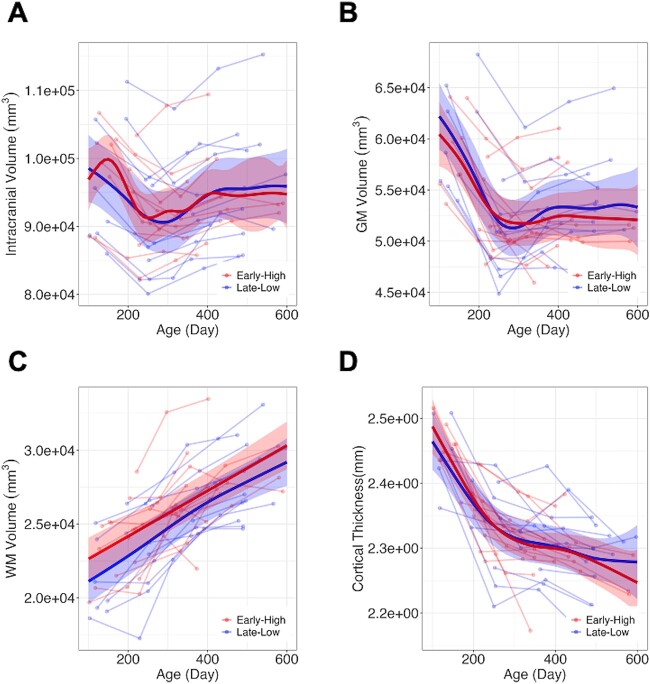
The longitudinal trajectories of intracranial and gray matter volumes exhibited nonlinear trends, while the white matter volume patterns maintained linearity from 100 to 600 days post-birth, as depicted in Fig. 2A–C. No statistically significant TNE_Group effect was observed in intracranial, gray matter, white matter, and cerebrospinal fluid volumes. Also, at the ROI level, no TNE_Group effect was observed except prefrontal white matter volume after corrections for multiple comparison (Table 1) demonstrating lower white matter volume in the “Late-Low” group compared to Early High group (correct p for TNE_Group =0.01).
Fig. 2.
Longitudinal trajectories in the intracranial volume (A), gray matter (B), white matter (C), and mean cortical thickness (D). No differences related to anesthesia exposure group were found in total, gray matter, and white matter volumes, as well as cortical thickness.
Table 1.
Summary of statistical analysis results of white matter volume. Significance codes:.: < 0.1 *P < 0.05, **P < 0.01, ***P < 0.001.
| WM Volume | s(Age_norm):Early High | s(Age_norm):Late-Low | R-sq.(adj) | Intercept | TNE_Group | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ROI | edf | Ref.df | F | P -value | edf | Ref.df | F | P -value | Estimate | Std. Error | t value | Pr(>|t|) | Estimate | Std. Error | t value | Pr(>|t|) | Corrrected p | ||
| Whole Brain | 1 | 1 | 91.75 | <2e − 16*** | 1.454 | 1.454 | 107.89 | <2e − 16*** | 0.409 | 26103.1 | 645.5 | 40.437 | <2e − 16*** | −1064.3 | 913.9 | −1.165 | 0.247 | NA | |
| Cerebellum | 1 | 1 | 1.934 | 0.167795 | 1.514 | 1.514 | 9.384 | 0.000565*** | 0.102 | 2749.765 | 97.006 | 28.346 | <2e − 16*** | 4.627 | 137.547 | 0.034 | 0.973 | 12.649 | |
| Cingulate | 1 | 1 | 21.43 | 1.26e − 05*** | 1 | 1 | 8.733 | 0.00399** | 0.236 | 392.61 | 16.45 | 23.87 | <2e − 16*** | −49.41 | 23.31 | −2.12 | 0.0368 | * | 0.4784 |
| Corpus Callosum | 1 | 1 | 50.48 | <2e − 16*** | 3.809 | 3.809 | 25.85 | <2e − 16*** | 0.188 | 840.14 | 34.11 | 24.633 | <2e − 16*** | −10.65 | 48.28 | −0.221 | 0.826 | 10.738 | |
| Frontal | 1 | 1 | 71.18 | <2e − 16*** | 1 | 1 | 50.7 | <2e − 16*** | 0.425 | 3924.9 | 130.1 | 30.169 | <2e − 16*** | −520.9 | 184.4 | −2.825 | 0.00582 | * * | 0.07566 |
| Insula | 2.3 | 2.3 | 2.953 | 0.0865 . | 2.224 | 2.224 | 6.763 | 0.0015** | 0.1 | 46.357 | 3.047 | 15.214 | <2e − 16*** | 1.298 | 4.314 | 0.301 | 0.764 | 9.932 | |
| Occipital | 1.196 | 1.196 | 2.463 | 0.0880 . | 1 | 1 | 8.446 | 0.0046** | 0.051 | 4205.2 | 109.3 | 38.471 | <2e − 16*** | −118.8 | 154.9 | −0.767 | 0.445 | 5.785 | |
| Parietal | 1 | 1 | 40.54 | <2e − 16*** | 1 | 1 | 33.29 | <2e − 16*** | 0.278 | 4247.1 | 129.9 | 32.69 | <2e − 16*** | −314.8 | 184.1 | −1.71 | 0.0907 | . | 1.1791 |
| Pons and Medulla | 5.675 | 5.675 | 56.58 | <2e − 16*** | 3.22 | 3.22 | 92.73 | <2e − 16*** | 0.674 | 1264.76 | 35.12 | 36.013 | <2e − 16*** | 38.78 | 49.26 | 0.787 | 0.433 | 5.629 | |
| Prefrontal | 1 | 1 | 72.9 | <2e − 16*** | 1 | 1 | 32.19 | 3.02e − 07*** | 0.447 | 1993.91 | 69.02 | 28.888 | <2e − 16*** | −340.17 | 97.85 | −3.476 | 0.000784 | * * * | 0.010192 |
| Subcortical | 1.593 | 1.593 | 6.68 | 0.00273** | 2.709 | 2.709 | 30.64 | <2e − 16*** | 0.303 | 2416.6 | 101.3 | 23.862 | <2e − 16*** | 244.6 | 143.4 | 1.706 | 0.0916 | . | 1.1908 |
| Temporal Audigory | 3.754 | 3.754 | 13.58 | <2e − 16*** | 3.093 | 3.093 | 79.43 | <2e − 16*** | 0.368 | 747.83 | 33.1 | 22.592 | <2e − 16*** | 61.46 | 46.8 | 1.313 | 0.193 | 2.509 | |
| Temporal Limbic | 1.921 | 1.921 | 12.53 | 1.77e − 05*** | 1.562 | 1.562 | 35.02 | <2e − 16*** | 0.206 | 464.71 | 23.25 | 19.985 | <2e − 16*** | 5.67 | 32.9 | 0.172 | 0.864 | 11.232 | |
| Temporal Visual | 2.162 | 2.162 | 9.51 | 0.000128*** | 1 | 1 | 94.74 | <2e − 16*** | 0.23 | 2765.427 | 85.319 | 32.41 | <2e − 16*** | −7.225 | 120.695 | −0.06 | 0.952 | 12.376 | |
Cortical thickness
Longitudinal trajectory of cortical thickness demonstrated exponential decay patterns (Fig. 2D). Effects of anesthesia configuration on longitudinal cortical thickness changes were not observed in both whole brain and ROI levels.
White mater integrity
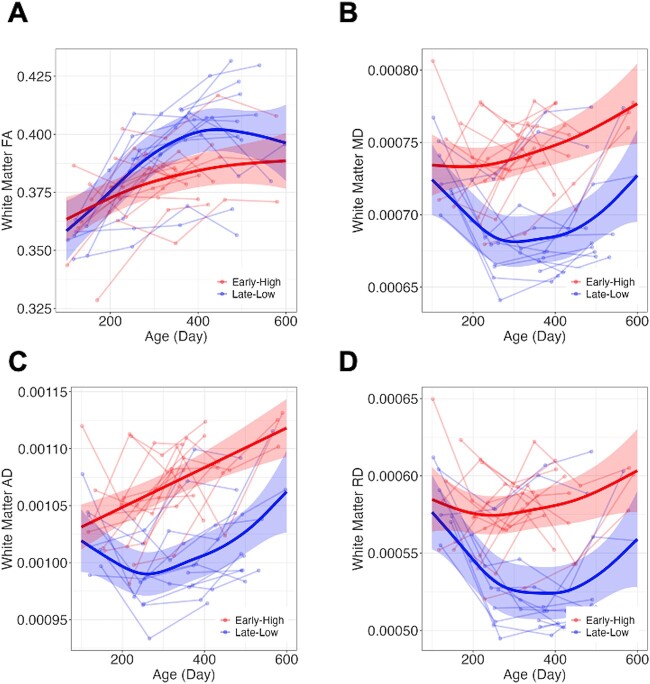
Rapid increases in whole white matter FA were observed until 300 days after birth, followed by slight increases. On the other hand, a rapid decrease in whole white matter MD until 7 mo (early high group after the second or third MRI scan) or 10 mo (late-low group after the second MRI scan) and rebounding patterns were observed (Fig. 3). AD and RD revealed similar patterns with MD. At the whole white matter level, TNE_Group effect was observed in longitudinal trajectories of MD, AD, and RD (corrected P-values for TNE_Group < 0.001) and subtle effect was observed in FA (uncorrected P = 0.075). Early High group (Red) demonstrated higher MD, AD, and RD compared to Late-Low group (Blue).
Fig. 3.
Longitudinal changes in FA, MD, AD, and RD in white matter. Differences related to anesthesia configuration were observed in longitudinal trajectories of MD, AD, and RD while only subtle effect was observed in FA.
Whereas all ROIs demonstrated TNE_Group effects for MD, AD, and RD, no TNE_Group effect was observed in FA. Details of statistical models and statistics were summarized in Table 2 for white matter and UNC Primate Atlas regions. Regional FA and MD plots are included in the Supplementary Figs. 1 and 2. Statistics for AD and RD are included in the Supplementary Table S2. The effective degrees of freedom (edf in Table 2) is a measure of the complexity of the smooth terms in the model. In Low-Late group demonstrated more complex curve shapes in all DTI measures compared to Early High group.
Table 2.
Summary of statistical analysis results of white matter integrity. Significance codes:.: < 0.1 *P < 0.05, **P < 0.01, ***P < 0.001.
| FA | s(Age_norm):Early-High | s(Age_norm):Late-Low | R-sq.(adj) | Intercept | TNE_Group | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ROI | edf | Ref.df | F | P -value | edf | Ref.df | F | P -value | Estimate | Std. Error | t value | Pr(>|t|) | Estimate | Std. Error | t value | Pr(>|t|) | Corrected p | ||
| Whole Brain | 1.813 | 1.813 | 7.698 | 0.000824*** | 3.032 | 3.032 | 26.628 | <2e-16*** | 0.391 | 0.379301 | 0.003771 | 100.572 | <2e-16*** | 0.009637 | 0.005345 | 1.803 | 0.0748 | . | NA |
| Cerebellum | 1.349 | 1.349 | 0.599 | 0.635907 | 2.687 | 2.687 | 6.908 | 0.000687*** | 0.204 | 0.378917 | 0.004269 | 88.759 | < 2e-16*** | 0.016125 | 0.006053 | 2.664 | 0.00918 | * * | 0.11934 |
| Cingulate | 1.922 | 1.922 | 20.8 | 1.34e-07*** | 2.274 | 2.274 | 20.68 | < 2e-16*** | 0.357 | 0.358988 | 0.005104 | 70.328 | <2e-16*** | −0.00614 | 0.007231 | −0.849 | 0.398 | 5.174 | |
| Corpus Callosum | 1.786 | 1.786 | 2.407 | 0.0604 . | 2.396 | 2.396 | 13.268 | 3.17e-06*** | 0.186 | 0.459242 | 0.006547 | 70.147 | <2e-16*** | 0.004986 | 0.009272 | 0.538 | 0.592 | 7.696 | |
| Frontal | 2.351 | 2.351 | 4.148 | 0.0185* | 2.812 | 2.812 | 12.764 | 1.48e-06*** | 0.253 | 0.394061 | 0.003953 | 99.685 | <2e-16*** | 0.009415 | 0.005598 | 1.682 | 0.0962 | . | 1.2506 |
| Insula | 2.241 | 2.241 | 8.912 | 0.000321*** | 2.525 | 2.525 | 14.418 | 1.1e-06*** | 0.324 | 0.269295 | 0.002878 | 93.557 | <2e-16*** | 0.007537 | 0.004077 | 1.849 | 0.0679 | . | 0.8827 |
| Occipital | 1.969 | 1.969 | 16.08 | 1.22e-06*** | 3.276 | 3.276 | 39.45 | <2e-16*** | 0.527 | 0.3864 | 0.003887 | 99.408 | <2e-16*** | 0.007903 | 0.005512 | 1.434 | 0.155 | 2.015 | |
| Parietal | 2.328 | 2.328 | 11.12 | 3.66e-05*** | 3.022 | 3.022 | 34.65 | <2e-16*** | 0.391 | 0.378698 | 0.004293 | 88.219 | <2e-16*** | 0.005923 | 0.006079 | 0.974 | 0.333 | 4.329 | |
| Pons and Medulla | 1 | 1 | 13.57 | 0.000396*** | 3.125 | 3.125 | 23.82 | <2e-16*** | 0.4 | 0.378703 | 0.004715 | 80.317 | <2e-16*** | 0.01753 | 0.006686 | 2.622 | 0.0103 | * | 0.1339 |
| Prefrontal | 1 | 1 | 10.8 | 0.00146** | 2.508 | 2.508 | 16.91 | 1.56e-07*** | 0.258 | 0.3549 | 0.004459 | 79.592 | <2e-16*** | 0.009183 | 0.006319 | 1.453 | 0.15 | 1.95 | |
| Subcortical | 1 | 1 | 19.18 | 3.31e-05*** | 2.972 | 2.972 | 23.82 | <2e-16*** | 0.369 | 0.357371 | 0.003898 | 91.686 | <2e-16*** | 0.010287 | 0.005527 | 1.861 | 0.0661 | . | 0.8593 |
| Temporal Audigory | 2.262 | 2.262 | 17 | 5.33e-07*** | 2.874 | 2.874 | 30.72 | <2e-16*** | 0.416 | 0.366904 | 0.004437 | 82.696 | <2e-16*** | 0.01196 | 0.006283 | 1.904 | 0.0603 | . | 0.7839 |
| Temporal Limbic | 1.553 | 1.553 | 0.245 | 0.63332 | 2.62 | 2.62 | 4.148 | 0.00754** | 0.125 | 0.360314 | 0.004483 | 80.378 | <2e-16*** | 0.012675 | 0.006352 | 1.995 | 0.0491 | * | 0.6383 |
| Temporal Visual | 1.535 | 1.535 | 3.386 | 0.0299* | 3.081 | 3.081 | 20.419 | <2e-16*** | 0.317 | 0.401309 | 0.003616 | 110.983 | <2e-16*** | 0.007141 | 0.005128 | 1.392 | 0.167 | 2.171 | |
| MD | s(Age_norm):Early-High | s(Age_norm):Late-Low | R-sq.(adj) | Intercept | TNE_Group | ||||||||||||||
| ROI | edf | Ref.df | F | P -value | edf | Ref.df | F | P-value | Estimate | Std. Error | t value | Pr(>|t|) | Estimate | Std. Error | t value | Pr(>|t|) | |||
| Whole Brain | 2.216 | 2.216 | 4.97 | 0.00826** | 3.077 | 3.077 | 5.635 | 0.00127** | 0.398 | 7.44E-04 | 6.68E-06 | 111.526 | < 2e-16*** | −5.05E-05 | 9.46E-06 | −5.335 | 7.52E-07 | * * * | NA |
| Cerebellum | 2.205 | 2.205 | 13.151 | 6.86e-06*** | 2.927 | 2.927 | 6.031 | 0.00129** | 0.426 | 6.72E-04 | 7.44E-06 | 90.28 | < 2e-16*** | −5.95E-05 | 1.05E-05 | −5.64 | 2.08E-07 | * * * | 2.70E-06 |
| Cingulate | 2.125 | 2.125 | 1.824 | 0.188 | 2.961 | 2.961 | 8.84 | 4.64e-05*** | 0.349 | 7.80E-04 | 7.79E-06 | 100.175 | < 2e-16*** | −4.48E-05 | 1.10E-05 | −4.055 | 0.000109 | * * * | 1.42E-03 |
| Corpus Callosum | 1.951 | 1.951 | 1.866 | 0.21046 | 2.562 | 2.562 | 4.519 | 0.00926** | 0.271 | 7.87E-04 | 6.13E-06 | 128.406 | < 2e-16*** | −3.33E-05 | 8.68E-06 | −3.837 | 0.000235 | * * * | 3.06E-03 |
| Frontal | 2.54 | 2.54 | 1.695 | 0.221197 | 3.216 | 3.216 | 7.127 | 0.000176*** | 0.35 | 7.30E-04 | 7.26E-06 | 100.595 | < 2e-16*** | −4.54E-05 | 1.03E-05 | −4.415 | 2.91E-05 | * * * | 3.78E-04 |
| Insula | 1 | 1 | 23.63 | 5.29e-06*** | 2.635 | 2.635 | 3.086 | 0.0292* | 0.384 | 7.82E-04 | 7.76E-06 | 100.766 | < 2e-16*** | −5.72E-05 | 1.10E-05 | −5.193 | 1.31E-06 | * * * | 1.70E-05 |
| Occipital | 1.194 | 1.194 | 12.352 | 0.000654*** | 2.838 | 2.838 | 4.944 | 0.004488** | 0.388 | 7.25E-04 | 6.67E-06 | 108.694 | < 2e-16*** | −5.14E-05 | 9.47E-06 | −5.428 | 4.99E-07 | * * * | 6.49E-06 |
| Parietal | 2.165 | 2.165 | 4.312 | 0.01553* | 2.928 | 2.928 | 5.801 | 0.00148** | 0.361 | 7.41E-04 | 7.52E-06 | 98.562 | < 2e-16*** | −5.11E-05 | 1.07E-05 | −4.796 | 6.64E-06 | * * * | 8.63E-05 |
| Pons and Medulla | 1 | 1 | 20.497 | 1.89e-05*** | 3.159 | 3.159 | 6.223 | 0.000576*** | 0.404 | 7.47E-04 | 7.31E-06 | 102.163 | < 2e-16*** | −5.42E-05 | 1.04E-05 | −5.217 | 1.20E-06 | * * * | 1.56E-05 |
| Prefrontal | 3.109 | 3.109 | 1.652 | 0.142 | 3.149 | 3.149 | 8.229 | 5.34e-05*** | 0.417 | 7.62E-04 | 7.21E-06 | 105.739 | < 2e-16*** | −5.07E-05 | 1.02E-05 | −4.966 | 3.44E-06 | * * * | 4.47E-05 |
| Subcortical | 2.027 | 2.027 | 6.646 | 0.00207** | 2.896 | 2.896 | 4.945 | 0.00415** | 0.398 | 7.54E-04 | 6.49E-06 | 116.129 | < 2e-16*** | −5.04E-05 | 9.21E-06 | −5.472 | 4.22E-07 | * * * | 5.49E-06 |
| Temporal Audigory | 1.717 | 1.717 | 12.814 | 0.00028*** | 2.771 | 2.771 | 3.654 | 0.01668* | 0.402 | 7.70E-04 | 7.33E-06 | 105.034 | < 2e-16*** | −5.65E-05 | 1.04E-05 | −5.434 | 4.90E-07 | * * * | 6.37E-06 |
| Temporal Limbic | 1 | 1 | 19.169 | 3.3e-05*** | 2.46 | 2.46 | 5.067 | 0.00468** | 0.451 | 7.98E-04 | 6.50E-06 | 122.735 | < 2e-16*** | −5.64E-05 | 9.22E-06 | −6.113 | 2.57E-08 | * * * | 3.34E-07 |
| Temporal Visual | 1.876 | 1.876 | 9.233 | 0.00125** | 2.968 | 2.968 | 5.044 | 0.00317** | 0.378 | 7.46E-04 | 6.85E-06 | 108.96 | < 2e-16*** | −5.00E-05 | 9.71E-06 | −5.152 | 1.59E-06 | * * * | 2.07E-05 |
Taken together, effects of TNE_Group on longitudinal morphometric development were not observed from 100 to 600 days after birth, while effects of Early High anesthesia were observed, resulting in relative increase in MD, AD, and RD with age.
Discussion
In this study, we analyzed the effects of multiple exposures to anesthesia on rhesus monkey brain development using longitudinal structural and diffusion MRI. We hypothesized that developmental trajectory of brain structure from late infancy to juvenile stages would be affected by earlier or higher dose exposure to anesthesia. Consistent with this hypothesis, longitudinal changes in brain development over the period studied were found in white matter integrity using the generalized additive mixed model with increases in mean, axial, and radial diffusivities associated with Early High-dose exposure of anesthesia. However, longitudinal changes in brain volume and cortical thickness were not affected by the timing and dose of anesthesia, nor was fractional anisotropy. Our results partially replicate the previous cross-sectional analysis results using the UNC-Wisconsin Neurodevelopmental Rhesus Data and Yerkes National Primate Research Center Cohort (Young et al. 2021): Widespread increases in MD previously reported agreed with our results whereas only subtle differences (not statistically significant after multiple comparison correction) were observed in FA from our longitudinal analysis. The lifespan trajectories of white matter development in NHP (Kubicki et al. 2019) also reported continuous increase in FA and decrease in MD from infancy to juvenile stages.
There are several mechanisms that could explain the white matter microstructure changes in response to repeated anesthesia (Tang et al. 2021)—(i) shrinking glial cells due to reduced activity(Anderson et al. 1996; Ostby et al. 2009), (ii) facilitated glymphatic transport and increased interstitial fluid volume fraction (DiNuzzo and Nedergaard 2017), (iii) microtubule instability and deformation of axons (Planel et al. 2009; Kohtala et al. 2016), and (iv) increased cerebrospinal fluid in extracellular space (Assaf et al. 2006). The observed lasting impairment of white matter integrity is most likely explained by microtubule instability and deformation because other mechanisms seem more relevant to transient change in white matter microstructure as observed in a recent human study (Tang et al. 2021).
From the study results, we observed that anesthetic timing/dosage impacted not FA but MD, RD, and AD. FA tends to decrease, and MD tends to increase in conditions that disrupt the integrity of white matter, such as in the presence of demyelination, axonal loss, or edema (Madden et al. 2012). Indeed, the relationship between FA and MD can be complex during brain development (Lebel and Beaulieu 2011). FA is linked to axon packing and myelination, and it has been observed to increase throughout brain white matter during childhood, adolescence, and into young adulthood. On the other hand, MD reflects water content and density. In our study, while we observed continuous increase in white matter FA as expected, the generalized additive mixed model captured the rebounding patterns of MD and RD in the early anesthesia exposure group ~6 mo (Early High-dose group) and 10 mo (Late-Low dose group) after birth when we incorporated longitudinal MRI data for the 2 yr after birth (Fig. 3). Fluctuations of MD, AD, and RD during brain development could mean decrease in axonal density or delays in white matter myelination and maturation (Feldman et al. 2010). The different rebound timings were associated with the difference in timing of the first anesthesia exposure and may also reflect the repeated and accumulated adverse effects of anesthesia on white matter integrity. Also, this difference in timing of directional diffusivity changes could make MD more sensitive to anesthetic timing/dosage compared to FA.
Early or repeated exposure to anesthesia can lead to damage in the white matter, affecting both memory functions and socio-emotional development. MRI and immunohistochemical studies using rhesus macaques with varying doses and types of anesthesia have been shown to have significant and sometimes lasting effects on white matter development in the brain (Brambrink et al. 2012a; Noguchi et al. 2017; Tounekti et al. 2018; Ikonomidou et al. 2019; Young et al. 2021; Tomlinson et al. 2023). These effects range from apoptosis to changes in microstructure and may be influenced by the duration and type of anesthesia used. Even low levels of repeated anesthesia exposure may delay white matter maturation and the effects on white matter are dose-dependent and may be influenced by the duration of anesthesia. In a study, rhesus monkeys were subjected to sevoflurane inhalation anesthesia around postnatal day 7, and subsequently on days 14 and 28, each session lasting 4 h. By the time these monkeys reached 12 to 18 and 24 to 30 mo of age, they displayed significant impairments in visual recognition memory, evident from their reduced attention to new images (Alvarado et al. 2017). Moreover, by 6 mo of age, this same group exhibited a notably higher frequency of anxiety-related behaviors compared to unexposed controls (Raper et al. 2015). This data suggests that early and repeated exposure to sevoflurane in infant nonhuman primates can induce an anxiety-prone disposition, noticeable from 6 mo and persisting for at least up to 2 yr (Raper et al. 2018). In human study, uncinate structural integrity at 3-mo old was the most robust predictor of negative emotionality at 9-mo old (Banihashemi et al. 2020) and increased RD and decreased FA in the cingulum-callosal regions linked to greater emotional dysregulation in the children among children (mean age = 9.53 yr old) (Hung et al. 2020). Our study observed relative increase in RD in the Early High anesthesia exposure group, suggesting a potential relationship between white matter disruption and adverse effects on emotional development. Anesthesia is routinely provided in children using volatile anesthetic gas. The isoflurane used in this NHP MRI study is the same anesthetic used for general anesthesia in young children. In clinical practice an inhalational induction with sevoflurane is routinely employed in children as sevoflurane is less pungent and causes less airway irritation and laryngospasm. Following induction with sevoflurane, the anesthetic is commonly changed to isoflurane for maintenance anesthesia. The inspired concentrations of 1.5 vol% isoflurane used for maintenance of anesthesia during the 2-hr MRI scans in this study are similar to concentrations used to maintain general anesthesia in children for procedures, imaging, and surgery (Rosenbaum et al. 2021). In this study, ketamine was also used to facilitate the induction of general anesthesia in the rhesus monkeys. Ketamine can be administered either intravenous or intramuscular for sedation or for general anesthesia and is more rapidly metabolized in the young and may require higher dosing (Brinck et al. 2018; Dadiomov and Lee 2019). In this NHP MRI study, a single intramuscular dose of ketamine of 10 mg/kg was used. This is comparable to the intramuscular dosing range of 9 to 13 mg/kg used in children to provide general anesthesia (Dadiomov and Lee 2019). However, the combination of ketamine at the higher surgical dose and isoflurane for general anesthesia is not very often employed in children in clinical practice. It is also important to note that this level of general anesthesia is commonly used to mitigate the effects of pain and surgery. Surgery and anesthesia may have a different outcome on brain development when compared to anesthesia alone, although this was not investigated here (Ririe 2015; Ririe et al. 2021).
In this study, the exposure timing and dose of the anesthetic were not associated with changes in longitudinal trajectories in brain volume or cortical thickness. This contradicts previously reported findings using a similar MRI dataset of potential effects of exposure to anesthesia on volumetric development during infancy with an volumetric dip between 5 and 9 mo of age (Malkova et al. 2006; Scott et al. 2016; Kim et al. 2020). Data from the current study confirms the previous findings and suggest that the volume dip during infancy of rhesus monkeys is likely a part of normal brain development and not related to anesthesia exposure. This difference in findings is partly a consequence of lack of a control group that was not exposed to anesthesia in the previous study leading to concluding the anesthesia was related to the volumetric dip. Furthermore, the previous study included a group of animals exposed to other types of sedatives that may have confounded the results. This part of the cohort was removed in this study to make the cohort anesthesia regimens for all animals similar. Additionally, in this study the varied exposures to and timing of anesthetics permitted resolving the effects of development from the effects related to anesthesia that were otherwise difficult to isolate because of the lack of control group not exposed to anesthesia. Explanations for the transient decrease in intracranial volume reported in previous cross-sectional studies have proposed weaning of infants as a potential etiology, with restricted access to nursing, along with increased physical activity and nutritional status changes (Vandeleest and Capitanio 2012). Other studies have also reported transient decreases in the intracranial volume between 5 and 9 mo of age regardless of the timing of weaning (after birth vs. 6–7 mo), housing conditions (individually housed vs. grouped with peers), size of the housing (half-acre enclosures vs. 0.25–0.73 m3) and the age of first MRI scan (Malkova et al. 2006; Scott et al. 2016). Further studies incorporating longer-term follow-up and less frequent and low anesthesia dose should confirm these findings on morphometric development in rhesus monkeys.
There are limitations of this study. Due to the age range differences between groups during the longitudinal MRI scans, we only focused on the limited age range between 100 and 600 days after birth for more reliable comparison. These limited data may not properly reflect the brain developmental trajectories earlier than 100 days or later than 600 days after birth. As reported in the previous study (Kim et al. 2020), for regional volume measures, we have only one source atlas that was derived from the study population itself and, therefore, we chose direct label propagation. However, it is known that the quality of label map propagation may be greatly improved when using multiple source atlases (Rohlfing et al. 2004; Klein et al. 2009), a process commonly referred to as multiatlas segmentation and label fusion. Robust tissue segmentation of the monkey brain is still challenging due to low signal-to-noise ratio around subcortical regions and strong susceptibility artifacts around the olfactory cortex and occipital lobes, which require manual segmentation. Segmentation of small fiber tracts around the occipital, temporal auditory, and internal and external capsule regions was less accurate, which could lead to underestimation of white matter volume. Learning-based algorithms based on reliable training dataset could improve the accuracy of tissue segmentation. Field maps to correct the geometric distortion of DWI were not available from the dataset; thus, registration errors could have occurred during ROI-wise analysis. In addition, even though all monkeys were oriented identically within a stereotaxic platform, geometric distortion mainly affected prefrontal and occipital regions, the impact of which may be worse on the younger brains than on the older ones. Temporal regions demonstrated a low signal-to-noise ratio, resulting in underestimation of FA. It is important to consider these limitations when interpreting the projected trajectories and the noise in these data. Other limitations are related to the anesthesia exposure. As noted, the combined exposure to both ketamine and isoflurane makes it difficult to understand the role of each or if the findings only occur when the drugs are used in combination. Furthermore, the inability to obtain imaging in the absence of anesthesia precludes the no anesthesia control group. Other limitations are the inability to understand how surgery may alter the brain integrity during development and the physiologic or behavioral impact of the changes in brain integrity.
In summary, Early High-dose exposure to multiple anesthesia events during infancy could impair white matter microstructural integrity. Whether this is due to ketamine, isoflurane, or the combination is not clear. Further studies to isolate effects of isoflurane and ketamine alone on the MD will be needed to better understand these changes. The behavioral implications of the differences in brain development structural integrity are not clear. This will require behavioral analysis to directly make correlations to clinical findings in the human related to outcomes such as attentional dysfunction, for example, related to multiple and early exposures to anesthesia. Furthermore, it is unclear how surgery combined with anesthesia may alter these findings on brain development. Despite these limitations, anesthesia dose and age of first exposure should be considered in clinical decision making for nontime sensitive surgery and procedures requiring anesthesia. Future clinical studies incorporating longer-term follow-up images, neuropsychological assessments, and specific anesthetics will elucidate more details of the effects of anesthesia on brain development.
Supplementary Material
Contributor Information
Jeongchul Kim, Radiology Informatics and Image Processing Laboratory (RIIPL), Wake Forest School of Medicine, Winston-Salem, NC, United States; Department of Radiology, Wake Forest School of Medicine, Winston-Salem, NC, United States; School of Biomedical Engineering and Sciences, Virginia Tech-Wake Forest University, Winston-Salem, NC, United States.
Richard Barcus, Radiology Informatics and Image Processing Laboratory (RIIPL), Wake Forest School of Medicine, Winston-Salem, NC, United States; Department of Radiology, Wake Forest School of Medicine, Winston-Salem, NC, United States.
Megan E Lipford, Radiology Informatics and Image Processing Laboratory (RIIPL), Wake Forest School of Medicine, Winston-Salem, NC, United States; Department of Radiology, Wake Forest School of Medicine, Winston-Salem, NC, United States; School of Biomedical Engineering and Sciences, Virginia Tech-Wake Forest University, Winston-Salem, NC, United States.
Hongyu Yuan, Radiology Informatics and Image Processing Laboratory (RIIPL), Wake Forest School of Medicine, Winston-Salem, NC, United States; Department of Radiology, Wake Forest School of Medicine, Winston-Salem, NC, United States.
Douglas G Ririe, Pain Mechanisms Lab, Department of Anesthesiology, Wake Forest School of Medicine, Winston-Salem, NC, United States.
Youngkyoo Jung, Department of Biomedical Engineering, University of California Davis, Davis, CA, United States.
Roza M Vlasova, Department of Psychiatry, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States.
Martin Styner, Department of Psychiatry, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States.
Michael A Nader, Department of Physiology and Pharmacology, Wake Forest School of Medicine, Winston-Salem, NC, United States; Center for Research on Substance Use and Addiction, Wake Forest School of Medicine, Winston-Salem, NC, United States; Clinical and Translational Science Institute, Wake Forest School of Medicine, Winston-Salem, NC, United States.
Christopher T Whitlow, Radiology Informatics and Image Processing Laboratory (RIIPL), Wake Forest School of Medicine, Winston-Salem, NC, United States; Department of Radiology, Wake Forest School of Medicine, Winston-Salem, NC, United States; School of Biomedical Engineering and Sciences, Virginia Tech-Wake Forest University, Winston-Salem, NC, United States; Center for Research on Substance Use and Addiction, Wake Forest School of Medicine, Winston-Salem, NC, United States; Clinical and Translational Science Institute, Wake Forest School of Medicine, Winston-Salem, NC, United States; Department of Biostatistics and Data Science, Wake Forest School of Medicine, Winston-Salem, NC, United States.
Credit taxonomy
CRediT TaxonomyJeongchul KimFormal analysis, Methodology, Supervision, Writing – original draftRichard BarcusData curation, Software, Validation, Writing – review & editingMegan LipfordFormal analysis, Validation, Writing – review & editingHongyu YuanData curation, Methodology, Software, Validation, Visualization, Writing – review & editingDoug RirieSupervision, Validation, Writing – review & editingYoungkyoo JungSupervision, Validation, Writing – review & editingRoza VlasovaResources, Validation, Writing – review & editingMartin StynerResources, Validation, Writing – review & editingMichael NaderSupervision, Writing – review & editingChristopher WhitlowConceptualization, Project administration, Supervision, Writing – review & editing
Funding
This study received partial support from several institutions. These include the National Institute of Neurological Disorders and Stroke (grants R03 NS118259 to JK and NS091602 to CTW), the National Institute of Aging (grant AG073199 to CTW), the National Institute of Mental Health (grant R01 MH116675 to CTW), and the National Institute of Drug Abuse (grant P50 DA006634 to MAN).
Conflict of interest statement: None declared.
References
- Aggarwal N, Moody JF, Dean DC, Tromp DPM, Kecskemeti SR, Oler JA, Alexander AL, Kalin NH. Spatiotemporal dynamics of nonhuman primate white matter development during the first year of life. NeuroImage. 2021:231:117825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado MC, Murphy KL, Baxter MG. Visual recognition memory is impaired in rhesus monkeys repeatedly exposed to sevoflurane in infancy. Br J Anaesth. 2017:119:517–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AW, Zhong J, Petroff OA, Szafer A, Ransom BR, Prichard JW, Gore JC. Effects of osmotically driven cell volume changes on diffusion-weighted imaging of the rat optic nerve. Magn Reson Med. 1996:35:162–167. [DOI] [PubMed] [Google Scholar]
- Andropoulos DB, Greene MF. Anesthesia and developing brains – implications of the FDA warning. N Engl J Med. 2017:376:905–907. [DOI] [PubMed] [Google Scholar]
- Assaf Y, Ben-Sira L, Constantini S, Chang LC, Beni-Adani L. Diffusion tensor imaging in hydrocephalus: initial experience. AJNR Am J Neuroradiol. 2006:27:1717–1724. [PMC free article] [PubMed] [Google Scholar]
- Banihashemi L, Bertocci MA, Alkhars HM, Versace A, Northrup JB, Lee VK, Schmithorst VJ, Samolyk A, Taylor M, English GE, et al. Limbic white matter structural integrity at 3 months prospectively predicts negative emotionality in 9-month-old infants: a preliminary study. J Affect Disord. 2020:273:538–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethlehem RAI, Seidlitz J, White SR, Vogel JW, Anderson KM, Adamson C, Adler S, Alexopoulos GS, Anagnostou E, Areces-Gonzalez A, et al. Brain charts for the human lifespan. Nature. 2022:604:525–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambrink AM, Back SA, Riddle A, Gong X, Moravec MD, Dissen GA, Creeley CE, Dikranian KT, Olney JW. Isoflurane-induced apoptosis of oligodendrocytes in the neonatal primate brain. Ann Neurol. 2012a:72:525–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambrink AM, Evers AS, Avidan MS, Farber NB, Smith DJ, Martin LD, Dissen GA, Creeley CE, Olney JW. Ketamine-induced neuroapoptosis in the fetal and neonatal rhesus macaque brain. Anesthesiology. 2012b:116:372–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambrink AM, Evers AS, Avidan MS, Farber NB, Smith DJ, Zhang X, Dissen GA, Creeley CE, Olney JW. Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology. 2010:112:834–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinck EC, Tiippana E, Heesen M, Bell RF, Straube S, Moore RA, Kontinen V. Perioperative intravenous ketamine for acute postoperative pain in adults. Cochrane Database Syst Rev. 2018:12:CD012033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman K, Robertson ND, Dissen GA, Neuringer MD, Martin LD, Cuzon Carlson VC, Kroenke C, Fair D, Brambrink AM. Isoflurane Anesthesia has long-term consequences on motor and Behavioral development in infant rhesus macaques. Anesthesiology. 2017:126:74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creeley C, Dikranian K, Dissen G, Martin L, Olney J, Brambrink A. Propofol-induced apoptosis of neurones and oligodendrocytes in fetal and neonatal rhesus macaque brain. Br J Anaesth. 2013:110(Suppl 1):i29–i38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creeley CE, Dikranian KT, Dissen GA, Back SA, Olney JW, Brambrink AM. Isoflurane-induced apoptosis of neurons and oligodendrocytes in the fetal rhesus macaque brain. Anesthesiology. 2014:120:626–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadiomov D, Lee K. The effects of ketamine on suicidality across various formulations and study settings. Ment Health Clin. 2019:9:48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNuzzo M, Nedergaard M. Brain energetics during the sleep-wake cycle. Curr Opin Neurobiol. 2017:47:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA . Drug safety communication: FDA review results in new warnings about using general anesthetics and sedation drugs in young children and pregnant women. In. 2017. Available from: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-approves-label-changes-use-general-anesthetic-and-sedation-drugs.
- Fehr T, Janssen WGM, Park J, Baxter MG. Neonatal exposures to sevoflurane in rhesus monkeys alter synaptic ultrastructure in later life. iScience. 2022:25:105685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman HM, Yeatman JD, Lee ES, Barde LH, Gaman-Bean S. Diffusion tensor imaging: a review for pediatric researchers and clinicians. J Dev Behav Pediatr. 2010:31:346–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flick RP, Katusic SK, Colligan RC, Wilder RT, Voigt RG, Olson MD, Sprung J, Weaver AL, Schroeder DR, Warner DO. Cognitive and behavioral outcomes after early exposure to anesthesia and surgery. Pediatrics. 2011:128:e1053–e1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatz P, Sandin RH, Pedersen NL, Bonamy AK, Eriksson LI, Granath F. Association of Anesthesia and Surgery during childhood with long-term academic performance. JAMA Pediatr. 2017:171:e163470. [DOI] [PubMed] [Google Scholar]
- Graham MR, Brownell M, Chateau DG, Dragan RD, Burchill C, Fransoo RR. Neurodevelopmental assessment in kindergarten in children exposed to general Anesthesia before the age of 4 years: a retrospective matched cohort study. Anesthesiology. 2016:125:667–677. [DOI] [PubMed] [Google Scholar]
- Hung Y, Uchida M, Gaillard SL, Woodworth H, Kelberman C, Capella J, Kadlec K, Goncalves M, Ghosh S, Yendiki A, et al. Cingulum-Callosal white-matter microstructure associated with emotional dysregulation in children: a diffusion tensor imaging study. Neuroimage Clin. 2020:27:102266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomidou C, Kirvassilis G, Swiney BS, Wang SH, Huffman JN, Williams SL, Masuoka K, Capuano S 3rd, Brunner KR, Crosno K, et al. Mild hypothermia ameliorates anesthesia toxicity in the neonatal macaque brain. Neurobiol Dis. 2019:130:104489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Istaphanous GK, Loepke AW. General anesthetics and the developing brain. Curr Opin Anaesthesiol. 2009:22:368–373. [DOI] [PubMed] [Google Scholar]
- Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003:23:876–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Jung Y, Barcus R, Bachevalier JH, Sanchez MM, Nader MA, Whitlow CT. Rhesus macaque brain developmental trajectory: a longitudinal analysis using tensor-based structural morphometry and diffusion tensor imaging. Cereb Cortex. 2020:30:4325–4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang MC, Christensen GE, Collins DL, Gee J, Hellier P, et al. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. NeuroImage. 2009:46:786–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohtala S, Theilmann W, Suomi T, Wigren HK, Porkka-Heiskanen T, Elo LL, Rokka A, Rantamaki T. Brief isoflurane Anesthesia produces prominent Phosphoproteomic changes in the adult mouse hippocampus. ACS Chem Neurosci. 2016:7:749–756. [DOI] [PubMed] [Google Scholar]
- Kubicki M, Baxi M, Pasternak O, Tang Y, Karmacharya S, Chunga N, Lyall AE, Rathi Y, Eckbo R, Bouix S, et al. Lifespan trajectories of White matter changes in rhesus monkeys. Cereb Cortex. 2019:29:1584–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Beaulieu C. Longitudinal development of human brain wiring continues from childhood into adulthood. J Neurosci. 2011:31:10937–10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Bennett IJ, Burzynska A, Potter GG, Chen NK, Song AW. Diffusion tensor imaging of cerebral white matter integrity in cognitive aging. Biochim Biophys Acta. 2012:1822:386–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkova L, Heuer E, Saunders RC. Longitudinal magnetic resonance imaging study of rhesus monkey brain development. Eur J Neurosci. 2006:24:3204–3212. [DOI] [PubMed] [Google Scholar]
- Noguchi KK, Johnson SA, Dissen GA, Martin LD, Manzella FM, Schenning KJ, Olney JW, Brambrink AM. Isoflurane exposure for three hours triggers apoptotic cell death in neonatal macaque brain. Br J Anaesth. 2017:119:524–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary JD, Janus M, Duku E, Wijeysundera DN, To T, Li P, Maynes JT, Crawford MW. A population-based study evaluating the association between surgery in early life and child development at primary school entry. Anesthesiology. 2016:125:272–279. [DOI] [PubMed] [Google Scholar]
- O'Leary JD, Janus M, Duku E, Wijeysundera DN, To T, Li P, Maynes JT, Faraoni D, Crawford MW. Influence of surgical procedures and general Anesthesia on child development before primary school entry among matched sibling pairs. JAMA Pediatr. 2019:173:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostby I, Oyehaug L, Einevoll GT, Nagelhus EA, Plahte E, Zeuthen T, Lloyd CM, Ottersen OP, Omholt SW. Astrocytic mechanisms explaining neural-activity-induced shrinkage of extraneuronal space. PLoS Comput Biol. 2009:5:e1000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planel E, Bretteville A, Liu L, Virag L, Du AL, Yu WH, Dickson DW, Whittington RA, Duff KE. Acceleration and persistence of neurofibrillary pathology in a mouse model of tauopathy following anesthesia. FASEB J. 2009:23:2595–2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbitts JA, Groenewald CB. Epidemiology of Pediatric surgery in the United States. Paediatr Anaesth. 2020:30:1083–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper J, Alvarado MC, Murphy KL, Baxter MG. Multiple Anesthetic exposure in infant monkeys alters emotional reactivity to an acute stressor. Anesthesiology. 2015:123:1084–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper J, De Biasio JC, Murphy KL, Alvarado MC, Baxter MG. Persistent alteration in behavioural reactivity to a mild social stressor in rhesus monkeys repeatedly exposed to sevoflurane in infancy. Br J Anaesth. 2018:120:761–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ririe DG. How long does incisional pain last: early life vulnerability could make it last a lifetime. Anesthesiology. 2015:122:1189–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ririe DG, Eisenach JC, Martin TJ. A painful beginning: early life surgery produces long-term behavioral disruption in the rat. Front Behav Neurosci. 2021:15:630889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlfing T, Russakoff DB, Maurer CR Jr. Performance-based classifier combination in atlas-based image segmentation using expectation-maximization parameter estimation. IEEE Trans Med Imaging. 2004:23:983–994. [DOI] [PubMed] [Google Scholar]
- Rosenbaum SB, Gupta V, Patel P, et al. Ketamine. [Updated 2023 May 26]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan. Available from: 10.1038/s41526-020-0103-2. [DOI]
- Scott JA, Grayson D, Fletcher E, Lee A, Bauman MD, Schumann CM, Buonocore MH, Amaral DG. Longitudinal analysis of the developing rhesus monkey brain using magnetic resonance imaging: birth to adulthood. Brain Struct Funct. 2016:221:2847–2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slikker W Jr, Zou X, Hotchkiss CE, Divine RL, Sadovova N, Twaddle NC, Doerge DR, Scallet AC, Patterson TA, Hanig JP, et al. Ketamine-induced neuronal cell death in the perinatal rhesus monkey. Toxicol Sci. 2007:98:145–158. [DOI] [PubMed] [Google Scholar]
- Sorensen O, Walhovd KB, Fjell AM. A recipe for accurate estimation of lifespan brain trajectories, distinguishing longitudinal and cohort effects. NeuroImage. 2021:226:117596. [DOI] [PubMed] [Google Scholar]
- Styner M, Knickmeyer R, Joshi S, Coe C, Short SJ, Gilmore J. Automatic brain segmentation in rhesus monkeys, medical imaging. Proc. SPIE 6512, Medical Imaging 2007: Image Processing, 65122L (8 March 2007); 2007. p. 8. [Google Scholar]
- Tang CY, Wang VX, Lun MY, Mincer JS, Ng JC, Brallier JW, Schwartz AE, Ahn H, McCormick PJ, Nir T, et al. Transient changes in white matter microstructure during general anesthesia. PLoS One. 2021:16:e0247678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson C, Vlasova R, Al-Ali K, Young JT, Shi Y, Lubach GR, Alexander AL, Coe CL, Styner M, Fine J. Effects of anesthesia exposure on postnatal maturation of white matter in rhesus monkeys. Dev Psychobiol. 2023:65:e22396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tounekti S, Troalen T, Bihan-Poudec Y, Froesel M, Lamberton F, Ozenne V, Clery J, Richard N, Descoteaux M, Ben Hamed S, et al. High-resolution 3D diffusion tensor MRI of anesthetized rhesus macaque brain at 3T. NeuroImage. 2018:181:149–161. [DOI] [PubMed] [Google Scholar]
- Tustison N, Avants B, Cook P, Song G, Das S, Strien N, Stone J, Gee J. The ANTs cortical thickness processing pipeline. Proc. SPIE 8672, Medical Imaging 2013: Biomedical Applications in Molecular, Structural, and Functional Imaging, 86720K (29 March 2013). 2013. [Google Scholar]
- Vandeleest JJ, Capitanio JP. Birth timing and behavioral responsiveness predict individual differences in the mother-infant relationship and infant behavior during weaning and maternal breeding. Am J Primatol. 2012:74:734–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder RT, Flick RP, Sprung J, Katusic SK, Barbaresi WJ, Mickelson C, Gleich SJ, Schroeder DR, Weaver AL, Warner DO. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology. 2009:110:796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JT, Shi Y, Niethammer M, Grauer M, Coe CL, Lubach GR, Davis B, Budin F, Knickmeyer RC, Alexander AL, et al. The UNC-Wisconsin rhesus macaque neurodevelopment database: a structural MRI and DTI database of early postnatal development. Front Neurosci. 2017:11:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JT, Vlasova RM, Howell BR, Knickmeyer RC, Morin E, Kuitchoua KI, Lubach GR, Noel J, Hu X, Shi Y, et al. General anaesthesia during infancy reduces white matter micro-organisation in developing rhesus monkeys. Br J Anaesth. 2021:126:845–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.