Abstract
Recent epidemiological studies indicate that Escherichia coli strains which exhibit the diffuse-adherence phenotype (DAEC strains) represent a potential cause of diarrhea in infants. We investigated the interaction of DAEC strains isolated from diarrhea patients in Brazil and in Germany with epithelial cells in tissue culture. The investigated strains were identified as DAEC strains by (i) their attachment pattern, (ii) presence of genes associated with the Dr family of adhesins, and (iii) lack of genetic markers for other diarrhea-associated E. coli categories. Several clinical DAEC isolates were shown to secrete similar patterns of proteins into tissue culture medium. Protein secretion was found to be regulated by environmental parameters, namely, medium, temperature, pH, and iron concentration. DAEC strains secreting these proteins induced accumulation of actin and tyrosine-phosphorylated proteins at sites of bacterial attachment, leading to the formation of pedestals and/or extended surface structures. These changes were phenotypically similar to the attaching and effacing (A/E) lesions observed with enteropathogenic and some enterohemorrhagic E. coli strains carrying the locus of enterocyte effacement (LEE) pathogenicity island. Proteins homologous to the EspA, EspB, and EspD proteins, necessary for signal transduction events inducing A/E lesions, were identified by sequence analysis and cross-reaction of specific antibodies. However, initially nonadhering strains secreting these proteins induced signal transduction events only after prolonged infection. These results indicate that secretion of the Esp proteins alone is not sufficient for efficient signal transduction. This study further shows that some DAEC strains are likely to contain a homolog(s) of the LEE locus which may contribute to the pathogenic potential of DAEC.
Escherichia coli strains remain a major cause of acute and persistent diarrhea contributing to the high mortality rate among infants in developing countries. Diarrhea-associated E. coli strains are currently classified in the six categories of enteropathogenic (EPEC), enterohemorrhagic (EHEC), enteroaggregative (EAggEC), enterotoxigenic (ETEC), enteroinvasive (EIEC), and diffusely adhering (DAEC) E. coli strains. Classification is based on known or putative virulence factors, clinical syndromes, or other characteristic markers, such as the adherence phenotype.
Three typical adherence phenotypes in E. coli strains have been distinguished. They are characteristic for EPEC (localized), EAggEC (aggregative), and DAEC (diffuse) strains (38, 39, 47, 56). Whereas for the first two E. coli categories epidemiological studies established a clear association with diarrhea, in the past diarrheal outbreaks and DAEC strains seemed to be difficult to correlate by epidemiological means (10). Thus, DAEC strains represent the E. coli category which has been least characterized. Until now they have been solely defined by their diffuse adherence pattern exhibited upon incubation with HeLa or HEp-2 cells and by the absence of markers typical for other categories. Consequently, DAEC strains are regarded as a heterogeneous group, most likely comprising strains with and without pathogenic potential (23). However, recent studies in several countries clearly established a link between the presence of DAEC strains and diarrheal diseases (19, 22, 23). DAEC strains were most frequently associated with persistent diarrhea in infants older than 24 months of age (2).
To date, four adhesins capable of mediating the diffuse-adherence phenotype have been identified in E. coli strains isolated from patients with diarrhea. AIDA-I, isolated from DAEC strain 2787 of the classical EPEC serotype O126:H27, is a plasmid-encoded ∼100-kDa protein located on the surface of the outer membrane (3–5). Recent studies showed that the adhesin belongs to the protein family of outer membrane autotransporters (26) where the transporter function is located in the cleaved C-terminal portion of the precursor molecule (51). A fimbrial adhesin isolated from DAEC strain F1845 (7) belongs to the Dr family of adhesins (40) and uses the decay-accelerating factor as receptor (6). Both aida and F1845 accessory gene daaC DNA probes have been used for the detection of DAEC strains. Recently, in a DAEC strain negative for the F1845 DNA probe, a 57-kDa protein which mediates the diffuse-adherence and hemagglutination phenotype has been identified (57). A recent report points towards the existence of a fourth adhesin of 16 kDa, CF16K (22).
Only a few reports describe the effects of DAEC strains on host cells which might contribute to their pathogenic potential. Yamamoto et al. found at the ultrastructural level that several DAEC strains could induce elongation of microvilli, apparently sometimes embedding the whole bacterium by forming “dimples” (55, 56). This observation was recently confirmed by two studies with the DAEC strain F1845. This strain adheres to intestinal epithelial cell lines and HEp-2 cells, which results in actin disassembly and the formation of cellular projections and of elongated microvilli partly surrounding the bacteria (6, 9).
In contrast, the effects of EPEC strains on host cells have been intensively studied and are well characterized. EPEC strains produce a characteristic histopathology in vitro and in vivo, termed the attaching and effacing (A/E) effect (11). This effect is accompanied by cytoskeletal rearrangements leading to microvillus effacement and often pedestal formation. All of the genes necessary for the development of the A/E effect, including the sep genes for the type III secretion apparatus, the esp genes necessary for signal transduction, and eaeA, the gene for intimin, are located on a 35.5-kb region on the bacterial chromosome, termed the locus of enterocyte effacement (LEE) (35, 36). Several other enteric pathogens like Citrobacter rodentium (48), Hafnia alvei (41), and EHEC serotype O157:H7 (13) are able to produce A/E phenotypes. In all of these bacteria, homologs of the genes necessary for A/E in EPEC have been found (35).
In this study, we investigated the interaction of clinical isolates of DAEC strains with epithelial cells. Several DAEC strains were identified to induce signal transduction leading to tyrosine-specific protein phosphorylation and actin rearrangements. Interestingly, among other proteins, these strains secreted homologs of the EspA, EspB, and EspD proteins.
(This study is part of the Ph.D. thesis work of C. Beinke and S. Laarmann.)
MATERIALS AND METHODS
Bacterial strains, tissue culture cell lines, and culture conditions.
Strains were isolated from patients with diarrhea in Germany (B6, B7, and 2129 [G. Peters, Institut für Medizinische Mikrobiologie, Münster]; 17-8 and 1469 [H. Karch, Institute für Hygiene und Medizinische Mikrobiologie, Würzburg]) or Brazil (3431, 0181, and 0391 [L. R. Trabulsi, Escola Paulista de Medicina, Saõ Paulo]). Reference strains DAEC strain 2787 (4) and EPEC strain 2348/69 (34) were taken from our strain collection. DAEC strain 2787 and E. coli K-12 strain C600 served as control strains. All strains were routinely stored at −70°C in Standard I medium (Merck, Darmstadt, Germany) with 15% glycerol.
HeLa cells (ATCC CCL 2; human cervical epitheloid carcinoma) were routinely grown at 37°C in 10% CO2 atmosphere in Dulbecco’s minimal essential medium (DMEM) supplemented with 10% (vol/vol) fetal calf serum (FCS), containing 1 mM glutamine, penicillin (100 U/ml), and streptomycin (100 μg/ml). Caco-2 cells (ATCC HTB 37; human colon adenocarcinoma) were grown at 37°C in 5% CO2 atmosphere in Eagle’s minimal essential medium supplemented with 10% (vol/vol) FCS, containing 2 mM glutamine, 25 mM glucose, 1% nonessential amino acids, penicillin (100 U/ml), and streptomycin (100 μg/ml).
Adherence assay.
Adherence of E. coli strains to HeLa cells was assessed as described by Vial et al. (53), with minor modifications. Briefly, HeLa cells were seeded at a density of 0.7 × 105 cells per well in 24-well tissue culture plates on round glass coverslips and grown in DMEM without antibiotics for 24 h to 50 to 75% confluence. Bacteria from −70°C stock cultures were grown statically in Standard I medium (Merck) for 15 h at 37°C. HeLa cells were washed once with Dulbecco’s phosphate-buffered saline (D-PBS) containing 1 mM MgCl2 and 0.5 mM CaCl2 before being infected with 5 × 106 bacteria per well (∼50 bacteria per HeLa cell) in 1 ml of low-serum DMEM (2% [vol/vol] FCS, 2 mM glutamine) containing 1% (wt/vol) methyl-α-d-mannoside (to block adhesion mediated by type I fimbriae) for 1.5, 3, or 6 h at 37°C in a 10% CO2 atmosphere as indicated for the relevant experiment. Nonadherent bacteria were removed by washing four times with D-PBS, and cells were fixed for 20 min at 37°C with 4% (wt/vol) paraformaldehyde in D-PBS. HeLa cells were washed three times before they were used for the fluorescence actin staining (FAS) assay and for the detection of tyrosine-phosphorylated proteins. Subconfluent or 11-day-postconfluent Caco-2 cells were treated the same way except that infection was carried out in Eagle’s minimal essential medium supplemented with 2% (vol/vol) FCS, containing 2 mM glutamine, 25 mM glucose, and 1% nonessential amino acids.
Isolation of secreted proteins.
Starting with an optical density at 600 nm of 0.02, bacteria were grown without shaking for 12 h in DMEM at 37°C in a 10% CO2 atmosphere. Bacteria were removed by two centrifugation steps (3,000 × g, 4°C, 15 min; 16,000 × g, 4°C, 15 min). To precipitate proteins released into the supernatant, 10% (wt/vol) trichloroacetic acid (TCA) was added, and the mixture was left on ice for at least 1 h. Precipitated proteins were collected by centrifugation (16,000 × g, 4°C, 15 min), washed once with ice-cold 90% acetone, resuspended in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer (10% glycerol, 1.5% SDS, 4% 2-mercaptoethanol, 30 mM Tris-HCl [pH 6.8]), and boiled for 15 min. After separation by SDS-PAGE, proteins were stained with Coomassie brilliant blue.
Influence of growth conditions (medium, pH, iron concentration, temperature, and pCO2) on protein secretion.
To test for the effect of culture medium or temperature on secretion of proteins, bacteria were grown without shaking either in Luria broth (LB) at 37°C or in DMEM at 30 or 37°C. To investigate the effect of CO2, bacteria were grown without shaking in an atmosphere of either 10 or 0.03% CO2 in DMEM at 37°C. To investigate the influence of pH on the secretion of proteins, DMEM was either supplemented with 100 mM MES (2-[N-morpholino]ethanesulfonic acid) and the pH was adjusted to pH 5.8 or supplemented with 100 mM HEPES and the pH was adjusted to pH 7.2 or 8.5. Depletion of iron during growth was achieved by adjusting DMEM to 18 mM nitrilotriacetic acid trisodium salt and to 1 mM MgCl2, CaCl2, MnCl2, and ZnCl2, each as described previously (16). For comparison of the amount of secreted proteins under the different growth conditions, the amounts of proteins separated by SDS-PAGE were based on equal numbers of bacterial cells.
FAS assay.
Actin rearrangements were detected by fluorescence microscopy of fixed cells after staining with fluorescein isothiocyanate-coupled phalloidin (FITC-phalloidin) as described previously (31). Fluorescence and phase-contrast micrographs of the same field were recorded. To evaluate the proportion of bacteria involved in signal transduction that was detectable with the FAS assay, the total number of adherent bacteria on 75 HeLa cells was determined and compared to the number of bacteria detected by the FAS assay.
Detection of tyrosine-phosphorylated proteins.
Proteins specifically phosphorylated at tyrosine residues were detected by immunofluorescence microscopy of fixed cells. The assays were performed as described elsewhere (42). The primary antibody used was antiphosphotyrosine monoclonal antibody PT-66 (Sigma Biosciences) at 10 μg/ml (in D-PBS–0.1% bovine serum albumin), and the secondary antibody was rhodamine-labeled anti-mouse immunoglobulin G (IgG) and IgM (heavy plus light chain) (Dianova Inc.).
Transmission electron microscopy.
HeLa cells were grown in DMEM supplemented with 10% FCS and 1 mM glutamine for 24 h in 35-mm-diameter culture dishes to about 75% confluence. Cells were infected with about 50 bacteria per HeLa cell and incubated for 3 h. HeLa cells were washed, subsequently fixed with 2% (vol/vol) glutaraldehyde in D-PBS (electron microscopy grade, pH 7.4), postfixed for 1 h in osmium tetroxide (0.5% in D-PBS), washed with D-PBS, and dehydrated in a series of increasing ethanol concentrations. After the cells were embedded in Epon, ultrathin sections were cut, stained with uranyl acetate, and analyzed by transmission electron microscopy (Philips EM 410).
Amino-terminal sequence analysis of proteins and database searches.
Secreted proteins were separated by SDS-PAGE and electrotransferred onto polyvinylidene difluoride membranes in 10 mM CAPS (3-[cyclohexylamino]-1-propanesulfonic acid; pH 11) containing 10% methanol. Proteins were visualized with 0.1% Coomassie blue R in 50% methanol–7% acetic acid. Protein bands of interest were cut out and analyzed by amino-terminal Edman degradation using an automatic sequencer model 373A (Applied Biosystems). Database searches with the obtained sequences were performed with the BLASTP and FASTA programs incorporated in the HUSAR program package of the Deutsches Krebsforschungszentrum (Heidelberg, Germany).
Generation of polyclonal antibodies specific for secreted proteins and immunodetection.
Proteins secreted in DMEM by DAEC strain 3431 were separated by SDS-PAGE (10 to 15% polyacrylamide gel), electrotransferred onto nitrocellulose membranes, and stained with Ponceau S in 0.3% TCA. Bands of interest were excised, washed several times to remove the dye, and powdered carefully in a mortar cooled by liquid nitrogen. The material was thoroughly suspended in D-PBS, and 8- to 10-week-old female BALB/c mice were immunized intraperitonally with the antigen suspension in an equal volume of MPL-TDM (monophosphoryl lipid A-trehalose dicorynomycolate) adjuvant (Sigma Biosciences). After four booster injections in 3-week periods, the mice were bled by retro-orbital puncture and the sera were collected. Secreted proteins were separated and electroblotted as described above. After electrotransfer, the membranes were blocked with 5% nonfat powdered milk in PBS for 1 h and then incubated for 1 h with the primary antibodies at a dilution of 1:7,500 in Tris-buffered saline with 3% nonfat powdered milk. Membranes were washed three times for 10 min, incubated for 1 h with alkaline phosphatase-conjugated goat anti-mouse IgG and IgM antibodies (Dianova) diluted 1:10,000 in Tris-buffered saline with 3% nonfat powdered milk, washed again three times for 10 min, and developed with p-nitrophenyl phosphate (Sigma) as the substrate.
DNA manipulation and DNA probes.
Routine DNA manipulation and cloning techniques were performed as described by Sambrook et al. (46). Preparation and Southern hybridization with the daaC gene probe (7) and the aida gene probe (4) were done as described elsewhere. Hybridization experiments were carried out with a digoxigenin labeling and detection kit (Boehringer Mannheim). Hybridization and PCR experiments (analyzed genetic markers: EIEC, EAggEC, EHEC [-hemolysin], EAF, heat-labile enterotoxin, Shiga-like toxins I, II, and IIe, the plasmid-encoded catalase of EHEC, and eaeA) and serotyping of the strains were performed as described previously (1, 27, 45, 49).
Data imaging.
Immunofluorescence and phase-contrast images were recorded with a 3CCD video camera (XC-003P; Sony). Electronic images were mounted in Adobe Photoshop and printed with a thermosublimation printer (UP-D8800; Sony). Coomassie blue-stained gels and immunoblots were scanned (Microtek Scanmaker III) and processed as described above.
RESULTS
Secretion of proteins by DAEC strains into tissue culture medium.
We investigated the interaction of clinical DAEC isolates from Brazil and Germany with epithelial cells. The diffuse-adherence phenotype of clinical E. coli isolates was first determined with HeLa cells and subsequently confirmed with the intestinal epithelial like cell line Caco-2. Strains exhibiting the diffuse-adherence phenotype after a 3-h infection with HeLa cells were analyzed further. Additionally, two E. coli strains (B7 and 2129) which adhered to HeLa cells only after 6 h of infection were included in this study. We were interested to see whether the expression of potential virulence genes would be influenced by different environmental conditions. Thus, the DAEC strains were incubated under different conditions, achieved by varying the composition of the medium, temperature, pH, pCO2, or Fe concentration. As a number of bacterial pathogens release virulence factors into the environment, we also analyzed these DAEC strains for the secretion of proteins into the supernatant.
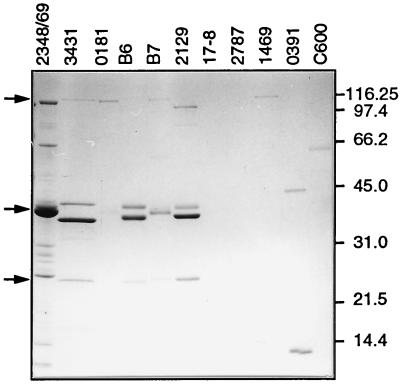
For this purpose, bacteria were grown in tissue culture medium (DMEM) to the late logarithmic phase and subsequently removed by repeated centrifugations. After precipitation with TCA, the proteins in the supernatant were analyzed by SDS-PAGE (Fig. 1). The DAEC strains were divided into three groups according to their secretion patterns. In the first group, no proteins could be detected in the supernatant. Strains 2787 and 17-8 belonged to this group. The heterogeneous second group, exemplified by strains 0181, 1469, and 0391 and also the laboratory K-12 strain C600, secreted low levels of proteins and showed no discernible common pattern among the individual proteins. In contrast, the third group, consisting of strains 3431, B6, B7, and 2129, showed high-level secretion of four proteins exhibiting similar patterns. One of these was a 100- to 110-kDa protein, two ranged in size between 37 and 43 kDa, and one was a protein of 25 kDa. In strain B7, the two proteins secreted in the 37- to 43-kDa range migrated very close to each other in SDS-PAGE. All strains secreted ∼50 μg of total protein per ml, with the protein of about 37 kDa being the most abundant, followed by the protein of about 42 kDa.
FIG. 1.
Secretion of characteristic proteins of DAEC strains into tissue culture medium. Bacteria were grown as static cultures in DMEM for 12 h at 37°C. Bacteria were removed from the supernatants by centrifugation; proteins were precipitated, separated by gradient SDS-PAGE (9 to 16% gel), and visualized by Coomassie blue staining. Protein samples loaded had been normalized based on the optical density at 600 nm of the corresponding bacterial cultures. The bacterial strains used are indicated at the top. EPEC strain 2348/69 and the laboratory K-12 strain C600 are shown for comparison. Bands of similar-size secreted proteins of several strains are marked by arrows on the left. Molecular mass markers in kilodaltons are indicated on the right.
This pattern of secreted proteins was reminiscent of the secretion pattern described for the model EPEC strain 2348/69 (Fig. 1), which we therefore used for comparison throughout this study. During incubation in tissue culture medium, the model EPEC strain 2348/69 secretes at least six proteins into the supernatant (29, 32). However, the individual proteins of the DAEC strains had distinctly different molecular weights. We could detect several other, yet undescribed proteins in the supernatant of EPEC strain 2348/69. One protein of about 62 kDa, two in the 30-kDa size range, and two proteins which were smaller than 14 kDa were identified.
Environmental parameters regulate the secretion of proteins in DAEC strains.
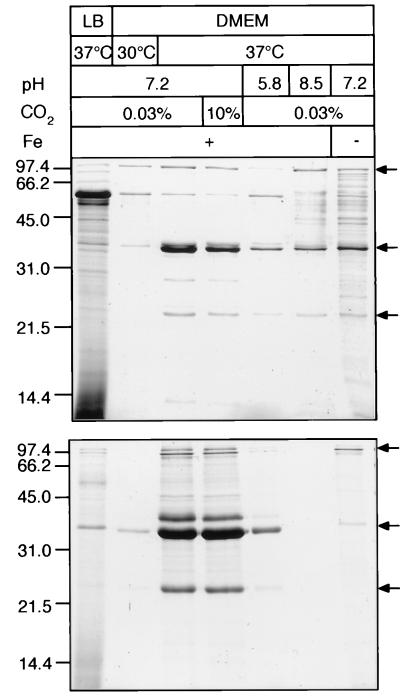
We were interested in determining whether the release of the characteristic protein pattern into the supernatant would be influenced by different environmental conditions which might modulate the interaction of DAEC with intestinal epithelia. Therefore, the effect of growth medium, temperature, pCO2, pH, and low iron concentration on protein secretion was investigated. DAEC strains 3431, B6, B7, 2129, and 2787, and for comparison EPEC strain 2348/69 and the K-12 strain C600, were incubated in different conditions, and the secreted proteins were isolated as described in Materials and Methods. To compare the relative amounts of secreted proteins, equal proportions of the culture supernatants, normalized on the basis of bacterial cell numbers, were separated by SDS-PAGE. Strains 2787 and C600 were apparently not able to secrete proteins under the conditions tested. Results for two exemplary secreting strains, EPEC strain 2348/69 (top panel) and DAEC 3431 (bottom panel), are depicted in Fig. 2.
FIG. 2.
Regulation of protein secretion by different growth conditions. Profiles of proteins secreted into the culture supernatant by EPEC strain 2348/69 (top) and DAEC strain 3431 (bottom) are shown. Bacterial strains were incubated for 12 h in LB at 37°C or in DMEM at 30 or 37°C at pH 7.2, 5.8, or 8.5. Growth was in air containing 0.03 or 10% CO2 and in the presence or absence of iron. Growth conditions with respect to medium, temperature, pH, CO2 concentration, and presence of iron are indicated at the top. Proteins were isolated as described in the legend to Fig. 1. Loaded protein samples were normalized according to the number of bacteria in the corresponding cultures. The characteristic pattern of secreted proteins is marked by arrows on the right. Optimal conditions for protein secretion were observed in DMEM in the presence of iron at 37°C and pH 7.2. Molecular mass markers are indicated in kilodaltons on the left.
Growth in LB medium or at a temperature of 30°C strongly downregulated the secretion of the major proteins of DAEC strain 3431 as has also been found with EPEC strain 2348/69 with the exception of a prominent protein of about 62 kDa which was upregulated in EPEC strain 2348/69.
Adjusting the partial CO2 content in the atmosphere to 0.03 or 10% during growth in DMEM had only minor effects on the amount of secreted proteins. However, deviation of the pH in DMEM from physiological values to acidic or basic growth conditions (pH 5.8 or 8.5) resulted in profound changes. The amounts of most proteins detectable in the supernatant for DAEC strain 3431 as well as for EPEC strain 2348/69 were markedly decreased. A comparable effect was observed in DMEM at low iron concentrations. Secretion of the characteristic proteins in the other strains investigated was regulated in a similar manner, with minor variations in the amount of individual secreted proteins (data not shown). In conclusion, maximal protein secretion for all strains was observed at 37°C and pH 7.2 and in the presence of iron in tissue culture medium. Conditions found to be necessary for the secretion of proteins in these DAEC strains are also encountered in the gastrointestinal tract.
DAEC strains accumulate actin at bacterial attachment sites.
Secreted proteins had been shown to be involved in the generation of A/E effects and signal transduction events that lead to actin rearrangements and the expression of characteristic morphological features like pedestal formation. We sought to investigate whether DAEC strains were able to induce similar effects.
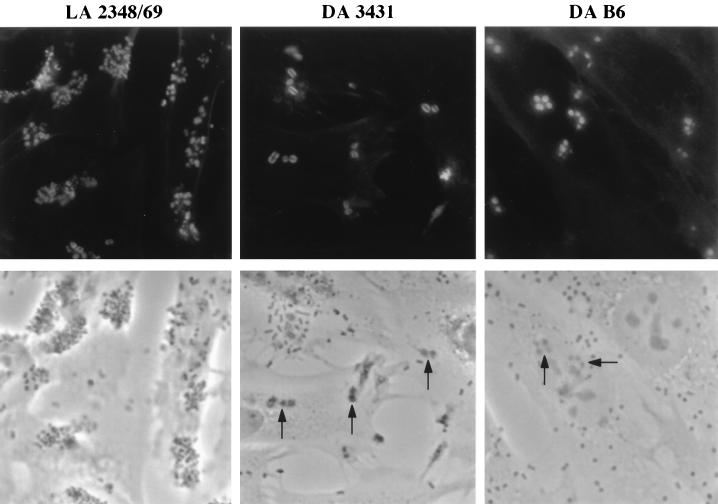
All strains shown in Fig. 1 were analyzed for induction of actin accumulation by using the FAS assay with FITC-phalloidin after a 3-h incubation. DAEC strains 3431, B6, and 0181 were found to induce actin accumulation at sites of bacterial attachment on HeLa and Caco-2 cells. Bright actin staining around adhering bacteria, phenotypically similar to the signals of the EPEC strain 2348/69 exhibiting localized adherence, was observed in the FAS assay (Fig. 3). Actin accumulation induced by these DAEC strains could be detected as well with subconfluent or 11-day-postconfluent Caco-2 cells (data not shown). Interestingly, actin-rich extended structures protruding from the surface (Fig. 4a) or lying on the cell surface of HeLa cells (Fig. 4b to d) associated with bacteria were occasionally observed. DAEC strains included as controls (e.g., 2787, 1469, 0391, and 17-8) and the E. coli K-12 strain C600 did not induce actin rearrangements.
FIG. 3.
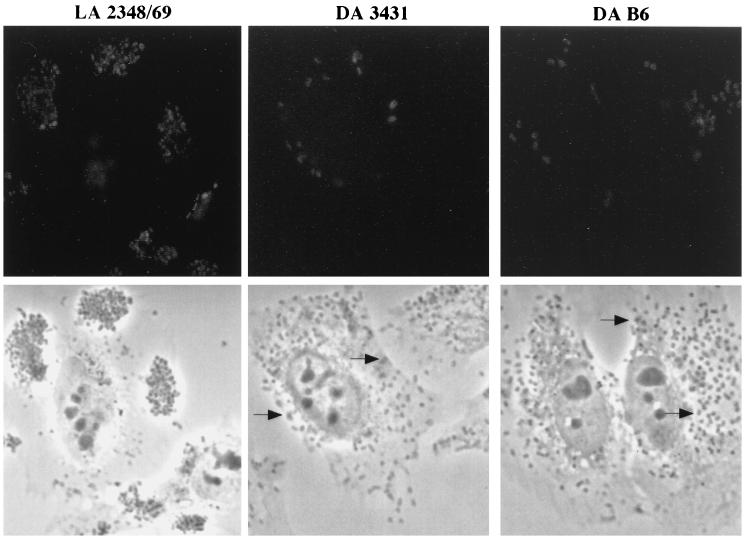
Accumulation of actin at sites of adherent DAEC. HeLa cells were infected for 3 h with DAEC strain 3431 or B6 or the localized adhering (LA) EPEC strain 2348/69 and stained for filamentous actin with FITC-phalloidin. Fluorescence (top) and corresponding phase-contrast (bottom) micrographs are shown. Bacteria are detectable in the phase-contrast micrographs as enlarged, darker, plaque-like structures (indicated by arrows). Bacteria showing actin staining were often seen in small groups. Magnification, ×165.
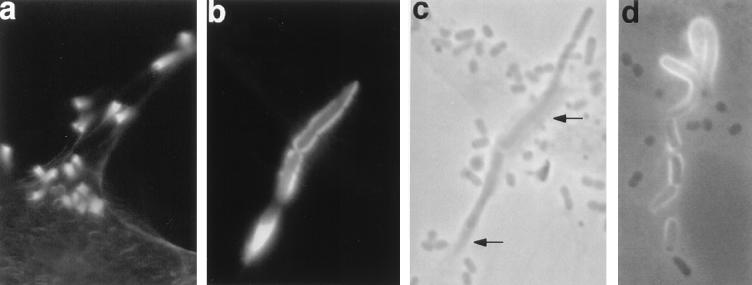
FIG. 4.
Bacteria induce complex extended structures of polymerized actin. HeLa cells were infected with DAEC strain 3431 for 6 h (a) or 3 h (b and c) or with DAEC strain B6 for 3 h (d) and processed as for Fig. 3. Fluorescence (a and b), phase-contrast (c), and simultaneous fluorescence and phase-contrast (d) micrographs are shown. Micrographs b and c are taken from identical areas. Actin accumulates in long horn-like structures protruding from the surfaces of the HeLa cells with a single bacterium on the top (a) or in long tubes associated with bacteria (b to d). Accumulated actin can be detected in phase-contrast micrographs as darker areas (indicated by arrows in panel c). Magnifications: a, ×260; b to d, ×410.
Among the FAS assay-positive DAEC strains, differences in the ratio of the number of adhering bacteria versus the number of bacteria inducing actin polymerization could be detected (Table 1). Upon prolonged incubation of the bacteria with tissue culture cells (1.5, 3, and 6 h), the number of adhering bacteria and the number of bacteria inducing actin polymerization increased, reaching a plateau at about 3 h of incubation. Strains 3431 and B6 adhered equally well. Up to about one-third of the bacteria of strain B6 developed FAS signals after 3 h of incubation, compared to only 10% of the strain 3431 bacteria. Strain 0181 adhered most efficiently, but only ≤1% of the bacteria were able to develop FAS signals. After 3 h of incubation, the majority of the FAS assay-positive bacteria of all strains were found in clustered groups of three or more bacteria (Table 1).
TABLE 1.
Quantification of adherent and FAS-positive bacteria of DAEC strainsa
| Strain | No. of adherent bacteriab
|
% FAS-positive bacteriac
|
% FAS-positive bacteria in groupsd
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| 1.5 h | 3 h | 6 h | 1.5 h | 3 h | 6 h | 1.5 h | 3 h | 6 h | |
| 3431 | 4.8 ± 4.4 | 37.8 ± 24.6 | 36.4 ± 23.1 | 3 | 10 | 10 | 23 | 63 | 67 |
| B6 | 8.4 ± 6.3 | 43.1 ± 25.4 | 47.5 ± 38.5 | 9 | 29 | 37 | 14 | 62 | 79 |
| 0181 | 26 ± 16.1 | 110 ± 57.7 | NDe | 1 | 0.3 | ND | 52 | 77 | ND |
The adherence assay for the indicated strains and time points and the detection of accumulated actin by the FAS assay were done as described in Materials and Methods. Adherent bacteria on HeLa cells were counted with a phase-contrast light microscope. Identical areas were also viewed under fluorescence, and the number of bright FAS signals associated with bacteria was determined. The proportion of FAS signals in groups of three or more bacteria was also recorded.
Mean ± standard deviation of two independent experiments counting bacteria on 75 HeLa cells for each time point.
Determined by dividing the total number of adherent bacteria by the total number of FAS signals.
Proportion of bacteria in groups of three or more bacteria of the total number of FAS assay-positive bacteria, determined by dividing the total number of FAS assay-positive bacteria in groups with three or more bacteria in close contact by the total number of FAS assay-positive bacteria.
ND, not determined.
Most of the adherent bacteria of EPEC strain 2348/69 seemed to be FAS assay positive after a 3-h incubation (Fig. 3), although microcolonies with few or almost no actin-staining- positive bacteria were observed as well (data not shown). Due to the formation of microcolonies in the localized adhering EPEC strain 2348/69, it was not possible to reliably quantitate the proportion of FAS assay-positive bacteria for this strain.
Strains 2129 and B7 were considered to be nonadhering, as very few adhering bacteria were detected after incubation with HeLa and Caco-2 cells for 3 h. Some of those few adhering bacteria induced actin accumulation and assembled phosphorylated proteins at their attachment sites, phenotypically indistinguishable from DAEC strain B6 or 3431 (data not shown). However, after prolonged infection of HeLa cells for 6 h, the number of bacteria of strains 2129 and B7 attaching in a diffuse pattern increased substantially. The majority of the attaching bacteria showed actin accumulation at the site of attachment. This finding is reminiscent of the results reported for EHEC strains (31, 37), which also had to be incubated at least for 5 h with HEp-2 cells to induce actin rearrangements. However, the EHEC strains showed a localized-adherence pattern.
Accumulation of tyrosine-phosphorylated proteins by FAS assay-positive DAEC strains.
To investigate whether the investigated DAEC strains might be able to induce signal transduction pathways, we further analyzed these strains for the accumulation of phosphorylated proteins at their attachment sites. Using a phosphotyrosine-specific antibody, we detected accumulated tyrosine-phosphorylated proteins underneath adhering bacteria for DAEC strains 3431, B6, and 0181 after 3 h of incubation with HeLa cells (Fig. 5) and Caco-2 cells (data not shown). Comparable signals were observed with EPEC strain 2348/69 (Fig. 5). In contrast to the evaluation of the FAS assay, signals obtained for phosphotyrosine fluorescence with the different strains could not be quantitated because the staining was much weaker. But the fact that only those DAEC strains which were FAS assay positive accumulated tyrosine-phosphorylated proteins at their attachment sites suggests that tyrosine phosphorylation is part of the signal transduction events leading to actin rearrangements.
FIG. 5.
Tyrosine-phosphorylated proteins accumulate underneath adherent bacteria. HeLa cells were infected for 3 h with DAEC strain 3431 or B6 or with EPEC strain 2348/69, washed, fixed, labeled with antiphosphotyrosine monoclonal antibody PT-66, and examined by immunofluorescence microscopy. Fluorescence (top) and corresponding phase-contrast (bottom) micrographs are shown. Concentrations of host tyrosine-phosphorylated proteins can be seen as bright spots or rings at sites of adherent bacteria. Fewer bacteria with fluorescence spots are observed for DAEC strains 3431 and B6 than for strain 2348/69. Magnification, ×165.
DAEC strains induce pedestal formation, microvillus extension, and membranous blisters on HeLa cells.
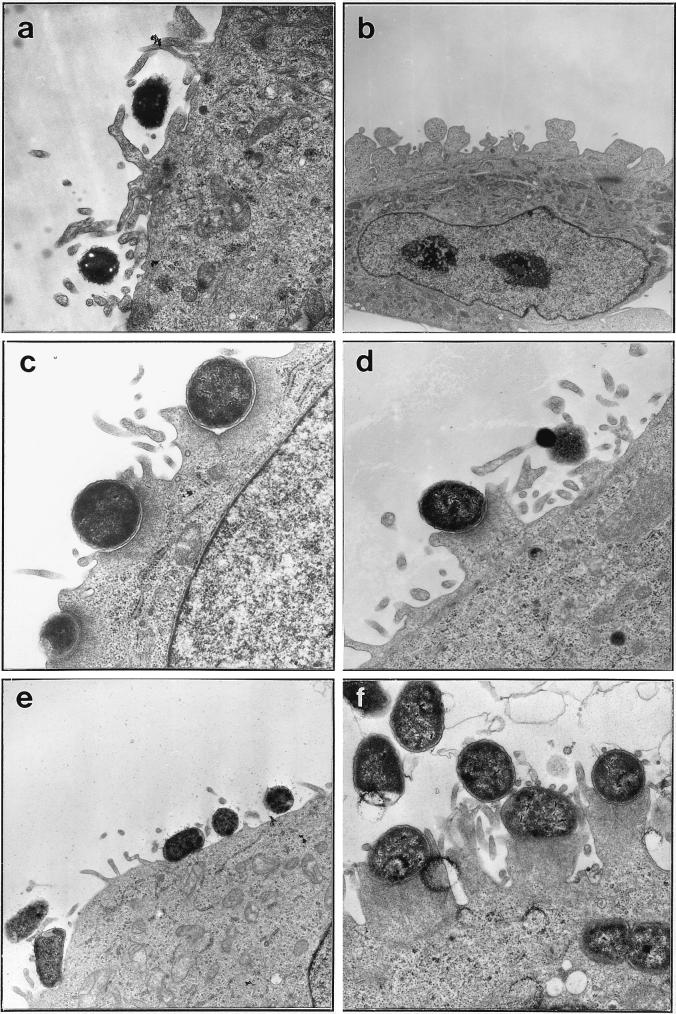
As DAEC strains had been found to affect HeLa cells upon adherence, we investigated this interaction at the ultrastructural level. HeLa cells were infected with DAEC strains 3431, B6, and 0181 and EPEC strain 2348/69 for 3 h and then processed for transmission electron microscopy (Fig. 6). Depending on the DAEC strain investigated, different effects on HeLa cells were observed. Adhering 3431 bacteria were only rarely found on pedestals but were often embedded or surrounded by elongated microvillus structures (Fig. 6a). Incubation with this strain also resulted in intensive blistering of HeLa cell membranes in many infected cells (Fig. 6b). In contrast, adhering bacteria of strain B6 frequently induced close contact zones with HeLa cell membranes either embedded (Fig. 6c) or protruding from the apical membrane as typical pedestals (Fig. 6d). Occasionally they were also found to be surrounded by microvilli as observed for strain 3431. Strain 0181 seemed to attach to HeLa cells without obvious morphological changes on the target cells (Fig. 6e). EPEC strain 2348/69 adhered in microcolonies, inducing the formation of numerous pedestals underneath attached bacteria, and was also found inside the target cells (Fig. 6f). Interestingly, often numerous microvillus structures at or between pedestals could be seen (Fig. 6f). These results show that upon adherence to their target cells, some of the investigated DAEC strains were able to induce morphological changes which are similar but not identical to the typical A/E effects as exemplified by EPEC strain 2348/69.
FIG. 6.
DAEC strains induce morphological changes on HeLa cells. Transmission electron micrographs show adherence phenotypes after a 3-h incubation of DAEC strains secreting characteristic proteins with HeLa cells. DAEC strains 3431 (a and b), B6 (c and d), and 0181 (e), and EPEC strain 2348/69 (f) are shown. Magnifications: a, d, and f, ×14,400; b, ×4,400; c, ×16,900; e, ×7,100.
Secreted homologs of EspD, EspB, and EspA in DAEC strains.
The secretion of similarly sized and regulated proteins in DAEC strains compared to EPEC strain 2348/69 prompted us to further analyze the secreted proteins. Amino-terminal amino acid sequences of several secreted proteins were determined. After separation by SDS-PAGE and electrophoretic transfer to polyvinylidene difluoride membranes, proteins were subjected to amino-terminal sequencing by automated Edman degradation. Sequences of various lengths were obtained for the ∼42-, ∼38-, and 25-kDa proteins of strains 3431, B6, and 2129 and were compared to protein sequences deposited in the databases. Sequence alignments with corresponding secreted proteins of EPEC strain 2348/69, EHEC strain EDL933, and EHEC strain 413.89-1 are depicted in Fig. 7.
FIG. 7.
Amino-terminal sequence homologies of secreted proteins of DAEC strains 3431 and B6 and E. coli 2129 to EspD (top), EspB (middle), and EspA (bottom) proteins. Amino-terminal sequences of various lengths for the proteins were aligned with sequences of EPEC strain 2348/69 (30, 32), EHEC strain EDL933 (15), and EHEC strain 413.89-1 (15). The apparent molecular masses, E. coli strains, and positions of amino acids (aa) are indicated. Amino acids are abbreviated by the single-letter code, and those which could not be determined are indicated by X’s. Gaps were allowed for optimal alignment.
Comparison of the sequences obtained for the 25-kDa proteins revealed that the amino-terminal sequences of these proteins were almost identical to those of several EspA proteins. The sequence of the DAEC strain B6 product was even identical to the amino-terminal EspA protein sequence of EHEC strain 413.89-1. Likewise, high levels of sequence homology with the amino-terminal regions of several EspB proteins were identified for the ∼38-kDa secreted proteins. Again, the sequences of DAEC strain B6 and of EHEC strain 413.89-1 were identical, as were the amino-terminal sequences of strain 2129 and EHEC strain EDL933. The 43-kDa secreted proteins exhibited amino-terminal sequences highly homologous to the amino-terminal sequence of EspD recently characterized in EPEC 2348/69 (32). In addition, amino acid sequences of two internal fragments of the 43-kDa protein from strain 3431 showed 70 to 80% identity to that of EspD, indicating that the observed amino-terminal sequence homology might well continue throughout the protein (data not shown).
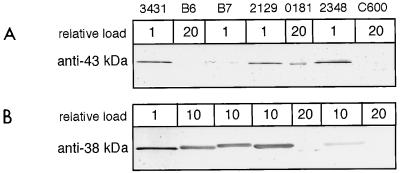
To further characterize the putative Esp protein homologs, we raised polyclonal antisera in mice against the 43- and 38-kDa proteins of DAEC strain 3431. Recognition of proteins derived from different DAEC strains on Western blots was assessed. The antiserum against the 43-kDa protein of DAEC strain 3431 (Fig. 8A) recognized not only the 42-kDa protein of EPEC 2348/69 but also the 43-kDa secreted proteins of the strains B7 and 2129. In strain B6, however, we could detect only a very faint band after loading a much larger amount of protein (data not shown). This finding indicates that the secreted 43-kDa protein of strain B6 is immunologically distinct. Interestingly, in DAEC strain 0181, which also induced actin accumulation at the attachment sites with low frequency, a secreted protein of slightly smaller size cross-reacted with the anti-43-kDa protein antibodies. The content of this protein in the supernatant must have been very low since much more protein has to be loaded to detect the cross-reaction and no prominent protein of that size could be detected after SDS-PAGE and Coomassie blue staining.
FIG. 8.
Recognition of secreted proteins by antisera directed at the secreted 43-kDa (A) and 38-kDa (B) proteins of DAEC strain 3431. Secreted proteins of the indicated strains were prepared as described in the legend to Fig. 1 and separated by SDS-PAGE (13% gel). Samples were analyzed by immunoblotting with the appropriate antisera. No signals besides those shown could be detected. Low cross-reactivity was detected by loading larger amounts of the secreted proteins for the corresponding strain as indicated in relative numbers at the top.
The antiserum against the 38-kDa protein of strain 3431 (Fig. 8B) cross-reacted with the 39-kDa EspB protein of EPEC strain 2348/69 and further showed strong cross-reactivity to similar-size proteins of the other DAEC strains. Again, strain 0181 showed weak cross-reactivity to a secreted protein of smaller size. In E. coli K-12 strain C600 and all other tested strains, no signal could be detected with either antiserum. When probing total bacterial extracts of the DAEC strains with the antisera, we detected only small amounts of the corresponding proteins (data not shown), which indicates that the EspA, EspB, and EspD homologs are apparently efficiently secreted.
Sequence information and the cross-reactivity analysis by Western blotting experiments represent two complementary lines of evidence that DAEC strains 3431, B6, B7, 2129, and presumably also 0181 secrete homologs of EspA, EspB, and EspD proteins.
Relationship of the investigated DAEC strains to other E. coli categories associated with diarrhea.
In EPEC strain 2348/69, the genes encoding the secreted Esp proteins are located on the LEE pathogenicity island. Several other enteric bacterial pathogens, like EHEC serotype O157:H7, produce A/E lesions, and all of them possess homologs of the genes required for the production of the A/E phenotype. As the results obtained in this study indicate the LEE to be present also in DAEC strains, we further characterized these strains by serotyping and by probing them for the presence of genetic markers specific for different E. coli categories. Positive results of hybridization experiments with these probes are listed in Table 2. Flagellar (H) antigens could be determined in only a few cases. Strains B7, 2129, and 0181 showed serogroups appearing in the EPEC category and strain B6 in EHEC. Other serogroups or K antigens could not be correlated to a specific group of pathogenic bacteria or DAEC.
TABLE 2.
Characteristics of the strains investigated in this study
| Strain | Adherence patterna | Serotype | Signalb
|
Secretion of Esp proteins | DNA probe(s)c | |
|---|---|---|---|---|---|---|
| FAS | Phosphotyrosine | |||||
| 2348/69 | LA | O127:H6 | + | + | + | eaeA, EAF |
| 3431 | DA | O8:H− | + | + | + | eaeA, daaC |
| B6 | DA | O26:K60 | + | + | + | eaeA, daaC |
| 0181 | DA | O119:H9:K61 | + | + | + | eaeA, daaC |
| B7d | DA | O86:K61 | + | + | + | eaeA |
| 2129d | DA | O55:K59 | + | NDe | + | eaeA |
| 2787 | DA | O126:H27 | − | − | − | aida |
| 0391 | DA | O17:H− | − | − | − | daaC |
| 17-8 | DA | O95:H− | − | − | − | daaC |
| 1469 | DA | O128:K67 | − | − | − | ND |
LA, localized adherence; DA, diffuse adherence.
Detected by immunofluorescence. +, bacteria showed positive signals; −, no positive signals were detected.
Listed are only relevant genetic markers with positive results. All other genetic markers tested were negative for the investigated strains (see Results). eaeA, probe of the gene for intimin, located on the LEE locus; EAF, probe of the 60-MDa EAF plasmid of strain 2348/69 carrying the genes for bundle-forming pili which mediate localized adherence (18); daaC, probe of accessory gene daaC of the Dr family of adhesins (7); aida, SphI-ClaI fragment of pIB264 which encodes the AIDA-I adhesin mediating diffuse adherence (3).
After 6 h of infection (see Results).
ND, not determined.
All strains were negative for the EIEC, EAggEC, and EHEC (-hemolysin) genetic markers tested (data not included in Table 2). Interestingly, all DAEC strains investigated proved to be negative for the EAF probe, indicating the absence of genes coding for the expression of bundle-forming pili generally associated with EPEC strains. All strains tested negative for the presence of the virulence factors heat-labile enterotoxin of ETEC, Shiga-like toxins I, II, and IIe, and the plasmid-encoded catalase of EHEC (data not included in Table 2). All strains which secreted Esp homologs were also positive with a probe for the eaeA gene, mediating intimate attachment, which is located in the middle of the LEE in EPEC strain 2348/69. DAEC strains 3431, B6, 0181, and 17-8 hybridized with the DNA probe of the accessory gene daaC of strain F1845, which is frequently used to detect DAEC strains. Only strain 2787 hybridized with the DNA probe for the afimbrial adhesin AIDA-I.
These results confirmed the initial classification of the investigated strains based on the adherence phenotype as DAEC since factors and phenotypes necessary for classification as EPEC, ETEC, EHEC, EIEC, or EAggEC strains were not detectable. Instead, the presence of the daaC gene of the Dr family of adhesins in most of the strains indicates the presence of factors which mediate the diffuse-adherence phenotype. The detection of the eaeA gene in the strains secreting the Esp homologs further supports the presence of the LEE, whose presence in DAEC has not been reported as yet, in these strains.
DISCUSSION
This study describes the interactions of DAEC strains with epithelial cells in tissue culture (HeLa and Caco-2). In a number of epidemiological studies, DAEC strains have been associated with diarrheal diseases in different geographic areas (2, 19, 22, 23), but no pathogenic mechanism has been identified.
To investigate whether the expression of genes which might be involved in interactions with target cells potentially contributing to pathogenesis would be induced or modulated by environmental factors, DAEC clinical isolates were analyzed in conditions where parameters such as the composition of the medium, pH, pCO2, temperature, and iron concentration had been varied. When grown in tissue culture medium, several strains were found to secrete proteins in the supernatant. DAEC strains 3431, B6, B7, 2129, and presumably also 0181 induced A/E effects, actin rearrangements, and accumulation of tyrosine-phosphorylated proteins in a manner similar to that described for the model EPEC strain 2348/69, which therefore served as a reference strain in our study. These DAEC strains secreted proteins which are apparent homologs of the EspA, EspB, and EspD proteins. All three proteins have been shown to be essential for signal transduction, leading to intimate attachment and actin accumulation in EPEC strain 2348/69 (14, 17, 30, 32).
These findings are supported by four lines of evidence: (i) the identified proteins were similar in size to the proteins secreted by the EPEC strain 2348/69; (ii) the secretion of these proteins was regulated by the same environmental parameters; (iii) the amino-terminal sequences of the three proteins obtained from strain 3431, B6, and 2129 as well as internal sequences of the 43-kDa protein from strain 3431 were highly homologous to the reported sequences of EspD, EspA, and EspB; and (iv) antibodies raised against the 43- and 38-kDa proteins of strain 3431 cross-reacted with the corresponding proteins of EPEC 2348/69 and of the other DAEC strains.
Interestingly, the short sequences of the EspA and EspB homologs of DAEC strain B6 (O26:H60) were identical to the protein sequence of EHEC strain 413.89-1 (O26:H−) isolated from calves, and that of the EspB homolog of E. coli 2129 (EPEC serotype O55:K59) was identical to that of one of the classical EHEC strains, EDL933 (O157:H7), isolated from humans. Aside from their O serotypes, however, these strains exhibit no genetic markers of EPEC or EHEC. Although the obtained sequences are short and cannot be extrapolated to the complete protein, it is tempting to speculate that the Esp proteins are derived from a common ancestor. Further sequence analysis of the complete genes and comparison of several of these homologous proteins will help to elucidate the specific properties and functions which at present are not known for any of these proteins.
The EspD protein was recently characterized in EPEC strain 2348/69 as the third secreted protein necessary for signal transduction. The gene is located between the espA and espB genes on the LEE (32). We identified a homolog of EspD as the second-most-abundant secreted protein also in the DAEC strains, supporting these results.
It has been discussed whether EspA, EspB, and EspD might be the only proteins necessary for the induction of A/E lesions. In favor of this notion is the observation that DAEC strain B6 efficiently induces actin accumulation and secretes only homologs of the EspA, EspB, and EspD proteins in detectable levels. The proteins detected in the DAEC strains with sizes of approximately 100 to 110 kDa are putative homologs of the 110-kDa secreted EspC protein reported for EPEC strain 2348/69. EspC shows homology to members of the IgA protease family, is probably not encoded on the LEE, and is not necessary for EPEC-induced signal transduction, invasion, or adherence (50). In several O157 and O26 EHEC strains, a homolog of EspC termed EspP was recently identified and shown to represent an extracellular serine protease cleaving human coagulation factor V (8).
The amino-terminal sequences of the DAEC Esp homologs which begin with the amino acid methionine indicate that they are secreted by a type III secretion system (24, 25, 33). Furthermore, strains inducing a FAS assay-positive reaction have been shown to react with a probe specific for the eaeA gene encoding the intimin protein in EPEC strains (Table 2).
Thus, we conclude that these DAEC strains harbor homologs of the LEE pathogenicity island (12, 35, 36) as has been reported now for a number of A/E pathogens including EPEC and EHEC strains (35, 36), C. rodentium (48), and some strains of H. alvei (41). Therefore, the association of DAEC strains with diarrheal outbreaks might be explained at least in part by the expression of virulence factors encoded on homologs of the LEE pathogenicity island.
The secretion of proteins by the DAEC strains investigated here was influenced by environmental factors like temperature and culture medium. We could further show that secretion of the Esp proteins is decreased by low and high pH as well as by iron depletion in the medium. Secretion was found to be optimal at 37°C and pH 7.2 and in the presence of iron. These environmental parameters are also found in the gastrointestinal tract and thus, by influencing the expression of esp genes in the intestine, might contribute to the development of the histopathological A/E lesions. In EPEC strain 2348/69, secretion of the Esp proteins is similarly increased by environmental parameters as exemplified by tissue culture medium and temperature (references 28, 29, and 43 and this study). In this study, a significant effect of carbon dioxide on the secretion of the Esp proteins in EPEC strain 2348/69 as well as in the DAEC strains was not apparent. This had also been reported for EPEC strain 2348/69 by Kenny et al. (28–30) and is in contrast to earlier data (21). The per region on the 60-MDa EAF plasmid of EPEC strain 2348/69 carrying the bfp operon has been shown to positively regulate espB, eaeA, and bfp gene expression (20, 52). However, as the DAEC strains do not hybridize with the EAF probe, further studies concerning the regulation of secretory protein expression in DAEC strains are needed.
Compared to EPEC strain 2348/69, which attaches in microcolonies to eukaryotic cells, thereby exhibiting the typical localized-adherence phenotype, a lower proportion of the diffusely adhering bacteria seemed to be able to transfer signals leading to the accumulation of actin at their attachment sites. Additionally, differences in the capacities of the DAEC strains to induce actin accumulation could be observed, although the amounts of the secreted Esp proteins of the DAEC strains and EPEC 2348/69 are comparable (with the exception of strain 0181). The reason for this is not known. One potential explanation might be that the secreted proteins need to be present at the surface of the eukaryotic cell in a concentration high enough to allow signal transduction. The formation of microcolonies by EPEC strain 2348/69 may lead to concerted effects or to sufficiently high concentrations of locally required secreted or surface-bound factors. Due to the different adherence patterns (localized versus diffuse), this might be the case only if DAEC organisms group together on the surface of the target cell. This possibility is supported by two observations. After 3 h of infection, the majority of FAS assay signals induced by DAEC strains were detected in groups of three or more bacteria. Also, DAEC strain 0181 adhered well, but only very few of the adherent bacteria developed signals in the FAS assay. This correlated well with the apparently very low abundance of the characteristic secreted proteins and the low level of cross-reactivity with the EspB and EspD antisera in DAEC strain 0181.
Actin-rich surface extensions associated with bacteria like “horns and tubes” were occasionally found on HeLa cells, emphasizing the tremendous potential of the bacteria to rearrange the host cell cytoskeleton (44). Evaluation of the interaction of the secreting DAEC strains by electron microscopy revealed subtle but significant differences in their effects induced on HeLa cells. DAEC strain 3431 preferentially induced elongation of microvilli and membrane blistering, and the bacteria seemed to be often captured by microvilli. DAEC strain B6 was more frequently found on pedestals typical for EPEC strain 2348/69, whereas DAEC strain 0181 seemed to attach to the membrane without any obvious effect on the HeLa cells. Although some of the observed morphological phenotypes like elongation of microvilli and actin rearrangements have also been found in other DAEC strains (55, 56), e.g., F1845 (6, 9), nothing is known about the presence of the LEE in those strains. Interestingly, Yamamoto et al. had reported on one DAEC strain (D2) that adhered after 6 h of infection in a diffuse-adherence pattern and accumulated actin, which completely surrounded the bacteria. Strain D2 was shown to be F1845 DNA probe positive but eaeA probe negative (55). The results obtained in this study indicate that the D2 DAEC strain probably belongs to a different group of DAEC strains capable of actin accumulation.
Diarrhea-associated E. coli strains exhibiting mixed phenotypes have been reported in a number of studies (22, 23, 54, 55, 57). DAEC strains used in this study have been analyzed for serotypes and for the presence of genetic markers encoding specific virulence factors or for markers characteristic for other known diarrhea-associated E. coli categories. No markers other than serotypes also found in EHEC and EPEC serotypes and markers of the LEE could be detected. This further validates the classification of the strains in this study as DAEC (Table 2).
All strains found to be FAS assay positive carry the eaeA gene probe, but only EPEC strain 2348/69 reacted with the probe used for detection of the bfp gene. This indicates that for the initial attachment preceding intimin binding, probably many, albeit preferentially fimbrial, adhesins like bundle-forming pili or the F1845 fimbrial adhesin, would probably be functional and support the effects generated by LEE-encoded factors. In support of this notion, the EAF plasmid and daaC-negative strains B7 and 2129 develop significant adherence and accumulation of actin and tyrosine-phosphorylated proteins comparable to that of DAEC strains 3431 and B6 only after prolonged incubation for 6 h. Probably due to the lack of a suitable adhesin(s) for the initial adherence, this property was not manifest at early time points of infection. This is reminiscent of reports on EHEC strains (31, 37), where also incubation for at least 5 h is needed for detection of actin accumulation after eaeA-dependent adherence. The increase in adherence and in the accumulation of actin and tyrosine-phosphorylated proteins upon prolonged incubation in some DAEC strains apparently lacking initial adhesins suggests an adaptation process which might potentially involve the synthesis or even transfer of proteins facilitating signal transduction. This notion is supported by the presence of the type III secretion system known to mediate the transfer of bacterial proteins of other pathogens into host cells (33). It will be interesting to see whether also in DAEC strains bacterial proteins are able to enter host cells. The observed time course is also remarkable, as potentially the sole acquisition of an additional adherence factor(s) might convert these strains in a single step to more virulent pathogens. Following this proposal, we further suggest that the acquisition of the LEE seems to be quite independent from the large virulence plasmid common to EPEC strains. Furthermore, the secretion of proteins homologous to the LEE-encoded EspA, EspB, and EspD in DAEC strains isolated in geographically distant areas of the world indicates that homologs of the LEE pathogenicity island are widely distributed. How these genes in DAEC strains differ from their counterparts in classical EPEC strains, EHEC strains, or other bacterial pathogens and how they are regulated may not only shed some light on their pathway of distribution but also help to elucidate the molecular details of their interaction with host cells.
ACKNOWLEDGMENTS
We are indebted to A. Bosserhoff und R. Frank (ZMBH, Heidelberg, Germany) for amino-terminal amino acid analysis and to E. M. Figuerol (Barcelona) for help with the initial screening experiments.
This study was supported in part by grant (SFB 310) from the German Research Foundation.
S. Laarmann and C. Wachter contributed equally to this study.
REFERENCES
- 1.Aleksic S, Karch H, Bockemuhl J. A biotyping scheme for Shiga-like (Vero) toxin-producing Escherichia coli O157 and a list of serological cross-reactions between O157 and other gram-negative bacteria. Int J Med Microbiol Virol Parasitol Infect Dis. 1992;276:221–230. doi: 10.1016/s0934-8840(11)80009-5. [DOI] [PubMed] [Google Scholar]
- 2.Baqui A H, Sack R B, Black R E, Haider K, Hossain A, Alim A R, Yunus M, Chowdhury H R, Siddique A K. Enteropathogens associated with acute and persistent diarrhea in Bangladeshi children less than 5 years of age. J Infect Dis. 1992;166:792–796. doi: 10.1093/infdis/166.4.792. [DOI] [PubMed] [Google Scholar]
- 3.Benz I, Schmidt M A. AIDA-I, the adhesin involved in diffuse adherence of the diarrhoeagenic Escherichia coli strain 2787 (O126:H27), is synthesized via a precursor molecule. Mol Microbiol. 1992;6:1539–1546. doi: 10.1111/j.1365-2958.1992.tb00875.x. [DOI] [PubMed] [Google Scholar]
- 4.Benz I, Schmidt M A. Cloning and expression of an adhesin (AIDA-I) involved in diffuse adherence of enteropathogenic Escherichia coli. Infect Immun. 1989;57:1506–1511. doi: 10.1128/iai.57.5.1506-1511.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benz I, Schmidt M A. Isolation and serologic characterization of AIDA-I, the adhesin mediating the diffuse adherence phenotype of the diarrhea-associated Escherichia coli strain 2787 (O126:H27) Infect Immun. 1992;60:13–18. doi: 10.1128/iai.60.1.13-18.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernet-Camard M F, Coconnier M H, Hudault S, Servin A L. Pathogenicity of the diffusely adhering strain Escherichia coli C1845: F1845 adhesin decay-accelerating factor interaction, brush border microvillus injury, and actin disassembly in cultured human intestinal epithelial cells. Infect Immun. 1996;64:1918–1928. doi: 10.1128/iai.64.6.1918-1928.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bilge S S, Clausen C R, Lau W, Moseley S L. Molecular characterization of a fimbrial adhesin, F1845, mediating diffuse adherence of diarrhea-associated Escherichia coli to HEp-2 cells. J Bacteriol. 1989;171:4281–4289. doi: 10.1128/jb.171.8.4281-4289.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunder W, Schmidt H, Karch H. EspP, a novel extracellular serine protease of enterohaemorrhagic Escherichia coli O157:H7 cleaves human coagulation factor V. Mol Microbiol. 1997;24:767–778. doi: 10.1046/j.1365-2958.1997.3871751.x. [DOI] [PubMed] [Google Scholar]
- 9.Cookson S T, Nataro J P. Characterization of HEp-2 cell projection formation induced by diffusely adherent Escherichia coli. Microb Pathog. 1996;21:421–434. doi: 10.1006/mpat.1996.0073. [DOI] [PubMed] [Google Scholar]
- 10.Cravioto A, Tello A, Navarro A, Ruiz J, Villafan H, Uribe F, Eslava C. Association of Escherichia coli HEp-2 adherence patterns with type and duration of diarrhoea. Lancet. 1991;337:262–264. doi: 10.1016/0140-6736(91)90868-p. [DOI] [PubMed] [Google Scholar]
- 11.Donnenberg M S, Kaper J B, Finlay B B. Interactions between enteropathogenic Escherichia coli and host epithelial cells. Trends Microbiol. 1997;5:109–114. doi: 10.1016/S0966-842X(97)01000-7. [DOI] [PubMed] [Google Scholar]
- 12.Donnenberg M S, Lai L C, Taylor K A. The locus of enterocyte effacement pathogenicity island of enteropathogenic Escherichia coli encodes secretion functions and remnants of transposons at its extreme right end. Gene. 1997;184:107–114. doi: 10.1016/s0378-1119(96)00581-1. [DOI] [PubMed] [Google Scholar]
- 13.Donnenberg M S, Tzipori S, McKee M L, O’Brien A D, Alroy J, Kaper J B. The role of the eae gene of enterohemorrhagic Escherichia coli in intimate attachment in vitro and in a porcine model. J Clin Invest. 1993;92:1418–1424. doi: 10.1172/JCI116718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donnenberg M S, Yu J, Kaper J B. A second chromosomal gene necessary for intimate attachment of enteropathogenic Escherichia coli to epithelial cells. J Bacteriol. 1993;175:4670–4680. doi: 10.1128/jb.175.15.4670-4680.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ebel F, Deibel C, Kresse A U, Guzman C A, Chakraborty T. Temperature- and medium-dependent secretion of proteins by Shiga toxin-producing Escherichia coli. Infect Immun. 1996;64:4472–4479. doi: 10.1128/iai.64.11.4472-4479.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eichenbaum Z, Green B D, Scott J R. Iron starvation causes release from the group A streptococcus of the ADP-ribosylating protein called plasmin receptor or surface glyceraldehyde-3-phosphate-dehydrogenase. Infect Immun. 1996;64:1956–1960. doi: 10.1128/iai.64.6.1956-1960.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foubister V, Rosenshine I, Donnenberg M S, Finlay B B. The eaeB gene of enteropathogenic Escherichia coli is necessary for signal transduction in epithelial cells. Infect Immun. 1994;62:3038–3040. doi: 10.1128/iai.62.7.3038-3040.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giron J A, Ho A S, Schoolnik G K. An inducible bundle-forming pilus of enteropathogenic Escherichia coli. Science. 1991;254:710–713. doi: 10.1126/science.1683004. [DOI] [PubMed] [Google Scholar]
- 19.Giron J A, Jones T, Millan Velasco F, Castro Munoz E, Zarate L, Fry J, Frankel G, Moseley S L, Baudry B, Kaper J B, Schoolnik G K, Riley L W. Diffuse-adhering Escherichia coli (DAEC) as a putative cause of diarrhea in Mayan children in Mexico. J Infect Dis. 1991;163:507–513. doi: 10.1093/infdis/163.3.507. [DOI] [PubMed] [Google Scholar]
- 20.Gomez Duarte O G, Kaper J B. A plasmid-encoded regulatory region activates chromosomal eaeA expression in enteropathogenic Escherichia coli. Infect Immun. 1995;63:1767–1776. doi: 10.1128/iai.63.5.1767-1776.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haigh R, Baldwin T, Knutton S, Williams P H. Carbon dioxide regulated secretion of the EaeB protein of enteropathogenic Escherichia coli. FEMS Microbiol Lett. 1995;129:63–67. doi: 10.1016/0378-1097(95)00136-S. [DOI] [PubMed] [Google Scholar]
- 22.Jallat C, Darfeuille Michaud A, Rich C, Joly B. Survey of clinical isolates of diarrhoeogenic Escherichia coli: diffusely adhering E. coli strains with multiple adhesive factors. Res Microbiol. 1994;145:621–632. doi: 10.1016/0923-2508(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 23.Jallat C, Livrelli V, Darfeuille Michaud A, Rich C, Joly B. Escherichia coli strains involved in diarrhea in France: high prevalence and heterogeneity of diffusely adhering strains. J Clin Microbiol. 1993;31:2031–2037. doi: 10.1128/jcm.31.8.2031-2037.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jarvis K G, Giron J A, Jerse A E, McDaniel T K, Donnenberg M S, Kaper J B. Enteropathogenic Escherichia coli contains a putative type III secretion system necessary for the export of proteins involved in attaching and effacing lesion formation. Proc Natl Acad Sci USA. 1995;92:7996–8000. doi: 10.1073/pnas.92.17.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jarvis K G, Kaper J B. Secretion of extracellular proteins by enterohemorrhagic Escherichia coli via a putative type III secretion system. Infect Immun. 1996;64:4826–4829. doi: 10.1128/iai.64.11.4826-4829.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jose J, Jahnig F, Meyer T F. Common structural features of IgA1 protease-like outer membrane protein autotransporters. Mol Microbiol. 1995;18:378–380. doi: 10.1111/j.1365-2958.1995.mmi_18020378.x. [DOI] [PubMed] [Google Scholar]
- 27.Karch H, Bohm H, Schmidt H, Gunzer F, Aleksic S, Heesemann J. Clonal structure and pathogenicity of Shiga-like toxin-producing, sorbitol-fermenting Escherichia coli O157:H- J Clin Microbiol. 1993;31:1200–1205. doi: 10.1128/jcm.31.5.1200-1205.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kenny B, Abe A, Stein M, Finlay B B. Enteropathogenic Escherichia coli protein secretion is induced in response to conditions similar to those in the gastrointestinal tract. Infect Immun. 1997;65:2606–2612. doi: 10.1128/iai.65.7.2606-2612.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kenny B, Finlay B B. Protein secretion by enteropathogenic Escherichia coli is essential for transducing signals to epithelial cells. Proc Natl Acad Sci USA. 1995;92:7991–7995. doi: 10.1073/pnas.92.17.7991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kenny B, Lai L C, Finlay B B, Donnenberg M S. EspA, a protein secreted by enteropathogenic Escherichia coli, is required to induce signals in epithelial cells. Mol Microbiol. 1996;20:313–323. doi: 10.1111/j.1365-2958.1996.tb02619.x. [DOI] [PubMed] [Google Scholar]
- 31.Knutton S, Baldwin T, Williams P H, McNeish A S. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect Immun. 1989;57:1290–1298. doi: 10.1128/iai.57.4.1290-1298.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lai L C, Wainwright L A, Stone K D, Donnenberg M S. A third secreted protein that is encoded by the enteropathogenic Escherichia coli pathogenicity island is required for transduction of signals and for attaching and effacing activities in host cells. Infect Immun. 1997;65:2211–2217. doi: 10.1128/iai.65.6.2211-2217.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee C A. Type III secretion systems: machines to deliver bacterial proteins into eucaryotic cells? Trends Microbiol. 1997;5:148–156. doi: 10.1016/S0966-842X(97)01029-9. [DOI] [PubMed] [Google Scholar]
- 34.Levine M M, Nataro J P, Karch H, Baldini M M, Kaper J B, Black R E, Clements M L, O’Brien A D. The diarrheal response of humans to some classic serotypes of enteropathogenic Escherichia coli is dependent on a plasmid encoding an enteroadhesiveness factor. J Infect Dis. 1985;152:550–559. doi: 10.1093/infdis/152.3.550. [DOI] [PubMed] [Google Scholar]
- 35.McDaniel T K, Jarvis K G, Donnenberg M S, Kaper J B. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc Natl Acad Sci USA. 1995;92:1664–1668. doi: 10.1073/pnas.92.5.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McDaniel T K, Kaper J B. A cloned pathogenicity island from enteropathogenic Escherichia coli confers the attaching and effacing phenotype on E. coli K 12. Mol Microbiol. 1997;23:399–407. doi: 10.1046/j.1365-2958.1997.2311591.x. [DOI] [PubMed] [Google Scholar]
- 37.McKee M L, O’Brien A D. Investigation of enterohemorrhagic Escherichia coli O157:O7 adherence characteristics and invasion potential reveals a new attachement pattern shared by intestinal E. coli. Infect Immun. 1995;63:2070–2074. doi: 10.1128/iai.63.5.2070-2074.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nataro J P, Kaper J B, Robins Browne R, Prado V, Vial P, Levine M M. Patterns of adherence of diarrheagenic Escherichia coli to HEp-2 cells. Pediatr Infect Dis J. 1987;6:829–831. doi: 10.1097/00006454-198709000-00008. [DOI] [PubMed] [Google Scholar]
- 39.Nataro J P, Scaletsky I C, Kaper J B, Levine M M, Trabulsi L R. Plasmid-mediated factors conferring diffuse and localized adherence of enteropathogenic Escherichia coli. Infect Immun. 1985;48:378–383. doi: 10.1128/iai.48.2.378-383.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nowicki B, Labigne A, Moseley S, Hull R, Hull S, Moulds J. The Dr hemagglutinin, afimbrial adhesins AFA-I, AFA-II, and AFA-III, and F1845 fimbriae of uropathogenic and diarrhea-associated Escherichia coli belong to a family of hemagglutinins with Dr receptor recognition. Infect Immun. 1990;58:279–281. doi: 10.1128/iai.58.1.279-281.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ridell J, Siitonen A, Paulin L, Mattila L, Korkeala H, Albert M J. Hafnia alvei in stool specimens from patients with diarrhea and healthy controls. J Clin Microbiol. 1994;32:2335–2337. doi: 10.1128/jcm.32.9.2335-2337.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosenshine I, Donnenberg M S, Kaper J B, Finlay B B. Signal transduction between enteropathogenic Escherichia coli (EPEC) and epithelial cells: EPEC induce tyrosine phosphorylation of host cell proteins to initiate cytoskeletal rearrangement and bacterial uptake. EMBO J. 1992;11:3551–3560. doi: 10.1002/j.1460-2075.1992.tb05438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosenshine I, Ruschkowski S, Finlay B B. Expression of attaching/effacing activity by enteropathogenic Escherichia coli depends on growth phase, temperature, and protein synthesis upon contact with epithelial cells. Infect Immun. 1996;64:966–973. doi: 10.1128/iai.64.3.966-973.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosenshine I, Ruschkowski S, Stein M, Reinscheid D J, Mills S D, Finlay B B. A pathogenic bacterium triggers epithelial signals to form a functional bacterial receptor that mediates actin pseudopod formation. EMBO J. 1996;15:2613–2624. [PMC free article] [PubMed] [Google Scholar]
- 45.Russmann H, Kothe E, Schmidt H, Franke S, Harmsen D, Caprioli A, Karch H. Genotyping of Shiga-like toxin genes in non-O157 Escherichia coli strains associated with haemolytic uraemic syndrome. J Med Microbiol. 1995;42:404–410. doi: 10.1099/00222615-42-6-404. [DOI] [PubMed] [Google Scholar]
- 46.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 47.Scaletsky I C A, Silva M L, Trabulsi L R. Distinctive patterns of adherence of enteropathogenic Escherichia coli to HeLa cells. Infect Immun. 1984;43:534–536. doi: 10.1128/iai.45.2.534-536.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schauer D B, Falkow S. Attaching and effacing locus of a Citrobacter freundii biotype that causes transmissible murine colonic hyperplasia. Infect Immun. 1993;61:2486–2492. doi: 10.1128/iai.61.6.2486-2492.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmidt H, Knop C, Franke S, Aleksic S, Heesemann J, Karch H. Development of PCR for screening of enteroaggregative Escherichia coli. J Clin Microbiol. 1995;33:701–705. doi: 10.1128/jcm.33.3.701-705.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stein M, Kenny B, Stein M A, Finlay B B. Characterization of EspC, a 110-kilodalton protein secreted by enteropathogenic Escherichia coli which is homologous to members of the immunoglobulin A protease-like family of secreted proteins. J Bacteriol. 1996;178:6546–6554. doi: 10.1128/jb.178.22.6546-6554.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suhr M, Benz I, Schmidt M A. Processing of the AIDA-I precursor: removal of AIDAC and evidence for the outer membrane anchoring as a β-barrel structure. Mol Microbiol. 1996;22:31–42. doi: 10.1111/j.1365-2958.1996.tb02653.x. [DOI] [PubMed] [Google Scholar]
- 52.Tobe T, Schoolnik G K, Sohel I, Bustamante V H, Puente J L. Cloning and characterization of bfpTVW, genes required for the transcriptional activation of bfpA in enteropathogenic Escherichia coli. Mol Microbiol. 1996;21:963–975. doi: 10.1046/j.1365-2958.1996.531415.x. [DOI] [PubMed] [Google Scholar]
- 53.Vial P A, Mathewson J J, DuPont H L, Guers L, Levine M M. Comparison of two assay methods for patterns of adherence to HEp-2 cells of Escherichia coli from patients with diarrhea. J Clin Microbiol. 1990;28:882–885. doi: 10.1128/jcm.28.5.882-885.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamamoto T, Echeverria P. Detection of the enteroaggregative Escherichia coli heat-stable enterotoxin 1 gene sequences in enterotoxigenic E. coli strains pathogenic for humans. Infect Immun. 1996;64:1441–1445. doi: 10.1128/iai.64.4.1441-1445.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamamoto T, Kaneko M, Changchawalit S, Serichantalergs O, Ijuin S, Echeverria P. Actin accumulation associated with clustered and localized adherence in Escherichia coli isolated from patients with diarrhea. Infect Immun. 1994;62:2917–2929. doi: 10.1128/iai.62.7.2917-2929.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamamoto T, Koyama Y, Matsumoto M, Sonoda E, Nakayama S, Uchimura M, Paveenkittiporn W, Tamura K, Yokota T, Echeverria P. Localized, aggregative, and diffuse adherence to HeLa cells, plastic, and human small intestines by Escherichia coli isolated from patients with diarrhea. J Infect Dis. 1992;166:1295–1310. doi: 10.1093/infdis/166.6.1295. [DOI] [PubMed] [Google Scholar]
- 57.Yamamoto T, Wakisaka N, Nakae T, Kamano T, Serichantalergs O, Echeverria P. Characterization of a novel hemagglutinin of diarrhea-associated Escherichia coli that has characteristics of diffusely adhering E. coli and enteroaggregative E. coli. Infect Immun. 1996;64:3694–3702. doi: 10.1128/iai.64.9.3694-3702.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]