Abstract

Zerovalent scandium, zirconium, hafnium, and manganese nanoparticles are prepared by reduction of ScCl3, ZrCl4, HfCl4, and MnCl2 with lithium or sodium naphthalenide in a one-pot, liquid-phase synthesis. Small-sized monocrystalline nanoparticles are obtained with diameters of 2.4 ± 0.2 nm (Sc), 4.0 ± 0.9 nm (Zr), 8.0 ± 3.9 nm (Hf) and 2.4 ± 0.3 nm (Mn). Thereof, Zr(0) and Hf(0) nanoparticles with such size are shown for the first time. To probe the reactivity and reactions of the as-prepared Sc(0), Zr(0), Hf(0), and Mn(0) nanoparticles, they are exemplarily reacted in the liquid phase (e.g., THF, toluene, ionic liquids) with different sterically demanding, monodentate to multidentate ligands, mainly comprising O–H and N–H acidic alcohols and amines. These include isopropanol (HOiPr), 1,1′-bi-2-naphthol (H2binol), N,N′-bis(salicylidene)ethylenediamine (H2salen), 2-mercaptopyridine (2-Hmpy), 2,6-diisopropylaniline (H2dipa), carbazole (Hcz), triphenylphosphane (PPh3), N,N,N′,N′-tetramethylethylenediamine (tmeda), 2,2′-bipyridine (bipy), N,N′-diphenylformamidine (Hdpfa), N,N′-(2,6-diisopropylphenyl)-2,4-pentanediimine ((dipp)2nacnacH), 2,2′-dipydridylamine (Hdpa), and 2,6-bis(2-benzimidazolyl)pyridine (H2bbp). As a result, 22 new compounds are obtained, which frequently exhibit a metal center coordinated only by the sterically demanding ligand. Options and restrictions for the liquid-phase syntheses of novel coordination compounds using the oxidation of base-metal nanoparticles near room temperature are evaluated.
Short abstract
Zerovalent scandium, zirconium, hafnium, and manganese nanoparticles (2−8 nm in size) are prepared by the reduction of ScCl3, ZrCl4, HfCl4, and MnCl2 with lithium or sodium naphthalenide. Reactivity and reactions of the as-prepared metal nanoparticles are exemplarily probed by the reaction with sterically demanding, monodentate to multidentate ligands near room temperature in the liquid phase at quasi-homogeneous conditions and results in 22 new coordination compounds.
Introduction
The knowledge about metal nanoparticles becomes smaller, hence, the more basic the respective metal. In particular, this relates to the great number of base metals with electrochemical potentials (E0) of −1.0 to −2.5 V (taking E0 of the respective bulk metal as an indicator).1 The limited knowledge about base-metal nanoparticles can be illustrated by annual publications on selected metal nanoparticles. Thus, more than 1500 publications addressed the synthesis of Au(0) nanoparticles (E0(bulk-Au) = +1.5 V)2 in 2022, and about 500 publications were related to the synthesis of Co(0) nanoparticles (E0(bulk-Co) = −0.3 V),2 whereas no report in 2022 addressed the synthesis of, for instance, Zr(0) nanoparticles (E0(bulk-Zr) = −1.5 V).2 Current knowledge can also be directly correlated to the synthesis of the base-metal nanoparticles, which is more demanding the higher their reactivity and the smaller their size.
With regard to Sc(0), Zr(0), Hf(0), and Mn(0) metal nanoparticles, the number of publications is limited, in general. So far, physical methods (e.g., evaporation techniques, laser ablation, sonochemical methods) or solid-state reactions were used most often,3−7 which, however, usually resulted in large particles (>50 nm) with certain agglomeration, broad size distributions and/or significant oxygen contaminations. Only few publications report on a liquid-phase synthesis of Sc(0), Zr(0), Hf(0), and Mn(0) metal nanoparticles. Herein, protection of the reactive metal with a dense shell (e.g., metal carbides, metal oxides, metal sulfides, polymers) was required to avoid oxidation by moisture or air.8−12 In the case of Mn(0) nanoparticles, the thermal decomposition of Mn2(CO)10 is most suitable.13 Finally, some reports even claim aqueous syntheses,14−18 which, however, seems hardly traceable with regard to the position of the respective metals in the voltage series (E0(bulk-Mn) = −1.2 V to E0(bulk-Sc) = −2.0 V).2 In this respect, it must be noticed that the reactivity of metal nanoparticles is expected to be significantly higher as expressed by the electrochemical potential of the bulk metals.19
Due to their high reactivity, base-metal nanoparticles can have fundamental advantages over the respective bulk metals. They exhibit a large number of surface atoms with incomplete coordination and nonoptimal packing, and they are not passivated by a dense oxide layer.19 For small nanoparticles (<5 nm), moreover, the diffusion paths in the solid state are short due to the small number of atoms. As a result, base-metal nanoparticles can be promising starting materials to perform reactions with low activation energy and to obtain new, metastable compounds near room temperature (≤100 °C). With this aim, we have prepared Sc(0), Zr(0), Hf(0), and Mn(0) nanoparticles in the liquid phase (THF or DME). Thereafter, the as-prepared metal nanoparticles were exemplarily treated in the liquid phase with different sterically demanding, predominately O–H and N–H acidic reactants (e.g., monodentate and multidentate alcohols and amines) to probe the reactivity and reactions with quasi-homogeneous conditions. As a result, 22 novel coordination compounds were obtained.
Experimental Methods
Due to the high reactivity of the Sc(0), Zr(0), Hf(0), and Mn(0) nanoparticles as well as due to the sensitivity of the coordination compounds to moisture, all reactions and sample handling were carried out with inert conditions (dried argon or nitrogen, vacuum), using standard Schlenk techniques or glove boxes. This also includes all of the centrifugation and washing steps. Moreover, all sample transfer for analytical characterization was performed with strict inert conditions (e.g., using suited transfer modules).
Detailed information related to the analytical equipment, the synthesis of the Sc(0), Zr(0), Hf(0), and Mn(0) nanoparticles as well as the synthesis of the novel coordination compounds 1–22 can be found in the Supporting Information (Tables S1–S22, Figures S1–S28). Further details of the crystal structure analysis may be also obtained from the joint CCDC/FIZ Karlsruhe deposition service by quoting the depository numbers 2286533–2286537, 2286545–2286559, 2286561, 2286562.
Results and Discussion
Base-Metal Nanoparticles
The synthesis of base-metal nanoparticles, in general, comprises several prerequisites. Due to the high reactivity of base-metal nanoparticles (e.g., spontaneous combustion when in contact with air, explosion when in contact with water), which is comparable to the heavy bulk alkali metals rubidium or cesium, all syntheses and sample handling must be performed with inert conditions (dried argon or nitrogen, vacuum), including centrifugation and transfer to analytical equipment. Metal halides are applied throughout as the most simple starting materials. Lithium naphthalenide [LiNaph] and sodium naphthalenide [NaNaph] were used as powerful reducing agents.20 They exhibit an even higher reducing power than the bulk alkali metals themselves and can be handled in tetrahydrofuran (THF) or 1,2-dimethoxyethane (DME). The crust of oxides, carbonates and/or hydroxides on the bulk alkali metal can be easily removed by centrifugation since this crust is insoluble in THF/DME. Based on the intense green color of [LiNaph] and [NaNaph] in THF/DME, moreover, the concentration of the reducing agent can be quantified by photometry.21 Finally, the instantaneous reduction of the metal and the insolubility of the as-formed metal in THF/DME offer ideal conditions for particle nucleation and particle growth, resulting in uniform and very small metal nanoparticles.
Taking the aforementioned prerequisites into account, the synthesis of Sc(0), Zr(0), Hf(0), and Mn(0) nanoparticles follows our recently developed strategy for obtaining base-metal nanoparticles.22,23 However, the synthesis of Zr(0), Hf(0), and Mn(0) nanoparticles and their use as starting materials for syntheses near room temperature are here shown for the first time. In detail, ScCl3, ZrCl4, HfCl4, and MnCl2 were used as the starting materials. Due to the insufficient solubility of ScCl3 and MnCl2 in THF/DME, the metal nanoparticles were prepared in a one-pot approach. Thus, lithium or sodium, naphthalene, and the respective metal chloride were simultaneously added to THF/DME. Thereafter, different reactions occur, including (i) the dissolution of Li/Na and the formation of [LiNaph]/[NaNaph]; (ii) the dissolution of the metal chloride; (iii) the reduction of the metal chloride by [LiNaph]/[NaNaph]. Due to the high reducing power of [LiNaph]/[NaNaph], the reduction of the metal halide is very fast and results in very small metal nanoparticles although the nonhomogeneous conditions for particle nucleation seem disadvantages at first sight.24 Aiming at Hf(0) nanoparticles, the reduction of HfCl4 with [LiNaph] in DME resulted in colloidally very stable black suspensions, which did not show any precipitate even after high-power centrifugation (25.000 rpm, 55.200 g). Therefore, Hf(0) nanoparticles were reduced with [LiNaph] in toluene (Tol), using N,N,N′,N′-tetramethylethylenediamine (TMEDA) in addition to dissolved HfCl4 in Tol. After 12 h of intense stirring, the reduction and nucleation of the metal nanoparticles were completed and resulted in deep-black suspensions of Sc(0), Zr(0), Hf(0), or Mn(0) nanoparticles (Figure 1a).
Figure 1.

Overview of reactants to probe the reactivity and reactions of the as-prepared Sc(0), Zr(0), Hf(0), or Mn(0) nanoparticles in the liquid phase (THF, Tol, ionic liquids): (a) exemplary Zr(0) nanoparticles in DME; (b) exemplary reaction of Zr(0) nanoparticle powder in air; (c) selected monodentate and multidentate ligands for liquid-phase reactions near room temperature (≤100 °C).
For purification, the as-prepared base-metal nanoparticles were centrifuged/redispersed in/from THF and Tol to remove the remaining starting materials, naphthalene and LiCl. In contrast to LiCl, NaCl is not soluble in THF and cannot be removed. The dissolution of NaCl is possible with methanol, which, however, oxidizes the metal nanoparticles. Finally, the base-metal nanoparticles were redispersed in THF, DME or Tol to obtain colloidally stable suspensions. Alternatively, they can be dried at room temperature in a vacuum to obtain powder samples with a yield of about 80%. Losses mainly occur due to incomplete centrifugation. Whereas suspensions and powder samples are chemically stable under inert conditions (argon, nitrogen), special attention must be paid if the metal nanoparticles come into contact with air, water, or other oxidizing agent. This can cause violent combustions (with air, Figures 1b and S1) or explosions (with water).
For characterization, first of all, the particle size and particle size distribution of the as-prepared Sc(0), Zr(0), Hf(0), or Mn(0) nanoparticles were examined by transmission electron microscopy (TEM). TEM images show spherical particles with uniform size and low degree of agglomeration (Figures 2, 3, S2, and S3). A statistical evaluation of >200 nanoparticles on TEM images reveals mean diameters of 2–8 nm (Table 1). High-resolution (HR)TEM images confirm the size and evidence the crystallinity of the as-prepared base-metal nanoparticles with lattice fringes extending through the whole particle (Figures 2, 3, S2, and S3). The lattice plane distances are in good agreement with the respective bulk metals (Table 1). This finding is also confirmed by Fourier-transform (FT) analysis, which is in accordance with the calculated diffraction pattern of hexagonal bulk-Sc(0) (P63/mmc, a = 3.296, c = 5.275 Å) in the [211] zone axis,22,25 hexagonal bulk-Zr(0) (P63/mmc, a = 3.233, c = 5.147 Å) in the [001] zone axis,26 a slightly distorted hexagonal closed packed structure of bulk-Hf(0) (P63/mmc, a = 3.198, c = 5.061 Å, γ = 115 ± 3°) in the [001] zone axis,27 and cubic bulk-α-Mn(0) (I4̅3m, a = 8.911 Å) in the [425] zone axis.28 The intensity of the Bragg reflections is low due to the small size of the base-metal nanoparticles.
Figure 2.

Size and size distribution of the as-prepared Zr(0) nanoparticles with (a) TEM overview image (single nanoparticle indicated by red-dotted circle), (b) size distribution based on a statistical evaluation of >200 nanoparticles on TEM images, (c) HRTEM image of Zr(0) nanoparticle with lattice fringes, and (d) FT pattern of the single nanoparticle shown in (c), which agrees with the simulated diffraction pattern of bulk hexagonal Zr in the [001] zone axis (red circles; zero-order beam (ZB) marked by white circle).
Figure 3.
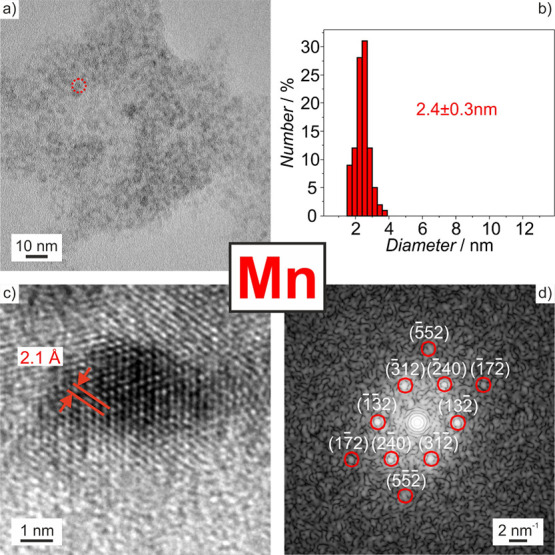
Size and size distribution of the as-prepared Mn(0) nanoparticles with (a) TEM overview image (single nanoparticle indicated by red-dotted circle), (b) size distribution based on a statistical evaluation of >200 nanoparticles on TEM images, (c) HRTEM image of Mn(0) nanoparticle with lattice fringes, (d) FT pattern of the single nanoparticle shown in (c), which agrees with the simulated diffraction pattern of bulk cubic α-Mn in the [425] zone axis (red circles; zero-order beam (ZB) marked by white circle).
Table 1. Mean Size and Lattice Plane Distances of Sc(0), Zr(0), Hf(0), or Mn(0) Nanoparticles.
| metal | mean size/nm | measured lattice plane distance/Å | lattice plane distance of bulk reference/Å |
|---|---|---|---|
| Sc(0) | 2.4 ± 0.2 | 2.5 | hexagonal Sc(0): d01̅1 with 2.5 Å22,25 |
| Zr(0) | 4.0 ± 0.9 | 2.9 | hexagonal β-Zr(0): d100 with 2.8 Å26 |
| Hf(0) | 8.0 ± 3.9 | 2.6 | hexagonal Hf(0): d1̅10 with 2.7 Å27 |
| Mn(0) | 2.4 ± 0.3 | 2.0 | cubic α-Mn(0): d2̅40 with 2.0 Å28 |
While the deep black color of the suspensions and the high reactivity of the Sc(0), Zr(0), Hf(0), or Mn(0) nanoparticles already point to zerovalent metals, their presence was exemplary, substantiated by the X-ray absorption near edge structure (XANES) of Zr(0) and Mn(0) nanoparticles (Figure 4). Specifically, K absorption edge (K-edge) XANES spectra were compared to the spectra of suitable references, including zirconium metal foil and ZrO2 (Figure 4a) as well as manganese metal foil, MnCl2, Mn(acac)3 and MnO2 (Figure 4c) representing the formal oxidation states ±0 and + IV for zirconium and ±0, +II, +III, and +IV for manganese. In the case of zirconium, the intensity and energy position of the rising absorption edge of the Zr(0) nanoparticles XANES spectrum, at first sight, is located between Zr(0) foil and ZrO2 (Figure 4a). The absorption edge of the Zr(0) nanoparticles is nevertheless clearly different from that of ZrO2, which is underlined by the first derivative of the XANES spectrum (Figure 4b). In fact, the pre-edge peak in the XANES spectrum of the Zr(0) nanoparticles at 17,998 eV appears less distinct in comparison to the respective bulk-Zr(0) sample. This pre-edge peak can be attributed to Zr-1s → Zr-4d electron transitions, which are dipole-forbidden but visible as a well-distinguishable peak in the spectrum of bulk-Zr(0) due to the band-mixing of d- and p-like states in the conduction band.29 For the nanoparticles, this hybridization is less pronounced, whereupon the pre-edge peak appears as only a weak shoulder (Figure 4a). In addition, the absorption edge for the Zr(0) nanoparticles (18,010 eV) is shifted to higher energies in comparison to bulk-Zr(0) (18,008 eV) due to more localized states and therefore located between the absorption edge of bulk-Zr(0) and ZrO2 (18,013 eV). This finding can be rationalized by taking the different behavior of volume atoms and surface atoms in the nanoparticle into account. With an average size of 4.0 ± 0.9 nm of the Zr(0) nanoparticles, about 20% of all zirconium atoms are located at the surface,19,22,29 where they are insufficiently coordinated by metal atoms (in comparison to volume atoms) but with THF/DME molecules adhered on the surface (Figure 4d). Overall, the differences between the spectra of Zr(0) nanoparticles and bulk-Zr(0) are to be expected and confirm the presence of zerovalent zirconium in the nanoparticles.
Figure 4.
XANES-M-L3 spectra of XANES spectra of (a) Zr(0) nanoparticles with Zr(0) foil and ZrO2 as references; (b) 1st derivative of XANES spectra in (a); (c) Mn(0) nanoparticles with Mn(0) powder, MnCl2, Mn(acac)3, and MnO2 as references; (d) Scheme of metal nanoparticle with volume atoms and surface atoms with adhered THF.
In the case of manganese, the similarity of the spectra of the Mn(0) nanoparticles and bulk Mn(0) is obvious and confirms the presence of zerovalent manganese in the nanoparticles (Figure 4c). Here, the position of the absorption edges of the Mn(0) nanoparticles and of bulk-Mn(0) is clearly different from the references MnCl2, Mn(acac)3, and MnO2. Similar to the Zr(0) nanoparticles, the high number of surface atoms in relation to the volume atoms again resulted in a certain reduction of the intensity of the spectral features (Figure 4c).
In regard to the reactivity and reactions of the as-prepared base-metal nanoparticles, their surface functionalization is important. Due to the liquid-phase synthesis, the solvent is of course adhered to the surface. Such nanoparticles are designated as “quasi-naked” in the literature.30 For the Zr(0), Mn(0), and Sc(0) nanoparticles, Fourier-transformed infrared (FT-IR) spectra recorded after the washing process of the nanoparticles only show vibrations of THF (Figures 5 and S4), whereas only weak absorptions of naphthalene and Tol occur. In the case of the Hf(0) nanoparticles, the synthesis was performed in Tol with tmeda to dissolve HfCl4 in Tol. Therefore, FT-IR spectra here indicate the vibrations of tmeda with low intensity (Figure S5). Moreover, elemental analysis (C/H/N analysis) shows C/H contents of 37.3/4.3 wt % for the Zr(0) nanoparticles and 8.1/0.9 wt % for the Mn(0) nanoparticles. The C/H ratios of 8.6 and 8.9 are close to the expected ratio for THF (C/H = 6), so that FT-IR and C/H/N analysis point to THF adhering on the nanoparticle surface. In the case of Hf(0), the particle surface is dominated by tmeda and Tol (observed: 20.2 wt % C, 2.8 wt % H, 2.4 wt % N; calculated: 6.2 wt % C, 1.4 wt % H, 2.4 wt % N due to tmeda, 14.0 wt % C, 1.4 wt % H due to Tol). The instantaneous combustion of base-metal nanoparticle powders when in contact with air already indicates their high reactivity and the absence of passivating surface layers (e.g, strongly coordinating and/or high-molecular-weight ligands, metal oxide layers; Figures 1b and S1). The high reactivity and the absence of strong binding and/or high-molecular-weight surface functionalization offer the option for reactions with specifically selected reactants using Sc(0) Zr(0), Hf(0) or Mn(0) nanoparticles as the starting material.
Figure 5.

Surface functionalization of Zr(0) and Mn(0) nanoparticles according to FT-IR spectra: (a) Zr(0) nanoparticles, (b) Mn(0) nanoparticles (with THF, DME, naphthalene as references). For spectra of Sc(0) and Hf(0) nanoparticles see Figures S4 and S5.
For the synthesis of the Sc(0), Zr(0), Hf(0), or Mn(0) nanoparticles, finally, it needs to be noted that the reduction with [LiNaph] and [NaNaph] leads to identical results in terms of particle size and colloidal stability. However, the removal of LiCl is much easier than for NaCl due to the good solubility of LiCl in THF or DME. Dissolution and removal of NaCl would need more polar solvents such as methanol, which, however, leads to hydrogen formation with the base-metal nanoparticles at room temperature. Most of the precipitated NaCl can be removed by low-power centrifugation (5,000 rpm). Nevertheless, NaCl partially remained in the nanoparticle suspensions. If the LiCl or NaCl remains, NaCl can be nevertheless advantageous due to its larger size and the less strong coordination of Na+ in comparison to Li+.
Reactivity and Reactions of Sc(0), Zr(0), Hf(0), or Mn(0) Metal Nanoparticles
The reactivity and reactions of the as-prepared base-metal nanoparticles were evaluated after centrifugation and redispersion in THF, Tol, or ionic liquids (Figure 1c). To this concern, the base-metal nanoparticles were treated in the liquid phase with different sterically demanding reactants, mainly including O–H and N–H acidic monodentate and multidentate alcohols and amines. Specifically, the reactants include isopropanol (HOiPr), 1,1′-bi-2-naphthol (H2binol), N,N′-bis(salicylidene)ethylenediamine (H2salen), 2-mercaptopyridine (2-Hmpy), 2,6-diisopropylaniline (H2dipa), carbazole (Hcz), triphenylphosphane (PPh3), N,N,N′,N′-tetramethylethylenediamine (tmeda), 2,2′-bipyridine (bipy), N,N′-diphenylformamidine (Hdpfa), N,N′-(2,6-diisopropylphenyl)-2,4-pentanediimine ((dipp)2nacnacH), 2,2′-dipydridylamine (Hdpa), and 2,6-bis(2-benzimidazolyl)pyridine (H2bbp) (Figure 1c).
Specific questions regarding reactivity and reactions address the following: (i) Can new coordination compounds be obtained using base-metal nanoparticles as starting materials? (ii) Can redox reactions be performed in the liquid phase near room temperature (≤100 °C) with quasi-homogeneous conditions? (iii) As Li+ and Na+ from the reducing agents [LiNaph] and [NaNaph] cannot be removed completely, are they present in (all) products? In this regard, it must be noted LiCl can be removed by washing with THF. NaCl, however, is more-or-less insoluble in THF, so that only larger crystals of NaCl can be separated by centrifugation. iv) THF, DME, and naphthalene may remain adsorbed on the surface of the as-prepared base-metal nanoparticles, are they present in (all) products? Upon an explorative evaluation of the synthesis conditions, 22 new compounds were realized. For other reactions, only the starting materials could be identified or no specific crystalline product could be identified until now (Table 2).
Table 2. Reaction of Sc(0), Zr(0), Hf(0), or Mn(0) Nanoparticles with Different Reactants, Resulting in the Novel Compounds 1–22a.
| reactant | abbreviation | reaction
with base-metal nanoparticle |
|||
|---|---|---|---|---|---|
| Sc(0) | Zr(0) | Hf(0) | Mn(0) | ||
| isopropanol | HOiPr | / | 1 | / | ncc |
| 1,1′-bi-2-naphthol | H2binol | ncc | ncc | 2 | / |
| N,N′-bis(salicylidene)ethylenediamine | H2salen | ncc | ncc | ncc | 3 |
| 2-mercaptopyridine | 2-Hmpy | 4 | ncc | ncc | nr |
| 2,6-diisopropylaniline | H2dipa | / | / | / | 5 |
| carbazole | Hcz | 6 | ncc | ncc | nr |
| triphenylphosphane | PPh3 | / | 7b | / | / |
| N,N,N′,N′-tetramethylethylenediamine | tmeda | / | / | / | 8 |
| 2,2′-bipyridine | bipy | 18 | 19b | 19b | nr |
| N,N′-diphenylformamidine | Hdpfa | 9 | 20 | 21 | nr |
| N,N′-bis(2,6-diisopropylphenyl)formamidine | Hdippfa | 22b | 22b | 22b | nr |
| N,N′-(2,6-diisopropylphenyl)-2,4-pentanediimine | (dipp)2nacnacH | nr | nr | nr | 10 |
| 2,2′-dipydridylamine | Hdpa | ncc | ncc | ncc | 11 |
| 2,6-bis(2-benzimidazolyl)pyridine | H2bbp | 12 | ncc | ncc | ncc |
| ethylmethylimodazolium chloride | [EMIm]Cl | 13b | 13b | / | nr |
| butylmethylimodazolium chloride | [BMIm]Cl | 14 | 15 | 16,17 | nr |
nr: no reaction observed; ncc: no crystalline compound obtained; /: not examined.
Metal of respective nanoparticles not present in identified product.
Follow-Up Reactions with Alcohols
To probe the reactivity of Sc(0), Zr(0), Hf(0) or Mn(0) nanoparticles, first of all, alcohols were selected as O–H acidic reactants. Here, a reaction with the base-metal nanoparticles is to be expected with regard to their strong oxophilic character (e.g., Zr, Hf). Exemplarily, isopropanol (HOiPr) was chosen as a simple monodentate alcohol, 1,1′-bi-2-naphthol (H2binol) as a bidentate alcohol, and N,N′-bis(salicylidene)ethylenediamine (H2salen) as a more complex multidentate ligand with O- and N-based linkers.
The reaction of HOiPr with the most oxophilic Zr(0) nanoparticles surprisingly results in [Li3Zr(OiPr)5(HOiPr)5(OMe)F] (1), which was obtained as colorless, transparent plates (space group P21/c; Table S1, Figure S6). As the central building unit, 1 contains an unusual distorted Li3ZrO3F heterocubane (Figure 6). Two Li+ atoms (due to two crystallographically independent heterocubane sites: Li(1), Li(3), Li(4), and Li(6)) are coordinated by two (OiPr)− and one (OMe)− ligand, with two O atoms being part of the heterocubane (Li–O: 190.0(14)–203.6(15) pm). The third Li+ atom (Li(2), Li(5)) is only coordinated by two (OiPr)− ligands with the O atoms being part of the heterocubane (Li–O: 185.3(15)–191.1(14) pm). Together with one F– in the heterocubane (Li–F: 162.5(15)–221.5(13) pm) and one additional HOiPr ligand (Li–O: 183.0(15)–189.3(15) pm), Li+ exhibits a distorted square-pyramidal (Li(1), Li(3), Li(4), Li(6)) or tetrahedral (Li(2), Li(5)) coordination. Zr4+ is coordinated by two (OiPr)− ligands (Zr–O: 216.9(4)–220.0(4) pm) and one (OMe)− ligand (Zr–O: 215.0(4)–215.3(4) pm) with the O atoms being part of the heterocubane (Figure 6). Moreover, Zr4+ is coordinated by a terminal (OiPr)− ligand (Zr–O: 192.2(5)–193.1(5) pm) and two HOiPr ligands (Zr–O: 203.1(5)–205.0(5) pm), in sum, resulting in a distorted octahedral coordination. The differentiation of (OiPr)− and HOiPr is possible, on the one hand, based on the M–O–C angles (>150° for (OiPr)−; < 150° for HOiPr) and, on the other hand, based on the Zr–O distances (≤193 pm for (OiPr)−; ≥ 203 pm for HOiPr). The formation of compound 1 can be rationalized as follows:
Figure 6.
Crystal structure of [Li3Zr(OiPr)5(HOiPr)5(OMe)F] (1) with (a) selected angles of the OiPr groups and (b) a Li3ZrO3F heterocubane-type building unit with hydrogen bonding (OiPr shown as wires and sticks; H atoms bound to C and partial disorder of methyl groups not shown for clarity).
LiCl remained as a byproduct of the synthesis of Zr(0) nanoparticles. The methoxy group can be assigned to the decomposition of DME adsorbed on the metal surface. Finally, F originates from poly(tetrafluoroethylene) (PTFE) grease used to seal the Schlenk flasks. The formation of such complex structure and composition is ascribed to the obviously high stability of the heterocubane unit and the specific spatial requirements with the availability of larger (OiPr–, HOiPr) and smaller ligands (OMe–, F–). The incorporation of F– as a side reaction as well as the almost quantitative high yield of the side reaction also point to the stability of 1. A comparable structure and composition is yet only known from [Zr2(OiPr)8(HOiPr)2].31 Interestingly, this known compound was also obtained by us, if no fluorine source was available in the reaction. Based on the knowledge of the existence of 1, moreover, this compound was also obtained after the addition of a stoichiometric amount of NaF. Based on the fluorine incorporation in 1, all further reactions were performed in glass ampoules sealed under argon (see SI).
As a second example, the bidentate 1,1′-bi-2-naphthol (H2binol) was reacted with Hf(0) nanoparticles in THF and resulted in the dinuclear complex [Hf(binol)(thf)2Cl2]2 (2) (P1̅; Table S2, Figure S7). The synthesis can be ascribed to the following reaction with evolution of hydrogen, which was visually recognized:
In 2, Hf4+ is coordinated by distorted octahedra with two Cl– on the axial positions (Hf–Cl: 246.9(2)–251.9(3) pm) (Figure 7). Moreover, two μ2-coordinating (binol)2– ligands bridge the Hf4+ centers (Hf–O: 191.7(7)–193.1(6) pm), which are each additionally coordinated by two thf molecules (Hf–O: 228.5(6)–229.3(7) pm). Although binolate complexes are well-known,32,33 such compound is prepared by a redox reaction for the first time, using metal nanoparticles as a starting material.
Figure 7.

Crystal structure of [Hf(binol)(thf)2Cl2]2: (a) dinuclear complex with octahedral arrangement around Hf4+, (b) coordination of Hf4+ with selected distances (pm) and angles (deg) (thf/binol shown as wires and sticks; H atoms and partial disorder of thf not shown for clarity).
As another sterically demanding O–H acidic ligand with different coordination sites, H2salen was selected. Due to the presence of hydroxyl groups and nitrogen atoms, the less oxophilic Mn(0) nanoparticles were used here to probe the reactivity and reaction. As a result, orange plates of [Mn3(salen)2(salan)] × Tol (3) were obtained in Tol at 80 °C with visible H2 evolution (P21/n; Table S3, Figure S8):
In the trinuclear complex, the central Mn2+ shows a distorted octahedral coordination, whereas the terminal Mn2+ atoms exhibit a distorted trigonal bipyramidal arrangement (Figure 8). The three ligand molecules interestingly coordinate Mn2+ with both the O atom and the N atoms. Thereof, the terminal Mn2+ atoms are each η4-coordinated by one salen ligand (Mn–O: 204.8(2) pm; Mn–N: 218.8(2)–219.9(2) pm). The central Mn2+ shows μ2-coordination via two bridging O atoms to both terminal Mn2+ atoms (Mn–O: 210.3(2)–224.3(2) pm) (Figure 8). Remarkably, the ligand at the central Mn2+ was reduced to salan, which is also reflected by the planarity of its salicylimine unit as well as by the different Mn–N distances (224.2(2)–225.1(2) pm). Multinuclear salen complexes are generally rare and, to the best of our knowledge, have not been reported for manganese before. Moreover, coordination compounds with salen as the sole ligand coordinating the metal center are rare. Until now, only {Fe(CN)4[CNMn(salen)(MeOH)]2}−34 and [Mn(salen)]2[Ni(CN)4] × 0.5H2O35 were described as mononuclear complexes.
Figure 8.
Crystal structure of [Mn3(salen)2(salan)] × Tol (3): (a) trinuclear complex, (b, c) coordination polyhedra around Mn2+ (thf, salen, salan shown as wires and sticks; H atoms not shown for clarity).
Follow-Up Reactions with Amines
Since the aforementioned alcohols, although sterically demanding, show very fast reactions (partially too fast) with the base-metal nanoparticles, we have focused on N–H acidic amines as milder oxidation agents in the following. Again, sterically demanding mono- and multidentate amines were selected to probe the reactivity and reactions of the base-metal nanoparticles. The formation of H2 and the resulting amide, on the one hand, make the reactions irreversible and, on the other hand, lead to strong binding with the respective metal cation, which also can be an advantage in comparison to conventionally applied Lewis-acid–base reactions, transmetalation, and metathesis reactions.
As a first example, 2-mercaptopyridine (2-Hmpy) with an amine and a thiol functionality was reacted with Sc(0) nanoparticles in Tol and resulted in [Sc(2-mpy)3]2 (4) (P21/c; Table S4, Figure S9) according to the reaction:
4 represents a dinuclear complex with unusual 7-fold coordination of Sc3+ (Figure 9). All 2-mpy ligands show bidentate coordination via nitrogen and sulfur. Two 2-mpy ligands are η2-coordinated to each of the Sc3+ centers (Sc–N: 228.9(3)–229.1(3) pm; Sc–S: 253.8(2)–259.8(1) pm). Two additional 2-mpy ligands bridge the two Sc3+ with sulfur as the bridging atom (Sc–S: 266.4(1)–278.5(2) pm) and nitrogen binding to only a single Sc3+ center (Sc–N: 229.0(3) pm) (Figure 9). Such 7-fold coordination of Sc3+ is rare but was reported before.36 With regard to 2-mpy as a ligand, only [Sc(C5Me4H)2(2-mpy)] is known.37 A 7-fold coordination of Sc3+ only by nitrogen and sulfur is here shown for the first time, in general.
Figure 9.
Crystal structure of [Sc(2-mpy)3]2 (4): (a) dinuclear complex, (b) 7-fold coordination of Sc3+ (2-mpy shown as wires and sticks; H atoms not shown for clarity).
As 2-Hmpy shows good reactivity with complete deprotonation of the NH-group, we switched to reactants with only N–H-acidic functionalities only. In this regard, Mn(0) nanoparticles were reacted with 2,6-diisopropylaniline (H2dipa) as a sterically demanding monodentate amine. This resulted in the dinuclear Mn(II) complex [Mn2Na2(2,6-Hdipa)4(OMe)2(dme)2] (5) (P21/n; Table S5, Figure S10). Here, Na+ as well as the (OMe)− ligands stem from metal-nanoparticle synthesis according to the following reaction:
The dinuclear Mn(II) complex exhibits a distorted tetrahedral coordination of Mn2+ with two O atoms of two bridging (OMe)− ligands (Mn–O: 212.3(1)–212.6(1) pm) as well as two nitrogen atoms of two η1-binding 2,6-Hdipa ligands (Mn–N: 203.9(1)–204.8(1) pm) (Figure 10). Here, it needs to be noted that the NH2 group of 2,6-H2DIPA was once deprotonated to result in a (2,6-Hdipa)− ligand with an NH group remaining. This is confirmed by the angles observed at the nitrogen atoms (C–N–Na: 102.1, 118.7°; C–N–Mn: 131.5, 142.4°; Na–N–Mn: 88.6, 89.2°), which together indicate a distorted tetrahedral arrangement. Besides Mn2+, 5 contains two Na+ cations with distorted trigonal bipyramidal coordination (Figure 10). Na+ is coordinated by three oxygen atoms of (OMe)− (Na–O: 239.5(1) pm) and dme (Na–O: 235.4(1) pm) as well as by two nitrogen atoms of (2,6-Hdipa)− (Na–N: 251.1(1)–256.1(1) pm).
Figure 10.
Crystal structure of [Na2Mn2(2,6-Hdipa)4(OMe)2(dme)2] (5) with (a) molecular structure and (b) coordination around Mn2+ and Na+ (2,6-Hdipa, OMe, dme shown as wires and sticks; H atoms only partially shown for clarity).
Based on the successful reaction of Mn(0) nanoparticles with H2dipa, carbazole (Hcz) was selected as another sterically demanding N–H-acidic amine. By reaction in Tol, [Sc(cz)3(thf)2]2 × 3 Tol (6) was obtained with colorless crystals (P21/n; Table S6, Figure S11). Herein, Sc3+ is coordinated by three (cz)− ligands (Sc–N: 210.7(4), 211.0(4), 211.7(3) pm) and two thf molecules (Sc–O: 219.2(3), 220.0(3) pm) (Figure 11a). These distances are shorter than that in [Sc(ctp)Cl(thf)] (ctp: carbazole-triazol-ylidene porphyrin, Sc–N: 220.4, 221.0 pm; Sc–O: 225.8 pm),38 which points to a more rigid coordination in 6. THF originates from the synthesis of the Sc(0) nanoparticles and remains absorbed on the nanoparticle surface. Moreover, three Tol molecules are located between the noncharged [Sc(cz)3(thf)2]2 molecules. In summary, Sc3+ exhibits a distorted trigonal bipyramidal coordination (Figure 11b). Such a sole coordination of Sc3+ with cz is unknown. So far, only pincer-type complexes were reported with even bulkier carbazole derivatives.39 The synthesis of 6 can be rationalized based on the following reaction:
Figure 11.
Crystal structure of [Sc(cz)3(thf)2]2 × 3 Tol (6): (a) molecular structure, (b) coordination around Sc3+, (c) excitation and emission spectra (with excitation/emission of HCz as a reference/black; cz, thf shown as wires and sticks; H atoms not shown for clarity).
The colorless crystals of 6 show blue emission after excitation with 366 nm (Figure 11c). The free molecule Hcz was examined for comparison. Whereas the emission of 6 is very comparable to that of free Hcz, the excitation is much narrower for the title compound, which can be related to the coordination to Sc3+.
In addition to the reaction of base-metal nanoparticles with monodentate amines, a single phosphane ligand was studied. Accordingly, Zr(0) nanoparticles were reacted with triphenylphosphane (PPh3) in Tol with CuI for activation. As a result, [MePPh3]3[Li6I9] × Tol (7) was obtained according to the reaction:
In this reaction, LiCl originates from the synthesis of Zr(0) nanoparticles. The unexpected addition of a methyl group to PPh3 resulted from a decomposition of DME as the solvent used to prepare the Zr(0) nanoparticles. The formation of copper was validated by the visible formation of a copper film on the glass wall after the reaction.
Although 7 does not contain any zirconium, its formation and structure are nevertheless interesting. Thus, 7 crystallizes in the noninversion symmetric, monoclinic space group Cc (Table S7, Figure S12) and contains infinite anionic [Li6I9]3– chains (Figure 12a). Herein, all Li+ species exhibit a distorted tetrahedral coordination. Most remarkably, all LiI4 tetrahedra share three edges with three other LiI4 tetrahedra. One iodine atom (I6) belongs to four LiI4 tetrahedra, four iodine atoms (I2, I3, I5, I9) are part of three different LiI4 tetrahedra and the remaining four iodine atoms (I1, I4, I7, I8) belong to two LiI4 tetrahedra. Such a ∞1[(LiI1/4I2/3I1/2)2(LiI1/4I1/3I2/2)2(LiI3/3I1/2)2]3– chain is observed here for the first time. The coordination is also reflected by the Li–I distances, which are shorter for μ2-I (269(2)–275(2) pm) than for μ3-I (270(3)–285(3) pm) and μ4-I (279(3)–297(3) pm) (Figure 12b). These distances compare to literature data of edge-sharing LiI4 tetrahedra (e.g., [LiI(bipy)0.5] with 271–283 pm). The I–Li–I angles (95.5–125.0°) are in agreement with the distorted tetrahedral arrangement.40 Such chains of highly condensed tetrahedra, to the best of our knowledge, are unknown so far. Its formation can be related to the specific conditions of synthesis applied here.
Figure 12.
Crystal structure of [MePPh3]3[Li6I9] × Tol (7): (a) infinite ∞1[(LiI1/4I2/3I1/2)2(LiI1/4I1/3I2/2)2(LiI3/3I1/2)2]3– chain, (b) connectivity of LiI4 tetrahedra with selected distances (pm).
After some exemplary monodentate amines were used to probe the reactivity of the base-metal nanoparticles. First of all, N,N,N′,N′-tetramethylethylenediamine (tmeda) as a sterically demanding bidentate ligand was reacted with Mn(0) nanoparticles. Similar to PPh3, again, no reaction occurred near room temperature, so iodine was added to Tol for activation. This results in the formation of [Li(tmeda)]2[MnI4] (8) with colorless, octahedron-shaped crystals (P42/n;Table S8, Figure S13):
Li+ stems from [LiNaph] used to obtain the Mn(0) nanoparticles. 8 consists of [Li(tmeda)]+ cations (Li–N: 210.3(1)–221.0(1) pm) and isolated tetrahedral [MnI4]2– anions (Mn–I: 271.7(1) pm) (Figure 13a). Upon excitation with UV light (366 nm), the compound shows a remarkably intense green emission (Figure 13b). While green emission is well known for Mn2+, the high emission intensity can be ascribed to the presence of the unusual isolated [MnI4]2– anion with large distances between these tetrahedra (Mn···Mn: 1164.8 pm), so that concentration quenching is avoided.41 The absence of any reaction of Mn(0) nanoparticles and tmeda without, e.g., I2-driven activation, finally confirms the role of N–H-acidic functionalities. Therefore, only N–H-acidic amines were investigated in the following.
Figure 13.
Crystal structure of [Li(tmeda)]2[MnI4] (8): (a) unit cell (tmeda shown as wires and sticks; H atoms not shown for clarity) and (b) excitation and emission spectra with photo of green emitting single crystals.
The reaction of Sc(0) nanoparticles with N,N′-diphenylformamidine (Hdpfa) as a bidentate N–H-acidic amine in Tol resulted in colorless crystals of [Sc(dpfa)3] (9), which crystallize in the noninversion symmetric orthorhombic space group Pna21 (Table S9, Figure S14) according to the reaction:
Here, Sc3+ is coordinated by three η2-binding dpfa ligands (Sc–N: 217.8(4)–221.8(4) pm), resulting in a distorted antiprismatic coordination (Figure 14). Coordination and structure compare to [Fe(dpfa)3] showing slightly shorter Fe–N distances (Fe–N: 208.0–213.3 pm).429 also shows fluorescence, which is discussed together with the related Zr and Hf compounds 15 and 16.
Figure 14.

Crystal structure of [Sc(dpfa)3] (9): (a) molecular structure, (b) coordination around Sc3+ with selected distances (pm) (dpfa shown as wires and sticks; H atoms not shown for clarity).
As a sterically most demanding bidentate amine, N,N′-(2,6-diisopropylphenyl)-2,4-pentanediimine ((dipp)2nacnacH) was reacted with Mn(0) nanoparticles in Tol. Here, greenish rectangular crystals of [Mn2((dipp)2nacnac)2(OMe)2] × 2 Tol (10) were formed according to the reaction (C2/m; Table S10, Figure S15):
In 10, Mn2+ is coordinated distorted tetrahedrally by two oxygen atoms of bridging (OMe)− ligands originating from DME (Mn–O: 202.8(2)–205.5(2) pm) as well as by a η2-binding ((dipp)2nacnac)− ligand (Mn–N: 210.4(2) pm) at each Mn2+ center (Figure 15). In principle, such dinuclear nacnac complexes of Mn2+ are known but contain Br– or H– as bridging atoms (e.g., [(LDepMn)2(μ-Br)2], [(LDepMn)2(μ-H)2]; LDEP: (dep)2nacnac; dep: 2.6-diethylphenyl).43,44
Figure 15.

Crystal structure of [Mn2((dipp)2nacnac)2(OMe)2 × 2Tol] (10) with (a) molecular structure and (b) coordination around Mn2+ ((dipp)2nacnac, OMe shown as wires and sticks; H atoms not shown for clarity).
As a tridentate N–H acidic ligand, 2,2′-dipyridylamine (Hdpa) was reacted with Mn(0) nanoparticles in Tol. Since no reaction occurred, iodine was again added for activation, which led to the formation of yellow transparent crystals of [Mn2(dpa)3I] × Tol (11) at 50 °C (P1̅;Table S11, Figure S16):
11 is again a dinuclear complex with Mn2+ bridged by three (dpa)− ligands (Figure 16a). Interestingly, one Mn2+ is η2-coordinated by three dpa ligands resulting in a distorted octahedral arrangement (Mn–N: 219.8(3)–230.2(3) pm) (Figure 16b). In contrast, the other Mn2+ is η1-coordinated by all dpa ligands (Mn–N: 214.3(3)–217.4(3) pm) as well as by I– (Mn–I: 282.7(1) pm), leading to a distorted tetrahedral arrangement (Figure 16b). The Mn–N and Mn–I distances are in good agreement with MnIII-amine complexes.45
Figure 16.
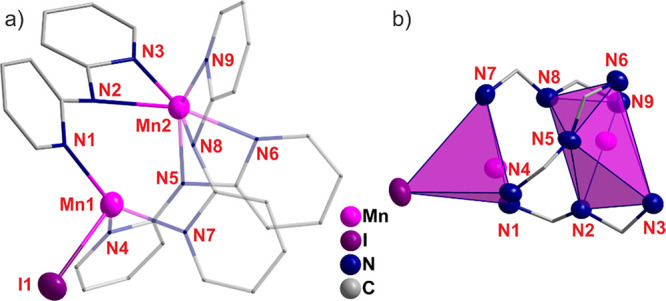
Crystal structure of [Mn2(dpa)3I] × Tol (11): (a) molecular structure, (b) coordination around Mn2+ (dpa shown as wires and sticks; H atoms not shown for clarity).
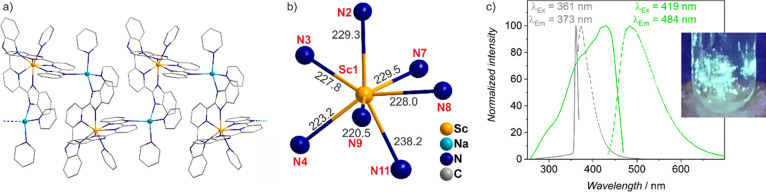
Finally, Sc(0) nanoparticles were exemplarily reacted with 2,6-bis(2-benzimidazolyl)pyridine (H2bbp) as a multidentate ligand in pyridine (Py) at room temperature, which results in the crystallization of [NaSc(bbp)2(py)3]2 × 5 Py (12) (C2/c; Table S12, Figures S17 and S28). Herein, zigzag chains of [Sc(bbp)2/2(py)] units and [Na(bbp)2/2(py)2] units are oriented along the crystallographic b-axis (Figure 17a,b). Thus, Sc3+ is coordinated by seven nitrogen atoms of two η3-binding (bbp)2– ligands (Sc–N: 220.5(2)–229.5(2) pm) and one py ligand (Sc–N: 238.2(2) pm). Na+ shows a distorted tetrahedral coordination with nitrogen of two η1-binding (bbp)2– ligands (Na–N: 236.0(2)–238.5(3) pm) and two py ligands (Na–N: 238.4(5)–241.1(6) pm). Furthermore, additional Py molecules are noncoordinating and located between the [NaSc(bbp)2(py)3]2 chains (Figure S17). The synthesis of 12 can be ascribed to the following reaction with Na+ stemming from [NaNaph] to prepare the Sc(0) nanoparticles:
Figure 17.
Crystal structure of [NaSc(bbp)2(py)3]2× 5 Py (12): (a) chain-type structure, (b) distorted single-capped, trigonal prismatically coordination around Sc3+ with selected distances (pm), (c) excitation and emission spectra (with excitation/emission of H2bbp as a reference/black; bbp/py shown as wires and sticks; H atoms and disorder of py not shown for clarity).
In contrast to Hdpa in Tol (see compound 11), the reaction of H2bbp in Py occurs already at room temperature. However, Py is also a much stronger coordinating ligand than THF or Tol. Therefore, Py was not considered further in our studies. Interestingly, the yellow crystals of 12 show intense green fluorescence with UV excitation (Figure 17c), which is clearly different from pure H2bbp. Excitation and emission of 12 are both significantly broader and red-shifted with a much stronger Stokes shift as for pure H2bbp. This finding can be attributed to the more rigid binding of bbp by the Sc3+ cation, which is known to decrease the energy between π and π* molecular orbitals of the ligand.46
Follow-Up Reactions in Ionic Liquids
Subsequent to the reaction of Sc(0), Zr(0), Hf(0), and Mn(0) nanoparticles with alcohols and amines, we have finally examined the reactivity and reactions of the base-metal nanoparticles in ionic liquids. In comparison to THF or Tol, ionic liquids as low-melting salts are much more polar than Tol but much less strongly coordinating than THF.47 [EMIm]Cl, [BMIm]Cl, and [BMPyr]Cl (EMIm: ethylmethylimodazolium; BMIm: butylmethylimodazolium; BMPyr: buthylmethylpyrrolidinium) were used as exemplary ionic liquids, which already turned out to be suitable for synthesis in regard of their chemical and thermal stability.48 First of all, Sc(0), Zr(0) and Hf(0) nanoparticles were reacted in [EMIm]Cl. These reactions, however, only resulted in the formation of [Li4(C6H10N2)4Cl4] (13) independent of the type of metal (Pbcn; Table S13, Figure S18). With Sc(0) as an example, the reaction can be attributed to
LiCl, again, stems from the synthesis of base-metal nanoparticles.
13 contains a distorted Li4Cl4 heterocubane (Li–Cl: 236.9(5)–244.5(6) pm; Cl–Li–Cl: 98.3–101.4°) with each Li+ additionally coordinated by an EMIm-ylide ligand (i.e., deprotonated EMIm) (Figure 18). The Li–C distances of the N-heterocyclic carbene (NHC) with 211.8(6) and 214.3(6) pm compare to other Li-NHC compounds, such as [Li4(C≡CtBu)4(NHC)4]·C7H8 (Li–C: 220.7 pm; NHC: 1,3,4,5-tetramethylimidazole-2-ylidene)49 or [Li4(OC10N2H18)2(OEt2)2I2] (Li–C: 213.1 pm).50 Heterocubane-type structures of lithium halides are well-known so that the Li4Cl4 unit in 13 compares to [Li4(Et2O)4Cl4] (Li–Cl: 235–240 pm; Cl–Li–Cl: 97.5–102.0°).51
Figure 18.

Crystal structure of heterocubane-type [Li4(C6H10N2)4Cl4] (13) (C6H10N2 shown as wires and sticks; H atoms are not shown for clarity).
Moreover, Sc(0) and Zr(0) nanoparticles were reacted in [BMIm]Cl/AlCl3 as Lewis acid ionic liquid, and Zr(0) and Hf(0) nanoparticles were reacted in eutectic mixtures of [BMIm]Cl/[BMPyr]Cl as room-temperature ionic liquid. This resulted in colorless plates of [BMIm]5[AlCl4]2[ScCl6] (14), [BMIm][BMPyr][ZrCl6] (15), and [BMIm][BMPyr][HfCl6] (16) (P1̅; Figures 19a–c and S19–S21; Tables S14–S16) according to
Figure 19.

Unit cells of (a) [BMIm][BMPyr][ZrCl6] (15), (b) [BMIm][BMPyr][HfCl6] (16), (c) [BMIm]5[AlCl4]2[ScCl6] (14), and (d) 1∞[NaZrCl6]− chain in [BMIm][NaZrCl6] (17) (BMIm, BMPyr as wires and sticks; H atoms not shown for clarity).
The reactivity of Sc(0), Zr(0), and Hf(0) nanoparticles with these conditions is obviously even high enough to reduce Al, which is confirmed by the occurrence of a grayish solid subsequent to the reaction. If the time of reaction with Zr(0) nanoparticles was increased to 6 weeks, furthermore, [BMIm][NaZrCl6] was obtained (17) (P21/c; Table S17, Figure S22). Herein, an infinite ∞1[(NaCl4/3Cl2/2)(ZrCl2/3Cl2/2Cl2/1)]− chain occurs, which consists of distorted edge-sharing ZrCl6 and NaCl6 octahedra with part of the chlorine atoms being part of three different octahedra, which, as expected, is more often observed for the NaCl6 octahedra with the low-charged Na+ (Figure 19d).
The synthesis of 13–17 mainly shows that base-metal nanoparticles can be handled in ionic liquids without, e.g., complete decomposition of the cation of the ionic liquid. In fact, the base-metal nanoparticles tend to reduce imidazolium-type cations to the respective ylide or even reduce [AlCl4]− anions to elemental aluminum. Based on these observations, again, amines were added as reactants. As a less bulky ligand, first of all, bipy was reacted with Sc(0) nanoparticles in [BMIm]Cl/GaCl3 as an ionic liquid. The less Lewis-acidic GaCl3 was applied instead of AlCl3. Here, colorless crystals of [BMIm][Sc(bipy)Cl4] (18) were obtained (P21/c; Table S18, Figure S23) by oxidation of Sc(0) with formation of Ga(0):
Sc3+ is η2-coordinated by one bipy ligand as well as by four Cl–, resulting in a distorted octahedral environment (Sc–N: 229.5(2), 230.4(2) pm; Sc–Cl: 241.3(1)–248.7(1) pm) (Figure 20a). In contrast to Sc(0), the reaction of Zr(0) or Hf(0) nanoparticles with bipy in [BMIm]Cl/GaCl3 resulted in colorless crystals of [Ga(bipy)2Cl2][GaCl4] (19) (Figures 20b and S24; Table S19), which, although first realized via our synthesis approach, was already described in the literature.52 For our compound, however, crystallization in a different space group was observed (19: P212121) than reported in the literature (ref (52): Pccn). Interestingly, the smaller Ga3+ (62 pm) is coordinated under similar conditions of synthesis with two bipy ligands, whereas the larger Sc3+ (75 pm) in 18 is coordinated by only one bipy ligand (Figure 20).
Figure 20.
Structure of (a) [Sc(bipy)Cl4]− anion in 18, (b) [Ga(bipy)2Cl2][GaCl4] (19) (bipy shown as wires and sticks; H atoms not shown for clarity).
The reaction of Sc(0) nanoparticles with Hdpfa in Tol resulted in colorless crystals of [Sc(dpfa)3] (9), Zr(0) and Hf(0) nanoparticles were too reactive, and the respective reaction was too fast to grow suitable single crystals. Therefore, both metal nanoparticles were reacted in [BMIm]Cl/GaCl3 as an ionic liquid. This leads to the formation of greenish crystals of [Zr(dpfa)4] (20) (P21/c; Table S20, Figure S25) and yellow crystals of [Hf(dpfa)4] (21) (P21/c; Table S21, Figure S26). The reaction follows the equation:
In contrast to [Sc(dpfa)3] (9) with 6-fold coordination of Sc3+ by three dpfa ligands, 20 and 21 exhibit an 8-fold coordination of Zr4+/Hf4+ by four η2-binding (dpfa)− ligands (Zr–N: 223.9(1)–233.4(1) pm; Hf–N: 221.8(4)–233.5(4) pm) (Figure 21a). The resulting distorted, double-capped trigonal prism as a coordination polyhedron (Figure 21b) compares to [Zr(NH3)8]4+ (Zr–N: 223.9–233.4 pm)53 or [Nb(dpfa)4 (Nb–N: 221.5–226.9 pm).54
Figure 21.

Crystal structure of [Zr(dpfa)4] (20): (a) molecular structure, (b) 8-fold coordination around Zr4+ (dpfa shown as wires and sticks; H atoms not shown for clarity).
All compounds [M(dpfa)4] (M = Sc, Zr, and Hf) show intense emission. Thus, the colorless crystals of 9 (Sc) exhibit blue emission (Figure 22a), whereas the greenish crystals of 20 (Zr) and the yellow crystals of 21 (Hf) show emission of green and yellow light (Figure 22b,c) with blue-light excitation (400–500 nm). In comparison to pure Hdpfa (Figure 22d), excitation and emission of 9, 20, and 21 are significantly broadened and red-shifted, which, again, can be correlated to the charge of the respective cation. Since solid Hdpfa does not show any emission, a solution of Hdpfa in THF was used as a reference.
Figure 22.
Fluorescence spectra of (a) [Sc(dpfa)3], (b) [Zr(dpfa)4], (c) [Hf(dpfa)4], and (d) pure Hdpfa (solution in THF) as a reference.
When N,N′-bis(2,6-diisopropylphenyl)formamidine (Hdippfa) was used as a ligand instead of Hdpfa, surprisingly, only a butylated formamidine cation [CH(ndipp)2C4H8]+ was obtained in [BMIm]Cl/GaCl3 instead of the intended metal complexes. Obviously, the ligand is too bulky for an exclusive coordination of Sc3+, Zr4+, and Hf4+. As a result, [CH(Ndipp)2C4H8][GaCl4] (22) was obtained with colorless plates (P21/n; Table S22, Figure S27):
[GaCl4]− originates from the ionic liquid. The butyl group stems from the decomposition of the [BMIm]+ cation, which obviously can be transferred under the reducing conditions of the base-metal nanoparticles.
Conclusions
Scandium, zirconium, hafnium, and manganese are prepared as high-quality nanoparticles with small size, narrow size distribution, and high crystallinity (Sc: 2.4 ± 0.2 nm, Zr: 4.0 ± 0.9 nm, Hf: 8.0 ± 3.9 nm, Mn: 2.4 ± 0.3 nm). The synthesis of the Sc(0), Zr(0), Hf(0), and Mn(0) nanoparticles was performed by reduction of simple metal chlorides (ScCl3, ZrCl4, HfCl4, MnCl2) with lithium or sodium naphthalenide in a one-pot, liquid-phase synthesis in THF or DME. While the knowledge of a liquid-phase synthesis of these metal nanoparticles is rare anyway, specifically Zr(0) and Hf(0) nanoparticles are shown here for the first time.
To probe their reactivity and reactions, the Sc(0), Zr(0), Hf(0), and Mn(0) nanoparticles were treated with different sterically demanding reactants in THF, toluene, or ionic liquids, predominately comprising monodentate and multidentate alcohols and amines. In detail, this includes isopropanol (HOiPr), 1,1′-bi-2-naphthol (H2binol), N,N′-bis(salicylidene)ethylenediamine (H2salen), 2-mercaptopyridine (2-Hmpy), 2,6-diisopropylaniline (H2dipa), carbazole (Hcz), triphenylphosphane (PPh3), N,N,N′,N′-tetramethylethylenediamine (tmeda), 2,2′-bipyridine (bipy), N,N′-diphenylformamidine (Hdpfa), N,N′-(2,6-diisopropylphenyl)-2,4-pentanediimine ((dipp)2nacnacH), 2,2′-dipydridylamine (Hdpa), and 2,6-bis(2-benzimidazolyl)pyridine (H2bbp). These reactions were performed at moderate temperatures (20–120 °C) and resulted in 22 new coordination compounds. Although the remains of the starting materials (e.g., Li+, Na+) can be present in the products, and although some compounds do not contain the respective metal of the nanoparticles, the majority of compounds show the metal coordinated by the sterically demanding ligand. When the different conditions for the synthesis of the base-metal nanoparticles and their follow-up reactions were compared, a reduction with [LiNaph] in THF as the solvent as well as follow-up reactions in toluene turned out to be most promising.
In summary, Sc(0), Zr(0), Hf(0), and Mn(0) nanoparticles turned out to be promising novel starting materials to perform reactions with quasi-homogeneous conditions in the liquid phase near room temperature. The oxidative approach, specifically reactions of the base-metal nanoparticles with O–H and N–H-acidic reactants, can be a useful strategy to obtain novel coordination compounds in addition to the yet most often used Lewis-acid–base reactions, transmetalations, and metathesis reactions.
Acknowledgments
L.-P.F, A.R., and C.F. acknowledge financial support from the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) through the Collaborative Research Centre “4f for Future” (CRC 1573, project number 471424360), project A4. We acknowledge the Karlsruhe Nano Micro Facility (KNMF), Dr. A. Eichhöfer and especially Prof. Dr. D. Fenske for data collection on a Stoe StadiVari diffractometer with Ga-metal-jet source. Moreover, we thank Dr. M. T. Gamer and Prof P. W. Roesky for data collection on a Stoe StadiVari diffractometer with Mo-microfocus source.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.inorgchem.3c03074.
Details related to analytical equipment, general aspects of synthesis, synthesis of base-metal nanoparticles, characterization of base-metal nanoparticles, synthesis of metal compounds, details on structure analysis, characterization of metal compounds (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Wiberg N.; Wiberg E.; Holleman A. F.. Anorganische Chemie; de Gruyter: Berlin, 2017, 103. Ed., Vol. 1, Annex III/IV. [Google Scholar]
- The American Chemical Society (ACS) . Program package Scifinder; ACS: Washington, 2023. [Google Scholar]
- Michelakaki I.; Boukos N.; Dragatogiannis D. A.; Stathopoulos S.; Charitidis C. A.; Tsoukalas D. Synthesis of hafnium nanoparticles and hafnium nanoparticle films by gas condensation and energetic deposition. Beilstein J. Nanotechnol. 2018, 9, 1868–1880. 10.3762/bjnano.9.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sniekers J.; Malaquias J. C.; Van Meervelt L.; Fransaer J.; Binnemans K. Manganese-containing ionic liquids: synthesis, crystal structures and electrodeposition of manganese films and nanoparticles. Dalton Trans. 2017, 46, 2497–2509. 10.1039/C6DT04781E. [DOI] [PubMed] [Google Scholar]
- Eshed M.; Pol S.; Gedanken A.; Balasubramanian M. Zirconium nanoparticles prepared by the reduction of zirconium oxide using the RAPET method. Beilstein J. Nanotechnol. 2011, 2, 198–203. 10.3762/bjnano.2.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokushige M.; Nishikiori T.; Ito Y. Plasma-induced cathodic discharge electrolysis to form various metal/alloy nanoparticles. Russ. J. Electrochem. 2010, 46, 619–626. 10.1134/S1023193510060042. [DOI] [Google Scholar]
- Si P. Z.; Brück E.; Zhang Z. D.; Tegus O.; Zhang W. S.; Buschow K. H. J.; Klaasse J. C. P. Structural and magnetic properties of Mn nanoparticles prepared by arc-discharge. Mater. Res. Bull. 2005, 40, 29–37. 10.1016/j.materresbull.2004.09.010. [DOI] [Google Scholar]
- Tiwari G.; Vinod C. P.; Jagirdar B. R. Controlled exchange bias behavior of manganese nanoparticles. J. Magn. Magn. Mater. 2022, 559, 169504 10.1016/j.jmmm.2022.169504. [DOI] [Google Scholar]
- Abdelkader A. M.; Fray D. J. Synthesis of self-passivated, and carbide-stabilized zirconium nanopowder. J. Nanopart. Res. 2013, 15, 2112. 10.1007/s11051-013-2112-5. [DOI] [Google Scholar]
- Bondi J. F.; Oyler K. D.; Ke X.; Schiffer P.; Schaak R. E. Chemical Synthesis of Air-Stable Manganese Nanoparticles. J. Am. Chem. Soc. 2009, 131, 9144–9147. 10.1021/ja901372q. [DOI] [PubMed] [Google Scholar]
- Purdy A. P.; Sorber R.; Epshteyn A.; Pettigrew K. A.; Miller J. B. The synthesis of hafnium nanomaterials by alkali metal reduction of hafnium tetrachloride. J. Nanopart. Res. 2001, 13, 5435. 10.1007/s11051-011-0531-8. [DOI] [Google Scholar]
- Bönnemann H.; Braun G.; Brijoux W.; Brinkmann R.; Schulze Tilling A.; Seevogel K. Nanoscale colloidal metals and alloys stabilized by solvents and surfactants. Preparation and use as catalyst precursors. J. Organomet. Chem. 1996, 520, 143–162. 10.1016/0022-328X(96)06273-0. [DOI] [Google Scholar]
- Schütte K.; Barthel J.; Endres M.; Siebels M.; Smarsly B. M.; Yue J.; Janiak C. Synthesis of Metal Nanoparticles and Metal Fluoride Nanoparticles from Metal Amidinate Precursors in 1-Butyl-3-Methylimidazolium Ionic Liquids and Propylene Carbonate. Chem. Open 2017, 6, 137–148. 10.1002/open.201600105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil S.; Hosapete S. S.; Irkal S.; Rajput S. Synthesis and characterisation of zirconium nano particles from orange juice and orange peel. J. Appl. Chem. 2019, 12, 29–37. 10.9790/5736-1207012937. [DOI] [Google Scholar]
- Li K.; Li H.; Xiao T.; Zhang G.; Liang A.; Zhang P.; Lin L.; Chen Z.; Cao X.; Long J. Zero-valent manganese nanoparticles coupled with different strong oxidants for thallium removal from wastewater. Front. Environ. Sci. Eng. 2020, 14, 34. 10.1007/s11783-019-1213-5. [DOI] [Google Scholar]
- Li C.; Zhang Y.; Li M.; Zhang H.; Zhu Z.; Xue Y. Fumaria officinalis-assisted synthesis of Manganese nanoparticles as an anti-human gastric cancer agent. Arab. J. Chem. 2021, 14, 103309 10.1016/j.arabjc.2021.103309. [DOI] [Google Scholar]
- Pradhan Amatya S.; Shrestha S. Biosynthesis of Manganese Nanoparticles (MnNPs) from Brassica oleraceae (Cabbage leaves) and its Antibacterial Activity. Asian J. Chem. Sci. 2021, 9, 1–11. [Google Scholar]
- Nador F.; Moglie Y.; Vitale C.; Yus M.; Alonso F.; Radivoy G. Reduction of polycyclic aromatic hydrocarbons promoted by cobalt or manganese nanoparticles. Tetrahedron 2019, 66, 4318–4325. 10.1016/j.tet.2010.04.026. [DOI] [Google Scholar]
- Sergeev G. B.; Klabunde K. J. Nanochemistry. 2nd Ed., Elsevier: Amsterdam, 2013; Vol 55, pp. 275. [Google Scholar]
- Connelly N. G.; Geiger W. E. Chemical Redox Agents for Organometallic Chemistry. Chem. Rev. 1996, 96, 877–910. 10.1021/cr940053x. [DOI] [PubMed] [Google Scholar]
- Reiß A.; Donsbach C.; Feldmann C. Insights of the Naphthalenide-driven Synthesis and Reactivity of Zerovalent Iron Nanoparticles. Dalton Trans. 2021, 50, 16343–16352. 10.1039/D1DT02523F. [DOI] [PubMed] [Google Scholar]
- Bartenbach D.; Wenzel O.; Popescu R.; Faden L.-P.; Reiß A.; Kaiser M.; Zimina A.; Grunwaldt J.-D.; Gerthsen D.; Feldmann C. Liquid-Phase Synthesis of Highly Reactive Rare-Earth Metal Nanoparticles. Angew. Chem., Int. Ed. 2021, 60, 17373–17377. 10.1002/anie.202104955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöttle C.; Bockstaller P.; Popescu R.; Gerthsen D.; Feldmann C. Sodium-Naphthalenide-driven Synthesis of Base Metal Nanoparticles and Specific Follow-up Reactions. Angew. Chem., Int. Ed. 2015, 54, 9866–9870. 10.1002/anie.201503269. [DOI] [PubMed] [Google Scholar]
- LaMer V. K.; Dinegar R. H. J. Theory, production, and mechanism of formation of monodispersed hydrosols. J. Am. Chem. Soc. 1950, 72, 4847–4854. 10.1021/ja01167a001. [DOI] [Google Scholar]
- Hajek B.; Brozek V.; Duvigneaud P. H. Thermal expansion of scandium oxidation products. J. Less-Common Metals 1973, 33, 385–386. 10.1016/0022-5088(73)90191-4. [DOI] [Google Scholar]
- Hull A. W. Crystal structure of titanium, zirconium, cerium, thorium and osmium. Phys. Rev. 1921, 18, 88–89. [Google Scholar]
- Romans P. A.; Paasche O. G.; Kato H. The transformation temperature of hafnium. J. Less-Common Metals 1965, 8, 213–215. 10.1016/0022-5088(65)90048-2. [DOI] [Google Scholar]
- Oberteuffer J. A.; Ibers J. A. Refinement of the atomic and thermal parameters of α-manganese from a single crystal. Acta Crystallogr. 1970, 26, 1499–1504. 10.1107/S0567740870004399. [DOI] [Google Scholar]
- Magnuson M.; Eriksson F.; Hultman L.; Hoegberg H. Bonding Structures of ZrHx Thin Films by X-ray Spectroscopy. J. Phys. Chem. C 2017, 121, 25750–25758. 10.1021/acs.jpcc.7b03223. [DOI] [Google Scholar]
- Zopes D.; Stein B.; Mathur S.; Graf C. Improved Stability of Naked Gold Nanoparticles Enabled by in Situ Coating with Mono and Multivalent Thiol PEG Ligands. Langmuir 2013, 29, 11217–11226. 10.1021/la4012058. [DOI] [PubMed] [Google Scholar]
- Seisenbaeva G. A.; Gohil S.; Kessler V. G. Influence of heteroligands on the composition, structure and properties of homo- and heterometallic zirconium alkoxides. Decisive role of thermodynamic factors in their self-assembly. J. Mater. Chem. 2004, 14, 3177–3190. 10.1039/b404303k. [DOI] [Google Scholar]
- Kobayashi S.; Yazaki R.; Seki K.; Ueno M. An air-stable chiral Hf-based catalyst for asymmetric Mannich-type reactions. Tetrahedron 2007, 63, 8425–8429. 10.1016/j.tet.2007.05.115. [DOI] [Google Scholar]
- Blay G.; Fernández I.; Muñoz M. C.; Pedro J. R.; Vila C. Enantioselective Friedel-Crafts Alkylation of Indoles with (E)-1-Aryl-4-benzyloxybut-2-en-1-ones Catalyzed by an (R)-3,3′-Br2BINOLate-Hafnium(IV) Complex. Eur. J. Org. Chem. 2013, 2013, 1902–1907. 10.1002/ejoc.201201636. [DOI] [Google Scholar]
- Choi H. J.; Sokol J. J.; Long J. R. High-spin metal-cyanide clusters: species incorporating [Mn(salen)]+ complexes as a source of anisotropy. J. Phys. Chem. Solids 2004, 65, 839–844. 10.1016/j.jpcs.2003.11.029. [DOI] [Google Scholar]
- Yuan A. H.; Shen X. P.; Wu Q. J.; Huang Z. X.; Xu Z. Synthesis, crystal structure and magnetic properties of a two-dimensional heterometallic assembly [Mn(salen)]2[Ni(CN)4]·1/2H2O. J. Coord. Chem. 2002, 55, 411–420. 10.1080/00958970211905. [DOI] [Google Scholar]
- McRitchie D. D.; Palenik R. C.; Palenik G. J. A pentagonal bipyramidal scandium(III) complex: synthesis and characterization of diaqua(2,6-diacetylpyridine bis-semicarbazone)scandium(III) hydroxide dinitrate. Inorg. Chim. Acta 1976, 20, L27–L28. 10.1016/S0020-1693(00)94066-0. [DOI] [Google Scholar]
- Demir S.; Mueller T. J.; Ziller J. W.; Evans W. J. σ Bond Metathesis Reactivity of Allyl Scandium Metallocenes with Diphenyl dichalcogenides, PhEEPh (E = S, Se, Te). Organometallics 2011, 30, 3083–3089. 10.1021/om2001876. [DOI] [Google Scholar]
- Maulbetsch T.; Kunz D. Carbenaporphyrins: No Longer Missing Ligands in N-Heterocyclic Carbene Chemistry. Angew. Chem., Int. Ed. 2021, 60, 2007–2012. 10.1002/anie.202013434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. R. D.; Hayes P. G. Yttrium and scandium complexes of a bulky bis(phosphinimine)carbazole ligand. Inorg. Chim. Acta 2014, 422, 209–217. 10.1016/j.ica.2014.05.045. [DOI] [Google Scholar]
- Fischer E.; Hummel H.-U. Investigations in the quasi-binary system LiI/2,2‘bipyridine. Z. Anorg. Allg. Chem. 1997, 623, 483–486. 10.1002/zaac.19976230176. [DOI] [Google Scholar]
- Merzlyakova E.; Wolf S.; Lebedkin S.; Bayarjargal L.; Neumeier B. L.; Bartenbach D.; Holzer C.; Klopper W.; Winkler B.; Kappes M.; Feldmann C. 18-Crown-6 Coordinated Metal Halides with Bright Luminescence and Nonlinear Optical Effects. J. Am. Chem. Soc. 2021, 143, 798–804. 10.1021/jacs.0c09454. [DOI] [PubMed] [Google Scholar]
- Cotton F. A.; Daniels L. M.; Maloney D. J.; Murillo C. A. Transition metal complexes with amidinato ligands: the ubiquitous tris-chelated structural motif. Inorg. Chim. Acta 1996, 242, 31–42. 10.1016/0020-1693(95)04846-4. [DOI] [Google Scholar]
- Yao S.; Xiong Y.; Driess M. Facile Metalation of Silicon and Germanium Analogues of Thiocarboxylic Acids with Manganese(II) Hydride Precursor. Chem.—Eur. J. 2012, 18, 11356–11361. 10.1002/chem.201201335. [DOI] [PubMed] [Google Scholar]
- Wu L. P.; Yamamoto M.; Kuroda-Sowa T.; Maekawa M.; Fukui J.; Munakata M. Crystal structure and magnetic properties of manganese(II) mellitate, [Mn2{C6(COO)6}(H2O)6][Mn(H2O)6]·2H2O with two-dimensional layered structure and three-dimensional hydrogen bonding networks. Inorg. Chim. Acta 1995, 239, 165–169. 10.1016/0020-1693(95)04713-1. [DOI] [Google Scholar]
- Mantel C.; Baffert C.; Romero I.; Deronzier A.; Pécaut J.; Collomb M. N.; Duboc C. Structural Characterization and Electronic Properties Determination by High-Field and High-Frequency EPR of a Series of Five-Coordinated Mn(II) Complexes. Inorg. Chem. 2004, 43, 6455–6463. 10.1021/ic049650k. [DOI] [PubMed] [Google Scholar]
- Vosough Razavi B.; Badiei A.; Lashgari N.; Mohammadi Ziarani G. 2,6-Bis(2-Benzimidazolyl)Pyridine Fluorescent Red-Shifted Sensor for Recognition of Zinc(II) and a Calorimetric Sensor for Iron Ions. J. Fluoresc. 2016, 26, 1723–1728. 10.1007/s10895-016-1863-7. [DOI] [PubMed] [Google Scholar]
- Wasserscheid P.; Welton T.. Ionic Liquids in Synthesis; Wiley-VCH: Weinheim, 2008. [Google Scholar]
- Liebertseder M.; Donsbach C.; Feldmann C. Reactions of Noble-Metal Oxides in Ionic Liquids Near Room Temperature. RSC Adv. 2023, 13, 11441–11449. 10.1039/D3RA00892D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasch A.; Sarish S. P.; Roesky H. W.; Meindl K.; Dali Antonia F.; Schulz T.; Stalke D. Synthesis and characterization of alkynyl complexes of Group 1 and 2. Chem.—Asian J. 2009, 4, 1451–1457. 10.1002/asia.200900178. [DOI] [PubMed] [Google Scholar]
- Arnold P. L.; Rodden M.; Davis K. M.; Scarisbrick A. C.; Wilson C. Asymmetric lithium(i) and copper(ii) alkoxy-N-heterocyclic carbene complexes; crystallographic characterisation and Lewis acid catalysis. Chem. Commun. 2004, (14), 1612–1613. 10.1039/B404614E. [DOI] [PubMed] [Google Scholar]
- Mitzel N. W.; Lustig C. Crystal structure of a lithium chloride cubane cluster solvated by diethyl ether. Z. Naturforsch. B 2001, 56, 443–445. 10.1515/znb-2001-4-521. [DOI] [Google Scholar]
- Restivo R.; Palenik G. J. Crystal structure of cis-dichlorobis(2,2’-bipyridyl) gallium(III)tetrachlorogallate(III). J. Chem. Soc., Dalton Trans. 1972, 341–344. 10.1039/DT9720000341. [DOI] [Google Scholar]
- Huang J.; Tan Y.; Su J.; Gu Q.; Černý R.; Ouyang L.; Sun D.; Yu X.; Zhu M. Synthesis, structure and dehydrogenation of zirconium borohydride octaammoniate. Chem. Commun. 2015, 51, 2794–2797. 10.1039/C4CC09317H. [DOI] [PubMed] [Google Scholar]
- Cotton F. A.; Matonic J. H.; Murillo C. A.; Wang X. The reduction of pentavalent Group 5 compounds with KC8 or LiBH4: a potpourri of oxidation states. Bull. Soc. Chim. Fr. 1996, 133, 711–720. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.