Abstract
BACKGROUND
Monoclonal antibodies that target amyloid-beta (Aβ) have the potential to slow cognitive and functional decline in persons with early Alzheimer’s disease. Gantenerumab is a subcutaneously administered, fully human, anti-Aβ IgG1 monoclonal antibody with highest affinity for aggregated Aβ that has been tested for the treatment of Alzheimer’s disease.
METHODS
We conducted two phase 3 trials (GRADUATE I and II) involving participants 50 to 90 years of age with mild cognitive impairment or mild dementia due to Alzheimer’s disease and evidence of amyloid plaques on positron-emission tomography (PET) or cerebrospinal fluid (CSF) testing. Participants were randomly assigned to receive gantenerumab or placebo every 2 weeks. The primary outcome was the change from baseline in the score on the Clinical Dementia Rating scale−Sum of Boxes (CDR-SB; range, 0 to 18, with higher scores indicating greater cognitive impairment) at week 116.
RESULTS
A total of 985 and 980 participants were enrolled in the GRADUATE I and II trials, respectively. The baseline CDR-SB score was 3.7 in the GRADUATE I trial and 3.6 in the GRADUATE II trial. The change from baseline in the CDR-SB score at week 116 was 3.35 with gantenerumab and 3.65 with placebo in the GRADUATE I trial (difference, −0.31; 95% confidence interval [CI], −0.66 to 0.05; P = 0.10) and was 2.82 with gantenerumab and 3.01 with placebo in the GRADUATE II trial (difference, −0.19; 95% CI, −0.55 to 0.17; P = 0.30). At week 116, the difference in the amyloid level on PET between the gantenerumab group and the placebo group was −66.44 and −56.46 centiloids in the GRADUATE I and II trials, respectively, and amyloid-negative status was attained in 28.0% and 26.8% of the participants receiving gantenerumab in the two trials. Across both trials, participants receiving gantenerumab had lower CSF levels of phosphorylated tau 181 and higher levels of Aβ42 than those receiving placebo; the accumulation of aggregated tau on PET was similar in the two groups. Amyloid-related imaging abnormalities with edema (ARIA-E) occurred in 24.9% of the participants receiving gantenerumab, and symptomatic ARIA-E occurred in 5.0%.
CONCLUSIONS
Among persons with early Alzheimer’s disease, the use of gantenerumab led to a lower amyloid plaque burden than placebo at 116 weeks but was not associated with slower clinical decline. (Funded by F. Hoffmann−La Roche; GRADUATE I and II ClinicalTrials.gov numbers, NCT03444870 and NCT03443973, respectively.)
Monoclonal antibodies that target different amyloid-beta (Aβ) protein species in persons with Alzheimer’s disease have been developed, but phase 2 and phase 3 clinical trials of these drugs have had mixed results.1–7 Trials in which removal of amyloid plaques was reported to a level below the threshold for amyloid positivity, as assessed by means of positron-emission tomography (PET), showed a benefit with respect to the slowing of cognitive and functional decline.1–3,5,8,9 However, trials in which incomplete removal of amyloid plaques was reported showed little to no benefit.4,6,7
Gantenerumab is a subcutaneously administered, fully human, anti-Aβ IgG1 monoclonal antibody with highest affinity for aggregated Aβ, including oligomers, fibrils, and plaques.10,11 It removes Aβ through microglia-mediated phagocytosis, promotes amyloid plaque clearance, and has been shown to have effects on biomarkers of Alzheimer’s disease and neurodegeneration.10,12 We conducted two phase 3 trials (GRADUATE I and II) to determine the clinical and biologic effects and safety of the use of gantenerumab in persons with early symptomatic Alzheimer’s disease, defined as mild cognitive impairment or mild dementia due to Alzheimer’s disease.13
METHODS
TRIAL DESIGN
The GRADUATE I and II trials were phase 3, multicenter, randomized, double-blind, placebo-controlled, parallel-group trials. Participants were recruited from 288 sites in 30 countries (156 sites in 15 countries in the GRADUATE I trial; 152 sites in 18 countries in the GRADUATE II trial) on five continents. Recruitment methods differed across sites and included review of patient databases and local advertising. After screening, eligible participants entered a double-blind treatment period. Some participants were enrolled in substudies that involved longitudinal cerebrospinal fluid (CSF) evaluation for amyloid, PET evaluation for amyloid, or PET evaluation for tau; these substudies were conducted to evaluate the effect of gantenerumab on brain amyloid and tau levels. CSF evaluation was conducted only at sites where lumbar puncture could be performed.
All the participants were evaluated for adverse events, concomitant medication use, and vital signs at every visit. Visits took place every 2 to 4 weeks (depending on dosing frequency), either at a trial site or at home (when applicable). Personnel who prepared and administered gantenerumab or placebo were not involved in any efficacy or safety assessments. All clinical assessments were completed by raters who were unaware of the trial-group assignments. The Clinical Dementia Rating (CDR) scale was used by raters who had appropriate training and experience, were not involved in safety assessments, and did not receive any data regarding amyloid-related imaging abnormalities (ARIA). Efforts were made to keep the CDR rater consistent throughout the trial for each participant. Different raters were involved in the other clinical assessments. Long-term safety follow-up visits were conducted 14 and 50 weeks after the last dose of gantenerumab or placebo was administered. Additional details regarding the design of the trials and substudies are provided in the protocol, available with the full text of this article at NEJM.org.
TRIAL OVERSIGHT
The GRADUATE I and II trials were designed and funded by the sponsor, F. Hoffmann–La Roche. The sponsor provided the trial drug and placebo. Two authors employed by the sponsor analyzed the data in collaboration with academic authors. The first, second, and last authors wrote the first draft of the manuscript with professional medical writing assistance, which was funded by the sponsor. All the authors contributed to subsequent drafts. The authors vouch for the completeness and accuracy of the data and for the fidelity of the trials to the protocol. Confidentiality agreements were in place between the authors and the sponsor, and the sponsor could not interdict or delay the publication of results.
The trials were conducted in accordance with the International Council for Harmonisation E6 Guideline for Good Clinical Practice and the principles of the Declaration of Helsinki, as well as the laws and regulations of the country in which the research was conducted. The protocol and any subsequent amendments were approved by the relevant institutional review board or ethics committee and by regulatory authorities. All the trial participants provided written informed consent. An independent data and safety monitoring committee, which consisted of experts in Alzheimer’s disease and statistics, reviewed unblinded safety data during the trials.
ELIGIBILITY CRITERIA
Persons 50 to 90 years of age were eligible for inclusion in the trials if they had mild cognitive impairment due to Alzheimer’s disease or had mild dementia due to Alzheimer’s disease (conditions previously referred to as prodromal Alzheimer’s disease and mild Alzheimer’s disease, respectively), in accordance with National Institute on Aging−Alzheimer’s Association diagnostic criteria.14,15 Specifically, participants needed to have a CDR−Global Score (CDR-GS) of 0.5 or 1, indicating very mild or mild dementia, respectively. The CDR-GS is derived from an algorithm with six domains; possible scores are 0, 0.5, 1, 2, or 3, with higher scores indicating greater cognitive impairment.16 Participants also needed to have a score on the Mini−Mental State Examination (MMSE) of 22 or higher (range, 0 to 30, with lower scores indicating greater impairment)17; cognitive impairment as manifested by abnormal memory, with a Free and Cued Selective Reminding Test (FCSRT) cueing index of 0.67 or lower (range, 0 to 1, with higher values indicating better performance) and an FCSRT free recall score of 27 or lower (range, 0 to 48, with higher values indicating better performance); and the presence of amyloid plaque on visual reading of PET or a ratio of phosphorylated tau 181 to Aβ42 of more than 0.024 on CSF testing.
Persons were excluded from the trials if they were taking anticoagulants or GV-971 (an oligosaccharide intended to reduce inflammation in the brain by regulating the gut microbiota)18 or if they had clinically significant findings on magnetic resonance imaging (MRI) at screening that could cause cognitive impairment, such as more than five microhemorrhages, more than two lacunar infarcts, or a Fazekas score of 3, indicating that confluent areas of the brain are affected by white-matter hyperintensity. Details are provided in the Supplementary Appendix, available at NEJM.org.
Participants needed to have amyloid-positive status on the basis of PET or CSF testing at screening. If amyloid was detected on PET, the participant was eligible for enrollment in the amyloid PET substudy. If amyloid was detected on CSF testing, the participant was eligible for enrollment in the amyloid CSF substudy. There were no restrictions on eligibility for enrollment in the tau PET substudy.
RANDOMIZATION AND TREATMENT
Participants were randomly assigned in a 1:1 ratio to receive gantenerumab or placebo, administered at a trial site or at home by a nurse. Randomization was stratified according to clinical stage (mild cognitive impairment due to Alzh eimer’s disease vs. mild dementia due to Alzheimer’s disease); apolipoprotein E (APOE) ε4 genotype (no ε4 allele vs. one or two ε4 alleles); use of medication for Alzheimer’s disease symptoms, such as donepezil or memantine (use vs. no use); geographic region (Western Europe and Australia vs. North America vs. other regions); and participation in the amyloid PET substudy or the tau PET substudy (participation vs. no participation).
During the double-blind treatment period, the gantenerumab dose was increased over a period of 36 weeks to a target level of 510 mg every 2 weeks, regardless of APOE ε4 genotype. Participants received a minimum of three doses at each level: the dose was started at 120 mg every 4 weeks (for three doses) and was increased to 255 mg every 4 weeks (for three doses), then to 510 mg every 4 weeks (for three doses), and finally to 510 mg every 2 weeks. At weeks 12, 24, and 36, participants underwent MRI for confirmation of the safety of dose escalation, performed with the use of an algorithm for the management of ARIA, before the next dose level was administered (Table S1 in the Supplementary Appendix); MRI monitoring continued throughout the trial (weeks 48, 60, 76, 104, and 116). MRIs were assessed by independent neuroradiologists who were unaware of the trial-group assignments. The double-blind treatment period was initially planned to be 104 weeks and was extended to 116 weeks in response to delayed and missed visits due to the coronavirus disease 2019 (Covid-19) pandemic. After completion of the 116-week double-blind treatment period, eligible participants could receive open-label gantenerumab, either under the GRADUATE protocol or after enrollment in the PostGRADUATE trial.
EFFICACY
The primary outcome was the change from baseline in the score on the CDR−Sum of Boxes (CDR-SB; range, 0 to 18, with higher scores indicating greater cognitive impairment) at week 116. Three outcomes were defined in the statistical analysis plan as confirmatory secondary outcomes: the change from baseline in the score on the 13-item cognitive subscale of the Alzheimer’s Disease Assessment Scale (ADAS-Cog13; range, 0 to 85, with higher scores indicating greater cognitive impairment), in the total score on the Alzheimer’s Disease Cooperative Study−Activities of Daily Living Inventory (ADCS-ADL; range, 0 to 78, with lower scores indicating greater functional impairment), and in the score on the Functional Activities Questionnaire (FAQ; range, 0 to 30, with higher scores indicating greater functional impairment) at week 116. Additional secondary and exploratory outcomes are listed in the protocol.
SAFETY
Safety outcomes included the incidence, nature, severity, and timing of adverse events, serious adverse events, and ARIA, including ARIA with edema (ARIA-E) and ARIA with hemosiderosis (ARIA-H). Additional safety outcomes included injection-site reactions, findings on physical examination, vital signs, results of blood tests, findings on electrocardiography, the score on the Columbia−Suicide Severity Rating Scale, and the presence of antidrug antibodies. All safety assessments were completed by raters who were unaware of the trial-group assignments.
BIOMARKERS
Exploratory biomarker outcomes included the change from baseline in plasma levels of phosphorylated tau 181 and Aβ42, as well as the change from baseline in the volume of the whole brain, ventricles, and hippocampi on volumetric MRI. Plasma and MRI biomarkers were assessed in all the participants. Additional biomarker outcomes included the change from baseline in CSF levels of total tau, phosphorylated tau 181, Aβ42, Aβ40, neurogranin, and neurofilament light. CSF biomarkers were assessed in the participants in whom amyloid had been detected on CSF testing at screening and a CSF sample had been obtained at week 116.
In the amyloid PET substudy, the main outcome was the change from baseline to week 116 in the amyloid level. The amyloid level was assessed on florbetaben or flutemetamol PET and was measured as a standardized uptake value ratio (SUVR), which is the ratio of the standardized uptake value in the composite region of interest to the value in the inferior cerebellar cortex; the SUVR results were converted to centiloids. In the tau PET substudy, the main outcome was the change from baseline to week 116 in the tau level. The tau level was assessed in medial temporal, lateral temporal, frontal, and parietal composite regions on PET with 18F-GTP1 (Genentech tau probe 1, an investigational radioligand for in vivo imaging of tau protein aggregates) and was measured as an SUVR. Details are provided in the Supplementary Appendix.
STATISTICAL ANALYSIS
We calculated that a sample of approximately 1016 participants per trial group (in both trials combined) would provide the trials with 90% power to detect a change from baseline in the CDR-SB score in the gantenerumab group that was 30% lower than the change in the placebo group, at a two-sided alpha level of 0.05. Assumptions for the power calculation, which were based on earlier studies such as the SCARLET ROAD trial19 and the Alzheimer’s Disease Neuroimaging Initiative study (ClinicalTrials.gov numbers, NCT00106899, NCT01231971, and NCT01078636), included the following: a mean change from baseline in the CDR-SB score at week 104 of 2.5 points with placebo, on the basis of expected disease progression and earlier studies; a common standard deviation across trial groups for the change from baseline in the CDR-SB score at week 104 of approximately 2.97; and a true effect of a 30% relative reduction in the deterioration of the CDR-SB score with gantenerumab. A decrease in sample size of up to 35% was also assumed. It was estimated that because of the Covid-19 pandemic, participants would miss a mean of 8 weeks of the double-blind treatment period (i.e., two to four visits during that period) over the course of the trials, which would reduce the power from 90% to 80%. The protocol was therefore amended to extend the double-blind treatment period to a total of 116 weeks to mitigate the effect of missed visits.
The efficacy analysis included participants who had received at least one dose of gantenerumab or placebo. Primary and secondary outcomes were analyzed according to a fixed hierarchical testing procedure,20 in the order provided above, for control of the overall type I error at 5%. The primary outcome was the first outcome analyzed in the hierarchical analysis. If the between-group difference in the primary outcome was not significant, the difference for all subsequent outcomes was considered to be not significant. Control of the type I error was performed at the trial level, and each trial had a separate analysis.
In line with the estimand framework,21 primary and secondary outcomes were analyzed with conditional mean imputation followed by analysis of covariance,22 as implemented in the R package for reference-based mean imputation (R Project for Statistical Computing).23 In statistical models that used change from baseline as the dependent variable, there was no imputation of the baseline value; therefore, participants missing the baseline value did not contribute to the analysis. When data were missing for a participant after the occurrence of a prespecified intercurrent event that was deemed by an independent adjudication committee to be unrelated to the trial drug or condition, the data were imputed with the standard “missing at random” assumption; when data were missing for a participant after the occurrence of a prespecified intercurrent event that was deemed to be related to the trial drug or condition, the data were imputed on the basis of the trajectory in the placebo group, with the “copy increment from reference” assumption. Sensitivity analyses of the primary outcome were conducted, including a mixed model for repeated measures (MMRM) analysis with adjustment for the baseline CDR-SB score, stratification factors, and key prognostic factors (see the statistical analysis plan, available with the protocol). Statistical analyses were performed with SAS software, version 9.4 (SAS Institute).
The safety analysis included participants who had received at least one dose of gantenerumab or placebo; those who had received at least one dose of gantenerumab were included in the gantenerumab group, regardless of the trial-group assignment. Amyloid PET, tau PET, and plasma biomarker outcomes were analyzed with MMRM. CSF biomarker outcomes were analyzed with analysis of covariance. For plasma and CSF biomarker outcomes, a logarithmic transformation was applied before model fitting.
Results
Trial Population
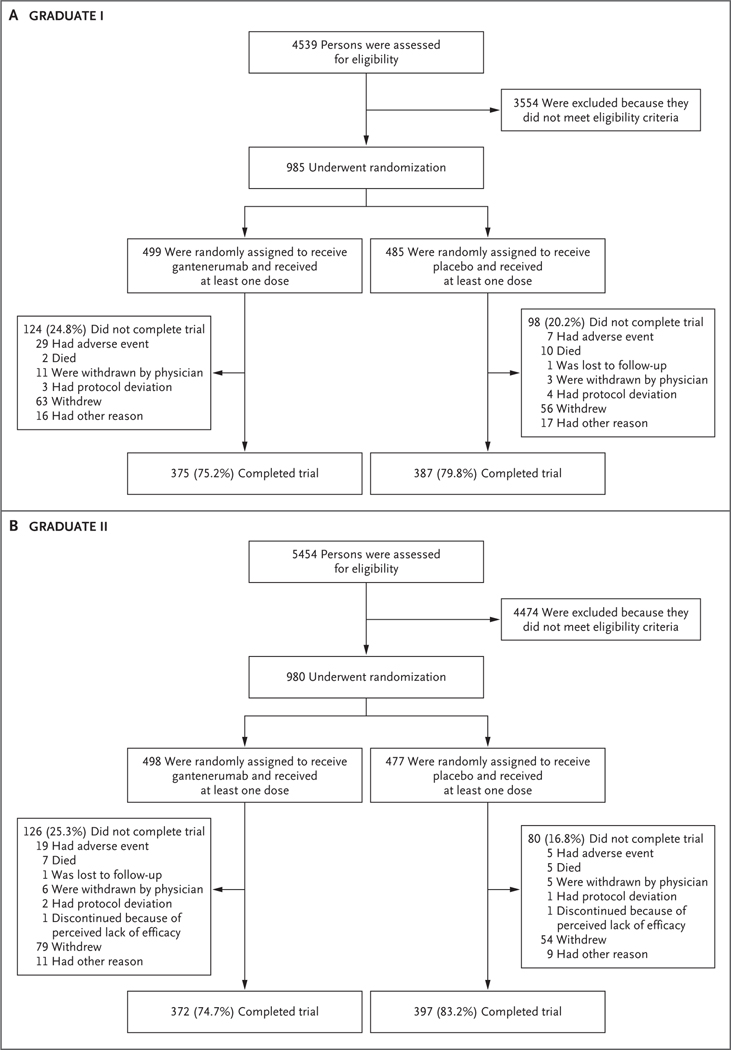
Of the 9993 persons who underwent screening, 1965 were enrolled in the trials; 985 were enrolled in the GRADUATE I trial and 980 in the GRADUATE II trial (Fig. 1). Most screening failures were due to participants’ not meeting FCSRT or MMSE inclusion criteria. In the GRADUATE I trial, 499 and 485 participants were randomly assigned to receive gantenerumab and placebo, respectively, and received at least one dose of the trial drug or placebo. In the GRADUATE II trial, 498 and 477 participants were randomly assigned to receive gantenerumab and placebo, respectively, and received at least one dose of the trial drug or placebo. The characteristics of the participants were similar between trials and trial groups (Table 1 and Table S2). The distribution of the participants with regard to sex, age, and race and ethnic group indicates the representativeness of the trial population as compared with the general population in the United States and other regions (Table S3).
Figure 1. Screening, Randomization, Treatment, and Follow-up.
Overall, 6 participants (1 in the GRADUATE I trial and 5 in the GRADUATE II trial) were randomly assigned to receive placebo but did not receive at least one dose.
Table 1.
Characteristics of the Participants at Baseline.*
| Characteristic | GRADUATE I | GRADUATE II | ||
|---|---|---|---|---|
| Gantenerumab (N = 499) | Placebo (N = 485) | Gantenerumab (N = 498) | Placebo (N = 477) | |
| Age — yr | 71.1±7.9 | 72.1±7.8 | 71.6±7.8 | 71.8±7.4 |
| Female sex — no. (%) | 290 (58.1) | 255 (52.6) | 288 (57.8) | 285 (59.7) |
| Use of medication for Alzheimer’s disease symptoms — no. (%) | 312 (62.5) | 295 (60.8) | 331 (66.5) | 315 (66.0) |
| Clinical stage — no. (%) | ||||
| Mild cognitive impairment due to Alzheimer’s disease | 275 (55.1) | 263 (54.2) | 269 (54.0) | 266 (55.8) |
| Mild dementia due to Alzheimer’s disease | 224 (44.9) | 222 (45.8) | 229 (46.0) | 211 (44.2) |
| CDR-GS — no. (%)† | ||||
| 0.5 | 344 (68.9) | 359 (74.0) | 344 (69.2) | 360 (75.5) |
| 1 | 149 (29.9) | 123 (25.4) | 150 (30.2) | 116 (24.3) |
| 2 | 6 (1.2) | 3 (0.6) | 3 (0.6) | 1 (0.2) |
| CDR-SB score‡ | 3.71±1.67 | 3.71±1.57 | 3.67±1.61 | 3.52±1.54 |
| ADAS-Cog13 score§ | 28.1±7.1 | 28.1±6.8 | 28.1±6.9 | 28.2±7.0 |
| ADCS-ADL total score¶ | 67.9±7.2 | 68.2±6.8 | 68.3±7.3 | 68.9±7.2 |
| FAQ score‖ | 8.0±5.9 | 7.8±5.7 | 7.7±5.8 | 6.8±5.3 |
| MMSE score** | 23.5±3.3 | 23.6±3.0 | 23.6±3.1 | 23.8±3.2 |
| APOE ε4 genotype — no. (%) | ||||
| No ε4 allele | 173 (34.7) | 157 (32.4) | 165 (33.1) | 156 (32.7) |
| One or two ε4 alleles | 326 (65.3) | 328 (67.6) | 333 (66.9) | 321 (67.3) |
| One ε4 allele | 235 (47.1) | 241 (49.7) | 242 (48.6) | 254 (53.2) |
| Two ε4 alleles | 91 (18.2) | 87 (17.9) | 91 (18.3) | 67 (14.0) |
| Amyloid burden on PET — centiloids†† | 94.44±26.48 | 96.07±31.47 | 95.62±30.76 | 90.70±30.80 |
Plus-minus values are means ±SD. Percentages may not total 100 because of rounding. Data for baseline characteristics are shown for participants who received at least one dose of gantenerumab or placebo. Data for additional baseline characteristics, including race, ethnic group, and education level, are provided in Table S2.
The Clinical Dementia Rating scale-Global Score (CDR-GS) is derived from an algorithm with six domains; possible scores are 0, 0.5, 1, 2, or 3, with higher scores indicating greater cognitive impairment. A CDR-GS of 0.5 or 1 at screening was a criterion for eligibility. In the gantenerumab group of the GRADUATE II trial, CDR-GS data were available for 497 participants.
On the Clinical Dementia Rating scale-Sum of Boxes (CDR-SB), scores range from 0 to 18, with higher scores indicating greater cognitive impairment.
On the 13-item cognitive subscale of the Alzheimer’s Disease Assessment Scale (ADAS-Cog 13), scores range from 0 to 85, with higher scores indicating greater cognitive impairment.
On the Alzheimer’s Disease Cooperative Study-Activities of Daily Living Inventory (ADCS-ADL), total scores range from 0 to 78, with lower scores indicating greater functional impairment.
On the Functional Activities Questionnaire (FAQ), scores range from 0 to 30, with higher scores indicating greater functional impairment.
On the Mini-Mental State Examination (MMSE), scores range from 0 to 30, with lower scores indicating greater impairment.
Data for amyloid burden on positron-emission tomography (PET) are shown for participants enrolled in the amyloid PET substudy: 123 participants in the GRADUATE I trial (65 in the gantenerumab group and 58 in the placebo group) and 114 participants in GRADUATE II trial (58 in the gantenerumab group and 56 in the placebo group).
Clinical Outcomes
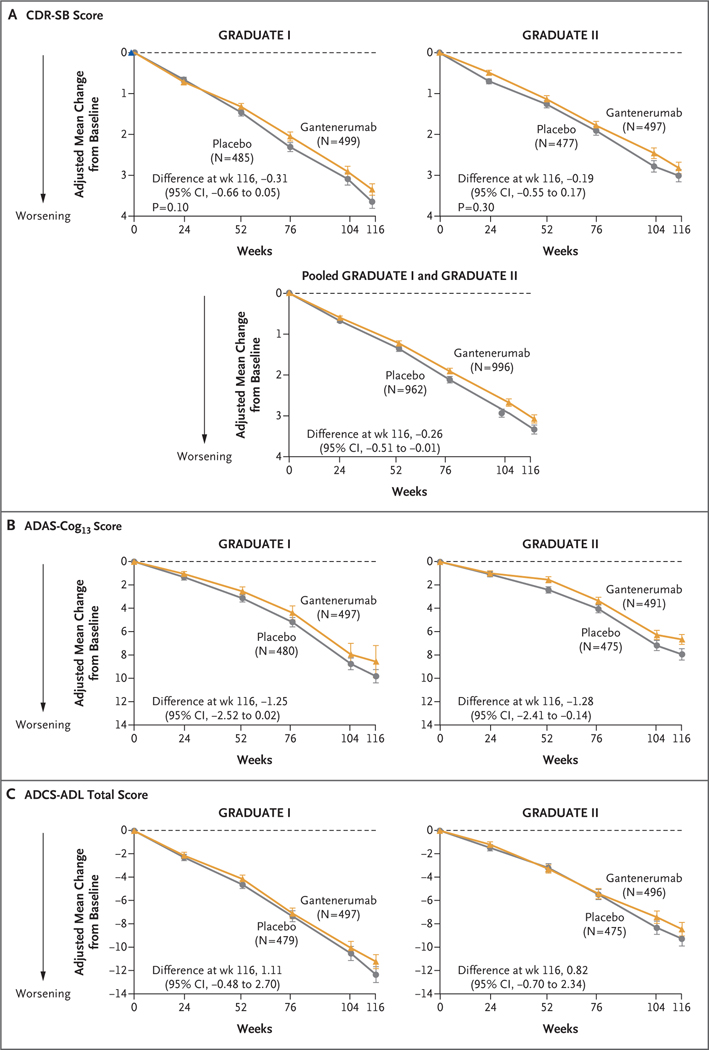
In the GRADUATE I trial, the estimated mean change from baseline in the CDR-SB score at week 116 was 3.35 in the gantenerumab group and 3.65 in the placebo group (difference, −0.31; 95% confidence interval [CI], −0.66 to 0.05; P = 0.10) (Fig. 2A and Table 2). In the GRADUATE II trial, the change was 2.82 in the gantenerumab group and 3.01 in the placebo group (difference, −0.19; 95% CI, −0.55 to 0.17; P = 0.30). A prespecified analysis of pooled data from both trials showed a difference in clinical decline at week 116 that directionally favored gantenerumab over placebo (difference, −0.26; 95% CI, −0.51 to −0.01); the pooled analysis was not part of the hierarchical analysis, and no definitive conclusions can be drawn from the findings.
Figure 2 (facing page). Clinical Outcomes.
Shown is the adjusted mean change from baseline in the score on the Clinical Dementia Rating scale-Sum of Boxes (CDR-SB; range, 0 to 18, with higher scores indicating greater cognitive impairment) (Panel A), in the score on the 13-item cognitive subscale of the Alzheimer’s Disease Assessment Scale (ADAS-Cog13; range, 0 to 85, with higher scores indicating greater cognitive impairment) (Panel B), and in the total score on the Alzh eimer’s Disease Cooperative Study-Activities of Daily Living Inventory (ADCS-ADL; range, 0 to 78, with lower scores indicating greater functional impairment) (Panel C) through week 116. I bars indicate 95% confidence intervals. The analyses were performed with conditional mean imputation followed by analysis of covariance. The primary outcome was the change from baseline in the CDR-SB score at week 116; secondary outcomes included the change from baseline in the ADAS-Cog13 and ADCS-ADL scores at week 116. Data are shown for participants who had available baseline values for a given outcome.
Table 2.
Primary and Secondary Outcomes.*
| Outcome | GRADUATE I | GRADUATE II | ||||
|---|---|---|---|---|---|---|
| Gantenerumab (N = 499) | Placebo (N = 485) | Difference (95% CI) | Gantenerumab (N = 498) | Placebo (N = 477) | Difference (95% CI) | |
| Primary outcome | ||||||
| Change from baseline in the CDR-SB score at week 116† | 3.35±0.14 | 3.65±0.16 | −0.31 (−0.66 to 0.05)‡ | 2.82±0.14 | 3.01±0.15 | −0.19 (−0.55 to 0.17)§ |
| Secondary outcomes | ||||||
| Change from baseline in the ADAS-Cog13 score at week 116¶ | 8.57±0.47 | 9.82±0.57 | −1.25 (−2.52 to 0.02) | 6.66±0.42 | 7.94±0.49 | −1.28 (−2.41 to −0.14) |
| Change from baseline in the ADCS-ADL total score at week 116‖ | −11.21±0.60 | −12.32±0.69 | 1.11 (−0.48 to 2.70) | −8.44±0.58 | −9.26±0.62 | 0.82 (−0.70 to 2.34) |
| Change from baseline in the FAQ score at week 116** | 7.28±0.30 | 8.13±0.33 | −0.86 (−1.68 to −0.03) | 5.86±0.31 | 6.72±0.33 | −0.86 (−1.70 to −0.02) |
Plus−minus values are adjusted means ±SE. The efficacy analysis included participants who had received at least one dose of gantenerumab or placebo. Data are shown for participants who had available baseline values for a given outcome. Data for exploratory outcomes, including amyloid PET, tau PET, cerebrospinal fluid, and plasma biomarker outcomes, are provided in Table S5.
CDR-SB scores range from 0 to 18, with higher scores indicating greater cognitive impairment.
P = 0.10.
P = 0.30.
ADAS-Cog13 scores range from 0 to 85, with higher scores indicating greater cognitive impairment.
ADCS-ADL total scores range from 0 to 78, with lower scores indicating greater functional impairment.
FAQ scores range from 0 to 30, with higher scores indicating greater functional impairment.
The results of sensitivity and supplementary analyses, including the MMRM analysis of the primary outcome, were generally consistent with the results of the primary analysis (Figs. S1 and S2). Estimates of the treatment effect with respect to the secondary outcomes — the change from baseline in the ADAS-Cog13, ADCS-ADL, and FAQ scores at week 116 in the gantenerumab group as compared with the placebo group — for the GRADUATE I and II trials (Fig. 2B and 2C, Table 2, and Fig. S3) were not significant because the hierarchical analysis had failed with the analysis of the primary outcome in each trial.
BIOMARKER OUTCOMES
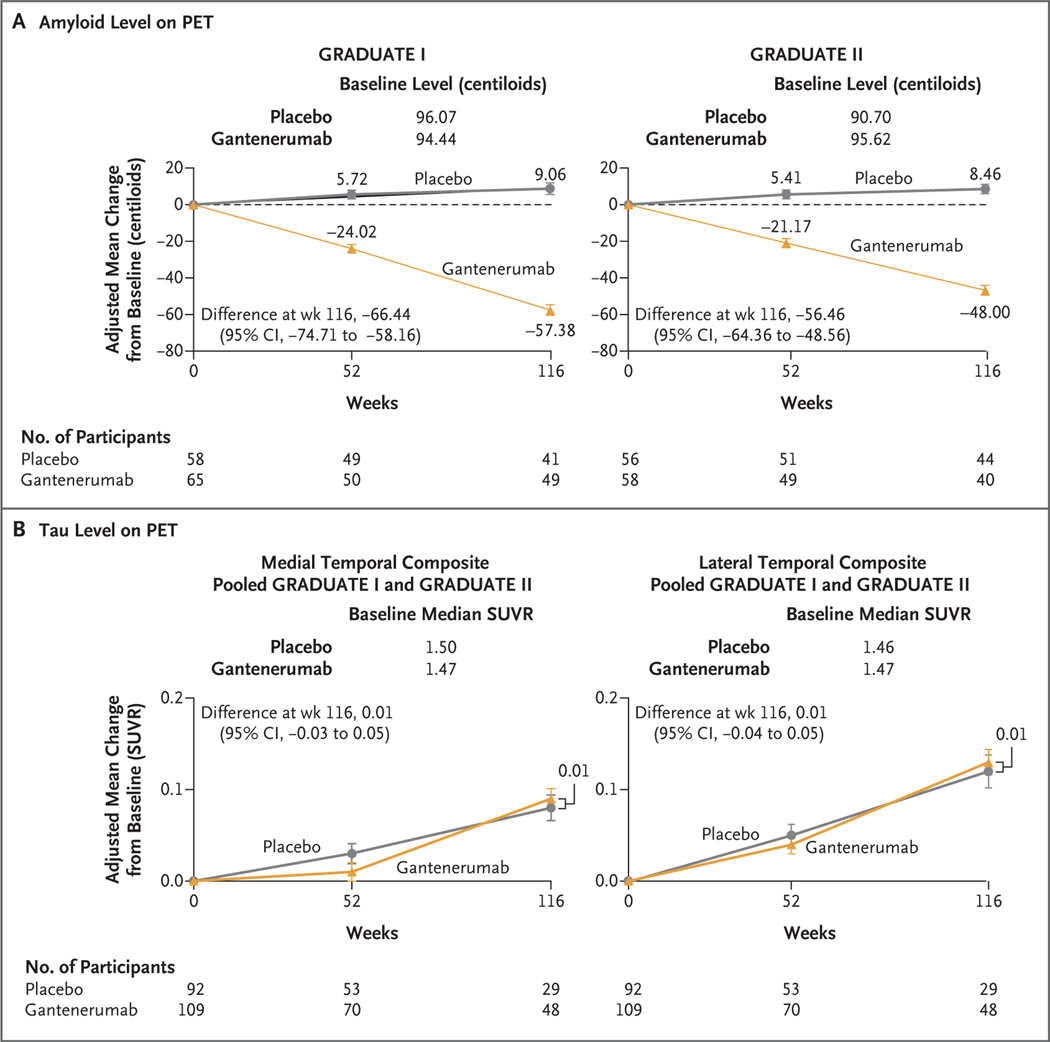
The amyloid level on PET at week 116 among participants receiving gantenerumab was lower than the level among those receiving placebo (Fig. 3A). The difference in the adjusted mean (±SE) amyloid level between the gantenerumab group and the placebo group was −66.44±4.17 centiloids (95% CI, −74.71 to −58.16) in the GRADUATE I trial and −56.46±3.98 centiloids (95% CI, −64.36 to −48.56) in the GRADUATE II trial. The mean (±SD) amyloid level at week 116 was 40.68±27.39 and 104.44±33.15 centiloids in the gantenerumab and placebo groups, respectively, in the GRADUATE I trial and 44.85±26.67 and 99.52±27.72 centiloids in the gantenerumab and placebo groups, respectively, in the GRADUATE II trial.
Figure 3. Biomarker Outcomes.
Shown is the adjusted mean change from baseline in the amyloid level (Panel A) and the tau level (Panel B) on positron-emission tomography (PET) through week 116. I bars indicate 95% confidence intervals. In the amyloid PET substudy, the main outcome was the change from baseline to week 116 in the amyloid level. The amyloid level was assessed on florbetaben or flutemetamol PET and was measured as a standardized uptake value ratio (SUVR), which is the ratio of the standardized uptake value in the composite region of interest to the value in the inferior cerebellar cortex; the SUVR results were converted to centiloids. In the tau PET substudy, the main outcome was the change from baseline to week 116 in the tau level. The tau level was assessed in medial temporal, lateral temporal, frontal, and parietal composite regions on PET with 18F-GTP1 (Genentech tau probe 1, an investigational radioligand for in vivo imaging of tau protein aggregates) and was measured as an SUVR.
At week 116, amyloid-negative status (amyloid level, ≤24 centiloids) was attained in 28.0% and 2.4% of the participants receiving gantenerumab and placebo, respectively, in the GRADUATE I trial and in 26.8% and none of the participants receiving gantenerumab and placebo, respectively, in the GRADUATE II trial (Table S4). A post hoc exploratory analysis of clinical response in participants who had attained amyloid-negative status was performed (Fig. S4); no definitive conclusions can be drawn from the findings.
On volumetric MRI performed at week 116, participants in the gantenerumab group had a greater decrease in the whole-brain volume and a greater increase in the ventricular volume than those in the placebo group in both trials (Fig. S5). Participants in the gantenerumab group had a greater decrease in the left hippocampal volume than those in the placebo group in the GRADUATE I trial, but this finding was not observed in the GRADUATE II trial. The change in the right hippocampal volume was similar in the gantenerumab and placebo groups in both trials.
There was no appreciable difference between the gantenerumab group and the placebo group in the tau level assessed in any of the four composite regions on PET at week 116 (Fig. 3B and Fig. S6). For example, the between-group difference in the median SUVR assessed in the medial temporal composite region, which did not include the hippocampus, was −0.02 (95% CI, −0.06 to 0.03) in the GRADUATE I trial and 0.04 (95% CI, −0.03 to 0.09) in the GRADUATE II trial.
On CSF testing performed at week 116, participants receiving gantenerumab had lower geometric mean levels of total tau, phosphorylated tau 181, and Aβ40 and had a higher geometric mean level of Aβ42 than those receiving placebo across both trials (Table S5). Participants receiving gantenerumab had a greater decrease in the level of neurogranin (a CSF biomarker of synaptic integrity) and a lesser increase in the level of neurofilament light (a CSF biomarker of neurodegeneration) than those receiving placebo. On plasma testing performed at week 116, participants receiving gantenerumab had a lower geometric mean level of phosphorylated tau 181 and a higher geometric mean level of Aβ42 than those receiving placebo (Fig. S7).
PHARMACOKINETIC OUTCOMES
The administration of gantenerumab at 2-week intervals was associated with a higher trough level and a lower maximum level in serum than the administration of similar doses at 4-week intervals. The mean overall levels were not affected, which indicates that the overall exposure was consistent with that observed in modeling (Fig. S8).
ADVERSE EVENTS
The safety profile of gantenerumab did not differ substantially between the GRADUATE I trial and the GRADUATE II trial. Therefore, pooled safety results are described (Table 3 and Table S6). Overall, 10 deaths occurred in the gantenerumab group, and 14 deaths occurred in the placebo group. In the safety population, serious adverse events were reported in 13.6% and 16.5% of the participants in the gantenerumab and placebo groups, respectively. At least one adverse event was reported in 90.1% of those receiving gantenerumab and 87.1% of those receiving placebo. Discontinuation of gantenerumab or placebo due to an adverse event occurred in 9.1% of the participants in the gantenerumab group, as compared with 1.8% of those in the placebo group, a difference predominantly driven by protocol-specified discontinuation criteria for ARIA-H. Injection-site reactions, which were typically mild (as assessed by the investigator) and were not typically associated with discontinuation, occurred in 16.8% of the participants in the gantenerumab group, as compared with 7.7% of those in the placebo group. Intraparenchymal macrohemorrhages (>10 mm) and subarachnoid hemorrhages were reported in 1.4% and 1.0% of the participants receiving gantenerumab and placebo, respectively. The incidence of ARIA-E reported as an adverse event was 21.8% with gantenerumab and 1.8% with placebo.
Table 3.
Summary of Adverse Events and ARIA.*
| Variable | GRADUATE I | GRADUATE II | Pooled | |||
|---|---|---|---|---|---|---|
| Gantenerumab (N = 503) | Placebo (N = 481) | Gantenerumab (N = 501) | Placebo (N = 474) | Gantenerumab (N = 1004) | Placebo (N = 955) | |
| Adverse events | ||||||
| Any adverse event — no. (%) | 454 (90.3) | 423 (87.9) | 451 (90.0) | 409 (86.3) | 905 (90.1) | 832 (87.1) |
| Serious adverse event — no. (%)† | 76 (15.1) | 95 (19.8) | 61 (12.2) | 63 (13.3) | 137 (13.6) | 158 (16.5) |
| Adverse event that led to discontinuation of gantenerumab or placebo — no. (%)‡ | 47 (9.3) | 10 (2.1) | 44 (8.8) | 7 (1.5) | 91 (9.1) | 17 (1.8) |
| Death — no. (%)§ | 3 (0.6) | 10 (2.1) | 7 (1.4) | 4 (0.8) | 10 (1.0) | 14 (1.5) |
| ARIA ¶ | ||||||
| ARIA-E — no./total no. (%) | ||||||
| Any ARIA-E | 119/497 (23.9) | 8/476 (1.7) | 128/496 (25.8) | 18/470 (3.8) | 247/993 (24.9) | 26/946 (2.7) |
| ARIA-E according to APOE ε4 genotype | ||||||
| No ε4 allele | 20/172 (11.6) | 2/155 (1.3) | 24/163 (14.7) | 7/155 (4.5) | 44/335 (13.1) | 9/310 (2.9) |
| One ε4 allele | 57/236 (24.2) | 4/237 (1.7) | 60/242 (24.8) | 6/249 (2.4) | 117/478 (24.5) | 10/486 (2.1) |
| Two ε4 alleles | 42/89 (47.2) | 2/84 (2.4) | 44/91 (48.4) | 5/66 (7.6) | 86/180 (47.8) | 7/150 (4.7) |
| Symptomatic ARIA-E‖ | 26/497 (5.2) | 0/476 | 24/496 (4.8) | 2/470 (0.4) | 50/993 (5.0) | 2/946 (0.2) |
| Symptomatic ARIA-E according to APOE ε4 genotype‖ | ||||||
| No ε4 allele | 7/172 (4.1) | 0/155 | 6/163 (3.7) | 1/155 (0.6) | 13/335 (3.9) | 1/310 (0.3) |
| One ε4 allele | 10/236 (4.2) | 0/237 | 8/242 (3.3) | 1/249 (0.4) | 18/478 (3.8) | 1/486 (0.2) |
| Two ε4 alleles | 9/89 (10.1) | 0/84 | 10/91 (11.0) | 0/66 | 19/180 (10.6) | 0/150 |
| Serious symptomatic ARIA-E** | 7/497 (1.4) | 0/476 | 4/496 (0.8) | 0/470 | 11/993 (1.1) | 0/946 |
| Recurrent ARIA-E | 48/497 (9.7) | 0/476 | 47/496 (9.5) | 3/470 (0.6) | 95/993 (9.6) | 3/946 (0.3) |
| Radiologic severity of ARIA†† | ||||||
| BGTS score | 9.4±7.6 | 2.8±2.5 | 8.5±7.6 | 4.0±3.2 | 9±7.6 | 3.7±3.0 |
| BGTS score ≥4 — no./total no. (%) | 155/191 (81.2) | 2/8 (25.0) | 138/189 (73.0) | 9/21 (42.9) | 293/380 (77.1) | 11/29 (37.9) |
| Concurrent ARIA-E and ARIA-H — no./total no. (%) | 69/497 (13.9) | 3/476 (0.6) | 65/496 (13.1) | 4/470 (0.9) | 134/993 (13.5) | 7/946 (0.7) |
| ARIA-H — no./total no. (%) | ||||||
| Any new ARIA-H | 118/497 (23.7) | 59/476 (12.4) | 109/496 (22.0) | 57/470 (12.1) | 227/993 (22.9) | 116/946 (12.3) |
| New isolated ARIA-H | 46/497 (9.3) | 55/476 (11.6) | 39/496 (7.9) | 53/470 (11.3) | 85/993 (8.6) | 108/946 (11.4) |
Plus−minus values are means ±SD. Percentages may not total 100 because of rounding. The safety analysis included participants who had received at least one dose of gantenerumab or placebo; those who had received at least one dose of gantenerumab were included in the gantenerumab group, regardless of the trial-group assignment. Data for additional adverse events are provided in Table S6. ARIA denotes amyloid-related imaging abnormalities, ARIA-E ARIA with edema, and ARIA-H ARIA with hemosiderosis.
In accordance with Medical Dictionary for Regulatory Activities preferred terms, the most frequently reported serious adverse events (occurring in ≥1% of the participants in either trial group across both trials) were coronavirus disease 2019, fall, pneumonia, pulmonary embolism, and ARIA-E.
The most frequently reported adverse events that led to discontinuation of gantenerumab or placebo were ARIA-H, cerebral hemorrhage, ARIA-E, asthenia, cerebral infarction, confusional state, delirium, and subdural hematoma. Such events were predominantly driven by protocol-specified discontinuation criteria for ARIA-H (the accumulation of >15 ARIA-H or >3 focal areas of leptomeningeal hemosiderosis, including baseline findings, during the trials).
All deaths that occurred in the gantenerumab group were considered by the primary investigator and sponsor to be unrelated to the trial drug, including deaths that occurred in the double-blind treatment period and safety follow-up period.
Data for ARIA findings are shown for participants who underwent magnetic resonance imaging after baseline; those who discontinued the trial before assessment are not included.
Symptomatic ARIA-E was defined as ARIA-E temporally associated with central nervous system (CNS) symptoms.
Serious symptomatic ARIA-E was defined as a case of symptomatic ARIA-E in which ARIA-E or the associated CNS symptom was reported as a serious adverse event. Of the 11 participants in the gantenerumab group who had serious symptomatic ARIA-E, 9 fully recovered and 2 recovered with sequelae during the reporting period (with 1 of these 2 participants fully recovering after the reporting period).
Confirmation of the safety of dose escalation was performed with the use of an algorithm for the management of ARIA, shown in Table S1. The radiologic severity of ARIA was assessed with the score on the Barkhof Grand Total Scale (BGTS; range, 0 to 60, with higher scores indicating a greater extent of ARIA-E). If the BGTS score was 4 or higher, dose escalation was suspended; if the BGTS score was less than 4 and the participant was asymptomatic, dose escalation was continued.
ARIA
MRI findings were reported independently of adverse events. In accordance with the protocol, some MRI findings were not required to be reported as adverse events by the investigator but were still analyzed. The incidence of ARIA-E overall was 24.9% with gantenerumab and 2.7% with placebo, and the incidence approximately doubled with each APOE ε4 allele present (Table 3). Most cases of ARIA-E were asymptomatic; 5.0% of the participants receiving gantenerumab had ARIA-E temporally associated with central nervous system (CNS) symptoms, whereas only 0.2% of the participants receiving placebo had symptomatic ARIA-E. The most common symptoms associated with ARIA-E were headache and dizziness (Table S7). Serious symptomatic ARIA-E (cases in which ARIA-E or the associated CNS symptom was reported as a serious adverse event) occurred only in participants receiving gantenerumab (1.1%). The median time to the resolution of ARIA-E in the gantenerumab group was 9 weeks (range, 3.0 to 82.9); the median time to the resolution of CNS symptoms associated with ARIA-E was 2 weeks (range, 0.1 to 50.6). No cases of fatal ARIA-E were reported.
The incidence of any new ARIA-H was 22.9% with gantenerumab and 12.3% with placebo, but the incidence of new isolated ARIA-H was 8.6% and 11.4%, respectively. The higher incidence of concurrent ARIA-E and ARIA-H in the gantenerumab group than in the placebo group (13.5% vs. 0.7%) can account for these findings.
DISCUSSION
In two randomized trials of gantenerumab (a human monoclonal antibody with high affinity for aggregated amyloid) for the treatment of early symptomatic Alzheimer’s disease, there was no significant difference between the gantenerumab group and the placebo group in the primary clinical outcome, the change from baseline in the CDR-SB score at week 116. The results for secondary clinical outcomes were not supportive of a beneficial clinical effect of the drug. When the analysis of the primary outcome was based on pooled data from both trials, the results were generally consistent with the results from the primary analysis in each trial.
The use of gantenerumab led to partial removal of amyloid plaques and improvement in some soluble biomarkers of Alzheimer’s disease. However, the magnitude of amyloid plaque removal was smaller than expected on the basis of previous trials and prespecified modeling predictions.12,24 The mean amyloid level on PET remained elevated after treatment, and only approximately one quarter of the participants receiving gantenerumab had amyloid plaque removal to a level below the threshold for amyloid positivity (i.e., attained amyloid-negative status).1 The results of the GRADUATE I and II trials of gantenerumab, taken together with results of trials of other anti-Aβ monoclonal antibodies, suggest the hypothesis that rapid plaque reduction, probably to a level below the threshold of detection, may be necessary to show clinical efficacy within the time frame of 18 to 27 months. However, the current trials did not assess this hypothesis.
The CSF and plasma levels of phosphorylated tau 181 in the gantenerumab group were lower than the levels in the placebo group, findings consistent with data observed in previous trials of gantenerumab.19,25 However, there was no treatment effect with respect to the accumulation of tau in the brain on PET; the absence of such an effect may be due to the limited amyloid plaque removal observed.
Volumetric MRI showed greater decreases in whole-brain volume and greater increases in ventricular volume in the gantenerumab group than the levels observed in the placebo group. These results are similar to those reported in other trials and studies of monoclonal antibodies and vaccines that target amyloid plaque removal and may not reflect neurodegeneration, given that amyloid plaque removal and fluid shifts could affect brain volume.3,26 Among participants receiving gantenerumab, the levels of biomarkers of synaptic and axonal integrity in CSF moved in a normalizing direction, as compared with the levels observed among participants receiving placebo.
Participants receiving gantenerumab had a higher incidence of ARIA-E than those receiving placebo, and homozygous carriers of the APOE ε4 allele were more likely to have ARIA-E than heterozygous carriers or noncarriers. Most cases of ARIA-E were asymptomatic; CNS symptoms associated with ARIA-E occurred in 5.0% of the participants receiving gantenerumab. In the gantenerumab group, there were cases of serious symptomatic ARIA-E (in 11 participants), ARIA-E that led to permanent discontinuation of gantenerumab (in 2 participants), and ARIA-E that led to permanent discontinuation of the trial (in 4 participants), but no deaths were associated with ARIA-E in either trial group. ARIA-E led to interruptions in the administration of gantenerumab or placebo or delays in dose escalation in 21.2% of the participants receiving gantenerumab and 1.5% of the participants receiving placebo. Results of sensitivity analyses performed to evaluate whether the occurrence of ARIA-E affected the trial outcomes suggest that the observed findings in the primary analysis did not result from unblinding of safety data (Table S8).
The dose-escalation scheme was introduced in the trial design to limit the increase in ARIA-E that was expected to result from the gantenerumab dose used in the GRADUATE I and II trials, which was 5 times as high as the dose used in the SCARLET ROAD and MARGUERITE ROAD trials.24 On the basis of modeling, an ARIA-E incidence of approximately 25% was predicted at the trial population level. The finding that the observed ARIA-E incidence in the GRADUATE I and II trials was in line with the prediction of 25% but amyloid plaque removal was lower than predicted suggests that different mechanisms may be involved in driving ARIA-E and plaque removal.10,27,28
Limitations of the GRADUATE I and II trials were the lack of racial diversity in the trial population from the United States, which may affect the generalizability of our findings, and the multiple differences between the protocol for these trials and the protocols for earlier trials of anti-amyloid monoclonal antibodies (including the previously untested dose-escalation scheme), which make it difficult to draw comparisons between trials.
The use of the antiamyloid antibody gantenerumab did not lead to a slower decline in cognitive function than placebo over a period of 116 weeks among participants with early symptomatic Alzheimer’s disease.
Supplementary Material
Acknowledgments
We thank all the participants and their families, as well as the site staff and trial team, for their time and commitment to the trials; the early developers and supporters of the gantenerumab program; the members of the gantenerumab steering committee for their critical advice throughout the trial; and the members of the independent data and safety monitoring committee and the cross-functional trial teams at F. Hoffmann-La Roche, Chugai, and Genentech. Medical writing support was provided by Mar Ferreró of F. Hoffmann-La Roche and Rachel Johnson and Jack O’Neill of Nucleus Global.
Supported by F. Hoffmann-La Roche.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
Footnotes
A complete list of the GRADUATE I and II Investigators and members of the Gantenerumab Study Group is provided in the Supplementary Appendix, available at NEJM.org.
References
- 1.van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med 2023; 388: 9–21. [DOI] [PubMed] [Google Scholar]
- 2.Swanson CJ, Zhang Y, Dhadda S, et al. A randomized, double-blind, phase 2b proof-of-concept clinical trial in early Alzheimer’s disease with lecanemab, an anti-Aβ protofibril antibody. Alzheimers Res Ther 2021;13: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mintun MA, Lo AC, Duggan Evans C, et al. Donanemab in early Alzheimer’s disease. N Engl J Med 2021; 384: 1691–704. [DOI] [PubMed] [Google Scholar]
- 4.Honig LS, Vellas B, Woodward M, et al. Trial of solanezumab for mild dementia due to Alzheimer’s disease. N Engl J Med 2018; 378:321–30. [DOI] [PubMed] [Google Scholar]
- 5.Budd Haeberlein S, Aisen PS, Barkhof F, et al. Two randomized phase 3 studies of aducanumab in early Alzheimer’s disease. J Prev Alzheimers Dis 2022; 9: 197210. [DOI] [PubMed] [Google Scholar]
- 6.Ostrowitzki S, Bittner T, Sink KM, et al. Evaluating the safety and efficacy of crenezumab vs placebo in adults with early Alzheimer disease: two phase 3 randomized placebo-controlled trials. JAMA Neurol 2022;79: 1113–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salloway S, Sperling R, Fox NC, et al. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N Engl J Med 2014; 370: 322–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Budd Haeberlein S, Castrillo-Viguera C, Chen T, et al. Aducanumab titration dosing regimen: 36-month analyses from PRIME, a phase 1b study in patients with early Alzheimer’s disease. J Prev Alzheimers Dis 2018; 5: Suppl 1: S42–S43 (https://investors.biogen.com/static-files/8ba5ad20-42ff-4045-b0bb-2042c6d3d4cf ). [Google Scholar]
- 9.Sims JR, Zimmer JA, Evans CD, et al. Donanemab in early symptomatic Alzheimer disease: the TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA 2023; 330: 512–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bohrmann B, Baumann K, Benz J, et al. Gantenerumab: a novel human anti-Aβ antibody demonstrates sustained cerebral amyloid-β binding and elicits cell-mediated removal of human amyloid-β. J Alzheimers Dis 2012; 28: 49–69. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, Wei G, Zhao J, Nussinov R, Ma B. Computational investigation of gantenerumab and crenezumab recognition of Aβ fibrils in Alzheimer’s disease brain tissue. ACS Chem Neurosci 2020; 11: 323344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klein G, Delmar P, Kerchner GA, et al. Thirty-six-month amyloid positron emission tomography results show continued reduction in amyloid burden with subcutaneous gantenerumab. J Prev Alzheimers Dis 2021; 8: 3–6. [DOI] [PubMed] [Google Scholar]
- 13.Bateman RJ, Cummings J, Schobel S, et al. Gantenerumab: an anti-amyloid monoclonal antibody with potential disease-modifying effects in early Alzheimer’s disease. Alzheimers Res Ther 2022; 14: 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011;7: 270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on AgingAlzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011; 7: 263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43: 2412–4. [DOI] [PubMed] [Google Scholar]
- 17.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12: 189–98. [DOI] [PubMed] [Google Scholar]
- 18.Xiao S, Chan P, Wang T, et al. A 36-week multicenter, randomized, doubleblind, placebo-controlled, parallel-group, phase 3 clinical trial of sodium oligomannate for mild-to-moderate Alzheimer’s dementia. Alzheimers Res Ther 2021; 13: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ostrowitzki S, Lasser RA, Dorflinger E, et al. A phase III randomized trial of gantenerumab in prodromal Alzheimer’s disease. Alzheimers Res Ther 2017; 9: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Westfall PH, Krishen A. Optimally weighted, fixed sequence and gatekeeper multiple testing procedures. J Stat Plan Inference 2001; 99:25–40 (https://www.sciencedirect.com/science/article/abs/pii/S0378375801000775?via%3Dihub ). [Google Scholar]
- 21.International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. Addendum on estimands and sensitivity analysis in clinical trials-to the guideline on statistical principles for clinical trials. E9(R1). November 2019. (https://database.ich.org/sites/default/files/E9-R1_Step4_Guideline_2019_1203.pdf ).
- 22.Wolbers M, Noci A, Delmar P, GowerPage C, Yiu S, Bartlett JW. Standard and reference-based conditional mean imputation. Pharm Stat 2022; 21: 1246–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gower-Page C, Noci A, Wolbers M. rbmi: An R package for standard and reference-based multiple imputation methods. J Open Source Softw 2022; 7: 4251 (https://www.theoj.org/joss-papers/joss.04251/10.21105.joss.04251.pdf ). [Google Scholar]
- 24.Retout S, Gieschke R, Serafin D, Weber C, Frey N, Hofmann C. Disease modeling and model-based meta-analyses to define a new direction for a phase III program of gantenerumab in Alzheimer’s disease. Clin Pharmacol Ther 2022;111: 857–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bittner T, Scelsi MA, Kollmorgen G, et al. Gantenerumab treatment increases plasma beta-amyloid(1−42) and decreases plasma pTau. Presented at the Alzheimer’s Association International Conference, San Diego, CA, July 31-August 4, 2022. [Google Scholar]
- 26.Fox NC, Black RS, Gilman S, et al. Effects of Abeta immunization (AN1792) on MRI measures of cerebral volume in Alzheimer disease. Neurology 2005; 64: 1563–72. [DOI] [PubMed] [Google Scholar]
- 27.Ostrowitzki S, Deptula D, Thurfjell L, et al. Mechanism of amyloid removal in patients with Alzheimer disease treated with gantenerumab. Arch Neurol 2012; 69:198–207. [DOI] [PubMed] [Google Scholar]
- 28.Sperling RA, Jack CR Jr, Black SE, et al. Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: recommendations from the Alzheimer’s Association Research Roundtable Workgroup. Alzheimers Dement 2011; 7: 367–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.