Abstract
We analyzed our challenging experience with a randomized controlled trial of misoprostol for prevention of recurrent C. difficile. Despite careful prescreening and thoughtful protocol modifications to facilitate enrollment, we closed the study early after enrolling just 7 participants over 3 years. We share lessons learned, noting the importance of feasibility studies, inclusion of biomarker outcomes, and dissemination of such findings to inform future research design and implementation successes.
Keywords: C. difficile, misoprostol, BioVU, PheWAS, Clostridioides difficile, trial design
C. difficile infection affects approximately 500,000 people in the United States each year, with billions of dollars annually in health care costs.1 Recurrence of symptoms affects approximately 25% of patients despite use of available treatments, with considerable adverse effects on quality of life and unending recurrence cycles in a notable subset.2-8 Further, those at highest risk of recurrence often present a fairly complex population clinically, with factors including advanced age, frailty, and comorbidities. 2-7
Misoprostol, a safe, low cost, generic prostaglandin analogue approved by the FDA for the prevention of NSAID-induced gastric ulcers (due to its mucosal protective and gastric acid inhibitory properties), has improved recovery from C. difficile infection in animal models.9 Misoprostol leads to an increase in prostaglandin pathway signaling, which human genetic data obtained from a phenome wide association study (PheWAS) indicate could help to reduce risk for C. difficile recurrence.10 Based on these (and other) independent lines of evidence, the PROCLAIM (Prevent Recurrence of Clostidioides difficile Infection with Misoprostol, NCT03617172) study was designed to compare the rate and severity of recurrent C. difficile infection in patients treated with oral misoprostol versus placebo, in addition to standard oral antibiotic therapy, in adults after diagnosis of a primary C. difficile infection.
Three study sites, Vanderbilt University Medical Center (Nashville, Tennessee), Washington University in St. Louis (St. Louis, Missouri) and The University of North Carolina at Chapel Hill (Chapel Hill, North Carolina) participated in the study. The study was reviewed by a central IRB (Vanderbilt IRB #181319) with reliance granted by the other sites’ IRBs before patient enrollment began. Additionally, an independent, three-member data and safety monitoring board (DMSB) oversaw the conduct of the study. Procedures were similar across sites.
Medical records of inpatients and outpatients with a C. difficile positive laboratory test were reviewed for eligibility, and potentially eligible patients were contacted to assess interest in study participation. All participants had to be enrolled 5 to 8 days after their positive laboratory test. Study participants were randomized 1:1 to receive 200 mcg oral misoprostol or an identical appearing placebo two times per day (200 mcg by mouth twice daily) for 14 days. Randomized patients were monitored for recurrence on study days 3, 7, 14, 28, 35, 42 and 49 by telephone, with a follow-up in person visit on days 62–66 where blood and stool samples were acquired.
Our initial power calculation estimated a sample size of 440 participants with 80% power to detect a 40% reduction in recurrence compared to a control group recurrence estimate of 30% and allowing for 10% overall attrition. Our final protocol amendment (version 8.1, November 2021) revised our primary outcome to fecal calprotectin, with a corresponding change in our sample size calculation to 50 total participants with 80% power to detect an 81% reduction in fecal calprotectin, a biomarker shown to correspond with disease severity in C. difficile infection.11,12
Despite screening 20–30 patients on average per month, the first 6 months of the PROCLAIM study yielded no eligible participants (Figure 1). Table 1 illustrates our ongoing deliberations about various inclusion and exclusion criteria, with overall evolution toward a more inclusive patient population reflective of those at risk for recurrence, without compromising safety. Protocol modifications (4.0, 5.0, 6.0, and 7.0) were made to significantly simplify both inclusion and exclusion criteria and this resulted in approximately 10% of patients pre-screened appearing eligible for the PROCLAIM study. Specifically, nearly all exclusion criteria were eliminated except those pertaining to the possibility of pregnancy, which would be a patient safety concern. Additionally, inclusion criteria were broadened to include patients over age 18, from an initial age threshold of 50 years (Table 1).
Figure 1. PROCLAIM Study Screening Timeline from 2018–2022.
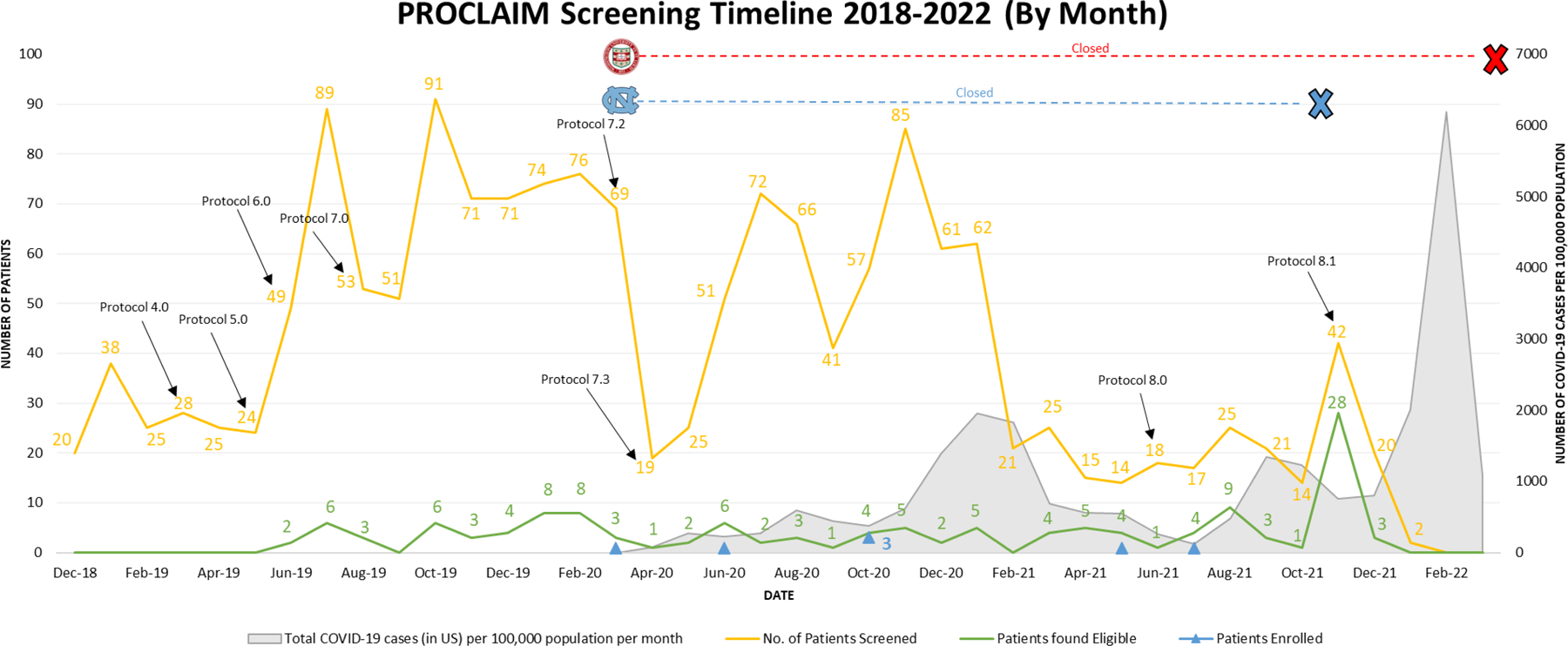
Timeline depicting the number of patients screened (yellow), identified as eligible based on pre-screening (green) and enrolled (indicated by the blue triangles) on a monthly basis during the screening period starting in December 2018 and ending in February 2022 (left y-axis). The total COVID-19 cases in the United States per 100,000 population per month is depicted in gray starting in March 2020 (right y-axis). Significant changes to the study protocol are marked by black arrows and the associated protocol version number at the respective months these amendments were approved. Site closures (no patients were screened or enrolled at the site) resulting from the COVID-19 pandemic are depicted at the top of the timeline for the University of North Carolina at Chapel Hill (light blue) and Washington University in St. Louis (red) with their respective logos and corresponding lines marking the duration of study site closure and the reopening of UNC in November 2021.
Table 1.
Table summarizing the major changes made to the PROCLAIM study protocol.
| Protocol Version | Month of IRB Approval | Protocol Modifications |
|---|---|---|
| Protocols 1 and 2 | -- | Protocol refinements preceding commencement of study start |
| Protocol 3.0 | August 2018 | Quality of life questionnaire, patient diary and phone assessments added. First version of protocol under which study was initiated and screening began in December 2018. |
| Protocol 4.0 | March 2019 | 1. Removed exclusion criteria for: • Oral or parenteral antibacterial therapy • Immunodeficiency disorder 2. Timing of Day 13 visit modified. 3. Courier transportation of samples added. |
| Protocol 5.0 | May 2019 | 1. Inclusion criteria modified: • 3 unformed stools changed to no longer need to be 2 consecutive days for 24 hours prior and continuing at time of randomization. 2. Exclusion criteria modified: • Abdominal discomfort changed to more than mild. |
| Protocol 6.0 | June 2019 | Neutrophil criteria changed from within 90 days of screening to 30. |
| Protocol 7.0 | August 2019 | 1. Day 13 study visit removed. 2. Inclusion criteria changed: • Age lowered to 18. • From oral vancomycin to other antibiotics. 3. Exclusion criteria removed: • Have had more than 1 episode of C. difficile within 6 months. • Myocardial infarction within past 6 months. • Have taken any investigational drug in 30 days. 4. Neutrophil count changed from 1000/mm3 to 500/mm3. |
| Protocol 7.2 | February 2020 | 1. Added possibility of home visits. 2. Diagnostic sample only analyzed when available. |
| Protocol 7.3 | April 2020 | New flyers and brochures. |
| Protocol 8.0 | June 2021 | 2. Removed Inclusion Criteria: • ≥3 unformed stools in 24-hour period • Oral antibiotic treatment at time of enrollment. 3. Removed Exclusion Criteria: • ≥3 unformed stools 24 hours prior to randomization. • Received or plans to use immunotherapy or toxin binding therapy. • Diarrhea likely to be caused by another infection or underlying gastrointestinal disorder. • Absolute neutrophil count < 500/mm3 • Need for mechanical ventilation or vasopressors hemodynamic support. • Inability to be seen for routine clinical care. |
| Protocol 8.1 | November 2021 | 1. Study endpoint changed to fecal calprotectin level analysis. 2. Patient number reduced to 50. 3. Study sample at end of study drug treatment incorporated. |
The number of patients screened decreased significantly in the months of April 2020 and May 2020 coinciding with the COVID-19 pandemic and the resulting closure of the Washington University in St. Louis (WashU) and University of North Carolina at Chapel Hill (UNC) study sites in March 2020; WashU resumed recruitment in November 2021. The number of patients screened dropped to its nadir (15 to 25 patients screened per month) from February 2021 to October 2021. Screening was stopped at VUMC in January 2022 when, upon recommendation by the DSMB, the PROCLAIM study was closed due to low enrollment. Participants were enrolled in March, June, and October (3 participants) in 2020 as well as May and July of 2021 (Figure 1).
A total of 1572 patients were pre-screened during the PROCLAIM study, 226 (14%) of these individuals were found to be eligible. The reasons for exclusion included a participant’s inability to understand or comply with study requirements (21%) or the inability to provide informed consent (17%), as well exclusion criteria specific to the PROCLAIM trial in earlier stages of the trial. For example, in the initial design, exclusion criteria of less than 3 unformed stools within a 24-hour period or having a low absolute neutrophil count were occurring at rates of 24% and 10% respectively and were first altered then ultimately removed entirely in Protocol 8.0 (Table 1). Having other causes of diarrhea (18%), or more than 1 episode of C. difficile infection in a 6-month period (15%) were also removed. Other clinically or medically significant conditions preventing patients from participating in the study (13%), was retained for safety reasons. As illustrated in Figure 1, these modifications to inclusion and exclusion criteria led to a modest increase in number of patients potentially eligible for the study.
An overwhelming majority of the 226 patients found eligible were not enrolled; only 7 patients (3% of those eligible) were included. Many patients declined to participate in the study (42%). Factors including antibiotic taper schedules (26%), inability to enroll due to COVID-19 (15%), lack of response to multiple contact attempts (12%) and recommendations from the treating physicians for their patients not to participate in the study (5%).
In those who volunteered a reason for study decline, reasons for decline included that they did not want to participate in any research (27%) or take additional medication (24%), were unable to return to the clinic for an enrollment visit window (11%) or felt too unwell (11%). Additionally, 33% of patients did not share a reason for declining, which makes drawing strong conclusions about the relative importance of patient reported reasons for declining challenging. While we know that variability in willingness to provide stool samples, perceived risk of further decline in health status, and other factors have been reported to adversely affect trial participation in patients affected by C. difficile infection or other inflammatory bowel diseases,13-15 systematic investigation to identify modifiable factors may facilitate improved success in trials involving such patients. Further, our trial was not alone in being negatively impacted by the COVID-19 pandemic; a growing number of reports share significant recruitment issues and early study closure brought about during this challenging time.16-18
We made several key errors in the design of this study, the most obvious of which was initially modeling the inclusion and exclusion criteria too closely to industry-sponsored studies with >300 sites (MODIFY I and MODIFY II).19 While these studies were a reasonable starting point, our goal did not need to be an endpoint or study population suitable for FDA registration; rather we simply intended to show clinical proof of concept. In hindsight, a smaller study with a biomarker or other more mechanism-focused outcome measure would have been far more practical to complete with only a handful of sites and the available funding. Ultimately, to enroll any participants at all, we had to dramatically simplify our inclusion and exclusion criteria. While we correctly estimated the number of C. difficile cases per month at each health system, we were surprised at just how sick many of these patients were. A nontrivial percentage of “C. difficile patients” we screened had complex comorbidities including cancer or organ transplants, beyond factors we expected such as older age and immune compromise; 2-7 a notable subset of the patients screened had complex combinations of several severe and active health issues (for example, hemodynamic instability, extreme immunosuppression, multiple concurrent infections, severe nausea or ileus, hospice care) leading to investigator judgment that trial participation was not a worthwhile option to explore with them and their care teams. The other clear challenge in this study was the relatively short time window, enrollment within 5–8 days after a positive laboratory test, during which a patient must enroll in the study. While we had extremely effective communication with the clinical microbiology labs and pre-screened every patient with a positive C. difficile lab test at the sites, this problem plagues all studies of acute illnesses.
A more reasonable design for a proof-of-concept study might have focused on a biomarker response, such as the fecal calprotectin we amended to in the last months of the study, and would have had an enrollment goal of approximately 50 to 80 participants from ~3 to 10 study sites. With reasonable inclusion/exclusion criteria, our experience suggests it would be possible to enroll sufficient participants to be informative from a handful of sites in a few years of time. Our critical lesson was the importance of matching the study design to the scale of resources available, and we recommend investigators conduct realistic feasibility studies so that only studies most likely to achieve their goal for informativeness will proceed. Additionally, given that many C. difficile patients had complex medical comorbidities we should have more proactively established relationships with clinicians involved in organ transplants and oncology, for example, to facilitate inclusion of the patients in these subsets.
Our original hypothesis remains untested, and the rationale for the target, the drug, the dose, and the patient population remain strong. We hope our identification of several specific barriers to recruitment can serve to help others in planning studies in C. difficile and other infectious diseases.
Supplementary Material
1. Study protocol
Figure 2. PROCLAIM Study Consort Diagram.
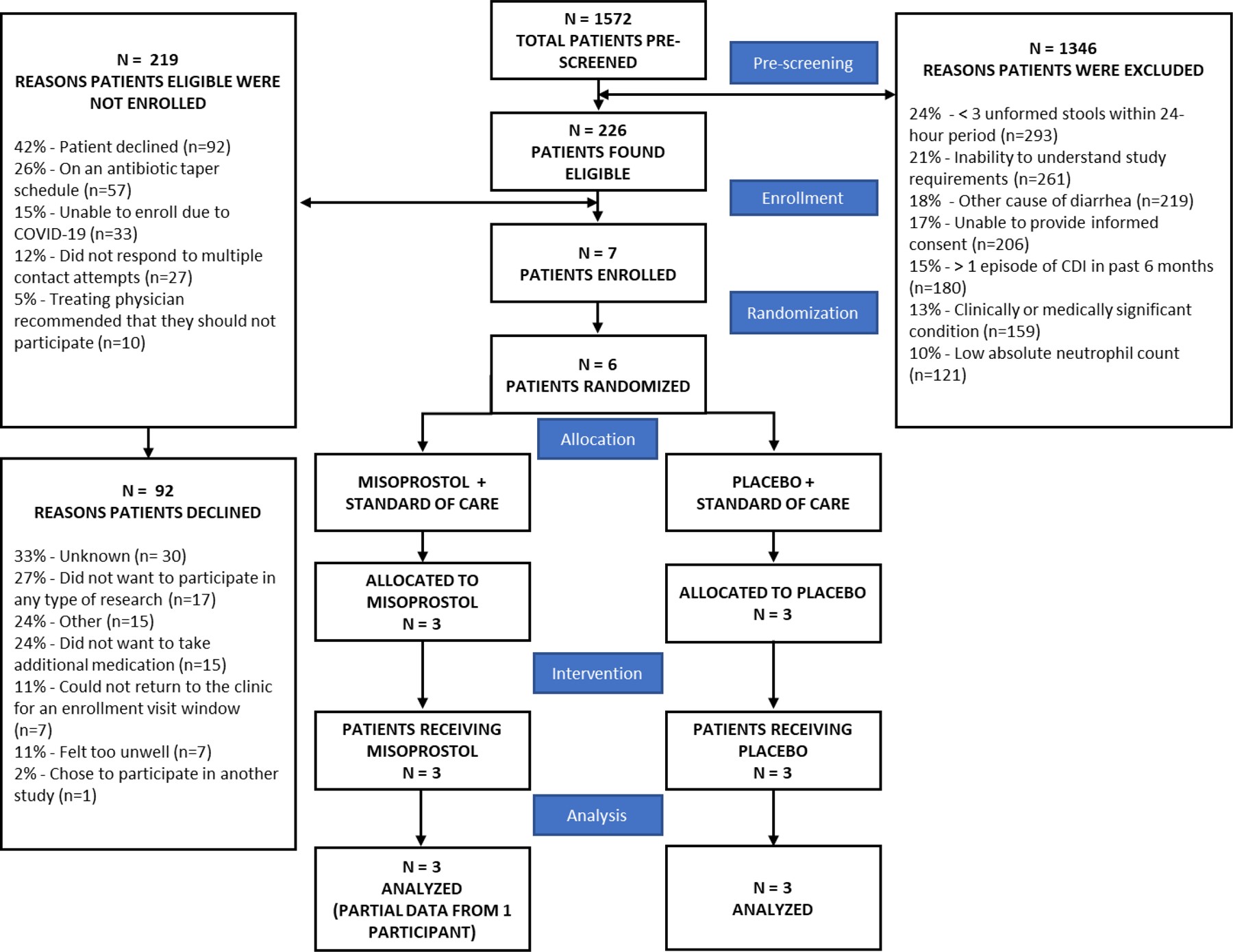
Consort diagram summarizing the PROCLAIM study from pre-screening to data analysis. The reasons for the exclusion of potential study subjects following pre-screening are shown in the top right of the diagram. Of the 1346 subjects that were excluded, the reason for exclusion was only collected for 1236 patients. In some instances, a single patient met multiple exclusion criteria. The reasons patients found eligible were not enrolled are detailed in the top left of the diagram with the reasons eligible patients declined to participate detailed below.
Highlights.
Recurrent Clostridioides difficile is a major public health problem
We attempted a randomized controlled trial of misoprostol for C. difficile
Of 1572 patients pre-screened, 226 were eligible, but only 7 enrolled
C. difficile patients often have significant barriers to study participation
Knowing these barriers could help future studies succeed
Acknowledgment
The authors acknowledge all patients who volunteered their time to learn about the study and the 7 patients who volunteered to participate in the study, staff at all 3 study sites, the 3 members of the DSMB including Leslie J. Crofford, M.D., Dawn B. Beaulieu, MD, and Jody D. Ciolino, PhD., and current and previous NCATS program officials including Dr. Bobbie Ann Mount, Dr. Soju Chang, and Dr. Eugene Passamani.
Source of Funding
This research was primarily supported by the National Institutes of Health (NCATS 5U01TR002398–03). Additional support for the project was provided by Vanderbilt’s CTSA award (5UL1TR002243–0). R.S.D. is supported by NIAID career development award (1K08AI151100).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
S.K.M.: Research funding from Finch Therapeutics.
E.R.D: Research funding provided by Ferring, Synthetic Biologics, and Pfizer; Consultant for Summit, Seres, Abbott, Pfizer, Merck, Ferring.
References
- 1.Hughes M, Qazi T, Berg A, Weinberg J, Chen X, Kelly CP, Farraye FA. Host Immune Response to Clostridium difficile Infection in Inflammatory Bowel Disease Patients: Inflammatory Bowel Diseases 2016. Apr;22(4):853–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelly CP. Can we identify patients at high risk of recurrent Clostridium difficile infection? Clinical Microbiology and Infection 2012;18:21–27. [DOI] [PubMed] [Google Scholar]
- 3.McFarland LV. A Randomized Placebo-Controlled Trial of Saccharomyces boulardii in Combination With Standard Antibiotics for Clostridium difficile Disease. JAMA 1994. Jun 22;271(24):1913. [PubMed] [Google Scholar]
- 4.Sheitoyan-Pesant C, Abou Chakra CN, Pépin J, Marcil-Héguy A, Nault V, Valiquette L. Clinical and Healthcare Burden of Multiple Recurrences of Clostridium difficile Infection. Clin Infect Dis 2016. Mar 1;62(5):574–580. PMID: 26582748 [DOI] [PubMed] [Google Scholar]
- 5.Garey KW, Aitken SL, Gschwind L, Goddu S, Xie Y, Duff C, Barbut F, Shah DN, DuPont HL. Development and Validation of a Clostridium difficile Health-related Quality-of-Life Questionnaire. Journal of Clinical Gastroenterology 2016. Sep;50(8):631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olsen MA, Yan Y, Reske KA, Zilberberg MD, Dubberke ER. Recurrent Clostridium difficile infection is associated with increased mortality. Clin Microbiol Infect 2015. Feb;21(2):164–170. PMID: 25658560 [DOI] [PubMed] [Google Scholar]
- 7.Wilcox MH, Ahir H, Coia JE, Dodgson A, Hopkins S, Llewelyn MJ, Settle C, Mclain-Smith S, Marcella SW. Impact of recurrent Clostridium difficile infection: hospitalization and patient quality of life. J Antimicrob Chemother 2017. Sep 1;72(9):2647–2656. PMID: 28633368 [DOI] [PubMed] [Google Scholar]
- 8.Johnson S, Lavergne V, Skinner AM, Gonzales-Luna AJ, Garey KW, Kelly CP, Wilcox MH. Clinical Practice Guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 Focused Update Guidelines on Management of Clostridioides difficile Infection in Adults. Clin Infect Dis 2021. Sep 7;73(5):e1029–e1044. PMID: 34164674 [DOI] [PubMed] [Google Scholar]
- 9.Zackular JP, Kirk L, Trindade BC, Skaar EP, Aronoff DM. Misoprostol protects mice against severe Clostridium difficile infection and promotes recovery of the gut microbiota after antibiotic perturbation. Anaerobe 2019. Aug;58:89–94. PMCID: PMC6697607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Challa AP, Zaleski NM, Jerome RN, Lavieri RR, Shirey-Rice JK, Barnado A, Lindsell CJ, Aronoff DM, Crofford LJ, Harris RC, Alp Ikizler T, Mayer IA, Holroyd KJ, Pulley JM. Human and Machine Intelligence Together Drive Drug Repurposing in Rare Diseases. Front Genet [Internet]. Frontiers; 2021. [cited 2021 Jul 30];12. Available from: 10.3389/fgene.2021.707836/full [DOI] [PMC free article] [PubMed]
- 11.Kim J, Kim H, Oh HJ, Kim HS, Hwang YJ, Yong D, Jeong SH, Lee K. Fecal Calprotectin Level Reflects the Severity of Clostridium difficile Infection. Ann Lab Med 2017. Jan;37(1):53–57. PMCID: PMC5107618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallo A, Vallone C, Sabatelli L, Ventura G, Covino M, Cammarota G, Gasbarrini A, Landolfi R, Montalto M. Fecal calprotectin in management of Clostridium difficile infection: a longitudinal study. Scandinavian Journal of Gastroenterology 2018. May 4;53(5):567–572. [DOI] [PubMed] [Google Scholar]
- 13.Bolte LA, Klaassen MAY, Collij V, Vich Vila A, Fu J, van der Meulen TA, de Haan JJ, Versteegen GJ, Dotinga A, Zhernakova A, Wijmenga C, Weersma RK, Imhann F. Patient attitudes towards faecal sampling for gut microbiome studies and clinical care reveal positive engagement and room for improvement. PLoS One 2021;16(4):e0249405. PMCID: PMC8031379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gehrmann U, Berger U, Teich N, Bruns T, Stallmach A, Weber M. Motivation of patients with inflammatory bowel disease to participate in a clinical trial. Z Gastroenterol 2016. Oct;54(10):1123–1129. PMID: 27723903 [DOI] [PubMed] [Google Scholar]
- 15.Ravikoff JE, Cole EB, Korzenik JR. Barriers to enrollment in inflammatory bowel disease randomized controlled trials: an investigation of patient perspectives. Inflamm Bowel Dis 2012. Nov;18(11):2092–2098. PMID: 22241674 [DOI] [PubMed] [Google Scholar]
- 16.Chen Z, Chen L, Chen H. The impact of COVID-19 on the clinical trial. PLOS ONE. Public Library of Science; 2021. May 11;16(5):e0251410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ledford H The COVID pandemic’s lingering impact on clinical trials. Nature 2021. Jun 28;595(7867):341–342. [DOI] [PubMed] [Google Scholar]
- 18.Sathian B, Asim M, Banerjee I, Pizarro AB, Roy B, van Teijlingen ER, do Nascimento IJB, Alhamad HK. Impact of COVID-19 on clinical trials and clinical research: A systematic review. Nepal J Epidemiol 2020. Sep 30;10(3):878–887. PMCID: PMC7538012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilcox MH, Gerding DN, Poxton IR, Kelly C, Nathan R, Birch T, Cornely OA, Rahav G, Bouza E, Lee C, Jenkin G, Jensen W, Kim YS, Yoshida J, Gabryelski L, Pedley A, Eves K, Tipping R, Guris D, Kartsonis N, Dorr MB, MODIFY I and MODIFY II Investigators. Bezlotoxumab for Prevention of Recurrent Clostridium difficile Infection. N Engl J Med 2017. Jan 26;376(4):305–317. PMID: 28121498 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1. Study protocol


