Abstract
PURPOSE
Clinical trials are valuable evidence for managing urologic malignancies. Early termination of clinical trials is associated with a waste of resources and may substantially affect patient care. We sought to study the termination rate of urologic cancer clinical trials and identify factors associated with trial termination.
METHODS
A cross-sectional search of ClinicalTrials.gov identified completed and terminated kidney, prostate, and bladder cancer clinical trials started. Trials were assessed for reasons for termination. Multivariable analyses were conducted to determine the significant factors associated with the termination.
RESULTS
Between 2000 and 2020, 9,145 oncology clinical trials were conducted, of which 11.30% (n = 1,033) were urologic cancer clinical trials. Of the urologic cancer clinical trials, 25.38% (n = 265) were terminated, with low patient accrual being the most common reason for termination, 52.9% (n = 127). Multivariable analysis showed that only the university funding source odds ratio (OR) of 2.20 (95% CI, 1.45 to 3.32), single-center studies OR of 2.11 (95% CI, 1.59 to 2.81), and sample size of <50 were significant predictors of clinical trial termination OR of 5.26 (95% CI, 3.85 to 7.69); all P values are <.001.
CONCLUSION
The termination rate of urologic cancer clinical trials was 25%, with low accrual being the most frequently reported reason. Trials funded by a university, single-center trials, and small trials (sample size <50) were associated with early termination. A better understanding of these factors might help researchers, funding agencies, and other stakeholders prioritize resource allocations for multicenter trials that aim to recruit a sufficient number of patients.
Urological cancer trial termination at 25% due to low accrual. Factors: university funding, single-center, small sample.
INTRODUCTION
In the past 30 years, there has been a global trend toward increasing incidence of kidney, bladder, and prostate cancers, collectively representing the most common genitourinary cancers.1 It was estimated that the incidence was 145,910, 234,750, and 524,110 for kidney, bladder, and prostate cancer cases, respectively, between 1990 and 2019.2 Consequentially, the socioeconomic burden of treatment for genitourinary (GU) malignancies is soaring.3 For example, bladder cancer's economic burden is considered the highest per patient of all cancers, with almost 8.6 billion euros per year in the United States and Europe.4,5
CONTEXT
Key Objective
What are the factors associated with the termination rate of urologic cancer clinical trials?
Knowledge Generated
The overall termination rate of urologic cancer clinical trials was 25.4%. The most frequent causes for trial termination were low patient accrual and safety concerns. Trials that received university funds, were based in a single center, and aimed to recruit <50 patients were more likely to end up being terminated.
Relevance
A better understanding of these factors might help researchers, funding agencies, and other stakeholders prioritize resource allocations for multicenter trials that aim to recruit a large number of patients.
Randomized clinical trials remain the cornerstone in advancing cancer care as they provide high-level evidence that influences the management of thousands of patients globally.6 For example, landmark clinical trials investigating the use of novel hormonal therapy in advanced prostate cancer led to a significant increase in overall survival rates for patients.7 Nevertheless, randomized control trials (RCTs) require a robust institutional infrastructure, support personnel, funding, and time and effort from the recruiting physician.6,8 Moreover, they may result in psychological, physical, and financial burdens for participants.9
Clinical trial termination because of safety issues, financial strains, and other logistical factors is well studied. Around 22% of oncologic clinical trials experience termination, whereas for nononcology clinical trials, the termination rate is approximately 19%.10 While clinical trial termination might be associated with a sense of loss, anxiety, and disappointment for patients, it also has an economic impact associated with the cost, utilization of research infrastructure, and loss of opportunity to extend life or improve life quality. However, most studies that assessed clinical trial termination were not focused on GU cancer clinical trials.
In this context, we sought to study the termination rate of GU cancer clinical trials, specifically among kidney, bladder, and prostate cancers. The study aimed to identify factors associated with clinical trial termination that can be improved for future trial design.
METHODS
Data Source and Search Study
ClinicalTrials.gov is the most comprehensive clinical trial register that provides extensive information to the public.11 The investigators independently conducted a thorough review of clinical trials of kidney, bladder, and prostate cancers available on the registry. The investigators initiated the search on March 11, 2023, focusing on identifying completed trials and having their results posted before December 2020. Only trials that had a recruitment status identified as “completed”; “active, not recruiting”; “terminated”; “suspended”; and “Withdrawn” on ClinicalTrials.gov have been included.
Data Extraction
Data Related to Clinical Trial
The extracted data included several parameters such as clinical trial date, funding source, intervention, enrollment, sample size, trial completion date, phase, clinical trial status, the reason for discontinuation, masking, center(s) (single or multicenter), area of the clinical trial, and primary outcome of the trial. Phases I/II and II/III were defined as phases II and III, respectively. The funding source was categorized into National Institutes of Health (NIH), non-NIH US funding or other (non-NIH, non-US) funding, industrial companies, private institutions/hospitals, or universities. The intervention was classified into medical, surgical, diagnostic, palliative, and other. Moreover, the area of conducting the clinical trials was categorized on the basis of high-income countries (HICs) or low- and middle-income countries (LMICs), according to the 2022-2023 World Bank Atlas country's income-level classification.
Data Related to the Principal Investigator
We used the Wiki-Gendersort to check the sex of the principal investigator (PI), either female or male,12 the age of the PI, the total number of citations, and the level of experience of the PI, which has been evaluated by subtracting the year of graduation of the PI from medical school or any similar-level degree.
Two independent investigators conducted the data extraction, and a third investigator was consulted to resolve any disagreements. In the case of clinical trial termination, the reason was extracted as provided on ClinicalTrials.gov. Reasons for termination included financial constraints, administrative reasons, informative decisions, low accrual, patients' safety, and other/unclear reasons.
Ethical Statement
As a result of the nature of the study, no IRB approval was needed to conduct this scientific work.
Consent for Publication
The data that have been collected from the clinical trials are published and publicly available, and thus, no informed consent was needed.
Statistical Analysis
The study categorized trials as completed or discontinued and reported continuous variables as median with IQR and categorical variables as percentages (%). Chi-square tests were used to determine differences between categorical variables, whereas the Shapiro-Wilks test assessed normal distribution for continuous variables. If variables were not normally distributed, logarithmic conversion was applied to adjust for normal distribution. Independent T-tests were used to compare two groups, and one-way ANOVA was used for more than two groups. A multivariable logistic regression analysis was conducted to identify the most factors associated with clinical trial termination, including centers (single v multiple), funding source, type of intervention, number of agents, masking of the clinical trial, number of participants, and the phase of the trial. R software was used for all statistical analyses, with P values <.05 considered statistically significant.
RESULTS
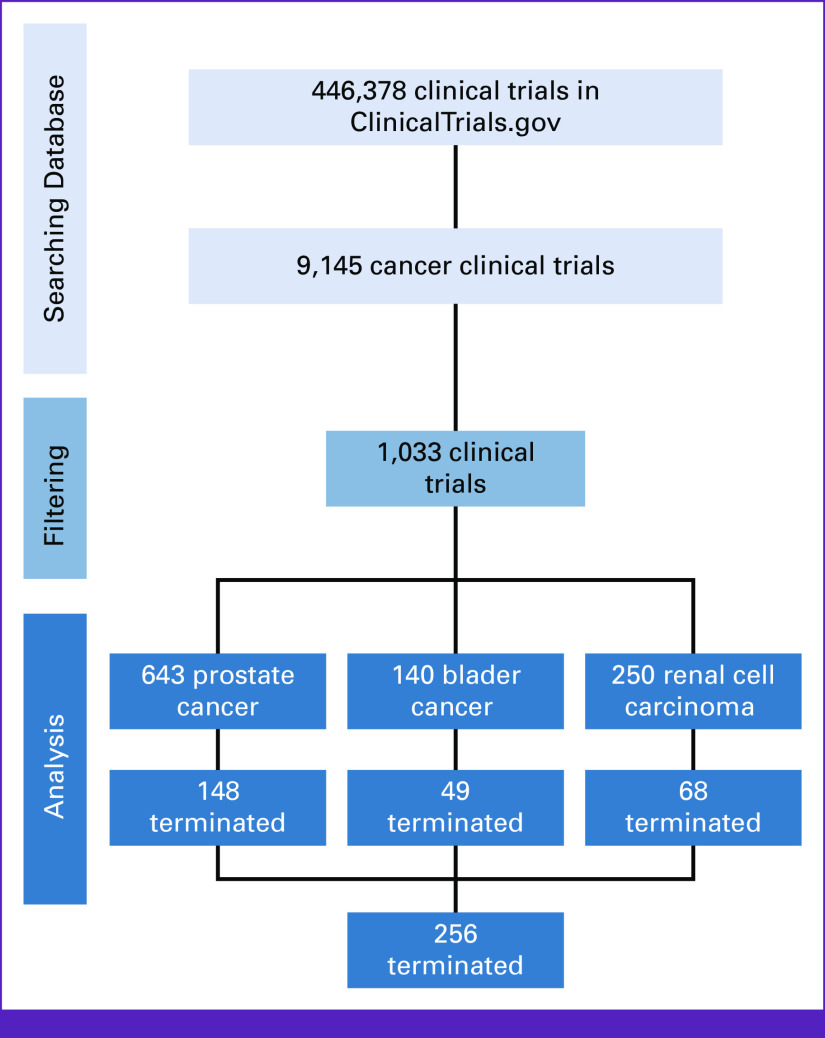
Between 2000 and 2020, 9,145 oncology clinical trials were conducted, of which 11.30% (n = 1,033) focused on urologic cancers. Prostate cancer clinical trials constituted the majority, with 62.24% (n = 643), followed by renal cell carcinoma clinical trials with 24.20% (n = 250) and bladder cancer clinical trials with 13.41% (n = 140; Fig 1). These clinical trials recruited 5,093,717 patients, with a median sample size of 41 and IQR 17-107, and more than half, 55.5% (n = 573), recruited <50 patients. Single-arm interventions were conducted in 45.7% (n = 402) of the trials, and medical intervention was the most common type of intervention in 79.5% (n = 816), with most of them being phase II clinical trials in 76.2% (n = 645). Industrial companies were the most common funding source, 51.2% (n = 528), followed by NIH 27.8% (n = 287). Throughout the trial conduction, most trials had no masking, 83.6% (n = 851), and more than half were conducted in more than one center, 55.5% (n = 578). Only 2.27% (n = 20) of clinical trials were conducted in LMICs. Table 1 summarizes the characteristics of the urologic clinical trials included in the study.
FIG 1.
Flowchart of included clinical trials collection. This figure presents a flowchart depicting the systematic process for the collection of included clinical trials in a comprehensive review. The flowchart comprises a series of sequential steps, each of which contributes to the selection of relevant clinical trials for analysis and synthesis.
TABLE 1.
Full Characteristics of the Clinical Trials
| Characteristic | All Trials (n = 1,033), No. (%) |
|---|---|
| Sex of the PI | |
| Female | 137 (16.8) |
| Male | 679 (83.2) |
| Experience in the medical field | 20.2 (9.71) |
| PI age, years | 59.0 (52.0; 64.5) |
| PI number of citations | 7,407 (2,525; 17,985) |
| Funding source | |
| Industrial | 528 (51.2) |
| NIH | 287 (27.8) |
| Other | 2 (0.19) |
| Private | 90 (8.73) |
| University | 124 (12.0) |
| Intervention | |
| Device | 31 (3.02) |
| Medication | 816 (79.5) |
| Medication/surgery | 41 (3.99) |
| Medication/radiation | 25 (2.43) |
| Other | 57 (5.55) |
| Radiation | 34 (3.31) |
| Surgery | 23 (2.24) |
| No. of agents | |
| 1 | 402 (45.7) |
| 2 | 298 (33.9) |
| 3 | 113 (12.9) |
| More than 3 | 66 (7.51) |
| Phase | |
| I | 45 (5.31) |
| II | 645 (76.2) |
| III | 135 (15.9) |
| IV | 22 (2.60) |
| Reason for discontinuation | |
| Administrative reasons | 26 (10.8) |
| Funding | 27 (11.2) |
| Low accrual | 127 (52.9) |
| Other | 12 (5.00) |
| Patient safety | 48 (20.0) |
| Masking | |
| None | 851 (83.6) |
| Single | 26 (2.55) |
| Double | 55 (5.40) |
| Triple | 41 (4.03) |
| Quadruple | 45 (4.42) |
| Centers | |
| Multi | 558 (55.5) |
| Single | 448 (44.5) |
| Country | |
| HICs | 860 (97.7) |
| LMICs | 20 (2.27) |
| Sample | |
| Less than 50 | 573 (55.5) |
| More than 50 | 460 (44.5) |
Abbreviations: HICs, high-income countries; LMICs, low- and middle-income countries; NIH, National Institutes of Health; PI, principal investigator.
Of all the urologic cancer clinical trials, 25.38% (n = 265) were terminated; low patient accrual was the most common reason, 52.9% (n = 127), followed by patient safety concerns, 20.0% (n = 48; Fig 1). A total of 12,280 patients were enrolled in these trials (Tables 2 and 3). Univariable analysis revealed significant associations between university funding of the clinical trials (18.9% v 9.66%; P < .001, for terminated v completed), surgical intervention (3.02% v 1.97%; P = .020, for terminated v completed), a higher number of arms with more than three interventions (9.40% v 6.82%; P < .001, for terminated v completed), phase II trials (84.8% v 73.0%; P = .005, for terminated vs completed), absence of masking (90.1% v 81.3%; P = .014, for terminated v completed), single-center clinical trials (58.2% v 39.7%; P < .001, for terminated v completed), and clinical trials recruiting less than 50 patients (82.3% v 46.2%; P < .001, for terminated vs completed). More details are shown in Table 1. On multivariable analysis, university-based funded trials (odds ratio [OR], 2.20 [95% CI, 1.45 to 3.32]; P < .001), single-center studies (OR, 2.11 [95% CI, 1.59 to 2.81]; P < .001), and trials with a sample size <50 (OR, 5.26 [95% CI, 3.85 to 7.69]; P < .001) were associated with termination (Table 1).
TABLE 2.
Univariate Analysis for the Difference Between Completed and Terminated Clinical Trials
| Factor | Completed Trial (n = 768) | Terminated Trial (n = 265) | P |
|---|---|---|---|
| Sex of the PI, No. (%) | .585 | ||
| Female | 102 (17.3) | 35 (15.4) | |
| Male | 487 (82.7) | 192 (84.6) | |
| Experience in the medical field, No. (%) | 19.6 (9.57) | 21.5 (9.94) | .056 |
| PI age, years | 59.0 (52.0; 65.0) | 59.0 (53.0; 64.0) | .640 |
| PI number of citations | 7,687 (2,865; 17,985) | 6,230 (2,101; 18,157) | <.001* |
| Funding source, No. (%) | |||
| Industrial | 404 (52.7) | 124 (46.8) | |
| NIH | 212 (27.7) | 75 (28.3) | |
| Other | 1 (0.13) | 1 (0.38) | |
| Private | 75 (9.79) | 15 (5.66) | |
| University | 74 (9.66) | 50 (18.9) | |
| Intervention, No. (%) | .020* | ||
| Device | 24 (3.15) | 7 (2.64) | |
| Medication | 602 (79.0) | 214 (80.8) | |
| Medication/surgery | 26 (3.41) | 15 (5.66) | |
| Medication/radiation | 19 (2.49) | 6 (2.26) | |
| Other | 53 (6.96) | 4 (1.51) | |
| Radiation | 23 (3.02) | 11 (4.15) | |
| Surgery | 15 (1.97) | 8 (3.02) | |
| No. of agents, No. (%) | <.001* | ||
| 1 | 300 (46.5) | 102 (43.6) | |
| 2 | 218 (33.8) | 80 (34.2) | |
| 3 | 83 (12.9) | 30 (12.8) | |
| More than 3 | 44 (6.82) | 22 (9.40) | |
| Phase, No. (%) | .005* | ||
| I | 36 (5.78) | 9 (4.02) | |
| II | 455 (73.0) | 190 (84.8) | |
| III | 113 (18.1) | 22 (9.82) | |
| IV | 19 (3.05) | 3 (1.34) | |
| Masking, No. (%) | .014* | ||
| None | 615 (81.3) | 236 (90.1) | |
| Single | 23 (3.04) | 3 (1.15) | |
| Double | 44 (5.82) | 11 (4.20) | |
| Triple | 37 (4.89) | 4 (1.53) | |
| Quadruple | 37 (4.89) | 8 (3.05) | |
| Centers, No. (%) | <.001* | ||
| Multi | 448 (60.3) | 110 (41.8) | |
| Single | 295 (39.7) | 153 (58.2) | |
| Country, No. (%) | .009* | ||
| HICs | 610 (96.8) | 250 (100) | |
| LMICs | 20 (3.17) | 0 (0.00) | |
| Sample, No. (%) | <.001* | ||
| Less than 50 | 355 (46.2) | 218 (82.3) | |
| More than 50 | 413 (53.8) | 47 (17.7) |
Abbreviations: HICs, high-income countries; LMICs, low- and middle-income countries; NIH, National Institutes of Health; PI, principal investigator.
Signifies statistical significance.
TABLE 3.
Multivariate Analysis for the Difference Between Completed and Terminated Clinical Trials
| Multivariate Analysis | OR (95% CI) | P |
|---|---|---|
| Funding source | ||
| Industrial | Ref | Ref |
| NIH | 1.15 (0.83 to 1.60) | .402 |
| Other | 3.25 (0.08 to 127) | .472 |
| Private | 0.66 (0.35 to 1.16) | .150 |
| University | 2.20 (1.45 to 3.32) | <.001* |
| Intervention | ||
| Device | Ref | Ref |
| Medication | 1.20 (0.53 to 3.09) | .675 |
| Medication/surgery | 1.94 (0.69 to 5.95) | .215 |
| Medication/radiation | 1.08 (0.29 to 3.89) | .901 |
| Other | 0.27 (0.06 to 0.99) | .049 |
| Radiation | 1.62 (0.53 to 5.18) | .398 |
| Surgery | 1.80 (0.53 to 6.30) | .344 |
| No. of agents | ||
| 1 | Ref | Ref |
| 2 | 1.08 (0.77 to 1.52) | .661 |
| 3 | 1.07 (0.65 to 1.70) | .794 |
| More than 3 | 1.47 (0.83 to 2.56) | .183 |
| Phase | ||
| I | Ref | Ref |
| II | 1.65 (0.81 to 3.73) | .176 |
| III | 0.77 (0.33 to 1.93) | .568 |
| IV | 0.65 (0.13 to 2.56) | .557 |
| Masking | ||
| None | 1.52 (0.80 to 3.15) | .213 |
| Single | 0.54 (0.11 to 1.98) | .373 |
| Double | Ref | Ref |
| Triple | 0.45 (0.11 to 1.45) | .185 |
| Quadruple | 0.87 (0.30 to 2.41) | .789 |
| Centers | ||
| Multi | Ref | Ref |
| Single | 2.11 (1.59 to 2.81) | <.001* |
| Sample | ||
| Less than 50 | Ref | Ref |
| More than 50 | 0.19 (0.13 to 0.26) | <.001* |
Abbreviations: NIH, National Institutes of Health; OR, odds ratio.
Signifies statistical significance.
DISCUSSION
To our knowledge, this is the first comprehensive study that examined the factors associated with GU cancer clinical trial termination. The overall termination rate of these trials was 25.4%. The most frequent causes for trial termination in this study were low patient accrual and safety concerns. Trials that receive university funds, were based in a single center, and aimed to recruit <50 patients were more likely to end up being terminated.
We observed that almost a quarter of clinical trials accounting for urologic malignancies conducted between 2000 and 2020 were terminated, which is consistent with the worldwide reported termination rate of nonurology oncologic clinical trials.13 This finding emphasizes the sophistication encountered in the designing and successfully conducting this specialized subset of clinical trials, given the complexity of urologic cancers. We observed that the predominant reason leading to the termination of urologic malignancies' clinical trials was low patient accrual (53%), followed by patient safety concerns (20%) of the trials. These findings are distinctly different from the predominant reasons leading to the termination of nononcologic trials, where the main reason for termination is the lack of efficacy.14-17 This could be of particular importance given that malignancies, including urologic malignancies, progress at a widely variable and, in certain instances, unexpected rate and high patient accrual is mandated to achieve high evidence-based and accurate findings.18-20 In addition, safety concerns are paramount, especially in malignancies, as patients tend to be immunocompromised and frail, making them prone to rapid deconditioning. Therefore, tailored protocols with rigorous safety measures must be implemented to ensure the safety of enrolled participants.21
We observed the presence of several factors associated with the termination of GU clinical trials across the conducted trials between 2000 and 2020. The funding source was found to have a notable connection with the increased probability of clinical trial termination. As such, in university-funded trials, 18.9% (n = 50) were terminated, while only 9.66% (n = 74) were completed. However, it is noteworthy that there are considerable discrepancies and a lack of consensus regarding the impact of the funding source and the outcome of the trials in terms of successful completion or termination and the currently available literature does not completely explain the wide variation witnessed.14,15,22,23 Furthermore, our study revealed that the setting of the clinical trial and whether it is a multicenter trial or a single-center trial are significantly associated with the outcome of the trial as only 19.7% of the multicenter clinical trials were terminated in contrast to 31.4% of the single-center clinical trials. Indeed, our finding of a lower termination rate in multicenter clinical trials when compared with a single center is in line with previously reported literature.24-27 While multicenter clinical trials are challenging, require robust infrastructure, and necessitate intricate logistics in contrast to single-center trials, the lower termination among multicenter trials suggests that single centers may collaborate with others to improve study completion.
Notably, a staggeringly low number of clinical trials were conducted in LMICs, accounting for only 2.27% of all the reported trials during 2000-2020. When compared with clinical trials conducted in HICs, our study demonstrated that there is a statistically significant association between the likelihood of completion of the clinical trial and whether it is conducted in an LMIC or HIC as surprisingly, none of the trials conducted in LMICs were terminated, in contrast to 250 trials (29%) of those conducted in HICs were terminated. Nonetheless, this is postulated to be due to a multitude of factors including the small number of clinical trials conducted in LMICs in contrast to HICs and thus the potential lack of generalizability; in addition to that, further emphasis on stringent and vigorous protocols might have been placed in the studies conducted in the LMICs given the scarcity of resources and thus the desirability to complete the trials.
This study is limited by a multitude of factors, notably the restricted time of 2000-2020 and thus the exclusion of clinical trials conducted before and after the allocated period, in addition to the exclusion of non-English reported trials. Furthermore, it is noteworthy that this work focused on establishing the predominant factors behind the termination of the clinical trials on the basis of the publicly available data and did not count for external factors that could potentially be contributing factors to the termination of trials. Nonetheless, the presented results are of significant and paramount importance toward improving successful conduction of GU cancer clinical trials as evidenced by their consistency in certain aspects with previously conducted studies evaluating the termination of nonurologic condition clinical trials, including oncologic trials.14,15,28 In addition, the absence of studies evaluating the driving factors leading to the termination of urologic malignancies' clinical trials further augments the significance of the findings reported in this work because of the importance of clinical trials in the progression and advancement of care in urologic malignancies. Accordingly, the reported findings can be of significant value in designing and conducting future clinical trials targeting urologic malignancies.
In conclusion, the termination rate of urologic cancer clinical trials was 25%, with low accrual being the most frequently reported reason. Trials funded by a university, single-center trials, and small trials (sample size <50) were associated with early termination. A better understanding of these factors might help researchers, funding agencies, and other stakeholders to prioritize resource allocations for multicenter trials that aim to recruit a large number of patients.
ACKNOWLEDGMENT
We cordially thank the participants of this study for their time and valuable contributions and ClinicalTrials.gov for their continuous and outstanding work in helping improve the health care of the patients in all aspects.
David I. Lee
Honoraria: Intuitive Surgical
No other potential conflicts of interest were reported.
DATA SHARING STATEMENT
All data generated or analyzed during this study are fully included in this published article. Researchers or interested parties who are seeking access to the raw data sets used and/or analyzed during the current study can obtain them from the corresponding author upon a reasonable request.
AUTHOR CONTRIBUTIONS
Conception and design: Abdulrahman Alhajahjeh, Faris Al-Abbadi, Mohammed Shahait
Financial support: Abdulrahman Alhajahjeh, Mohammed Shahait
Administrative support: Abdulrahman Alhajahjeh, Majedah Hmeidan, Mus’ab Elatrsh, Razan Sukerji, Mohammad Salah, Mohammed Shahait
Provision of study materials or patients: Abdulrahman Alhajahjeh, Mohammed Shahait
Collection and assembly of data: Abdulrahman Alhajahjeh, Majedah Hmeidan, Mus’ab Elatrsh, Faris Al-Abbadi, Diala Kakish, Razan Sukerji, Mohammad Salah, Mohammed Shahait
Data analysis and interpretation: Abdulrahman Alhajahjeh, Bashir Al Hussein Al Awamlh, David I. Lee, Mohammed Shahait
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
David I. Lee
Honoraria: Intuitive Surgical
No other potential conflicts of interest were reported.
REFERENCES
- 1. Dy GW, Gore JL, Forouzanfar MH, et al. Global burden of urologic cancers, 1990–2013. Eur Urol. 2017;71:437–446. doi: 10.1016/j.eururo.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 2. Zi H, He S-H, Leng X-Y, et al. Global, regional, and national burden of kidney, bladder, and prostate cancers and their attributable risk factors, 1990–2019. Mil Med Res. 2021;8:60. doi: 10.1186/s40779-021-00354-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen S, Cao Z, Prettner K, et al. Estimates and projections of the global economic cost of 29 cancers in 204 countries and territories from 2020 to 2050. JAMA Oncol. 2023;9:465–472. doi: 10.1001/jamaoncol.2022.7826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Richters A, Aben KKH, Kiemeney LALM. The global burden of urinary bladder cancer: An update. World J Urol. 2020;38:1895–1904. doi: 10.1007/s00345-019-02984-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Michaeli JC, Boch T, Albers S, et al. Socio-economic burden of disease: Survivorship costs for bladder cancer. J Cancer Policy. 2022;32:100326. doi: 10.1016/j.jcpo.2022.100326. [DOI] [PubMed] [Google Scholar]
- 6.Patlak M, Nass S, editors. Improving the Quality of Cancer Clinical Trials. Washington, DC: National Academies Press; 2008. [Google Scholar]
- 7. Schaeffer EM, Srinivas S, Adra N, et al. NCCN Guidelines® Insights: Prostate cancer, version 1.2023. J Natl Compr Canc Netw. 2022;20:1288–1298. doi: 10.6004/jnccn.2022.0063. [DOI] [PubMed] [Google Scholar]
- 8. Pascarella G, Capasso A, Nardone A, et al. Costs of clinical trials with anticancer biological agents in an Oncologic Italian Cancer Center using the activity-based costing methodology. PLoS One. 2019;14:e0210330. doi: 10.1371/journal.pone.0210330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Naidoo N, Nguyen VT, Ravaud P, et al. The research burden of randomized controlled trial participation: A systematic thematic synthesis of qualitative evidence. BMC Med. 2020;18:6. doi: 10.1186/s12916-019-1476-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Khan MS, Shahid I, Asad N, et al. Discontinuation and non-publication of heart failure randomized controlled trials: A call to publish all trial results. ESC Heart Fail. 2021;8:16–25. doi: 10.1002/ehf2.13099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.ClinicalTrials.gov . ClinicalTrials.gov Background. 2020. https://clinicaltrials.gov/ct2/about-site/background [Google Scholar]
- 12. Bérubé N, Ghiasi G, Sainte-Marie M, et al. Wiki-Gendersort: Automatic gender detection using first names in Wikipedia. SocArXiv, 2020. doi: 10.31235/osf.io/ezw7p. [DOI] [Google Scholar]
- 13. Chen EY, Joshi SK, Tran A, et al. Estimation of study time reduction using surrogate end points rather than overall survival in oncology clinical trials. JAMA Intern Med. 2019;179:642–647. doi: 10.1001/jamainternmed.2018.8351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pica N, Bourgeois F. Discontinuation and nonpublication of randomized clinical trials conducted in children. Pediatrics. 2016;138:e20160223. doi: 10.1542/peds.2016-0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Roddick AJ, Chan FTS, Stefaniak JD, et al. Discontinuation and non-publication of clinical trials in cardiovascular medicine. Int J Cardiol. 2017;244:309–315. doi: 10.1016/j.ijcard.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 16. Fogel DB. Factors associated with clinical trials that fail and opportunities for improving the likelihood of success: A review. Contemp Clin Trials Commun. 2018;11:156–164. doi: 10.1016/j.conctc.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Institute of Medicine (US) Public Engagement and Clinical Trials: New Models and Disruptive Technologies. Washington, DC: National Academies Press; 2011. [PubMed] [Google Scholar]
- 18. Paul K, Sathianathen N, Dahm P, et al. Variation in accrual and race/ethnicity reporting in urological and nonurological related cancer trials. J Urol. 2019;202:385–391. doi: 10.1097/JU.0000000000000294. [DOI] [PubMed] [Google Scholar]
- 19. Lara PN, Higdon R, Lim N, et al. Prospective evaluation of cancer clinical trial accrual patterns: Identifying potential barriers to enrollment. J Clin Oncol. 2001;19:1728–1733. doi: 10.1200/JCO.2001.19.6.1728. [DOI] [PubMed] [Google Scholar]
- 20. Manini C, López JI. Insights into urological cancer. Cancers (Basel) 2021;13:204. doi: 10.3390/cancers13020204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fountzilas E, Tsimberidou AM, Vo HH, et al. Clinical trial design in the era of precision medicine. Genome Med. 2022;14:101. doi: 10.1186/s13073-022-01102-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Caruana DL, Gouzoulis MJ, McLaughlin WM, et al. Analysis of the frequency, characteristics, and reasons for termination of shoulder- and elbow-related clinical trials. J Shoulder Elbow Surg. 2022;31:1922–1928. doi: 10.1016/j.jse.2022.02.030. [DOI] [PubMed] [Google Scholar]
- 23. Sakate R, Fukagawa A, Takagaki Y, et al. Trends of clinical trials for drug development in rare diseases. Curr Clin Pharmacol. 2018;13:199–208. doi: 10.2174/1574884713666180604081349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang E, DuBois SG. Early termination of oncology clinical trials in the United States. Cancer Med. 2023;12:5517–5525. doi: 10.1002/cam4.5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McDonald AM, Knight RC, Campbell MK, et al. What influences recruitment to randomised controlled trials? A review of trials funded by two UK funding agencies. Trials. 2006;7:9. doi: 10.1186/1745-6215-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Treweek S, Pitkethly M, Cook J, et al. Strategies to improve recruitment to randomised trials. Cochrane Database Syst Rev. 2018;2:MR000013. doi: 10.1002/14651858.MR000013.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hines SE, Barker EA, Robinson M, et al. Cross-sectional study of respiratory symptoms, spirometry, and immunologic sensitivity in epoxy resin workers. Clin Transl Sci. 2015;8:722–728. doi: 10.1111/cts.12341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jones CW, Handler L, Crowell KE, et al. Non-publication of large randomized clinical trials: Cross sectional analysis. BMJ. 2013;347:f6104. doi: 10.1136/bmj.f6104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are fully included in this published article. Researchers or interested parties who are seeking access to the raw data sets used and/or analyzed during the current study can obtain them from the corresponding author upon a reasonable request.