Abstract
We studied the antibody response including antibody-secreting cells (ASC) in the female genital tract of mice after mucosal immunizations with the recombinant B subunit of cholera toxin (rCTB) perorally, intraperitoneally, vaginally, and intranasally (i.n.). The strongest genital antibody responses as measured with a novel perfusion-extraction method were induced after vaginal and i.n. immunizations, and these routes also gave rise to specific immunoglobulin A (IgA) and IgG ASC in the genital mucosa. Specific ASC in the iliac lymph nodes, which drain the female genital tract, were seen only after vaginal immunization. Progesterone treatment increased the ASC response in the genital tissue after all mucosal immunizations but most markedly after vaginal immunization. We also tested rCTB as a carrier for human gamma globulin (HGG) and the effect of adding cholera toxin (CT) as an adjuvant for the induction of systemic and genital antibody responses to HGG after vaginal and i.n. immunizations. Vaginal immunizations with HGG conjugated to rCTB resulted in high levels of genital anti-HGG antibodies whether or not CT was added, while after i.n. immunization the strongest antibody response was seen with the conjugate together with CT. In summary, vaginal and i.n. immunization give rise to a specific mucosal immune response including ASC in the genital tissue, and vaginal immunization also elicits ASC in the iliac lymph nodes. We have also shown that rCTB can act as an efficient carrier for a conjugated antigen for induction of a specific antibody response in the genital tract of mice after vaginal or i.n. immunization.
Sexually transmitted viral and bacterial infections of the genital tract are common and cause significant morbidity. Notable examples of such infections are those caused by herpes simplex virus (HSV), human papillomavirus, human immunodeficiency virus, Chlamydia trachomatis, Neisseria gonorrhoeae and Haemophilus ducreyi. It is a high priority to develop vaccines that induce protection from these infections and their associated diseases. Both antibodies in the cervicovaginal fluid, especially secretory immunoglobulin A (IgA) but also IgG antibodies, and cell-mediated immunity are likely to be important in the protection against many sexually transmitted infections (5, 6, 18, 36). The most investigated route for stimulation of the mucosal immune system is oral immunization. It is well known that orally administered antigens can stimulate antigen-specific B and T cells, including IgA precursor cells in the gut-associated lymphoid tissues, leading to a dissemination of activated B and T cells to various preferred mucosal effector sites, mainly the lamina propria of the gut but also the mammary and salivary glands (1, 10, 20, 23). Oral immunization with live organisms has been described to give rise to significant antibody responses in the genital tract (7, 11), whereas the oral route has been less efficient in inducing a genital immune response to nonreplicating antigens (22, 35).
There are some major differences between the mucosal immune system in the genital tract and that in the intestinal immune system. No lymphoid nodules have been detected in the genital tracts of mice, and no M-like cells similar to those found in the intestine have been reported. Furthermore, IgG is the predominant immunoglobulin in the cervicovaginal fluid, in contrast to the intestine, where secretory IgA is by far the most abundant antibody isotype (28). Several studies have shown that there is a compartmentalization of the mucosal immune system, so that mucosal immunization induces stronger immune responses at or adjacent to the site of induction than at distant sites, consistent with the observation that the dissemination of mucosal lymphocytes to more remote sites is a ligand-receptor restricted rather than a random process (13, 14, 29, 30). However, immunizations at the vaginal mucosa to induce a local antibody response have given variable results (13, 12, 34). Instead, it has recently been shown that intranasal (i.n.) immunizations can give rise to a genital antibody response (4, 12, 31).
We have used the stable and highly immunogenic molecules cholera toxin (CT) and its B subunit (CTB) as model immunogens in mice for studying the efficiency of various immunization routes to stimulate an antibody response in the female genital tract. CTB has also been found to function as a useful carrier protein for induction of mucosal IgA antibodies in the intestine and salivary glands after oral immunizations, as well as in the airways after i.n. immunizations against chemically or genetically coupled foreign antigens (3, 9, 21). We therefore evaluated the ability of a model protein antigen conjugated to CTB to elicit a local antibody response in the female genital tract.
MATERIALS AND METHODS
Antigens.
Recombinant CTB (rCTB) was produced and purified from Vibrio cholerae 358 as described previously (16). CT was obtained from List (Campbell, Calif.).
Preparation of CTB-HGG conjugate.
Commercially available, purified human gamma globulin (HGG; Kabi Pharmacia AB, Uppsala, Sweden) was further purified by gel filtration chromatography on a column (16 by 600 mm) of Sephacryl S-300 HR (Pharmacia). HGG was then chemically coupled to CTB by using N-succinimidyl (3-[2-pyridyl]-dithio)propionate (SPDP; Pharmacia) as a bifunctional coupling reagent, according to the manufacturer’s instructions. Briefly, CTB and HGG were separately derivatized with SPDP at molar ratios of 1:5 and 1:3, respectively. The SPDP-derivatized HGG was reduced with dithiothreitol, and excess dithiothreitol and pyridine-2-thione was excluded by separation via Sephadex G-25 chromatography (Pharmacia). Final ratios of 2-pyridyl disulfide on CTB and HGG were 3 and 1.5, respectively. SPDP-derivatized CTB and HGG were mixed at a weight/weight ratio of 1:5 for 24 h at 23°C. The resulting CTB-HGG conjugate was freed of released pyridine-2-thione by gel filtration on Sephadex G-25 chromatography. By means of a solid-phase enzyme-linked immunosorbent assay (ELISA) using GM1 as capture system and enzyme-labeled antibodies to CTB and HGG as detection reagents, the conjugate was shown to have GM1-binding capacity and to retain both CTB and HGG serological reactivities.
Animals.
C57BL/6 female mice, 6 to 7 weeks old, were obtained from B&K Universal (Stockholm, Sweden; Bomholtsgård, Denmark) and from our own breeding. All mice were treated with medroxyprogesteroneacetate (Depo-Provera; purchased from the Upjohn Company, Kalamazoo, Mich.), 70 μl subcutaneously (10 mg), on days 10 and 3 prior to immunization unless otherwise indicated.
Immunizations. (i) Active immunization.
The immunization routes used were intragastric (referred to as peroral [p.o.]), vaginal, i.n., intraperitoneal (i.p.), and intravenous (i.v.). CTB and CT were diluted in 0.15 M NaCl to a volume of 200 μl for i.p. immunization, 100 μl for i.v. immunization, and 25 μl for vaginal and i.n. immunizations. For the p.o. immunization, CTB was diluted to a volume of 0.5 ml in 3% Na2CO3. The time between the first and second immunizations was 12 to 14 days, with 7 to 10 days between the second and the third immunizations. The CTB and CT doses used varied between experiments as specified for the various tables and figures.
(ii) Passive immunization.
Mice were immunized i.v. with two doses of 100 μl, given 1 h apart, of pooled hyperimmune serum from syngeneic mice that previously had been actively immunized with CTB plus CT. Two hours after the second dose, when preliminary tests had indicated that the tissue distribution of antibodies had reached steady state, the mice were killed and serum and tissues were collected.
Perfusion-extraction method (PERFEXT).
Mice were euthanized 1 week after the last immunization, and 0.1 ml of 1% heparin (Lövens Kemiske Fabrik, Ballerup, Denmark) in phosphate-buffered saline (PBS) was injected i.p. in the mouse before blood was drawn from the subclavian vein. Immediately after the bleeding, the mice were killed and at least 20 ml of 0.1% heparin–PBS was infused into the heart to maximally remove blood from the tissues. The organs were collected and weighed before storage in the freezer at −20°C in a PBS solution (1 ml per g of tissue) containing 2 mM phenylmethylsulfonyl fluoride, 0.1 mg of trypsin inhibitor from soybean (Sigma Chemical Co., St. Louis, Mo.) per ml, and 0.05 M EDTA. Saponin (Sigma) was added to a final concentration of 2% (wt/vol) to permeabilize the cell membranes, and the samples were stored at 4°C overnight. The organs were spun down at 16,000 × g, and the supernatant was analyzed for antibody content by ELISA.
ELISA.
To detect anti-CTB antibodies, a GM1 ELISA was used (32). Briefly, 96-well low-binding polystyrene plates (Nunc, Roskilde, Denmark) were coated with GM1 ganglioside (0.3 nmol/ml in PBS) overnight and then incubated with 0.5 μg of CTB/ml. Test samples were added, and incubation continued for 1 h. Horseradish peroxidase-conjugated anti-mouse IgG or IgA antibodies (Southern Biotechnology Associates, Inc., Birmingham, Ala.) were added, and the ELISA was finally developed with o-phenylenediamine (Sigma) and H2O2. The antibody titers were expressed as the reciprocal sample dilution giving an absorbance of 0.4 above the background level. All antibody titers are given as the geometric mean (GM) ± 1 standard error of the mean (SEM).
Preparation of cells for ELISPOT assay.
Spleen and lymph node cells were isolated by passing the organs through a 150/250-μm nylon net to obtain a single cell suspension, and the cells were then washed in PBS. Splenic erythrocytes were lysed with ammonium chloride.
Lamina propria lymphocytes (LPL) were isolated as described elsewhere (19). In short; the small intestines were washed in Hanks’ buffered salt solution (HBSS; Gibco BRL) to remove fecal contents. The Peyer’s patches were carefully excised and put in calcium- and magnesium-free HBSS (CMF-HBSS) (Gibco BRL) with 5 mM EDTA. The sedimented Peyer’s patches were then minced through a 150/250-μm nylon net. The intestines were opened up lengthwise and cut into 5-mm pieces. The pieces were washed six times in CMF-HBSS and incubated four times for 15 min each in prewarmed (37°C) CMF-HBSS containing 5 mM EDTA. The tissue pieces were then digested with collagenase (300 U/ml; Sigma) for 1 h to extract the LPL. This incubation was repeated twice; after each incubation, LPL were collected. Finally LPL from three cycles were pooled and purified on a 40%/100% discontinuous Percoll (Pharmacia, Uppsala, Sweden) gradient by centrifugation (650 × g for 20 min). Lymphocytes were recovered from the 40%/100% interface and washed twice in PBS. The number and viability of lymphocytes were determined by trypan blue exclusion.
Cells were prepared from the genital tissue and lungs by cutting the tissues in small pieces. The tissue pieces were incubated in HBSS supplemented with collagenase-dispase (1 mg/ml; Sigma), gentamicin (0.1 mg/ml), and DNase (0.2 mg/ml) (Boehringer Mannheim) for 30 to 45 min on a magnetic stirrer at 37°C. The supernatant was decanted and saved, and the collagenase treatment was repeated once with fresh medium. The cells were washed and centrifuged (5 min, 375 × g) three times, and cell numbers and viability were determined by trypan blue exclusion. This procedure releases approximately 90% of the lymphocytes from the genital tissue and all lymphocytes from the lungs.
ELISPOT.
Cell suspensions from genital tissue, lungs, lymph nodes, spleen, lamina propria, and Peyer’s patches were analyzed for CTB-specific antibody-secreting cells (ASC) by an ELISPOT assay as described previously (22), with some modifications. Briefly, nitrocellulose-bottom 96-well plates (Millipore) were coated overnight with GM1 ganglioside (3 nmol/ml) followed by CTB (2.5 μg/ml) and were then blocked with 0.5% (wt/vol) bovine serum albumin. Immediately after isolation, the mononuclear cells (MNC) were added in triplicate to the antigen-coated wells and incubated overnight at 37°C in a moist atmosphere with 5% CO2. Biotin-labeled goat anti-mouse IgG and IgA antibodies (Southern Biotechnology) were added, followed by horseradish peroxidase-labeled egg-avidin (Extravidin; 4 μg/ml; Sigma). Spots were developed by using 0.3 mg of 3-amino-9-ethylcarbazole (Sigma) per ml and 0.015% (vol/vol) H2O2 in 0.1 M sodium acetate (pH 5.0).
Statistical methods.
Before calculations, all values were log10 transformed. GM and SEM or standard deviation were calculated. Student’s t test with Bonferroni correction was used to compare mean values of different groups. In the CTB-HGG study, analysis of variance was used as appropriate for analysis of the significances of differences in titers, and post hoc comparisons of the individual groups were performed with Scheffe’s test. The software Statistica 4.0 for Windows (Softstat, Tulsa, Okla.) was used for the calculations.
RESULTS
Antibody response in the female genital tract.
To determine the best route of immunization for induction of high antibody titers in the female genital tract, mice were immunized three times i.p., vaginally, i.n., or p.o. with rCTB mixed with a low amount of CT. One week after the last immunization, the genital tissue was collected and divided into fallopian tubes, uterus, and vagina before extraction of the immunoglobulins.
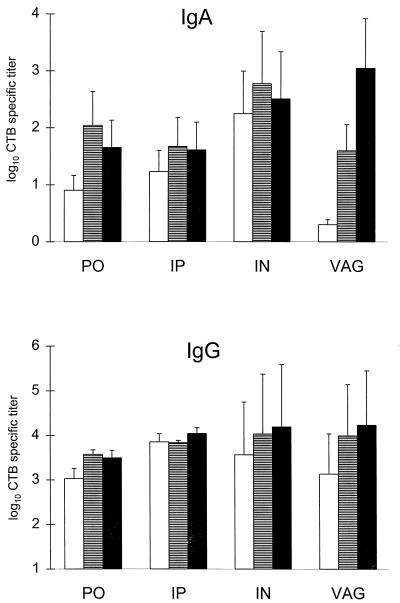
The vaginal and i.n. routes of immunization were significantly (P < 0.01) more efficient than the i.p. and p.o. routes in stimulating a specific IgA response to CTB in the vaginal mucosa (Fig. 1). The i.n. immunizations induced high specific titers in the vagina, the uterus, and the fallopian tubes, while vaginal immunization gave the highest specific IgA titers in the vagina but lower titers in the uterus and no specific titers in the fallopian tubes. The i.p. and p.o. immunizations also resulted in significant genital titer responses, but these were in both cases approximately 10 times lower than those seen after the i.n. immunizations (Fig. 1). The IgG antibody levels were relatively high in all parts of the genital tract irrespective of the immunization route (Fig. 1).
FIG. 1.
CTB-specific IgA and IgG titers in the genital mucosa after three p.o., i.p., i.n., or vaginal (VAG) immunizations with rCTB admixed with a small amount of CT. Antibody titers are given as log10 of the GM of titers ± SEM. Each group contain 8 to 12 mice. White bars, titers in the fallopian tubes; striped bars, titers in the uterus; black bars, titers in the vagina. The CTB and CT doses used were 135 μg of CTB plus 5 μg of CT (p.o.), 20 μg of CTB plus 1.25 μg of CT (i.p.), and 85 μg of CTB plus 5 or 2.5 μg of CT (vaginal or i.n., respectively). Student’s t test corrected for multiple comparisons shows that vaginal IgA titers after i.n. or vaginal immunizations were significantly (P < 0.01) higher than after p.o. or i.p. immunizations.
A separate experiment performed with a much lower CTB dose, 6 μg, given together with 2 μg of CT gave a pattern very similar to that for the high-dose experiment illustrated in Fig. 1, irrespective of immunization route (data not shown).
The PERFEXT method.
We used the PERFEXT method combined with ELISA for measuring antibody levels in different mucosal tissues. Antibodies were extracted in vitro from the tissues by freezing and thawing in a solution containing saponin. The tissues had been collected after extensive perfusion of the animals to maximally remove blood from the tissues. To determine whether the antibodies measured by this method result from the transudation of serum antibodies, we compared the tissue-to-serum antibody titer ratios after different routes of mucosal immunizations with those after passive i.v. immunization with immune serum. The results are shown in Table 1. The specific IgA titers in the small intestine after p.o. immunization, in the lungs and genital tissue after i.n. immunization, and in the genital tissue after vaginal immunization in a selective route-tissue-related manner exceed the corresponding serum titers and could thus not be explained by transudation of serum antibodies. In addition, the results show that only a few percent of the passively administered antibodies are found in the mucosal tissues, thereby supporting the notion that the substantially higher levels of specific IgA seen in the vagina/uterus tissue after p.o. immunization and of specific IgG after vaginal immunization also to a significant extent represent local synthesis rather than mere serum transudation. Altogether, the results strongly indicate that the PERFEXT method largely measures the local IgA antibody response in the tissue and that the contribution of IgA antibodies resulting from maintained blood in the tissue represents only a very small part of the measured antibody titers.
TABLE 1.
CTB-specific antibody titers in tissues
| Route of immunizationa | GM % of serum titerb (GM ± 1 SD)
|
|||||
|---|---|---|---|---|---|---|
| Lungs
|
Vagina/uterus
|
Small intestine
|
||||
| IgA | IgG | IgA | IgG | IgA | IgG | |
| p.o. | 18 (6.2–53) | 2.5 (0.72–8.8) | 15 (6.3–33) | 5.4 (1.8–16) | 131 (105–164) | 1.9 (1.4–2.7) |
| Vaginal | 0.76 (0.39–1.51) | 2.2 (1.6–3.0) | 221 (204–240) | 40 (30–53) | 7.1 (5.0–10) | 4.0 (3.1–5.0) |
| i.n. | 220 (162–300) | 2.3 (0.88–6.2) | 144 (60–343) | 4.7 (3.4–6.7) | 11 (6.4–20) | 0.66 (0.52–0.84) |
| Passive i.v. | 0.71 (0.32–1.60) | 0.87 (0.29–2.59) | 1.95 (0.52–7.26) | 4.14 (1.3–12.7) | 1.48 (0.45–4.93) | 0.78 (0.67–0.90) |
The CTB and CT doses used in this experiment were 6 μg of CTB plus 2 μg of CT. Each group contained four mice.
GM% = CTB-specific antibody titer in tissue/corresponding serum titers × 100.
ASC in genital tract tissue.
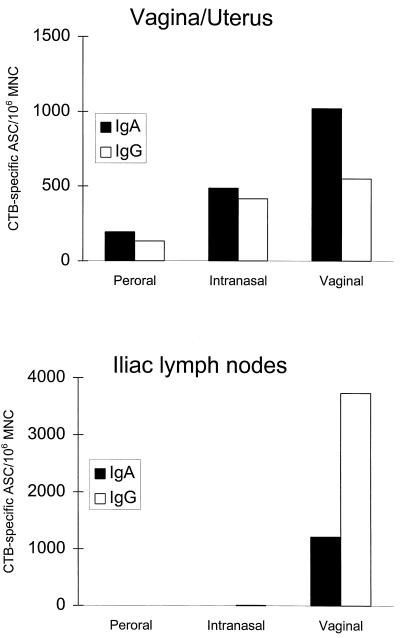
To further establish the potency with which the different mucosal immunizations induced a local antibody production in the genital tissue, we used the ELISPOT assay to enumerate CTB-specific IgA and IgG ASC after three p.o., vaginal, or i.n. immunizations. One week after the last immunization, MNC were isolated from combined vagina, uterus, and fallopian tube tissues as well as from the iliac lymph nodes (ILN) and examined for specific anti-CTB IgA and IgG ASC. As shown in Fig. 2, both the vaginal and to a lesser extent the i.n. immunizations induced high numbers of CTB-specific IgA as well as IgG ASC in the female genital tissue, thus confirming the results from the PERFEXT studies of local production of specific antibodies after either of these routes of immunization. In further agreement with the PERFEXT studies, p.o. immunization induced only modest numbers of CTB-specific ASC in the genital tissue. Upon examination of the ASC in the ILN that drain the female genital tract, it was evident that only the vaginal immunization induced CTB-specific ASC at this site (Fig. 2).
FIG. 2.
CTB-specific IgA and IgG ASC in the vagina and uterus and ILN after p.o. or vaginal immunization with 20 μg of CTB plus 5 μg of CT or i.n. immunization with 20 μg of CTB plus 2.5 μg of CT. Each group consisted of a pool of three mice, and values are given as the GM of specific ASC/106 MNC from two independent experiments.
Effect of progesterone treatment on the specific ASC.
All mice used in our study had been treated with progesterone. To assess the possible effect of such a treatment on the antibody response, we compared the number of specific ASC in groups of progesterone-treated or untreated animals following immunization with CTB. As evident from Table 2, the progesterone treatment increased both the anti-CTB IgA and IgG ASC in the female genital tract after all mucosal immunizations, although the increase was most marked after vaginal immunization. No specific IgA ASC were detected in the genital tract following i.v. immunization (not shown).
TABLE 2.
Effect of progesterone treatment expressed as the ratio between CTB-specific ASC in mice treated with progesterone and in untreated micea
| Route of immunization | Tissue | GM
|
|
|---|---|---|---|
| IgA | IgG | ||
| p.o. | Small intestine | 0.55 | 0.87 |
| Large intestine | 0.55 | 0.71 | |
| Vagina/uterus | 18 | 3.5 | |
| Spleen | 0.85 | 0.69 | |
| i.n. | Vagina/uterus | 10 | 32 |
| Lungs | 1.8 | 1.5 | |
| Spleen | 3.9 | 1.5 | |
| Vaginal | Vagina/uterus | 129 | 18 |
| ILN | 3.8 | 3.1 | |
| Spleen | 1.6 | 0.94 | |
| i.v. | Spleen | 1.6 | 0.94 |
The doses of CTB and CT used in these experiments were 20 μg of CTB plus 5 μg of CT for p.o. and vaginal immunizations, 20 μg of CTB plus 2.5 μg of CT for i.n. immunizations, and 8 μg of CTB plus 0.2 μg of CT for i.v. immunizations. Each group consists of a pool of three mice, and the results are expressed as the GM of two independent experiments.
Antibody response in the genital tract to a CTB-protein antigen conjugate.
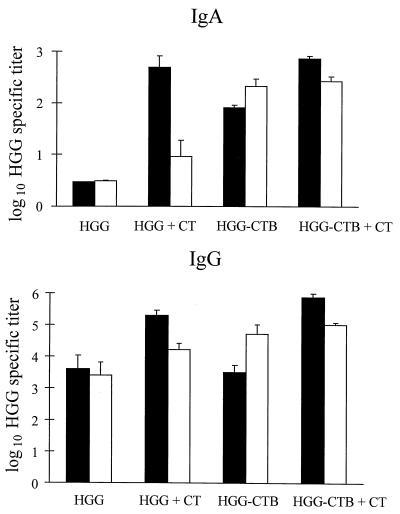
The potential of using CTB as a carrier protein for the induction of a systemic as well as a local genital tract antibody response to a conjugated protein antigen was evaluated by using an HGG-CTB conjugate. We also analyzed the effect of adding free CT as an adjuvant to either HGG or the HGG-CTB conjugate. The antibody response in the serum and the female genital tract to HGG was measured after three i.n. or vaginal immunizations. In serum, no specific IgA titers could be detected after immunization with HGG alone (Fig. 3). In contrast, when using CT as an adjuvant and/or rCTB as a carrier protein for HGG, we observed a significant HGG-specific serum IgA response after i.n. immunization (P < 0.001). Coupling of HGG to CTB was also found necessary to induce a significant HGG-specific IgA serum response after vaginal immunization (P < 0.001), but in contrast to the i.n. immunizations, the addition of CT had no effect on the HGG-specific IgA titers. All immunization regimens induced high IgG titers to HGG in serum (Fig. 3), which were further increased in the i.n. immunization group if CT was used as an adjuvant (P = 0.003) and in the vaginal immunization group by using CTB as a carrier and CT as an adjuvant (P = 0.008).
FIG. 3.
HGG-specific IgA and IgG titers in serum after i.n. or vaginal immunizations with 12 μg of HGG ± 3 μg of CTB ± 2 μg of CT. Antibody titers are given as log10 of the GM of titers ± SEM. Each group contained four mice. Black bars, i.n. immunization; white bars, vaginal immunization.
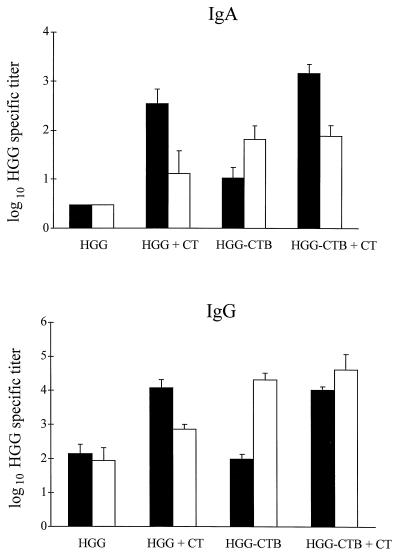
In the genital tract, after i.n. immunization, it was necessary to use CT as an adjuvant to obtain a significant IgA response (P < 0.001) (Fig. 4). In contrast, the addition of CT had no significant effect on the local IgA response following the vaginal immunization, which instead was dependent on the conjugation of HGG to CTB (P < 0.05 for the IgA response in the HGG-CTB group versus the HGG alone group). This difference between the vaginal and i.n. immunizations was also obvious in the induction of a specific IgG response in the genital tract. The addition of CT increased the genital IgG titers to HGG significantly after i.n. immunization (P < 0.001) but not after vaginal immunization (Fig. 4). In contrast, the conjugation of HGG to CTB markedly increased the genital IgG titers after vaginal immunization (P < 0.05), while the conjugate was no better than HGG alone in stimulating a genital tract IgG response after i.n. immunization unless CT was added as an adjuvant.
FIG. 4.
HGG-specific IgA and IgG titers in vagina and uterus after i.n. or vaginal immunizations with 12 μg of HGG ± 3 μg of CTB ± 2 μg of CT. Antibody titers are given as log10 of the GM of titers ± SEM. Each group contained four mice. Black bars, i.n. immunization; white bars, vaginal immunization.
DISCUSSION
An important component in research efforts to develop vaccines against viral or bacterial sexually transmitted diseases (STDs) is the definition of routes and other conditions of immunization that stimulate mucosal immune responses in the genital tract, and ideally also systemic immune responses. Our results for female mice after immunization with CTB and CT or a CTB-protein antigen conjugate show that (i) both vaginal and i.n. immunizations give rise to strong antibody responses in the genital tract mucosa, while p.o. or parenteral (i.p. or i.v.) immunizations are less efficient in this regard; (ii) there is a strong hormonal influence on the immune response, progesterone being found to increase the mucosal antibody response in the genital tract; and (iii) conjugation to CTB markedly increases the systemic as well as the genital tract immunogenicity of a model protein antigen (HGG) when administered by either the vaginal or the i.n. route. After i.n. immunization, the immunogenicity is much further enhanced by the addition of CT as an adjuvant, while the immune response after vaginal immunization with the CTB-HGG conjugate is strong both in the presence and in the absence of CT.
The local antibody response after either vaginal or i.n. immunization was documented both with a novel PERFEXT method based on extraction of antibodies from mucosal tissues of extensively perfused animals and with the ELISPOT method enumerating specific ASC in isolated MNC from the tissues. The PERFEXT results provided evidence for a significant although relatively modest local IgA formation in the genital tract after p.o. immunization and a more substantial local antibody response after vaginal and i.n. immunizations. These results were confirmed by the ELISPOT assays of ASC responses in the different tissues. We also found specific IgG ASC in addition to IgA ASC in the genital tissue, supporting previous observations (12) that there is a local production of IgG in the genital tract after mucosal immunizations. However, in contrast to the specific IgA antibody titers, high IgG antibody titers in the range observed for serum transudation were found in the genital tract independently of the immunization route. This indicates that despite the evidence for some local production, the bulk of IgG antibodies in the genital tract may come from transudation of serum.
The fact that the vaginal immunization induced a large number of specific IgA and IgG ASC in the draining lymph nodes in addition to the ASC response in the genital mucosa is a clear indication that the genital tract can act not only as an expression site but also as an initiation site of an immune response. The induction of specific ASC, mainly IgG but also IgA, in the ILN after vaginal immunization probably contributes to both the local antibody response in the genital tract and the systemic antibody response seen after this route of immunization. In our study, CTB-specific ASC in the ILN were found exclusively after vaginal immunization and not after i.n. immunization, neither 7 nor 14 days (not shown) after the third immunization. Gallichan and Rosenthal (12) found HSV type 2 (HSV-2)-specific ASC in the ILN following i.n. immunization with a recombinant adenovirus vector expressing HSV-2 antigen followed by an intravaginal challenge with HSV-2. This discrepancy may be explained by the use of nonreplicating CTB rather than replicating adenovirus antigens for i.n. immunization and the fact that the animals in the study by Gallichan and Rosenthal were intravaginally challenged with live virus.
Although the strongest local IgA antibody and ASC responses in the cervicovaginal mucosa was achieved by the vaginal immunizations, almost as strong cervicovaginal responses associated with an even higher response in the uterus could be obtained after i.n. immunization. It is not clear why the i.n. route, much better than the p.o. and parenteral immunizations, induces such a strong antibody response in the genital tract. One possibility is the differential expression of homing receptors on migrating antigen-activated immunocytes from different mucosal induction sites. Such a difference was recently found in human subjects after immunization with CTB by the i.n., p.o. and rectal routes, where the circulating ASC following p.o. or rectal immunizations expressed α4β7 and only a minor fraction of these cells expressed L-selectin, while the ASC after i.n. immunization coexpressed L-selectin and α4β7 (30). However, it is not known which ligand-receptor specificities are involved in the homing of lymphocytes to the genital tract. We have recently been able to confirm the capacity of i.n. immunization with rCTB to elicit a specific IgA and IgG antibody response in vaginal secretions of humans (4), and Wassén et al. (35) found that in humans, vaginal immunization with CTB and to a lesser extent p.o. immunization could induce specific antibodies in the genital tract. Thus, it appears that both vaginal and i.n. immunizations would be efficient administration routes for future STD vaccines in women, and work is in progress to determine if the i.n. route may also be useful for vaccination of males. Arguably, it cannot be excluded that after the i.n. immunization there might be some spillage from the nasal passages into the lungs, which could possibly affect the antibody response, including its tissue distribution. However, in a recent study of humans, we have confirmed the induction of CTB-specific antibodies in the vaginal secretions after i.n. vaccination under conditions where pulmonary immunization practically could be excluded.
The finding that CTB induces a strong antibody response in the female genital tract after vaginal immunization as shown in this study and others (35) differ from results reported by Haneberg et al. (13), who found that vaginal immunization with CTB did not induce any vaginal antibody response. A likely explanation for this discrepancy is the influence of estrogen and progesterone on the immune system in the female genital tract. The animals used by Haneberg et al. were not treated with progesterone, while our mice were treated with progesterone throughout the study. The ability to respond to an antigen varies greatly with the estrous cycle. When animals are in the estrous stage, the epithelium can act as a barrier to protein movement and may interfere with the antigen uptake (27). Further, it has been shown that the ability of APC to present antigens in vagina and uterus varies with the stage of the estrous cycle (38, 39). The treatment with progesterone before immunization reduces variability in the results due to hormonal influence. Progesterone maintains the animals in the midestrus-diestrous stage of the cycle, where the influence of estrogen is low; an additional advantage of progesterone treatment is easier administration of the antigen by the vaginal route. Progesterone is, however, known to affect the immune system, although the effects described are not consistent. Wira and Sullivan (37) found in rats that progesterone blocked estradiol-stimulated increases in uterine IgA and IgG antibody levels and that progesterone either alone or in combination with estradiol inhibited the cervicovaginal IgA and IgG response. Parr and Parr (26), on the other hand, found that in the mouse uterus both estradiol and progesterone increased the number of IgA plasma cells, and Gallichan and Rosenthal (12) recently reported a selective increase in IgG in vaginal secretions after progesterone treatment. Our results presented here show that treatment with progesterone before immunization markedly increased the number of specific IgA and IgG ASC in the vagina and uterus after all mucosal immunizations and that this effect was most pronounced after vaginal immunization and was also evident in the ILN.
An important reason for studying the genital tract immune response after various mucosal routes of immunization using CTB as a model antigen relates to the promising features of CTB as a carrier-delivery system for future STD vaccine antigens (22, 31). The induction of a mucosal immune response to most nonreplicating antigens generally requires that the antigen be given together with a strong mucosal adjuvant and/or be administered physically linked to a mucosa-binding lectin-like carrier molecule (2). CTB, with a strong binding affinity to mucosal surfaces, has proved to be an exceptionally efficient mucosal immunogen, especially in humans, after either p.o., i.n., rectal, or vaginal immunizations (4, 30, 33, 35). CTB has also been shown in animals to function as an efficient carrier for other antigens, especially when coadministered with CT (3, 9, 24). In the present study, we show that rCTB can act as a carrier for HGG even without the use of CT as an adjuvant and that such a conjugate induces a substantial genital mucosal IgA and IgG anti-HGG response after vaginal immunization. Conjugation of HGG to CTB also enhanced the specific IgA antibody titers both in serum and in the genital tract after i.n. immunization. The adjuvant properties of CT appear related to the route of immunization, but the reason for this is not clear. Whereas addition of free CT in the i.n.-immunized group markedly enhanced the antibody response to both free and coupled antigen, no such effect of CT was observed in vaginally immunized animals. The much stronger immunogenicity and adjuvant effect of CT compared with those of CTB in mice is in contrast to the effects seen after vaccination of humans, where CTB alone is strongly immunogenic after p.o., vaginal, and i.n. vaccinations and antibody levels in the gut after oral vaccination are comparable to the levels seen in convalescents after severe cholera disease (4, 33, 35). In preliminary studies, we have shown a significant increase in the vaginal IgA antibody response in mice to a specific C. trachomatis candidate vaccine peptide (a colinear T-B-cell epitope sequence derived from the major outer membrane protein of C. trachomatis) after conjugating this peptide to CTB and administering the conjugate by the vaginal route (15). Altogether, our results support the concept that CTB is a promising carrier/immunomodulating molecule for inducing local genital immunity to relevant proteins or peptide antigens conjugated to CTB and that either the vaginal or the i.n. route of administration may be useful for this purpose.
ACKNOWLEDGMENTS
This work was supported by the Swedish Medical Research Council (16x-3383), the Sida-SAREC Sweden Special Program for AIDS and Related Diseases, the Commission of the European Communities for Biomedical and Health Research (Biomed 1), the National Institutes of Health (grant RO1-AI35543), and Maxim Pharmaceuticals.
REFERENCES
- 1.Ahlstedt S, Carlsson B, Fällström S P, Hansson L Å, Holmgren J, Lidin-Janson G, Lindblad B S, Jodal U, Kaijser B, Sohl-Åkerlund A, Wadsworth C. Antibodies in human serum and milk induced by enterobacteria and food proteins. Proc Ciba Found Symp. 1977;46:115–134. doi: 10.1002/9780470720288.ch6. [DOI] [PubMed] [Google Scholar]
- 2.Aizpurua H J, Russell-Jones G J. Oral vaccination. Identification of classes of proteins that provoke an immune response upon oral feeding. J Exp Med. 1988;167:440–451. doi: 10.1084/jem.167.2.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergquist C, Lagergård T, Lindblad M, Holmgren J. Local and systemic antibody responses to dextran-cholera toxin B subunit conjugates. Infect Immun. 1995;63:2021–2025. doi: 10.1128/iai.63.5.2021-2025.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergquist C, Johansson E-L, Lagergård T, Holmgren J, Rudin A. Intranasal vaccination of humans with recombinant cholera toxin B subunit induces systemic and local antibody responses in the upper respiratory tract and the vagina. Infect Immun. 1997;65:2676–2684. doi: 10.1128/iai.65.7.2676-2684.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunham R C, Kuo C-C, Cles L, Holmes K K. Correlation of host immune response with quantitative recovery of Chlamydia trachomatis from the human endocervix. Infect Immun. 1983;39:1491–1494. doi: 10.1128/iai.39.3.1491-1494.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cotter T W, Meng Q, Shen Z-L, Zhang Y-X, Su H, Caldwell H D. Protective efficacy of a major outer membrane protein-specific immunoglobulin A (IgA) and IgG monoclonal antibodies in a murine model of Chlamydia trachomatis genital tract infection. Infect Immun. 1995;63:4704–4714. doi: 10.1128/iai.63.12.4704-4714.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui Z D, Tristram D, LaScolea L J, Kwiatowski T, Jr, Kopti S, Ogra P L. Induction of antibody response to Chlamydia trachomatis in the genital tract by oral immunization. Infect Immun. 1991;59:1465–1469. doi: 10.1128/iai.59.4.1465-1469.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Czerkinksky C, Nilsson L-Å, Nygren H, Ouchterlony Ö, Tarkowski A. A solid-phase enzyme-linked immunospot (ELISPOT) assay for enumeration of specific antibody-secreting cells. J Immunol Methods. 1983;65:109–121. doi: 10.1016/0022-1759(83)90308-3. [DOI] [PubMed] [Google Scholar]
- 9.Czerkinsky C, Russell M W, Lycke N, Lindblad M, Holmgren J. Oral administration of a streptococcal antigen coupled to cholera toxin B subunit evokes strong antibody responses in salivary glands and extramucosal tissues. Infect Immun. 1989;57:1072–1077. doi: 10.1128/iai.57.4.1072-1077.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Czerkinsky C, Svennerholm A-M, Quiding M, Jonsson R, Holmgren J. Antibody-producing cells in peripheral blood and salivary glands after oral cholera vaccination of humans. Infect Immun. 1991;59:996–1001. doi: 10.1128/iai.59.3.996-1001.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fidel P L, Jr, Lynch M E, Conaway D H, Tait L, Sobel J D. Mice immunized by primary vaginal Candida albicans infection develop acquired vaginal mucosal immunity. Infect Immun. 1995;63:547–553. doi: 10.1128/iai.63.2.547-553.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallichan G S, Rosenthal K L. Specific secretory immune responses in the female genital tract following intranasal immunization with a recombinant adenovirus expressing glycoprotein B of herpes simplex virus. Vaccine. 1995;13:1589–1595. doi: 10.1016/0264-410x(95)00100-f. [DOI] [PubMed] [Google Scholar]
- 13.Haneberg B, Kendall D, Amerongen H M, Apter F M, Kraehenbuhl J-P, Neutra M R. Induction of specific immunoglobulin A in the small intestine, colon-rectum and vagina measured by a new method for collection of secretions from local mucosal surfaces. Infect Immun. 1994;62:15–23. doi: 10.1128/iai.62.1.15-23.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hopkins S, Kraehenbuhl J-P, Schödel F, Potts A, Peterson D, de Gandi P, Nardelli-Haefliger D. A recombinant Salmonella typhimurium vaccine induces local immunity by four different routes of immunization. Infect Immun. 1995;63:3279–3286. doi: 10.1128/iai.63.9.3279-3286.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johansson E, Fredriksson M, Lindblad M, Caldwell H, Czerkinsky C, Holmgren J. Cholera toxin and its B-subunit as mucosal immunogens and carrier/adjuvant molecules for inducing genital immunity. Clin Immunol Immunopathol. 1995;76:S43–S44. [Google Scholar]
- 16.Lebens M, Johansson S, Osek J, Lindblad M, Holmgren J. Large-scale production of Vibrio cholerae toxin B subunit for use in oral vaccines. Bio Technology. 1993;11:1574–1578. doi: 10.1038/nbt1293-1574. [DOI] [PubMed] [Google Scholar]
- 17.Lehner T, Panagiotidi C, Bergmeier L A, Tao L, Brookes R, Gearing A, Adams S E. Genital-associated lymphoid tissue in female non-human primates. Adv Mucosal Immunol. 1995;371A:357–365. doi: 10.1007/978-1-4615-1941-6_75. [DOI] [PubMed] [Google Scholar]
- 18.Lehner T, Wang Y, Cranage M, Bergmeier L A, Mitchell E, Tao L, Hall G, Dennis M, Cook N, Brookes R, Klavinskis L, Jones I, Doyle C, Ward R. Protective mucosal immunity elicited by targeted iliac lymph node immunization with a subunit SIV envelope and core vaccine in macaques. Nature Med. 1996;2:767–775. doi: 10.1038/nm0796-767. [DOI] [PubMed] [Google Scholar]
- 19.Lycke N. A sensitive method for the detection of specific antibody production in different isotypes from single lamina propria plasma cells. Scand J Immunol. 1986;24:393–403. doi: 10.1111/j.1365-3083.1986.tb02127.x. [DOI] [PubMed] [Google Scholar]
- 20.McDermott M R, Bienenstock J. Evidence for a common mucosal immune system. I. Migration of B immunoblasts into intestinal, respiratory and genital tissues. J Immunol. 1979;122:1892–1898. [PubMed] [Google Scholar]
- 21.McKenzie S, Halsey J F. Cholera toxin B-subunit as a carrier protein to stimulate a mucosal immune response. J Immunol. 1984;133:1818–1824. [PubMed] [Google Scholar]
- 22.Menge A C, Michalek S M, Russell M W, Mestecky J. Immune response of the female rat genital tract after oral and local immunization with keyhole limpet hemocyanin conjugated to the cholera toxin B subunit. Infect Immun. 1993;61:2162–2171. doi: 10.1128/iai.61.5.2162-2171.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mestecky J. The common mucosal immune system and current strategies for induction of immune responses in external secretions. J Clin Immunol. 1987;7:265–276. doi: 10.1007/BF00915547. [DOI] [PubMed] [Google Scholar]
- 24.Nedrud J G, Liang X P, Lamm M E. Oral immunization with Sendai virus in mice. Curr Top Microbiol Immunol. 1989;146:117–122. doi: 10.1007/978-3-642-74529-4_13. [DOI] [PubMed] [Google Scholar]
- 25.Parr E L, Parr M B, Thapar M. A comparison of specific antibody responses in mouse vaginal fluid after immunization by several routes. J Reprod Immun. 1988;14:165–176. doi: 10.1016/0165-0378(88)90067-8. [DOI] [PubMed] [Google Scholar]
- 26.Parr M B, Parr E L. Effects of oestradiol-17β and progesterone on the number of plasma cells in uteri of ovariectomized mice. J Reprod Fertil. 1986;77:91–97. doi: 10.1530/jrf.0.0770091. [DOI] [PubMed] [Google Scholar]
- 27.Parr M B, Parr E L. Antigen recognition in the female reproductive tract: uptake of intraluminal protein tracers in the mouse vagina. J Reprod Immunol. 1990;17:101–114. doi: 10.1016/0165-0378(90)90029-6. [DOI] [PubMed] [Google Scholar]
- 28.Parr M B, Parr E L. Mucosal immunity in the female and male reproductive tracts. In: Ogra P L, Mestecky J, Lamm M E, Strober W, McGhee J R, Bienenstock J, editors. Handbook of mucosal immunology. San Diego, Calif: Academic Press, Inc.; 1994. pp. 677–689. [Google Scholar]
- 29.Quiding-Järbrink M, Granström G, Nordström I, Holmgren J, Czerkinsky C. Induction of compartmentalized B-cell responses in human tonsils. Infect Immun. 1995;63:853–857. doi: 10.1128/iai.63.3.853-857.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quiding-Järbrink M, Nordström I, Granström G, Kilander A, Jertborn M, Butcher E C, Lazarovits A I, Holmgren J, Czerkinsky C. Differential expression of tissue-specific adhesion molecules on human circulating antibody-forming cells after systemic, enteric, and nasal immunizations a molecular basis for the compartmentalization of effector B cell responses. J Clin Invest. 1997;99:1281–1286. doi: 10.1172/JCI119286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Russell M W, Moldoveanu Z, White P L, Sibert G J, Mestecky J, Michalek S. Salivary, nasal, genital, and systemic antibody responses in monkeys immunized intranasally with a bacterial protein antigen and the cholera toxin B subunit. Infect Immun. 1996;64:1272–1283. doi: 10.1128/iai.64.4.1272-1283.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Svennerholm A-M, Holmgren J. Identification of Escherichia coli heat-labile enterotoxin by means of a ganglioside immunosorbent assay (GM1-ELISA) procedure. Curr Microbiol. 1978;1:19–23. [Google Scholar]
- 33.Svennerholm A M, Sack D A, Holmgren J, Bardhan P K. Intestinal antibody responses after immunisation with cholera B subunit. Lancet. 1982;6:305–308. doi: 10.1016/s0140-6736(82)91568-9. [DOI] [PubMed] [Google Scholar]
- 34.Thapar M A, Parr E L, Parr M B. Secretory immune responses in mouse vaginal fluid after pelvic, parenteral or vaginal immunization. Immunology. 1990;70:121–125. [PMC free article] [PubMed] [Google Scholar]
- 35.Wassén L, Schön K, Holmgren J, Jertborn M, Lycke N. Local intravaginal vaccination of the female genital tract. Scand J Immunol. 1996;44:408–414. doi: 10.1046/j.1365-3083.1996.d01-320.x. [DOI] [PubMed] [Google Scholar]
- 36.Whaley K J, Zeitlin L, Barratt R A, Hoen T E, Cone R A. Passive immunization of the vagina protects mice against vaginal transmission of genital herpes infections. J Infect Dis. 1994;169:647–649. doi: 10.1093/infdis/169.3.647. [DOI] [PubMed] [Google Scholar]
- 37.Wira C R, Sullivan D A. Estradiol and progesterone regulation of immunoglobulin A and G and secretory component in cervicovaginal secretions of the rat. Biol Reprod. 1985;32:90–95. doi: 10.1095/biolreprod32.1.90. [DOI] [PubMed] [Google Scholar]
- 38.Wira C R, Rossoll R M. Antigen presenting cells in the female reproductive tract: influence of the estrous cycle on antigen presentation by uterine epithelial and stromal cells. Endocrinology. 1995;136:4526–4534. doi: 10.1210/endo.136.10.7664673. [DOI] [PubMed] [Google Scholar]
- 39.Wira C R, Rossoll R M. Antigen presenting cells in the female reproductive tract: influence of sex hormones on antigen presentation in the vagina. Immunology. 1995;84:505–508. [PMC free article] [PubMed] [Google Scholar]