Abstract
Background:
Liver transplantation (LT) is the gold standard for end-stage liver disease, yet postoperative complications challenge patients and physicians. Indocyanine green (ICG) clearance, a quantitative dynamic test of liver function, is a rapid, reproducible, and reliable test of liver function. This study aimed to systematically review and summarize current literature analyzing the association between ICG tests and post-LT outcomes.
Methods:
This systematic review was conducted according to PRISMA guidelines. MEDLINE and Cochrane Library, as main databases, and other sources were searched until August 2022 to identify articles reporting the prognostic value of postoperative ICG tests associated with outcomes of adult LT recipients.
Risk of bias of included articles was assessed using Quality In Prognosis Studies tool. Methodological quality varied from low to high across risk of bias domains.
Results:
Six studies conducted between 1994 and 2018 in Europe, America, and Asia were included. The study population ranged from 50 to 332 participants. ICG clearance on the first postoperative day was associated with early allograft dysfunction, graft loss, 1-month and 3-month patient survival probability, prolonged ICU, and hospital stay. The dichotomized ICG plasma disappearance rate (PDR) provided a strong association with medium-term and long-term outcomes: PDR less than 10%/min with 1-month mortality or re-transplantation (odds ratio: 7.89, 95% CI 3.59–17.34, P<0.001) and PDR less than 16.0%/min with 3-month patient survival probability (hazard ratio: 13.90, 95% CI 4.67–41.35, P<0.01). The preoperative model for end-stage liver disease and body mass index were independent prognostic factors for early allograft dysfunction, early complications, and prolonged ICU stay; post-LT prothrombin time and INR were independently associated with graft loss and bilirubin with a prolonged hospital stay.
Conclusion:
This review shows that ICG clearance tests are associated with graft function recovery, suggesting that a potential prognostic role of ICG test, as an aid in predicting the post-LT course, could be considered.
Keywords: evidence in transplantation, graft dysfunction, ICG, liver function, liver transplant prognosis
Introduction
Highlights
Only six studies explored the prognostic value of indocyanine green tests after liver transplantation.
ICG tests correlate with graft function recovery.
Dichotomizing plasma disappearance rate stratifies the risk of postoperative events.
Liver transplantation (LT) is the standard therapy for curing patients with end-stage liver disease1. To date, patient survival rates 1 year and 5 years after transplantation have been achieved at over 90% and slightly above 80%, respectively2. Despite the ongoing improvements, LT still has a non-negligible rate of postoperative complications3. LT-related outcomes depend on the preoperative clinical condition of the patient, the graft quality, and the surgical intervention1,4. Identifying indices able to predict future events in transplant recipients could contribute to a timely recognition of the patient critical condition, guiding early postoperative management and clinical decision-making. Monitoring of post-transplant graft function recovery is mainly based on blood tests and clinical assessment5. Beyond static tests, which supply indirect measures of hepatic function, and clinical signs, that often are not sufficient in prediction of post-LT outcomes6, dynamic tests (ICG, LiMAX, MEGX, etc.) directly quantify hepatic function providing an estimation of liver activity together with a general prognostic assessment7,8.
Indocyanine green (ICG) clearance has aroused particular interest in recent decades as a quantitative dynamic test of liver function5,6,9. Indocyanine green, a water-soluble and inert compound, is a carbocyanine molecule with amphiphilic properties. ICG is taken up exclusively by hepatocytes and is secreted unchanged into the bile without entering the enterohepatic recirculation. ICG clearance depends on hepato-splanchnic blood flow, functional hepatocytes, and bile secretion, thus reflecting liver function reserve and hepatic blood flow9. Various techniques are used to evaluate liver function in relation to plasma levels of ICG. The analysis of ICG concentration can be performed by blood sampling using serum spectrophotometry or an optical transcutaneous sensor using pulse dye densitometric technology10. ICG clearance is reported as ICG plasma disappearance rate (ICG-PDR) per min (%/min), as retention rate (%) at fifteen minutes (ICG-R15), or as ICG clearance constant (KICG), calculated between 5 and 15 min after ICG injection (min−1)6,9,11.
Several studies reported the ability of ICG clearance to predict patient outcomes in mainly two fields: critically ill patients with or without liver failure and in hepatic surgery (resection and transplantation)11–13.
ICG has been investigated in terms of prognostic value in the setting of liver transplantation. However, only few studies have been published so far14–19. Uncertainty about the utility of ICG measurement therefore motivated this systematic review. The study aimed to synthesize evidence on the association between ICG clearance tests and specific post-LT outcomes and provide evidence to base future prospective studies.
Methods
This systematic review was conducted and reported according to the PRISMA 2020, Supplemental Digital Content 1, http://links.lww.com/JS9/B143, Supplemental Digital Content 2, http://links.lww.com/JS9/B144 (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) Statement and its extension, PRISMA 2020 Explanation and Elaboration (E&E), and under the guidelines for systematic reviews and meta-analyses for prognostic factor studies20–22. A self-evaluation of the systematic review was performed using AMSTAR-2 (Assessing the Methodological quality of Systematic reviews) criteria23.
Two authors independently used the AMSTAR-2 online checklist, Supplemental Digital Content 3, http://links.lww.com/JS9/B145, and any disagreements were resolved with discussion together with a third author. We answered the seven critical domains and the nine non-critical ones with “yes”, “no” or “partial yes”. A “weak” label was provided if the answer was “no” to a specific domain. Subsequently, we rated the SR as high-quality, moderate-quality, low-quality, or critically low-quality based on these 16 domains23.
The systematic review protocol was registered with the PROSPERO international prospective register of systematic reviews [The systematic review protocol was registered with the PROSPERO international prospective register of systematic reviews, available at: (CRD42020222261], available at: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020222261
Search strategy and eligibility criteria for studies
Objective
The main goal of this review was to systematically analyze and summarize evidence relating to the prognostic value of ICG tests for predicting fatal and non-fatal events related to LT in transplant patients.
Review question: Does Indocyanine green test predict post-LT outcomes in patients who underwent liver transplantation?
Search strategy
Medline (PubMed) and Cochrane Library databases were searched through August 2022 for relevant published original articles using the following keywords: “liver transplantation” AND “Indocyanine Green”. In addition, we consulted other sources such as indexed databases and repositories (Ovid, Google scholar, DOAJ). Google scholar was consulted for the first 10 pages for relevance to the topic. At least, we also searched the reference lists of included studies. Two authors independently reviewed the found records based on titles, abstracts, and the full text against the eligibility criteria (Fig. 1). Any conflict regarding study selection was resolved by consensus or by a third reviewer.
Figure 1.
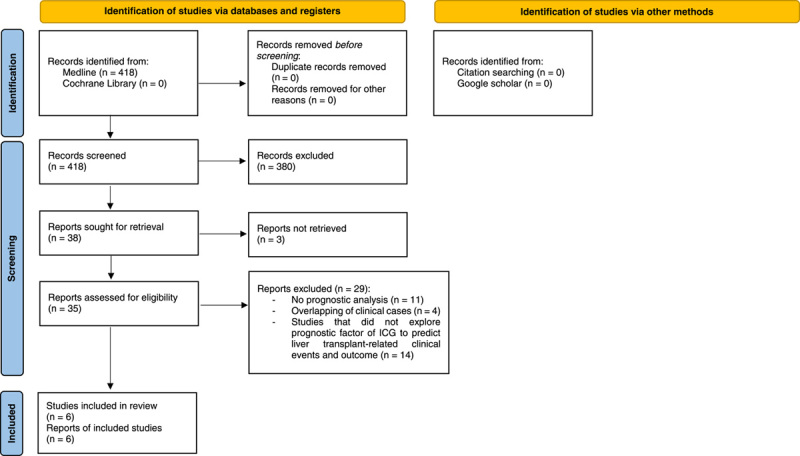
Preferred Reporting Items for Systematic Reviews and Meta-Analyses. (PRISMA) 2020 flow diagram. Selection of studies to review the prognostic role of indocyanine green in liver transplantation22. ICG, indocyanine green.
MEDLINE (PubMed) search strategy:
#1 “liver transplantation” [MeSH Terms] OR “liver transplant*“ [Title/Abstract] OR “Grafting, Liver” [Title/Abstract] OR “Liver Grafting” OR “Transplantation, Liver” [Title/Abstract] OR “Hepatic Transplant*“ [Title/Abstract]” OR “Transplantation, Hepatic” [Title/Abstract]” OR “Transplant, Liver” [Title/Abstract]
#2 “Indocyanine Green” [MeSH Terms] OR “Indocyanine Green” [Title/Abstract] OR “Green, Indocyanine” [Title/Abstract] OR “Wofaverdin” [Title/Abstract] OR “Vophaverdin” [Title/Abstract] OR “Vofaverdin” [Title/Abstract]
#3 #1 AND #2”
Eligibility criteria
This review focused on retrospective and prospective observational studies, which evaluate liver transplant outcomes concerning ICG measurement taken after LT in adults over 18 years of age. Studies were included if they investigated the prognostic value of ICG relating to any event following LT. Case series, case reports, literature reviews, or studies without adequate prognostic analyses were excluded. Studies were selected based on the PICOTS framework (Supplementary File 1, Supplemental Digital Content 4, http://links.lww.com/JS9/B146). No geographic, linguistic, year of publication or follow-up restrictions were applied. Where a study featured multiple eligible articles, we chose the most recent paper with the most significant number of participants and the most extended duration of follow-up for post-LT recorded outcomes. Any non-English study that are identified were translated by a native speaker translation service.
Data extraction
Two independent reviewers identified and collected data using the modified CHARMS-PF checklist13. Information extracted included: author name, country and study period, study design, number of patients at baseline in the analysis, sample demographics, the frequency of primary disease, preoperative indices [Model for End-stage Liver Disease (MELD) score), surgical procedure, ICG dose administered, ICG timing, type of ICG assay and ICG measurement, type of outcome events, and study follow-up.
Risk of bias assessment
To assess the risk of bias in the included studies, two reviewers evaluated articles using Quality In Prognosis Studies (QUIPS) tool and figured out the QUIPS assessment results with the Robvis tool24. The validity of studies was judged for six potential bias domains: (1) study participation, (2) study attrition, (3) prognostic factor measurement, (4) outcome measurement, (5) study confounding, and (6) statistical analysis and reporting25. We considered information regarding the baseline characteristics of study participants, reasons for loss to follow-up or whether this might have influenced differences in reported associations between prognostic factor and outcome, methods for measuring PF and outcome, the definition of potential predefined confounders and their adjustment in analyses, methods for analyzing and reporting results. For each domain, the sub-item reporting prompts help guide and inform the judgment of potential bias. The adequacy of reporting by a study was rated as yes, partial, or no. Items not relevant to the review question were not considered. The overall risk of bias for each domain was rated using a three-grade scale (low, moderate, or high quality). A low risk of bias was assigned if the majority (≥75%) were satisfied, a moderate risk of bias was assigned when 50–74% coverage of items was achieved, and a high risk of bias if less than 50% of items were met. Two authors assessed included studies, and the inter-rater agreement using the weighted Kappa and percent agreement was evaluated. Any discrepancies were resolved by consensus or by a third reviewer. Information supporting studies assessment was directly taken from the primary study and recorded in the QUIPS spreadsheet, as provided by Hayden et al.26 (Supplementary File 2, Supplemental Digital Content 5, http://links.lww.com/JS9/B147).
Analysis
We developed a qualitative summary of the evidence from the included studies on the prognostic value of ICG for predicting patient outcomes after liver transplantation. The narrative description of the results is enriched by tables and figures27. We organized ICG test results based on the timing of post-transplant outcome measurement diagnosed using standard procedures. There was high heterogeneity among the selected studies regarding patient sampling, the timing of ICG measurement, outcome assessment, follow-up durations, methods of analysis, and reporting of results. Therefore, conducting a meta-analysis was inappropriate.
Results
Study selection process and study characteristics
The study selection process is summarized through the PRISMA flow diagram22 (Fig. 1). The search strategy identified 820 records and no records from reference lists. After the deduplication process, 499 records were screened based on the selection by title/abstract. Four hundred and sixty-one records were excluded because they were irrelevant to the review question or did not adhere to the inclusion criteria. Between 1988 and 1992, three reports were not recoverable. Of the remaining eligible records (n=35), 29 full-text articles were discarded for several reasons (Fig. 1). In detail, the reasons for discard were: lack of multivariate prognostic analyses such as linear or logistic regression (n=11), studies carried out on the same population or from the same centre with overlapping of the study period (n=4), studies did not explore prognostic factor of ICG to predict liver transplant-related clinical events and outcome in adults (n=14). The list of these studies with the relative reasons for exclusion is itemized in the table of Supplementary File 3, Supplemental Digital Content 6, http://links.lww.com/JS9/B148.
From the selection process, the pool of studies for qualitative synthesis was reduced to 628–33. Key characteristics of the included studies are illustrated in Table 1. Four of the six single-centre observational studies were prospective, and two were retrospective cohort studies. Only two studies tested the predictive ability of the prognostic factor in an internal validation cohort29,30. The recruitment method of participants was not always reported. Only Klinzing S. and colleagues specified that they enroled consecutive patients in their study31.
Table 1.
Characteristics of studies.
| Study | Country and study period | Design and follow-up | Study population and sampling frame | Sample demographics | Underlying disease (%) | Preoperative indices | Type of surgical approach | ICG dose administered | ICG setting/timing | IGT assay type | Outcomes reported |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cherchi et al.28 | Italy January 2010–June 2018 | Retrospective cohort 5 years | N=77 | 54 M, 23 F 57 (53–58) years |
HBV/HCV-related HCC (37.7) Alcoholic cirrhosis (27.3) Other (35.0) |
MELD score: 16.57±6.80 | Whole LT from DBD | 0.5 mg/kg | POD1 | ICG-PDR by non-invasive pulse-densitometric method | EAD, patient survival probability, overall graft-survival |
| Yunhua et al. 29 | China January 2015–February 2016 | Prospective cohort 1 year | N=61 and internal validation cohort (n=26); consecutive patients | 50 M, 11 F 47.3±8.1 years |
HBV + HCC + cirrhosis (41.0) Other (59.0) |
MELD score: 18.3±11.4 | Whole LT from DBD (80.4) and DCD (19.6) | 0.5 mg/kg | 12 h post-LT | ICG-R15 by non-invasive pulse-densitometric method | EAD and early post-LT complications |
| Olmedilla et al.30 | Spain February 2002–February 2012 | Prospective cohort 1 month | N=332 and internal validation cohort (n=77) | 252 M, 80 F 51±9 years |
Alcoholic cirrhosis (20.5) HCV + HCC (13.6) Other (65.9) |
MELD score: 15.8±6 | Whole LT from DBD | 0.5 mg/kg | POD1 | ICG-PDR by non-invasive pulse-densitometric method | Mortality or Re-LT |
| Klinzing et al.31 | Switzerland September 2007–June 2009 | Retrospective cohort 3 months | N=50; consecutive patients | 37 M, 13 F 51.3±11.1 years |
HCV (34) HCC (20) Others (46) |
MELD score: 21±10.4 (6–40) | Whole LT from DD (88) - LDLT (12) | 0.25 mg/kg | Within 6 h after admission to the ICU | ICG-PDR by non-invasive pulse-densitometric method | ICU stay, hospital stay and Hospital Mortality |
| Lock et al.32 | Germany August 2005–May 2007 | Prospective cohort 3 months | N=99 | 66 M, 33 F 55 (27–69) years |
Alcoholic cirrhosis (32.3) HCV (28.3) Other (39.4) |
NA | Whole LT from DD | 0.5 mg/kg | Directly after LT (POD 0), PODs 1 and 3 | ICG-PDR by non-invasive pulse-densitometric method | IDF |
| Tsubono et al.33 | USA May 1994–October 1994 | Prospective cohort 3 months | N=50 | 26 M, 21 F 53 (31–73) years |
NA | NA | Whole LT from DD | 0.5 mg/kg | PODs 1, 3, and 7 | SM: ICG elimination rate constant (KICG) by serial blood-sampling (KICG-B) and by non-invasive pulse-densitometric (KICG-F) methods | Graft losses, prolonged postoperative liver dysfunction |
DBD, donation after brain death; DCD, donation after cardiac death; EAD, early graft dysfunction; F, females; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; ICG-PDR, indocyanine green plasma disappearance rate; ICG-R15, ICG retention rate at 15 min; IDF, initial graft dysfunction; KICG-B, measurement by spectrophotometric method; KICG-F, measurement by finger-piece method; LDLT, living-donor liver transplantation; LT, liver transplantation; M, males; MELD, Model for End-stage Liver Disease; NA, not applicable; POD, postoperative day; SM, simultaneous measurement.
Studies were conducted between 1994 and 2018 in six countries: the USA (n=1), Germany (n=1), Switzerland (n=1), Spain (n=1), China (n=1), and Italy (n=1). The study population ranged from 5031,33 to 33230 participants. The median patient age range was 27–73 years, and 72.5% were male. The leading cause of LT was alcoholic cirrhosis in Spain (n=68/332, 20.5%)30 and Germany (n=32/99, 32.3%)32 study populations, while diseases related to viral infections showed a greater incidence in Italy (HCV cirrhosis: n=28/77, 36.4%)28, China (HCC caused by HBV-related cirrhosis: n=25/61, 41.0%)29 and Switzerland (HCV cirrhosis: n=17/50, 34.0%)31. The prognostic value of ICG was evaluated in studies as a predictor of LT-related outcomes or endpoints in the early (Table 2), medium (Table 3), and long-term (Table 4) postoperative period. The duration of study follow-up ranged from 1 month to 5 years. Measurements of ICG were performed by non-invasive pulse-densitometric (PDR) (n=5) or both PDR and serial blood-sampling method (n=1), following intravenous injection of a 0.25 mg/kg (n=1) or 0.5 mg/kg (n=5) dose of indocyanine green, in the postoperative period between 6 h and 7 days. ICG concentration was calculated as RR1529 or PDR28,30–32, whereas Tsubono et al.33 presented ICG results as elimination rate constant (KICG).
Table 2.
ICG and short-term (≤ 7 d) outcomes in liver transplants.
| Study | Study variable(s) | Short-term outcome measure (n/N) | Prognostic value analysis test | Prognostic value for short-term outcome |
|---|---|---|---|---|
| Cherchi et al.28 | Postoperative. ICG-PDR ≥16%/min on POD1 | EAD (18/77) | Univariate logistic regression model | EAD: ICG-PDR OR 0.88 (0.79–0.97), P <0.01. |
| Yunhua et al.29 | Preoperative. MELD score Postoperative. ICG-R15 on 12 h post-LT |
EAD (25/61) | Logistic regression | EAD: MELD score (β=0.128, P=0.013), ICG-R15 (β=0.117, P=0.002). |
| Lock et al.32 | Postoperative. ICG-PDR (%/min), AST (U/L), ALT (U/L), GLDH (U/L), Bilirubin (mg/dl), Ammonia (μmol/l), Albumin (g/dl), INR, Factor II (%), Factor VII (%) on POD 1 and 3 | IDF (8a/99) | Multivariate forward and backward logistic regression analysis | IDF: ICG-PDR NS. |
| Tsubono et al.33 | Postoperative. KICG-B, KICG-F, total bilirubin, highest PT. | Graft loss (12a/50) | Univariate and forward stepwise multivariate analysis | Univariate analysis. Hepatic graft loss: KICG-B on POD1,3,7, KICG-F on POD1,3,7, total bilirubin on POD 3,7, highest PT. P <0.01 multivariate analysis. Hepatic graft loss: KICG-B on POD1, P=0.0003; highest PT, P=0.0815 |
8: 3 cases of re-transplantation on postoperative days (PODs) 4–6 due to primary non-function (PNF), 5 cases of surgical reintervention on postoperative day (POD) 1 due to severe anastomotic bleeding, Hepatic artery thrombosis (HAT), or portal vein thrombosis.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; EAD, early graft dysfunction; GLDH, glutamate dehydrogenase; ICG-PDR, indocyanine green plasma disappearance rate; ICG-R15, ICG retention rate at 15 minutes; IDF, initial graft dysfunction; INR, international normalized ratio; KICG-B, measurement by spectrophotometric method; KICG-F, measurement by finger-piece method; MELD, Model for End-Stage Liver Disease; NS, Not Statistically Significant; OR, odd ratio; POD, postoperative day; PT, prothrombin time.
12: 7 cases of re-transplantation or death due to hepatic causes and 5 cases of re-transplantation or death for technical or extrahepatic reasons.
Table 3.
ICG and medium-term (≤3 mo) outcomes in liver transplants.
| Study | Study variable(s) | Medium-term outcome measure (n/N) | Prognostic value analysis test | Prognostic value for medium-term outcome |
|---|---|---|---|---|
| Cherchi et al.28 | Postoperative. ICG-PDR <16.0%/min on POD1 | 3-months patient survival probability | Univariate survival analysis | 3-month patient survival probability: ICG-PDR HR 13.90 (4.67–41.35), P <0.01. |
| Yunhua et al.29 | Preoperative. MELD score Postoperative. ICG-R15 on 12 h post-LT |
Early post-LT complications (11a/61) | Logistic regression | Early post-LT complications: MELD (β=0.081, P=0.097), ICG-R15 (β=0.092, P=0.005). |
| Olmedilla et al.30 | Intraoperative data. High duration of warm ischaemia time Postoperative data. Lower ICG-PDR (%/min), greater INR, greater AST (IU/l) on POD1 |
1-month mortality or re-transplantation (33a/332) | Univariate logistic regression Stepwise backward multivariate regression model |
Univariate analysis. 1-month mortality or re-transplantation: Warm ischaemia time OR 1.02 (1.01–1.04), P=0.002; ICG-PDR OR 0.83 (0.78–0.89), P <0.001; INR OR 1.57 (1.29–1.91), P <0.001; AST OR 1.02 (1.01–1.03) P <0.001. Multivariate analysis. 1-month mortality or re-transplantation: ICG-PDR OR 0.85 (0.79–0.92), P <0.001 and INR OR 1.45 (1.17–1.82), P=0.002. PF categorized: 1-month mortality or re-transplantation: ICG-PDR <10%/min OR 7.89 (3.59–17.34), P <0.001; INR ≥2.2 OR 2.91 (1.30–6.53), P=0.009. |
| Klinzing et al.31 | Preoperative. MELD score > 25, greater age, greater BMI Postoperative. ICG-PDR <20%/min within 6 h from admission to the ICU, peak bilirubin > 100 μmol/l within 7 days |
ICU stay > 4 days (27/50) Hospital stay > 37 days (13/50) |
Multivariate logistic regression model | ICU stay > 4 days: MELD score OR 4.12 (1.2–13.8), P=0.024; ICG-PDR OR 3.54 (1.1–11.8), P=0.047. Hospital stay > 37 days: MELD score OR 13 (2.5–68.6), P=0.001; ICG-PDR OR 4.67 (1.20–18.34), P=0.027; Bilirubin OR 0.063 (0.007–0.54), P=0.01. PF combined: ICU stay > 4 days: MELD score/ICG-PDR combination; BMI OR 9.61 (1.88–26.5), P=0.007. Hospital stay >37 days: MELD score/ICG-PDR combination OR 64.17 (3.3–1253), P=0.006; Age OR 22.63 (1.08–415.2), P=0.045. |
| Tsubono et al.33 | Postoperative. lower KICG-B, lower KICG-F, total bil, highest PT, highest AST, Highest ALT. | ICU stay > 7 days (30/50) Hospital stay > 30 days (29/50) Prolonged graft dysfunction (15/50) Preservation injury (20/50) Sepsis (12/50) |
Univariate and forward stepwise multivariate analysis | Univariate analysis. ICU stay > 7 days: KICG-B on POD 3,7; KICG-F on POD 3,7; P <0.01. Hospital stay > 30 d: KICG-B on POD1,3,7; KICG-F on POD1,3,7; P <0.01. Prolonged graft dysfunction: KICG-B on POD7; KICG-F on POD7; T. Bil on POD3, 7; P <0.01. Preservation injury: KICG-B on POD 1, 3, 7; KICG-F on POD 1, 3, 7; T. Bil on POD 3, 7; highest AST highest ALT; highest PT; P <0.01. Sepsis: KICG-B on POD 1, 3, 7; KICG-F on POD 1, 3, 7; T. Bil on POD 3, 7; P <0.01. Multivariate analysis. ICU stay > 7 days: KICG-F on POD 7, P=0.004. Hospital stay > 30 days: KICG-F on POD 7, P <0.0001. Prolonged graft dysfunction: T. Bil on POD7, P=0.0001. Preservation injury: KICG-F on POD3, P <0.0001; Highest ALT, P=0.0004. Sepsis: KICG-B on POD7, P=0.0001 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; Bil., Bilirubin; HR, hazard ratio; ICG-PDR, indocyanine green plasma disappearance rate; ICG-R15, ICG retention rate at 15 min; INR, international normalized ratio; KICG-B, measurement by spectrophotometric method; KICG-F, measurement by finger-piece method; MELD, Model for End-Stage Liver Disease; OR, odd ratio; POD, postoperative day.
11: 3 cases of primary non-function (PNF), or graft failure within 14 days; 2 cases of acute rejection; 4 cases of early ischaemic biliary complications within a year; 2 cases of hepatic artery thrombosis (HAT).
33: 25 cases of re-transplantation within the first 7 days. 5 of the 25 patients who underwent re-transplantation died within the first month after surgery. The reason for re-transplantation was hepatic artery thrombosis (HAT) in 14 cases, severe graft dysfunction in 10 cases, and graft infection in 1 case. 8 cases of death within the first month. The causes of death were severe graft dysfunction (7 patients), arterial thrombosis (5 patients), and pulmonary embolism (1 patient).
Table 4.
ICG and long-term (> 3 months) outcomes in liver transplants.
| Study | Study variable(s) | Long-term outcome measure (n/N) | Prognostic value analysis test | Prognostic value for long-term outcome |
|---|---|---|---|---|
| Cherchi et al.28 | Postoperative. ICG-PDR <16.0%/min on POD1 MELD score High volume of packed red blood cells on POD1 Hyperlactatemia on POD1 High bilirubin levels on POD1 |
One-year mortality One-year patient survival probability One-year overall Graft-Survival (55/77) Five-year patient survival |
Univariate survival analysis, univariate regression and stepwise backward Cox multivariate regression model | Univariate Cox regression analysis. One-year mortality: ICG-PDR HR 0.92 (0.86–0.98), P <0.02; MELD HR 1.09 (1.02–1.16), P=0.01; Packed red blood cells HR 1.07 (1.00–1.15), P <0.04; Hyperlactatemia HR 1.65 (1.25–2.19), P <0.01; Bilirubin HR 1.14 (1.07–1.21), P <0.01. Multivariate Cox regression analysis. One-year mortality: ICG-PDR HR 0.92 (0.86–0.99), P <0.04; MELD HR 1.07 (1.01–1.15), P=0.02. One-year patient survival probability: ICG-PDR HR 3.67 (1.52–8.87), P <0.01. One-year overall Graft-Survival: ICG-PDR HR 3.35 (1.44–7.76), P <0.01. 5-year patient survival: ICG-PDR HR 2.84 (1.18–6.86), P <0.03. |
HR, hazard ratio; ICG-PDR, indocyanine green plasma disappearance rate; MELD, Model for End-Stage Liver Disease; POD, postoperative day.
Quality assessment
The ROB assessment is depicted in Figure 2 using the QUIPS tool, and detailed information with judgment assignments for all risk-of-bias domains is presented in Supplementary File 2, Supplemental Digital Content 5, http://links.lww.com/JS9/B147. Studies selected for review showed variability across the various quality assessment domains. At least one domain per study was rated as moderate, and no study had a low risk of bias across all domains. Most studies have been judged poorly due to incomplete analysis of the last two domains, adjustment for other prognostic factors, and statistical analysis and reporting28,29,31,32. The percent agreement was 82%, and the inter-rater agreement was moderate (Kappa=0.56).
Figure 2.
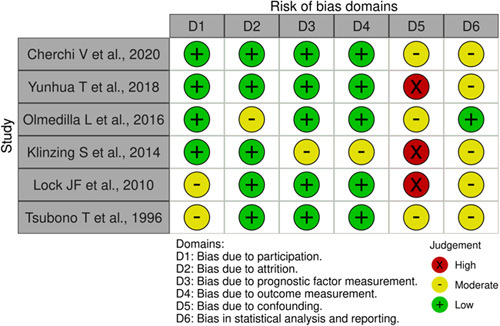
Risk of bias assessment of the included studies according to the quality in prognosis studies tool26.
We rated the systematic review as moderate-quality since it has more than one weakness but no critical flaws23.
Post-LT outcome
ICG value test, together with patient-related preoperative, intraoperative, and postoperative variables, were investigated for their relationship to liver transplant outcomes.
Four of the six articles reported data for short-term post-LT event prediction. Table 2 illustrates the statistical analysis results used to assess the predictive ability of the study variables within 7 days after transplantation. EAD, defined according Olthoff criteria34 was evaluated in two studies. Cherchi et al. 28 recorded 18 cases of EAD in 77 transplanted patients. Patients with EAD showed lower mean ICG-PDR value on the first postoperative day (POD1) (12.9%/min±4.3) versus patients without EAD (18.3%/min±7.6). They reported that the ICG-PDR value of greater than or equal to 16.0%/min on the POD1 was negatively associated with the risk of developing EAD28. Yunhua et al. 29 estimated in a regression model whether the MELD score and ICG-R15 influence the risk of EAD. Authors proposed a combination of ICG-R15-MELD as a predictive variable showing a positive relationship with the probability of EAD (χ2=24.506, P<0.001)29. In the article by Lock and colleagues, of the 99 patients who received deceased-donor LT, eight cases of initial graft dysfunction (IDF) were recorded. IDF conditions refer to liver-related causes requiring re-surgery within two days or leading to death / retransplant within 14 days after LT. Among the different variables measured directly after liver transplantation, the authors, thanks to a multivariate forward and backward logistic regression analysis, observed that the liver maximum capacity (LiMAx) breath test is the only independent predictor of IDF, a dynamic test for determining quantitative enzymatic liver function32. To determine graft quality and resultant clinical outcome, Tsubono and colleagues measured the elimination of indocyanine green in 50 transplant patients with the serial blood-sampling method (KICG-B) and the spectrophotometric method (KICG-F). The ICG measurements on days 1, 3, and 7 after LT, performed by both tests, showed a highly significant linear correlation. In the study population, twelve grafts were lost due to re-transplantation or patient death, of which five were for technical or extrahepatic reasons. Lower KICG levels and higher PT showed a significant association with graft loss. No statistically significant association was found with these factors in predicting non-liver-related graft loss and rejection33.
Table 3 summarizes the association between ICG tests and outcomes within three months of liver transplantation. Cherchi et al. 28 revealed that the ICG-PDR cut-off value <16.0%/min at POD1 was associated with a high mortality risk three months post-transplant. In Yunhua’s study, early postoperative complications were used as an aggregate outcome, including primary dysfunction, defined as graft failure leading to re-transplantation or death within 14 days of LT, acute rejection, venous or biliary complications within 1 year of LT treated with radiological intervention or re-transplantation. They reported that MELD score and ICG-R15 were statistically associated with the risk of early postoperative complications, and the proposed ICG-R15-MELD combined variable showed a positive relationship with the probability of developing early post-LT complications (χ2=19.984, P<0.001)29. In the study by Olmedilla et al. 30, the primary endpoint refers to liver-related events leading to re-transplantation within the first 7 days or death within the first month after the surgical procedure. Thirty-three patients who reached the endpoint presented a mean value of ICG-PDR on POD of 10.5±7%/min versus 18.5±8%/min in the group of patients who did not undergo re-transplantation or died. Among the variables analyzed in the univariate regression model, the Authors found that a high duration of warm ischaemic time, a low ICG-PDR, and high INR and AST values were associated with the endpoint. In contrast, only ICG-PDR and INR covariates continued to be associated with the response variable in a multivariate logistic regression analysis. The relationship with the endpoint was also confirmed when ICG-PDR (< 10%/min) and INR (≥2.2) were introduced as categorized variables30. Klinzing and colleagues evaluated the duration of post-transplant events such as ICU stay (11.6±21.9 days) and hospitalization (31.4±28.0 days) in association with preoperative and postoperative patient data. MELD score greater than 25 and the ICG-PDR less than 20%/min were associated with a higher risk of a prolonged ICU and hospital stay, while peak bilirubin >100 μmol/l within the first postoperative week is for hospitalization only. In multivariate analysis, the combination of MELD score greater than 25 with ICG-PDR <20%/min and the higher BMI were independently associated with the length of stay in the ICU, while for the hospital stay, the combination of MELD score greater than 25 with ICG-PDR less than 20%/min and older age were significant in the analysis31. Tsubono and colleagues revealed that a low KICG was statistically associated with an ICU stay of greater than 7 days, a hospital stay of greater than 30 days, sepsis, and preservation injury. A high ALT was also associated with the latter clinical result. Total bilirubin was independently associated with prolonged graft dysfunction defined by total bilirubin greater than 5 mg/dl at postoperative day 1433.
Table 4 summarizes the predictive value of ICG tests with long-term outcomes. Cherchi et al. 28 showed that the ICG-PDR cut-off value less than 16.0%/min at POD1 was associated with 1-year patient survival probability and the overall graft-survival, considering re-transplantation and mortality at 12 months after transplantation (55/77 liver transplants). In a univariate analysis, ICG-PDR value on POD1 less than 16%/min, high MELD score, high volume of packed red blood cells (POD1), hyperlactatemia (POD1), and high bilirubin level (POD1) were significantly associated with 1-year mortality. Graft and patient survival analysis curves showed that in the group with ICG-PDR less than 16%, the event tends to occur earlier than in the group with ICG-PDR greater than 16% in a period of 1-year and 5-year post-LT. The Mantel–Cox test confirmed that the survival curves are significantly different28.
Discussion
Indocyanine green is a molecular dye approved by the Food and Drug Administration (FDA) as a powerful tool for fluorescence imaging of lymph nodes, blood flow, and tissue perfusion during surgery and intraoperative tumour localization35,36. In hepatic surgery, the kinetic parameters of ICG clearance have been the subject of extensive research for the quantitative assessment of liver function in critically ill patients and as valuable predictors of liver failure after resection37–40.
This systematic review is the first to provide a summary of prognostic research evidence for the association of ICG with liver transplantation outcomes. The results of five out of six studies included in the review highlighted an association between ICG clearance tests and post-LT outcomes, but it is necessary to make some considerations. The outcome measures were variegated across the studies, with some patients requiring re-transplantation while others were developing only transient graft dysfunction. Worse ICG clearance values were associated with EAD regarded as a predictor of mortality and allograft failure28,29, or with events that requires surgery, re-transplantation or leads to death28,33.
In the multivariable analysis of patient survival, MELD score was identified as clinical variable that were highly prognostic of EAD, early postoperative complications associated with graft dysfunction29 and of 1-year mortality28. Tsubono et al. 33 identified three clinical variables (Total bilirubin level, PT within 7 postoperative days, levels of ALT) as indicators of overall and liver-related graft loss, or preservation injury. Only one study reported INR reflects the incidence of graft loss30. Regarding morbidity, BMI, MELD score, and age were significantly associated with longer ICU stay and hospitalization31.
ICG clearance in the context of liver transplantation shows a very relevant negative predictive value. Patients with successful transplantation showed ICG test results within a normal range17,30,31, and satisfactory levels of ICG clearance are associated with a low risk of complications18,41. Conversely, since the causes of primary non-function and dysfunction in liver allografts are multifactorial, careful evaluation and interpretation of ICG test results6 are necessary for patients at high risk of developing postoperative complications. In this regard, several studies, to increase the predictive power of ICG, proposed composite tools using only independent factors associated with the endpoint. Yunhua et al. 29 combined preoperative MELD with ICG-RR15 12 hours post-LT to predict EAD with an AUC of 0.87. Klinzing and colleagues proposed a combination of the low value of a single ICG-PDR measurement at 6 h post-transplant and a high MELD score. This combination correctly predicted a significantly more prolonged ICU and hospital stay (9 vs. 4 days, 42 vs. 22 days, respectively) and significantly higher hospital mortality (40% vs. 0%) compared to other combinations31. Olmedilla and colleagues proposed a risk score combining objective variables INR and ICG-PDR, obtained within the first 24 h after surgery. High INR and low ICG-PDR correctly stratified patients with a high risk of graft loss or overall mortality (90% [86–93]). The risk score was also significantly associated with other relevant outcomes after LT, such as duration of mechanical ventilation and length of ICU stay30.
The application of ICG was heterogeneous between studies. Some studies used it only once per patient (spot use), while others adopted repeat measures to allow the drawing of ICG clearance trends. Sequential changes in indocyanine green clearance rates help identify when a difference in ICG values becomes relevant in risk stratification and outcome prediction9. From postoperative day four onward, Schneider et al. 17 observed a significant difference in ICG-PDR in patients who had complications, died, or underwent re-transplantation, within 30 days after LT, compared to the eventless group. This difference was evident on the seventh postoperative day, with an ICG-PDR cut-off level of 12.3%/min to predict events.
In brief, the most relevant findings of this review are: ICG clearance on POD 1 and MELD are the independent predictors associated with 1-year mortality28, whereas ICG and highest PT are the independent predictors associated with overall and liver-related graft loss, longer ICU and hospital stay33. The combined variable of ICG, on POD1 and POD7, and preoperative MELD, together with BMI, were found to be independent predictors of EAD, early postoperative complications29 and length of ICU stay31, while ICG on POD1 and INR independently predict the overall cause of mortality or re-LT, duration of mechanical ventilation and length of ICU stay30.
The prediction of graft function recovery is a flourishing topic in liver transplantation. Different predictive models have been proposed, from simple binary definitions such as EAD by Olthoff et al. 34 to sophisticated algorithms culminating in the most recent L-GrAFT by Agopian et al. 42 and EASE score by Avolio et al. 43. Incorporating ICG clearance into these models might improve their performance and be a field of further investigation. Among the included studies of this systematic review, the study by Lock et al. 32 compared LIMAx with ICG clearance, demonstrating improved performance with the former in predicting graft dysfunction. However, out of 15 patients with end-of-LT LIMAx values suggestive of initial dysfunction, seven false positive cases showing a significant increase in LiMAx readouts on days 1 and 3 post-LT, suggesting the ability of LIMAx to capture the trajectory towards recovery. Similarly with ICG, the Authors noticed a correlation between the use of cathecolaminic support and false positive LIMAx values, thus suggesting a role of liver hypoperfusion in impairing the reliability of the test, particularly in the initial post-LT phase.
While LIMAx is based on a liver metabolic process, ICG clearance is purely dependent on a secretory process and is notoriously subject to liver flow changes that can impact its reliability6. Restoration of stable hemodynamic conditions is a prerequisite for valuable test readouts, which is an inherent limitation as there will be situations when this is not achievable. In a study investigating the relationship between ICG clearance and the development of post-LT complications, Levesque et al. 19 recognize that in the early postoperative period, ICG-PDR accurately evaluates liver dysfunction but cannot differentiate the underlying causes. As a test for global hepato-splanchnic blood flow and biliary extraction, anything that disturbs blood flow will change ICG-PDR19. Similarly, in the presence of conditions affecting liver flow, such as sepsis and cardiac failure, ICG clearance has been demonstrated to be impaired44.
The present study has some limitations. Most of the studies available in the literature have a small population size and are single-centre. Studies included in the review showed high heterogeneity in terms of the definition of outcomes, and the set of adjustment factors differed across studies. Furthermore, categorizing covariates into study-specific levels prevented comparison effect estimates and provided a cumulative analysis. Another potential limitation is related to the presence of only one study that investigated the value of ICG in long-term prognosis. This review allowed us to define and highlight the current knowledge and the most relevant results regarding the potential use of the ICG test in the context of liver transplantation. The reported results provide a starting point for the design of prospective studies with a sample size defined a priori and the use of clearly defined, reliable, and validated clinical outcome indicators that can be used as endpoints45. Further studies are required to address the considerations emerging from this systematic review and to provide an unbiased estimate of the effect of ICG tests on risk stratification in the transplant recipient population.
In conclusion, the association between ICG clearance tests and early graft function recovery suggests the role of ICG as a possible prognostic tool to help physicians evaluate LT patients in the early stages of postoperative care. Nevertheless, prospective studies should clarify the mechanisms behind the association between ICG clearance and specific post-LT outcomes.
Ethical approval
Not applicable for systematic reviews.
Consent
Not applicable.
Sources of funding
This work was supported by a grant from the Italian Ministry of Health, “Bando Ricerca Finalizzata 2019”, Research Type: Change-promoting. Project Type: Young Researchers (under 40 years). Grant number GR-2019-12369666.
Author contribution
Conception and design: M.C., G.B., A.C. and G.S. Administrative support: G.M., Q.L. and S.A. Provision of study materials or patients: M.C., G.B., A.C., G.M. and G.S. Collection and assembly of data: M.C., G.B., A.C. and Q.L. Data analysis and interpretation: M.C., Q.L. and G.S. Manuscript writing: all authors. Final approval of manuscript: all authors.
Conflicts of interest disclosure
All Authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Research registration unique identifying number (UIN)
Name of the registry: PROSPERO.
Unique Identifying number or registration ID: CRD42020222261.
Hyperlink to PROSPERO registration: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=222261.
Guarantor
Dr Gabriele Spoletini, PhD.
Data availability
The raw data supporting the conclusions of this article will be made available by the authors, upon request to the corresponding author.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Supplementary Material
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website, www.lww.com/international-journal-of-surgery.
Published online 4 October 2023
Contributor Information
Miriam Caimano, Email: miriam.caimano492@gmail.com.
Giuseppe Bianco, Email: giuseppe.bianco@policlinicogemelli.it.
Alessandro Coppola, Email: alessandro.coppola85@gmail.com.
Giuseppe Marrone, Email: giuseppe.marrone@policlinicogemelli.it.
Salvatore Agnes, Email: salvatore.agnes@unicatt.it.
Quirino Lai, Email: quirino.lai@uniroma1.it.
Gabriele Spoletini, Email: gabriele.spoletini@policlinicogemelli.it.
References
- 1. Burra P, Burroughs A, Graziadei I, et al. EASL Clinical Practice Guidelines: liver transplantation. J Hepatol 2016;64:433–485. [DOI] [PubMed] [Google Scholar]
- 2. Kwong AJ, Ebel NH, Kim WR, et al. OPTN/SRTR 2020 Annual Data Report: Liver. Am J Transplant 2022;22(Suppl 2 S2):204–309. [DOI] [PubMed] [Google Scholar]
- 3. Hudcova J, Scopa C, Rashid J, et al. Effect of early allograft dysfunction on outcomes following liver transplantation. Clin Transplant 2017;31:10. [DOI] [PubMed] [Google Scholar]
- 4. Moreno R, Berenguer M. Post-liver transplantation medical complications. Ann Hepatol 2006;5:77–85. [PubMed] [Google Scholar]
- 5. Vos JJ, Wietasch JKG, Absalom AR, et al. Green light for liver function monitoring using indocyanine green? An overview of current clinical applications. Anaesthesia 2014;69:1364–1376. [DOI] [PubMed] [Google Scholar]
- 6. de Gasperi A, Mazza E, Prosperi M. Indocyanine green kinetics to assess liver function: Ready for a clinical dynamic assessment in major liver surgery? World J Hepatol 2016;8:355–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bolondi G, Mocchegiani F, Montalti R, et al. Predictive factors of short term outcome after liver transplantation: a review. World J Gastroenterol 2016;22:5936–5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dubray BJ, Zarrinpar A. Quantification of hepatic functional capacity: a call for standardization. Expert Rev Gastroenterol Hepatol 2016;10:9–11. [DOI] [PubMed] [Google Scholar]
- 9. Levesque E, Martin E, Dudau D, et al. Current use and perspective of indocyanine green clearance in liver diseases. Anaesth Crit Care Pain Med 2016;35:49–57. [DOI] [PubMed] [Google Scholar]
- 10. Purcell R, Kruger P, Jones M. Indocyanine green elimination: a comparison of the LiMON and serial blood sampling methods. ANZ J Surg 2006;76(1–2):75–77. [DOI] [PubMed] [Google Scholar]
- 11. Lau NS, Ly M, Liu K, et al. Current and potential applications for indocyanine green in liver transplantation. Transplantation 2022;106:1339–1350. [DOI] [PubMed] [Google Scholar]
- 12. Hemming AW, Scudamore CH, Shackleton CR, et al. Indocyanine green clearance as a predictor of successful hepatic resection in cirrhotic patients. Am J Surg 1992;163:515–518. [DOI] [PubMed] [Google Scholar]
- 13. Sakka SG, Reinhart K, Meier-Hellmann A. Prognostic value of the indocyanine green plasma disappearance rate in critically ill patients. Chest 2002;122:1715–1720. [DOI] [PubMed] [Google Scholar]
- 14. Zarrinpar A, Lee C, Noguchi E, et al. A rapid, reproducible, noninvasive predictor of liver graft survival. J Surg Res 2015;197:183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tang Y, Han M, Chen M, et al. Donor indocyanine green clearance test predicts graft quality and early graft prognosis after liver transplantation. Dig Dis Sci 2017;62:3212–3220. [DOI] [PubMed] [Google Scholar]
- 16. Asencio JM, Cortese S, López Baena JA, et al. Evaluation of plasma disappearance rate indocyanine green clearance as a predictor of liver graft rejection in donor brain death. Transplant Proc 2020;52:1472–1476. [DOI] [PubMed] [Google Scholar]
- 17. Schneider L, Spiegel M, Latanowicz S, et al. Noninvasive indocyanine green plasma disappearance rate predicts early complications, graft failure or death after liver transplantation. Hepatobiliary Pancreat Dis Int 2011;10:362–368. [DOI] [PubMed] [Google Scholar]
- 18. Cherchi V, Vetrugno L, Zanini V, et al. Association between indocyanine green clearance test and ischemic type biliary lesions within one year after orthotopic liver transplantation. Gastroenterol Hepatol 2021;44:687–695. [DOI] [PubMed] [Google Scholar]
- 19. Levesque E, Saliba F, Benhamida S, et al. Plasma disappearance rate of indocyanine green: a tool to evaluate early graft outcome after liver transplantation. Liver Transpl 2009;15:1358–1364. [DOI] [PubMed] [Google Scholar]
- 20. Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ 2021;372:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Riley RD, Moons KGM, Snell KIE, et al. A guide to systematic review and meta-analysis of prognostic factor studies. BMJ 2019;364:k4597. [DOI] [PubMed] [Google Scholar]
- 22. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg 2021;88:105906. [DOI] [PubMed] [Google Scholar]
- 23. Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017;358:4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hayden JA, Côté P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med 2006;144:427–437. [DOI] [PubMed] [Google Scholar]
- 25. Grooten WJA, Tseli E, Äng BO, et al. Elaborating on the assessment of the risk of bias in prognostic studies in pain rehabilitation using QUIPS-aspects of interrater agreement. Diagn Progn Res 2019;3:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hayden JA, van der Windt DA, Cartwright JL, et al. Assessing bias in studies of prognostic factors. Ann Intern Med 2013;158:280–286. [DOI] [PubMed] [Google Scholar]
- 27. Altman DG. Systematic reviews of evaluations of prognostic variables. BMJ 2001;323:224–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cherchi V, Vetrugno L, Zanini V, et al. Indocyanine green dye clearance test: early graft (dys)-function and long-term mortality after liver transplant. Should we continue to use it? An observational study. J Clin Monit Comput 2021;35:505–513. [DOI] [PubMed] [Google Scholar]
- 29. Yunhua T, Weiqiang J, Maogen C, et al. The combination of indocyanine green clearance test and model for end-stage liver disease score predicts early graft outcome after liver transplantation. J Clin Monit Comput 2018;32:471–479. [DOI] [PubMed] [Google Scholar]
- 30. Olmedilla L, Lisbona CJ, Pérez-Peña JM, et al. Early measurement of indocyanine green clearance accurately predicts short-term outcomes after liver transplantation. Transplantation 2016;100:613–620. [DOI] [PubMed] [Google Scholar]
- 31. Klinzing S, Brandi G, Stehberger PA, et al. The combination of MELD score and ICG liver testing predicts length of stay in the ICU and hospital mortality in liver transplant recipients. BMC Anesthesiol 2014;14:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lock JF, Schwabauer E, Martus P, et al. Early diagnosis of primary nonfunction and indication for reoperation after liver transplantation. Liver Transpl 2010;16:172–180. [DOI] [PubMed] [Google Scholar]
- 33. Tsubono T, Todo S, Jabbour N, et al. Indocyanine green elimination test in orthotopic liver recipients. Hepatology 1996;24:1165–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Olthoff KM, Kulik L, Samstein B, et al. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transpl 2010;16:943–949. [DOI] [PubMed] [Google Scholar]
- 35. Gilmore DM, Khullar O v, Jaklitsch MT, et al. Identification of metastatic nodal disease in a phase 1 dose-escalation trial of intraoperative sentinel lymph node mapping in non-small cell lung cancer using near-infrared imaging. J Thorac Cardiovasc Surg 2013;146:562–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jiang JX, Keating JJ, Jesus EM, et al. Optimization of the enhanced permeability and retention effect for near-infrared imaging of solid tumors with indocyanine green. Am J Nucl Med Mol Imaging 2015;5:390. [PMC free article] [PubMed] [Google Scholar]
- 37. Sakka SG. Assessment of liver perfusion and function by indocyanine green in the perioperative setting and in critically ill patients. J Clin Monit Comput 2018;32:787–796. [DOI] [PubMed] [Google Scholar]
- 38. de Liguori Carino N, O’Reilly DA, Dajani K, et al. Perioperative use of the LiMON method of indocyanine green elimination measurement for the prediction and early detection of post-hepatectomy liver failure. Eur J Surg Oncol 2009;35:957–962. [DOI] [PubMed] [Google Scholar]
- 39. Ohwada S, Kawate S, Hamada K, et al. Perioperative real-time monitoring of indocyanine green clearance by pulse spectrophotometry predicts remnant liver functional reserve in resection of hepatocellular carcinoma. Br J Surg 2006;93:339–346. [DOI] [PubMed] [Google Scholar]
- 40. Greco E, Nanji S, Bromberg IL, et al. Predictors of peri-opertative morbidity and liver dysfunction after hepatic resection in patients with chronic liver disease. HPB (Oxford) 2011;13:559–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Levesque E, Hoti E, Azoulay D, et al. Non-invasive ICG-clearance: a useful tool for the management of hepatic artery thrombosis following liver transplantation. Clin Transplant 2011;25:297–301. [DOI] [PubMed] [Google Scholar]
- 42. Agopian VG, Harlander-Locke MP, Markovic D, et al. Evaluation of Early allograft function using the liver graft assessment following transplantation risk score model. JAMA Surg 2018;153:436–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Avolio AW, Franco A, Schlegel A, et al. Development and validation of a comprehensive model to estimate early allograft failure among patients requiring early liver retransplant. JAMA Surg 2020;155:e204095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kimura S, Yoshioka T, Shibuya M, et al. Indocyanine green elimination rate detects hepatocellular dysfunction early in septic shock and correlates with survival. Crit Care Med 2001;29:1159–1163. [DOI] [PubMed] [Google Scholar]
- 45. Coppola A, Bianco G, Lai Q, et al. Indocyanine green clearance test in liver transplantation: defining cut-off levels for graft viability assessment during organ retrieval and for the prediction of post-transplant graft function recovery—the Liver Indocyanine Green (LivInG) Trial Study Protocol. BMJ Open 2022;12:e063081. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, upon request to the corresponding author.


