Abstract
Background:
Surgical site infections (SSIs) pose a global challenge, impacting patients and healthcare expenditures. This second-order meta-analysis endeavors to assess the efficacy of antibiotic sutures in averting SSIs by amalgamating data from various meta-studies.
Materials and methods:
This research adhered to the PRISMA 2020 guidelines. The quality and comprehensiveness of the encompassed meta-analyses were assessed through the QUOROM checklist and AMSTAR techniques. The primary study overlap was evaluated via measures such as pairwise intersection heat maps, corrected covered area, and the citation matrix of evidence. The statistical power at the study-level was determined utilizing the meta-meta package. Data synthesis employed random and fixed effects models at a 95% CI. A meta-regression analysis was conducted to explore potential correlations between the CDC classification of SSIs, trial types, and the observed effect sizes in the studies.
Results:
This investigation revealed a significant reduction in SSI rates due to antimicrobial-coated sutures, evidenced by a relative risk (RR) of 0.68 (95% CI: 0.59–0.76), with a prediction interval of 0.38–1.19. The analysis encompassed 18 studies with 22 meta-analyses, demonstrating a median QUOROM score of 13.6 out of 18 and an AMSTAR score of 9.1 out of 11. The presence of moderate heterogeneity was noted (Q=106.611, I2=54.038%), with nonrandomized controlled trials exhibiting an RR of 0.56 (95% CI: 0.39–0.80), and RCTs displaying an RR of 0.71 (95% CI: 0.63–0.81). Subgroup analysis unveiled variable RR reductions for specific surgical procedures.
Conclusion:
Antimicrobial-coated sutures offer a promising approach to mitigating SSIs risk. However, their efficacy is optimally realized when employed in conjunction with other robust practices.
Keywords: antimicrobial agent, antimicrobial-coated sutures, bacterial infections, hospital-acquired infections, surgical site infections, triclosan
Introduction
Highlights
Surgical site infections are the third severe hospital-acquired infections in the healthcare system.
Triclosan-coated sutures can be used in reducing the severity of such infections.
A statistically significant result in favor of antimicrobial-coated sutures was found.
Different surgery groups failed to achieve statistical significance.
Several factors are related to surgical site infections and research needs to be conducted further.
Surgical site infections (SSIs) affect millions of patients every year worldwide. In Asia and low-income to middle-income countries, over 11% of surgery patients and 20% of African cesarean recipients suffer from SSIs annually1. Even developed nations face this predicament; the WHO report underscores that SSIs lead to an extra 0.4 million hospital days and an added US$10 billion yearly expenses in the USA2. Infections of this nature commonly manifest within a 30-day postoperative period, specifically where the surgical intervention occurred. The severity of these infections may vary3. Microbial pathogens, including gram-positive bacteria such as Staphylococcus aureus [both methicillin-resistant (MRSA) and methicillin-sensitive (MSSA)], as well as gram-negative bacteria like Escherichia coli, Pseudomonas aeruginosa, Acinetobacter species, and Enterococcus species, can cause SSIs4. Furthermore, prior research has established that Staphylococcus epidermidis can establish biofilm colonization on percutaneous sutures4. The dominance of S. aureus in diverse surgical procedures suggests the potential variability of bacterial types based on the surgery’s nature and location4.
One potential solution to SSI is to use antibacterial agents on medical devices, including surgical sutures. Triclosan, a lipid-soluble chlorinated phenoxy phenol agent, has been in use for over three decades in numerous everyday products, exhibiting bacteriostatic properties at low concentrations (0.025–1.000 mg/ml) and bactericidal properties at higher concentrations (7.5–8.0 mg/ml)5–8. While triclosan-coated sutures have demonstrated efficacy against a broad-spectrum of bacteria, concerns regarding developing triclosan-resistant bacteria have arisen due to its extensive usage in daily life and clinical settings, particularly in S. aureus 9.
Foreign materials, such as sutures and medical implants, are employed as supportive structures during surgical procedures, inadvertently creating a potential reservoir for exogenous bacteria. Studies have indicated that sutures can instigate SSIs across diverse hosts and environments. To address this concern, diverse types of sutures are presently under exploration, incorporating biotechnological techniques such as dip-coating, surface modification, and blending involving antiseptics, nanoparticles, and antibiotics. In 2002, the US Food and Drug Administration (FDA) approved incorporating the initial antibacterial agent with surgical sutures, specifically the polychlorophenoxyphenol (triclosan) coating. In vitro and in vivo investigations have demonstrated its broad-spectrum biocidal action against both gram-positive and gram-negative bacteria. Moreover, the antimicrobial coating can effectively diminish bacterial adhesion, inhibit biofilm formation, and impede the development of drug-resistant pathogens at surgical sites, presenting a promising approach to mitigate bacterial infections10.
The occurrence of SSI is influenced by a range of patient-related factors, encompassing patient status (new or old), sociodemographic characteristics (sex, age, education, religious affiliation, marital status, and income status), as well as comorbidities like type 2 diabetes and heart disease. For instance, research has revealed that abdominal surgery patients encounter SSIs in 22.1% of cases within a 30-day period. Orthopedic patients are also susceptible to developing SSIs due to the potential impairment of proper physical function11. Furthermore, specific risk factors, including diabetes mellitus, smoking habits, surgeries extending beyond three hours, the absence of antibiotic prophylaxis, and a history of prior surgeries, significantly contribute to an escalated risk of SSIs. Beyond individual patient factors, parameters such as hospital hygiene conditions, the education level of hospital staff, infrastructure, and obesity hold significance in the context of SSI occurrence.
In this study, a second-order meta-analysis, a method that quantitatively synthesizes findings from multiple meta-analyses addressing analogous research inquiries, will be implemented. This approach aims to consolidate outcomes concerning the impact of antimicrobial-coated sutures on surgical sites and wound infections into a coherent body of information12. Through the application of quantitative synthesis, our objective is to offer a comprehensive assessment of the potential benefits and efficacy associated with using antimicrobial-coated sutures to prevent SSIs.
Methods
Literature search strategy and study selection
The present study followed the requirements established by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA 2020, Supplemental Digital Content 1, http://links.lww.com/JS9/B192, Supplemental Digital Content 2, http://links.lww.com/JS9/B193) to conduct a comprehensive and rigorous literature evaluation13,14. A thorough and extensive search was performed across four databases: Scopus, PubMed, Web of Science, and Google Scholar. The search used various combinations of relevant keywords, including ʻmeta-analysisʼ, ʻsystematic reviews and meta-analysisʼ, ʻtriclosanʼ, ʻquantitative synthesisʼ, ʻantisepticʼ, ʻPDS Plusʼ, ʻAntibacterial agentsʼ, ʻSurgical site infectionʼ, ʻVicryl Plusʼ, ʻsutureʼ, ʻMonocryl Plusʼ, ʻAnti-infective agentsʼ, ʻCoated materialsʼ, ʻPDSʼ, ʻBiomimetic materialʼ, ʻinfect*ʼ, ʻpatients*ʼ, ʻSurgical Wound infectionʼ, ʻbiocompatibleʼ, ʻbiocideʼ, ʻnosocomialʼ, ʻPostoperative Complicationsʼ, ʻSSIʼ. The specific search criteria used for each database are presented in Supplementary Table 1 (Supplemental Digital Content 4, http://links.lww.com/JS9/B195). The protocol registration for this review can be found on the Open Science Framework (OSF) at osf.io/827r4.
The inclusion criteria for this study were centered around meta-analyses that particularly examined the effects of antimicrobial-coated sutures on surgical site or wound infections. To ensure the selection of high-quality studies, strict exclusion criteria were applied. Excluded from consideration were systematic reviews without data pooling or meta-analysis, narrative reviews, reviews without a specified search algorithm, and reviews lacking clear selection criteria for included studies.
Two authors (S.A. and A.I.) meticulously reviewed and assessed the titles and abstracts of potential studies, diligently identifying relevant manuscript titles and abstracts. Any discrepancies among the published articles were thoroughly investigated and resolved through consensus.
The authors applied the following inclusion criteria to select studies for the current meta-meta-analysis:
The study must be a meta-analysis examining antimicrobial-coated sutures’ impact on surgical sites or wound infections.
The study must provide the necessary statistical data or effect sizes required for a meta-analysis of effect sizes.
The study must be a meta-analysis of controlled trials, either randomized or nonrandomized.
Studies considered for inclusion were required to present statistical data that allowed for calculating effect sizes related to the effectiveness of antibiotic-infused sutures in preventing SSIs. In cases where essential information was not immediately apparent in an article, attempts were made to contact the corresponding authors via e-mail to request the necessary data.
Studies were excluded from the analysis if they met any of the following exclusion criteria:
The study was classified as a systematic review without a meta-analysis.
The review did not provide precise criteria for the inclusion of studies.
No restrictions were placed on the type of environment in the included research, and no limitations were made on the year of publication. In instances of discord, both authors engage in reassessing the studies to arrive at a mutually agreed-upon conclusion. The authors conducted an in-depth evaluation of the whole texts of all the included studies. They also reached out to the respective authors for any more information that was needed. Subsequently, the authors determined the inclusion or exclusion of each study. The research management tools utilized in this study were Zotero (Version 6.0.26) and Herzing’s Publish or Perish (Version 8.2). The data extraction form has been included in Supplementary Table 2 (Supplemental Digital Content 4, http://links.lww.com/JS9/B195).
Data extraction and quality assessment
The data obtained from the meta-analyses included a range of important components, including the first author, primary studies, confidence intervals, relative risk (RR) or odds ratio (OR), study years, sample size for both treatment and control groups, language, database searches, publication dates, journals, protocols, models, and software15,16. To evaluate the validity and robustness of the included meta-analyses, two well-established techniques were employed: the Quality of Reporting of Meta-analyses (QUOROM) checklist and the Assessment of Multiple Systematic Reviews (AMSTAR)17 (Supplemental Digital Content 3, http://links.lww.com/JS9/B194). Additionally, several measures were utilized to assess the primary study overlap, including pairwise intersection heat maps, corrected covered area (CCA), and a citation matrix of evidence18–21.
To quantify the study-level statistical power across a range of true effect sizes, a ʻFirepowerʼ plot was generated using functions from the ‘metameta’ package (Supplementary Fig. 2, Supplemental Digital Content 5, http://links.lww.com/JS9/B196)22. This analysis provided valuable insights into the statistical robustness of the included meta-analyses22.
For the meta-analytical computations, the Comprehensive Meta-Analysis program (V4) was employed to combine the outcomes of meta-analyses modeled with random and fixed effects and 95% CI23. The calculations involved various specifics, such as the prediction interval, the DerSimonian–Laird estimate for tau2, the Jackson technique for computing the CI of tau2 and tau, and the Clopper–Pearson CI for individual studies.
Cochran’s Q statistic was employed in order to evaluate the consistency of impact sizes across studies. Furthermore, Higgins and Thompson’s I2 index quantified the heterogeneity between studies. Sensitivity analysis was conducted to balance the influence of index studies on the overall summary effect, thereby enhancing the robustness of the findings. Meta-regression was performed to explore potential associations between CDC classification of SSI or trial type and study effect sizes24,25. This analysis allowed for identifying any significant links between these variables, providing further insights into the factors influencing the study outcomes. Finally, data extraction and quality assessment were carried out rigorously using established methodologies, and statistical analyses were performed to ensure the reliability and validity of the results obtained from the included meta-analyses.
Results
Evaluation of meta-analytic studies on antibiotic sutures for preventing SSIs
Initially, 149 abstracts were identified based on the search criteria for studies exploring the effects of antibiotic-coated sutures on SSIs. Following a rigorous selection process adhering to PRISMA guidelines, 18 studies (encompassing 22 meta-analyses) fulfilled the eligibility criteria and were included in the analysis5,9,10,26–38. Elaborate information about the chosen studies is available in Table 1, and the study selection procedure is depicted in Figure 1.
Table 1.
Characteristics of included studies.
| Paper ID | Meta-analysis | Publication Year | Studies included in meta-analysis | TCSs (n) | TCSs (N)2 | Uncoated(n) | Uncoated(N)3 | RR | lower | upper | Type of study | Protocol | Quality and bias assessment | Model | Software | Included Studies (Range) | Journal |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | Ademuyiwa et al.27 | 2022 | 5 | 733 | 4360 | 784 | 4259 | 0.93 | 0.84 | 1.02 | RCTs | PRISMA | ROBIS | REM | R software | 2013–2021 | Lancet Infect Dis |
| P2 | Apisarnthanarak et al.28 | 2015 | 26 | 322 | 3376 | 446 | 3554 | 0.74 | 0.61 | 0.89 | RCTs | NR | ROBIS | REM/FEM | STATA | 1986–2014 | Infection Control & Hospital Epidemiology |
| P3 | Apisarnthanarak et al.28 | 2015 | 7 | 127 | 2426 | 276 | 2586 | 0.53 | 0.42 | 0.66 | nRCTs | NR | NOS | REM/FEM | STATA | 2009–2014 | Infection Control & Hospital Epidemiology |
| P4 | Wang et al.29 | 2013 | 17 | 149 | 1726 | 227 | 1994 | 0.7 | 0.57 | 0.85 | RCTs | PRISMA | ROBIS | FEM | RevMan | 2008–2012 | British Journal of Surgery |
| P5 | Chang et al.26 | 2012 | 7 | 30 | 469 | 35 | 419 | 0.78 | 0.49 | 1.25 | RCTs | PRISMA | NR | REM | RevMan | 2005–2010 | Annals of Surgery |
| P6 | Ahmed et al.5 | 2019 | 25 | 420 | 6008 | 581 | 5949 | 0.73 | 0.65 | 0.82 | RCTs | PRISMA | GRADE | FEM | RevMan | 2011–2019 | BMJ Open |
| P7 | Edmiston | 2013 | 13 | 126 | 1654 | 190 | 1914 | 0.69 | 0.53 | 0.92 | RCTs | NR | GRADE/ROBIS | REM/FEM | CMA | 2005–2013 | Surgery |
| P8 | Elsolh et al.10 | 2017 | 5 | 168 | 1606 | 196 | 1511 | 0.78 | 0.49 | 1.25 | RCTs | PRISMA | GRADE | REM | RevMan | 2011–2015 | Journal of Gastrointestinal Surgery |
| P9 | Guoet al.36 | 2016 | 13 | 262 | 2592 | 349 | 2664 | 0.76 | 0.65 | 0.88 | RCTs | PRISMA | ROBIS | REM/FEM | RevMan | 2009–2014 | Journal of Surgical Research |
| P10 | Henriksen et al.9 | 2017 | 8 | 179 | 1797 | 230 | 1705 | 0.76 | 0.63 | 0.92 | RCTs | PRISMA | SIGN | REM/FEM | RevMan | 2009–2015 | Hernia |
| P11 | konstantelias | 2017 | 19 | 330 | 3311 | 427 | 3436 | 0.75 | 0.63 | 0.91 | RCTs | PRISMA | Jadad | REM | RevMan | 2005–2016 | Acta Chirurgica Belgica |
| P12 | konstantelias | 2017 | 11 | 196 | 3623 | 555 | 5015 | 0.57 | 0.4 | 0.81 | nRCTs | PRISMA | NOS | REM | RevMan | 2009–2016 | Acta Chirurgica Belgica |
| P13 | Sandini et al.30 | 2016 | 6 | 129 | 1102 | 143 | 1066 | 0.89 | 0.71 | 1.11 | RCTs | PRISMA | ROBIS | REM | ProMeta | 2011–2014 | Medicine |
| P14 | Daoud | 2013 | 15 | 180 | 2323 | 273 | 2477 | 0.67 | 0.54 | 0.84 | RCTs | PRISMA | CEBM | REM | CMA/STATA | 2005–2013 | Surgical Infections |
| P15 | Uchino | 2018 | 10 | 160 | 1798 | 205 | 1690 | 0.67 | 0.48 | 0.94 | RCTs | PRISMA | GRADE/ROBIS | REM | RevMan | 2011–2017 | Journal of Gastrointestinal Surgery |
| P16 | Uchino | 2018 | 5 | 77 | 1091 | 184 | 1124 | 0.47 | 0.36 | 0.6 | nRCTs | PRISMA | GRADE/ROBIS | REM | RevMan | 2011–2016 | Journal of Gastrointestinal Surgery |
| P17 | wu | 2016 | 13 | 267 | 2661 | 345 | 2685 | 0.8 | 0.69 | 0.93 | RCTs | PRISMA | GRADE /NOS | REM | RevMan | 2005–2014 | Eur J Clin Microbiol Infect Dis |
| P18 | wu | 2016 | 5 | 84 | 965 | 164 | 1147 | 0.64 | 0.5 | 0.82 | nRCTs | PRISMA | GRADE/NOS | REM | RevMan | 2011–2014 | Eur J Clin Microbiol Infect Dis |
| P19 | Sajid | 2012 | 7 | 43 | 760 | 38 | 871 | 0.61 | 0.37 | 0.99 | RCTs | PRISMA | GRADE/jadad | REM | RevMan | 2005–2011 | Gastroenterology Report |
| P20 | Leaper et al.34 | 2017 | 34 | NR | 252 | NR | 241 | 0⋅61 | 0⋅52 | 0⋅73 | RCTs | PRISMA | NR | REM | NR | 2011–2017 | BJS Society |
| P21 | dejonge | 2017 | 21 | 330 | 3208 | 450 | 3254 | 0.72 | 0.6 | 0.86 | RCTs | PRISMA | GRADE/ROBIS | REM | CMA/ TSA | 2005–2014 | Wiley Online Library |
| P22 | Hunger | 2018 | 6 | 76 | 1101 | 169 | 1856 | 0.62 | 0.29 | 1.31 | RCTs | PRISMA | ROBIS | REM | R software | 2013–2016 | Springer Nature |
Figure 1.
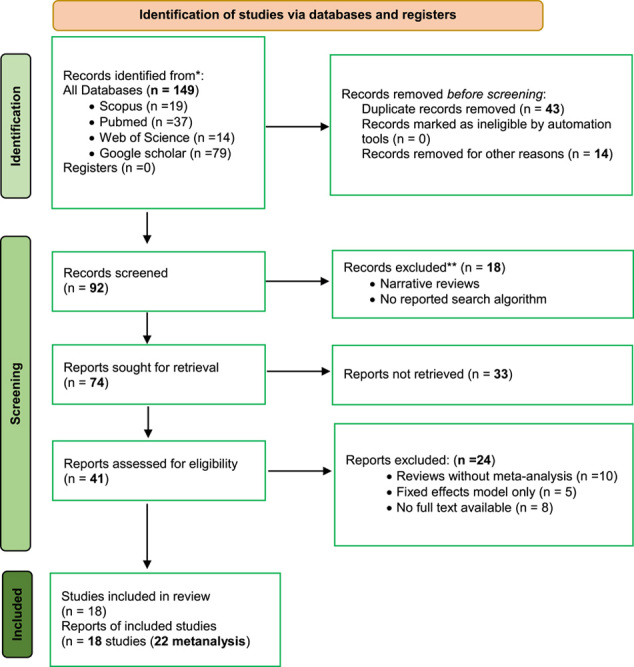
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA model) flow map of article selection from the previous literature search.
The selected studies were published between 2012 and 2022, encompassing a broad time range to ensure a comprehensive overview of the research landscape. Concerning the employed meta-analysis models, the DerSimonian–Laird random effects model was the prevailing choice, utilized in 15 out of the included meta-analyses (68.18%). In the study, a total of five examples, accounting for 22.73% of the sample, employed a combined approach utilizing both the random effects model and the fixed-effect model. Additionally, two cases, representing 9.09% of the sample, exclusively utilized the fixed-effect model (Supplementary Data 1, Supplemental Digital Content 6, http://links.lww.com/JS9/B197). The Risk of Bias in Systematic Reviews (ROBIS) tool was frequently applied in six meta-analyses (27.27%) for evaluating the quality of primary research. Several assessment tools were utilized in the study. These included GRADE/ROBIS in four instances, accounting for 18.18% of the cases. GRADE was employed in two cases, representing 9.09% of the sample. The Newcastle–Ottawa Scale (NOS) was utilized in two cases, accounting for 9.09%. The Center for Evidence-Based Medicine (CEBM) was employed in six cases, making up 4.55% of the sample. GRADE/NOS, GRADE/Jadad, Jadad, Scottish Intercollegiate Guidelines Network (SIGN), and not reported (NR) were each used in one case, representing 4.55% of the cases.
In terms of the software utilized for data synthesis, the most often utilized tool was RevMan, accounting for 59.09% of the cases (n=13). STATA and R software were utilized in 9.09% of the cases (n=2). ProMeta, CMA, and CMA/STATA were each used in 4.55% of the cases (n=1), while the software used was not reported in 4.55% of the cases (n=1). The meta-analyses used in this study drew upon primary studies published from 2005 to 2019, including a significant research timeframe in this particular field. The range of primary studies referenced per meta-analysis varied from 5 to 34, with an average of 13 primary studies cited per meta-analysis included in the study (Table 1).
The coated and uncoated sample sizes exhibited variations, ranging from 252 to 6008 and 241 to 5949, respectively, with an average of 2191 coated and 2337 uncoated samples per included meta-analysis. Among the 18 meta-analyses, 12 (66.7%) reported that antimicrobial-coated sutures significantly reduced the risk of postoperative SSI, providing substantial evidence of their efficacy. However, six meta-analyses (33.3%) underscored the necessity for more evidence prior to definitive conclusions, underscoring the importance of further research in this domain. In sum, this extensive analysis of the selected studies yields valuable insights into the characteristics and outcomes of meta-analyses scrutinizing the impact of antibiotic-coated sutures on SSIs. The incorporation of multiple meta-analyses enhances the dependability and applicability of the conclusions drawn from this investigation.
Study quality and power evaluation
The literature search conducted in the papers included in this analysis predominantly relied on web/Medline resources, except for Daoud et al. 2013 (Supplementary Figure 1, Supplemental Digital Content 5, http://links.lww.com/JS9/B196). Although there was significant variation in the utilization of supplementary resources, every study conducted a search on a minimum of two electronic databases. It is worth mentioning that a considerable percentage of the studies included in the analysis (94.4%) did not impose any restrictions on the languages used in their search criteria17.
The QUOROM scores ranged from 11 to 15, with a median score of 13.6 out of a maximum of 18, indicating a moderate-to-high level of quality in reporting. Similarly, the AMSTAR scores ranged from 8 to 10, with a median score of 9.1 out of a maximum of 11, suggesting a good overall methodological quality of the systematic reviews. However, it is worth noting that the included studies did not consistently provide detailed information about the analyzed studies’ features. Nevertheless, the majority of studies conducted comprehensive literature searches and evaluated the methods of study selection and potential publication bias. Additional details can be found in Table 2.
Table 2.
AMSTAR criteria and QUOROM score.
| Paper ID | Items/ study | Priori design? | Duplicate study selection and data extraction? | Comprehensive search? | Publication status used as an inclusion criterion? | Studies list provided? | Characteristics studies provided? | Scientific quality assessed and documented? | Scientific quality used conclusions? | Methods used to combine findings, correct? | Publication bias assessed? | Conflict of interest stated? | Total AMSTAR | QUOROM score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | Ademuyiwa et al.27, 2022 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | 15 |
| P2 | Apisarnthanarak et al.28, 2015 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | 15 |
| P3 | Wang et al.29, 2013 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | 12 |
| P4 | Chang et al.26, 2012 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | 14 |
| P5 | Ahmed et al.5, 2019 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | 11 |
| P6 | Edmiston, 2013 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | 13 |
| P7 | Elsolh et al.10, 2017 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | 14 |
| P8 | Guo et al.36, 2016 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | 14 |
| P9 | Henriksen et al.9, 2017 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | 13 |
| P10 | konstantelias, 2017 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | 14 |
| P11 | Sandiniet al.30, 2016 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | 13 |
| P12 | Daoud, 2013 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | 15 |
| P13 | Uchino, 2018 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | 14 |
| P14 | wu, 2016 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | 12 |
| P15 | Sajid, 2012 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | 14 |
| P16 | Leaper et al.34, 2017 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | 14 |
| P17 | dejonge, 2017 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | 14 |
| P18 | Hunger, 2018 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | 13 |
The included studies showed a range of statistical power ratings from 0.25 to 1. Most studies fell within the range of 0.5 (medium-powered) to 1.0 (high-powered), assuming the summary effect size represents the true effect size. These findings indicate that most of the included studies possess sufficient statistical power to detect a wide range of meaningful effects, especially when considering studies drawn from a pool of potential studies. Figure 3 visually represents the distribution of power values among the included studies.
Figure 3.
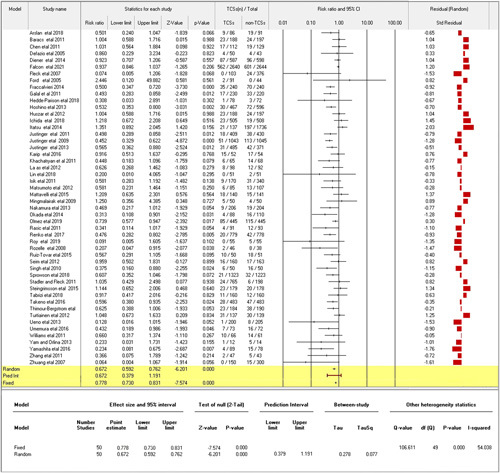
Forest plot of pooled study estimates.
The literature search methods used in the included studies primarily utilized web/Medline databases, except for Daoud et al. 2013 (Supplementary Figure 1, Supplemental Digital Content 5, http://links.lww.com/JS9/B196). Each study searched at least two electronic databases, demonstrating a consistent and rigorous approach. Remarkably, a substantial proportion of the included studies (94.4%) imposed no language limitations on their search criteria, ensuring a comprehensive review of relevant literature16.
To evaluate the quality of reporting, the QUOROM scores ranged from 11 to 15, with a median score of 13.6 out of a possible maximum of 18. This suggests a moderate-to-high level of reporting quality. Similarly, the methodological quality of the systematic reviews was assessed using AMSTAR scores, which ranged from 8 to 10, with a median score of 9.1 out of a maximum of 11. This indicates a good overall methodological quality. It’s worth noting that while some of the included studies lacked consistent and detailed information about the features of the analyzed studies, the majority conducted comprehensive literature searches and evaluated methods for study selection and potential publication bias. Further information is provided in Table 2.
Regarding statistical power, the included studies exhibited a range of values from 0.25 to 1.0. Most studies fell within the range of 0.5 (medium-powered) to 1.0 (high-powered), assuming the summary effect size represents the true effect size. These findings indicate that the majority of included studies possess sufficient statistical power to detect a wide range of meaningful effects, especially when considering studies drawn from a pool of potential studies. Figure 3 visually represents the distribution of power values among the included studies.
In summary, the study quality and power evaluation results indicate that the included meta-analyses exhibit good reporting and methodological quality and adequate statistical power to detect meaningful effects. The comprehensive nature of the literature search and assessment of potential biases further strengthen the credibility and reliability of the findings.
RR of surgical site infection in various surgical procedures
Most of the included meta-analyses cited similar index studies, resulting in a high overlap in primary studies with a CCA score greater than 15% (20.51%). Therefore, a direct summary meta-analysis of meta-analyses was not performed, and data were pooled directly from index studies instead (Fig. 2). Studies that computed effects in risk ratios were converted to odd ratios to ensure uniformity. Both fixed effects and random effects models were used for data pooling.
Figure 2.
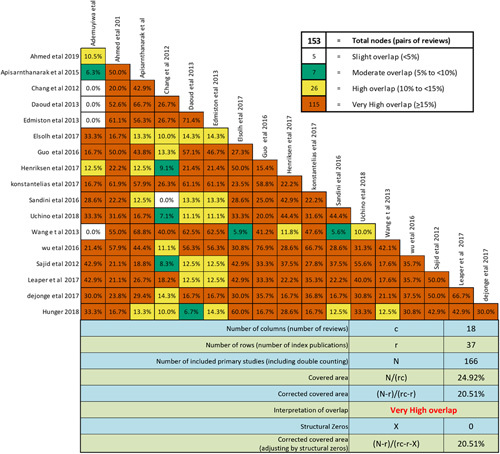
Pair wise intersection heat maps of index study overlaps.
The combined analysis encompassed fifty trials of varying quality (Fig. 3). Antimicrobial-coated sutures exhibited a statistically significant reduction in SSI rates, as reflected by a random effects RR of 0.68 (95% CI: 0.59–0.76; P=0.0000), along with a prediction interval of 0.68 (95% CI: 0.38–1.19). Notably, the study displayed moderate heterogeneity with Q=106.611 and I2=54.038%. In this context, Q signifies evidence of variation in the RR, while I2 represents the proportion of variation in true effects beyond sampling error. Further differentiation based on study type revealed a RR of 0.56 (95% CI: 0.39–0.80; P=0.00) for nonrandomized controlled trials (non-RCTs) and a RR of 0.71 (95% CI: 0.63–0.81; P=0.00) for randomized controlled trials (RCTs).
The computation of summary analysis using OR yielded a random effect OR of 0.63 (95% CI: 0.54–0.73; I2=52.56%, Q=84.62, P=0.0000), accompanied by a fixed-effect OR of 0.72 (95% CI: 0.67–0.79), both favoring antimicrobial-coated sutures (Supplementary Figure 3, Supplemental Digital Content 5, http://links.lww.com/JS9/B196). Subgroup analysis (Fig. 4) unveiled the statistical significance of antimicrobial-coated sutures in reducing SSI rates across diverse surgical procedures. This included abdominal surgery (RR 0.64, 95% CI: 0.51–0.82; P=0.00), arthroplasty (RR 0.59, 95% CI: 0.43–0.81; P=0.001), cerebrospinal fluid shunt surgery (RR 0.21, 95% CI: 0.05–0.92; P=0.04), coronary artery bypass grafting (RR 0.55, 95% CI: 0.36–0.85; P=0.01), hepatobiliary surgery (RR 0.45, 95% CI: 0.33–0.62; P=0.00), multiple surgical wound types (RR 0.48, 95% CI: 0.33–0.70; P=0.00), and spinal surgery (RR 0.13, 95% CI: 0.02–1.02; P=0.05).
Figure 4.
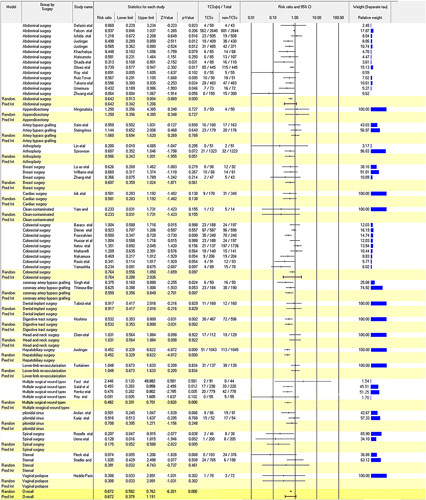
Subgroup analysis by suture site.
Despite the overall reduction in the pooled risk of developing SSIs within the antimicrobial-coated group, certain types of surgeries did not yield statistically significant evidence, including breast surgery, cardiac surgery, clean-contaminated surgery, colorectal surgery, dental implant surgery, digestive tract surgery, gastric cancer surgery, head and neck surgery, hip and knee arthroplasty, knee arthroplasty, lower-limb revascularization, pilonidal sinus surgery, sternal surgery, umbilical surgery, and vaginal prolapse surgery39,40. Furthermore, the aggregated RR for appendicectomy and artery bypass grafting did not provide substantial support for a reduction in SSIs through the utilization of antimicrobial-coated sutures.
An in-depth subgroup analysis based on CDC classification unveiled varying degrees of statistical significance for different site categories. Specifically, antimicrobial-coated sutures exhibited a statistically significant impact in reducing the rates of SSIs at a risk ratio of 0.70 for clean sites (95% CI: 0.56–0.88, P=0.00), 0.86 for clean-contaminated and contaminated sites (95% CI: 0.23–3.23, P=0.82), 0.71 for clean-contaminated sites (95% CI: 0.60–0.84, P=0.00), and 0.63 for dirty/infected sites (95% CI: 0.48 to 0.84, P=0.00).
Further exploration through meta-regression analysis did not reveal any statistically significant associations between CDC classification or trial type and the cohort 2×2 effect sizes (P≤0.05) (Supplementary Figures 3a to f, Supplemental Digital Content 5, http://links.lww.com/JS9/B196).
Discussion
In the pursuit of preventing postoperative wound infections, which result from the complex interplay between introduced microorganisms, their infectivity, and the host’s immune response, the conventional approach of administering broad-spectrum antibiotics has been a common practice41. However, recent research has shifted towards innovative biomaterial designs to impede microbial colonization. Studies have consistently shown that biomaterials coated with antibacterial agents exhibit significantly reduced bacterial colonization and adhesion compared to noncoated materials42. In this context, the use of antibacterial-coated sutures stands out as a promising strategy to mitigate SSIs. Microbial colonization on surgical sutures has consistently shown a strong link to the chemical and structural composition of the sutures, in line with findings from other biomaterial studies41. Researchers are actively working on developing suture materials with improved tensile strength and controlled absorption properties to minimize epithelial reactivity and enhance infection prevention.
One crucial antibacterial coating used on surgical sutures is triclosan, which has been in use since 2003. As supported by previous research, triclosan coating promotes an optimal environment for invasive procedures and supports the healing process43. The ongoing efforts to refine biomaterial design and coating techniques will likely lead to a significant reduction in SSIs and improve patient outcomes. These advancements hold significant potential for enhancing the safety and success of surgical interventions.
Research into the impact of suture materials containing triclosan has been conducted across controlled trials involving subjects with diverse patient-related and procedural risk factors. However, trial quality and potential biases have hampered the interpretation of these findings and the ability to draw definitive conclusions. To address these limitations, establishing rigorous inclusion and exclusion criteria and subgroup analyses on homogenous cohorts is crucial. The utilization of meta-analyses and systematic reviews can further help overcome these limitations.
Despite a multitude of studies and meta-analyses assessing the efficacy of triclosan-coated sutures in reducing SSIs since their inception, methodological shortcomings have hindered conclusive findings. While guidelines from reputable bodies such as NICE (2019) and WHO (2018) recommend the use of triclosan-coated sutures and alcoholic chlorhexidine to lower SSI rates based on favorable findings from small RCTs, these findings have been assessed as primarily low or extremely low in reliability using the GRADE approach27.
In this study, we conducted the first second-order meta-analysis and the most extensive meta-analysis of trials investigating the impact of antimicrobial-coated sutures on surgical outcomes and wound healing. Our approach involved the inclusion of trials with varying quality, ranging from low to moderate-to-high, based on quality assessment and study-level statistical power evaluations, resulting in a total of fifty studies. This approach was adopted to address the significant overlap of included meta-analyses and avoid excluding well-conducted research that may not have achieved the highest quality reporting standards, considering potential cost differences compared to alternatives.
Through a quantitative synthesis of evidence from 50 primary studies (trials), derived from 18 studies and 22 meta-analyses with considerable primary study overlap, we assessed the overall impact of antimicrobial-coated sutures on the risk of patients developing SSI. Our random effects meta-analysis of these fifty trials yielded an aggregated RR of 0.68 (95% CI: 0.59–0.76; P=0.0000), with a prediction interval of 0.68 (95% CI: 0.38–1.19), favoring the use of triclosan-coated sutures. This suggests that triclosan reduces organism adhesion, leading to fewer infections compared to uncoated sutures. Notably, patients undergoing multiple surgical wound types, including abdominal surgery, arthroplasty, cerebrospinal fluid shunt surgery, coronary artery bypass grafting, hepatobiliary surgery, and spinal surgery, experienced a significant reduction in RR44.
The variability observed in the reduction of RR across different surgical/wound site categories might be attributed to the substantial variation in the microbiota’s composition across different populations, mainly influenced by patient habits and environmental conditions45. Therefore, the presence of various bacterial strains with different susceptibilities to triclosan’s antimicrobial effects might contribute to the variation in results among trials. Understanding these factors will be crucial in tailoring the use of antimicrobial-coated sutures to specific patient populations and surgical contexts to optimize their efficacy in reducing SSIs.
However, it is important to consider the potential risk of widespread use leading to the selection of triclosan-resistant strains with cross-resistance to antibiotics26. This concern should be actively monitored to address any emerging challenges effectively.
In our study, certain moderators examined did not show significant effects of triclosan-impregnated sutures on the rate of SSIs in patients. However, pooling the results of potentially homogeneous trials based on the level of wound contamination and surgery type revealed moderate heterogeneity among studies. Nonetheless, we did not find evidence of publication bias, as indicated by the symmetric distribution in the funnel plot. This is significant because, despite the higher costs associated with coated sutures, the potential cost benefits of reduced SSIs could be substantial.
As we continue to delve into the effectiveness of triclosan-coated sutures, it is crucial to account for patient-specific factors, surgical context, and resistance patterns to optimally tailor the use of antimicrobial-coated sutures. By integrating these considerations into clinical decision-making, we can maximize the benefits of triclosan-coated sutures in preventing SSIs while minimizing the risk of resistance development. Further research and ongoing vigilance will be paramount in refining our understanding of these factors and optimizing patient outcomes.
Based on our investigation, the use of coated sutures demonstrates a pronounced average effect size, with a mean of 0.68 and confidence intervals spanning from 0.59 to 0.76 in studies utilizing random-effect modeling. Likewise, studies employing fixed effects modeling also reveal a favorable mean effect size of 0.78 (95% CI: 0.73–0.831; P=0.000). Our synthesis of estimates suggests that the RR of all comparable studies should lie within a prediction interval of 0.382 to 1.193 at a 95% confidence level, assuming a normal distribution of the logarithm of true RR. These findings align with a recent meta-analysis conducted by46 (unaccounted for in our aggregated data), which reported a mean effect size of 0.76, falling within this specified range.
A multitude of clinically based research studies and literature reviews have consistently underscored the efficacy of triclosan-coated sutures in mitigating SSIs. This finding harmonizes with endorsements from esteemed international organizations, including WHO, CDC, NICE, APSIC, SHEA/IDSA, and ACS/SIS. Despite these encouraging indications, it is imperative to acknowledge that there remains a scarcity of studies focused specifically on assessing triclosan’s efficacy against SSIs. Thus, the conduct of additional randomized controlled trials (RCTs) is a pivotal endeavor, offering invaluable evidence to reinforce the effectiveness of triclosan-coated sutures in preventing SSIs. Such studies would fortify the existing knowledge repository and enrich patient care and outcomes.
The escalating incidence of SSIs and the concomitant healthcare expenditures underscore the urgency of a holistic and efficacious strategy involving diverse stakeholders, encompassing hospital personnel, physicians, surgeons, and healthcare practitioners. This comprehensive approach is pivotal in averting and managing these infections47. Preoperative interventions play a pivotal role in diminishing the SSI risk. These interventions encompass preoperative hair removal, skin disinfection employing antiseptics, and prophylactic antibiotic administration. Rigorous sterilization of the operating environment and surgical instruments is equally paramount48.
Encouraging preoperative showers with soap among impending surgery recipients can significantly curtail the likelihood of S. aureus contamination. The hand hygiene of the surgical team, using alcoholic hand rubs or antibiotics, is of paramount importance prior to commencing any procedure. Optimal skin preparation before surgery might necessitate dual sets of sterile gloves and gowns2.
For minor surgeries, the adoption of 0.5% chlorhexidine in 70% alcohol is endorsed, while invasive procedures should employ 2.0% chlorhexidine in 70% alcohol. Recognizing the skin as a primary source of S. aureus, patients are advised to prioritize preoperative soap showers to attenuate bacterial loads. Cephalosporins are recommended for surgical patients as a prophylaxis against prevalent skin pathogens such as S. aureus and Streptococcal species. In wound infection cases, flucloxacillin is an effective treatment option against gram-positive cocci that produce β-lactamases, encompassing streptococci and staphylococci49. Maintaining euglycemia (blood glucose levels between 70 and 150 mg/dl) after the surgery is crucial, and wound dressings should be changed and disposed of after 48 h. Daily wound cleaning and chlorhexidine baths should be administered before discharge to ensure proper healing and reduce the risk of infection. By implementing these preventive measures and adopting strict infection control protocols, healthcare providers can make significant strides in reducing the incidence of SSIs and the associated complications and costs.
Limitations
Several limitations inherent to our analysis warrant acknowledgement. As a meta-analysis, our conclusions derive from previously published articles, which in turn relied on observational data rather than originating from original research. This reliance on existing literature restricts our capacity for individual patient data analysis and exposes us to potential heterogeneity across studies, as well as the inherent risk of incorporating errors or biases inherent in the original studies50.
While the fundamental concept of SSI was generally well-defined and established across most included trials, the classification of SSIs remained subject to subjective determination. Several studies employed outcome classifications that diverged from or only partially adhered to the globally acknowledged standards stipulated by the CDC. This divergence in outcome assessment introduces the potential for misclassification and inconsistency in delineating and identifying SSIs. Additionally, considerable disparities existed among the included studies in terms of trial design, reliability, suture materials implemented, wound closure methodologies applied, and the specific anatomical sites where triclosan-coated sutures were administered. These methodological variations and intervention disparities introduce clinical heterogeneity, rendering the comprehensive accounting for all conceivable sources of bias in our analysis challenging.
The selected studies spanned diverse healthcare settings, consequently exposing patients to heterogeneous care practices. This clinical practice heterogeneity, exemplified by the variability in routine antibiotic prophylaxis encompassing the choice of agents and timing, could potentially obscure certain effects and introduce supplementary sources of variability into our findings. Lastly, the utilization of triclosan-coated sutures exhibited variability across studies, ranging from their implementation in full-thickness abdominal wall closure to their exclusive use in superficial layers. This variability in applying triclosan-coated sutures introduces an additional layer of complexity and potential heterogeneity into our analysis. In light of these limitations, a cautious approach is advised when interpreting our outcomes.
To surmount these limitations, heighten the reliability of outcomes, and reinforce the overall evidence corpusconducting further research through meticulously planned randomized controlled trials is imperative. Such research should encompass studies included in the previous meta-analysis as well as other previously published works that were not taken into account previously.
Conclusion
In culmination, this exhaustive study represents a secondary meta-analysis, standing as the most comprehensive endeavor to date in scrutinizing the preventive efficacy of antimicrobial-coated sutures. While the findings underscore a significant reduction in the risk of postoperative SSIs, it is imperative to approach these results judiciously and contextualize them within the broader landscape of infection prevention strategies. The amalgamated RR delineating the decrease in SSI incidence is substantively noteworthy, albeit moderately impactful. This collective RR underscores compelling evidence supporting the potential benefits of antimicrobial-coated sutures, albeit within a moderate scope. However, it remains pivotal to recognize that the effectiveness of these sutures is most optimally evaluated in conjunction with established infection mitigation approaches. An efficacious strategy for SSI prevention necessitates a multifaceted approach that encompasses meticulous equipment sterilization, appropriate antibiotic prophylaxis, and stringent adherence to procedural checklists.
Among the eighteen encompassed meta-analyses, a significant reduction in SSIs’ risk through the utilization of antimicrobial-coated sutures was evidenced in twelve (66.7%). Nevertheless, it is noteworthy that six meta-analyses (constituting 33.3% of the total) indicated the necessity for further evidence before definitive conclusions can be drawn. The inclusion of substantial participant numbers in these meta-analyses bolsters results in confidence. The robust participant count enhances statistical power, thus elevating the credibility of the findings.
Integral to comprehensive infection control strategies, antimicrobial-coated sutures deserve a strategic place due to their SSI risk mitigation capacity. Nevertheless, the onus remains on rigorous research to fill existing knowledge gaps and establish more conclusive insights. Healthcare providers must meticulously weigh potential benefits against associated costs and logistical complexities when contemplating the integration of coated sutures into clinical practices. By incorporating coated sutures within a broader infection control framework, clinicians possess the potential to elevate patient outcomes and curtail the prevalence of SSIs, thereby enhancing the quality of care delivered.
Ethical approval
No applicable.
Consent
No applicable.
Sources of funding
This research did not receive specific grants from public, commercial, or not-for-profit funding agencies.
Author contribution
A.S.S.: conceptualization, data curation, investigation, writing – original draft, writing – review and editing; M.A.: validation and reviewing; M.H.: validation and reviewing; P.B.: validation and reviewing; M.A.I.: conceptualization, data curation, investigation, writing – original draft, writing – review and editing, validation, and reviewing.
Conflicts of interest disclosure
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Research registration unique identifying number (UIN)
Name of the registry: not applicable.
Unique identifying number or registration ID: not applicable.
Hyperlink to your specific registration (must be publicly accessible and will be checked): not applicable.
Guarantor
Md. Aminul Islam,
Lecturer, Department of Microbiology.
President Abdul Hamid Medical College Hospital, Kishoreganj (PAHMCH).
Senior Research Assistant, NSTU COVID-19 Lab, Department of Microbiology, NSTU.
Lab Incharge, Advance Molecular Lab, PAHMCH.
Data Assistant, MIS, DGSH, ERP Project, Bangladesh.
COVID-19 Lab Consultant, DGSH (ERP Project).
Phone/Whatsapp: +880 1734452665.
E-mail: aminulmbg@gmail.com, aminul@pahmc.edu.bd.
Google Scholar: https://scholar.google.com/citations?user=GFDJ70oAAAAJ&hl=en.
Research Gate: https://www.researchgate.net/profile/Md-Islam-1376.
Web address: https://nstu.academia.edu/AMINULISLAM.
ORCID ID: 0000-0003-1091-9726 (https://orcid.org/my-orcid?orcid=0000-0003-1091-9726).
Data availability statement
The data in this meta-meta-analysis is not sensitive in nature and is accessible in the public domain. The data is therefore available and not of a confidential nature.
Supplementary Material
Footnotes
Adeiza Shuaibu Suleiman and Md. Aminul Islam contributed equally as the first authors.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website, www.lww.com/international-journal-of-surgery.
Published online 12 October 2023
Contributor Information
Adeiza S. Suleiman, Email: shuaibusuleiman60@yahoo.com.
Mortada Abbass, Email: abbassmortada7@gmail.com.
Maqsud Hossain, Email: Muhammad.Hossain1@nottingham.ac.uk.
Priyanka Choudhary, Email: priyankachoudhary@gadvasu.in.
Prosun Bhattacharya, Email: prosun@kth.se.
Md. Aminul Islam, Email: aminulmbg@gmail.com.
References
- 1. Owens CD, Stoessel K. Surgical site infections: epidemiology, microbiology and prevention. J Hosp Infect 2008;70:3–10. [DOI] [PubMed] [Google Scholar]
- 2. Seidelman JL, Mantyh CR, Anderson DJ. Surgical site infection prevention: a review. JAMA 2023;329:244–252. [DOI] [PubMed] [Google Scholar]
- 3. Chua R, Lim SK, Chee CF, et al. Surgical site infection and development of antimicrobial sutures: a review. Eur Rev Med Pharmacol Sci 2022;26:828–845. [DOI] [PubMed] [Google Scholar]
- 4. Malone DL, Genuit T, Tracy JK, et al. Surgical site infections: reanalysis of risk factors. J Surg Res 2002;103:89–95. [DOI] [PubMed] [Google Scholar]
- 5. Ahmed I, Boulton AJ, Rizvi S, et al. The use of triclosan-coated sutures to prevent surgical site infections: a systematic review and meta-analysis of the literature. BMJ Open 2019;9:e029727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alexiou K, Drikos I, Terzopoulou M, et al. A prospective randomized trial of isolated pathogens of surgical site infections (SSI). Ann Med Surg 2017;21:25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Birhanu Y, Endalamaw A. Surgical site infection and pathogens in Ethiopia: a systematic review and meta-analysis. Patient Saf Surg 2020;14:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. David MZ, Daum RS. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev 2010;23:616–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Henriksen NA, Deerenberg EB, Venclauskas L, et al. Triclosan-coated sutures and surgical site infection in abdominal surgery: the TRISTAN review, meta-analysis and trial sequential analysis. Hernia 2017;21:833–841. [DOI] [PubMed] [Google Scholar]
- 10. Elsolh B, Zhang L, Patel SV. The effect of antibiotic-coated sutures on the incidence of surgical site infections in abdominal closures: a meta-analysis. J Gastrointest Surg 2017;21:896–903. [DOI] [PubMed] [Google Scholar]
- 11. Renko M, Paalanne N, Tapiainen T, et al. Triclosan-containing sutures versus ordinary sutures for reducing surgical site infections in children: a double-blind, randomized controlled trial. Lancet Infect Dis 2017;17:50–57. [DOI] [PubMed] [Google Scholar]
- 12. Tamim RM, Bernard RM, Borokhovski E, et al. What forty years of research says about the impact of technology on learning: a second-order meta-analysis and validation study. Rev Educ Res 2011;81:4–28. [Google Scholar]
- 13. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev 2021;10:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg 2021;88:105906. [DOI] [PubMed] [Google Scholar]
- 15. Islam Md A, Ahammed T, Ahmed Noor ST, et al. An estimation of five-decade long monkeypox case fatality rate: systematic review and meta-analysis. J Pure Appl Microbiol 2022;16:3036–3047; Epub ahead of print. doi: 10.22207/JPAM.16.SPL1.16 [DOI] [Google Scholar]
- 16. Islam Md A, Hasan MN, Ahammed T, et al. Association of household fuel with acute respiratory infection (ARI) under-five years children in Bangladesh. Front Public Health 2022;10:985445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moher D, Cook DJ, Eastwood S, et al. Improving the quality of reports of meta-analyses of randomized controlled trials: the QUOROM statement. Lancet 1999;354:1896–1900. [DOI] [PubMed] [Google Scholar]
- 18. Shea BJ, Reeves BC, Wells G, et al. AMSTAR: a critical appraisal tool for systematic reviews that include randomized or non-randomized studies of healthcare interventions, or both. bmj 2017;21:358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bougioukas KI, Vounzoulaki E, Mantsiou CD, et al. Methods for depicting overlap in overviews of systematic reviews: an introduction to static tabular and graphical displays. J Clin Epidemiol 2021;132:34–45. [DOI] [PubMed] [Google Scholar]
- 20. Pérez‐Bracchiglione J, Meza N, Bangdiwala SI, et al. Graphical representation of overlap for overviews: groove tool. Res Synth Methods 2022;13:381–388. [DOI] [PubMed] [Google Scholar]
- 21. Pieper D, Antoine S-L, Mathes T, et al. Systematic review finds overlapping reviews were not mentioned in every other overview. J Clin Epidemiol 2014;67:368–375. [DOI] [PubMed] [Google Scholar]
- 22. Quintana DS. A guide for calculating study-level statistical power for meta-analyses. Adv Methods Pract Psychol Sci 2023;6:25152459221147260. [Google Scholar]
- 23. Borenstein M. Comprehensive meta-analysis software. Syst Rev Health Res: Meta-Analysis in Context 2022:535–548. [Google Scholar]
- 24. Adeiza SS, Ademola Onaolapo J, Olalekan, et al. Prevalence and Antimicrobial Susceptibility Profile of Methicillin-Resistant Staphylococcus aureus (MRSA) obtained from nares of patients and staff of sokoto state-owned hospitals in Nigeria. SSRN Elect J 2020;15:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Suleiman S, Onaolapo JA, Olayinka BO. Nasal colonization as a risk factor for staphylococcal infection: A systematic review and meta-analysis. J Microbiol 2018;32:4220–4235. [Google Scholar]
- 26. Chang WK, Srinivasa S, Morton R, et al. Triclosan-impregnated sutures to decrease surgical site infections: systematic review and meta-analysis of randomized trials. Ann Surg 2012;255:854–859. [DOI] [PubMed] [Google Scholar]
- 27. Ademuyiwa AO, Adisa AO, Bach S, et al. Alcoholic chlorhexidine skin preparation or triclosan-coated sutures to reduce surgical site infection: a systematic review and meta-analysis of high-quality randomized controlled trials. Lancet Infect Dis 2022;22:1242–1251. [DOI] [PubMed] [Google Scholar]
- 28. Apisarnthanarak A, Singh N, Bandong AN, et al. Triclosan-coated sutures reduce the risk of surgical site infections: a systematic review and meta-analysis. Infect Control Hosp Epidemiol 2015;36:169–179. [DOI] [PubMed] [Google Scholar]
- 29. Wang ZX, Jiang CP, Cao Y, et al. Systematic review and meta-analysis of triclosan-coated sutures for the prevention of surgical site infection. Br J Surg 2013;100:465–473. [DOI] [PubMed] [Google Scholar]
- 30. Sandini M, Mattavelli I, Nespoli L, et al. Systematic review and meta-analysis of sutures coated with triclosan for the prevention of surgical site infection after elective colorectal surgery according to the PRISMA statement. Medicine 2016;95:e4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Daoud FC, Coppry M, Moore N, et al. Do triclosan sutures modify the microbial diversity of surgical site infections? A systematic review and meta-analysis. Microorganisms 2022;10:927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. On behalf of the SSI Prevention Guideline Committee of the Japan Society for Surgical Infection. Uchino M, Mizuguchi T, Ohge H, et al. The efficacy of antimicrobial-coated sutures for preventing incisional surgical site infections in digestive surgery: a systematic review and meta-analysis. J Gastrointest Surg 2018;22:1832–1841. [DOI] [PubMed] [Google Scholar]
- 33. Sajid MS, Craciunas L, Sains P, et al. Use of antibacterial sutures for skin closure in controlling surgical site infections: a systematic review of published randomized, controlled trials. Gastroenterol Rep 2013;1:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Leaper DJ, Edmiston CE, Holy CE. Meta-analysis of the potential economic impact following introduction of absorbable antimicrobial sutures. Br J Surg 2017;104:e134–e144. [DOI] [PubMed] [Google Scholar]
- 35. Hunger R, Mantke A, Herrmann C, et al. Triclosan-beschichtete Nahtmaterialien in der kolorektalen Chirurgie. Chirurg 2019;90:37–46. [DOI] [PubMed] [Google Scholar]
- 36. Guo J, Pan L-H, Li Y-X, et al. Efficacy of triclosan-coated sutures for reducing risk of surgical site infection in adults: a meta-analysis of randomized clinical trials. J Surg Res 2016;201:105–117. [DOI] [PubMed] [Google Scholar]
- 37. Wu X, Kubilay NZ, Ren J, et al. Antimicrobial-coated sutures to decrease surgical site infections: a systematic review and meta-analysis. Eur J Clin Microbiol Infect Dis 2017;36:19–32. [DOI] [PubMed] [Google Scholar]
- 38. de Jonge SW, Atema JJ, Solomkin JS, et al. Meta-analysis and trial sequential analysis of triclosan-coated sutures for the prevention of surgical-site infection. Br J Surg 2017;104:e118–e133. [DOI] [PubMed] [Google Scholar]
- 39. Uchino M, Mizuguchi T, Ohge H, et al. The efficacy of antimicrobial-coated sutures for preventing incisional surgical site infections in digestive surgery: a systematic review and meta-analysis. J Gastrointest Surg 2018;22:1832–1841. [DOI] [PubMed] [Google Scholar]
- 40. Ceresoli M, Carissimi F, Piemontese A, et al. The clinical and economic value of triclosan-coated surgical sutures in abdominal surgery. Appl Sci 2020;10:1090. [Google Scholar]
- 41. Liu Y-F, Ni P-W, Huang Y, et al. Therapeutic strategies for chronic wound infection. Chin J Traumatol 2022;25:11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Allizond V, Comini S, Cuffini AM, et al. Current knowledge on biomaterials for orthopedic applications modified to reduce bacterial adhesive ability. Antibiotics 2022;11:529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Narasimhan AK, Rahul TS, Krishnan S. Thomas S, Coates P, Whiteside B, et al. Chapter 9 - Revisiting the properties of suture materials: an overview. Advanced Technologies and Polymer Materials for Surgical Sutures. Woodhead Publishing. 199–235. [Google Scholar]
- 44. Head SJ, Milojevic M, Daemen J, et al. Mortality after coronary artery bypass grafting versus percutaneous coronary intervention with stenting for coronary artery disease: a pooled analysis of individual patient data. Lancet. 2018;391:939–948. [DOI] [PubMed] [Google Scholar]
- 45. Krezalek MA, Alverdy JC. The role of the microbiota in surgical recovery. Curr Opin Clin Nutr Metab Care 2016;19:347–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. He P, Liu Z, Chen H, et al. The role of triclosan-coated suture in preventing surgical infection: A meta-analysis. Jt Dis Relat Surg 2023;34:42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fleck T, Moidl R, Blacky A, et al. Triclosan-coated sutures for the reduction of sternal wound infections: economic considerations. Ann Thorac Surg 2007;84:232–236. [DOI] [PubMed] [Google Scholar]
- 48. Ling ML, Apisarnthanarak A, Abbas A, et al. APSIC guidelines for the prevention of surgical site infections. Antimicrob Resist Infect Control 2019;8:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Purba AKR, Setiawan D, Bathoorn E, et al. Prevention of surgical site infections: a systematic review of cost analyses in the use of prophylactic antibiotics. Front Pharmacol 2018;9:776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Suleiman AS, Islam MA, Akter MS, et al. A comprehensive meta-meta-analysis of co-infection, secondary infections, and antimicrobial resistance among COVID-19 patients. J Infect Public Health 2023;16:1562–1590. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data in this meta-meta-analysis is not sensitive in nature and is accessible in the public domain. The data is therefore available and not of a confidential nature.


