Abstract
A monoclonal antibody (MAb) (MAb 10F3) directed against the CopB outer membrane protein of Moraxella catarrhalis previously was found to enhance pulmonary clearance of M. catarrhalis in an animal model (M. Helminen, I. Maciver, J. L. Latimer, L. D. Cope, G. H. McCracken, Jr., and E. J. Hansen, Infect. Immun. 61:2003–2010, 1993). In the present study, this same MAb was shown to exert complement-dependent bactericidal activity against this pathogen in vitro. Nucleotide sequence analysis of the copB gene from two MAb 10F3-reactive and two MAb 10F3-unreactive strains of M. catarrhalis revealed that the deduced amino acid sequences of these four CopB proteins were at least 90% identical. Comparison of the amino acid sequences of these proteins allowed localization of possible MAb 10F3 binding sites to five relatively small regions of the CopB protein from M. catarrhalis O35E. When five synthetic peptides representing these regions were tested for their ability to bind MAb 10F3 in a direct enzyme-linked immunosorbent assay system, an oligopeptide containing 26 amino acids was shown to bind this MAb. The actual binding region for MAb 10F3 was localized further through the use of overlapping decapeptides that spanned this 26-mer. A fusion protein containing the same 26-mer readily bound MAb 10F3 and was used to immunize mice. The resultant antiserum contained antibodies that reacted with the CopB protein of the homologous M. catarrhalis strain in Western blot analysis and bound to the surface of both homologous and heterologous strains of M. catarrhalis.
Moraxella (Branhamella) catarrhalis is an important respiratory tract pathogen. In children, this organism is the third most common etiologic agent of acute bacterial otitis media and accounts for up to 20% of cases (3, 11, 18). In a recent report, M. catarrhalis DNA could be detected by performing PCR on middle ear fluid from 46% of patients with chronic otitis media with effusion (41). In adults, M. catarrhalis is a frequent cause of acute exacerbations of chronic obstructive pulmonary disease (10, 24, 34, 38). Invasive infections with this organism, such as bacteremia, meningitis, skeletal infections, and endocarditis, are rare and occur mainly in immunocompromised individuals (12, 33).
The wide occurrence of M. catarrhalis infections and the rapid spread of β-lactamase production among clinical isolates have stimulated efforts to develop a vaccine against this pathogen (1). Several lines of evidence suggest that the induction of appropriate humoral immunity will likely be protective against respiratory tract disease caused by M. catarrhalis. (i) Acute M. catarrhalis infection induces the production of both serum and secretory antibodies against various antigenic determinants of this pathogen (17, 25, 44). (ii) The age-dependent development of the humoral response against M. catarrhalis is inversely related to the prevalence of nasopharyngeal colonization and incidence of otitis media involving M. catarrhalis (20, 45). (iii) Passive immunization with M. catarrhalis-directed antibodies as well as active immunization with M. catarrhalis outer membrane proteins enhanced pulmonary clearance of M. catarrhalis in an animal model (26, 27, 32).
The lack of a polysaccharide capsule surrounding M. catarrhalis indicates that surface-exposed outer membrane antigens are the likely targets for a protective immune response. Different M. catarrhalis strains have remarkably similar outer membrane protein profiles (4, 35), and at least three surface-exposed proteins of this organism appear to be well conserved antigenically (26, 27, 29, 36). One of these, the 80-kDa CopB protein (also designated OMP B2), is a potential vaccine candidate, based on the finding that a monoclonal antibody (MAb) (MAb 10F3) directed against a surface-exposed CopB epitope reacted with approximately 70% of M. catarrhalis strains and enhanced pulmonary clearance of M. catarrhalis in a murine model (26). It has been established that CopB expression is iron regulated (2, 5) and that CopB is involved at some level in the ability of M. catarrhalis to acquire iron from human transferrin and lactoferrin (2). Expression of CopB is apparently essential for virulence of M. catarrhalis, at least in an animal model, because an isogenic copB mutant was less able than its wild-type parent strain to resist clearance from the lungs of mice (28).
In this study, MAb 10F3 was shown to be bactericidal against MAb 10F3-reactive strains. Comparison of the deduced amino acid sequences of the CopB proteins from four strains of M. catarrhalis revealed a high degree of identity among these proteins, which in turn facilitated mapping of the MAb 10F3-reactive epitope. These data allowed construction of a fusion protein which bound MAb 10F3 and induced the synthesis of antibodies directed against the surface of M. catarrhalis.
(Part of this research was presented by C. Aebi et al. at the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy, New Orleans, La., 18 September 1996 [1a].)
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. M. catarrhalis O35E has been described in detail previously (26, 28). M. catarrhalis strains were routinely cultured at 37°C on brain heart infusion (BHI) agar plates (Difco Laboratories, Detroit, Mich.) in an atmosphere of 95% air–5% CO2 or in BHI broth. The Escherichia coli cloning strains RR1, HB101, and DH5α and recombinant strains were grown on Luria-Bertani medium (42) supplemented, when necessary, with an appropriate antimicrobial compound.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description | Source and/or reference |
|---|---|---|
| M. catarrhalis | ||
| O35E | Wild-type isolate from middle-ear fluid | 26 |
| O12E | Wild-type isolate from middle-ear fluid | J. Nelson |
| O46E | Wild-type isolate from middle-ear fluid | J. Nelson |
| TTA24 | Wild-type isolate from transtracheal aspirate | S. Berk (27) |
| E. coli | ||
| HB101 | Host for cloning experiments | 42 |
| RR1 | Host for cloning experiments | 42 |
| DH5α | Host for cloning experiments | 42 |
| Plasmids | ||
| pBR322 | Cloning vector; Ampr Tetr | 42 |
| pBluescript II SK+ | Cloning vector; Ampr | Stratagene |
| pTTA100 | pBR322 with a 7.8-kb PstI fragment of M. catarrhalis TTA24 chromosomal DNA containing the copB gene | This study |
| pTTA150 | pBluescript II SK+ with a 2.6-kb HinfI fragment from pTTA100 containing the copB gene | This study |
| pGEX-4T-2 | Cloning vector | Pharmacia |
| pEP10F3 | pGEX-4T-2 with a 78-bp insert encoding amino acids 275 to 300 of the CopB protein of M. catarrhalis O35E | This study |
Outer membrane protein preparations and Western blot analysis.
Outer membrane vesicles were prepared from BHI broth-grown M. catarrhalis strains as described previously (37). Proteins present in these outer membrane vesicles were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and detected by Western blot analysis as described previously (26).
MAbs and polyclonal antisera.
MAb 10F3 is a murine IgG2a antibody reactive with a surface-exposed epitope of the CopB outer membrane protein of M. catarrhalis O35E and O12E (26). This MAb does not bind to the CopB protein expressed by M. catarrhalis TTA24 and O46E (26). This MAb was used in the form of hybridoma culture supernatant fluid for enzyme-linked immunosorbent assays (ELISAs), epitope mapping, and Western blot analysis and in the indirect antibody accessibility assay. For use in bactericidal assays, MAb 10F3 was purified by using protein A-Sepharose CL-4B (Pharmacia Biotech Inc., Piscataway, N.J.) as described previously (15). MAb 17C7 is a murine immunoglobulin G (IgG) antibody directed against the UspA surface antigen of M. catarrhalis (27). MAb 3F12 is a murine IgG antibody specific for the major outer membrane protein (MOMP) of Haemophilus ducreyi (21). The last two of these MAbs were used as negative controls in the ELISA and indirect antibody accessibility assays, respectively. Polyclonal rabbit antiserum raised against M. catarrhalis TTA24 outer membrane vesicles was described previously (26).
Cloning and nucleotide sequence analysis of the copB gene of M. catarrhalis strain TTA24.
Chromosomal DNA obtained from this strain was partially digested with PstI (Gibco BRL, Gaithersburg, Md.) and then subjected to agarose gel electrophoresis. Fragments (6 to 9 kb) were purified by using the GeneCleanII kit (BIO 101 Inc., La Jolla, Calif.); these fragments were ligated overnight at 16°C into PstI-digested and alkaline phosphatase-treated pBR322 (New England Biolabs Inc., Beverly, Mass.) by using T4 DNA ligase (Gibco BRL). The ligation reaction mixture was used to transform competent E. coli RR1 cells. Colony material from tetracycline-resistant transformants was transferred to nitrocellulose pads and treated with UV light (UV Stratalinker; Stratagene, La Jolla, Calif.) to bind DNA to the nitrocellulose. These membranes were probed with a 32P-labeled 1.3-kb PvuII-XbaI fragment from the copB gene of M. catarrhalis O35E (26). Clones that hybridized this copB gene probe were tested for expression of the CopB protein by Western blot analysis.
PCR-based amplification and nucleotide sequence analysis of the copB genes of M. catarrhalis strains O12E and O46E.
Chromosomal DNA from M. catarrhalis strains O12E and O46E was extracted by standard methods and used as the template DNA in a PCR system together with primers derived from the nucleotide sequence of the copB gene from M. catarrhalis O35E (26). With a PTC 100 Programmable Thermal Controller (MJ Research, Inc., Cambridge, Mass.) and the GeneAmp PCR kit (Roche Molecular Systems Inc., Branchburg, N.J.), a 2.5-kb DNA fragment containing the copB gene was amplified from each strain. With Spin-X centrifuge tube filters (Corning Costar Corp., Cambridge, Mass.), these 2.5-kb PCR products were extracted from 0.7% agarose gel slices and resuspended in water. The nucleotide sequences of these two PCR products were determined with a model 373 DNA sequencer (Applied Biosystems, Inc., Foster City, Calif.), used according to the manufacturer’s protocol. The sequencing reactions were performed with the Applied Biosystems PRISM Ready Reaction DiDeoxy Terminator Cycle Sequencing Kit with AmpliTaq DNA Polymerase, according to the manufacturer’s directions (Applied Biosystems, Inc.). Sequencing primers were designed from the nucleotide sequence of the copB gene from M. catarrhalis O35E (26) or from the individual copB sequences.
Oligopeptide synthesis.
Oligopeptides were synthesized on a Symphony Peptide Synthesizer (Rainin Instrument Co., Inc., Woburn, Mass.) by the Biopolymers Facility, Howard Hughes Medical Institute, Dallas, Tex. The molecular weight and purity of the peptides were determined by high-performance liquid chromatography and mass spectrometry.
ELISA.
Flat-bottom microtiter ELISA plates (Corning Glass Ware, Corning, N.Y.) were coated with outer membrane vesicles (5 μg) from M. catarrhalis O35E or with synthetic peptides (10 μg) in 50 mM sodium carbonate buffer (pH 9.6) with or without 2% (vol/vol) fresh glutaraldehyde and incubated overnight at 4°C. After washing three times with phosphate-buffered saline (PBS)-Tween (PBS containing 0.05% [vol/vol] Tween 20 and 0.025% [wt/vol] sodium azide) and blocking with PBS-Tween containing 1% (wt/vol) bovine serum albumin for 1 h, 100-μl portions of MAb 10F3 or MAb 17C7 (negative control) or mouse antiserum in dilutions of 1:10 to 1:1,000 in blocking buffer were added and the plates were incubated at 37°C for 1 h. After washing, antibody binding to peptides was detected by using alkaline phosphatase-conjugated goat anti-mouse IgG (Organon Teknika Corp., West Chester, Pa.) with p-nitrophenylphosphate (Sigma Chemicals Co., St. Louis, Mo.) in 10% (vol/vol) diethanolamine buffer (pH 9.8) as the enzyme substrate. Absorbance at 410 nm was measured with an MR 700 Microplate reader (Dynatech Laboratories, Chantilly, Va.).
Fine mapping of the MAb 10F3-reactive epitope.
Overlapping synthetic decapeptides that were N-terminally bound to a membrane composed of derivatized cellulose were obtained from Research Genetics Inc., Huntsville, Ala. After five washes with PBS-Tween, the membrane was blocked for 1 h at room temperature in PBS-Tween containing 10% (wt/vol) nonfat dry milk and subsequently incubated with MAb 10F3 overnight at 4°C. Following three washes with PBS-Tween, the membrane was incubated overnight at 4°C with gentle rocking with 106 cpm of radioiodinated (specific activity, 2 × 107 cpm/μg protein), affinity-purified goat anti-mouse immunoglobulin. The membrane was then washed as described above and exposed to X-ray film (Fuji RX safety film; Fuji Industries, Tokyo, Japan).
Construction of a GST fusion protein expressing the MAb 10F3-reactive epitope.
The pGEX-4T-2 expression vector system (Pharmacia Biotech Inc.) was used to construct a fusion protein containing the MAb 10F3-reactive epitope at the C terminus of glutathione-S-transferase (GST). By using the oligonucleotide primers 5′-CGGGATCCCTAGATATAGAAAAAGAT-3′ and 5′-CCGCTCGAGCTTGCCTCGATATTTGTTATC-3′ derived from the copB gene sequence of M. catarrhalis O35E and containing a BamHI or an XhoI restriction site at their 5′ ends, respectively, a 78-bp fragment encoding amino acid residues 275 to 300 of the CopB protein (i.e., peptide R1 [see Fig. 2]) was amplified from M. catarrhalis O35E chromosomal DNA by PCR, digested with BamHI and XhoI, and ligated into BamHI- and XhoI-digested pGEX-4T-2 by using T4 DNA ligase. The ligation product was transformed into competent E. coli DH5α, and recombinant clones were screened for reactivity with MAb 10F3 by a colony blot assay described elsewhere (21). The plasmid construct present in one of the MAb 10F3-reactive clones was designated pEP10F3. The correct in-frame position and orientation of the 78-bp insert with respect to the GST open reading frame were confirmed by nucleotide sequence analysis of the relevant region of pEP10F3 with the vector-derived sequencing primers 5′-CAATGTGCCTGGATGCGTTC-3′ and 5′-CAGACAAGCTGTGACCGTCTCC-3′. Large quantities of this fusion protein, designated GST-26, and of GST alone were produced and purified according to the manufacturer’s directions for purification of GST fusion proteins.
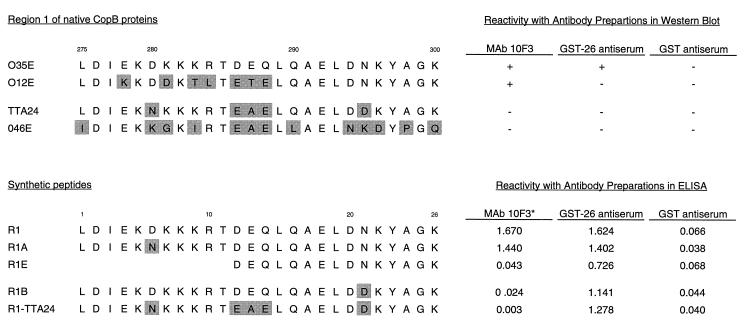
FIG. 2.
Amino acid sequences and reactivities with antibodies of both region 1 from CopB of four different M. catarrhalis strains and synthetic peptides derived from these sequences. The numbers (i.e., 275 to 300) above the region 1 sequences indicate the amino acid positions in the intact CopB protein. The numbers (i.e., 1 to 26) above the R1 synthetic peptide indicate residue positions in the R1 peptide and its derivatives. Shading indicates residues differing from those in O35E. The reactivities of MAb 10F3, GST-26 antiserum, and GST antiserum with the CopB proteins of these strains in Western blot analysis are indicated on the right; these two antisera were diluted 1:100 for use in this assay. The reactivities of the synthetic peptides with these same antibody preparations in the ELISA are indicated on the right opposite these peptides. The asterisk indicates that the ELISA with MAb 10F3 was performed after adsorption of the peptides to the microtiter wells in the presence of glutaraldehyde. The ELISA readings (optical densities at 410 nm) were recorded after 30 min as described in Materials and Methods.
Immunization protocol.
Groups of five 2-month-old female BALB/c mice (Charles River Breeding Laboratories, Wilmington, Mass.) were immunized on day 1 by intraperitoneal injection with 50 μg of GST-26 suspended in 0.2 ml of Freund’s complete adjuvant (Difco Laboratories) that had been diluted 1:1 in PBS. Control animals were immunized with GST only in adjuvant. These animals were given an intraperitoneal injection on day 28 with the same amount of protein in Freund’s incomplete adjuvant (Difco Laboratories) that had been diluted 1:1 in PBS. Blood for serum preparation was obtained by standard methods on day 42. All procedures involving animals received approval from the Institutional Animal Care and Use Committee; all animals were housed in accordance with guidelines from the United States Department of Agriculture and the Association for Assessment and Accreditation of Laboratory Animal Care International.
Indirect antibody accessibility assay.
An overnight BHI broth culture of M. catarrhalis O35E was diluted in PBS buffer containing 10% (vol/vol) fetal bovine serum and 0.025% (wt/vol) sodium azide to a density of 110 Klett units (ca. 109 CFU/ml) as measured with a Klett-Summerson colorimeter (Klett Manufacturing Co., New York, N.Y.). Identical portions (100 μl) of this suspension were added to 1 ml of MAb 10F3 or MAb 3F12 hybridoma culture supernatant or to 1 ml of the PBS buffer described above containing mouse antiserum at a dilution of 1:500. After incubation at 4°C with gentle agitation for 1 h, the bacterial cells were washed once and then resuspended in 1 ml of the buffer solution. Radioiodinated goat anti-mouse immunoglobulin was added, and the mixture was incubated for 1 h at 4°C with gentle agitation. The cells were then washed four times with 1 ml of the buffer solution, resuspended in 500 μl of triple detergent (22), and transferred to a 12-by-75-mm glass tube. The radioactivity present in each sample was determined by using a gamma radiation counter.
Bactericidal assay.
Complement-sufficient normal adult human serum was prepared by standard methods. Complement inactivation was achieved by heating the serum for 30 min at 56°C. An M. catarrhalis BHI broth culture was grown to early logarithmic phase and diluted in Veronal-buffered saline containing 0.15% (wt/vol) gelatin (Sigma Chemicals Co.) to a concentration of 2.5 × 104 CFU/ml (28). Portions (100 μl) of this cell suspension were added to 100 μl of native or heat-inactivated normal human serum containing either 15 μg of purified MAb 10F3 or heat-inactivated antiserum in final dilutions of 1:20 to 1:2,000. This mixture was then incubated at 37°C. At time zero and at 60 and 120 min after the start of the assay, 10-μl aliquots were removed, suspended in 75 μl of BHI broth, and spread onto prewarmed BHI agar plates which were then incubated overnight to determine the number of CFU in each sample.
Nucleotide sequence accession numbers.
The nucleotide sequences of the copB genes from M. catarrhalis O12E, TTA24, and O46E have been submitted to GenBank and assigned the accession numbers U69981, U69980, and U69982, respectively.
RESULTS
Cloning of the copB gene from M. catarrhalis TTA24.
As a first step in localizing the epitope that bound MAb 10F3, the amino acid sequence of the CopB protein from an M. catarrhalis strain unreactive with this MAb (i.e., strain TTA24) was determined. A 1.3-kb PvuII-XbaI fragment from the M. catarrhalis O35E copB gene (26) was used to screen a genomic library constructed from M. catarrhalis TTA24 in the plasmid vector pBR322. Whole-cell lysates prepared from recombinant E. coli clones that hybridized this DNA probe were probed in Western blot analysis with rabbit antiserum raised against outer membrane vesicles from M. catarrhalis TTA24. One of these recombinant clones which expressed an 80-kDa protein reactive with this antiserum was shown to contain a 7.8-kb PstI DNA insert (data not shown), as was expected from previous Southern blot-based analysis of the conservation of the copB gene among M. catarrhalis strains (26). The recombinant plasmid was designated pTTA100, and a 2.6-kb HinfI fragment from this plasmid that was predicted to contain the entire copB gene was subcloned into pBluescript to obtain the recombinant plasmid pTTA150. Nucleotide sequence analysis of both strands of this 2.6-kb DNA fragment confirmed that it contained the complete copB gene from strain TTA24.
Nucleotide sequence analysis of copB genes from additional M. catarrhalis strains.
To obtain information about the amino acid sequence of other CopB proteins, the copB genes from a MAb 10F3-reactive strain (O12E) and a MAb 10F3-unreactive strain (O46E) were amplified from the chromosome of each strain by PCR. The oligonucleotide primers (5′-CAAGCCTCATAATCGGAG-3′) and 5′-CCTCCAGTGAAATCGAATC-3′) were derived from the sequence of the copB gene of strain O35E (26) and were located just outside the open reading frame. Both strands of these two PCR products were sequenced in their entirety.
Comparison of the CopB proteins from MAb 10F3-reactive and -unreactive strains.
In an attempt to localize the epitope for MAb 10F3 within the CopB protein, the deduced amino acid sequences of the CopB proteins from these four strains were compared (Fig. 1). These sequences included the leader peptide, the existence of which was confirmed previously by N-terminal amino acid sequence analysis of the mature CopB protein from M. catarrhalis O35E (26). The overall identities among these four proteins were striking, with the level of identity between the two most dissimilar proteins (i.e., those of strains O35E and O46E) being 91%. Interestingly, the reactivity of these CopB proteins with MAb 10F3 was not indicative of the degree of identity among these proteins. For example, the MAb 10F3-reactive protein from strain O35E and 98% identical to the MAb 10F3-unreactive CopB protein from strain TTA24 and only 94% identical to the MAb 10F3-reactive protein from strain O12E (Fig. 1).
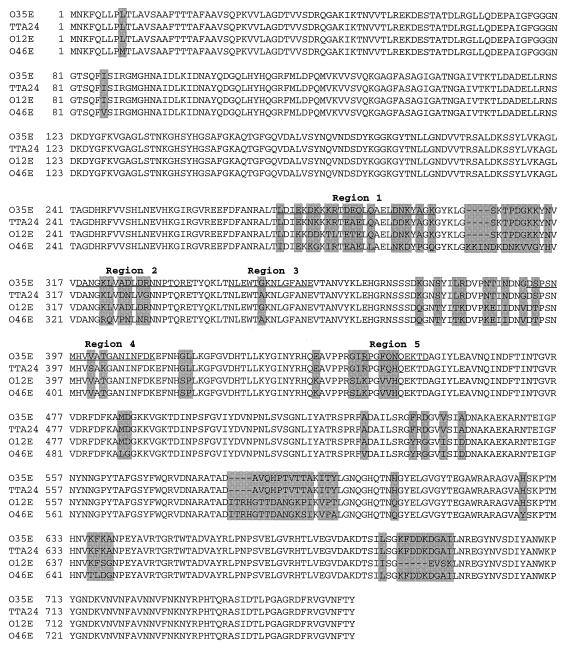
FIG. 1.
Comparison of the deduced amino acid sequences of the CopB proteins from M. catarrhalis O35E, O12E, TTA24, and O46E. Shaded areas indicate positions of amino acid differences. Regions 1 to 5 demarcate the general areas where the amino acid sequences of the CopB proteins from strains O35E and TTA24 differ. The underlined amino acid sequences correspond to the synthetic peptides R1, R2, R3, R4, and R5, respectively.
The fact that the CopB proteins of strains O35E and TTA24 had the highest degree of identity but differed in their abilities to bind MAb 10F3 provided the necessary information to begin localizing the MAb 10F3-reactive epitope. Sequence deviations between these two CopB proteins were confined to five distinct regions (regions 1 to 5 [Fig. 1]) located between amino acid residues 280 and 450. Because MAb 10F3 was known to bind to both native and denatured CopB (26) and thus in all likelihood to a linear epitope, only one of these five regions was expected to be involved in the binding of this MAb.
Mapping of the MAb 10F3-reactive epitope.
Oligopeptides spanning the five regions of dissimilarity between the CopB proteins of strains O35E and TTA24 were synthesized; these contained the amino acid sequences from the MAb 10F3-reactive CopB protein of strain O35E (underlined sequences in Fig. 1). These peptides were designated R1 through R5 and had amino acid sequences as follows: R1, LDIEKDKKKRTDEQLQAELDNKYAGK; R2, DANGKLVADLDRNNPTQRE; R3, NLEWTGKNLGFANE; R4, DSPSNMHVVATGANINFDK; and R5, RPGFQNQEKTD.
The abilities of these peptides to bind MAb 10F3 were investigated by means of a direct ELISA. When sodium carbonate coating buffer was used to affix the five peptides to the microtiter wells, none of them bound MAb 10F3. Variations in the pH and salt concentration of this coating buffer and variation of the coating temperature did not result in detectable binding of MAb 10F3 to any of these peptides. When 100-μl portions of MAb 10F3 hybridoma culture supernatant were preincubated with various concentrations of the five peptides and then used to probe microtiter wells coated with outer membrane vesicles of strain O35E, there was no concentration-dependent reduction of MAb 10F3-binding by any of the five peptides (data not shown). However, when glutaraldehyde was added to a final concentration of 2% to the five peptides dissolved in coating buffer, wells coated with the 26-residue peptide R1 (Fig. 2) readily bound MAb 10F3. In contrast, none of the other four peptides bound this MAb. The negative control MAb 17C7 did not bind any of these peptides (data not shown).
Amino acid sequence differences between strains O35E and TTA24 within region 1 corresponded to residues 6, 12, 13, 14, and 21 of peptide R1 (Fig. 2). Because the amino acids in positions 12, 13, and 14 also varied between the two MAb 10F3-reactive strains, O35E and O12E (Fig. 2), these residues were considered less likely to be essential for binding this MAb. The peptides R1A and R1B (Fig. 2) were synthesized to determine whether the aspartic acid (D) in position 6 or the asparagine (N) in position 21 was required for binding of MAb 10F3. As shown in Fig. 2, changing N to D in position 21 (peptide R1B) resulted in the loss of MAb 10F3 reactivity, while replacing D with N in position 6 (peptide R1A) did not affect binding of this MAb. The presence of N in position 21 of peptide R1 (corresponding to residue 295 in the CopB protein) thus appeared critical for binding of MAb 10F3. As expected, a peptide (R1-TTA24) containing the amino acid sequence from the same region of the CopB protein from M. catarrhalis TTA24 did not bind this MAb (Fig. 2).
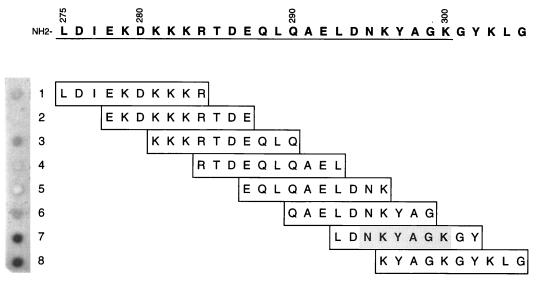
Based on the assumption that sequential B-cell epitopes are typically 5 to 7 amino acids in length (30, 43), an oligopeptide (R1E in Fig. 2) which contained 5 amino acids of the CopB sequence of strain O35E on either side of the N in position 21 of peptide R1 was expected to bind MAb 10F3. However, peptide R1E exhibited no binding of this MAb (Fig. 2). Therefore, the binding site for MAb 10F3 within R1 was mapped by using eight overlapping decapeptides (Fig. 3) bound (via the N terminus) to a solid-phase support composed of derivatized cellulose. Probing these eight decapeptides with MAb 10F3 revealed that peptides 7 and 8 readily bound MAb 10F3 (Fig. 3), with peptide 7 exhibiting the strongest binding. The sequence LDNKYAGKGY (residues 293 to 302 of the CopB protein of strain O35E) thus allowed maximal binding of MAb 10F3.
FIG. 3.
Fine mapping of the MAb 10F3-reactive epitope by a dot blot assay involving immobilized synthetic decapeptides. Binding of MAb 10F3 to individual peptides was detected by incubating the membrane with radioiodinated goat anti-mouse immunoglobulin. The autoradiograph of the dot blot (peptides 1 to 8) is shown on the left; the corresponding amino acid sequence of each of the overlapping decapeptides with their respective positions in the CopB protein is listed on the right. The numbers written vertically denote the amino acid positions in the intact CopB protein; the R1 region is underlined.
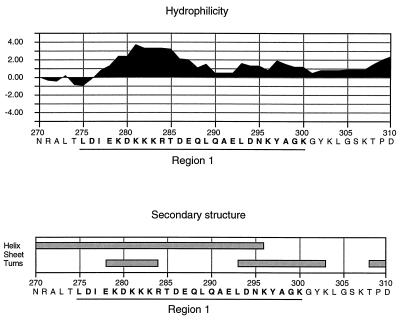
By the method of Kyte and Doolittle (31), further analysis of region 1 within the context of the CopB protein of strain O35E revealed that this region is part of a highly hydrophilic domain (Fig. 4). Use of the Chou-Fasman method (9) for predicting secondary structure indicated that this region likely displays a primarily α-helical structure, while the binding site for MAb 10F3 is predicted to form a β-turn (Fig. 4).
FIG. 4.
Hydropathy plot and secondary-structure analysis of region 1 from the CopB protein of M. catarrhalis O35E. The hydropathy plot was generated by the method of Kyte and Doolittle (31), with a window size of seven residues, and secondary-structure predictions were made by the method of Chou and Fasman (9) as found in MacVector sequence analysis software (version 6.0). The numbers on the horizontal scale denote the amino acid positions in the intact CopB protein; the R1 region is underlined.
Immunogenicity of the GST-26 fusion protein.
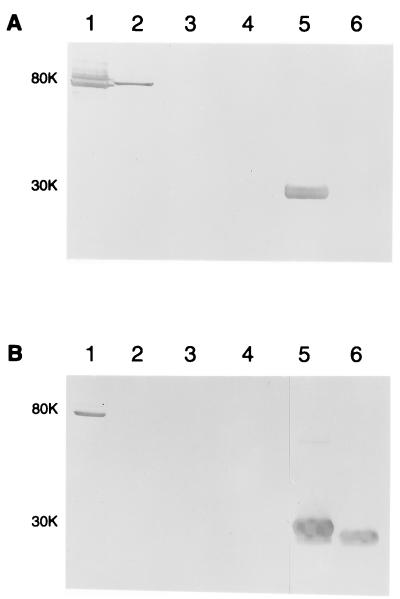
To determine whether the peptide sequence of R1 could induce the synthesis of antibodies to CopB that would bind to the surface of M. catarrhalis, this 26-residue peptide was expressed in a fusion construct (GST-26) at the C terminus of the 26-kDa GST protein and used to immunize mice. As expected, the molecular mass of purified GST-26 was approximately 30 kDa (Fig. 5, lanes 5) and was strongly reactive with MAb 10F3 in Western blot analysis (Fig. 5A, lane 5), while GST alone was unreactive with this MAb (Fig. 5A, lane 6). GST-26 was also strongly reactive with MAb 10F3 when used as the antigen in the direct ELISA system (data not shown).
FIG. 5.
Western blot analysis of the reactivities of MAbs and polyclonal serum antibodies with outer membrane proteins of M. catarrhalis strains, the GST-26 fusion protein, and GST alone. Proteins present in outer membrane vesicles from strains O35E (lane 1), O12E (lane 2), TTA24 (lane 3), and O46E (lane 4) and purified GST-26 (lane 5) and GST (lane 6) were resolved by SDS-PAGE, transferred to nitrocellulose, and probed with MAb 10F3 (A) or with mouse GST-26 antiserum (dilution 1:1,000) (B). The CopB protein from strain O35E gave rise to a doublet reactive with MAb 10F3 in this experiment. The positions of the molecular mass markers (in kilodaltons) are indicated to the left of each panel.
The presence of CopB-specific antibody in mouse antiserum raised against GST-26 was assessed by ELISA using the synthetic peptides R1, R1A, R1E, R1B, and R1-TTA24 (Fig. 2) and by Western blot analysis using outer membrane vesicles of M. catarrhalis O35E, O12E, TTA24, and O46E as well as GST-26 and GST as antigens. In the ELISA, the GST-26 antiserum, but not the GST antiserum, reacted strongly with R1, R1A, R1E, and R1B (Fig. 2). Interestingly, GST-26 antiserum also reacted with R1-TTA24 (Fig. 2). Binding occurred regardless of whether glutaraldehyde was included with the peptides for coating of the microtiter plate wells (described above).
In Western blot analysis, both the CopB-specific MAb 10F3 (Fig. 5A, lane 1) and antibodies in the GST-26 antiserum (Fig. 5B, lane 1) bound to an 80-kDa antigen of strain O35E. MAb 10F3 also bound to an 82-kDa antigen from strain O12E (Fig. 5A, lane 2); the CopB protein of strain O12E is known to migrate slightly more slowly during SDS-PAGE than the CopB protein of strain O35E. The GST-26 antiserum did not react with any antigens in the 80- to 82-kDa range from strains O12E, TTA24, and O46E (Fig. 5B, lanes 2 to 4, respectively). As expected, antibodies in the GST-26 antiserum bound to both GST-26 and GST (Fig. 5B, lane 5 and 6, respectively).
Binding of GST-26 antibodies to the cell surface of M. catarrhalis.
The MAb 10F3-reactive epitope of CopB has been shown to be expressed on the bacterial surface (26). Because the GST-26 antiserum was shown to contain antibodies specifically binding to the CopB protein from strain O35E and also to region 1 of strain TTA24 (by ELISA with R1-TTA24 [Fig. 2]), it was of interest to determine whether antibodies derived from this antiserum would bind to the cell surface of M. catarrhalis. The use of the indirect antibody accessibility assay with whole cells of M. catarrhalis O35E, TTA24, O12E, and O46E as antigen demonstrated that, as expected, MAb 10F3 readily bound to the surface of strains O35E and O12E but not strains TTA24 and O46E (Table 2). Similarly, antibodies in the GST-26 antiserum exhibited specific binding to whole cells of strain O35E, while antibodies present in the control antiserum raised against GST did not (Table 2).
TABLE 2.
Binding of antibodies to surface determinants of whole cells of M. catarrhalis strains
| M. catarrhalis strain | Primary antibodya
|
|||
|---|---|---|---|---|
| GST-26 antiserum | GST antiserumb | MAb 10F3c | MAb 3F12d | |
| O35E | 62,712a | 6,081 | 107,332 | 3,437 |
| O12E | 50,099 | 4,293 | 63,830 | 3,817 |
| TTA24 | 40,480 | 4,795 | 4,231 | 2,651 |
| O46E | 14,223 | 16,205 | 3,952 | 3,544 |
Counts per minute of 125I-labeled goat anti-mouse immunoglobulin bound to murine antibodies attached to the bacterial cell surface, as determined by the indirect antibody accessibility assay. The results are the means of two independent experiments.
Antiserum raised in mice against GST was included as a negative control.
MAb 10F3, specific for the M. catarrhalis CopB outer membrane protein, was included as a positive control.
MAb 3F12, a murine IgG antibody specific for an H. ducreyi MOMP, was included as a negative control.
As expected from the ELISA data, antibodies in the GST-26 antiserum also reacted with whole cells of strains O12E and TTA24 (Table 2), although no binding of the same antiserum to outer membrane vesicles of these strains was detected by Western blot analysis (Fig. 5B, lanes 2 and 3, respectively). High levels of binding of the GST control antiserum to whole cells of strain O46E (Table 2) precluded determination of whether the GST-26 antiserum would bind specifically to this organism.
Bactericidal activity of MAb 10F3 and of GST-26 antiserum.
MAb 10F3 was previously shown to enhance pulmonary clearance of M. catarrhalis from the lungs of mice passively immunized with this MAb (26). However, the functional basis for this MAb-accelerated elimination of bacteria from the lower respiratory tract was not determined. Therefore, the ability of MAb 10F3 to kill M. catarrhalis in the presence of human complement was evaluated by performing bactericidal assays with the MAb 10F3-reactive strains O35E and O12E and with the MAb 10F3-unreactive strains TTA24 and O46E. Similarly, the bactericidal activities of the antisera raised against GST-26 and GST (negative control) were tested, because GST-26 contained antibodies directed at the surface of strains O35E, O12E, and TTA24.
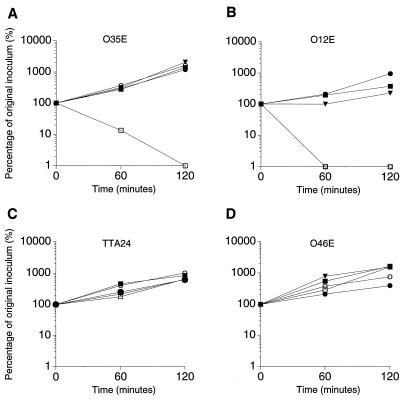
All four M. catarrhalis strains were resistant to killing by complement-sufficient normal human serum in the absence of specific antibody (Fig. 6). In the presence of normal human serum, strains O35E (Fig. 6A) and O12E (Fig. 6B) were readily killed by MAb 10F3. The MAb 10F3-unreactive strains TTA24 and O46E were not killed by MAb 10F3 (Fig. 6C and D). MAb 10F3 did not kill any of these four strains when heat-inactivated normal human serum was used in place of complement-sufficient normal human serum (Fig. 6).
FIG. 6.
Bactericidal activity of MAb 10F3 against M. catarrhalis strains. Suspensions of the MAb 10F3-reactive strains O35E (A) and O12E (B) and the MAb 10F3-unreactive strains TTA24 (C) and O46E (D) were incubated with normal human serum (solid triangles), normal human serum and MAb 10F3 (open squares), heat-inactivated normal human serum and MAb 10F3 (solid squares), normal human serum and GST-26 antiserum (open circles), or normal human serum and GST antiserum (solid circles). Portions of the reaction mixture were removed over time and spread on BHI agar plates to determine the number of viable bacteria. (B) Note that the open circles and open squares are superimposed.
The GST-26 antiserum did not exert detectable bactericidal activity against strains O35E, TTA24, and O46E (Fig. 6A, C, and D). However, the same GST-26 antiserum did kill strain O12E (Fig. 6B). The possibility that a prozone effect had prevented killing of strain O35E by the GST-26 antiserum was addressed by incubating M. catarrhalis O35E cells with dilutions of the GST-26 antiserum ranging from 1:20 to 1:2,000, none of which showed bactericidal activity (data not shown). Control antiserum raised against GST did not cause complement-mediated killing of any of these M. catarrhalis strains (Fig. 6). Western blot analysis revealed that antibodies reactive with M. catarrhalis were present in the normal human serum used as the source of complement in this bactericidal activity system (Fig. 7). Antibodies in this serum bound antigens of both M. catarrhalis O35E (Fig. 7, lane 1) and O12E (Fig. 7, lane 2); the former strain had more reactive antigens than did the latter strain.
FIG. 7.
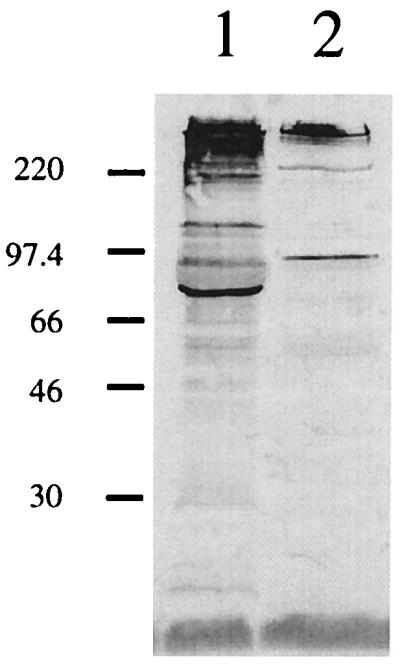
Detection of M. catarrhalis-reactive antibodies in normal human serum by Western blot analysis. Proteins present in EDTA-extracted outer membrane vesicles of M. catarrhalis O35E (lane 1) and O12E (lane 2) were resolved by SDS-PAGE, transferred to nitrocellulose, and probed in Western blot analysis with 100 μl of the normal human serum used as the source of complement in the bactericidal activity assay. The positions of the molecular mass markers (in kilodaltons) are shown to the left.
DISCUSSION
It has been documented that infections caused by M. catarrhalis induce an antibody response directed against this pathogen (6, 8, 16, 17, 19, 20, 44) and that some of these antibodies have the potential to exert biologic activity via complement-dependent killing (6) or opsonophagocytosis (16). However, with a few exceptions (20, 44), the identity of the target antigen(s) remains unclear, because in most studies, whole bacteria or crude outer membrane preparations were used as antigens to detect M. catarrhalis-directed antibodies. To date, the only M. catarrhalis outer membrane antigens that have been shown to be targets for antibodies that can exert protective effects in an animal model are the CopB and UspA proteins (7, 26, 27) while antibodies to protein CD have been reported to be bactericidal in vitro (46).
Several lines of evidence indicate that portions of the CopB protein are exposed on the surface of M. catarrhalis. These include (i) the reactivity of the CopB-specific MAb 10F3 with a surface-exposed epitope (26), (ii) the bactericidal activity of MAb 10F3 against strains that react with this MAb (Fig. 6), and (iii) the likely function of the CopB protein in a TonB-dependent physiologic process (2). In addition, FrpB, an iron-regulated outer membrane protein of Neisseria meningitidis that is 49% identical to the CopB protein of strain O35E, has also been shown to be a target for bactericidal antibody (39, 40).
Comparison of the deduced amino acid sequences of the CopB proteins from four M. catarrhalis strains revealed that reactivity with MAb 10F3 was not an indicator of similarity between CopB proteins. Furthermore, the primary amino acid sequence of this protein was very well conserved among these four strains. The level of identity among these four CopB proteins proved to be greater than 90%, a finding which is somewhat encouraging from the standpoint of vaccine development. A similar level of amino acid sequence conservation has been reported for the CD outer membrane protein of M. catarrhalis (29).
The facts that the MAb 10F3-reactive epitope was detected in the majority of M. catarrhalis strains (26) and that this epitope induced the synthesis of antibodies that were biologically active both in vivo (26) and in vitro (Fig. 6) made the determination of this epitope’s location within the CopB protein an important step in the evaluation of CopB as a potential vaccine candidate. This task was facilitated considerably by the finding that the deduced amino acid sequence differences between the CopB protein of the MAb 10F3-reactive strain O35E and that of the MAb 10F3-unreactive strain TTA24 were limited to five well-defined regions (Fig. 1), one of which had to contain the MAb 10F3-reactive epitope. The analyses of the copB genes from two other M. catarrhalis strains reinforced the likelihood that one of these five regions bound MAb 10F3 and prompted the synthesis of oligopeptides that contained the corresponding amino acid sequences from the CopB protein of strain O35E.
The use of these synthetic peptides in an ELISA identified region 1 as the sequence containing the MAb 10F3-reactive epitope. Unexpectedly, binding of MAb 10F3 to peptide R1 (Fig. 2) required the presence of the cross-linking agent glutaraldehyde during the coating of the polystyrene surface of the ELISA plate with this peptide. At first, it was thought that either inherently poor binding of R1 to the microtiter well or binding of R1 to the well via the MAb 10F3-reactive residues was responsible for this finding. However, the failure of soluble R1 to bind MAb 10F3 in an inhibition assay as well as the subsequent observation that GST-26 antiserum readily bound to microtiter wells coated with R1 in the absence of glutaraldehyde invalidated these hypotheses and made conformational changes of R1 caused by glutaraldehyde a more plausible explanation. Glutaraldehyde preferentially cross-links amino groups of lysine residues such as those present in both the N- and C-terminal portions of R1 (Fig. 2). Cross-linking of these lysine residues may have led to the formation of a loop-like structure that more closely resembled the native conformation of the MAb 10F3-reactive epitope. This hypothesis is supported by the lack of binding of MAb 10F3 by peptide R1E (Fig. 2) which, as was subsequently determined, contained the binding site for MAb 10F3 but lacked the lysine-rich N-terminal portion of R1.
Differential binding of MAb 10F3 to the peptides R1A and R1B (Fig. 2) indicated that this MAb likely bound the C-terminal portion of R1 and that the asparagine (N) residue in position 21 was essential for binding. This assumption was confirmed by fine mapping of the MAb 10F3-reactive epitope by using overlapping decapeptides (Fig. 3), which demonstrated optimal binding of MAb 10F3 to a decapeptide that corresponded to residues 19 to 28 of region 1 (i.e., residues 293 to 302 of the CopB protein). This epitope comprises the C-terminal portion of a highly hydrophilic domain when analyzed by means of the hydropathy algorithm of Kyte and Doolittle (31) (Fig. 4). This finding is in keeping with the proven, bacterial cell surface-exposed location of the binding site for MAb 10F3. It is also noteworthy that the MAb 10F3-reactive epitope appears to be part of the largest variable region of the otherwise highly conserved CopB proteins studied here (Fig. 1). This finding is reminiscent of those for other well-studied bacterial outer membrane proteins, such as the MOMP P2 of nontypeable Haemophilus influenzae, for which it has been demonstrated that some surface-exposed domains exhibit strain-specific antigenicity (13, 14, 23, 47).
Characterization of the immune response directed against the GST-26 fusion protein containing the MAb 10F3 binding site revealed that, in Western blot analysis, antibodies in the GST-26 antiserum bound the CopB protein of strain O35E but not the CopB protein of the equally MAb 10F3-reactive strain O12E (Fig. 5B). This result suggested that these polyclonal antibodies did not possess the same antigenic specificity as MAb 10F3. In fact, the strong reactivity of the GST-26 antiserum with the MAb 10F3-unreactive peptides R1B and R1-TTA24 (Fig. 2) suggested that most of these polyclonal antibodies are directed against epitopes of peptide R1 other than that which binds MAb 10F3. The reduced reactivity of GST-26 antiserum with peptide R1E, which consisted of the 15 C-terminal residues of peptide R1, indicated that, not unexpectedly, some of the antibodies in the GST-26 antiserum are directed against epitopes located in the N-terminal portion of peptide R1.
Antibodies raised against GST-26 bound to the surface of whole cells of M. catarrhalis O35E, O12E, and TTA24 (Table 2) but exerted bactericidal activity against only strain O12E (Fig. 6). That these polyclonal antibodies, raised against this strain O35E-derived region 1 sequence (Fig. 2) could bind to strains O12E and TTA24 is not surprising when one considers that this same region of the CopB proteins of these other two strains was 73 to 80% identical to that of strain O35E (Fig. 2). It is also likely that these polyclonal antibodies had relatively low binding affinities which precluded their detection in Western blot analysis. However, the fact that these same surface-directed antibodies killed only strain O12E and not the homologous strain O35E or the heterologous strain TTA24 is more difficult to explain.
One possibility is the presence of an immunodominant determinant in the R1 region that induced the synthesis of blocking antibodies that bound to the R1 region of CopB of strains O35E and TTA24 but which were unreactive with the R1 region of CopB of strain O12E. Alternatively, there may have been other blocking antibodies in the mouse antiserum or in the normal human serum used as the source of complement in these experiments; these blocking antibodies could be directed against a surface determinant of strains O35E and TTA24 that was absent on strain O12E. In fact, Western blot analysis of the normal human serum used as the source of complement revealed that it contained antibodies that bound M. catarrhalis antigens (Fig. 7). The fact that antigens of strain O35E apparently bound more of these normal human serum antibodies than did strain O12E (Fig. 7) gives some credence to the possibility that blocking antibodies may be involved in the inability of the GST-26 antiserum to kill strain O35E. In this regard, noted that the existence of normal human serum antibodies reactive with one or more M. catarrhalis outer membrane proteins with apparent molecular masses of approximately 80 kDa was reported some time ago by Goldblatt and colleagues (20). Investigation of the possible involvement of blocking antibodies in this inability of the GST-26 antiserum to kill strain O35E is beyond the scope of the present study.
In summary, comparison of the deduced amino acid sequences of the CopB proteins from four strains of M. catarrhalis revealed a striking degree of identity among these macromolecules. This conservation of the primary amino acid sequence of the CopB protein among strains of this pathogen was crucial for localization of the protective epitope on the CopB protein of M. catarrhalis O35E that is recognized by the bactericidal MAb 10F3. Immunization with a fusion protein reactive with MAb 10F3 induced the synthesis of CopB-specific antibodies bactericidal for at least one MAb 10F3-reactive strain of M. catarrhalis. Further work will be necessary to determine (i) whether immunization with larger CopB peptides or with purified CopB protein can induce an immune response that is bactericidal as well as cross-reactive and (ii) whether blocking antibodies will affect the biological activity of polyclonal CopB antibodies.
ACKNOWLEDGMENTS
This study was supported by U.S. Public Health Service Grant AI-36344 and by an Advanced Technology Project award (003660-087) from the Texas Higher Education Coordinating Board to E.J.H. C.A. was supported by grants from the National Research Foundation of Switzerland and from Novartis AG, Basel, Switzerland.
We thank Steven Berk and John D. Nelson for providing the clinical isolates of M. catarrhalis used in this study.
REFERENCES
- 1.Anonymous. Consensus. Pediatr Infect Dis J. 1989;8:S94–S97. [Google Scholar]
- 1a.Aebi C, Cope L D, Latimer J L, Thomas S E, Slaughter C A, McCracken G H, Hansen E J. Abstracts of the 36th International Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Moraxella catarrhalis outer membrane protein CopB: identification of a peptide that contains a protective epitope, abstr. 783; p. 158. [Google Scholar]
- 2.Aebi C, Stone B, Beucher M, Cope L D, Maciver I, Thomas S E, McCracken G H, Jr, Sparling P F, Hansen E J. Expression of the CopB outer membrane protein by Moraxella catarrhalis is regulated by iron and affects iron acquisition from transferrin and lactoferrin. Infect Immun. 1996;64:2024–2030. doi: 10.1128/iai.64.6.2024-2030.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aspin M M, Hoberman A, McCarty J, McLinn S E, Aronoff S, Lang D J, Arrieta A. Comparative study of the safety and efficacy of clarithromycin and amoxicillin-clavulanate in the treatment of acute otitis media in children. J Pediatr. 1995;126:677–678. doi: 10.1016/s0022-3476(94)70140-7. [DOI] [PubMed] [Google Scholar]
- 4.Bartos L C, Murphy T F. Comparison of the outer membrane proteins of 50 strains of Branhamella catarrhalis. J Infect Dis. 1988;158:761–765. doi: 10.1093/infdis/158.4.761. [DOI] [PubMed] [Google Scholar]
- 5.Campagnari A A, Shanks K L, Dyer D W. Growth of Moraxella catarrhalis with human transferrin and lactoferrin: expression of iron-repressible proteins without siderophore production. Infect Immun. 1994;62:4909–4914. doi: 10.1128/iai.62.11.4909-4914.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chapman A J, Jr, Musher D M, Jonsson S, Clarridge J E, Wallace R J., Jr Development of bacterial antibody during Branhamella catarrhalis infection. J Infect Dis. 1985;151:878–882. doi: 10.1093/infdis/151.5.878. [DOI] [PubMed] [Google Scholar]
- 7.Chen D, McMichael J C, van der Meid K R, Hahn D, Mininni T, Cowell J, Eldridge J. Evaluation of purified UspA from Moraxella catarrhalis as a vaccine in a murine model after active immunization. Infect Immun. 1996;64:1900–1905. doi: 10.1128/iai.64.6.1900-1905.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chi D S, Verghese A, Moore C, Hamati F, Berk S L. Antibody response to P-protein in patients with Branhamella catarrhalis infections. Am J Med. 1990;88(5A):25S–27S. doi: 10.1016/0002-9343(90)90257-e. [DOI] [PubMed] [Google Scholar]
- 9.Chou P Y, Fasman G D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- 10.Davies B I, Maesen F P V. The epidemiology of respiratory tract pathogens in Southern Netherlands. Eur Respir J. 1988;1:415–420. [PubMed] [Google Scholar]
- 11.Del Beccaro M A, Mendelman P M, Inglis A F, Richardson M A, Duncan N O, Clausen C R, Stull T L. Bacteriology of acute otitis media: a new perspective. J Pediatr. 1992;120:81–84. doi: 10.1016/s0022-3476(05)80605-5. [DOI] [PubMed] [Google Scholar]
- 12.Doern G V, Miller M J, Winn R E. Branhamella (Neisseria) catarrhalis systemic disease in humans. Arch Intern Med. 1981;141:1690–1692. [PubMed] [Google Scholar]
- 13.Duim B, van Alphen L, Eijk P P, Jansen H M, Dankert J. Antigenic drift of non-encapsulated Haemophilus influenzae major outer membrane protein P2 in patients with chronic bronchitis is caused by point mutations. Mol Microbiol. 1994;11:1181–1189. doi: 10.1111/j.1365-2958.1994.tb00394.x. [DOI] [PubMed] [Google Scholar]
- 14.Duim B, Vogel L, Puijk W, Jansen H M, Meloen R H, Dankert J, van Alphen L. Fine mapping of outer membrane protein P2 antigenic sites which vary during persistent infection by Haemophilus influenzae. Infect Immun. 1996;64:4673–4679. doi: 10.1128/iai.64.11.4673-4679.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ey P I, Prowse S J, Jenkin C R. Isolation of pure IgG1, IgG2a, and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978;15:429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- 16.Faden H, Hong J J, Pahade N. Immune response to Moraxella catarrhalis in children with otitis media: opsonophagocytosis with antigen-coated latex beads. Ann Otol Rhinol Laryngol. 1994;103:522–524. doi: 10.1177/000348949410300704. [DOI] [PubMed] [Google Scholar]
- 17.Faden H S, Hong J J, Murphy T F. Immune response to outer membrane antigens of Moraxella catarrhalis in children with otitis media. Infect Immun. 1992;60:3824–3829. doi: 10.1128/iai.60.9.3824-3829.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gan V N, Kusmiesz H, Shelton S, Nelson J D. Comparative evaluation of loracarbef and amoxicillin-clavulanate for acute otitis media. Antimicrob Agents Chemother. 1991;35:967–971. doi: 10.1128/aac.35.5.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldblatt D, Seymour N D, Levinsky R J, Turner M W. An enzyme-linked immunosorbent assay for the determination of human IgG subclass antibodies directed against Branhamella catarrhalis. J Immunol Methods. 1990;128:219–225. doi: 10.1016/0022-1759(90)90213-f. [DOI] [PubMed] [Google Scholar]
- 20.Goldblatt D, Turner M W, Levinsky R J. Branhamella catarrhalis: antigenic determinants and the development of the IgG subclass response in childhood. J Infect Dis. 1990;162:1128–1135. doi: 10.1093/infdis/162.5.1128. [DOI] [PubMed] [Google Scholar]
- 21.Gulig P A, Frisch C F, Hansen E J. A set of two monoclonal antibodies specific for the cell surface-exposed 39K major outer membrane protein of Haemophilus influenzae type b defines all strains of this pathogen. Infect Immun. 1983;42:516–524. doi: 10.1128/iai.42.2.516-524.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gulig P A, McCracken G H, Jr, Frisch C F, Johnston K H, Hansen E J. Antibody response of infants to cell surface-exposed outer membrane proteins of Haemophilus influenzae type b after systemic Haemophilus disease. Infect Immun. 1982;37:82–88. doi: 10.1128/iai.37.1.82-88.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haase E M, Yi K, Morse G D, Murphy T F. Mapping of bacterial epitopes on the P2 porin protein of nontypeable Haemophilus influenzae. Infect Immun. 1994;62:3712–3722. doi: 10.1128/iai.62.9.3712-3722.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hager H, Verghese A, Alvarez S, Berk S L. Branhamella catarrhalis respiratory infections. Rev Infect Dis. 1987;9:1140–1149. doi: 10.1093/clinids/9.6.1140. [DOI] [PubMed] [Google Scholar]
- 25.Helminen M E, Beach R, Maciver I, Jarosik G P, Hansen E J, Leinonen M. Human immune response against outer membrane proteins of Moraxella (Branhamella) catarrhalis determined by immunoblotting and enzyme immunoassay. Clin Diagn Lab Immunol. 1995;2:35–39. doi: 10.1128/cdli.2.1.35-39.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Helminen M E, Maciver I, Latimer J L, Cope L D, McCracken G H, Jr, Hansen E J. A major outer membrane protein of Moraxella catarrhalis is a target for antibodies that enhance pulmonary clearance of the pathogen in an animal model. Infect Immun. 1993;61:2003–2010. doi: 10.1128/iai.61.5.2003-2010.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Helminen M E, Maciver I, Latimer J L, Klesney-Tait J, Cope L D, Paris M M, McCracken G H, Jr, Hansen E J. A large, antigenically conserved protein on the surface of Moraxella catarrhalis is a target for protective antibodies. J Infect Dis. 1994;170:867–872. doi: 10.1093/infdis/170.4.867. [DOI] [PubMed] [Google Scholar]
- 28.Helminen M E, Maciver I, Latimer J L, Lumbley S R, Cope L D, McCracken G H, Jr, Hansen E J. A mutation affecting expression of a major outer membrane protein of Moraxella catarrhalis alters serum resistance and survival of this organism in vivo. J Infect Dis. 1993;168:1194–1201. doi: 10.1093/infdis/168.5.1194. [DOI] [PubMed] [Google Scholar]
- 29.Hsiao C B, Sethi S, Murphy T F. Outer membrane protein CD of Branhamella catarrhalis—sequence conservation in stains recovered from the human respiratory tract. Microb Pathog. 1995;19:215–225. doi: 10.1016/s0882-4010(95)90272-4. [DOI] [PubMed] [Google Scholar]
- 30.Kabat E A. Heterogeneity and structure of antibody-combining sites. Ann N Y Acad Sci. 1970;169:43–54. doi: 10.1111/j.1749-6632.1970.tb55978.x. [DOI] [PubMed] [Google Scholar]
- 31.Kyte J, Doolittle R F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 32.Maciver I, Unhanand M, McCracken G H, Jr, Hansen E J. Effect of immunization on pulmonary clearance of Moraxella catarrhalis in an animal model. J Infect Dis. 1993;168:469–472. doi: 10.1093/infdis/168.2.469. [DOI] [PubMed] [Google Scholar]
- 33.Malkamaki M, Honkanen E, Leinonen M, Makela P H. Branhamella catarrhalis as a cause of bacteremic pneumonia. Scand J Infect Dis. 1983;15:125–126. doi: 10.3109/inf.1983.15.issue-1.21. [DOI] [PubMed] [Google Scholar]
- 34.McLeod D T, Ahmad F, Capewell S, Croughan M J, Calder M A, Seaton A. Increase in bronchopulmonary infection due to Branhamella catarrhalis. Br Med J Clin Res. 1986;292:1103–1105. doi: 10.1136/bmj.292.6528.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murphy T F, Bartos L C. Surface exposed and antigenically conserved determinants of outer membrane proteins of Branhamella catarrhalis. Infect Immun. 1989;57:2938–2941. doi: 10.1128/iai.57.10.2938-2941.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murphy T F, Kirkham C, Lesse A J. The major heat-modifiable outer membrane protein CD is highly conserved among strains of Branhamella catarrhalis. Mol Microbiol. 1993;10:87–97. doi: 10.1111/j.1365-2958.1993.tb00906.x. [DOI] [PubMed] [Google Scholar]
- 37.Murphy T F, Loeb M R. Isolation of the outer membrane of Branhamella catarrhalis. Microb Pathog. 1989;6:159–174. doi: 10.1016/0882-4010(89)90066-1. [DOI] [PubMed] [Google Scholar]
- 38.Nicotra B, Rivera M, Liman J I, Wallace R J. Branhamella catarrhalis as a lower respiratory tract pathogen in patients with chronic lung disease. Arch Intern Med. 1986;146:890–893. [PubMed] [Google Scholar]
- 39.Pettersson A, Kuipers B, Pelzer M, Verhagen E, Tiesjema R H, Tommassen J, Poolman J T. Monoclonal antibodies against the 70-kilodalton iron-regulated protein of Neisseria meningitidis are bactericidal and strain specific. Infect Immun. 1990;58:3036–3041. doi: 10.1128/iai.58.9.3036-3041.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pettersson A, Maas A, van Wassenaar D, van der Ley P, Tommassen J. Molecular cloning of FrpB, the 70-kilodalton iron-regulated outer membrane protein of Neisseria meningitidis. Infect Immun. 1995;63:4181–4184. doi: 10.1128/iai.63.10.4181-4184.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Post J C, Preston R A, Aul J J, Larkins-Pettigrew M, Rydquist-White J, Anderson K W, Wadowsky R M, Reagan D R, Walker E S, Kingsley L A, Magit A E, Ehrlich G D. Molecular analysis of bacterial pathogens in otitis media with effusion. JAMA. 1995;273:1598–1604. [PubMed] [Google Scholar]
- 42.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 43.Schechter I. Mapping of the combined sites of antibodies specific to polyalanine chains. Ann N Y Acad Sci. 1971;190:394–419. doi: 10.1111/j.1749-6632.1971.tb13551.x. [DOI] [PubMed] [Google Scholar]
- 44.Sethi S, Hill S L, Murphy T F. Serum antibodies to outer membrane proteins (OMPs) of Moraxella (Branhamella) catarrhalis in patients with bronchiectasis: identification of OMP B1 as an important antigen. Infect Immun. 1995;63:1516–1520. doi: 10.1128/iai.63.4.1516-1520.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vaneechoutte M, Verschraegen G, Claeys G, van den Abeele A M. Respiratory tract carrier rates of Moraxella (Branhamella) catarrhalis in adults and children and interpretation of the isolation of M. catarrhalis from sputum. J Clin Microbiol. 1990;28:2674–2680. doi: 10.1128/jcm.28.12.2674-2680.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang Y-P, Myers L E, Mcguinness U, Chong P, Kwok Y, Klein M H, Harkness R E. The major outer membrane protein, CD, extracted from Moraxella (Branhamella) catarrhalis is a potential vaccine antigen that induces bactericidal antibodies. FEMS Immunol Med Microbiol. 1997;17:187–199. doi: 10.1111/j.1574-695X.1997.tb01012.x. [DOI] [PubMed] [Google Scholar]
- 47.Yi K, Murphy T F. Mapping of a strain-specific bacterial epitope to the surface-exposed loop 5 on the P2 porin protein of non-typeable Haemophilus influenzae. Microb Pathog. 1994;17:277–282. doi: 10.1006/mpat.1994.1073. [DOI] [PubMed] [Google Scholar]