Abstract
Current theories of attention differentiate exogenous from endogenous orienting of visuospatial attention. While both forms of attention orienting engage different functional systems, endogenous and exogenous attention are thought to share resources, as shown by empirical evidence of their functional interactions. The present study aims to uncover the neurobiological basis of how salient events that drive exogenous attention disrupts endogenous attention processes. We hypothesize that interference from exogenous attention over endogenous attention involves alpha-band activity, a neural marker of visuospatial attention. To test this hypothesis, we contrast the effects of endogenous attention across two experimental tasks while we recorded electroencephalography (n = 32, both sexes): a single cueing task where endogenous attention is engaged in isolation, and a double cueing task where endogenous attention is concurrently engaged with exogenous attention. Our results confirm that the concurrent engagement of exogenous attention interferes with endogenous attention processes. We also found that changes in alpha-band activity mediate the relationship between endogenous attention and its effect on task performance, and that the interference of exogenous attention on endogenous attention occurs via the moderation of this indirect effect. Altogether, our results substantiate a model of attention, whereby endogenous and exogenous attentional processes involve the same neurophysiological resources.
Significance Statement
Scientists differentiate top-down from bottom-up visuospatial attention processes. While bottom-up attention is rapidly engaged by emerging demands from the environment, top-down attention in contrast reflects slow voluntary shifts of spatial attention. Several lines of research substantiate the idea that top-down and bottom-up attentional processes involve distinct functional systems. An increasing number of studies, however, argue that both attention systems share brain processing resources. The current study examines how salient visual events that engage bottom-up processes interfere with top-down attentional processes. Using neurophysiological recordings and multivariate pattern classification techniques, the authors show that these patterns of interference occur within the alpha-band of neural activity (8–12 Hz), which implies that bottom-up and top-down attention processes share this narrow-band frequency brain resource. The results further demonstrate that patterns of alpha-band activity explains, in part, the interference between top-down and bottom-up attention at the behavioral level.
Keywords: Visuospatial attention, Exogenous orienting, Endogenous orienting, Electroencephalography, Multivariate statistics, Pattern classification, Event-related potentials, Alpha patterns
Introduction
We rely on visuospatial attention to accomplish many everyday tasks. For example, when reading a journal article, we use goal-oriented attention to select and process the information written on the page. However, this process can be interrupted by a salient stimulus, like a notification on our phone. Salient events can therefore engage attention resources and interfere with ongoing goal-oriented attention. The present work explores the neural substrate of these interference effects by investigating how salient events that engage bottom-up attention disrupt top-down attention.
Research typically differentiates between at least two modes of control for visuospatial attention: Endogenous (i.e. top-down) orienting that reflects the voluntary control of attention and exogenous (i.e. bottom-up) orienting that corresponds to the involuntary shift of attention triggered by salient sensory events (Carrasco 2011). Exogenous and endogenous attention are distinct functional processes that engage overlapping brain systems, as evidenced by a large body of findings (Corbetta and Shulman 2002; Corbetta et al. 2008; Chica et al. 2013; Buschman and Kastner 2015). Indeed, several differences distinguish exogenous and endogenous control of attention (Wright and Ward 2008). For example, while endogenous attention is slow to engage a target stimulus, exogenous attention shifts rapidly. Exogenous attention is also uniquely characterized by a significant delay before re-engaging to the same stimulus twice—a phenomenon called inhibition of return (Klein 2000). Conversely, cognitive load manipulations that draw upon top-down resources selectively impair endogenous attention (Jonides 1981). The prevailing view is therefore of separable visuospatial attention systems at the functional level.
Still, several studies substantiate instances where exogenous and endogenous attention processes interact with each other (Berger et al. 2005; Grubb et al. 2015). For example, the abrupt onset of a salient event that engages exogenous attention can disrupt endogenous attention processes–like a notification on your phone in the middle of reading this paragraph (Yantis and Jonides 1990). These disruptions occur within and across sensory modalities (van der Lubbe and Postma 2005). In the same vein, one study reports interactions between exogenous and endogenous attention when task demands are high, e.g. when a visual target is difficult to discriminate. Behavioral responses of participants in this context are compatible with the idea that exogenous and endogenous attention represent distinct functional systems capable of mutual interference (Berger et al. 2005). Evidence from electroencephalography (EEG) further corroborates these findings. Specifically, both endogenous and exogenous attention influence the same EEG components during visual processing, thereby revealing that the underlying neural correlates are not fully separable (Hopfinger and West 2006; Landry et al. 2023). Specifically, evidence indicates that these attention processes have distinct effects on early target-locked event-related potentials (ERPs) such as P1 and N1. However, they both influence later visual ERP components like N2 and P3 in a similar manner. These findings align with previous work showing that the concurrent engagement of exogenous and endogenous attention results in interactions within the extrastriate visual area (Busse et al. 2008). This overlap between them at the neural level putatively accounts for patterns of mutual interference when they are both concurrently engaged. They both recruit the same neural resources, which may result in interference. In sum, evidence reveals that both attention processes interact with each other in various contexts, thereby implying that they share common neurophysiological resources.
The present study aims to better understand interactions between both attention processes by focusing on the interference of exogenous attention on endogenous attention. We hypothesized that this interference effect would involve alpha-band (8–12 Hz) brain activity. This hypothesis derives from previous work showing that when participants orient their attention towards one hemi-field, both attention systems exhibit similar patterns of lateralized alpha-band brain activity (Foxe et al. 1998; Störmer et al. 2016; Foster et al. 2017; Keefe and Störmer 2021). Here, alpha-band activity is thought to reflect modulations of cortical excitability, a central neural mechanism for the selection of attended sensory inputs and the suppression of unattended ones (Haegens et al. 2011; Händel et al. 2011; Klimesch 2012; Gutteling et al. 2022). Evidence support this viewpoint, including findings from a recent study indicating that changes in alpha power in the occipital cortex plays a causal role in visuospatial attention (Bagherzadeh et al. 2020).
In Bagherzadeh and colleagues’ study, participants were trained to alter lateralized patterns of alpha power over the posterior regions of the brain using neurofeedback. Critically, neurofeedback training was done independently from task-based shifts of visuospatial attention. Participants showed overall shifts of visuospatial attention thereafter, which implies that these changes in alpha-band activity led to implicit and automatic shifts of visuospatial attention. This outcome is consistent with studies showing that neural entrainment in the range of alpha rhythms using transcranial altering current stimulation modulates visuospatial attention (Kasten et al. 2020; Kemmerer et al. 2022a; Kemmerer et al. 2022b). This work emphasizes the centrality and potential causality of alpha-band activity for visuospatial attention. In contrast to this view, however, some have interrogated the methodology and design of Bagherzadeh and colleagues’ study (Gundlach and Forschack 2020). This challenge aligns with recent findings that undermine the notion that alpha-band activity is causality related to visuospatial attention (Morrow et al. 2023). In particular, leveraging frequency-tagging techniques, some of these investigations suggest that modulations of alpha power, which occur concurrently with shifts in visuospatial attention, are distinct from the active processes underlying selective attention (Antonov et al. 2020; Gundlach et al. 2020). As a result, sensory-level alpha-band activity might be more accurately described as a consequence, rather than the cause, of visuospatial attention (Peylo et al. 2021).
Cause or consequence, alpha-band activity remains a reliable neurophysiological marker of visuospatial attention (Wang et al. 2023). We therefore expect that exogenous attention will interact with endogenous attention at the level of alpha-band neurophysiological signaling because both attention processes engage this marker. To test our hypothesis, we evaluated the effects of endogenous attention across two different tasks: A first one, the single-cueing task, where endogenous attention was engaged in isolation, and a second one, the double cueing task, where endogenous attention was concurrently engaged with exogenous attention (Fig. 1). We expected to observe interference when comparing the effects of endogenous attention between the single and double tasks. This expectation derives from previous research showing that the presence of a task-irrelevant salient event hampers performance relying on endogenous attention processes (e.g. Theeuwes 1992). It also follows from results that highlight interactions between exogenous and endogenous attention processes, suggesting that both attention processes are capable of mutual interference (Berger et al. 2005). Furthermore, based on our hypothesis, we also expected that changes in alpha power would mediate the relationship between endogenous cueing and task performance, while the concurrent engagement of exogenous attention would moderate this mediation effect. We therefore tested this model. We expected that evidence would show that exogenous attention interferes with endogenous attention processing due to conflicting activity patterns within the same neural population when both attention processes are directed towards opposite hemifields. Specifically, while endogenous attention leads to decreased power in alpha rhythms at the contralateral posterior region (indicating increased neural excitation), the orienting of exogenous attention to the opposite hemifield induces increased alpha power in the same neural region (indicating increased neural inhibition). We designed our approach to evaluate this hypothesis.
Fig. 1.
(A. Single-cueing of endogenous attention. Each trial began with a fixation screen, followed by the onset of the endogenous cue after a random interval. The endogenous cue remained on screen until the end of trial. Next, a Gabor stimulus was shown at the left or right location. (B) Double cueing task comprising both exogenous and endogenous cueing. The trials began with a fixation screen followed by the endogenous cue, then the exogenous cue, and finally the target event onset at the left or right location. The endogenous cue was predictive of the target’s location in both cueing tasks, while exogenous cue validity was set to chance level. For both tasks (A and B), each trial ended with the participant’s discrimination response for the orientation of the Gabor stimulus (clockwise versus counter-clockwise). In both cueing tasks, onsets of the endogenous cue, the exogenous cue, and the target stimulus were time jittered. Diagram at the top right corner shows that the double cueing task divides into trials where both cues indicated the same location (i.e. congruent exogenous cues) and trials where both cues indicated opposite locations (i.e. incongruent exogenous cues). The plots show group (dots) and participants (gray lines) averaged accurate RTs for endogenous cue validity across single cueing (C), double cueing with congruent exogenous cues (D), and double cueing with incongruent exogenous cues (E). Error bars represent bootstrapped 95% C.I. Bottom graphs indicate single-trial hierarchical regression model coefficients and corresponding 95% C.I. For single cueing (F), we included endogenous cue validity (dummy coding was 0 for cue invalid and 1 for cue valid) as a fixed predictor for predicting correct RTs. For double cueing, we evaluated endogenous cue validity across single versus double cueing with congruent exogenous cues (G) and incongruent exogenous cues (H). In both instances, we included endogenous cue validity, cueing task (i.e. single versus double) and their interaction as fixed factors.
Methods
Participants
We recruited 42 participants based on convenience sampling. Participants received monetary compensation of $10/hour for completing 4 different tasks. Each task consisted of 384 trials. Participants performed 10 practice trials before each task. In the current study, we analyzed data from 3 tasks: the non-cueing task, the endogenous cueing task, and the double cueing task comprising both exogenous and endogenous cues (Fig. 1). The experiment was a single testing session and lasted approximately 2 hours. All participants had normal or corrected-to-normal vision and provided consent. The experimental protocol was approved by the McGill Research Ethics Board.
Seven participants were excluded due to poor EEG data quality. One participant did not complete all tasks. We also excluded one participant due to below chance discrimination accuracy rate (~40% averaged across all tasks) and one participant due to a high volume of timeout errors (response times > 1500 ms on ~ 11% of trials overall). The final sample included 32 individuals (24 females and 8 males, Mean age = 21.9 (SD = 2.6)). Our sample size follows from previous work using the double cueing experimental approach: Effect size estimates of task performance suggest that 6 participants are required to achieve a power of 0.8 for the within-individual cueing effects of exogenous and endogenous orienting in a target discrimination task for alpha = 0.05 (Landry et al. 2021). The sample size of the current study is therefore adequate to detect the effect of attention at the behavioral level. Furthermore, our sample size is almost twice that of previous EEG experiments investigating exogenous and endogenous orienting together (Hopfinger and West 2006; Keefe and Störmer 2021).
Stimuli, Apparatus & Design. Participants viewed stimuli on a 24-in BenQ G2420HD monitor sitting approximately 60 cm away. Stimulus presentation used MATLAB R2015b (Mathworks Inc., Natick, MA, USA) and the third version of the Psychophysics toolbox (Brainard 1997; Pelli 1997; Kleiner et al. 2007). The screen’s refresh rate was set to 75 Hz. Except for the target, all stimuli were black (i.e. RGB values of 0, 0, 0; 1.11 cd/m2) and white (i.e. RGB values of 255, 255, 255; 218.8 cd/m2) drawings on a gray background (i.e. RGB values of 128, 128, 128; 70.88 cd/m2). The fixation marker was an empty circle made from a black line drawing with a radius of 1.2° located in the center of the screen. Two target placeholders were located at 8.7° on each side of the fixation marker on the left and right side of the screen. The placeholders were located close to the fixation to minimize saccades. These placeholders were made from black line drawings of empty circles with a 2.4° radius. The targets were sinusoidal black and white gratings combined with a Gaussian envelope. Spatial frequency was set to 3 cpd. Target stimuli were tilted 5° degrees clockwise or counter-clockwise. We cued endogenous attention by coloring the inside of the fixation marker, wherein the right or left half of the circle was shaded in black, and the other half in white. The side of the fixation marker that turned white indicated where the target was likely to occur. For example, if the right side turned white, the target was 66.6% likely to appear in the right placeholder. We opted to color the inside of the fixation marker to avoid using overlearned directional cues, like an arrow, to engage endogenous attention (Ristic and Landry 2015). Participants were aware of these cue-target contingencies. In our previous work, we had observed robust cueing effects using similar cue-target contingency (66.6% predictive cue validity) for endogenous attention in the context of target discrimination task (Landry et al. 2021). We opted for 66.6% cue-target contingency to maximize the number of endogenous invalid trials. Note that a different cue-target contingency for endogenous attention could have influenced the degree of interference from exogenous attention (e.g. Sawaki and Luck 2013). However, the present work aimed to assess whether such interference encompass modulations of alpha-band activity—a neural marker of visuospatial attention. In this way, our experimental approach was reliable in generating an endogenous cueing effect and interference from exogenous attention. We cued exogenous attention by briefly changing the line drawing from one of the placeholders to white. To ensure that this cue solely engaged exogenous orienting, the cue-target spatial contingency was set to 50%, such that the cue was only predictive of the target’s location at chance-level. The exogenous cue was therefore non-informative and occurred at the periphery. This is consistent with the standard approach for eliciting exogenous attention in the lab (Chica et al. 2014).
Procedure
For each task, participants were asked to maintain their fixation at the center of the screen throughout the experiment, while we assessed their eye movements using electro-oculogram. We jittered the latencies between fixation and attention cues, between both attention cues, and between the cues and the target. We used a uniform distribution of latencies. The purpose of this jitter was to minimize the effects of temporal prediction following spatial cueing, a potential confounding factor for studies of visuospatial attention. Cue-target latencies were adjusted following the temporal profiles of exogenous and endogenous attention so that the benefit of attention processing would be optimized for both attention types (Chica et al. 2014). Moreover, the latencies in the current experiment match that of a previous double cueing experiment (Landry et al. 2021).
In the non-cueing task, the timing between the fixation circle and target stimulus was jittered from 1027 ms to 1280 ms. The target stimulus stayed on the screen until participants responded. We used the non-cueing task as a baseline against which we regressed the sensory effects of cue stimuli for the target-related analysis (see the Electroencephalography section below).
In the endogenous orienting task, the timing between the fixation circle and target stimulus was similarly jittered between 1027 ms and 1280 ms. The endogenous cue preceded the target stimulus. The timing between the onset of the endogenous cue and target stimulus was jittered between 613 ms and 926 ms. The target stimulus remained on screen until participants responded (Fig. 1A).
The double cueing task was similar to the single cueing task, with the exception that we interleaved a peripheral cue between the endogenous cue and the target events. The timing between the fixation circle and the target event was jittered between 1027 ms and 1280 ms. The endogenous cue would onset after the fixation after a random delay. Next, the peripheral cue would onset following a random time interval between 507 ms to 620 ms. The peripheral cue was always displayed for 106 ms. The target stimulus would then onset after a random jitter between 0 ms and 200 ms. Critically, the timing between the endogenous cue and the target stimulus was the same as the single cueing task and varied between 613 ms and 926 ms. The timing between the onset of peripheral cue and the onset target stimulus was jittered between 106 ms and 307 ms (Fig. 1B).
Participants were instructed to complete a target discrimination task and indicate the orientation of the Gabor target as quickly and accurately as possible on a QWERTY keyboard by pressing the F key for counter-clockwise orientation and the J key for clockwise orientation. Inter-trial period was set to 1 s.
Data analyses for behavioral performance
Participants’ discrimination performance was near the ceiling; the average accuracy rate was ~ 93%. We examined whether accuracy rates varied across cueing tasks using single-trial hierarchical logistic regression modeling for predicting accuracy. We included endogenous cue validity (i.e. endogenous cue valid versus endogenous cue invalid) as a fixed factor for the single cueing task, and the double cueing task for congruent and incongruent exogenous cues. In both analyses we included participants as a random factor.
Given that accuracy rates did not vary across tasks, we examined performance via accurate response times (RTs; see behavior in Results section). Here, we removed trials where participants made anticipation (i.e. RTs < 150 ms) or timeout (i.e. RTs > 1500 ms) errors, which accounted for less than 2% of the trials. We additionally removed wrong key presses, which corresponded to less than 1% of the trials. We used hierarchical linear regression models (Gelman and Hill 2006) to test the effects of cueing (i.e. valid versus invalid) and task (i.e. single versus double cueing). We added fixed factors in a stepwise fashion and we used a chi-square goodness-of-fit test over the deviance to determine whether they significantly improved the fit. We computed the Bayesian information criterion (BIC) to select the most parsimonious model. Lastly, we calculated Bayes factors to weight evidence for the alternative against the null hypothesis based on the BIC approximation (Wagenmakers 2007):
 |
For the single cueing endogenous task, we included endogenous cue validity (i.e. endogenous cue valid versus endogenous cue invalid) as a fixed factor. For the double cueing task, we included endogenous cue validity, exogenous cue validity, and their interaction as fixed factors. Given that our study aimed to compare single and double cueing tasks, we examined if the cueing effect of endogenous attention varied as a function of task (i.e. single and double tasks), wherein we included cue validity (i.e. endogenous cue valid versus endogenous cue invalid) and task (i.e. single versus double cueing) as fixed factors. For all regression models, we added participants as a random factor to account for the within-subject design of the study.
We performed two additional analyses. First, we examined whether RT for endogenous cue valid trials only differed as a function of single cueing and double cueing with incongruent exogenous cues. This additional analysis aimed to determine if incongruent exogenous cues impact the engagement of endogenous attention towards target events. Second, we performed the same analysis where we examined RT for endogenous cue invalid trials only across single cueing and double cueing with incongruent exogenous cues. This analysis evaluated if the onset of the exogenous cue to the opposite location benefitted the re-orienting of visuospatial attention for endogenous invalid trials. In both additional analyses, we used a single-trial hierarchical regression model with task (single vs. double cueing with incongruent exogenous cues) as fixed factor and participants as a random factor for predicting response times.
Electroencephalography
We recorded EEG signals using 64 Ag/AgCl active electrodes at a sampling rate of 1000 Hz (ActiCap System; Brain Products GmbH; Gilching, Germany). We monitored eye blinks and eye movements through additional bipolar electrodes placed at the outer canthi, as well as the superior and inferior orbits of the left eye. We kept impedances of all electrodes below 10 kΩ. Electrodes were referenced online to C4. We re-referenced the electrodes offline to the average of all channels. Preprocessing and analysis were conducted in BrainVision Analyzer (ActiChamp System; Brain Products GmbH Inc.; Gilching, Germany) and MATLAB (R2020a; Mathworks Inc., Natick, MA) using Brainstorm (Tadel et al. 2011) and custom scripts. We downsampled the data to 250 Hz and visually inspected EEG signals to identify activity exceeding ±200 μV. We applied two IIR Butterworth filters: a first High-pass 0.1 Hz filter of 8th order and a 60 Hz notch filter. We interpolated bad channels topographically (1.12% of channels across participants) and identified artifacts related to eye movements and blinks using independent component analysis via the BrainVision Analyzer Ocular correction ICA tool. We mitigated (i.e. corrected the EEG signal) the effects of ocular artifacts using ICA analysis. Trials containing artifacts were manually labeled and removed from subsequent analyses. Note that on average 5% (sd = 3%) of trails were removed; the number of bad trials did not differ between conditions (two-tailed paired t-test, P = 0.49). Furthermore, we conducted a comparative analysis of endogenous cue-locked time series for both VOEG and HEOG signals between single and double cueing tasks. This analysis was performed separately for the two variations of double cueing: one with congruent exogenous cues and the other with incongruent exogenous cues, for both right and left endogenous orienting. The results are depicted in Supplementary Fig. 1, which demonstrates that there were no significant differences in VEOG and HEOG signals between single and double cueing conditions.
For target-related events, we removed event-related responses from exogenous and endogenous cues by regressing-out the effects of the task. We took the residuals of the linear regression model using the non-cueing task as a baseline. We ran a hierarchical linear regression in MATLAB with cueing task as a dummy coded fixed factor, and subjects as a random factor. “Raw” Residuals were obtained from all channels and subjects. We used this approach instead of the ADJAR algorithm (Woldorff 1993).
Event-related potentials
We analyzed target-related event-related potentials (ERPs). We first applied a FIR bandpass-pass filter between 0.1 and 15 Hz, consistent with approaches to best estimate ERPs (Kappenman and Luck 2010), and then divided the EEG into epochs spanning -200 ms and 1000 ms. All triggers were realigned according to a photodiode stimulation linked to the onset of the target event. ERPs were baseline corrected from -100 ms to 0 ms.
Lateralized alpha index
We computed the lateralized alpha index (LAI) based on time-resolved alpha power from target-locked epochs for every channel from -1000 ms and 500 ms. We first estimated the instantaneous amplitude of alpha waves (i.e. 8 to 12 Hz) from the real part of the Hilbert transform. Note, the alpha-band encompassed all participants’ individual peak alpha frequencies (mean 10.0 Hz, std 1.1 Hz). Next, we computed the LAI with respect to the target location at each time t point using:
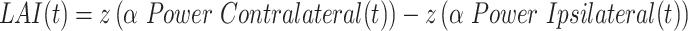 |
In one series of analyses, we defined contra- and ipsilateral components relative to the direction of endogenous orienting. In another, we defined it relative to the location of the target event. See multivariate analyses and hierarchical linear regression modeling sections for details. We computed LAI for channels left/right of the midline electrodes of the EEG cap. Every channel was paired with its homologous channel on the other side of the cap. For each trial, we used z-scores of alpha power changes relative to the baseline period of the endogenous cue (from -400 ms to 0 ms) for computing LAI. In this, our baseline correction included 4 cycles of alpha-band activity. In the multivariate analyses centered on LAI, we determined the index for all lateral channels, purposefully excluding the midline channels. This approach involved pairing each channel from one hemisphere with its equivalent on the other, such as aligning PO7 with PO8. This yielded 28 LAI time series that were used for multivariate pattern classification. In our moderated mediation analysis, we derived LAI from the most prominent channels of the SVM classification to discriminate single and double cueing conditions based on LAI (PO3/PO4 and P3/P4; see Fig. 6B).
Fig. 6.
(A) Group averages of decoding accuracy from target-locked changes in lateralized alpha power (LAI) relative to the orienting direction of endogenous attention. SVM classifiers were trained on each time point of the time series to separate single cueing from double cueing with incongruent exogenous cues. We tested these models on left-out data using one sample t-tests, random cluster permutation and mass t-statistics to test for significant differences in classification performances against chance-level (50%). Horizontal lines indicate the temporal segments of significant differences in decoding accuracy. Shaded areas represent the 95% C.I. (B) the corresponding averaged SVM coefficients derived from the LAI time series that were used for training the model, as well as their topographies. Gray band represents the jittered timing for the onset of the endogenous and exogenous cues in both graphs.
Multivariate analyses
We leveraged multivariate statistics and cross-validation techniques to evaluate the influence of exogenous attention on the cueing effects of endogenous attention. We performed multivariate classification using linear support vector machine (SVM) and MATLAB’s fitcsvm and predict functions. The training and testing phases were completed at the participant’s level. Our approach, which mainly follows that of Bae and Luck (2018), was twofold (Fig. 2). In contrast to univariate analyses, multivariate approaches enhance the sensitivity for the detection of effects by examining the combined relationship of multiple EEG channels relative to our variables of interests: The classification of cue validity (cue valid vs. cue invalid) and of cueing task (single vs. double cueing; Grootswagers et al. 2017). Moreover, the weights can be made interpretable in the context linear modeling (Haufe et al. 2014). Within our framework, we used a within-subject 3-fold cross-validation procedure to prevent model overfitting.
Fig. 2.
(A) Linear SVM classification procedure to decode endogenous cue validity. We used a three-fold cross-validation procedure whereby we trained classifiers at each time point using two ERP for valid trials and two for invalid ones from the single cueing task, and then tested the same classifiers on a third held-out ERP for valid trials and invalid trials from the single cueing task. Next, we assessed the performance of these SVM classifiers in the context of double cueing. The same procedure was used when we tested these models in the context of the double cueing task with congruent exogenous cues. (B) Linear SVM classification to decode single versus double cueing. Again, we used a three-fold cross-validation procedure whereby we trained classifiers at each time point of two EEG time series from single- and double cueing tasks, and then tested these models using left-out data from a third time series. In this example, we exhibit how classifiers trained and tested to decode single cueing (Brown) vs. double cueing with incongruent exogenous cues (purple).
In our first analysis, multivariate pattern classification and cross-validation techniques enabled us to evaluate the performance of a linear model, trained to decode the cueing effect of endogenous attention in the context single cueing, when subsequently tested in the context of double cueing (i.e. transfer learning from the single cueing task to the double cueing task). Here, we trained classifiers to decode the effects of endogenous cue validity during the single cueing task and then evaluated their performance in the context of the double cueing task (Fig. 2A). Specifically, we validated the classifiers’ capacity to decode the cueing effects of endogenous attention alone based on target-locked ERPs during the single cueing task (endogenous cue valid vs. endogenous cue invalid trials). We then assessed whether the performance of these classifiers was impaired in the double cueing task. We reasoned that a significant drop in classification performance would indicate that the addition of the exogenous attention cue in the double cueing task interferes with the cueing effect of endogenous attention. We tested the performance of the SVM models separately for trials with congruent and incongruent exogenous cues relative to endogenous cues. We used a three-fold cross-validation procedure wherein trials from the single cueing task were separated into three different bins per class—i.e. three bins of trials for endogenous cue valid and three for endogenous cue invalid. We equated trials per bins (n = 14) for all tasks and participants. We flipped the entire topography from left to right hemisphere for trials with right target locations to avoid the target’s location acting as a confounding feature (Landry et al. 2023). In this way, the same electrodes always remained contra- and ipsilateral relative to the target location for all trials, while central electrodes remained unchanged. Target location was therefore orthogonal to the classifiers’ performance. We decoded the cueing effect of endogenous attention from target-locked ERPs by averaging the EEG from each bin, and then baseline corrected them from -100 ms to 0 ms. Next, we trained SVM on each time point along the time series by including the EEG channels as features in our model. We trained the classifier using two ERPs from each class (i.e. two ERPs for invalid cueing and two for valid cueing), and then tested the model on the remaining ones (i.e. one ERP for invalid cueing and one for valid cueing). We iterated this process such that each ERP was used twice for training and once for testing. We repeated this procedure 50 times per participant while randomly shuffling trials across bins for each repetition. Lastly, we averaged classification accuracy rates for each participant across both target locations and smoothed these values across the time series via a five-sample sliding window—i.e. a 20 ms window (Hong et al. 2020). This procedure allowed us to assess the classification performance across the time series during single cueing. We tested these classifiers’ ability to classify endogenous cue validity in three separate contexts: (1) on the left-out data from single cueing task; (2) on the data from the double cueing task with congruent exogenous cues; and (3) on the data from the double cueing task with incongruent exogenous cues. We used one sample t-tests across the time series to determine whether classification performances were better than chance-level (i.e. 50% decoding accuracy). For all analyses, we controlled for family-wise errors via random permutations and mass t-statistics for cluster size. The cluster forming threshold was set to P < 0.05. We performed 1000 permutations where we randomly varied the classification labels to generate surrogate distributions. We contrasted the observed cluster sizes based on t-statistics against surrogate distributions of cluster size. Statistically significant clusters were thresholded at ≥95%.
We also contrasted the classification performances from all three separate contexts: (1) Single cueing vs. Double cueing with congruent exogenous cues; (2) Single cueing vs. Double cueing with incongruent exogenous cues; (3) Double cueing with congruent exogenous cues vs. Double cueing with incongruent exogenous cues. Again, we controlled for family-wise errors via random permutations and mass t-statistics for cluster size. The cluster forming threshold was set to P < 0.05. We performed 1000 permutations where we randomly varied the classification labels to generate surrogate distributions. We contrasted the observed cluster sizes based on t-statistics against surrogate distributions of cluster size. Statistically significant clusters were thresholded at ≥95%.
In our second analysis, we trained and tested classifiers in their ability to decode cueing tasks—i.e. single versus double cueing tasks (Fig. 2B). Here, we tested whether target-locked activity associated with endogenous attention varied from single to double cueing task. Given that our main hypothesis aimed to investigate the interference of exogenous attention on endogenous attention and that we observed this pattern of interference during double cueing for trials with incongruent exogenous cues, we trained and tested classifiers in their ability to accurately separate single vs. double cueing task with incongruent exogenous cues. Our training and validation procedures were similar to the ones we described previously using a three-fold cross-validation procedure. Specifically, we grouped trials into separate bins for valid and invalid trials across the single and double cueing tasks. Again, each bin comprised 14 trials. We then averaged the time series from each bin, which yielded three endogenous cue valid waveforms and three endogenous cue invalid waveforms separately for the single and double cueing tasks. For each cueing task, we then subtracted the cue valid waveform from the cue invalid waveform for each corresponding bin (e.g. cue valid bin one minus cue invalid bin one). This procedure generated three endogenous cue validity waveforms in the single cueing task and three endogenous cue validity waveforms in the double cueing task. Again, we trained the SVM classifier on 2 waveforms from each cueing task (i.e. single versus double cueing) and validated on the remaining one. Once more, we iterated this procedure such that each waveform was used twice for training and once for testing and repeated this process 50 times for each participant, while randomly selecting the trials for each bin on every repetition. We used one sample t-test against chance-level (i.e. 50%) and mass t-statistics to identify clusters where classifiers performed better than chance-level. Again, the cluster forming threshold was set to P < 0.05. We performed 1000 permutations where we randomly varied the classification labels and then contrasted observed cluster sizes based on t-statistics against surrogate distributions of cluster size. Statistically significant clusters were threshold at ≥95%.
In a separate analysis, we aimed to confirm that exogenous orienting of attention to the opposite hemifield interferes with endogenous attention during the processing of target events. We applied the same procedure as described previously yet solely focused on endogenous cue valid trials and tested whether SVM classification could discriminate the cueing task (i.e. single vs. double cueing) based on target-locked waveforms. Again, we only used trials with incongruent exogenous cue where we had observed interference from exogenous attention over endogenous cueing effects. Hence, this approach solely focused on the processing of target events following the engagement of endogenous attention. Again, we trained and tested classifiers across time channels as model features. Note that we performed separate analyses for contralateral and ipsilateral channels. Here we used the same procedure as described above. We then averaged the trials from each bin, wherein each waveform corresponding to trials where endogenous attention is engaged towards the target stimulus.
We adopted a similar approach to examine the modulations of LAI across single and double cueing tasks using multivariate statistics (Fig. 2B). Following our main hypothesis, we computed target-locked LAI during the pre-stimulus period following the direction of endogenous orienting. For the double cueing task, we only focused on trials with incongruent exogenous cues, in which we observed that exogenous attention interfered with the endogenous cueing effect. For this analysis, we separated trials where we computed time series of alpha power into bins of contralateral and ipsilateral channels. Each bin comprised 32 trials. Next, we computed the average from each bin and computed the LAI by matching the contra- and ipsilateral components of the first, second and third bins across single and double cueing tasks. This procedure yielded three time series of LAI for single cueing and three time series for double cueing. We used the same 3-fold cross-validation as before to evaluate the effects of the cueing task over LAI (single vs. double cueing task). We used one sample t-test against chance-level (i.e. 50%) and mass t-statistics to identify clusters where classifiers performed better than chance-level. Again, the cluster forming threshold was set to P < 0.05. We performed 1000 permutations where we randomly varied the classification labels and then contrasted observed cluster sizes based on t-statistics against surrogate distributions of cluster size. Statistically significant clusters were threshold at ≥95%. Lastly, to interpret the encoding model, we used the method described by Haufe et al. (2014) and multiplied the weights of the classifiers with the covariance matrix of the neural data along the time series.
Visual event-related potentials
To supplement our multivariate analyses and explore how exogenous orienting to the opposite location impacts endogenous cue validity, we conducted an univariate visual ERP analysis. We extracted target-locked ERPs at channel PO7 and PO8 based on previous work showing that visuospatial attention modulates early visual components of the target-locked waveforms (Luck et al. 2000; Hopfinger and West 2006). We extracted the waveform at the contralateral channel relative to the target location for each trial. For each participant, we averaged the waveforms at the contralateral channel separately for the following experimental conditions: Single cueing with endogenous valid cues; single cueing with endogenous invalid cues; double cueing with endogenous valid cues and incongruent exogenous cues; double cueing with endogenous invalid cues and incongruent exogenous cues. Here, we therefore tested whether the onset of the exogenous cue at the opposite location relative to the direction of the endogenous cue impacted the endogenous cue validity effect for EEG waveforms using hierarchical regression models. We tested this regression model at each time point for the waveforms where we included endogenous cue validity (endogenous cue valid vs. endogenous cue invalid), cueing task (single vs. double cueing), as well as their interaction as fixed factor, while participants were included as random factors:
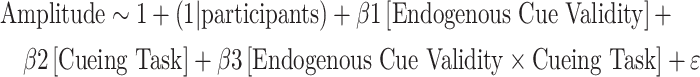 |
We then cluster-corrected across time using mass t-statistic for each regression coefficient.
Moderated mediation analysis
To test our hypothesis that exogenous attention interferes with endogenous attention through alpha activity, we first identified the channels where the SVM beta weight was maximal to discriminate between the single and double cueing tasks based on LAI when both cues indicate opposite locations during double cueing. We observed the maximal SVM weight value for channels PO3/PO4 and P3/P4 (Fig. 6B). We therefore extracted the LAI value for these channels and used a moderated mediation analysis to assess our hypothesis, wherein changes in LAI mediates the relationship between endogenous cue validity and RTs, and the onset of the exogenous cue moderates this mediation effect (Fig. 7A). We first evaluated the a path (β1), moderation of the a path (β3), and the b path (β6) through the time series based on the following single-trial regression analyses:
Fig. 7.
A. Moderated mediation model where the direct effect involves endogenous cue validity predicted response times (RT), while the cueing task moderates this relationship. The indirect effect involves mediation through averaged LAI at channels PO3/PO4 and P3/P4, wherein the cueing task moderates the relationship between endogenous cue validity and LAI. Coefficient of the a path (B), the b path (C) and the moderated a path (D) through the time series. Horizontal purple bar indicates the period where we observed a moderated mediation (from -152 ms to -68 ms). Panel E indicates observed coefficient value for the indirect effect of a moderated mediation relative to the null distribution from random permutations.
 |
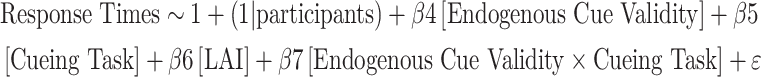 |
This analysis revealed that the statistically significant periods for the a path, the moderation of the a path, and the b path overlapped between -158 ms to -68 ms relative to target onset. We therefore evaluated the moderated mediation indirect effect for that period using the differential approach (i.e. total effect minus direct effect; Judd and Kenny 1981) based on the coefficients from prior regression analyses, including the regression model to evaluate the total effect. We accordingly averaged LAI for that period and then relied on random permutations where we randomly shuffled RTs, cue validity, cueing task, and averaged LAI values within each participant to determine whether the indirect effect was statistically significant against a surrogate distribution. Beforehand, we validated our assumption that LAI mediates the relationship between endogenous cue validity and RTs before testing the moderation effect. We used a simple mediation model based on the following regression analyses:
 |
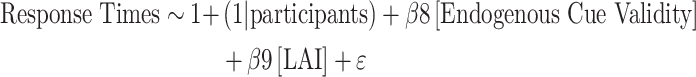 |
Results
Behavior. The participants identified the target orientation with near-ceiling performances (mean accuracy rate = 0.92, standard deviation = 0.001). In the single cueing task, we observed no statistical difference for accuracy rates between endogenous valid trials (mean accuracy rate = 0.93, 95% C.I. = [.91, 0.94]) and endogenous invalid trials (mean accuracy rate = 0.93, 95% C.I. = [.91, 0.95]). We used a single-trial hierarchical logistic regression model where endogenous cue validity (i.e. endogenous cue valid vs. endogenous cue invalid) was added as a fixed factor and participants as a random factor for predicting accuracy (endogenous cue validity effect; β = −.004, SE = 0.08, 95% CI [−.16, 0.15]). In the double cueing task, endogenous cue validity was not significant for trials with congruent exogenous cues (endogenous cue validity effect; β = −.13, SE = 0.10, 95% CI [−.06, 0.15]) and for trials with incongruent exogenous cues (endogenous cue validity effect; β = −.02, SE = 0.11, 95% CI [−.23, 0.19]). For trials with congruent exogenous cues, mean accuracy rate was 0.93 (95% C.I. = [.89, 0.95]) for endogenous valid trials and 0.93 (95% C.I. = [.90, 0.94]) for endogenous invalid trials. For trials with incongruent exogenous cues, the mean accuracy rate was 0.91 (95% C.I. = [.88, 0.93]) for endogenous valid trials] and 0.92 (95% C.I. = [.89, 0.94]) for invalid trials. Therefore, endogenous attention did not influence discrimination accuracy across single and double cueing tasks. This result also indicates that task difficulty was comparable across both tasks.
Consequently, our behavioral analyses focused on response times (RT) in correct trials. Here, we confirmed that the exogenous and endogenous cues facilitated discrimination performance in both cueing tasks. In the single-cueing task, endogenous attention decreased the mean RT by 36 ms (endogenous cue validity effect; β = −36.49, SE = 3.24, 95% CI [−42.84, −30.13]), thereby confirming that the endogenous cue yielded the desired cueing effect. In the double cueing task, we evaluated both the effects of the exogenous and endogenous cues to ensure that they produced their desired effects. We observed main effects of exogenous and endogenous cue validity whereby participants were 39 ms faster for valid exogenous cues (exogenous cue validity effect; β = −39.27, SE = 3.04 ms, 95% CI [−45.22, −33.32]) and 22 ms faster for valid endogenous cueing (endogenous cue validity effect; β = −22.42, SE = 3.22 ms, 95% CI [−28.73, −16.11]. Evidence weighted against the interaction between endogenous cue validity and exogenous cue validity in the double cueing task (Bayes factor, BF01 = 105.59). Therefore, both cues produced their expected effects.
Next, we also confirmed that the presence of the exogenous cue reduced the endogenous cueing effect by contrasting the cue validity effects of endogenous attention in the single cueing vs. double cueing tasks. We performed this analysis separately across congruent and incongruent exogenous cueing trials (Fig. 1). For trials with congruent exogenous cues, we observed a significant interaction between endogenous cue validity and cueing task (β = −25.2, SE = 5.68, 95% CI [−36.33, −14.06]; Fig. 1C). This interaction indicated that the cueing effect of endogenous attention increased from single to double cueing, thereby showing exogenous attention boosted endogenous attention. Conversely, for trials with incongruent exogenous cues, our results indicated that exogenous attention interfered with the cueing effect of endogenous attention, as evidenced by a significant interaction between endogenous cue validity and cueing task (β = 52.74, SE = 5.72, 95% CI [41.52 63.97]; Fig. 1D). Individually, 29 participants exhibited a decrease in the endogenous cue validity effect when contrasting single cueing to double cueing with incongruent exogenous cues. On average, this reduction was 16 ms, with a standard deviation of 59 ms. This effect includes 22 participants not showing any effect of endogenous cue validity for double cueing with incongruent exogenous cues. These results are consistent with the exogenous attention interfering with endogenous attention whenever they are engaged towards opposite locations.
Given the interference effect we observed for double cueing trials with incongruent exogenous cues, we also examined whether RT for endogenous cue valid trials were impacted whenever the exogenous cue occurred at the opposite location. We reasoned that slower discrimination responses between single cueing and double cueing with incongruent cues would provide additional evidence of interference, similar to the attentional capture phenomenon. Our analysis confirmed that participants were slower for double cueing with incongruent exogenous cues (β = 23.22, SE = 3.14, 95% CI [17.07, 29.36). Consistent with attentional capture, our results confirm that the peripheral cue to the opposite location hinders the engagement of endogenous attention towards the target event. In the same vein, we also examine endogenous cue invalid trials only as a function of single cueing and double cueing with incongruent exogenous cues. Here, we evaluated whether incongruent exogenous cues during double cueing would benefit re-orienting of visuospatial attention relative to single cueing. This analysis revealed that participants were not faster for the double cueing condition with incongruent exogenous cues (β = 6.57, SE = 4.11, 95% CI [−1.49., 14.62). Additionally, Bayes factor analysis strongly supported the null hypothesis for this analysis, BF01 = 32.03. Evidence showing a benefit for re-orienting was therefore limited.
Electrophysiology: Exogenous attention disrupts the cueing effect of endogenous attention over target-locked event-related potentials. Based on our behavioral results, we expected that exogenous attention would interfere with target-related processing. As described in the method section, we relied on multivariate statistics and cross-validation techniques to assess this hypothesis (Fig. 2).
We first validated that the performance of classifiers trained in the single cueing task to decode endogenous cue validity (i.e. valid versus invalid trials) based on target-locked ERPs was above chance level. We observed two significant clusters. The SVM classifiers performed above chance level between 132 ms and 716 ms (cluster size P < 0.001) relative to target onset, as well from 760 ms to 960 ms (cluster size P < 0.001; Fig. 3A). This confirmed the ability of the models to identify valid from invalid trials during this time period. We then evaluated the classifier performances when tested on double cueing data. Decoding was successful for trials with congruent exogenous cues, as evidenced by two significant clusters: A first one between 224 ms and 336 ms post-target onset (cluster size P < 0.01), and the second from 368 ms to 436 ms post-target onset (cluster size P < 0.05; Fig. 3B). Conversely, for trials with incongruent exogenous cues, the decoding of endogenous cue validity was unsuccessful and was even below chance-level between 240 ms and 400 ms (cluster size P < 0.001) and then between 768 ms and 832 ms relative to target onset (cluster size P < 0.001; Fig. 3C). These results are consistent with our behavioral findings and show that the occurrence of a salient event at the opposite location relative to endogenous attention prevents the classification of endogenous cue validity from target-locked ERP.
Fig. 3.
Group averages of decoding accuracy from target-locked ERPs in the singlecueing (A), double cueing with congruent exogenous cues (B), and double cueing with incongruent exogenous cues (C) tasks. SVM classifiers were trained for each time point of the time series to separate endogenous cue valid trials from endogenous cue invalid trials during single cueing. We then tested this model on left-out data from the single cueing task (A), on double cueing task with congruent exogenous cues (B), and double cueing task with incongruent exogenous cues (C). We used one sample t-tests, random cluster permutation and mass t-statistics to test for significant differences in classification performances against chance-level (50%). Horizontal color lines indicate the temporal segments of significant differences in decoding accuracy. Shaded areas represent the 95% C.I.
We further contrasted the classification performances from all three conditions: (1) Single cueing vs. double cueing with congruent exogenous cues; (2) single cueing vs. double cueing with incongruent exogenous cues; (3) double cueing with congruent exogenous cues vs. double cueing with incongruent exogenous cues. Our observations highlighted significant distinctions for the second and third contrast (Supplementary Fig. 2). However, the first contrast did not reveal any significant patterns. Our results show a significant difference was found between 132 ms and 424 ms post-target stimulus when comparing single cueing to double cueing with an incongruent exogenous cue. Additionally, there was a significant difference between 216 ms and 564 ms post-target stimulus when contrasting double cueing with congruent exogenous cues against double cueing with incongruent cues.
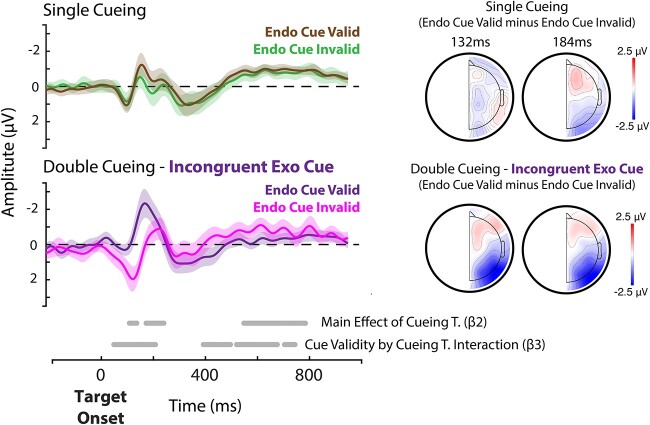
We also examined how the orienting of exogenous attention to the opposite location (i.e. trials with incongruent exogenous cues) impacts endogenous cue validity over target-locked ERPs at channels PO7 and PO8. This additional analysis aimed to better understand the impact of incongruent exogenous cues in the double cueing task over early visual processing. We focused on the contralateral target-locked ERPs and examined the interaction between endogenous cue validity (endogenous cue valid vs. endogenous cue invalid) and cueing task (single cueing vs. double cueing with incongruent exogenous cue) across the time series. This analysis returned a significant cluster for the interaction between cue validity (endogenous cue valid vs. endogenous cue invalid) and cueing task (single- vs. double cueing) at the time of the P1-N1 complex (from 36 ms to 196 ms post-target onset; cluster size, P < 0.001), thereby validating that the onset of the exogenous cue modulates the effect of endogenous attention across early visual ERP components (Fig. 4). This interaction further included the late positivity (P3) wherein we observed several significant clusters ranging from 380 ms to 732 ms relative to target onset.
Fig. 4.
Target-locked averaged waveforms for endogenous cue valid and endogenous cue invalid trials at the contralateral side relative to the location of the target for channels PO7 and PO8. Lines represent group averaged waveforms, shaded area represents the 95% C.I. We extracted waveform from channel PO8 when the target even occurred on the left side of the screen, and channel PO7 when it occurred on the right side. Top-row shows the data from the single cueing task, bottom row data from the double cueing with incongruent exogenous cues. We examined whether the endogenous cue validity effect varied as a function of cueing task using the following hierarchical regression model across the time series: Amplitude ~ 1 + (1|participants) + β1[endogenous cue validity] + β2[cueing task] + β3[endogenous cue validity × cueing task] + ε. We cluster-corrected the regression coefficients based on mass t-statistics. Significant clusters across the time series are shown at the bottom. Right side of the figure shows the corresponding topographies for the endogenous cue validity effect: Endogenous cue valid minus endogenous cue invalid for single cueing (top) and double cueing with incongruent exogenous cues (bottom).
Our behavioral and neural results align with our hypothesis that exogenous attention interferes with endogenous attention for trials with incongruent exogenous cues, per our expectations. The remainder of our analysis will accordingly solely focus on the comparison of single cueing and double cueing for trials with incongruent exogenous cues.
Our next analysis aimed to corroborate our behavioral findings and confirm that the onset of the exogenous cue to the opposite location alters endogenous processing of visual stimuli at the neural level. For this analysis, we solely focused on endogenous valid cueing trials (see Methods for more details). For contralateral channels, we observed one significant cluster from 36 ms to 960 ms relative to target onset. A similar pattern emerged for ipsilateral channels where we observed two significant clusters: A first one from -152 ms to -88 ms, and then from -20 ms to 960 ms, all relative to the target stimulus (Fig. 5B). All cluster sizes, P < 0.001. In short, the presence of the exogenous cue to the opposite location altered the processing of the target event by endogenous attention throughout almost the entire time series.
Fig. 5.
Group averages of decoding accuracy from target-locked ERP for endogenous cue valid trials only. We performed separate analysis for contralateral (A) and ipsilateral (B) channels relative to target location. SVM classifiers were trained across each time point of the time series to separate single cueing from double cueing with incongruent exogenous cues. We tested these classifiers on left-out data using one sample t-tests, random cluster permutation and mass t-statistics to test for significant differences in classification performances against chance-level (50%). Horizontal purple lines indicate the temporal segments of significant differences in decoding accuracy. Shaded areas represent the 95% C.I.
Electrophysiology: Modulations of alpha power distinguishes between single & double cueing. Following our main hypothesis, our multivariate approach further shows that alpha-band brain activity (LAI) relative to the orienting of endogenous attention varies as a function of single versus double cueing. Here, we observed two significant clusters: A first one occurring at the time of the exogenous cue from -420 ms and -232 ms (cluster size, P < 0.05) and a second one ranging from -128 ms to 424 ms (cluster size, P < 0.001), both of them relative to target onset (Fig. 6A). This outcome supports our hypothesis relative to alpha-band activity and shows that the presence of the exogenous cue to the opposite location of endogenous orienting alters alpha-band activity related to endogenous attention. Importantly, the corresponding decoding weights at that time highlighted posterior contributions (Fig. 6B).
Interference in alpha-band power explains behavioral interference between attention systems
The interference effect of exogenous attention over endogenous attention was therefore related to the modulations of lateralized alpha power, which supported our main hypothesis. We performed a moderated mediation analysis to further explore this relationship. For this analysis, we extracted target-locked LAI values for EEG channels where we observed the top features for separating single cueing and double cueing with incongruent exogenous cues. These corresponded to channels PO3/PO4 and P3/P4 (Fig. 6B).
Our moderated mediation model encompassed two hypotheses. First, consistent with previous findings, we hypothesized that changes in the target-locked LAI values we extracted would mediate the relationship between endogenous cue validity and response times (Supplementary Fig. 3). Second, we hypothesized that the onset of the exogenous cue to the opposite location relative to the orienting of endogenous attention would moderate this mediation (Fig. 7A). We tested this model by verifying overlap between the moderation of the a path where the interaction between endogenous cue validity and cueing task predicts LAI values, and the b path where LAI predicts response times across the time series for target-locked LAI. We then evaluated the moderation of the mediation using the differential approach (see Method section for details).
We observed that endogenous cue validity modulates LAI values between from -640 ms to -492 ms and then from -420 ms to 28 ms relative to target onset (a path of our model; both cluster sizes, P < 0.001; Fig. 7B). Next, we confirmed that changes in LAI predict response times from -276 ms to -28 ms relative to target onset (b path of our model; Fig. 7C). Importantly, we found that the a path of our model was moderated from -153 ms to 252 ms relative to target onset (cluster size, P < 0.001), and then again from 356 ms until the end of the time series (cluster size, P < 0.05; Fig. 7D). Our results supported our moderated mediation model, whereby endogenous cue validity predicted changes in LAI, the cueing task moderated this relationship, and LAI predicted response times. We confirmed our model by focusing on the significant time points where the moderated a path and the b path overlapped (i.e. from -152 ms to -68 ms relative to target onset; cluster size, P < 0.05). We verified that the mediation effect was statistically significant using the differential approach (i.e. total effect minus direct effect) of the mediation model and random permutations (indirect mediation effect: β = −29.95, P < 0.001). We also confirmed the moderated mediation using the same statistical approach (indirect moderated mediation effect: β = 0.21, P < 0.001; Fig. 7E). However, this was a partial moderated mediation since the direct effect was statistically significant (β = 46.65, P < 0.001).
Discussion
We investigated the neural correlates associated with the interference effect of exogenous attention on endogenous attention. We compared the effects of endogenous attention as a function of exogenous attention across single and double cueing tasks. Our experimental approach provided evidence that a salient peripheral cue, which captures exogenous attention, interferes with endogenous attention when both attention processes are directed to opposite locations in the visual field. This interference was observed at both the behavioral level (Fig. 1E) and the neural level (Fig. 3C). Furthermore, at the behavioral level, our results show that most participants exhibited a reduction in endogenous cue validity effect going from single cueing to double cueing for incongruent exogenous cues–many participants no longer exhibited an endogenous cueing effect altogether. These findings are consistent with previous studies demonstrating that task-irrelevant salient events can capture attention and disrupt endogenous attention processes (e.g. Theeuwes 1991). Overall, our findings support the notion that the dynamics of exogenous and endogenous attention are governed by competition, leading to interference between these two attentional processes (Berger et al. 2005; Hopfinger and West 2006; Busse et al. 2008; Grubb et al. 2015).
Our double cueing protocol involved the sequential presentations of an endogenous cue followed by an exogenous cue. This experimental approach entails that, for trials with incongruent exogenous cues, task-irrelevant peripheral cues triggered a reflexive re-orienting response of visuospatial attention to the opposite target location—a process that rests on key brain regions of visuospatial attention, including the temporo-parietal junction and fronto-parietal networks (Corbetta and Shulman 2002; Corbetta et al. 2008). In the present work, we hypothesized that this re-orienting response interfered with endogenous attention processes. However, evidence shows that the re-orienting of attention may benefit perception (Chica et al. 2010), which brings about the hypothesis that incongruent exogenous cues decrease the endogenous cue validity effect during double cueing by boosting task performance for endogenous cue invalid trials. This alternative hypothesis therefore emphasizes the flexibility of the re-orienting response rather than its cost. To arbitrate between these competing accounts, we evaluated the impact of incongruent exogenous cues on the endogenous cue validity effect. In support of the interference account, participants were slower to respond on endogenous cue valid trials for the double cueing task compared to the single cueing task. We observed no discernible difference in RT on endogenous cue invalid trials across single cueing and double cueing tasks with incongruent exogenous cues. Our results therefore indicate that the re-orienting of visuospatial attention interferes with the engagement of endogenous attention processing towards the target stimulus for endogenous cue valid trials, much like the attentional capture phenomenon (Luck et al. 2021).
Our neurophysiological results are consistent with this construal where the advent of a peripheral cue to the opposite location modulated early visual processing for endogenous cue valid trials, for both ipsi- and contralateral ERPs (Fig. 5). Together, these results indicate that the engagement of endogenous attention processes towards visual stimuli are compromised in the context of double cueing for trials with incongruent exogenous cues. They also align with the notion of capacity-limited attention processing, whereby salient events (such as the exogenous cue) can prevent endogenous attention from fully engaging visual stimuli (Alvarez and Franconeri 2007; Busse et al. 2008).
Our findings are consistent with the idea that, exogenous and endogenous attention represent distinct functional systems that are not fully separable (Berger et al. 2005). Examining neural activity related with this interference effect, we observed that SVM classifiers trained to decode endogenous cue validity effects based on target-locked ERPs immediately dropped to chance-level when tested in the context of double cueing with incongruent exogenous cues (Fig. 3C). Notably, classification dipped below the expected chance level between 250 ms and 320 ms relative to target onset. This suggests that the onset of the exogenous cue on the other side of the screen systematically misled classifiers that were trained to decode the effects of endogenous cue validity. Though this pattern is subtle and limited, it implies that the exogenous cue amplified the signal at the location where the endogenous cue was invalid, thereby mirroring the effects of endogenous attention during this time period. This finding aligns with previous results showing an overlap of both attention processes around the N2 and P3 ERP components (Landry et al. 2023). Thus, for trials with incongruent exogenous cues, evidence shows that visuospatial exogenous attention reflexively engaged the location indicated by the exogenous cue after the orienting of endogenous attention towards the opposite location beforehand. This outcome highlights the flexibility of attention resources to engage and disengage spatial locations as a function of exogenous and endogenous attention processes.
Importantly, our study suggests the early involvement of sensory areas in the interaction between exogenous and endogenous attention. This is revealed by evidence showing that the presence of the exogenous cue at the opposite location impacted the P1-N1 ERP complex at the contralateral site (Fig. 4), thereby highlighting interactions between exogenous and endogenous attention along the visual pathway (Hopfinger and West 2006). Source modeling relates these ERP components to extrastriate and parietal regions (Di Russo et al. 2002). These findings therefore provide evidence for overlap between exogenous and endogenous attention processing during early sensory processing (Müller and Ebeling 2008). Additionally, the early interaction between endogenous cue validity and the cueing task observed over the P1-N1 complex at the contralateral channels PO7 and PO8 suggests that incongruent exogenous cues influence endogenous attention during the initial stage of visual processing. Yet, this observation is inconsistent with prior EEG studies utilizing the double cueing task, where such early interactions between exogenous and endogenous attention were not observed (Hopfinger and West 2006). This interaction also encompass later stages of visual processing, such as the late positivity, which is often associated with perceptual decision making (Twomey et al. 2015). This suggests the impact of incongruent exogenous cues extend beyond early stages of sensory processing.
Our primary hypothesis revolved around the role of alpha power in the interference between exogenous and endogenous attention. This hypothesis was built upon a substantial body of research demonstrating that shifts of visuospatial attention correspond to decreased alpha power amplitude over the contralateral posterior brain region, while the ipsilateral region exhibits increased alpha power (Peylo et al. 2021). This lateralized pattern of alpha-band activity is widely considered to reflect changes in neural excitability and inhibition during visuospatial attention (Woodman et al. 2022; Jensen 2023). Recent evidence, however, challenges the idea that alpha rhythms cause shifts of attention (Peylo et al. 2021; Morrow et al. 2023). Nevertheless, one of the prevailing view is that reduced alpha-band activity is associated with heightened neural processing for events occurring in the contralateral attended visual field, while increased alpha power signifies the suppression of unattended ipsilateral sensory inputs (Klimesch 2012). This hypothesis therefore considers that patterns of alpha activity reflects a potential mechanisms of selective attention (van Moorselaar and Slagter 2020). We postulated that modulations of neuronal excitability and suppression, dependent on exogenous and endogenous attention, would play a crucial role in the emergence of interference patterns. The conflicting effects of attention orienting on neural activity give rise to interference, as endogenous attention engenders increased neuronal excitability in the contralateral posterior region (evidenced by decreased lateralized alpha power), whereas exogenous attention induces heightened inhibition within the same neuronal population (indicated by increased lateralized alpha power in the ipsilateral posterior region). Consistent with our hypothesis, we observed that the onset of the exogenous cue at the opposite location altered alpha rhythms associated with endogenous attentional orienting (Fig. 6A), with this effect observed primarily in the posterior region of the brain (Fig. 6B). Hence, both forms of attention orienting involve modulations of this neurophysiological marker of visuospatial attention and that interference between them arises due to their contrasting effects on alpha-band activity. Importantly, it should be noted that these findings do not discount the involvement of additional frequency bands in explaining interference effects originating from exogenous attention.
We investigated whether the observed effects on alpha power were related to the interference effects at the behavioral level. For this endeavor, we first confirmed that modulations of alpha-band activity were associated with the cueing effect of endogenous attention through a mediation analysis, which demonstrated a partial mediation of the endogenous cueing effect for lateralized alpha power. We then evaluated the moderation of this mediation to test our main hypothesis linking the interference effect of exogenous attention on endogenous attention to alpha-band activity. Consistent with our main hypothesis, the results showed that the cueing task moderated the mediation path relating endogenous cue validity and response times through alpha-band activity prior to target onset (Fig. 7). Specifically, we found that the onset of the exogenous cue to the opposite location modulated the mediation of alpha-band activity that relate endogenous attention to behavioral performance. These findings support the notion that modulations of alpha-band synchrony reflect core mechanisms of visuospatial attention that are shared between exogenous and endogenous processes, leading to interference between them.
We, however, only observed a partial moderated mediation effect. In addition, our results do not conclusively determine if the modulations of alpha-band activity observed in our moderated mediation analysis signify a causal relationship or merely an incidental correlation in relation to shifts in visuospatial attention, particularly in light of our partial moderated mediation. Therefore, while our findings identify a neural marker of the interference of exogenous orienting over endogenous attention processes, further research is necessary to ascertain the exact causal nature of this relationship.
Our recent work demonstrated that classifiers trained to decode the cueing effect of endogenous attention based on target-locked EEG could also successfully decode the cueing effect of exogenous attention, and vice versa (Landry et al. 2023), which supports the idea that these attention processes share similar neural mechanisms. However, this overlap between exogenous and endogenous attention excluded early ERP components of the target evoked response. In contrast to these findings, the current study shows that incongruent exogenous cues interfere with endogenous attention modulations of early visual processing (Fig. 4). This outcome suggests that interference between endogenous and exogenous attention arises when both attention systems engage the same sensory processes, leading to limitations in processing (Busse et al. 2008). This phenomenon can be attributed to conflicting patterns of neuronal excitation and inhibition when both attention processes are directed to opposite sides of the visual field. Specifically, our results demonstrate that when endogenous attention modulates the excitability of neuronal populations involved in visual processing at the contralateral side, as evidenced by alpha-band activity, this modulation is disrupted by exogenous attention oriented in the opposite hemifield, resulting in neuronal inhibition within the same neuronal population.
Recent findings suggests that attention modulations of alpha power reflect a gating mechanism operating downstream of the primary visual cortex (Jensen 2023). This viewpoint rests on mounting evidence showing that attention modulations of early sensory responses are uncorrelated with alpha-band activity at the trial level, as well as source localization based on magnetoencephalography showing that attention modulations of alpha power occurs beyond the primary visual cortex, at the level of the occipito-parietal sulcus (Antonov et al. 2020; Gundlach et al. 2020; Zhigalov and Jensen 2020). Hence, the present work provides empirical support for the hypothesis that exogenous and endogenous attention rely on the same neural resources for gating sensory information beyond the primary visual cortex, highlighting the finite processing resources of the brain that regulate the dynamics between these attentional systems. Accordingly, we may conjecture that modulations of alpha-band activity are unlikely to modulate early visual processing, as revealed by alterations of the P1-N1 complex.
The results of the present experiment should be interpreted alongside some methodological considerations. Several variables impact alpha-band activity in the context of perception. These include task difficulty, whereby greater perceptual load increases alpha power (Gutteling et al. 2022). In our experiment, a ceiling-level discrimination performance implies that the target discrimination task was relatively straightforward. This suggests that, if the task were more challenging, one might observe different alpha activity patterns. Furthermore, endogenous cue validity probability was set to 66.6% to maximize cue endogenous invalid trials across experimental conditions. However, different cue-target contingencies for endogenous attention is likely to influence the impact of exogenous attention on endogenous attention (Geng and Behrmann 2005; Kabata and Matsumoto 2012), including resistance to distracting events that may capture attention resources in a stimulus-driven fashion (Luck et al. 2021). Previous findings show that cue-target contingencies in the context of endogenous attention modulate patterns of lateralized alpha-band activity (Gould et al. 2011). Furthermore, existing studies indicate that attentional capture, and distractibility more broadly, exhibit considerable inter-individual variations (Sörqvist and Rönnberg 2014; Zhang et al. 2021). Although our findings exhibited limited inter-individual disparities, introducing greater task difficulty would likely increase such differences. Thus, one may expect that increasing the probability of endogenous cue validity would reduce the monitoring of uncued target locations, impact the task-irrelevant exogenous cue on endogenous attention processes, and lateralized patterns of alpha-band activity.
The impact of eye movements in spatial attention studies presents an important issue that needs addressing. Recent findings suggest that ICA corrections may sometimes be insufficient to correct eye movement-related artifacts (Quax et al. 2019). Despite this literature, we argue that the current body of results presented herein are not likely driven to eye movements. First, we argue that if the performance of our classifiers were the result of eye movements, the MPVA weights would emphasize anterior sensors as contributing the most to classification–which we do not observe. Second, we corrected the EEG signals across the entire recording, blind to the cueing condition marker. Third, the number of bad segments identified across single and double cueing did not statistically differ (two-tailed, paired t-test; P = 0.49). Last, our decoding analyses were consistent with the outcome of our mediation analysis where we observed that alpha-band activity particularly mediated the relationship between endogenous attention and task performance. Together, we argue that these findings do not corroborate the interpretation that the current findings are driven primarily by eye movements.
Overall, our findings support the notion that the dynamics of exogenous and endogenous attention are governed by competition, leading to interference between these two attentional processes. This outcome is consistent with previous studies that have linked the inhibition of processing task-irrelevant salient events to alpha oscillations (Gutteling et al. 2022). This convergence of results strengthens our understanding of the mechanisms underlying attention processing and resistance to distractions by showing that modulations of alpha-band activity, a neural marker of visuospatial attention, partly relates to the ongoing dynamics between exogenous and endogenous attention. Although the relationship between visuospatial attention and alpha-band remains unclear (Peylo et al. 2021; Morrow et al. 2023), evidence suggests that patterns of alpha activity reflect the gating of sensory processing beyond the primary visual cortex (Jensen 2023). The present findings accordingly suggest that the interference of exogenous attention over endogenous attention putatively encompasses this gating mechanism. It also opens new research avenues to delve deeper into the specific features and mechanisms involved in shaping attentional processing with respect to the dynamics between exogenous and endogenous attention, including in the context of attentional capture (Gaspelin and Luck 2018). By further investigating these aspects, we can gain a more comprehensive understanding of attentional control and its underlying neural mechanisms.
Supplementary Material
Acknowledgments
M.L. acknowledges support from the Fonds de Recherche du Québec—Nature et Technologies. J.D.C. acknowledges a doctoral fellowship from the Natural Science and Engineering Research Council of Canada.
Contributor Information
Mathieu Landry, Laboratoire de Sciences Cognitives et Psycholinguistique, ENS, PSL University, EHESS, CNRS, Paris 75005, France.
Jason da Silva Castanheira, Montreal Neurological Institute, McGill University, Montreal, Qc, H3A 2B4, Canada.
Amir Raz, Institute for Interdisciplinary Behavioral and Brain Sciences, Chapman University, Irvine, CA, 92618, USA.
Sylvain Baillet, Montreal Neurological Institute, McGill University, Montreal, Qc, H3A 2B4, Canada.
Jérôme Sackur, Laboratoire de Sciences Cognitives et Psycholinguistique, ENS, PSL University, EHESS, CNRS, Paris 75005, France; École Polytechnique, Palaiseau 91120, France.
CRediT author statement
Mathieu Landry (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Visualization, Writing—original draft, Writing—review & editing), Jason da Silva Castanheira (Conceptualization, Formal analysis, Investigation, Methodology, Validation, Writing—review & editing), Amir Raz (Funding acquisition, Investigation, Supervision, Writing—review & editing), Sylvain Baillet (Conceptualization, Funding acquisition, Investigation, Methodology, Resources, Supervision, Writing—review & editing), Jérôme Sackur (Conceptualization, Funding acquisition, Investigation, Methodology, Resources, Supervision, Writing—review & editing).
Funding
This work was supported by a grant from CIHR and tiers-2 Canada Research Chair to A.R. S.B. is supported by the NIH (R01 EB026299), a Discovery grant from the Natural Sciences and Engineering Research Council of Canada (436355-13), and the CIHR Canada research Chair in Neural Dynamics of Brain Systems. J.S. acknowledges grants from the ANR (France): ANR-16-ASTR-0014 and ANR-17-EURE-0017. We would like to thank Anna Prokusheva for her help collecting the data. Lastly, we would also like to thank Barry Giesbrecht, Mathieu Roy, Dave Saint-Amour, Vincent de Gardelle, Viola Störmer for helpful comments about this research project.
Conflict of interest statement: None declared.
Contributions
M.L. and J.D.C. designed and implemented the study. M.L. and J.D.C analyzed the data. J.S., S.B. and A.R. provided feedback throughout the project. All authors wrote the manuscript. A.R secured funding for this research project.
Competing interests
All authors declare no competing interest.
Data availability
Data for the analyses supporting the current study is publicly available on the Brain Imaging Center at the NEURO repository: https://box.bic.mni.mcgill.ca/s/BikhIax5qb9VXLX.
Code availability
Code for the analyses supporting the current study is publicly available on the Open Science Framework repository: https://osf.io/bn3g8/
References
- Alvarez GA, Franconeri SL. How many objects can you track?: evidence for a resource-limited attentive tracking mechanism. J Vis. 2007:7(13):14–14, 1410. [DOI] [PubMed] [Google Scholar]
- Antonov PA, Chakravarthi R, Andersen SK. Too little, too late, and in the wrong place: alpha band activity does not reflect an active mechanism of selective attention. NeuroImage. 2020:219:1–12, 117006. [DOI] [PubMed] [Google Scholar]
- Bae G-Y, Luck SJ. Dissociable decoding of spatial attention and working memory from eeg oscillations and sustained potentials. J Neurosci. 2018:38(2):409–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagherzadeh Y, Baldauf D, Pantazis D, Desimone R. Alpha synchrony and the neurofeedback control of spatial attention. Neuron. 2020:105(3):577–587. [DOI] [PubMed] [Google Scholar]
- Berger A, Henik A, Rafal R. Competition between endogenous and exogenous orienting of visual attention. J Exp Psychol Gen. 2005:134(2):207–221. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The psychophysics toolbox. Spat Vis. 1997:10(4):433–436. [PubMed] [Google Scholar]
- Buschman TJ, Kastner S. From behavior to neural dynamics: an integrated theory of attention. Neuron. 2015:88(1):127–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse L, Katzner S, Treue S. Temporal dynamics of neuronal modulation during exogenous and endogenous shifts of visual attention in macaque area mt. Proc Natl Acad Sci. 2008:105(42):16380–16385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M. Visual attention: the past 25 years. Vis Res. 2011:51(13):1484–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chica AB, Lasaponara S, Lupiáñez J, Doricchi F, Bartolomeo P. Exogenous attention can capture perceptual consciousness: Erp and behavioural evidence. NeuroImage. 2010:51(3):1205–1212. [DOI] [PubMed] [Google Scholar]
- Chica AB, Bartolomeo P, Lupiáñez J. Two cognitive and neural systems for endogenous and exogenous spatial attention. Behav Brain Res. 2013:237:107–123. [DOI] [PubMed] [Google Scholar]
- Chica AB, Martín-Arévalo E, Botta F, Lupiánez J. The spatial orienting paradigm: how to design and interpret spatial attention experiments. Neurosci Biobehav Rev. 2014:40:35–51. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002:3(3):201–215. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008:58(3):306–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Russo F, Martínez A, Sereno MI, Pitzalis S, Hillyard SA. Cortical sources of the early components of the visual evoked potential. Hum Brain Mapp. 2002:15(2):95–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JJ, Sutterer DW, Serences JT, Vogel EK, Awh E. Alpha-band oscillations enable spatially and temporally resolved tracking of covert spatial attention. Psychol Sci. 2017:28(7):929–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxe JJ, Simpson GV, Ahlfors SP. Parieto-occipital approximately 10 hz activity reflects anticipatory state of visual attention mechanisms. Neuroreport. 1998:9(17):3929–3933. [DOI] [PubMed] [Google Scholar]
- Gaspelin N, Luck SJ. The role of inhibition in avoiding distraction by salient stimuli. Trends Cogn Sci. 2018:22(1):79–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman A, Hill J. Data analysis using regression and multilevel/hierarchical models. Cambridge: Cambridge University Press; 2006 [Google Scholar]
- Geng JJ, Behrmann M. Spatial probability as an attentional cue in visual search. Percept Psychophys. 2005:67(7):1252–1268. [DOI] [PubMed] [Google Scholar]
- Gould IC, Rushworth MF, Nobre AC. Indexing the graded allocation of visuospatial attention using anticipatory alpha oscillations. J Neurophysiol. 2011:105(3):1318–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grootswagers T, Wardle SG, Carlson TA. Decoding dynamic brain patterns from evoked responses: a tutorial on multivariate pattern analysis applied to time series neuroimaging data. J Cogn Neurosci. 2017:29(4):677–697. [DOI] [PubMed] [Google Scholar]
- Grubb MA, White AL, Heeger DJ, Carrasco M. Interactions between voluntary and involuntary attention modulate the quality and temporal dynamics of visual processing. Psychon Bull Rev. 2015:22(2):437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundlach C, Forschack N. Commentary: alpha synchrony and the neurofeedback control of spatial attention. Front Neurosci. 2020:14:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundlach C, Moratti S, Forschack N, Müller MM. Spatial attentional selection modulates early visual stimulus processing independently of visual alpha modulations. Cereb Cortex. 2020:30(6):3686–3703. [DOI] [PubMed] [Google Scholar]
- Gutteling TP, Sillekens L, Lavie N, Jensen O. Alpha oscillations reflect suppression of distractors with increased perceptual load. Prog Neurobiol. 2022:214:1–13, 102285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegens S, Nácher V, Luna R, Romo R, Jensen O. Α-oscillations in the monkey sensorimotor network influence discrimination performance by rhythmical inhibition of neuronal spiking. Proc Natl Acad Sci. 2011:108(48):19377–19382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Händel BF, Haarmeier T, Jensen O. Alpha oscillations correlate with the successful inhibition of unattended stimuli. J Cogn Neurosci. 2011:23(9):2494–2502. [DOI] [PubMed] [Google Scholar]
- Haufe S, Meinecke F, Görgen K, Dähne S, Haynes J-D, Blankertz B, Bießmann F. On the interpretation of weight vectors of linear models in multivariate neuroimaging. NeuroImage. 2014:87:96–110. [DOI] [PubMed] [Google Scholar]
- Hong X, Bo K, Meyyappan S, Tong S, Ding M. Decoding attention control and selection in visual spatial attention. Hum Brain Mapp. 2020:41(14):3900–3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfinger JB, West VM. Interactions between endogenous and exogenous attention on cortical visual processing. NeuroImage. 2006:31(2):774–789. [DOI] [PubMed] [Google Scholar]
- Jensen O. Gating by alpha band inhibition revised: a case for a secondary control mechanism; 2023PsyArXiv
- Jonides J. Voluntary versus automatic control over the mind’s eye’s movement. Attention and performance IX. 1981:9:187–203. [Google Scholar]
- Judd CM, Kenny DA. Process analysis: estimating mediation in treatment evaluations. Eval Rev. 1981:5(5):602–619. [Google Scholar]
- Kabata T, Matsumoto E. Cueing effects of target location probability and repetition. Vis Res. 2012:73:23–29. [DOI] [PubMed] [Google Scholar]
- Kappenman ES, Luck SJ. The effects of electrode impedance on data quality and statistical significance in erp recordings. Psychophysiology. 2010:47(5):888–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasten FH, Wendeln T, Stecher HI, Herrmann CS. Hemisphere-specific, differential effects of lateralized, occipital–parietal α-versus γ-tacs on endogenous but not exogenous visual-spatial attention. Sci Rep. 2020:10(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe JM, Störmer VS. Lateralized alpha activity and slow potential shifts over visual cortex track the time course of both endogenous and exogenous orienting of attention. NeuroImage. 2021:225:1–13, 117495. [DOI] [PubMed] [Google Scholar]
- Kemmerer SK, Graaf TA, Oever S, Erkens M, De Weerd P, Sack AT. Parietal but not temporoparietal alpha-tacs modulates endogenous visuospatial attention. Cortex. 2022a:154:149–166. [DOI] [PubMed] [Google Scholar]
- Kemmerer SK, Sack AT, Graaf TA, Oever S, De Weerd P, Schuhmann T. Frequency-specific transcranial neuromodulation of alpha power alters visuospatial attention performance. Brain Res. 2022b:1782:1–15, 147834. [DOI] [PubMed] [Google Scholar]
- Klein RM. Inhibition of return. Trends Cogn Sci. 2000:4(4):138–147. [DOI] [PubMed] [Google Scholar]
- Kleiner M, Brainard D, Pelli D, Ingling A, Murray R, Broussard C. What’s new in psychtoolbox-3? Perception. 2007:36(14):1–16. [Google Scholar]
- Klimesch W. Alpha-band oscillations, attention, and controlled access to stored information. Trends Cogn Sci. 2012:16(12):606–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry M, Da Silva CJ, Sackur J, Raz A. Investigating how the modularity of visuospatial attention shapes conscious perception using type i and type ii signal detection theory. J Exp Psychol Hum Percept Perform. 2021:47(3):402–422. [DOI] [PubMed] [Google Scholar]
- Landry M, Silva CJ, Jerbi K. Differential and overlapping effects between exogenous and endogenous attention shape perceptual facilitation during visual processing. J Cogn Neurosci. 2023:35(8):1279–1300. [DOI] [PubMed] [Google Scholar]
- Lubbe RHJ, Postma A. Interruption from irrelevant auditory and visual onsets even when attention is in a focused state. Exp Brain Res. 2005:164(4):464–471. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Woodman GF, Vogel EK. Event-related potential studies of attention. Trends Cogn Sci. 2000:4(11):432–440. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Gaspelin N, Folk CL, Remington RW, Theeuwes J. Progress toward resolving the attentional capture debate. Vis Cogn. 2021:29(1):1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorselaar D, Slagter HA. Inhibition in selective attention. Ann N Y Acad Sci. 2020:1464(1):204–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow A, Elias M, Samaha J. Evaluating the evidence for the functional inhibition account of alpha-band oscillations during preparatory attention. J Cogn Neurosci. 2023:35(8):1195–1211. [DOI] [PubMed] [Google Scholar]
- Müller NG, Ebeling D. Attention-modulated activity in visual cortex—more than a simple ‘spotlight’. NeuroImage. 2008:40(2):818–827. [DOI] [PubMed] [Google Scholar]
- Pelli DG. The videotoolbox software for visual psychophysics: transforming numbers into movies. Spat Vis. 1997:10(4):437–442. [PubMed] [Google Scholar]
- Peylo C, Hilla Y, Sauseng P. Cause or consequence? Alpha oscillations in visuospatial attention. Trends Neurosci. 2021:44(9):705–713. [DOI] [PubMed] [Google Scholar]
- Quax SC, Dijkstra N, Staveren MJ, Bosch SE, Gerven MAJ. Eye movements explain decodability during perception and cued attention in meg. NeuroImage. 2019:195:444–453. [DOI] [PubMed] [Google Scholar]
- Ristic J, Landry M. Combining attention: a novel way of conceptualizing the links between attention, sensory processing, and behavior. Attention, Perception, & Psychophysics. 2015:77(1):36–49. [DOI] [PubMed] [Google Scholar]
- Sawaki R, Luck SJ. Active suppression after involuntary capture of attention. Psychon Bull Rev. 2013:20(2):296–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sörqvist P, Rönnberg J. Individual differences in distractibility: an update and a model. Psych J. 2014:3(1):42–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Störmer VS, Feng W, Martinez A, McDonald JJ, Hillyard SA. Salient, irrelevant sounds reflexively induce alpha rhythm desynchronization in parallel with slow potential shifts in visual cortex. J Cogn Neurosci. 2016:28(3):433–445. [DOI] [PubMed] [Google Scholar]
- Tadel F, Baillet S, Mosher JC, Pantazis D, Leahy RM. Brainstorm: a user-friendly application for meg/eeg analysis. Computational intelligence and neuroscience. 2011:2011:8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theeuwes J. Exogenous and endogenous control of attention: the effect of visual onsets and offsets. Percept Psychophys. 1991:49(1):83–90. [DOI] [PubMed] [Google Scholar]
- Theeuwes J. Perceptual selectivity for color and form. Percept Psychophys. 1992:51(6):599–606. [DOI] [PubMed] [Google Scholar]
- Twomey DM, Murphy PR, Kelly SP, O'Connell RG. The classic p300 encodes a build-to-threshold decision variable. Eur J Neurosci. 2015:42(1):1636–1643. [DOI] [PubMed] [Google Scholar]
- Wagenmakers E-J. A practical solution to the pervasive problems of p values. Psychon Bull Rev. 2007:14(5):779–804. [DOI] [PubMed] [Google Scholar]
- Wang J, Wang J, Sun J, Li C, Tong S, Hong X. The effects of pre-cue posterior alpha on post-cue alpha activity and target processing in visual spatial attention tasks with instructional and probabilistic cues. Cereb Cortex. 2023:33(7):4056–4069. [DOI] [PubMed] [Google Scholar]
- Woldorff MG. Distortion of erp averages due to overlap from temporally adjacent erps: analysis and correction. Psychophysiology. 1993:30(1):98–119. [DOI] [PubMed] [Google Scholar]
- Woodman GF, Wang S, Sutterer DW, Reinhart RMG, Fukuda K. Alpha suppression indexes a spotlight of visual-spatial attention that can shine on both perceptual and memory representations. Psychon Bull Rev. 2022:29(3):681–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright RD, Ward LM. Orienting of attention. New York, NY: Oxford University Press; 2008 [Google Scholar]
- Yantis S, Jonides J. Abrupt visual onsets and selective attention: voluntary versus automatic allocation. J Exp Psychol Hum Percept Perform. 1990:16(1):121–134. [DOI] [PubMed] [Google Scholar]
- Zhang H, Abagis TR, Jonides J. The malleability of attentional capture. Vis Cogn. 2021:29(9):571–574. [Google Scholar]
- Zhigalov A, Jensen O. Alpha oscillations do not implement gain control in early visual cortex but rather gating in parieto-occipital regions. Hum Brain Mapp. 2020:41(18):5176–5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data for the analyses supporting the current study is publicly available on the Brain Imaging Center at the NEURO repository: https://box.bic.mni.mcgill.ca/s/BikhIax5qb9VXLX.