Abstract
BACKGROUND:
Hypertensive disorders of pregnancy (HDP) are associated with long-term maternal risks for cardiovascular disease for reasons that remain incompletely understood.
METHODS:
The HCHS/SOL (Hispanic Community Health Study/Study of Latinos), a multi-center community-based cohort of Hispanic/Latino adults recruited 2008 to 2011, was used to evaluate the associations of history of de novo HDP (gestational hypertension, preeclampsia, eclampsia) with echocardiographic measures of cardiac structure and function in Hispanic/Latina women with ≥1 prior pregnancy and the proportion of association mediated by current hypertension (>140/90 mm Hg or antihypertensive therapy).
RESULTS.
The study cohort included 5168 Hispanic/Latina women with an average age (SD) of 58.7 (9.7) years at time of echocardiogram. Prior de novo HDP was reported by 724 (14%) of the women studied and was associated with lower left ventricle (LV) ejection fraction −0.66 (95% confidence interval [CI], −1.21 to −0.11), higher LV relative wall thickness 0.09 (95% CI, 0–0.18), and 1.39 (95% CI, 1.02–1.89) higher risk of abnormal LV geometry after adjusting for blood pressure and other confounders. The proportion of the association mediated by current hypertension between HDP and LV ejection fraction was 0.09 (95% CI, 0.03–0.45), LV relative wall thickness was 0.28 (95% CI, 0.16–0.51), abnormal LV geometry was 0.14 (95% CI, 0.12–0.48), concentric left ventricular hypertrophy was 0.31 (95% CI, 0.19–0.86), and abnormal LV diastolic dysfunction was 0.58 (95% CI, 0.26–0.79).
CONCLUSIONS.
In a large cohort of Hispanic/Latina women those with history of de novo HDP had detectable and measurable subclinical alterations in cardiac structure and both systolic and diastolic dysfunction that were only partially mediated by current hypertension.
Keywords: blood pressure, cardiovascular disease, cardiovascular pregnancy complication, Hispanic, hypertension, ventricular cardiac remodeling
NOVELTY AND RELEVANCE.
What Is New?
First study to evaluate the cardiac sequelae of de novo hypertensive disorder of pregnancy in a cohort of 5168 Hispanic/Latina women.
What Is Relevant?
Women with de novo hypertensive disorders of pregnancy had detectable and measurable subclinical alterations in cardiac structure and both systolic and diastolic dysfunction, above and beyond the effects of current hypertension.
Clinical/Pathophysiological Implications?
Our findings suggest that women with de novo hypertensive disorders of pregnancy have pathophysiologic cardiac sequelae decades later that likely play a role in modulating long-term cardiovascular risk in women.
The rates of hypertensive disorders of pregnancy (HDP), including preeclampsia and gestational hypertension, more than doubled from 2007 to 2019 in the United States with highest rates in Non-Hispanic Black and Hispanic/Latina women.1,2 Growing evidence demonstrates that history of HDP is associated with higher maternal risk for long-term cardiovascular disease (CVD) and CVD-related death;3,4 resulting in the addition of HDP as a risk-modifier in the 2019 American College of Cardiology/American Heart Association primary prevention guidelines.5
Prior investigations have found evidence of structural cardiac abnormalities during the antepartum and immediate postpartum period, attributable in part to the short-term hemodynamic effects of excess afterload in pregnancies complicated by HDP.6–8 These cardiac structural changes, including increased left ventricle (LV) wall thickness, mass index, diastolic dysfunction, and abnormalities in strain, have been shown to persist postpartum in pregnancies complicated by HDP.9–11 Up to 20% of women with pregnancies complicated by HDP remain hypertensive after 6 months postpartum and have a 3- to 10-fold lifetime risk of chronic hypertension.12,13 However, the role of chronic hypertension on adverse cardiac remodeling in women with history of HDP remains debatable. This is due to the mixed findings in studies to date, from some showing adverse cardiac remodeling driven by chronic hypertension regardless of HDP-history, to evidence that adverse remodeling is independent of the development of chronic hypertension, to reports on the cumulative effects of history of HDP and chronic hypertension on adverse remodeling.9–11 Yet, very little is known about the effects of HDP on cardiac abnormalities and the role of chronic hypertension in Hispanic/Latina women, one of the fastest-growing ethnic minority group in the United States with a diverse genetic architecture.14
The degree to which cardiac abnormalities occur well beyond the early postpartum period before the development of CVD decades later—notwithstanding the effects of postpartum or age-related chronic hypertension—has remained unclear. We hypothesize that history of de novo HDP is associated with pathological alterations in cardiac structure and function that are only partially mediated by current hypertension. We aimed to examine this hypothesis in a diverse cohort of Hispanic/Latina women in the United States.
METHODS
Study Sample
We studied participants of the HCHS/SOL (Hispanic Community Health Study/Study of Latinos), a multi-center community-based cohort of all Hispanic/Latino adults representing diverse backgrounds (Central American, Cuban, Dominican, Mexican, Puerto Rican, and South American).14 The HCHS/SOL sampling methods and design have been detailed previously.15,16 In brief, self-identified Hispanic/Latino men and women were recruited between March 2008 to June 2011 from 4 communities in the United States (Bronx, NY; Chicago, IL; Miami, FL; and San Diego, CA) using a multi-stage area probability sample design. At each stage of sampling, oversampling occurred, and sampling weights were generated to reflect the probabilities of selection. The institutional review board at each study site approved all protocols, and all study participants provided written informed consent. Data from the HCHS/SOL cohort is publicly available to researchers upon application to NHLBI BIOLINCC.
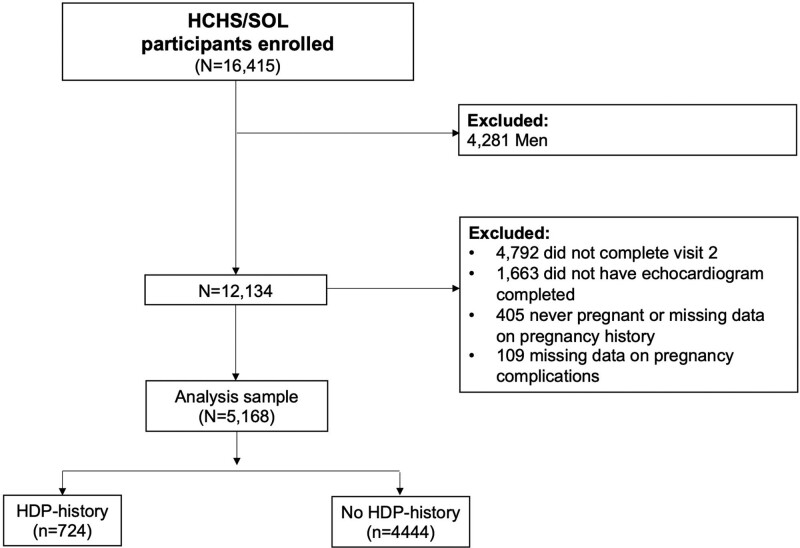
Of the 16 415 HCHS/SOL participants who enrolled in this study, we included women age ≥45 years who completed visit 2 and transthoracic echocardiography (TTE) and reported at least 1 prior pregnancy at baseline visit (2008–2011) or visit 2 (2014–2017). We excluded men (n=4281), participants that did not complete visit 2 (n=4792), did not complete TTE (n=1663), participants that were never pregnant or missing data on pregnancy history (n=405), and participants with missing data on HDP history (n=109). The final sample for this analysis included 5168 women (724 with HDP and 4444 without HDP; Figure).
Figure.
Sampling strategy and study design. Central illustration. Proportion of association between hypertensive disorders of pregnancy and measures of left ventricular structure and function mediated by current hypertension in Hispanic/Latina women. Figure created using BioRender. HCHS/SOL indicates Hispanic Community Health Study/Study of Latinos; and HDP, hypertensive disorders of pregnancy.
Clinical and Echocardiographic Data Collection
At each study visit, all study participants underwent a standardized assessment of demographic and clinical characteristics including questionnaires regarding medical and pregnancy history along with standardized measurements of blood pressure (BP), as previously described.16,17 Visit 2 questionnaire collected self-reported data on the history of gestational hypertension (collected as high BP or hypertension first diagnosed during pregnancy), preeclampsia (collected as preeclampsia or toxemia) and eclampsia (collected as seizures, convulsions, or eclampsia) for all their pregnancies. We defined composite de novo HDP status as any history of gestational hypertension, preeclampsia, or eclampsia.
At visit 2, participants age ≥45 years underwent comprehensive 2-dimensional TTE performed according to a previously detailed standardized protocol.18 In brief, TTE examination was performed with the participant in the partial left decubitus position with image acquisition techniques and measurements of cardiac structure and function performed according to American Society of Echocardiography guidelines.19,20 All image acquisition was performed by centrally trained and certified research sonographers and all imaging measurements were conducted by imaging technical specialists at the core HCHS/SOL Echocardiography Reading Center at Brigham and Women’s Hospital.18
As previously described, we defined concentric remodeling as LV mass index ≤95 gm/m2 and relative wall thickness (RWT) >0.42, concentric LV hypertrophy (LVH) as LV mass index >95 gm/m2 and RWT >0.42, and eccentric LVH as LV mass index >95 gm/m2 and RWT ≤0.42.19 Abnormal LV geometry was defined as presence of concentric remodeling, concentric LVH, or eccentric LVH. Diastolic function was graded according to an algorithm that integrates American Society of Echocardiography guidelines with Redfield criteria as previously described.17 In the analysis, LV diastolic dysfunction was dichotomized and grade I–IV diastolic dysfunction was compared with normal diastolic function.
Statistical Analyses
We compared demographic, clinical, and echocardiographic traits in Hispanic/Latina women with and without prior HDP using the Student t test for continuous variables and χ2 test for categorical variables. Holm-Bonferroni method was used to adjust for multiple comparisons.
We then used multivariable-adjusted regression models to examine the association of prior HDP status with established measures of cardiac structure and function: LV ejection fraction (EF), LV stroke volume, LV mass index, LV end-diastolic diameter, LV mass/end-diastolic volume ratio, LV RWT, peak tricuspid regurgitation velocity, lateral E/e′ ratio, concentric remodeling, concentric LVH, eccentric LVH, abnormal LV geometry, and abnormal LV diastolic function. We constructed linear and logistic regression models for continuous and categorical variables, respectively. For all echocardiographic traits, model 1 adjusted for age, study field center, and Hispanic/Latino background. Model 2 adjusted for the covariates in model 1, in addition to SBP and treatment with antihypertensive therapy at visit 2. Model 3 adjusted for the covariates in model 2, in addition to body mass index, diabetes, smoking, total number of prior pregnancies, total cholesterol/HDL (high-density lipoprotein) ratio, and urine albumin-to-creatinine ratio all assessed at visit 2. Covariates were selected based on prior studies demonstrating association with HDP and LV measures of structure and function.9–11 No adjustments were made for multiple testing. Stratified analyses were performed by type of HDP for gestational hypertension and preeclampsia, however, the sample size was too small to perform adjusted models for eclampsia.
In secondary analyses, we examined the extent to which current hypertension (defined as BP >140/90 mm Hg or antihypertensive therapy at visit 2) mediated the associations of HDP with echocardiography traits. Models are adjusted for age, field center, Hispanic/Latino background, and current hypertension. These analyses test the extent to which current hypertension mediate the associations of HDP with the given echocardiographic trait, whereby a mediation effect of 0 would indicate that current hypertension does not mediate the association and a mediation effect of 1 would indicate that current hypertension mediates all of the association (range of possible effect is from 0 to 1). We also assessed for potential interaction of current hypertension on HDP associations with each of the echocardiographic traits.
Reported values were survey-weighted, to account for the complex study design and the nonresponses for visit 2.15 Weights were trimmed and calibrated to the 2010 Census characteristics by age, sex, and Hispanic/Latino background. We considered statistical significance as a 2-tailed P value and Holm-Bonferroni adjusted P value of <0.05. All statistical analysis were conducted using R (v4.0.4).
RESULTS
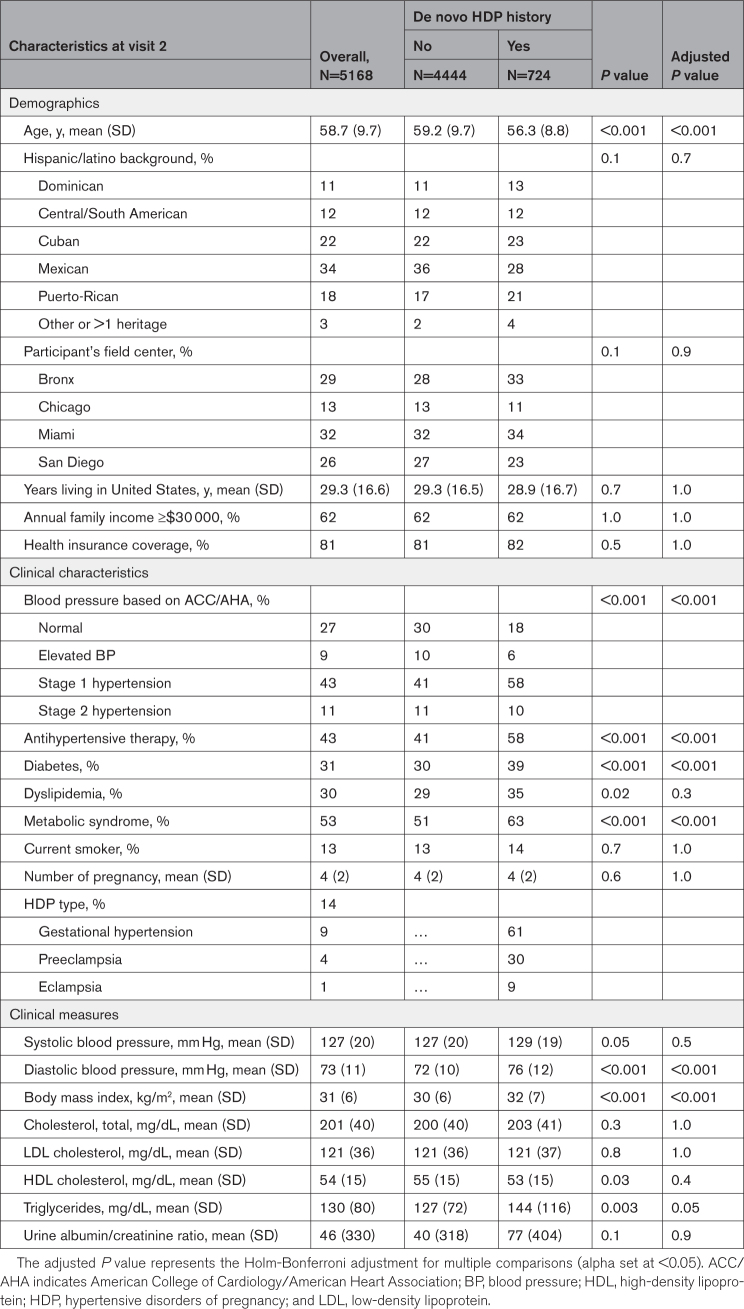
Of the total study sample including 5168 Hispanic/Latina women with prior pregnancy, 724 (14%) reported a history of de novo HDP including 439 (61%) gestational hypertension, 219 (30%) preeclampsia, and 66 (9%) eclampsia. The mean age (SD) at visit 2 was 58.7 (9.7) years. The demographic and clinical characteristics of the study sample at visit 2 are shown in Table 1. Hispanic/Latina women with a history of de novo HDP, compared with those without HDP were younger, had a higher level of education, greater body mass index, and more frequent history of dyslipidemia. After the Holm-Bonferroni adjustment, dyslipidemia and HDL-cholesterol levels were no longer statistically different between the groups. With respect to echocardiographic LV characteristics (Table S1), Hispanic/Latina women with prior HDP had lower LV EF, and higher LV stroke volume, LV mass index, LV RWT, and peak tricuspid regurgitation velocity. After the Holm-Bonferroni adjustment, LV mass index and peak tricuspid regurgitation velocity were not significantly different between the groups. HDP was associated with higher rates of concentric LVH, abnormal LV geometry, and abnormal LV diastolic function.
Table 1.
Demographics and Clinical Characteristics by De Novo Hypertensive Disorders of Pregnancy History
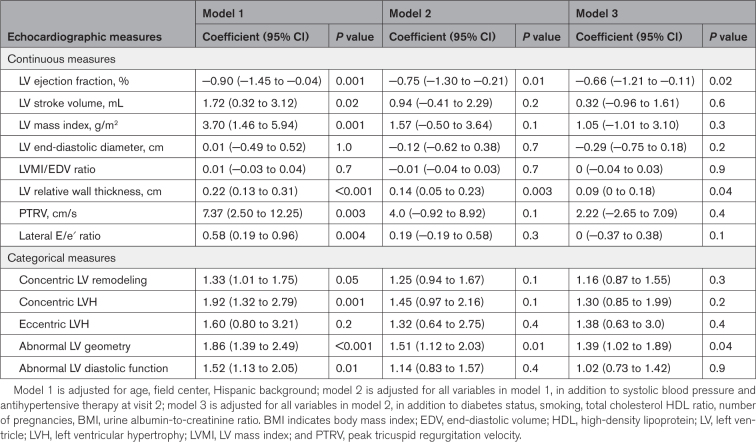
In multivariable-adjusted analyses, prior de novo HDP was associated with lower LV EF in all models after adjustment for systolic BP, antihypertensive therapy and confounders (P≤0.02; Table 2). Prior HDP was also associated with higher LV RWT in all models (P≤0.04). Prior HDP was associated with a 1.92-fold higher odds of LV stroke volume, LV mass index, LV relative wall thickness, peak tricuspid regurgitation velocity, and lateral E/e′ ratio after adjustment for age and demographic factors (model 1) but the associations were attenuated in subsequent models. Notably, prior HDP was significantly associated with higher risk of abnormal LV geometry in all models (P≤0.04). Prior HDP was associated with higher risk of concentric LVH and abnormal LV diastolic function after adjustment for age and demographic factors (model 1; P=0.001 and P=0.01, respectively); these associations were attenuated when adjusted for systolic BP and antihypertensive therapy. All other measures of cardiac structure and function were not significantly associated with history of HDP.
Table 2.
Associations Between De Novo Hypertensive Disorders of Pregnancy History and Measures of Left Ventricle Structure and Function.
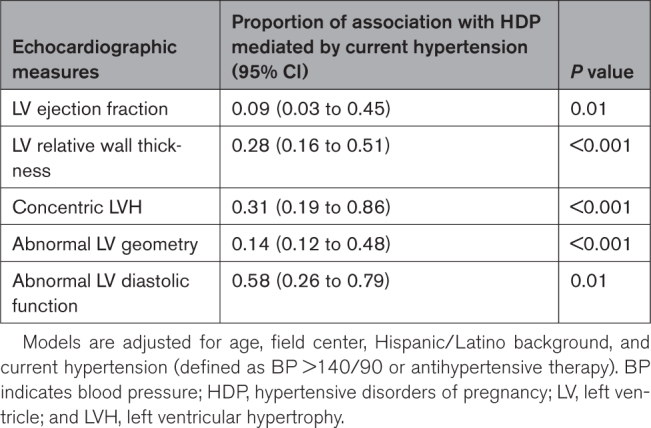
In secondary analyses, we examined the extent to which current hypertension mediated the associations of de novo HDP with echocardiography traits. Current hypertension at visit 2 was seen in 459 (63.4%) of Hispanic/Latina women with HDP-history and 1830 (41.2%) with normotensive pregnancies. We found that the proportion of the association between HDP and LV EF mediated by current hypertension was modest at 0.09 (95% confidence interval [CI], 0.03–0.45), with similar results seen for abnormal LV geometry (0.14 [95% CI, 0.12–0.48]); Table 3). The proportion of the association between HDP and other traits mediated by postpartum hypertension were higher for LV RWT (0.28 [95% CI, 0.16–0.51]), concentric LVH (0.31 [95% CI, 0.19–0.86]), and abnormal LV diastolic dysfunction (0.58 [95% CI, 0.26–0.79]). We also assessed for potential interaction of current hypertension on the associations of HDP with each of the echocardiographic traits and found no significant interactions (P>0.30 for all). We observed no material difference in any results of analyzing data using survey-weighted versus unweighted values.
Table 3.
Causal Mediation Analysis to Assess Mediation Effect of Current Hypertension for Observed Association Between De Novo Hypertensive Disorders of Pregnancy and Measures of Left Ventricle Structure and Function
In stratified analysis by type of HDP, there was a significant trend towards lowest EF and LV stroke volume and highest LV RWT in women with eclampsia history (Table S2). The proportion of concentric LVH was highest in women with history of gestational hypertension and the proportion of abnormal LV geometry was highest in those with history of eclampsia and gestational hypertension. In multivariable-adjusted analyses, gestational hypertension was associated with LV RWT and 1.79-fold higher risk of abnormal LV geometry across all models, whereas there was no association between preeclampsia and any of the measures of LV structure and function (Table S3).
DISCUSSION
In our study of over 5100 Hispanic/Latina women with prior pregnancy, 14% reported de novo HDP. Women with history of de novo HDP were significantly more likely to have measurable abnormalities in cardiac structure and function, including lower LV systolic function and higher rates of abnormal LV geometry than women without history of HDP. These cardiac alterations were in part mediated by the effects of current hypertension (Central Illustration).
The rate of de novo HDP in this study is consistent with the 12.5% (95% CI, 12.2–12.8) reported in the United States National Inpatient Sample between 2017 and 2019.21 To date, a scant number of studies have examined longer-term cardiac phenotypes following delivery before development of clinical CVD; these few studies, with sample sizes of <200 women with history of HDP report an association with LVH and LV diastolic dysfunction.22,23 In studies accounting for chronic hypertension, findings are mixed on whether or not the changes in cardiac structure and function associated with HDP history are present after adjusting for chronic hypertension —an obvious confounder of CVD risk.9–11 We extend previous work and analyze one of the largest samples of de novo HDP in 724 women and compared their cardiac phenotypes with 4444 similarly aged clinical controls (ie, women with prior pregnancy but no HDP). Importantly, we found that prior HDP was associated with higher LV RWT and higher risk of abnormal LV geometry—even after adjusting for current hypertension and cardiovascular risk factors—consistent with a recent study by Countouris et al11 in a cohort of non-Hispanic White and Black women. Our study extends these findings to Hispanic/Latina women who are underrepresented in research studies.
History of pregnancy complicated by de novo HDP was associated with higher risk of abnormal LV geometry (defined as concentric LV remodeling, concentric LVH, or eccentric LVH as determined by LV mass index and RWT) after adjusting for important confounders. These findings are of clinical significance because abnormal LV geometry, particularly LVH, is an independent predictor of adverse cardiovascular events including heart failure, ischemic heart disease, and sudden cardiac death.24 We also found an association between de novo HDP and abnormal LV diastolic function, which is linked to higher incidence of cardiovascular events in healthy cohorts and is a strong predictor of progression to heart failure.25 Further, the individual measures of LV geometry (LV mass index, LV RWT) that we found to be associated with history of de novo HDP have also been shown to be associated with cardiovascular events. For instance, in the Framingham Heart Study of Offspring in a cohort of adults free of CVD, each 10 g/m2 increment in LV mass index was associated with 33% increased risk of CVD and each 0.1 unit in LV RWT was associated with 59% increased risk of CVD.26
Further, this is the first known study to report de novo HDP history to be associated with decrements in LV systolic function. The finding of slightly lower LV EF despite greater concentric remodeling and higher stroke volume suggests a type of contractile inefficiency that is not typically seen in the setting of chronic hypertension alone, suggesting a pathophysiology distinct form of hypertensive heart disease. These findings and the LV diastolic dysfunction exhibited by these mothers all suggest a preheart failure with preserved EF remodeling phenotype, supported by the excess risk of heart failure with preserved EF in women with preeclampsia, that warrants further investigation.27,28
Hypertension results in chronic central pressure overload and myocardial ischemia that leads to the development of LVH and heart failure. Therefore, we evaluated the extent to which current hypertension mediated the association between history of de novo HDP and measures of cardiac structure and function. Hypertension is associated with significantly higher rates of concentric LVH and is a strong independent predictor of LV diastolic dysfunction, but the association with eccentric LVH and LV systolic dysfunction is less robust.29,30 This helps explain why current hypertension was a moderate mediator in the association between HDP and concentric LVH and LV diastolic dysfunction but a weaker mediator of LV EF (a measure of LV systolic function). Our results are consistent with large epidemiological data indicating that the association of HDP with later-life CVD is only partially mediated by current hypertension.4 These findings provide insights into HDP as a potential novel mechanism to explain the disproportionally higher risk of heart failure in women with a history of HDP.31,32
The mechanisms by which HDP may lead to abnormalities in cardiac structure and function beyond the effects of current hypertension remain to be elucidated.33 Diabetes was found to be higher in Hispanic/Latina women with history of de novo HDP and although we included diabetes in our adjusted models it may have contributed to some of our findings. In fact, glucose intolerance, insulin resistance, and diabetes have been shown to be associated with increased LV mass and wall thickness and reduced end-diastolic volume, stroke volume, and EF.34,35 Activation of pathways involving the antiangiogenic soluble fms-like tyrosine kinase 1 in preeclampsia can contribute to endothelial dysfunction and deranged lipid metabolism that can persist after delivery.36,37 Dysregulations of the renin-angiotensin system may also contribute to persistent postpartum cardiac abnormalities. Additionally, shared upstream factors such as cardiovascular risk factors and genetics may predispose women to both HDPs and later in life pathological LV remodeling and function. Further investigations are needed to identify the potential mechanisms contributing to cardiac abnormalities in women with history of HDP and how these may be different by race and ethnicity.
Study Limitations
Several limitations of our study merit consideration. The cross-sectional design of our study precludes inference of causality, although timing of reported prior HDP consistently preceded timing of assessed cardiac traits. Prior HDP status was based on self-report, which is subject to recall bias and limits precision with respect to subtypes of HDP; nonetheless, self-report has been evaluated as valid and thus applied in the vast majority of cohort studies on HDP.38,39 Data are not available on severity of preeclampsia, number of pregnancies complicated by HDP, or the exact timing of diagnosis of de novo HDP, or current hypertension. Therefore, we are unable to account for the time period between HDP pregnancy and development of hypertension, or performance of TTE at visit 2, or the length of time with hypertension diagnosis, which limits our understanding of how these important factors affect cardiac structure and function. Additionally, multiple echocardiographic measures were analyzed, which can lead to heightened type 1 error rate. Despite these limitations, our study offered several strengths including analyses from the largest study to date investigating the relations of prior HDP with cardiac traits in Hispanic/Latina women who represent a demographically important yet historically understudied population. In addition, all cardiac traits were assessed from echocardiographic protocols that involved standardized image acquisition and centralized measures performed at a core center with high interreader and intrareader reproducibility and BP was measured in a standardized method.
Conclusions
In a large cohort of previously pregnant Hispanic/Latina women, those with history of de novo HDP had detectable and measurable pathological alterations in cardiac structure and systolic and diastolic dysfunction. Our findings suggest that women with prior HDP have pathophysiologic cardiac sequelae decades later, not fully explained by hypertension postpartum, that likely play a role in modulating long-term cardiovascular risk. Notwithstanding the need for further investigations into the mechanisms driving HDP pathophysiology, our findings highlight the potential importance of targeted surveillance and interventions aimed at preventing later-life cardiovascular events in these at-risk understudied women.
Perspectives
Hispanic/Latina women are disproportionally affected by HDP, yet very little is known on how HDP affects cardiac structure and function in this growing population in the United States. In this study of a diverse cohort of Hispanic/Latina women, those with history of de novo HDP had higher rates of abnormal LV geometry, and alterations in cardiac structure (LV mass index, RWT) and function (LVEF, diastolic dysfunction), known predictors of cardiovascular events and mortality. Notably, these findings appear to be in part mediated by current hypertension—underscoring the importance of screening for and managing hypertension. Hypertension alone did not account for all the associations between history of de novo HDP and morphological and functional cardiac alterations. With the higher rates of heart failure, particularly heart failure with preserved EF, in women and the alterations in cardiac structure and function identified in this study; we hypothesize HDP may be a sex-specific risk factor for heart failure that warrants further investigation.
ARTICLE INFORMATION
Acknowledgments
The authors thank the participants and staff of HCHS/SOL (Hispanic Community Health Study/Study of Latinos) for their important contributions to this research. Also thank Christian Schmidt and Danielle Tapp for their work on the article. The views expressed in this article are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the US Department of Health and Human Services.
Sources of Funding
The HCHS/SOL (Hispanic Community Health Study/Study of Latinos) is a collaborative study supported by National Heart, Lung, and Blood Institute (NHLBI) contracts N01-HC65233, N01-HC65234, N01-HC65235, N01-HC65236, and N01-HC65237. The following institutes, centers, or offices contribute to the HCHS/SOL through a transfer of funds to the NHLBI: National Center on Minority Health and Health Disparities, the National Institute on Deafness and Other Communications Disorders, the National Institute of Dental and Craniofacial Research, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Neurological Disorders and Stroke, and the Office of Dietary Supplements. The authors report the following additional sources of funding: National Institutes of Health (NIH) K23-HL136853, NIH K23-HL151867, Erika J. Glazer Women’s Heart Research Initiative, and the Barbra Streisand Women’s Cardiovascular Research and Education Program.
Disclosures
None.
Supplementary Material
Nonstandard Abbreviations and Acronyms
- BP
- blood pressure
- CVD
- cardiovascular disease
- EF
- ejection fraction
- HCHS/SOL
- Hispanic Community Health Study/Study of Latinos
- HDL
- high-density lipoprotein
- HDP
- hypertensive disorders of pregnancy
- LV
- left ventricle
- LVH
- left ventricular hypertrophy
- RWT
- relative wall thickness
- TTE
- transthoracic echocardiography
For Sources of Funding and Disclosures, see page 262.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/HYPERTENSIONAHA.123.21248.
REFERENCES
- 1.Freaney PM, Harrington K, Molsberry R, Perak AM, Wang MC, Grobman W, Greenland P, Allen NB, Capewell S, O’Flaherty M, et al. Temporal trends in adverse pregnancy outcomes in birthing individuals aged 15 to 44 years in the United States, 2007 to 2019. J Am Heart Assoc. 2022;11:e025050. doi: 10.1161/JAHA.121.025050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shah NS, Harrington KA, Huang X, Cameron NA, Yee LM, Khan SS. Trends in De Novo hypertensive disorders of pregnancy among Asian and Hispanic population subgroups in the United States, 2011 to 2019. JAMA Cardiol. 2022;7:742–746. doi: 10.1001/jamacardio.2022.1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garovic VD, White WM, Vaughan L, Saiki M, Parashuram S, Garcia-Valencia O, Weissgerber TL, Milic N, Weaver A, Mielke MM. Incidence and long-term outcomes of hypertensive disorders of pregnancy. J Am Coll Cardiol. 2020;75:2323–2334. doi: 10.1016/j.jacc.2020.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Honigberg MC, Zekavat SM, Aragam K, Klarin D, Bhatt DL, Scott NS, Peloso GM, Natarajan P. Long-term cardiovascular risk in women with hypertension during pregnancy. J Am Coll Cardiol. 2019;74:2743–2754. doi: 10.1016/j.jacc.2019.09.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation. 2019;140:e596–e646. doi: 10.1161/CIR.0000000000000678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castleman JS, Ganapathy R, Taki F, Lip GY, Steeds RP, Kotecha D. Echocardiographic structure and function in hypertensive disorders of pregnancy: a systematic review. Circ Cardiovasc Imaging. 2016;9:e004888. doi: 10.1161/CIRCIMAGING.116.004888 [DOI] [PubMed] [Google Scholar]
- 7.Melchiorre K, Sutherland GR, Baltabaeva A, Liberati M, Thilaganathan B. Maternal cardiac dysfunction and remodeling in women with preeclampsia at term. Hypertension. 2011;57:85–93. doi: 10.1161/HYPERTENSIONAHA.110.162321 [DOI] [PubMed] [Google Scholar]
- 8.Vaught AJ, Kovell LC, Szymanski LM, Mayer SA, Seifert SM, Vaidya D, Murphy JD, Argani C, O’Kelly A, York S, et al. Acute cardiac effects of severe pre-eclampsia. J Am Coll Cardiol. 2018;72:1–11. doi: 10.1016/j.jacc.2018.04.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scantlebury DC, Kane GC, Wiste HJ, Bailey KR, Turner ST, Arnett DK, Devereux RB, Mosley TH, Jr, Hunt SC, Weder AB, et al. Left ventricular hypertrophy after hypertensive pregnancy disorders. Heart. 2015;101:1584–1590. doi: 10.1136/heartjnl-2015-308098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levine LD, Ky B, Chirinos JA, Koshinksi J, Arany Z, Riis V, Elovitz MA, Koelper N, Lewey J. Prospective evaluation of cardiovascular risk 10 years after a hypertensive disorder of pregnancy. J Am Coll Cardiol. 2022;79:2401–2411. doi: 10.1016/j.jacc.2022.03.383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Countouris ME, Villanueva FS, Berlacher KL, Cavalcante JL, Parks WT, Catov JM. Association of hypertensive disorders of pregnancy with left ventricular remodeling later in life. J Am Coll Cardiol. 2021;77:1057–1068. doi: 10.1016/j.jacc.2020.12.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Behrens I, Basit S, Melbye M, Lykke JA, Wohlfahrt J, Bundgaard H, Thilaganathan B, Boyd HA. Risk of post-pregnancy hypertension in women with a history of hypertensive disorders of pregnancy: nationwide cohort study. BMJ. 2017;358:j3078. doi: 10.1136/bmj.j3078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Podymow T, August P. Postpartum course of gestational hypertension and preeclampsia. Hypertens Pregnancy. 2010;29:294–300. doi: 10.3109/10641950902777747 [DOI] [PubMed] [Google Scholar]
- 14.Conomos MP, Laurie CA, Stilp AM, Gogarten SM, McHugh CP, Nelson SC, Sofer T, Fernandez-Rhodes L, Justice AE, Graff M, et al. Genetic diversity and association studies in us Hispanic/Latino populations: applications in the Hispanic community health study/study of Latinos. Am J Hum Genet. 2016;98:165–184. doi: 10.1016/j.ajhg.2015.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lavange LM, Kalsbeek WD, Sorlie PD, Aviles-Santa LM, Kaplan RC, Barnhart J, Liu K, Giachello A, Lee DJ, Ryan J, et al. Sample design and cohort selection in the Hispanic community health study/study of Latinos. Ann Epidemiol. 2010;20:642–649. doi: 10.1016/j.annepidem.2010.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sorlie PD, Aviles-Santa LM, Wassertheil-Smoller S, Kaplan RC, Daviglus ML, Giachello AL, Schneiderman N, Raij L, Talavera G, Allison M, et al. Design and implementation of the Hispanic community health study/study of Latinos. Ann Epidemiol. 2010;20:629–641. doi: 10.1016/j.annepidem.2010.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehta H, Armstrong A, Swett K, Shah SJ, Allison MA, Hurwitz B, Bangdiwala S, Dadhania R, Kitzman DW, Arguelles W, et al. Burden of systolic and diastolic left ventricular dysfunction among Hispanics in the United States: insights from the echocardiographic study of Latinos. Circ Heart Fail. 2016;9:e002733. doi: 10.1161/CIRCHEARTFAILURE.115.002733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah AM, Cheng S, Skali H, Wu J, Mangion JR, Kitzman D, Matsushita K, Konety S, Butler KR, Fox ER, et al. Rationale and design of a multicenter echocardiographic study to assess the relationship between cardiac structure and function and heart failure risk in a biracial cohort of community-dwelling elderly persons: the atherosclerosis risk in communities study. Circ Cardiovasc Imaging. 2014;7:173–181. doi: 10.1161/CIRCIMAGING.113.000736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the american society of echocardiography and the European association of cardiovascular imaging. European heart journal cardiovascular Imaging. 2015;16:233–270. doi: 10.1093/ehjci/jev014 [DOI] [PubMed] [Google Scholar]
- 20.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011 [DOI] [PubMed] [Google Scholar]
- 21.Ford ND, Cox S, Ko JY, Ouyang L, Romero L, Colarusso T, Ferre CD, Kroelinger CD, Hayes DK, Barfield WD. Hypertensive disorders in pregnancy and mortality at delivery hospitalization - United States, 2017-2019. MMWR Morb Mortal Wkly Rep. 2022;71:585–591. doi: 10.15585/mmwr.mm7117a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boardman H, Lamata P, Lazdam M, Verburg A, Siepmann T, Upton R, Bilderbeck A, Dore R, Smedley C, Kenworthy Y, et al. Variations in cardiovascular structure, function, and geometry in midlife associated with a history of hypertensive pregnancy. Hypertension. 2020;75:1542–1550. doi: 10.1161/HYPERTENSIONAHA.119.14530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bokslag A, Franssen C, Alma LJ, Kovacevic I, Kesteren FV, Teunissen PW, Kamp O, Ganzevoort W, Hordijk PL, Groot CJM, et al. Early-onset preeclampsia predisposes to preclinical diastolic left ventricular dysfunction in the fifth decade of life: An observational study. PLoS One. 2018;13:e0198908. doi: 10.1371/journal.pone.0198908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bluemke DA, Kronmal RA, Lima JA, Liu K, Olson J, Burke GL, Folsom AR. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52:2148–2155. doi: 10.1016/j.jacc.2008.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kane GC, Karon BL, Mahoney DW, Redfield MM, Roger VL, Burnett JC, Jr, Jacobsen SJ, Rodeheffer RJ. Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA. 2011;306:856–863. doi: 10.1001/jama.2011.1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsao CW, Gona PN, Salton CJ, Chuang ML, Levy D, Manning WJ, O’Donnell CJ. Left ventricular structure and risk of cardiovascular events: a Framingham heart study cardiac magnetic resonance study. J Am Heart Assoc. 2015;4:e002188. doi: 10.1161/JAHA.115.002188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams D, Stout MJ, Rosenbloom JI, Olsen MA, Joynt Maddox KE, Deych E, Davila-Roman VG, Lindley KJ. Preeclampsia predicts risk of hospitalization for heart failure with preserved ejection fraction. J Am Coll Cardiol. 2021;78:2281–2290. doi: 10.1016/j.jacc.2021.09.1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hansen AL, Sondergaard MM, Hlatky MA, Vittinghof E, Nah G, Stefanick ML, Manson JE, Farland LV, Wells GL, Mongraw-Chaffin M, et al. Adverse pregnancy outcomes and incident heart failure in the women’s health initiative. JAMA Netw Open. 2021;4:e2138071. doi: 10.1001/jamanetworkopen.2021.38071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yun M, Li S, Yan Y, Sun D, Guo Y, Fernandez C, Bazzano L, He J, Zhang T, Chen W. Blood pressure and left ventricular geometric changes: a directionality analysis. Hypertension. 2021;78:1259–1266. doi: 10.1161/HYPERTENSIONAHA.121.18035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verdecchia P, Angeli F, Gattobigio R, Sardone M, Porcellati C. Asymptomatic left ventricular systolic dysfunction in essential hypertension: prevalence, determinants, and prognostic value. Hypertension. 2005;45:412–418. doi: 10.1161/01.HYP.0000154822.37141.f6 [DOI] [PubMed] [Google Scholar]
- 31.Behrens I, Basit S, Lykke JA, Ranthe MF, Wohlfahrt J, Bundgaard H, Melbye M, Boyd HA. Association between hypertensive disorders of pregnancy and later risk of cardiomyopathy. JAMA. 2016;315:1026–1033. doi: 10.1001/jama.2016.1869 [DOI] [PubMed] [Google Scholar]
- 32.Honigberg MC, Riise HKR, Daltveit AK, Tell GS, Sulo G, Igland J, Klungsoyr K, Scott NS, Wood MJ, Natarajan P, et al. Heart failure in women with hypertensive disorders of pregnancy: insights from the cardiovascular disease in Norway project. Hypertension. 2020;76:1506–1513. doi: 10.1161/HYPERTENSIONAHA.120.15654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rana S, Lemoine E, Granger JP, Karumanchi SA. Preeclampsia: pathophysiology, challenges, and perspectives. Circ Res. 2019;124:1094–1112. doi: 10.1161/CIRCRESAHA.118.313276 [DOI] [PubMed] [Google Scholar]
- 34.Kishi S, Gidding SS, Reis JP, Colangelo LA, Venkatesh BA, Armstrong AC, Isogawa A, Lewis CE, Wu C, Jacobs DR, Jr, et al. Association of insulin resistance and glycemic metabolic abnormalities with LV structure and function in middle age: the CARDIA study. JACC Cardiovasc Imaging. 2017;10:105–114. doi: 10.1016/j.jcmg.2016.02.033 [DOI] [PubMed] [Google Scholar]
- 35.Rutter MK, Parise H, Benjamin EJ, Levy D, Larson MG, Meigs JB, Nesto RW, Wilson PW, Vasan RS. Impact of glucose intolerance and insulin resistance on cardiac structure and function: sex-related differences in the Framingham heart study. Circulation. 2003;107:448–454. doi: 10.1161/01.cir.0000045671.62860.98 [DOI] [PubMed] [Google Scholar]
- 36.Germain AM, Romanik MC, Guerra I, Solari S, Reyes MS, Johnson RJ, Price K, Karumanchi SA, Valdes G. Endothelial dysfunction: a link among preeclampsia, recurrent pregnancy loss, and future cardiovascular events? Hypertension. 2007;49:90–95. doi: 10.1161/01.HYP.0000251522.18094.d4 [DOI] [PubMed] [Google Scholar]
- 37.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stuart JJ, Bairey Merz CN, Berga SL, Miller VM, Ouyang P, Shufelt CL, Steiner M, Wenger NK, Rich-Edwards JW. Maternal recall of hypertensive disorders in pregnancy: a systematic review. J Womens Health (Larchmt). 2013;22:37–47. doi: 10.1089/jwh.2012.3740 [DOI] [PubMed] [Google Scholar]
- 39.Carter EB, Stuart JJ, Farland LV, Rich-Edwards JW, Zera CA, McElrath TF, Seely EW. Pregnancy complications as markers for subsequent maternal cardiovascular disease: validation of a maternal recall questionnaire. J Womens Health (Larchmt). 2015;24:702–712. doi: 10.1089/jwh.2014.4953 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.