Abstract
Background & objectives:
Microvessel density (MVD) is a surrogate measure of tumour angiogenesis, and is well known for over two decades to identify individuals with a high risk of recurrence with greater prevision than traditional markers. This study aims to assess the utility of MVD and its correlation with the Nottingham Prognostic Index (NPI) and other routine histopathological parameters in carcinoma breast.
Methods:
This two year retrospective, cross-sectional and analytical study evaluated 143 women with breast cancer presenting to rural tertiary hospital in central India. These women were graded histopathologically, the immunophenotype was determined using ER (estrogen receptor), PR (progesterone receptor), Her2 neu (human epidermal growth factor receptor 2 neu) and Ki-67 proliferation index (Kiel-67) immunohistochemical markers and anti-CD34 antibody to stain the endothelial cell clusters displaying the microvessels. The NPI was generated for each participant based on the tumour size, histologic grade and involvement of lymph node. The parameters were compared with the CD34 scores. Differential and inferential statistics, including the independent t test, analysis of variance, Pearson’s correlation coefficient, Spearman’s rank correlation coefficient and point biserial correlation coefficient, were used for statistical analysis.
Results:
This study showed that CD34 values ranged from 6-36 microvessels/hpf, with 24.16±6.77 microvessels/hpf as the mean. The mean microvessel counts showed a significant positive correlation with the Bloom–Richardson histological grade, vascular invasion, LN (lymph node) positivity and NPI. However, there was no significant correlation of CD34 values with the participant’s age, tumour size neither any significant association of CD34 values with the individual’s immunophenotype.
Interpretations & conclusions:
A positive linear correlation of the microvessel counts and the NPI scores suggest that with an increase in tumour angiogenesis, there was increased proliferative potential. Based on the significant correlation between the microvessel counts and the vascular invasion of the tumour masses in this study, it can be assumed that there will be vascular invasion if the microvessel count is higher and vice-versa. Although it is established that angiogenesis and neovascularization are required for the expansion of the solid tumour tissue, the heterogeneous nature of this entity makes it difficult for obtaining a linear correlation. Hence, it is suggested that though neovascularization permits advanced tumour spread it, however, does not guarantee it.
Keywords: Bloom–Richardson grade, breast carcinoma, CD34, immunohistochemistry, microvessel density, nottingham prognostic index, vascular invasion
Microvessel density (MVD) has been shown to have a predictive influence in a range of cancers, particularly in breast cancers1. It is a surrogate measure of tumour angiogenesis, and has been advocated for over 20 years to more precisely identify at high risk individuals. Carcinoma breast is thought to be the most common malignancy among women and is a major health issue. The incidence of breast cancer has increased worldwide, as per the World Health Organization1. Breast cancer is reportedly the top ranking cancer among Indian females with an age-adjusted rate of 25.8 per 100,000 women and mortality of 12.7 per 100,000 women1. The incidence of breast cancer contributes to 30 per cent of all cancers among females and 20 per cent of the overall cancer-related deaths among females1.
The diagnosis of breast cancer is based on clinical, radiological and histopathological assessment. The management of carcinoma breast involves a multimodality approach. Currently, the treatment modalities are decided based on the histological grade, immunophenotype status and the TNM (Tumour, Node, Metastasis) stage2. Nottingham prognostic index (NPI) is an essential tool to determine the prognosis following a breast cancer surgery. Its value is calculated using three fundamental pathological parameters namely, size of the tumour, number of metastatic lymph nodes and the histological grade of the tumour. It is calculated to select cases for adjuvant treatment3.
Among the various predictive and prognostic factors, angiogenesis is a major concern in tumour progression. Haematogenous spread of tumour cells is known to be quantitatively related to MVD. Evaluating this hallmark of cancer can be done by assessing the MVD in the tumour tissues using immunohistochemical stains to the endothelial cells lining the microvessels4. Oncologists can utilize this quantifiable parameter to provide targeted therapies against the proliferating new microvessels. It can also be used as an adjunct in the grading and staging of the tumour tissue.
The present study was designed as an analytical retrospective study to determine the value of MVD and its relationship to the NPI in individuals with breast cancer.
Material & Methods
The study included 143 primary cases of breast carcinoma diagnosed and operated at a rural tertiary care centre in central India over two years (September 2019 - November 2021). This study strictly adhered to ethical guidelines and received approval from the Institutional Ethics Committee. Informed consent was obtained from all the participants, ensuring confidentiality and anonymity throughout the research process.
Inclusion and exclusion criteria: Females of all ages who were diagnosed with carcinoma breast on fine-needle aspiration cytology (FNAC) or tru-cut biopsy were included in this study. In addition, those individuals who underwent modified radical mastectomy and their surgical specimens were subjected to immunohistochemical (IHC) staining and evaluation were also included in this study. The exclusion criteria included: male breast cancer cases, lobectomy specimens, wedge biopsy specimens, tru-cut biopsy tissues, cases where individuals already took neoadjuvant chemotherapy, cases of recurrent carcinoma breast and individuals with non-epithelial carcinoma and atypical ductal or lobular proliferative lesions.
A retrospective analysis of the case records was done to document the age of the individual, characteristics of the tumour such as the size, histological grade, vascular invasion, nodal status and IHC sensitivity of the tumour for PR (progesterone receptor), ER (estrogen receptor), Her-2 neu (human epidermal growth factor receptor 2 neu) and Ki-67 proliferation index (Kiel-67). In addition, the molecular subtype of the tumour was also established. The Bloom–Richardson (BR) grading system for breast cancer was used to assess the histological grade of breast cancer based on tubule formation, nuclear pleomorphism and mitotic rate. Each character is scored on a scale of 1-3 and the sum of three scores was evaluated to grade the tumour as well-differentiated (score ≤5), moderately-differentiated (score 6 or 7) and poorly differentiated (score 8 or 9). The NPI was scored using the formula:
NPI = 0.2 × tumour size (in cm) + BR Grade 1, 2 or 3 + lymph node status (1, 2 or 3), where 1 was added if all lymph nodes were free from infiltration by malignant epithelial cells, 2 was added if three or lesser number of nodes were involved and 3 if four or more lymph nodes were positive for metastasis.
Assessment of microvessel density (MVD): The microvessel count was calculated by IHC evaluation using a mouse monoclonal anti-CD34 antibody (Dako Omnis, Agilent, CA, USA) for endothelial cells showing membranous positivity. Weidner et al5 demonstrated the identification of hotspots on low power (×40 or ×100 magnification). The number of endothelial clusters was counted in high power (×400 magnification), where the microvessels were counted. A single brown dot or a cluster even without a patent lumen, was considered an individual microvessel, for assigning a CD34 score/hpf.
Statistical analysis: The clinical, demographic data, tumour size, lymph node status, histological grading, vascular invasion, immunophenotype status, NPI score and MVD were tabulated and mean and standard deviation (SD) were calculated using SPSS 24.00 for Windows (IBM SPSS Inc., Chicago, IL, USA). The data normality was checked using the Kolmogorov–Smirnov test. Non-normal data were analyzed using non-parametric tests. CD34 was correlated using Pearson’s correlation analysis for normally distributed data, using Spearman rank correlation for non-normally distributed data and point biserial correlation coefficient was used for correlation of CD34 with lymphovascular invasion (LVI). Association of CD34 with different study variables was analyzed using an independent t test (for two groups) and analysis of variance (ANOVA) (for more than two groups) followed by Bonferroni correction. P<0.05 was considered as significant.
Results
Microvessel counts/hpf and the age of the study participants were compared. Maximum cases were in the pre-menopausal age group (45.45%), i.e., below 48 yr, followed by the post-menopausal group (33.57%), i.e., ≥55 yr, and then peri-menopausal (20.98%), i.e., between 48 and 55 yr. The mean of values of the CD34 IHC staining was 24.2±6.45 microvessels/hpf in pre-menopausal women, 23.33±5.88 microvessels/hpf in peri-menopausal women and 24.64±7.72 microvessels/hpf in post-menopausal women with no significant association (P= 0.712) (Figure).
Figure.
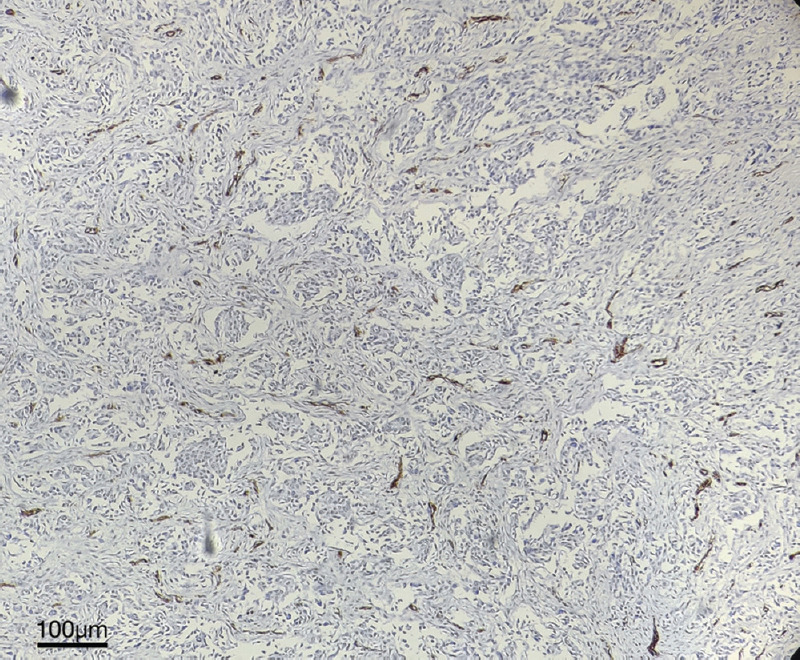
Tumour tissue stained with immunohistochemical stain-anti CD34 antibody (DAB + hematoxylin) in low power (×100 magnification) showing membranous positivity for endothelial cells as brown clusters identified as ‘hotspot’.
The tumour size of the cases was categorized according to the TNM classification. The majority of the study participants were in the T2 stage (69.2%). Cases that fell in the T1 category with the size <2 cm had a mean of 19±6.12 microvessels/hpf. Categories T2 (size 2-5 cm) and T3 (size >5 cm) had a mean of 24.45±7.01 microvessels/hpf and 24.83±5.74, respectively. Category T4, where the tumour tissue was found to involve the skin or chest wall, had a mean of 21±6.93 microvessels/hpf. There were only three cases of T4. ANOVA analysis showed no significant association between the tumour size and microvessel counts with a P value of 0.12.
Categorization according to BR histological grading showed maximum tumours was of Grade II (60.8%). Cases with BR Grade I had a mean CD34 count of 20.33±6.49 microvessels/hpf; Grade II cases had a mean value of 23.67±6.55 microvessels/hpf. Grade III cases had a mean value of 27.7±5.95 microvessels/hpf. From this it was apparent that the microvessel count increased significantly (P=0.002) with a higher grade. Furthermore, CD34 count in BR grade III was significantly higher as compared to Grade II (P=0.002) and Grade I (P<0.0001) and CD34 count in BR Grade II was significantly higher as compared to Grade I (P=0.034).
The cases were divided into two groups, depending on whether the participants were positive or negative for vascular invasion on histopathological examination, and 105/143 participants showed positivity. The mean value of the CD34 counts of cases positive for the vascular invasion was significantly higher (26.01±6.08 microvessels/hpf) than that of mean CD34 counts negative for vascular invasion (19.07±5.95 microvessels/hpf).
The grouping of lymph node status of the cases was done according to the TNM classification where N0 showed no lymph nodal spread, N1 showed up to 3 metastatic lymph nodes, N2 – 4 to 9 and N3 had 10 or more lymph nodes involved. Out of 143 cases, N0 (37.8%), followed by N1 (25.2%), then N2 (22.4%) and only 14.7 per cent of the cases were of N3. The mean value among cases in the N0 category was 21.99±5.65 microvessels/hpf, 23.08±7.53 microvessels/hpf in the N1 category, 27.44±5.96 microvessels/hpf in the N2 category and 26.62±6.92 microvessels/hpf in the N3 category. This shows that the mean value of the CD34 counts increased as the number of lymph nodes increased; (P=0.0006). CD34 count was significantly higher in N2 and N3 as compared to N0 and N1, however, no significant difference was seen in CD34 count between N0 and N1 (P=0.431) or between N2 and N3 (P=0.651).
The cases were subdivided into four immunophenotypes based on ER, PR, Her2neu and Ki-67 proliferation index status on IHC, where triple-negative breast cancer contributed to 29.37 per cent. Luminal A contributed to 28.67 per cent of cases, whereas Luminal B and Her – 2 enriched contributed to around 30 per cent of the cases each. The mean value of the CD34 counts of cases of Luminal A was 25.17±7.37 microvessels/hpf and Her-2 enriched was 22.65±7.74 microvessels/hpf. Luminal B and triple-negative breast cancer cases had a mean CD34 count of 24.07±5.63 and 24.33±6.17 microvessels/hpf, respectively. The ANOVA analysis was done, and the P value was 0.49, which showed no association of CD34-stained microvessels with the immunophenotype.
Finally, the NPI scores were calculated, and the cases were divided into four defined groups based on the scores. Group 1 had ≤2.4, group 2 had 2.5-3.4, group 3 had 3.5-5.4 and group 4 had scores of ≥5.5. Of the 143 cases NPI scores were highest in group 3 and group 4, where only 12.6 per cent of cases were of group 2 and not a single case was in group 1. Participants with NPI between 2.5-3.4 had a mean value of CD34 count of 19.61±6.36 microvessels/hpf, and those with NPI scores between 3.5 and 5.4 had a mean value of 22.61±6.38 and participants with NPI scores ≥5.5 had a mean value of 27.46±5.87. This significant (P<0.0001) increase in microvessel counts was observed with a higher NPI value. CD34 count was significantly higher in group 4 as compared to group 2 (P<0.0001) and group 3 (P<0.0001). No significant difference was seen in CD34 count between group 2 and group 3 (P=0.069).
A positive linear relationship was seen between CD34 count and BR score, LVI, LN positive and NPI score. A significant weak positive correlation was seen between CD34 count with BR score, LN positivity with a correlation coefficient (r) of 0.327 and 0.375, respectively. A moderately positive correlation was seen between CD34 score with LVI and NPI score with correlation coefficient (r) of 0.455 and 0.453, respectively. A weak positive correlation was seen between CD34 counts with tumour mass size in cm with correlation coefficient (r) of 0.129. No correlation was seen between CD34 score with age a correlation coefficient (r) of −0.018 (Table).
Table.
Correlation coefficient (r) and P values of various parameters in association with CD34 stained microvessels/hpf
| Variables | r | P |
|---|---|---|
| Age | −0.018 | 0.832† |
| Tumour mass size (cm) | 0.129 | 0.126* |
| BR score | 0.327 | 0.0001* |
| LVI +/− | 0.455 | <0.0001‡ |
| LN positive | 0.375 | <0.0001* |
| NPI score | 0.453 | <0.0001* |
†Pearson’s correlation coefficient; *Spearman’s rank correlation coefficient; ‡Point biserial correlation coefficient. BR, Bloom-Richardson score; LVI, lymphovascular invasion; NPI, nottingham prognostic index; LN, lymph node
Discussion
Carcinoma breast is one of the most common malignancy in women and is a major health problem1. The incidence of breast cancer has increased worldwide, as per the World Health Organization1. The diagnosis of carcinoma breast is based on clinical, radiological and histopathological assessment. The management of carcinoma breast involves a multimodality approach. Currently, the treatment modalities are targeted and decided based on the histological grade, immunophenotype status and the TNM stage. Trastuzumab or pertuzumab are specifically prescribed when the tumour shows Her-2 neu positivity; tamoxifen, letrozole or anastrozole are given when the hormone receptors are positive, similarly, anti-angiogenic drugs such as bevacizumab can be an adjunct if the MVD is considered significant2.
NPI is calculated to select potential individuals for adjuvant treatment. It takes into consideration the size, node number and histological grade of the tumour. Many parameters define the behaviour and aggressiveness of the tumours, such as age, size of the tumour, histological grading, vascular invasion and involvement of lymph nodes. These parameters ultimately define the disease-free survival and prognostic implications of the disease. Studies on new CD marker expression such as CD4/CD8 ratio and CD30 expression levels will be helpful for prognostication and possible therapeutic intervention in invasive breast carcinoma and of interest in the field of targeted therapy6.
Out of a variety of parameters, vascularization or angiogenesis is what feeds the tumour. One of the parameters that can help earmark or evaluate the blood vessel proliferation is the MVD. This includes mere counting of the new proliferating endothelial cells that can be immunostained with anti-CD34 antibody.
The current study showed a significant correlation of the histological grade of the tumour and the microvessel counts, which was in concordance with the studies7-15. Based on this it was inferred that the tumour’s aggressiveness leads to an increased microvessel count.
There was a significant correlation between the microvessel counts and the vascular invasion of the tumour masses as well, which were similar to prior studies13-17. Therefore, it can be assumed that there will be vascular invasion if the microvessel count is higher and vice-versa.
There was no significant association of the age of the individual, tumour size, lymph node metastasis and immunophenotype in the present study, which is concordant with the findings of other studies11-13,15,17-19 which also showed a positive correlation of the MVD with the tumour size and lymph node metastasis. These findings suggest that carcinoma breast is a heterogenic entity. Although it is established that angiogenesis and neovascularization are required for the expansion of the solid tumour tissue, the heterogeneous nature of this entity makes it difficult for a linear correlation to be obtained. It must be emphasised that though neovascularization permits advanced tumour spread but does not guarantee it. About 20-30 per cent of all lymph node-negative individuals can still develop a recurrence of the disease within 10 yr of initial treatment of primary tumour. Therefore, even in cases with high microvessel counts, individuals in this study had no metastatic deposits in the lymph nodes. The hormonal receptors are independent of the microvessel counts. We expect the aggressive triple-negative cancers to show higher microvessel counts and the luminal cancers to show lower ones. However, the current researchers have not proved it. A study with larger sample size and stringent IHC staining protocols should be undertaken to this effect.
NPI is the amalgamation of the essential histopathological parameters for the grading and staging of tumours. A positive linear correlation of the microvessel counts and the NPI scores suggest that with an increase in angiogenesis of the tumour, there is an increased proliferative potential. Furthermore, the correlation of the NPI and the microvessel count of the individual cases was found to be significant, similar to previous studies12,13,18 suggesting the importance of CD34 as an important marker, as an adjunct in the histopathological reporting system for the accurate treatment strategies.
There were a few technical and subjective limitations faced during this study. Improper tissue sampling, tissue heterogeneity, IHC expertise during antigen retrieval and the cost of the procedures were the primary technical predicaments. Subjective variability and keen know-how are pre-requisites for assessing CD34 interpretation of IHC. However, the anti-CD34 antibody does not have pre-defined cut-offs or ranges set, affecting the results and correlative interpretation.
CD34 detection using anti-CD34 antibodies can be incorporated as a part of the reporting protocol, thereby enhancing the overall effectiveness of the carcinoma breast reporting protocol. NPI also needs to be incorporated as an essential prognostic tool in carcinoma breast reporting format as it can help plan an effective management strategy. Further studies and research, with a larger sample size and for a more extended period, are needed to be carried out to evaluate the relationship between the MVD and other parameters for better efficacy, also for more accurate quantification methods of the same.
Overall, CD34 expression is a crucial IHC-based biomarker index, which can be used independently or in association with other histopathological parameters. CD34 biomarker can help predict tumour behaviour in cell proliferation, differentiation and metastatic tendency. The results of this biomarker can be harnessed to guide targeted therapies in conjunction with the presently employed chemotherapy regimens which can minimize the risk of recurrence and also provide a significant outcome for the individual. Therefore, CD34 can be utilized as an adjunct with other known histopathological parameters, improving patient care by risk stratification and better management protocols.
Financial support and sponsorship
This study received funding support by the Indian Council of Medical Research (ICMR) for MD/MS Thesis Projects (Grant no. 3/2/Dec-2019/PG-Thesis-HRD(9)).
Conflicts of interest
None.
Acknowledgment:
Authors acknowledge Dr Venkat Goyal and Dr Madhvi Goyal for their help in statistical analysis and literature review.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Weissenbacher TM, Zschage M, Janni W, Jeschke U, Dimpfl T, Mayr D, et al. Multicentric and multifocal versus unifocal breast cancer: Is the tumor-node-metastasis classification justified? Breast Cancer Res Treat. 2010;122:27–34. doi: 10.1007/s10549-010-0917-9. [DOI] [PubMed] [Google Scholar]
- 3.Lee AH, Ellis IO. The Nottingham prognostic index for invasive carcinoma of the breast. Pathol Oncol Res. 2008;14:113–5. doi: 10.1007/s12253-008-9067-3. [DOI] [PubMed] [Google Scholar]
- 4.Folkman J. Tumor angiogenesis. Adv Cancer Res. 1974;19:331–58. doi: 10.1016/s0065-230x(08)60058-5. [DOI] [PubMed] [Google Scholar]
- 5.Weidner N, Folkman J, Pozza F, Bevilacqua P, Allred EN, Moore DH, et al. Tumor angiogenesis: A new significant and independent prognostic indicator in early-stage breast carcinoma. J Natl Cancer Inst. 1992;84:1875–87. doi: 10.1093/jnci/84.24.1875. [DOI] [PubMed] [Google Scholar]
- 6.Jagtap SV. Evaluation of CD4+T-cells and CD8+T-cells in triple-negative invasive breast cancer. Indian J Pathol Microbiol. 2018;61:477–8. doi: 10.4103/IJPM.IJPM_201_18. [DOI] [PubMed] [Google Scholar]
- 7.Kwon GY, Lee SD, Park ES. Mast cell and macrophage counts and microvessel density in invasive breast carcinoma-comparison analysis with clinicopathological parameters. Cancer Res Treat. 2005;37:103–8. doi: 10.4143/crt.2005.37.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolat F, Kayaselcuk F, Nursal TZ, Yagmurdur MC, Bal N, Demirhan B. Microvessel density, VEGF expression, and tumor-associated macrophages in breast tumors:correlations with prognostic parameters. J Exp Clin Cancer Res. 2006;25:365–72. [PubMed] [Google Scholar]
- 9.Rani D, Somasundaram VH, Nair S, Koyakutty M. Advances in cancer nanomedicine. J Indian Inst Sci. 2012;92:187–218. [Google Scholar]
- 10.Safwat MD, Habib F, Elayat A, Oweiss N, Reffat S, Algaidi S. Morphometric and immunohistochemical study of angiogenic marker expressions in invasive ductal carcinoma of human breast. Folia Morphol (Warsz) 2009;68:144–55. [PubMed] [Google Scholar]
- 11.Vijayasree V, Ramana SV, Kumar RA, Singh R, Anil S. Correlation of microvessel density with tumor type, tumor size, histological grade and lymph node status of breast carcinoma. Indian J Pathol Oncol. 2018;5:318–25. [Google Scholar]
- 12.Kwatra A, Aggarwal D, Gupta R, Chaturvedi AK, Kudesia M, Singh S. Correlation of various histopathologic prognostic factors with Nottingham prognostic index and microvessel density in invasive breast carcinoma: A study of 100 cases. Indian J Cancer. 2015;52:110–3. doi: 10.4103/0019-509X.175594. [DOI] [PubMed] [Google Scholar]
- 13.Agnani B, Solanki R, Ansari M, Agnani S. Prognostic significance of microvessel density as assessed by anti CD34 monoclonal antibody in invasive ductal carcinoma of breast. Asian Pac J Cancer Biol. 2020;5:75–9. [Google Scholar]
- 14.Dhakal HP, Bassarova A, Naume B, Synnestvedt M, Borgen E, Kaaresen R, et al. Breast carcinoma vascularity: A comparison of manual microvessel count and Chalkley count. Histol Histopathol. 2009;24:1049–59. doi: 10.14670/HH-24.1049. [DOI] [PubMed] [Google Scholar]
- 15.Şener E, Şipal S, Gündoğdu C. Comparison of microvessel density with prognostic factors in invasive ductal carcinomas of the breast. Turk Patoloji Derg. 2016;32:164–70. doi: 10.5146/tjpath.2016.01366. [DOI] [PubMed] [Google Scholar]
- 16.Verma K, Kumar S, Srivastava AN. Prognostic significance of microvessel density in breast cancer of Indian Population. Int J Sci Eng Res. 2013;4((2)) [Google Scholar]
- 17.Bosari S, Lee AK, DeLellis RA, Wiley BD, Heatley GJ, Silverman ML. Microvessel quantitation and prognosis in invasive breast carcinoma. Hum Pathol. 1992;23:755–61. doi: 10.1016/0046-8177(92)90344-3. [DOI] [PubMed] [Google Scholar]
- 18.Pyakurel D, Karki S, Agrawal CS. A study on microvascular density in breast carcinoma. J Pathol Nepal. 2014;4:570–5. [Google Scholar]
- 19.Goulding H, Abdul Rashid NF, Robertson JF, Bell JA, Elston CW, Blamey RW, et al. Assessment of angiogenesis in breast carcinoma: An important factor in prognosis? Hum Pathol. 1995;26:1196–200. doi: 10.1016/0046-8177(95)90193-0. [DOI] [PubMed] [Google Scholar]


