Abstract
Woolly mammoths in mainland Alaska overlapped with the region’s first people for at least a millennium. However, it is unclear how mammoths used the space shared with people. Here, we use detailed isotopic analyses of a female mammoth tusk found in a 14,000-year-old archaeological site to show that she moved ~1000 kilometers from northwestern Canada to inhabit an area with the highest density of early archaeological sites in interior Alaska until her death. DNA from the tusk and other local contemporaneous archaeological mammoth remains revealed that multiple mammoth herds congregated in this region. Early Alaskans seem to have structured their settlements partly based on mammoth prevalence and made use of mammoths for raw materials and likely food.
A female mammoth’s lifetime movements are traced to a hotspot of early Alaskan hunting camps with remains of her relatives.
INTRODUCTION
Woolly mammoths (Mammuthus primigenius), an iconic ice age species, are undergoing a cultural, scientific, and perhaps even literal renaissance stemming from new evidence of late-surviving populations (1–5), ongoing debate about the causes of their extinction (6–9), and well-publicized efforts to “de-extinct” them (10, 11). However, their behavior remains largely enigmatic, despite its importance to our growing interest in how mammoths survived, why they became extinct, and what they might need to live in our modern world if rewilded.
Woolly mammoth populations in mainland Alaska peaked shortly after the Last Glacial Maximum (LGM), ~20 thousand calibrated years before present (ka), before the spread of shrub tundra, peatlands, and eventually the boreal forest (12–16). This late glacial period (20 to 12 ka) coincides with the earliest archaeological evidence for the spread of people from Eurasia through Beringia (17–20). Woolly mammoths and people coexisted on a regional scale at the close of the last ice age in Alaska for at least a millennium, demonstrated by the overlap in the earliest dated archaeological sites and the latest mammoth specimens in both archaeological and paleontological contexts. However, the precise nature of their interactions is unresolved.
The ~1000-year period of overlap between people and mammoths in Alaska highlights the potential for interaction, including hunting, which would have affected the dynamics of mammoth extinction, a key aspect of the broader debate on the causes of megafaunal extinctions in the Americas (21, 22). Evidence indicates that human roles in other megafaunal extinctions in the Americas may have been substantial (23). However, there has been limited direct evidence of mammoth predation in Beringia, contrary to a blitzkrieg overkill scenario that implicates people as the central cause for mammoth extirpation (22, 24). Contemporaneous mammoth remains are clearly present at some of the earliest archaeological sites in Alaska (25, 26) before a sharp population decline in mammoths after ~13 ka (12). However, human use of mammoth ivory and bones seen in these archaeological contexts could have derived from scavenging of either recently deceased or long-dead animals, and people may have thus played a less direct role in conjunction with climate and vegetation change (6). The post-LGM warmer and wetter conditions coincided with (and perhaps facilitated) human settlement of Alaska but, at the same time, activated changes in vegetation that would have been inhospitable to large grazers such as mammoths, confounding our ability to understand the role of people in the extinction process (6, 9, 12, 27). Mammoth population decline, whether the initial impetus was human or climate driven, may also have diminished their prominent role as ecosystem engineers (28, 29), possibly contributing to boreal forest expansion and peat formation in the former mammoth steppe (7).
Underlying these controversies are issues regarding mammoth ecology and behavior and, in particular, the question of how mammoths moved across the landscape in ways that influenced their susceptibility to human interaction. If mammoths made regular and predictable movements across a landscape also occupied by people, then it could have made them an attractive resource (30), in much the same way as caribou in Alaska (31, 32). Alternatively, erratic and widespread movements might have made mammoths less predictable for people. The expansion of shrub tundra in Alaska after the LGM, regardless of whether it can be attributed solely to climate change or exacerbated by megafaunal hunting, may have fragmented mammoths’ preferred open habitat, potentially decreasing movement and making them more vulnerable to human predation (6).
We conducted isotopic and genetic analyses of a complete woolly mammoth tusk (UA2009-177-21444, named Élmayųujey’eh by the Healy Lake Village Council, Supplementary Text) from the Swan Point archaeological site located in the Shaw Creek basin of the Tanana River valley in interior Alaska. This tusk is one of only two largely complete adult tusks from archaeological contexts in Alaska (table S1). The tusk was found in and dated to the same time as the initial human appearance in Swan Point’s Cultural Zone 4b (CZ4b) [14,177 to 13,900 calibrated years before present (ya)], the oldest known archaeological component in Alaska (33). CZ4b also contained a suite of other mammoth remains, some clearly from contemporaneous individuals, including a juvenile and neonate (Supplementary Text). Additional mammoth remains have also been found in a cluster of three other early archaeological sites (the Broken Mammoth, Holzman, and Mead sites) within ~10 km of the Swan Point site in the Shaw Creek basin (fig. S1 and Supplementary Text). The Swan Point adult mammoth tusk was previously interpreted as having been scavenged in a subfossilized state based on its slightly older age relative to other dated samples from the cultural occupation (25). However, a new radiocarbon date for this tusk produced a younger calibrated date between 13,810 and 14,068 ya (33), which overlaps with multiple hearth features, artifacts, and other mammoth remains in the same cultural zone (CZ4b) and indicates they were contemporaneous.
The Swan Point CZ4b component is interpreted as a seasonal workshop and hunting camp containing numerous organic and stone tools, including the tusk analyzed in this study (25). The tusk likely served multiple functions, both as an anvil and as a source of ivory fragments for later modification. The occupation of CZ4b coincides with the beginning of a rapid regional transition from herb- to shrub-dominated tundra (34). Graminoids and forbs, the preferred forage for mammoths, were still an important component of the local vegetation, but they were increasingly supplanted by birch and willow shrubs during this interval (34). Growing season temperatures were relatively stable at this time (35), suggesting a general increase in moisture as the main cause for this vegetation change. We studied the strontium (87Sr/86Sr), oxygen (δ18O), and sulfur (δ34S) isotope ratios of the complete adult tusk from Swan Point CZ4b to model the lifetime movement of the mammoth. To determine the sex and relatedness of the mammoths from Swan Point and other local archaeological sites, we extracted and analyzed ancient DNA (aDNA).
RESULTS
The DNA of the Swan Point tusk showed that this mammoth was female and closely related to the other mammoth individuals we analyzed from Swan Point (Fig. 1 and Supplementary Text) and more distantly related to the tusk from the nearby Holzman site (Supplementary Text). These specimens included the remains of a male neonate and male juvenile mammoth from Swan Point CZ4b. Mammoth remains at Swan Point number in the hundreds (see the Supplemental Materials), most being fragments of ivory from tusk reduction. At least three full tusks are present, including the adult and juvenile tusks sampled in this paper. More than a hundred cheek teeth fragments were documented, which together account for at least two cheek teeth from a juvenile or juveniles. At least twelve rib fragments are present and account for a minimum of eight elements, the sizes of which are consistent with having been obtained from a single neonate mammoth. If mammoths behaved in similar ways to elephants, the neonate and juvenile would have needed to travel with a matriarchal herd until their maturity (36). The mitochondrial genomes reconstructed from eight of the mammoths showed that, although mainly contemporaneous, the mammoths at these archaeological sites consisted primarily of members from at least two, closely related (ancestor and descendant) but distinct herds (Fig. 1 and Supplementary Text).
Fig. 1. Bayesian maximum clade credibility tree of dated Mammuthus spp. mitochondrial sequences.
(A) Heatmap comparison of nucleotide differences between haplogroup C mammoths from Eastern Beringia, (B) map of sample origins, (C) mapDamage nucleotide misincorporation plots showing terminal base modifications typical of aDNA, and (D) fragment length distributions from the eight reassembled mitochondrial genomes. Tip dates are estimated as calendar/calibrated years before present using a strict clock with the bModel test to average over substitution models, a Bayesian skyline tree prior, and 100 million iterations. Node labels indicate posterior support, and the blue bars show the 95% highest posterior distribution (HPD) for clade divergence age. Map of sample locations color-coded based on clade branches in the tree and geographic region. Sea level in the map set 126 m lower than present to approximate the land extent during the LGM.
Microscopic examination of growth layers exposed on the interior surface of the bisected Swan Point adult tusk indicated approximately 20 years represented in the tusk (Supplementary Text). This growth layer examination provides a minimum age for the mammoth, given the likely loss of a record from the animal’s early years due to potential tusk tip wear during the animal’s life. High-resolution 87Sr/86Sr (~47,000 individual 87Sr/86Sr ratio measurements) and lower-resolution δ34S and δ18O variations (97 and 293 sequential measurements, respectively) were used to model the geographic range and movements of the mammoth (Fig. 2 and Supplementary Text). We compared these measurements to a set of predictive isotope maps for eastern Beringia (Supplementary Text). An isotope-guided Markov chain Monte Carlo random walk approach (30) identified the most probable routes and most frequently visited areas to reveal the individual’s lifetime movement history (Supplementary Text) (Fig. 3). The female mammoth from the Swan Point site underwent relatively little movement during the beginning of her life, which she most likely spent in southeastern Beringia near the Cordilleran ice sheet (Fig. 3), as reflected by the minimal changes in the 87Sr/86Sr, δ18O, and δ34S values (Fig. 3). In the middle of her ~20-year life, the mammoth underwent a relatively large (~1000 km) movement over the span of approximately 2.5 years, as evidenced by large, simultaneous changes in 87Sr/86Sr, δ18O, and δ34S, with the model indicating likely northwestward movement through the White Mountains and as far as the southern extent of the Brooks Range (Figs. 2 and 3) (Supplementary Text). Last, the mammoth moved to and stayed in interior Alaska for ~3 years until the end of her life (Fig. 3).
Fig. 2. Sequential isotopic analyses along an entire ~60-cm-long transect of a female mammoth tusk from the Swan Point archaeological site, interior Alaska.
(A) strontium, (B) oxygen, (C) nitrogen, (D) sulfur, and (E) inorganic carbon isotope values. Vertical lines represent annual markers (peak winter) (Supplementary Text). VPDB, Vienna Pee Dee belemnite.
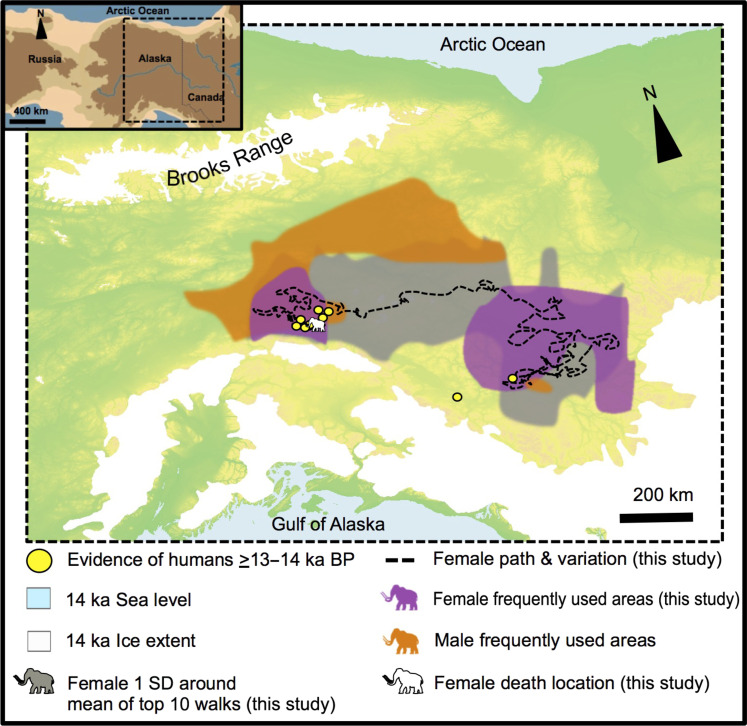
Fig. 3. Summary life history of this study’s woolly mammoth within the geographic, climatic, altitude, and early archaeology in Alaska.
The core movement areas correspond to those visited most frequently (purple polygons) (Supplementary Text). The black dashed lines between the most frequently used areas represent the route produced by the spatial modeling (representing the mean of the top 10 walks; Supplementary Text). The light gray polygon represents 1 SD around the mean of the top 10 walks. The orange polygons represent two frequently used areas of a male woolly mammoth from ~17 ka (30) that overlap with the mammoth in this study. The white mammoth symbol indicates the area where the female specimen was found (i.e., death location). Also shown are the locations of early archaeological sites in Alaska and Yukon, including Swan Point, Holzman, Mead, Broken Mammoth, Little John, and Britannia Creek (see also Supplementary Text). The small inset map of Beringia shows the study region (redrawn from US National Park Service map).
DISCUSSION
The lower 87Sr/86Sr variability in the female mammoth from Swan Point, relative to a male woolly mammoth from Alaska that lived closer to the time of the LGM (30), suggests much less lifetime movement. Most of her time was spent in interior Alaska and western Yukon (Fig. 3). However, despite the ~3000-year difference in age, the lifetime ranges of both the male and female mammoths overlapped in a frequently used area in interior Alaska (Fig. 3), implying potential long-term habitat fidelity. The relatively restricted movement of the female also suggests different behavioral patterns for the two sexes in mammoths, as observed in modern elephants. Modern elephant behavior is sexually dimorphic, with females and juveniles living in close-knit matriarchal herds and mature males traveling alone or in looser male groups, often with larger home ranges than their female counterparts (36). However, the male also appears to have had a more limited home range as a juvenile when he would have still been a part of a maternal herd (30). This juvenile portion of the male’s life would have followed the herd, and during this time, both mammoths’ ranges overlapped in frequently used areas (Fig. 3). The male also returned to this area as an adult, indicating some degree of regional fidelity.
Unlike the male, the Swan Point female appears to have preferred the use of highlands and avoided lowlands throughout her lifetime (Fig. 3 and fig. S5). This preference for highlands is shown in the best random walk results (driven by 87Sr/86Sr, δ34S, and δ18O values; Supplementary Text). Further evidence for a highlands preference is her elevated δ15N values relative to other contemporaneous herbivores (37) and relative to the male mammoth (30). Elevated δ15N values can indicate feeding on plants from more arid habitats (38, 39). Modern plants at higher elevations also tend to have higher δ15N values than those at lower elevations (40), as do herbs and grasses compared to trees and shrubs (41). The male lived ~3000 years earlier, when climate conditions were similar to the drier LGM. In the intervening millennia, Alaska’s climate grew warmer and wetter, eventually stimulating a “birch rise” in interior Alaska (34). The invasion of woody shrubs into the lowlands and river valleys would have begun to fragment the formerly massive pan-Beringian mammoth steppe habitat (6), thereby limiting the available range of the Swan Point mammoth, forcing her and other mammoths upland and creating a smaller and more easily exploited prey patch for potential human hunters.
The δ15N values from along the Swan Point tusk are driven by the movement of the female woolly mammoth across the landscape (Supplementary Text). Compound specific stable nitrogen isotope analyses of phenylalanine, an amino acid indicative of habitat baseline δ15N values, along the Swan Point tusk showed marked changes that correlated with the bulk δ15N values (Supplementary Text). This indicates that the incremental variation in the δ15N values along the tusk was caused by movement to different regions with different δ15N baselines during the mammoth’s life rather than physiological mechanisms or nutritional stress (30, 42). This δ15N pattern contrasts with some of the interpretation of δ15N values from the male woolly mammoth from 17 ka, which indicated starvation at the end of his life (30).
A common feature of both mammoths is the overlap between one of their most frequently used areas and early human occupations in interior Alaska (Fig. 3). Both mammoths also have frequently used areas further east in Yukon, Canada, close to or overlapping with two more early human occupations (Fig. 3) (43, 44). The frequent use of particular areas by mammoths could have made those areas attractive locations for seasonal hunting camps and/or ivory workshops.
The claim that humans hunted adult mammoths has been conservatively assessed in Alaska in the past due to an absence of direct evidence of mammoth hunting, such as mammoth kill sites (25, 45). By contrast, there is unambiguous evidence of adult mammoth hunting in Eurasia (46), Siberia (47, 48), and in the continental Americas (22), suggesting that coeval people in Alaska were likewise capable of hunting adult mammoths. Moreover, the weapon technology, seen in the stone tool kit at Swan Point, is essentially identical to the microblade technology and composite projectile points known from Upper Paleolithic Siberia (17), where mammoth hunting is documented (47).
Despite the Swan Point mammoth remains being found in an archaeological context, we have no direct evidence, such as embedded or closely associated weaponry, hunting lesions, or a clear primary kill site setting to verify that the human occupants of Swan Point—or any site in Alaska—actively hunted mammoths (25, 26, 45). Some mammoth individuals at Swan Point are represented only by cranial elements, which would be consistent with a scavenging scenario (25, 49, 50). We can evaluate competing hypotheses (hunting, scavenging, or both) for the processes that resulted in the remains of three mammoth individuals being present in the archaeological context at Swan Point. The remains were contemporary with hearth charcoal dates and directly associated with lithic weapon technology (microblades likely set into composite projectile points) and evidence for onsite consumption of megafauna and smaller taxa (horse, caribou, lagomorphs, waterfowl, and gamefowl) (25, 49). The adult mammoth individual is represented only by tusk remains and is thus consistent with scavenging for raw materials. However, exclusive scavenging for ivory is inconsistent with the presence of postcranial elements, namely, neonate ribs, which form part of a larger anatomical package (thoracic cavity) with high nutritional value and, unlike ivory, little or no raw material value. This suggests that at least young mammoths were probably hunted by the residents of Swan Point (25). Selective predation of young age classes among mammoth populations has been documented in Upper Paleolithic contexts in Eurasia (50). The ribs lack clear cut marks, but potential cut marks have likely been erased by postdepositional processes, as is the case for the rest of the faunal assemblage. When butchering elephants, ribs may be cut unintentionally during primary butchering, but cut marks are rare, in part to prevent dulling cutting implements (51). Because ribs may bear heavy traces of damage through carnivore feeding on carcasses, including those of elephants, these ribs may also indicate early access to the ribs before carnivore feeding (52), further suggesting a hunting scenario. Redating of the Swan Point mammoth tusk (33) showed that she was contemporary with the earliest human occupation of Swan Point, CZ4b. She was also around 20 years old at the time of death, which is in the prime of early adulthood, with no evidence of nutritional stress in the δ15N values (Supplementary Text). We estimate that the season of mortality was late summer/early autumn, based on the maintenance of relatively higher δ18O values for a period immediately before her death (Supplementary Text). This timing is consistent with the seasonal timing of occupation of CZ4b at Swan Point by humans, based on the presence of migratory waterfowl (25). She was also closely related, although not directly, to the juvenile and neonate mammoth remains found at the same site and in the same cultural layer, confirmed using the aDNA results (Supplementary Text). A plausible scenario is that useful ivory was transported back to the Swan Point camp, along with a juvenile mandible (possibly with the tongue), neonate ribs and associated meat.
Early North Americans, with a deep understanding of the lifeways of mammoths and the knowledge and technology to hunt them, were attracted to the habitats favored by mammoths (45), as late Pleistocene peoples were in Eurasia (48, 50). The highest density of documented early archaeological sites in Alaska falls within one of two areas frequently used by the Swan Point female mammoth and her genetic relatives (Fig. 3). The other frequently used area, earlier in her life, is close to two more early archaeological sites in Yukon, Canada (Fig. 3) (43, 44). The cooccurrence of both human and mammoth hotspot areas on the Beringian landscape is probably not coincidental. Instead, it more likely demonstrates people’s purposeful and strategic intent to map their behavior onto that of a mobile but highly visible and predictable megafaunal resource (29). At the Holzman site, close to Swan Point, mammoth ivory is present in multiple late Pleistocene cultural layers (Supplementary Text) (26). Mammoth remains were assuredly a source of valued raw material for tools, whether acquired through hunting, scavenging, or both (25, 26, 53). In addition to direct effects on mammoth populations from hunting, human activity on the landscape and especially human settlements (accompanied by their sounds, smells, fires and smoke, etc.) may also have indirectly affected mammoth populations by constraining their movements and access to preferred habitats (6). These novel human factors would have occurred against a backdrop of a changing landscape with increasingly reduced and fragmented grazing habitat.
MATERIALS AND METHODS
We conducted aDNA analyses of mammoth remains from 12 specimens found at the Swan Point [minimum number of individuals (MNI) = 3], Mead (MNI = 1), Broken Mammoth (MNI = 2), and Holzman (MNI = 1) archaeological sites in interior Alaska (Fig. 1 and table S1) to determine the sex and mitochondrial clade of the specimens (Supplementary Text). We split a complete adult tusk from Swan Point CZ4b lengthwise (Supplementary Text) and examined the central axis (~60 cm long from the tip of the pulp cavity to the tip of the tusk) of the cut surface for growth layers, confirming the presence of daily and weekly growth bands (Supplementary Text). This 60-cm-long central axis served as the focus for our isotopic analyses, which involved sequential sampling along the entire tusk following previously published protocols (Supplementary Text) (30). We measured high-resolution 87Sr/86Sr isotope ratios using a Laser Ablation Multi-Collector Inductively Coupled Plasma Mass Spectrometer (Supplementary Text). We used continuous flow stable isotope ratio mass spectrometry to generate stable oxygen, carbon, sulfur, and nitrogen isotope data along the tusk (Supplementary Text). We combined the 87Sr/86Sr, δ34S, and δ18O values in a tri-isotope spatial model (Supplementary Text) to compare with a bioavailable 87Sr/86Sr map of Beringia (54), a δ18O isoscape calibrated to the late Pleistocene (55, 56) and our new δ34S isoscape for Alaska (Supplementary Text). We used an isotopically guided random walk approach to probabilistically assess this mammoth’s geographic range, mobility, and the most frequently used areas over her entire life (Supplementary Text) (30, 57). The code and data have been made available at https://doi.org/10.5281/zenodo.8408732.
Acknowledgments
We thank T. Howe and S. Fields at the Alaska Stable Isotope Facility for assistance with stable isotopic analyses. We thank N. Bigelow for discussions regarding the vegetation history of the Swan Point site. T.J.M. and H.P. wish to thank the CANA Foundation for support of a PDF to T.J.M., along with thanks to B. Golding for providing access to computational resources. T.J.M., S.B., and H.P. also wish to thank all members and affiliates of the McMaster Ancient DNA Centre for ongoing support, as well as the admin and faculty of the Anthropology and Biochemistry departments at McMaster University. We acknowledge the assistance of J. Kari, P. Moore, and L. Jules in the spelling and etymology of the Tanacross, Kaska, and Proto-Dene etymology referenced here. We thank the anonymous reviewers for constructive and positive suggestions that improved our paper.
Funding: This study was supported by the MJ Murdock Charitable Trust, the National Science Foundation, the National Park Service (MCT SR-10 201811010, NSF DBI MRI 1625573, NSF ANS OPP 2310505, and NPS CESU P20AC00623 to M.J.W.). A.G.R. was additionally funded by the Hopkins Fellowship at the Alaska Quaternary Center (2020). S.B. is funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation #42555017). H.P. was funded through an NSERC Discovery Grant (grant no. 4184-15) (2020).
Author contributions: Conceptualization: A.G.R., M.J.W., K.J.S., and C.P.B.; methodology: A.G.R., M.J.W., K.J.S., and C.P.B.; formal analysis: A.G.R., M.J.W., K.J.S., and C.P.B.; investigation: A.G.R., M.J.W., K.J.S., C.P.B., F.L., J.T.R., C.E.H., H.P., T.J.M., and S.B.; resources: A.G.R., M.J.W., K.J.S., C.P.B., F.L., J.T.R., J.D.R., C.E.H., D.C.F., K.E.K., B.T.W., B.A.C., B.P., H.P., T.J.M., and S.B.; writing (original draft): A.G.R., M.J.W., K.J.S., and C.P.B.; writing (review and editing): all authors; visualization: A.G.R., M.J.W., K.J.S., T.J.M., H.P., S.B., and C.P.B.
Competing interests: M.J.W. serves on the scientific advisory board for Colossal Biosciences. The authors declare that they have no other competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. They may be found at https://doi.org/10.5281/zenodo.8408732.
Supplementary Materials
This PDF file includes:
Supplementary Text
Figs. S1 to S12
Tables S1 to S6
References
REFERENCES AND NOTES
- 1.Graham R. W., Belmecheri S., Choy K., Culleton B. J., Davies L. J., Froese D., Heintzman P. D., Hritz C., Kapp J. D., Newsom L. A., Rawcliffe R., Saulnier-Talbot É., Shapiro B., Wang Y., Williams J. W., Wooller M. J., Timing and causes of mid-Holocene mammoth extinction on St. Paul Island, Alaska. Proc. Natl. Acad. Sci. U. S. A. 113, 9310–9314 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arppe L., Karhu J. A., Vartanyan S., Drucker D. G., Etu-Sihvola H., Bocherens H., Thriving or surviving? The isotopic record of the Wrangel Island woolly mammoth population. Quat. Sci. Rev. 222, 57–76 (2019). [Google Scholar]
- 3.Wang Y., Pedersen M. W., Alsos I. G., De Sanctis B., Racimo F., Prohaska A., Coissac E., Owens H. L., Merkel M. K. F., Fernandez-Guerra A., Rouillard A., Lammers Y., Alberti A., Denoeud F., Money D., Ruter A. H., McColl H., Larsen N. K., Cherezova A. A., Edwards M. E., Fedorov G. B., Haile J., Orlando L., Vinner L., Korneliussen T. S., Beilman D. W., Bjørk A. A., Cao J., Dockter C., Esdale J., Gusarova G., Kjeldsen K. K., Mangerud J., Rasic J. T., Skadhauge B., Svendsen J. I., Tikhonov A., Wincker P., Xing Y., Zhang Y., Froese D. G., Rahbek C., Bravo D. N., Holden P. B., Edwards N. R., Durbin R., Meltzer D. J., Kjær K. H., Möller P., Willerslev E., Late Quaternary dynamics of Arctic biota from ancient environmental genomics. Nature 600, 86–92 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murchie T. J., Karpinski E., Eaton K., Duggan A. T., Baleka S., Zazula G., MacPhee R. D. E., Froese D., Poinar H. N., Pleistocene mitogenomes reconstructed from the environmental DNA of permafrost sediments. Curr. Biol. 32, 851–860.e7 (2022). [DOI] [PubMed] [Google Scholar]
- 5.Dehasque M., Pečnerová P., Muller H., Tikhonov A., Nikolskiy P., Tsigankova V. I., Danilov G. K., Díez-del-Molino D., Vartanyan S., Dalén L., Lister A. M., Combining Bayesian age models and genetics to investigate population dynamics and extinction of the last mammoths in northern Siberia. Quat. Sci. Rev. 259, 106913 (2021). [Google Scholar]
- 6.Fordham D. A., Brown S. C., Akçakaya H. R., Brook B. W., Haythorne S., Manica A., Shoemaker K. T., Austin J. J., Blonder B., Pilowsky J. A., Rahbek C., Nogues-Bravo D., Process-explicit models reveal pathway to extinction for woolly mammoth using pattern-oriented validation. Ecol. Lett. 25, 125–137 (2022). [DOI] [PubMed] [Google Scholar]
- 7.Zimov S. A., Chuprynin V. I., Oreshko A. P., Chapin F. S., Reynolds J. F., Chapin M. C., Steppe-Tundra transition: A herbivore-driven biome shift at the end of the pleistocene. Am. Nat. 146, 765–794 (1995). [Google Scholar]
- 8.Stuart A. J., Kosintsev P. A., Higham T. F. G., Lister A. M., Pleistocene to Holocene extinction dynamics in giant deer and woolly mammoth. Nature 431, 684–689 (2004). [DOI] [PubMed] [Google Scholar]
- 9.Mann D. H., Groves P., Gaglioti B. V., Shapiro B. A., Climate-driven ecological stability as a globally shared cause of Late Quaternary megafaunal extinctions: The Plaids and Stripes Hypothesis. Biol. Rev. Camb. Philos. Soc. 94, 328–352 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans Ogden L., Extinction is forever… or is it? Bioscience 64, 469–475 (2014). [Google Scholar]
- 11.Wrigley C. A., Ice and Ivory: The cryopolitics of mammoth de-extinction. J. Polit. Ecol. 28, 782–803 (2021). [Google Scholar]
- 12.Dale Guthrie R., New carbon dates link climatic change with human colonization and Pleistocene extinctions. Nature 441, 207–209 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Krasinski K., Haynes G., The eastern Beringian chronology of quaternary extinctions: A methodological approach to radiocarbon evaluation. Alsk. J. Anthropol. 8, 39–60 (2010). [Google Scholar]
- 14.MacDonald G. M., Beilman D. W., Kuzmin Y. V., Orlova L. A., Kremenetski K. V., Shapiro B., Wayne R. K., Van Valkenburgh B., Pattern of extinction of the woolly mammoth in Beringia. Nat. Commun. 3, 893 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mann D. H., Groves P., Reanier R. E., Gaglioti B. V., Kunz M. L., Shapiro B., Life and extinction of megafauna in the ice-age Arctic. Proc. Natl. Acad. Sci. U. S. A. 112, 14301–14306 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murchie T. J., Monteath A. J., Mahony M. E., Long G. S., Cocker S., Sadoway T., Karpinski E., Zazula G., MacPhee R. D. E., Froese D., Poinar H. N., Collapse of the mammoth-steppe in central Yukon as revealed by ancient environmental DNA. Nat. Commun. 12, 7120 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gómez Coutouly Y. A., Holmes C. E., The microblade industry from Swan Point Cultural Zone 4b: Technological and cultural implications from the earliest human occupation in Alaska. Am. Antiq. 83, 735–752 (2018). [Google Scholar]
- 18.J. Hoffecker, S. Elias, Human Ecology of Beringia (Columbia Univ. Press, 2007). [Google Scholar]
- 19.Moreno-Mayar J. V., Potter B. A., Vinner L., Steinrücken M., Rasmussen S., Terhorst J., Kamm J. A., Albrechtsen A., Malaspinas A.-S., Sikora M., Reuther J. D., Irish J. D., Malhi R. S., Orlando L., Song Y. S., Nielsen R., Meltzer D. J., Willerslev E., Terminal Pleistocene Alaskan genome reveals first founding population of Native Americans. Nature 553, 203–207 (2018). [DOI] [PubMed] [Google Scholar]
- 20.Potter B. A., Baichtal J. F., Beaudoin A. B., Fehren-Schmitz L., Haynes C. V., Holliday V. T., Holmes C. E., Ives J. W., Kelly R. L., Llamas B., Malhi R. S., Miller D. S., Reich D., Reuther J. D., Schiffels S., Surovell T. A., Current evidence allows multiple models for the peopling of the Americas. Sci. Adv. 4, eaat5473 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cannon M. D., Meltzer D. J., Early Paleoindian foraging: Examining the faunal evidence for large mammal specialization and regional variability in prey choice. Quat. Sci Rev. 23, 1955–1987 (2004). [Google Scholar]
- 22.Surovell T. A., Waguespack N. M., How many elephant kills are 14? Clovis mammoth and mastodon kills in context. Quat. Int. 191, 82–97 (2008). [Google Scholar]
- 23.Prates L., Perez S. I., Late Pleistocene South American megafaunal extinctions associated with rise of Fishtail points and human population. Nat. Commun. 12, 2175 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.P. Martin, "Prehistoric Overkill: The Global Model" in Quaternary Extinctions: A Prehistoric Revolution, P. Martin, R. Klein, Eds. (University of Arizona Press, 1984). [Google Scholar]
- 25.Lanoë F. B., Holmes C. E., Animals as raw material in Beringia: Insights from the site of swan point CZ4B, Alaska. Am. Antiq. 81, 682–696 (2016). [Google Scholar]
- 26.Wygal B. T., Krasinski K. E., Holmes C. E., Crass B. A., Smith K. M., Mammoth ivory rods in Eastern Beringia: Earliest in North America. Am. Antiq. 87, 59–79 (2022). [Google Scholar]
- 27.Monteath A. J., Gaglioti B. V., Edwards M. E., Froese D., Late Pleistocene shrub expansion preceded megafauna turnover and extinctions in eastern Beringia. Proc. Natl. Acad. Sci. U. S. A. 118, e2107977118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Owen-Smith N., Pleistocene extinctions: The pivotal role of megaherbivores. Paleobiology 13, 351–362 (1987). [Google Scholar]
- 29.Haynes G., Mammoth landscapes: Good country for hunter-gatherers. Quat. Int. 142–143, 20–29 (2006). [Google Scholar]
- 30.Wooller M. J., Bataille C., Druckenmiller P., Erickson G. M., Groves P., Haubenstock N., Howe T., Irrgeher J., Mann D., Moon K., Potter B. A., Prohaska T., Rasic J., Reuther J., Shapiro B., Spaleta K. J., Willis A. D., Lifetime mobility of an Arctic woolly mammoth. Science 373, 806–808 (2021). [DOI] [PubMed] [Google Scholar]
- 31.E. Burch, Caribou Herds of Northwest Alaska, 1850–2000 (University of Alaska Press, 2012). [Google Scholar]
- 32.L. D. Minc, K. P. Smith, The spirit of survival: Cultural responses to resource variability in North Alaska, in Bad Year Economics (Cambridge Univ. Press, 1989), pp. 8–39. [Google Scholar]
- 33.Reuther J. D., Holmes C. E., Smith G. M., Lanoe F. B., Crass B. A., Rowe A. G., Wooller M. J., The Swan Point Site, Alaska: The chronology of a multi-component archaeological site in Eastern Beringia. Radiocarbon 65, 693–720 (2023). [Google Scholar]
- 34.Bigelow N., Powers W. R., Climate, vegetation, and archaeology 14,000-9000 Cal Yr B.P. in Central Alaska. Arctic Anthropol. 38, 171–195 (2001). [Google Scholar]
- 35.Kielhofer J. R., Tierney J. E., Reuther J. D., Potter B. A., Holmes C. E., Lanoë F. B., Esdale J. A., Wooller M. J., Bigelow N. H., BrGDGT temperature reconstruction from interior Alaska: Assessing 14,000 years of deglacial to Holocene temperature variability and potential effects on early human settlement. Quat. Sci. Rev. 303, 107979 (2023). [Google Scholar]
- 36.Bonhof W. J., Pryor A. J. E., Proboscideans on Parade: A review of the migratory behaviour of elephants, mammoths, and mastodons. Quat. Sci. Rev. 277, 107304 (2022). [Google Scholar]
- 37.Lanoë F. B., Reuther J. D., Holmes C. E., Hodgins G. W. L., Human paleoecological integration in subarctic eastern Beringia. Quat. Sci. Rev. 175, 85–96 (2017). [Google Scholar]
- 38.Rabanus-Wallace M. T., Wooller M. J., Zazula G. D., Shute E., Jahren A. H., Kosintsev P., Burns J. A., Breen J., Llamas B., Cooper A., Megafaunal isotopes reveal role of increased moisture on rangeland during late Pleistocene extinctions. Nat. Ecol. Evol. 1, 125 (2017). [DOI] [PubMed] [Google Scholar]
- 39.Schwartz-Narbonne R., Longstaffe F. J., Metcalfe J. Z., Zazula G., Solving the woolly mammoth conundrum: Amino acid 15N-enrichment suggests a distinct forage or habitat. Sci. Rep. 5, 9791 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tanaka-Oda A., Kenzo T., Toriyama J., Matsuura Y., Variability in the growth rates and foliage δ15N values of black spruce trees across a slope gradient in the Alaskan Interior. Can. J. For. Res. 46, 1483–1490 (2016). [Google Scholar]
- 41.Tessone A., Srur A., Aranibar J. N., δ13C and δ15N of plants in a longitudinal transect from the Andes to the Atlantic coast: Terrestrial baseline for paleodietary and paleocological studies. J. Archaeol. Sci. Rep. 47, 103787 (2023). [Google Scholar]
- 42.Funck J., Kellam C., Seaton C. T., Wooller M. J., Stable isotopic signatures in modern wood bison (Bison bison athabascae) hairs as telltale biomarkers of nutritional stress. Can. J. Zool. 98, 505–514 (2020). [Google Scholar]
- 43.Altamira Consulting Ltd., Western Copper and Gold Corporation Casino Project Archaeological Resources Mitigation. (Whitehorse, Canada, 2014).
- 44.N. A. Easton, G. R. Mackay, P. B. Young, P. Schnurr, D. R. Yesner, Chindadn in Canada? Emergent Evidence of the Pleistocene Transition in Southeast Beringia as Revealed by the Little John Site, Yukon. in From the Yenisei to the Yukon: Interpreting Lithic Assemblage Variability in Late Pleistocene/Early Holocene Beringia, T. Goebel, I. Buvit, Eds. (Texas A&M Univ Press, 2011), pp. 289–307. [Google Scholar]
- 45.Holmes C., Tanana river valley archaeology circa 14,000 to 9000 B.P. Arctic. Anthropol. 38, 154–170 (2001). [Google Scholar]
- 46.Wojtal P., Haynes G., Klimowicz J., Sobczyk K., Tarasiuk J., Wroński S., Wilczyński J., The earliest direct evidence of mammoth hunting in Central Europe – The Kraków Spadzista site (Poland). Quat. Sci. Rev. 213, 162–166 (2019). [Google Scholar]
- 47.Zenin V. N., Leshchinskiy S. V., Zolotarev K. V., Grootes P. M., Nadeau M.-J., Lugovskoe: Geoarchaeology and culture of a paleolithic site. Archaeol. Ethnol. Anthropol. Eurasia 25, 41–53 (2006). [Google Scholar]
- 48.Nikolskiy P., Pitulko V., Evidence from the Yana Palaeolithic site, Arctic Siberia, yields clues to the riddle of mammoth hunting. J. Archaeol. Sci. 40, 4189–4197 (2013). [Google Scholar]
- 49.Hirasawa Y., Holmes C. E., The relationship between microblade morphology and production technology in Alaska from the perspective of the Swan Point site. Quat. Int. 442, 104–117 (2017). [Google Scholar]
- 50.Svoboda J., Péan S., Wojtal P., Mammoth bone deposits and subsistence practices during Mid-Upper Palaeolithic in Central Europe: Three cases from Moravia and Poland. Quat. Int. 126–128, 209–221 (2005). [Google Scholar]
- 51.Haynes G., Krasinski K., Butchering marks on bones of Loxodonta africana (African savanna elephant): Implications for interpreting marks on fossil proboscidean bones. J. Archaeol. Sci. Rep. 37, 102957 (2021). [Google Scholar]
- 52.Haynes G., Hutson J., African elephant bones modified by carnivores: Implications for interpreting fossil proboscidean assemblages. J. Archaeol. Sci. Rep. 34, 102596 (2020). [Google Scholar]
- 53.Gelvin-Reymiller C., Reuther J. D., Potter B. A., Bowers P. M., Technical aspects of a worked proboscidean tusk from Inmachuk River, Seward Peninsula, Alaska. J. Archaeol. Sci. 33, 1088–1094 (2006). [Google Scholar]
- 54.Funck J., Bataille C., Rasic J., Wooller M., A bio-available strontium isoscape for eastern Beringia: A tool for tracking landscape use of Pleistocene megafauna. J. Quat. Sci. 36, 76–90 (2021). [Google Scholar]
- 55.Ma C., Vander Zanden H. B., Wunder M. B., Bowen G. J., assignR: An R package for isotope-based geographic assignment. Methods. Ecol. Evol. 11, 996–1001 (2020). [Google Scholar]
- 56.Metcalfe J. Z., Proboscidean isotopic compositions provide insight into ancient humans and their environments. Quat. Int. 443, 147–159 (2017). [Google Scholar]
- 57.R Core Team, R: A Language and Environment for Statistical Computing (2023).
- 58.Cannon C., Justin W., Herbert P., Hubbard C., Neyelle C., Northern Dene constellations as worldview projections with case studies from the Ahtna, Gwich’in, and Sahtúot’ı̨nę. Arctic. Anthropol. 56, 1–26 (2019). [Google Scholar]
- 59.G. Smith, The Gift of the Middle Tanana (Lexington Books, 2022). [Google Scholar]
- 60.L. M. Johnson, Trail of Story, Traveller’s Path: Reflections on Ethnoecology and Landscape (Athabasca Univ. Press, 2010). [Google Scholar]
- 61.J. Kari, S. Tuttle, Eds., Yenida’a Tah, Ts’utsaede, K’adiide: Mythical Times, Ancient Times, Recent Times. An Anthology of Ahtna Narratives (Alaska Native Language Center, 2018). [Google Scholar]
- 62.Kaska Tribal Council, Guzāgi K’úgé’: Our language Book: Nouns: Kaska, Mountain Slavey and Sekani (Arctic Star Printing, 1997). [Google Scholar]
- 63.Kedrowski B. L., Crass B. A., Behm J. A., Luetke J. C., Nichols A. L., Moreck A. M., Holmes C. E., GC/MS Analysis Of fatty acids from ancient hearth residues at the swan point archaeological site. Archaeometry 51, 110–122 (2009). [Google Scholar]
- 64.B. Crass, B. Kedrowski, J. Baus, J. Behm, Residue analysis of bone-fueled Pleistocene hearths, in From the Yensi to the Yukon: Interpreting Lithic Assemblage Variability in Late Pleistocene/Early Holocene Beringia, T. Goebel, I. Buvit, Eds. (A&M Press, 2011). [Google Scholar]
- 65.B. Potter, C. Holmes, R. Yesner, Technology and Economy among the earliest prehistoric foragers in interior Eastern Beringia in Paleoamerican Odyssey, K. E. Graf, C. V. Ketron, M. R. Waters, Eds. (Texas A&M Univ Press, 2014), pp. 81–104. [Google Scholar]
- 66.Wygal B. T., Krasinski K. E., Holmes C. E., Crass B. A., Holzman south: A late Pleistocene archaeological site along shaw creek, Tanana Valley, Interior Alaska. PaleoAmerica. 4, 90–93 (2018). [Google Scholar]
- 67.C. Holmes, "Broken Mammoth" in American Beginnings: The Prehistory and Palaeoecology of Beringia, F. West, Ed. (University of Chicago Press, 1996), pp. 312–318. [Google Scholar]
- 68.Dabney J., Knapp M., Glocke I., Gansauge M.-T., Weihmann A., Nickel B., Valdiosera C., García N., Pääbo S., Arsuaga J.-L., Meyer M., Complete mitochondrial genome sequence of a Middle Pleistocene cave bear reconstructed from ultrashort DNA fragments. Proc. Natl. Acad. Sci. U. S. A. 110, 15758–15763 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Murchie T. J., Kuch M., Duggan A. T., Ledger M. L., Roche K., Klunk J., Karpinski E., Hackenberger D., Sadoway T., MacPhee R., Froese D., Poinar H., Optimizing extraction and targeted capture of ancient environmental DNA for reconstructing past environments using the PalaeoChip Arctic-1.0 bait-set. Quat. Res. 99, 305–328 (2021). [Google Scholar]
- 70.Kircher M., Sawyer S., Meyer M., Double indexing overcomes inaccuracies in multiplex sequencing on the Illumina platform. Nucleic Acids Res. 40, e3 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Renaud G., Stenzel U., Kelso J., leeHom: Adaptor trimming and merging for Illumina sequencing reads. Nucleic Acids Res. 42, e141 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J., Basic local alignment search tool. J. Mol. Biol. 215, 403–410 (1990). [DOI] [PubMed] [Google Scholar]
- 73.Huson D. H., Auch A. F., Qi J., Schuster S. C., MEGAN analysis of metagenomic data. Genome Res. 17, 377–386 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Katoh K., Standley D. M., MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 30, 772–780 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kearse M., Moir R., Wilson A., Stones-Havas S., Cheung M., Sturrock S., Buxton S., Cooper A., Markowitz S., Duran C., Thierer T., Ashton B., Meintjes P., Drummond A., Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28, 1647–1649 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bouckaert R., Heled J., Kühnert D., Vaughan T., Wu C.-H., Xie D., Suchard M. A., Rambaut A., Drummond A. J., BEAST 2: A software platform for Bayesian evolutionary analysis. PLoS Comput. Biol. 10, e1003537 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bouckaert R. R., Drummond A. J., bModelTest: Bayesian phylogenetic site model averaging and model comparison. BMC Evol. Biol. 17, 42 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Drummond A. J., Rambaut A., Shapiro B., Pybus O. G., Bayesian coalescent inference of past population dynamics from molecular sequences. Mol. Biol. Evol. 22, 1185–1192 (2005). [DOI] [PubMed] [Google Scholar]
- 79.Paradis E., Pegas: An R package for population genetics with an integrated–modular approach. Bioinformatics 26, 419–420 (2010). [DOI] [PubMed] [Google Scholar]
- 80.Gu Z., Complex heatmap visualization. iMeta 1, e43 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gu Z., Eils R., Schlesner M., Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 32, 2847–2849 (2016). [DOI] [PubMed] [Google Scholar]
- 82.Prohaska T., Irrgeher J., Zitek A., Simultaneous multi-element and isotope ratio imaging of fish otoliths by laser ablation split stream ICP-MS/MC ICP-MS. J. Anal. At. Spectrom. 31, 1612–1621 (2016). [Google Scholar]
- 83.Irrgeher J., Galler P., Prohaska T., 87 Sr/ 86 Sr isotope ratio measurements by laser ablation multicollector inductively coupled plasma mass spectrometry: Reconsidering matrix interferences in bioapatites and biogenic carbonates. Spectrochim. Acta Part B At. Spectrosc. 125, 31–42 (2016). [Google Scholar]
- 84.Misarti N., Finney B., Maschner H., Wooller M. J., Changes in northeast Pacific marine ecosystems over the last 4500 years: Evidence from stable isotope analysis of bone collagen from archeological middens. Holocene 19, 1139–1151 (2009). [Google Scholar]
- 85.Glassburn C. L., Potter B. A., Clark J. L., Reuther J. D., Bruning D. L., Wooller M. J., Strontium and Oxygen Isotope Profiles of Sequentially Sampled Modern Bison (Bison bison bison) Teeth from Interior Alaska as Proxies of Seasonal Mobility. Arctic 71, 183–200 (2018). [Google Scholar]
- 86.Funck J., Heintzman P. D., Murray G. G. R., Shapiro B., McKinney H., Huchet J.-B., Bigelow N., Druckenmiller P., Wooller M. J., A detailed life history of a pleistocene steppe bison (Bison priscus) skeleton unearthed in Arctic Alaska. Quat. Sci Rev. 249, 106578 (2020). [Google Scholar]
- 87.R. Verkouteren, D. Klinedinst, Value Assignment and Uncertainty Estimation of Selected Light Stable Isotope Reference Materials: RMs 8543–8545, RMs 8562–8564, and RM 8566 (2004).
- 88.Velivetskaya T. A., Smirnov N. G., Kiyashko S. I., Ignatiev A. V., Ulitko A. I., Resolution-enhanced stable isotope profiles within the complete tooth rows of Late Pleistocene bisons (Middle Urals, Russia) as a record of their individual development and environmental changes. Quat. Int. 400, 212–226 (2016). [Google Scholar]
- 89.K. Hoppe, P. Koch, The biogeochemistry of the Aucilla River Fauna, in The First Floridians and Last Mastodons: The Page-Ladson Site on the Aucilla River, Topics in Geobiology (Plenum Press, 2006). [Google Scholar]
- 90.Chikaraishi Y., Steffan S. A., Ogawa N. O., Ishikawa N. F., Sasaki Y., Tsuchiya M., Ohkouchi N., High-resolution food webs based on nitrogen isotopic composition of amino acids. Ecol. Evol. 4, 2423–2449 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.J. Meachen, M. J. Wooller, B. D. Barst, J. Funck, C. Crann, J. Heath, M. Cassatt-Johnstone, B. Shapiro, E. Hall, S. Hewitson, G. Zazula, Zhur: A mummified Pleistocene gray wolf pup (Canis lupus) from Yukon Territory, Canada, SSRN Electronic Journal (2020). [DOI] [PubMed]
- 92.Lachniet M. S., Lawson D. E., Stephen H., Sloat A. R., Patterson W. P., Isoscapes of δ18O and δ2H reveal climatic forcings on Alaska and Yukon precipitation. Water Resour. Res. 52, 6575–6586 (2016). [Google Scholar]
- 93.Bowen G. J., Wassenaar L. I., Hobson K. A., Global application of stable hydrogen and oxygen isotopes to wildlife forensics. Oecologia 143, 337–348 (2005). [DOI] [PubMed] [Google Scholar]
- 94.S. A. Yi-Balan, An investigation of the factors controlling the terrestrial sulfur cycle, University of California Berkeley (2013).
- 95.Karger D. N., Nobis M. P., Normand S., Graham C. H., Zimmermann N. E., CHELSA-TraCE21k – high-resolution (1 km) downscaled transient temperature and precipitation data since the Last Glacial Maximum. Clim. Past 19, 439–456 (2023). [Google Scholar]
- 96.Osipova L., Okello M. M., Njumbi S. J., Ngene S., Western D., Hayward M. W., Balkenhol N., Validating movement corridors for African elephants predicted from resistance-based landscape connectivity models. Landsc. Ecol. 34, 865–878 (2019). [Google Scholar]
- 97.Baltensperger A. P., Joly K., Using seasonal landscape models to predict space use and migratory patterns of an arctic ungulate. Mov. Ecol. 7, 18 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.D. S. Kaufman, W. F. Manley, Pleistocene Maximum and Late Wisconsinan glacier extents across Alaska, U.S.A. in (2004), pp. 9–27.
- 99.Szpak P., Gröcke D. R., Debruyne R., MacPhee R. D. E., Guthrie R. D., Froese D., Zazula G. D., Patterson W. P., Poinar H. N., Regional differences in bone collagen δ13C and δ15N of Pleistocene mammoths: Implications for paleoecology of the mammoth steppe. Palaeogeogr Palaeoclimatol Palaeoecol. 286, 88–96 (2010). [Google Scholar]
- 100.R. Stevens, H. Reade, J. Tripp, D. Fremondeau, A. Lister, I. Barnes, M. Germonpre, M. Street, J. Murton, S. Bottrell, T. Higham, Major excursions in sulfur isotopes linked to permafrost change in Eurasia during the last 50,000 Years. Res. Sq. (2023).
- 101.Passey B. H., Robinson T. F., Ayliffe L. K., Cerling T. E., Sponheimer M., Dearing M. D., Roeder B. L., Ehleringer J. R., Carbon isotope fractionation between diet, breath CO2, and bioapatite in different mammals. J. Archaeol. Sci. 32, 1459–1470 (2005). [Google Scholar]
- 102.DeNiro M. J., Epstein S., Influence of diet on the distribution of carbon isotopes in animals. Geochim. Cosmochim. Acta 42, 495–506 (1978). [Google Scholar]
- 103.Codron D., Clauss M., Codron J., Tütken T., Within trophic level shifts in collagen-carbonate stable carbon isotope spacing are propagated by diet and digestive physiology in large mammal herbivores. Ecol. Evol. 8, 3983–3995 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.L. L. Tieszen, T. Fagre, Effect of Diet Quality and Composition on the Isotopic Composition of Respiratory CO2, Bone Collagen, Bioapatite, and Soft Tissues in Prehistoric Human Bone (Springer Berlin Heidelberg, Berlin, Heidelberg, 1993), pp. 121–155.
- 105.Tejada-Lara J. V., MacFadden B. J., Bermudez L., Rojas G., Salas-Gismondi R., Flynn J. J., Body mass predicts isotope enrichment in herbivorous mammals. Proc. R. Soc. Lond. B Biol. Sci. 285, 1–10 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Behar D. M., Harmant C., Manry J., Van Oven M., Haak W., Martinez-Cruz B., Salaberria J., Oyharçabal B., Bauduer F., Comas D., Quintana-Murci L.; Genographic Consortium , The Basque paradigm: Genetic evidence of a maternal continuity in the Franco-Cantabrian region since pre-Neolithic times. Am. J. Hum. Genet. 90, 486–493 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Text
Figs. S1 to S12
Tables S1 to S6
References