Abstract
Coronary artery calcification (CAC) accompanies the development of advanced atherosclerosis. Its role in atherosclerosis holds great interest because the presence and burden of coronary calcification furnishes direct evidence of the presence and extent of coronary artery disease; furthermore, CAC predicts future events independently from concomitant conventional cardiovascular risk factors, and to a greater extent than any other non-invasive biomarker of this disease. Nevertheless, the relationship between CAC and the susceptibility of a plaque to provoke a thrombotic event remains incompletely understood. This review summarizes the current understanding and literature on CAC. It outlines the pathophysiology of CAC and reviews laboratory, histopathologic and genetic studies, as well as imaging findings, to characterize different types of calcification and elucidate their implications. Some patterns of calcification, such as microcalcification, portend increased risk of rupture and CV events, and may improve prognosis assessment non-invasively. However, contemporary CT cannot assess early microcalcification. Limited spatial resolution and blooming artifacts may hinder estimation of degree of coronary artery stenosis. Technical advances such as photon counting detectors and combination with nuclear approaches (e.g. NaF imaging) promise to improve the performance of cardiac CT. These innovations may speed achieving the ultimate goal of providing non-invasively specific and clinically actionable information.
Keywords: Atherosclerosis, coronary arteries, vascular calcification, microcalcification, CCTA, CAC score, PCCT, Photon-Counting CT, calcified nodule, vulnerable plaque, Agatston, atherosclerosis, imaging
Introduction
Atherosclerosis is a leading and growing cause of morbidity and mortality globally. Cardiovascular disease (CVD) accounted for 17.9 million deaths worldwide in 2019 [1]. Atherosclerosis represents the main cause of ischemic heart disease/coronary artery disease (CAD.) Coronary artery calcification (CAC) usually accompanies the development of advanced atherosclerosis: the presence and burden of CAC furnishes direct evidence of the presence and extent of CAD and predicts future events independently from concomitant CV risk factors, and to a greater extent than any other non-invasive biomarker of this disease [2]. Hence, several guidelines have incorporated CAC as an additional risk marker to assess an individual’s overall CV risk and inform management [3–4]. The extent and pattern of calcification have prognostic implications [5–6]. Yet, the relationship between CAC and the susceptibility of a plaque to provoke a thrombotic event remains incompletely understood. While some studies have highlighted microcalcification and spotty calcification as definable components of vulnerable plaque [7–9], others have suggested that increasing CAC extent and size represents an advanced stage of atherosclerosis and sheet calcification may render a plaque less likely to rupture [10].
The advent of more advanced and precise imaging techniques, such as dual-energy CT (DECT) and photon-counting CT (PCCT) permit not only quantitative assessment of coronary calcification, but also evaluation of the morphologic features of calcification within atherosclerotic plaques. Improvements in technology enable more comprehensive reporting on microscopic changes that heretofore required histopathologic study or deduction from macroscopic vascular calcification seen on clinical CT scans. Assessing calcification patterns together with low-attenuation components could enhance the identification of high-risk plaques and consequently high-risk patients that might merit more intensive therapies.
This review summarizes current understanding and literature of coronary artery calcification. It aims to correlate findings from histopathologic and imaging studies to understand the clinical implications of coronary artery calcification.
Mechanisms of Calcification
Mönckeberg calcification
Arterial calcification results from crystallization of calcium and phosphate in the form of hydroxyapatite, which can deposit in the extracellular matrix of the arterial wall. Multiple mechanisms contribute to this process. Depending on its location and the site of the deposition within the arterial wall, arterial calcification falls into two main groups, which have distinct etiologies and implications: 1) Mönckeberg medial calcification and 2) intimal atherosclerotic calcification.
Mönckeberg sclerosis affects primarily the tunica media of peripheral arteries of the lower extremities in individuals with longstanding diabetes. It derives mainly from the action of osteoblast-like cells. Specifically, intracellular signaling pathways and altered calcium-sensing receptors can lead to differentiation of vascular cells to osteoblast-like cells and to osteoid metaplasia [11–12]. In addition to diabetes, this type of medial calcification is associated with factors such as chronic kidney disease (CKD), hypercalcemia, high phosphate blood concentration and elevated parathyroid hormone levels. Mönckeberg sclerosis does not generally involve lipid deposition and inflammatory cell accumulation, at least in its advanced stages.
Intimal Calcification
Atherosclerotic intimal calcification usually associates with atherosclerosis progression. CAC reports primarily on this type of intimal calcification, thus this review focusses on this intimal process. Intimal calcification is an active process associated with the presence of CV risk factors, such as aging, diabetes mellitus and hyperlipidemia and the local factors that drive its formation differ from those of medial calcification. Specifically, intimal calcification results from dysmorphic calcium precipitation driven by chondrocyte-like cells, rather than osteoblast-like cells as seen in medial calcification [8], and by an inflammatory cascade, activated by macrophages and local cytokine release. In particular, death of inflammatory cells within the atheroma and subsequent release of apoptotic bodies nucleate crystal formation [13]; death of smooth muscle cells (SMCs) and of macrophages, can release matrix vesicles [14]; cholesterol accumulation in the intima can promote inflammation; and phenotypic modulation of SMCs to chondrocyte-like cells leads to bone deposition. These mechanisms promote oxidative stress, inflammation, and consequent calcification within the arterial intima [15–16].
Recent studies have shown that a variety of biochemical factors modulate SMC phenotype: for example, transforming growth factor-b1 (TGF-b 1) and platelet-derived growth factor (PDGF) can promote the switching from the so called contractile to secretory phenotype of SMCs and integrin-α9 can promote its proliferation. Such mediators may serve as therapeutic targets for regulating arterial remodeling [17]. Similarly, matrix-vesicles merit consideration as a target for interventions to treat arterial calcification, considering their participation in pathogenesis [18]. Matrix vesicles can initiate calcification by serving as nucleating foci [19]. These membrane-bound microparticles released by cells can contain different material, including protein, DNA, mRNA, microRNA (miRNA.) Their composition dictates different calcification potential [20]. After release, initial mineralization starts within the vesicles, until the mineral content grows causing rupture of the vesicle’s membrane, release of the content, and promote further local mineralization [21]. Moreover, extracellular vesicles can mediate intercellular communication in the calcifying milieu through miRNA. Certain vesicular miRNAs can induce pro-osteogenic gene expression and activate signaling programs in mesenchymal stem cells, which will then differentiate into osteoblast-like calcifying cells. Overall, extracellular matrix-vesicles seem to participate in all stages of the pathogenesis of arterial ossification, from its initiation to its progression [22].
Endothelial progenitor cells (EPCs) with an osteogenic phenotype (co-staining in flow cytometry for the osteoblast marker osteocalcin [OCN]; i.e. OCN (+) EPC]) may play an important role in CAC and may comprise a potential mechanism of and biomarker for coronary calcification. Patients with early and severe coronary atherosclerosis have high levels of circulating OCN (+) EPC [23]. This mediator may promote early, accelerated ossification not only in coronary arteries but also in other vascular beds as well as in aortic valves [24–25].
In patients with endothelial dysfunction, the osteogenic EPCs are retained within the coronary circulation for the repair of the injured coronary endothelium, however, this may lead to abnormal vascular repair, initiation, and progression of coronary artery disease and calcification rather than homeostatic endothelial repair [24]. In the early stage of coronary arterial plaque development, the retention of the osteogenic subset within lesions correlates with a larger extent of necrotic core and calcification [26–27].
Calcification: histopathology
Histopathologically, the classification of types of calcifications in atheromata depends on their diameter: microcalcification (0.5–15 µm); punctate calcification (15 µm-1 mm); fragment calcification (>1mm); sheet calcification (>3 mm); and nodular calcification. Nodular calcium deposits within the atherosclerotic lesions may extend to the media without disruption of the fibrous cap and can arise from fracture of calcified sheets by mechanical stress [28]. The difference among these types of calcification and the progression from one type to the other has undergone study for decades, especially by histological approaches [29–31]. Calcification, by light microscopic examination, appears to begin with microcalcification, foci may then coalesce and form punctate calcification that can aggregate and produce larger areas of fragmented calcification. The growing calcified structures can localize within the necrotic core and reach into the surrounding collagen- and elastin-rich extracellular matrix and form calcified sheets. Sheets of calcification that can encompass at least a quarter of the circumference of the coronary artery by histology, are the hallmarks of fibrocalcific plaques. Calcified sheets may generate calcified nodules that can colocalize with fibrin deposition [32].
Plaque morphology
Hence, various types of calcifications can complicate different types of atherosclerotic lesions, which, based on morphology, fall into several categories [33]:
Pathologic intimal thickening (PIT), characterized by SMC and lipid accumulation near the intimal-medial border, with hyaluronan and proteoglycan matrix, extracellular lipid pool and foci of microcalcification or mixed microcalcification/punctate calcification (Figure 1A–1B).
Fibroatheroma (FA), designated as “early” or “late” depending on the presence or absence, respectively, of macrophage accumulation in the lipid pool and on the relative extent of matrix proteoglycans in the necrotic core. “Early” fibroatheroma may contain punctate calcification (Figure 1C), while “late” fibroatheroma contains an acellular or paucicelluar necrotic core, deficient in extracellular matrix, with or without calcification, especially in the form of fragmented calcification (Figure 1D). The temporal characterization of these plaque types is inferential, due to limitations in serial histologic observations on human atheroma.
Thin-cap fibroatheroma (TCFA), characterized by fibrous caps <65µm thick, with macrophages, lymphocytes, rare SMCs, large necrotic core (>10% of plaque area), containing calcification (Figure 1E), with or without intraplaque hemorrhage.
Plaque rupture, due to degradation of fibrous cap by matrix metalloproteases (MMPs) and other proteolytic enzymes, including certain cysteinyl proteinases and mast-cell-derived proteases [34], high circumferential stress [35], microcalcification (Figure 1F) and iron accumulation within the cap [36]; ultimately complicated by thrombosis.
Plaque erosion, which occurs on PIT or FA, characterized by endothelial cell loss, but with intact fibrous caps. Luminal thrombi contact the denuded intimal layer directly.
Calcified nodule (range 2.5 mm2 [37]), fibrous plaque comprised by nodular calcification (Figure 1G), protruding through the fibrous cap into the lumen, an uncommon cause of luminal thrombosis [38].
Healed plaque, composed of SMCs, proteoglycans and collagen-rich matrix, with or without disrupted fibrous cap or a “buried cap” that presumably forms due to healing of a plaque fissure. The resulting plaques can contain large area of calcification, usually fragmented or sheet (Figure 1H), with few inflammatory cells and a smaller NC (fibrocalcific plaque) [39].
Figure 1.
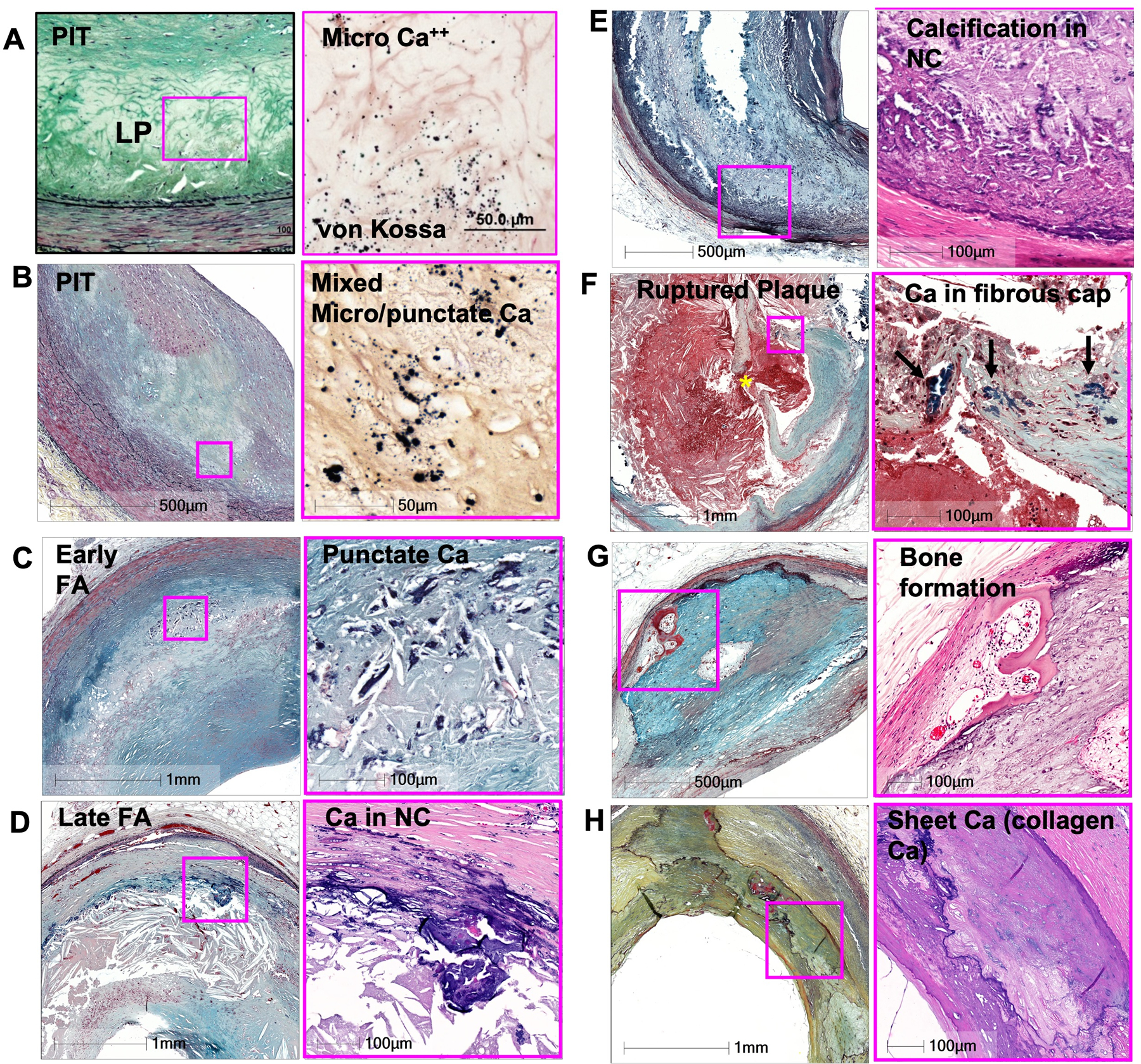
Calcification in various types of plaque.
(A) Microcalcification (varies from 0.5 to15um) in PIT.
(B) Mixed punctate and microcalcification may be observed in PIT.
(C) Punctate Calcification in early FA.
(D) Calcified in late FA, occurs as fragmented calcification and is seen near the media involving collagen and necrotic core.
(E) NC calcification.
(F) Plaque rupture showing calcification in the fibrous cap. Asterisk indicate the site of ruptured fibrous cap.
(G) Bone formation near the border of medial layer.
(H) Sheet collagen calcification (collagen Ca).
All low power images is shown with Movat pentachrome stain. High power images are shown with Von Kossa stain in A and B, Movat pentachrome stain in C and E, and H&E in D, F, G, and H. Abbreviations: PIT = pathologic intimal thickening, FA = fibro atheroma, NC = necrotic core. (Modified from Otsuka F, et al. Arterioscler Thromb Vasc Biol 2014;34:724-736)
Plaque visualization
In recent years, histopathologic analysis and visualization of coronary calcification has witnessed great advances in technology. Among these, micro-CT, even though not yet available for clinical use, allows much greater detailed imaging and offers a better understanding of calcification progression, especially when correlated with histologic and radiograph images (Figure 2.) Micro-CT is an imaging method that allows greater spatial resolution, up to 1–10 μm, which enables the detection of and differentiation between microscopic and macroscopic calcification. Micro-CT acquisition from biological specimens can be further implemented by using a radiopaque contrast agent, in order to better delineate adjacent tissues for histopathologic analysis. Therefore, iodine enhancement, paired with micro-CT scanning, can further characterize atherosclerotic plaques with specific emphasis on the vascularization in a manner comparable to routine histology, while providing whole volume data [40]. An additional method for plaque visualization and histopathologic analysis is electron microscopy. Together with classic histological imaging, it permits investigation of the detailed structure of atherosclerotic plaques and understanding the pathology of atherogenesis (Figure 3). Both scanning and transmission electron microscopy have been used to illustrate the timeline of atherosclerosis. The combined use of all these ex vivo histological imaging techniques provides deeper insights into atherogenesis and calcification development and progression. Hutcheson et al. [41], by using high-resolution micro-CT, electron microscopy and spectroscopic analyses -for mineral content evaluation, provided insight into calcific mineral formation and maturation. In their in vitro study, which involved using both human calcified plaque specimens and collagen hydrogels to mimic the plaque environment and structural features, they observed that microcalcification, and ultimately large calcification zones, results from progressive aggregation of calcifying extracellular matrix vesicles. More importantly, they suggested that the aggregation kinetics of vesicles may impact the stability of the fibrous cap and that calcification morphology and plaque’s collagen content are interlinked. Indeed, they showed that microcalcification promotes high plaque-destabilizing stress within the cap extracellular matrix, compromising its structural integrity and leading to its rupture. Additionally, they observed an inverse relationship between collagen content within the fibrous cap and microcalcification size, linked to plaque vulnerability: when surrounding collagen is degraded, vesicles can aggregate, nucleate and form microcalcified foci. These punctate collections of calcium of approximately 5 μm, in a collagen-poor fibrous cap, can increase mechanical stress in the surrounding hyperelastic tissue, favoring rupture.
Figure 2.
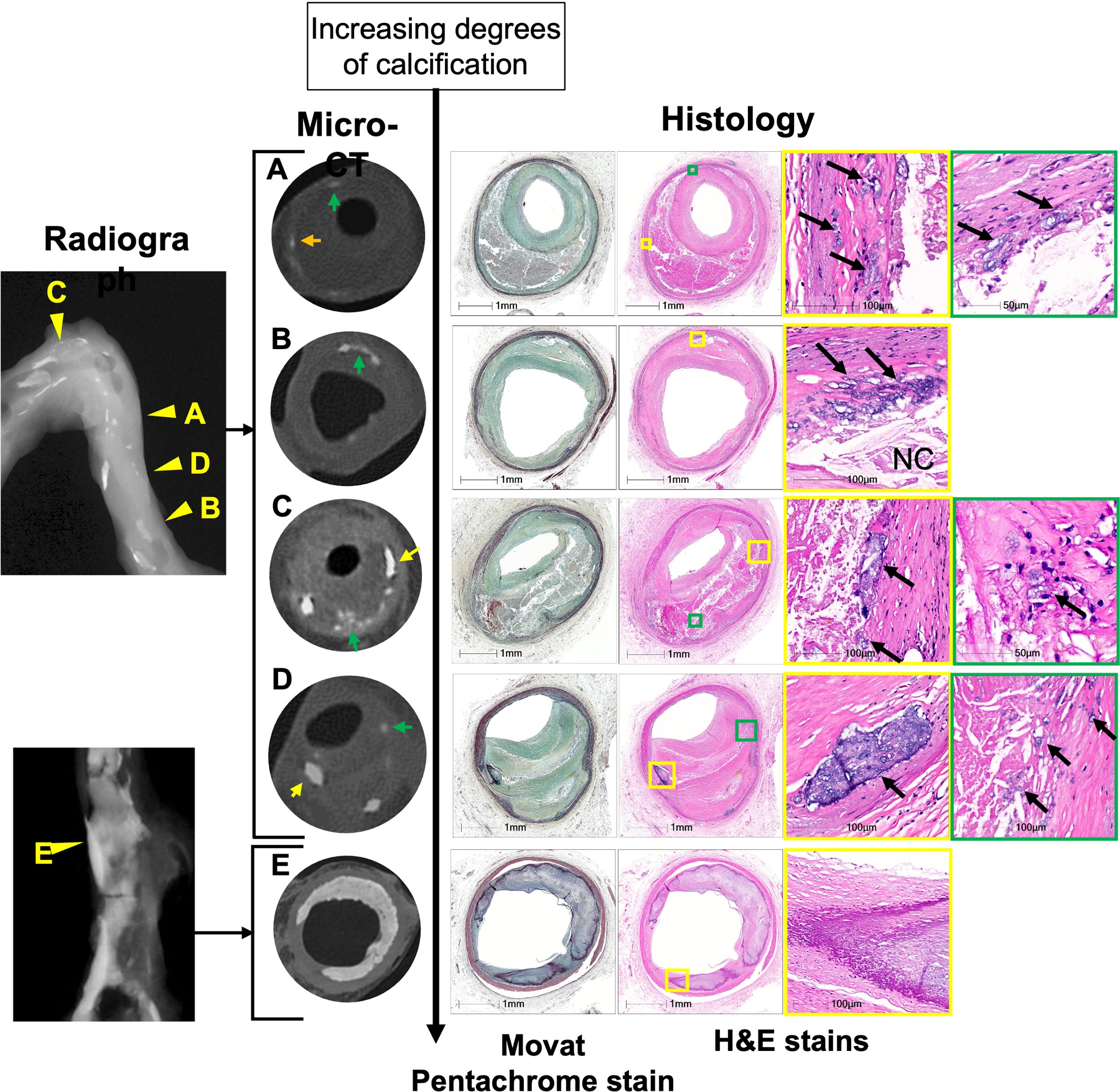
Detection of calcification by various modalities (radiograph, micro-CT, and histology).
Image A to E shows severity of calcification from punctate calcification to sheet calcification by micro-CT; on the right are shown the corresponding histologic images and on the left is shown the radiograph of the artery; yellow arrows show the area of calcification in micro-CT and arrowheads in radiograph show the matching areas.
(A) Micro-CT image can detect punctate calcification (on radiograph speckled) in the border area of a large NC with adjoining fibrous tissue in late FA.
(B) Micro-CT image shows speckled calcification composed of an aggregate of punctate calcification in the early FA.
(C, D) Micro-CT show varying sizes of fragmented calcification (yellow arrows) and speckled calcification (green arrows) in the late FA (C) and healed plaque rupture (D).
(E) Micro-CT showing sheet calcification.
Abbreviations: FA = fibroatheroma, NC = necrotic core.
Figure 3.
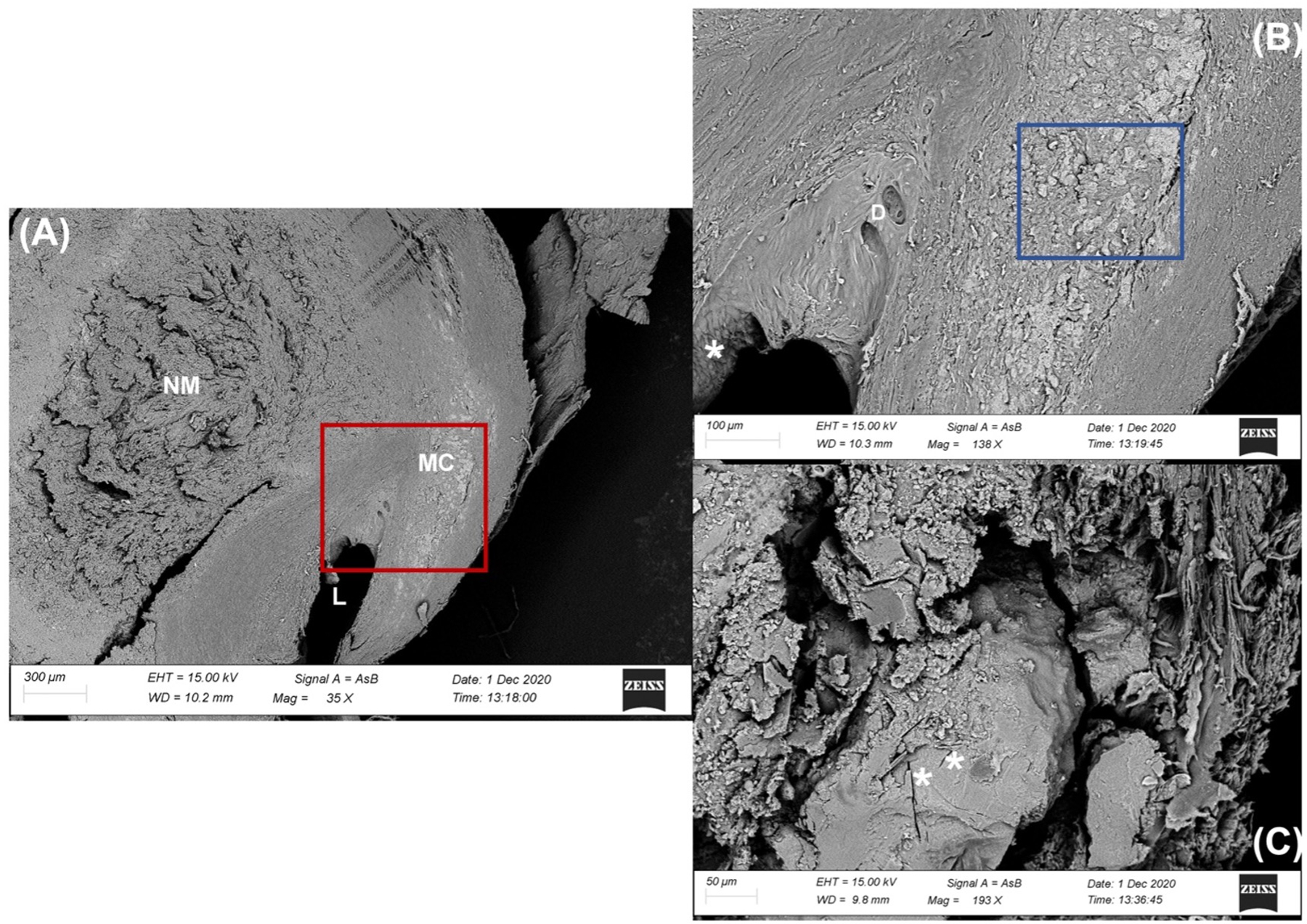
Electron microscopy images.
Low (A), medium (B [red boxed area in (A)]) and high (C [blue boxed area in (B)]) magnification scans from a transversal section of carotid artery observed by Back Scattered Electron probe (BSE).
(A) The lumen (L) of the vessel is remarkably reduced and eccentrically constricted by the large necrotic mass (NM) in the thickness of the Tunica Media. The Tunica Intima appears strongly altered even at low magnification. The BSE mode, utilized for analyzing differences in tissue density, highlights an area with numerous microcalcifications (MC) within the Tunica Media with a light gray-white tone.
(B) A greater enlargement of the vessel lumen shows the presence of small diverticula (D) and important differences in the shape of the endothelial cells, some of them in fact appear taller and less distended (white asterisk *) according to the blood flow. In the thickness of the tunica media, the micro-calcifications of the plaque are distinguished by definition and size.
(C) Partially sectioned microcalcification useful to show the internal structure. The calcified mass in calcium phosphate appears fairly homogeneous centrally, with some traces of the presence of cholesterol crystals (white asterisk *). In the periphery, small centers of crystalline aggregation are visible, which suggest a progressive growth of the calcified mass.
Moreover, Kelly-Arnold et al. [42] examined microcalcification in non-ruptured fibrous caps using high-resolution micro-CT. They suggested that potentially dangerous microcalcified structures localize in regions of elevated background stress, usually where the cap is thin. They argue that clusters of such structures in close proximity, parallel to the circumferential (tensile) axis of the fibrous cap, can contribute to high local mechanical stress. High resolution micro-CT study of human coronary arteries obtained at autopsy has also shown excellent correlation with histology, and can distinguish nodular from sheet calcification, allowing to observe plaque in greater details compared to radiography alone [10].
Genetic determinants of atheroma calcification
Understanding molecular mechanisms of vascular health underlying the genetic risk factors of atherosclerosis, may promote new management strategies of patients with or without known CAD [43]. Genetics have a strong influence on calcified atherosclerotic plaques, especially CAC score and calcified plaque volume, as demonstrated by Drobni et al. in a twin study [44]. The high prevalence of family history of coronary heart disease (CHD) among young adults (mean age [SD], 43,5 [4,5]) with CAC further supports genetic influences on CAC [45]. Additionally, genetic factors influence CAC progression, as shown by Cassidy-Bushrow et al. [46] who found that heritability of CAC progression was 40%, with 14% of the variation explained by genetic factors. Genome-wide association studies showed that three single nucleotide polymorphisms attained genome-wide significance for association with CAC [47], and two of them are also positively associated with CAC progression [48].
Additionally, Klenke et al. [49] observed that genetic variations in the G-protein signal pathways influence CAC progression. Particularly, they studied three single nucleotide polymorphisms and risk alleles in the signaling pathway, ADRB2, GNAS and GNB3 specifically, and they found that the presence of risk alleles was associated with increased 5-year CAC progression and accelerated increase of CAC over 5 years compared with what was expected with respect to baseline CAC. Thus, they suggested the importance of this pathway for genetic heritability of CAC.
Lastly, detection of CV calcification in children and adolescents can relate to underlying hereditary disorders linked to increased arterial calcification; thus, disorders caused by altered purine or phosphate metabolism, interferonopathies and Gaucher disease merit further evaluation in this specific population [50].
Laboratory tests
Recent studies have also investigated the potential role of specific serum biomarkers as predictors of coronary calcification, of its pattern and progression (Table 1.) Ren et al. [51] studied the association between serum alkaline phosphatase (ALP) and calcification patterns and plaque morphology. ALP is a membrane-bound metalloenzyme that catalyzes hydrolysis of pyrophosphate, an inhibitor of vascular calcification. They found that patients with higher ALP serum levels had higher risks of having coronary calcification, especially spotty calcification, and minimum lumen area <4.0 mm2, features that associate with plaque instability and increased risk of major adverse CV events. Hence, they proposed ALP as an independent predictor and biochemical marker for calcification and plaque vulnerability. Similarly, Li et al. [52] found an association between calcified nodules, a characteristic of some plaques that have ruptured, and high serum ALP level.
Table 1.
Literature regarding laboratory findings and their correlation with calcification
| Laboratory marker | Correlation | Ref. |
|---|---|---|
| ⬆ ALP | ⬆ Spotty calcification | [51] |
| ⬆ ALP | ⬆ Calcified nodule | [52] |
| ⬆ Phosphate | ⬆ CAC | [53] |
| ⬇ Irisin | ⬆ CAC progression | [54] |
| ⬆ MMPs | ⬆ CAC | [55] |
| ⬇ 1,5-AG | ⬆ Calcium index in patients with diabetes | [56] |
| ⬆ Lp(a) | ⬆ CAC volume and rapid progression | [59–60] |
| ⬆ Lp(a) in middle age | ⬆ CAC (>100) in older age | [61] |
ALP: alkaline phosphatase; CAC: coronary artery calcification; MMPs: metalloproteinases;
1,5-AG: 1,5-anhydro-D-glucitol; Lp(a): lipoprotein (a)
Additionally, Campos-Obando et al. [53] found that high serum phosphate levels correlate with CAC in the general population and that this association is also evident for persons with normal phosphate levels and in the absence of CKD, challenging the concept that only marked hyperphosphatemia in the setting of CKD promotes calcification.
Some studies have implicated other serum markers of arterial calcification. Hisamatsu et al. [54] examined serum irisin levels and their association with prevalence and progression of coronary atherosclerosis. Irisin is an exercise-induced hormone, secreted by skeletal muscle, and its levels associate inversely with CAC progression and could serve as a biomarker of coronary atherosclerotic burden in asymptomatic non-obese patients. Furthermore, other blood biomarkers including MMPs, especially MMP-2 and MMP-9, associate positively with CAC, perhaps related to their role in extracellular matrix degradation and initiation and development of calcification [55]; 1,5-anhydro-D-glucitol (1,5-AG), a marker for glycemic status in patients with diabetes, correlated inversely with calcium index, presence of fibrocalcific lesions, overall increased risk of CAC, and may predict of future major adverse CV events in patients with diabetes. [56] Moreover, serum uric acid may be increased in patients who showed higher prevalence of TCFA and macrophage accumulation and in those with plaques characterized by longer calcification length and thinner fibrous cap [57]. Another marker that may correlate with early atherosclerosis is the presence of osteogenic monocytes, cells involved in plaque development, within the coronary circulation. As Collin et al. [58] showed in their study, retention of such cells was associated with a larger extent of calcification and necrotic core. Subsequent maturation of monocytes into macrophages in the early phases of atherosclerosis is associated with an unbalanced turnover of cells, which contributes to inflammation and plaque expansion.
Finally, some studies have focused on lipoprotein (a) (Lp(a)), a cholesterol-rich LDL bound with apolipoproteinB100, which has pro-atherogenic, pro-inflammatory and pro-thrombotic activity. Elevated levels of Lp(a) are a highly prevalent genetic risk factor for CVD, that correlate positively with CAC. Indeed, Garg et al. [59] and Ong et al. [60] have shown that elevated Lp(a) is associated with a rapid CAC progression and an increase in CAC volume, especially in patients with higher levels of inflammation and coagulation markers, thus suggesting Lp(a) as a marker of CAC progression. Similarly, Obisesan et al. [61] suggested that high levels of Lp(a) in middle age (59.2 SD 4.3) are associated with elevated CAC (>100) in older age. They also found that increased Lp(a) is associated with increased aortic valve calcification and more rapid progression of aortic stenosis, underlying the importance of cardiac CT in the evaluation of cardiac calcifications among patients with high Lp(a) levels.
Imaging
Direct noninvasive detection of CAC through CT scanning started in the 1980s with the use of electron-beam CT (EBCT) scanning [62–64]; followed in the late 1990s by multidetector CT scanning, which allowed higher spatial resolution as compared with EBCT with inferior temporal resolution compensated by retrospective ECG-gated spiral acquisition technique. In the following decades, CT technologies evolved verry rapidly enhancing conditions for noninvasive coronary imaging. While evolving imaging techniques have offered constant improvements in CAC scanning, the methods for the assessment and quantifications of CAC underwent standardization in the early days of EBCT by Agatston’s method [65]; newer and more reliable/reproducible scores such as “calcium mass” and “calcium volume” were developed, but clinical adoption lagged because the mainstream epidemiological literature used the Agatston method, which continues today. This lack of interest initially limited efforts to improve calcium detection with alternative scores not based on EBCT. In the meantime, plaque imaging with CCTA developed rapidly and is providing more and more insight into qualitative and quantitative assessment of different plaque components.
Differentiating calcification subtypes using non-invasive imaging remains a challenge for contemporary cardiovascular imaging. On one hand, certain plaque features, including some patterns of calcification, which portend higher risk of rupture and CV events, may improve assessment of prognosis. On the other hand, CT scanning struggles to assess early microcalcification and grade of stenosis because of limited spatial resolution and the influence of blooming artifacts. Hence, there is a need for high spatial resolution non-invasive imaging modalities, with fewer artifacts and more accuracy. These innovations may help reach the ultimate goal of non-invasively providing specific and clinically actionable information regarding features delineated by direct histopathologic studies (Table 2).
Table 2.
Literature regarding imaging findings and their clinical correlation.
| CT | Definition | Clinical correlation | Ref. |
|---|---|---|---|
| Spotty calcification | <3 mm in diameter | Culprit lesion – risk of CAD death or nonfatal MI | [67] |
| Highly dense calcified plaques | >1000 HU | Stable disease – reduced event risk | [69] |
| Highly dense calcified plaques | >1000 HU | Higher risk of CVD, CHD, cancer, and all-cause mortality compared with CAC score 0 and 400–999 | [71, 73] |
| PET-CT | |||
| 18F-NaF uptake | Positive (focal uptake with TBR >25% than a proximal reference lesion) | Culprit lesion of MI patients and high-risk lesion on IVUS among stable angina patients | [76] |
| 18F-NaF uptake | Positive (focal uptake with TBRmax>1.25) | Rapid 1-year progression of coronary calcification in patients with stable CAD | [77] |
CAD: coronary artery disease; CHD: coronary heart disease; MI: myocardial infarction; TBR: tissue-to-background ratio
Coronary CT angiography
Plaque characterization using coronary computed tomography angiography (CCTA) has already yielded exceptional and detailed results. Well known high-risk plaque features delineated by CCTA include positive remodeling, low attenuation, the napkin-ring sign, and spotty calcification [66]. In fact, CT analyses among patients with acute coronary syndrome showed that culprit lesions tend to have spotty calcification (focal calcification <3 mm in diameter), while non-culprit lesions tend to have contiguous calcium deposits (>3 mm) [67–68] (Figure 4). While data are consistent in that the presence of a spotty pattern of calcium deposits characterizes high-risk plaques, discrepancies arise when highly dense calcified plaques (>1000 HU on CCTA) are considered. Some studies suggest that this type of calcification is associated with stable disease and lower risk of future acute coronary syndrome (ACS), as seen in a case-control study from the ICONIC (Incident Coronary Syndromes Identified by Computed Tomography) study, in which patients who experienced ACS exhibited less dense plaque, on a per-patient and per-lesion basis [69]. This finding also derives support from data that suggest that statin treatment was associated with increased calcification burden but reduced necrotic core volume on follow-up [70] (Figure 5). However, other authors [71–73] have recently suggested that CAC >1000 indicates higher all-cause and CVD mortality: in their study, which included 2,869 adults from the cohort CAC Consortium study, Peng et al. [71] suggested that highly dense plaque denotes high-risk. The discrepancy could reflect that CAC score combines CAC volume and density, and while isolated higher CAC density may be a marker of stable plaque, higher CAC volume signifies more plaque burden and higher CVD risk. These considerations highlight the need for guidelines that adopt a more fluid stratification algorithm for primary versus secondary prevention [73]. To reduce blooming artifacts, caused by calcification-induced beam hardening, the development of de-blooming algorithms has shown promise, and should lead to improvement of CCTA diagnostic accuracy [74].
Figure 4.
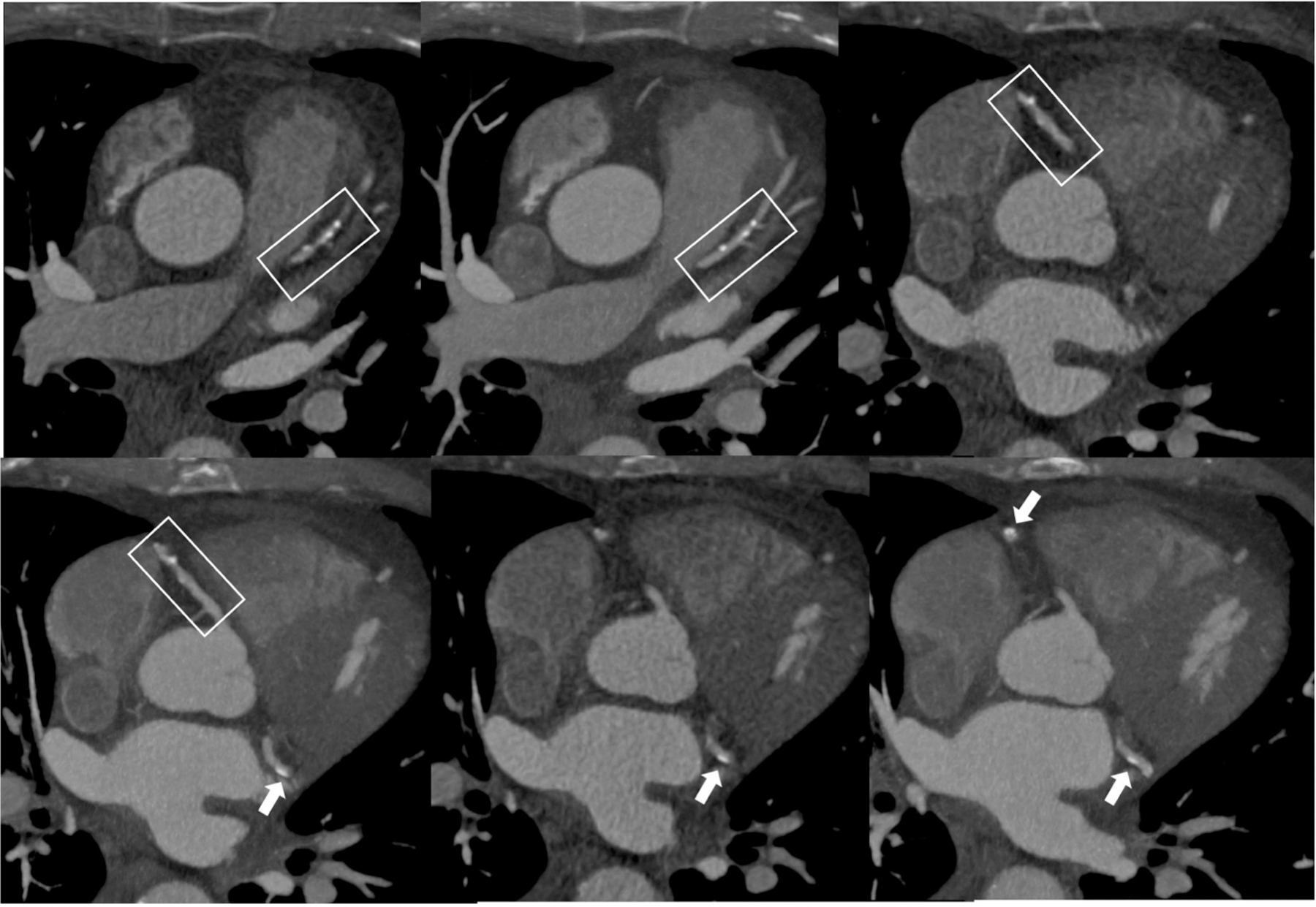
Atherosclerotic plaque as seen in CCTA.
Male patient, 67 years old, history of diabetes. CCTA images show diffuse mixed-atherosclerotic plaques, with a three-vessel distribution (box and arrows).
Figure 5.
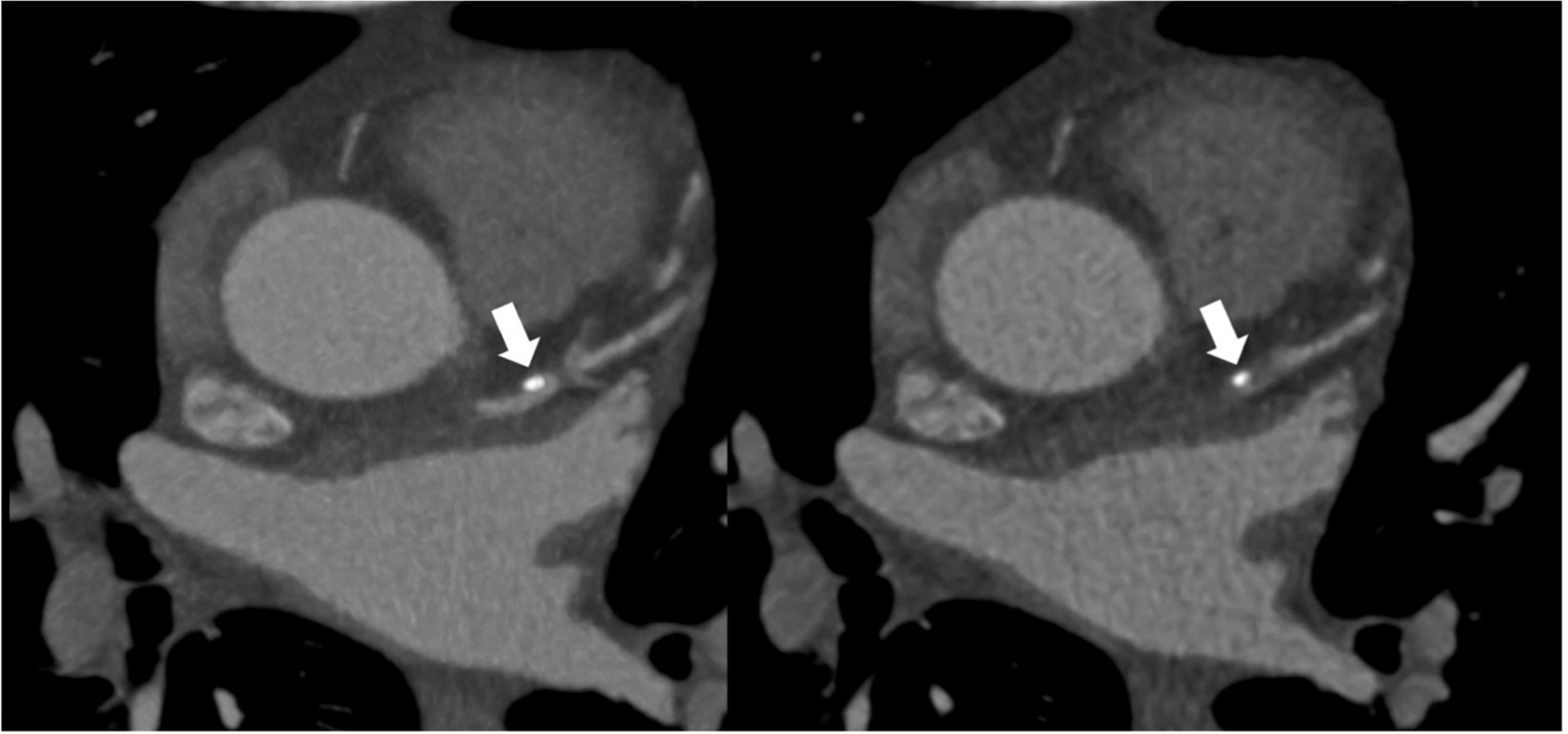
Atherosclerotic plaque as seen in CCTA.
Intermediate-risk 71-year-old patient presenting with acute chest pain and no known history of CAD. CCTA image shows highly dense calcified plaque (arrows).
High spatial resolution CCTA scanners have been developed to reduce partial volume and beam hardening artifacts. This type of scanner is designed to have higher in-plane spatial resolution (0.2–0.23 mm) compared to traditional 64-section multidetector CT with standard definition of 0.5–0.75 mm. Pontone et al. [75] compared image quality, evaluability, diagnostic accuracy and radiation exposure of high spatial resolution CCTA (HR, 0.23-mm) and standard spatial resolution CCTA (SR, 0.625-mm) among patients at high risk of CAD, using invasive coronary angiography as reference method. They found that HR CCTA compared with standard resolution improved evaluability and accuracy of calcified lesions in this clinical setting. CCTA discloses not only calcification, but also low attenuation regions of atheroma and positive arterial remodeling that also correlate with plaques that provoke ACS, features not apparent on invasive contrast luminograms.
Positron emission tomography
The use of positron emission tomography (PET) fluorine imaging to identify high-risk plaques has shown promising results in terms of spatial resolution and accuracy in the detection of calcium deposits that are below the resolution of CT (200–500 μm). 18F-NaF PET-CT localized recently ruptured plaques and identified high-risk coronary lesions among patients with stable CAD [76]. Similarly, Doris et al. [77] investigated the relationship between 18F-NaF uptake and coronary calcification progression in stable CAD and they identified 18F-NaF as a marker of future progression, able to identify patients, as well as coronary segments, with rapid progression. This modality also visualized areas interpreted as showing initial and ongoing calcification activity, providing complementary information to CT [78]. 18F-NaF PET-CT’s prognostic value as marker of coronary plaque vulnerability is being evaluated by ongoing prospective study PREFFIR (Prediction of Recurrent Events with 18F-Fluoride) [79].
Photon-counting CT and Dual-energy CT
Better spatial resolution, soft-tissue contrast, and radiation dose-efficiency are the main capabilities of photon-counting CT (PCCT), a recent advance in imaging technology. PCCT allows significantly better image quality and diagnostic confidence of CCTA when compared with conventional CT. Sandstedt et al. [80] compared the accuracy of coronary calcium quantification in an ex vivo study and they obtained better accuracy among PCCT images, which offered reduced partial volume averaging, better morphological depiction of CAC, and lower image noise. Similarly, VanMeter et al. [81] showed that PCCT images had fewer blooming artifacts, less volume overestimation when compared with micro-CT and greater volume quantification accuracy when compared with energy-integrating detector CT. Furthermore, in an in vivo study, Si-Mohamed et al. [82] compared PCCT to energy-integrating detector dual-layer CT in three independent blinded analyses. They found that CCTA obtained with PCCT demonstrated improved results in humans and better diagnostic quality of coronary calcification.
Dual-energy CT (DECT), also called spectral CT, is another emerging type of CT that potentially provides great anatomical information of arterial calcification. DECT enhances plaque visualization and enable an accurate assessment of high-risk plaque features, by combining information regarding vulnerable features on CT and effective atomic number [83–84]. This approach also permits subtraction of calcified plaques from the image, improving intracavity visualization of patients with severely calcified coronary arteries, a limitation of conventional CCTA [85]. Moreover, the use of multiple virtual monoenergetic images can reduce blooming artifacts caused by highly dense calcification [86].
From this perspective, PCCT holds great promise since the intrinsic capability of counting and classifying photon energies allows spectral imaging associated with mush higher spatial and contrast resolution altogether. Moreover, current clinical PCCT technologies are embedded into “cardiac-designed” CT scanner, which means dual source CT scanners with much higher temporal resolution (i.e. 66ms effective temporal resolution in hardware) compared with single source CT scanners (i.e. 120-125ms at best in hardware). The significant reduction of residual motion artifacts furnishes another factor that improves spatial and contrast resolution.
Clinical Implications
The identification of high-risk coronary plaque features through non-invasive imaging techniques has important clinical implications for accurate identification of patients at elevated risk of acute CV events. CCTA and CAC scoring play a crucial role in the identification and quantification of atherosclerotic disease, thus directing the intensification of preventive interventions, through lifestyle changes and risk factor management. As recently showed by Budoff et al. [87], a fine line separates primary and secondary prevention among patients with high CAC score. They demonstrated that patients without known CAD with CAC score >300 have a risk of MACE similar to that of stable high-risk patients with known CVD (post-MI). This finding has particular importance because high-risk patients with known CVD are treated with more intensive therapies (e.g. addition of nonstatin therapy such as ezetimibe), which hitherto have not been recommended for primary prevention. Thus, further studies are needed to clarify the role of CAC in treatment stratification, but high CAC score may serve as a secondary prevention risk equivalent, which would prompt to reconsider the current standard of care. However, when considering the role of CAC score in management and prevention of CVD, sex differences have to be taken into consideration to avoid bias and undertreatment of female population. It has been shown that the application of CAC score alone significantly underestimates the CV risk of women [88] and that CAC develops later in the female population when compared to men, with a comparable CAC score among the two groups with a 10-year difference [89]. Hence, risk stratification methods that apply sex-specific CAC cutoffs have been suggested in order to account for sex-based discrepancies in coronary calcium distribution [90–91]. Many studies have focused on sex differences in atherosclerosis, and in calcification specifically, but the overall key takeaway when considering CAC score in a clinical setting is that CAC exhibits significant differences between females and males, which cannot be denied or taken nonchalantly [92–93].
CCTA can assess and define the presence of features associated with greater propensity to provoke an ACS. Among these characteristics, calcification features mark an increased incidence of CVD and of CV mortality. CAC predicts future risk of ACS much better than any blood biomarker, and guidelines thus incorporate it as a risk enhancing feature. Advances in CT techniques allow better and more in-depth visualization of CAC, a welcome evolution because not all types of calcifications have the same implications for clinical outcome [94]. Several ongoing trials are examining whether the use of CAC improves clinical outcome. The results of such studies should clarify the role of CAC in the stratification of preventive treatment in future guidelines. The SCOT-HEART 2 trial (NCT03920176) investigates CCTA-guided management compared with current standard of care; the ROBINSCA trial examines whether CAC screening-guided preventive therapy is effective in reducing morbidity and mortality among asymptomatic adults; the CAC PREVENTABLE as part of the PRagmatic EValuation of evENTs And Benefits of Lipid lowering in the Elderly study (NCT04262206), which will evaluate the benefit of statin therapy among elderly without known CVD, and its correlation with CAC score; CorCal trial (NCT03439267), which tests the effectiveness of a proactive CV primary prevention strategy, with or without CAC screening, in preventing future MACE, compared with current standard of care.
Discussion and conclusions
Broadly, CAC progresses with plaque type, degree of luminal stenosis and it advances with atherosclerosis. It reliably predicts future major adverse atherosclerotic CV events in asymptomatic patients, and in the PROMISE study. [95] CAC can also predict ACS events in apparently stable patients who present with suspected CAD, thanks to its higher sensitivity for future CV events when compared with functional testing. Additionally, since most events occurred in patients with positive CAC, thanks to its discriminatory ability, CAC testing or CCTA can aid the initial evaluation of new onset chest discomfort. Even though CAC score correlates with coronary atherosclerotic burden, its evaluation with Agatston score by cardiac CT may permit identification of “vulnerable patients” who have increased risk of developing ACS, beyond characterizing a single “vulnerable” plaque [96]. Conversely, CCTA, with its higher discriminatory ability for CAD than CAC score and functional testing, may identify better plaques with propensity to rupture, and its use provides significantly better prognostic information compared with functional testing modalities alone [97–98].
Nonetheless, a debate persists as to whether CAC’s predictive ability relates to the presence of a specific calcified plaque as source of future events or reflects its excellent ability to assess the overall burden of coronary atherosclerosis, with many events actually arising from non-calcified plaques [99]. To address this question, combined pathologic and radiologic studies can relate calcification subtypes to different grades of plaque/patient vulnerability, and a combination of imaging techniques can provide better understanding of calcified plaque (Figure 6). Even though invasive imaging techniques, such as intravascular ultrasound (IVUS) and optical coherence tomography (OCT), have been used to understand and correlate the changes in atherosclerotic plaque with clinical events, the presence of calcification often affects plaque analysis, and it tends to be an exclusion criterion from progression/regression studies [100] (Figure 7). However, culprit lesions and ruptured plaques seem to have more spotty calcification, indicating that spotty calcium deposits within unstable lesion may represent a marker of ruptured, and subsequently healed, plaques [101–102].
Figure 6.
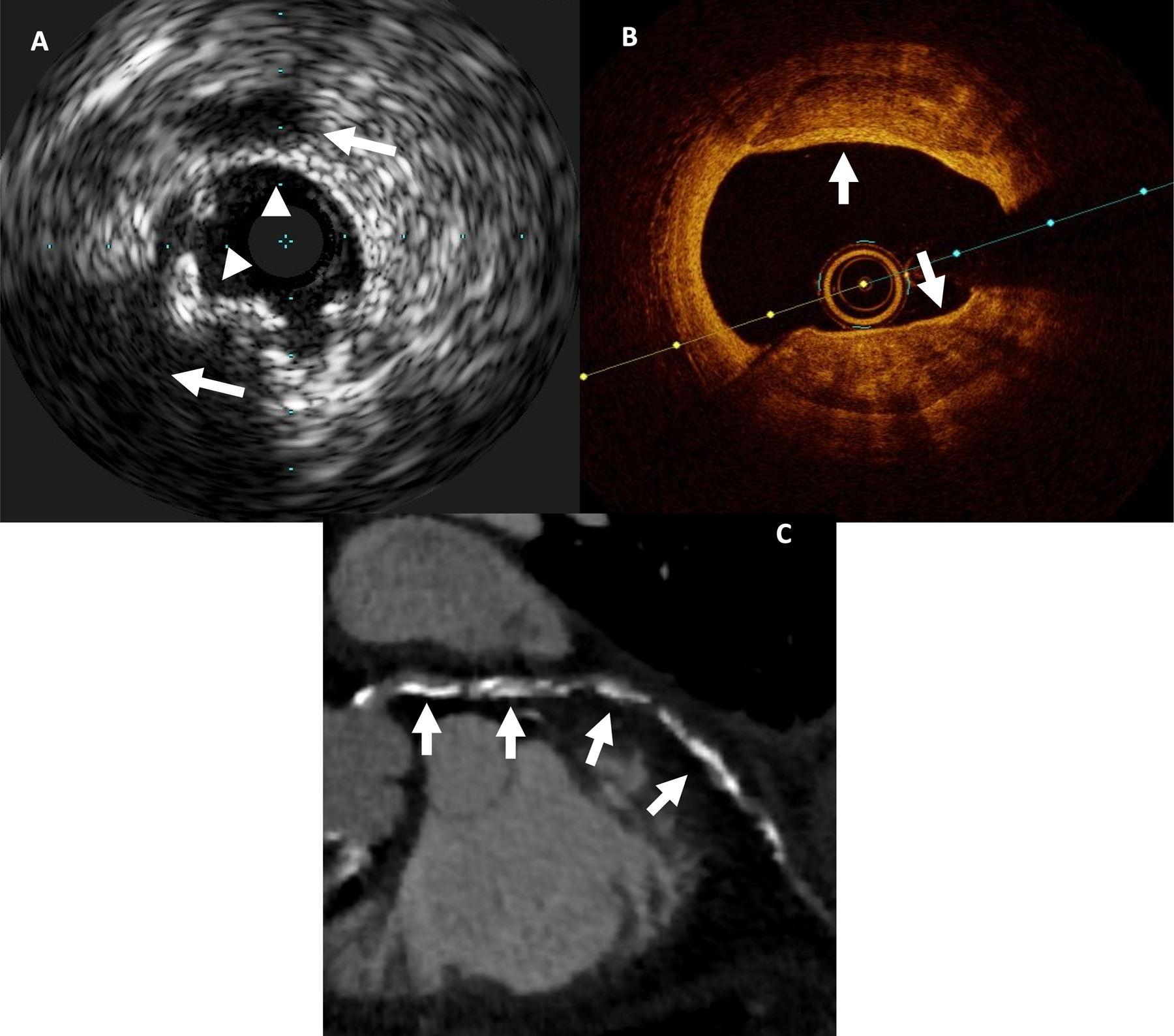
Invasive and non-invasive imaging of atherosclerotic plaque.
72-year-old male patient with CAC score of 357:
(A) IVUS: multiple calcifications (white arrowheads) with shadowing (white arrows)
(B) OCT: multiple superficial calcifications, seen as signal-poor heterogeneous regions with well delineated borders (white arrows)
(C) CCTA: diffuse mixed plaque, predominantly calcified (white arrows)
Figure 7.
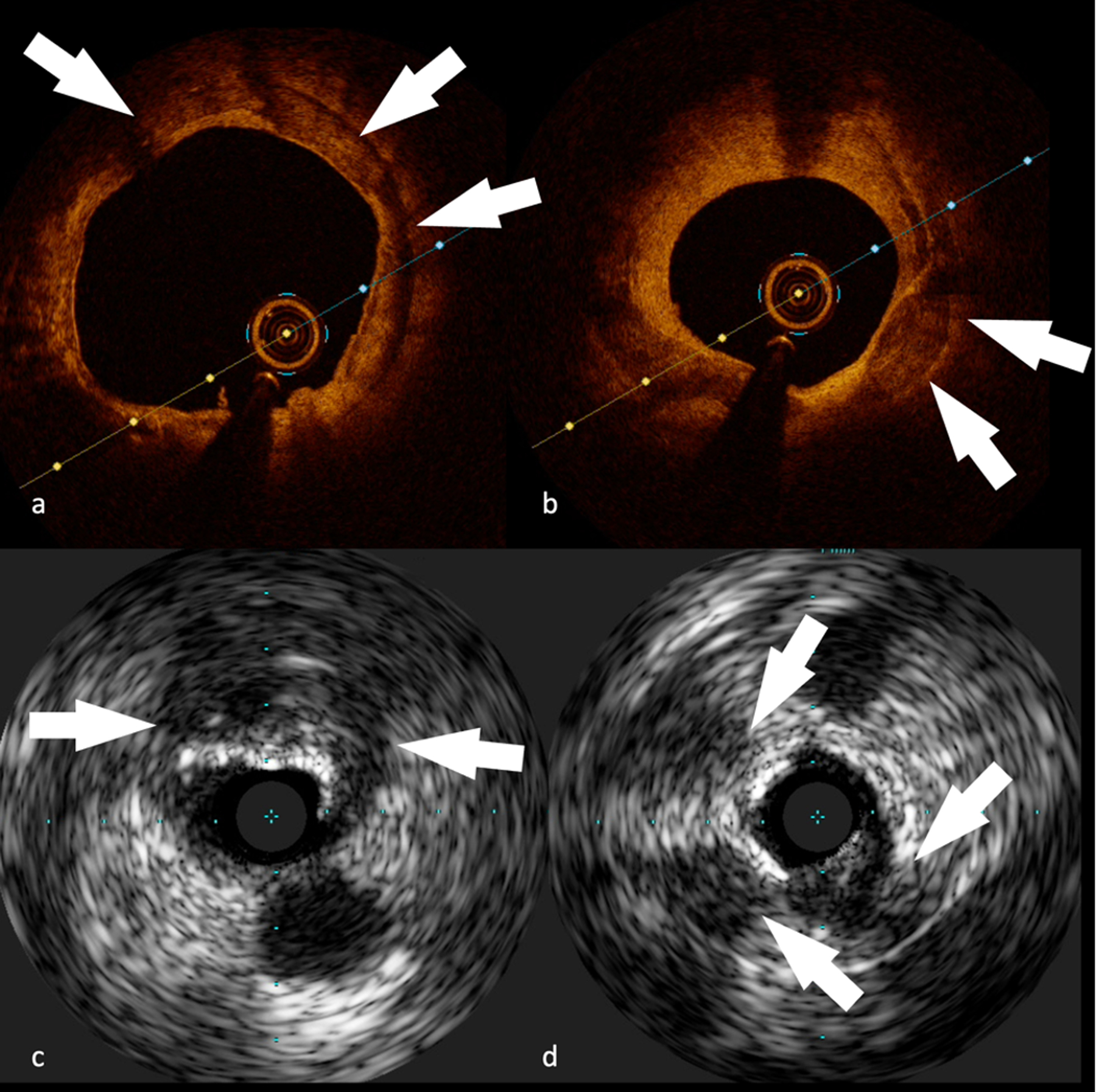
Coronary atherosclerotic plaque images obtained with invasive imaging techniques.
OCT (A-B) and IVUS (C-D), showing different types of calcifications:
(A) Concentric calcification (white arrows) on RCA in a 74-year-old male patient
(B) Superficial calcification (white arrows) on LAD in a 67-year-old male patient
(C) Big superficial calcification with posterior cone of shadow (white arrows) on LCx in a 72-year-old male patient
(D) Multiple clusters of calcifications (white arrows) on RCA in a 62-year-old male patient
Limitations of CAC scoring include its limited ability to track responses to interventions with serial imaging. For example, guidelines have stated that there is no clinical utility for CAC score among statin users, because statin treatment may increase CAC, but overall lower risk of ACS [103]. However, recent findings show that statin use does not weaken the prognostic utility of CAC and suggest that this limitation can be overcome [104], thus high CAC remains predictive of CVD and CHD mortality [105]. Debates continue regarding the decision to withhold therapies in those with low or no detectable coronary artery calcification, and the debate is extending to imaging as well. In fact, undergoing imaging tests might increase patient’s compliance to preventive treatment and therapy adherence. Despite the promising results regarding high discriminatory power of CAC for CVD risk prediction when compared with polygenic scores or high sensitivity C-reactive protein, still very few studies have compared outcomes based on allocation of therapy by CAC head-to-head with other non-imaging risk markers [106–107]. However, the role of polygenic scores among young adults, who have not developed CAC yet, requires further research. Finally, we need to recognize that no properly powered and rigorous randomized trial has allocated therapy based on CAC and shown a clinical benefit. The SCOT-HEART 2 trial currently underway may close this gap using CCTA, which however, because of its intrinsic limitations (e.g., use of contrast media, higher radiation dose compared with CAC scan), may limit the generalizability of the study.
The promise of ever more advanced imaging techniques should enable following the progression of calcification’s morphologic characteristics to contribute to prognostic assessment of high-risk, vulnerable patients, and may be helpful in the development of novel therapies [108]. For example, fragmented calcification on histology corresponds to spotty calcification on CCTA, which links to greater risk of rupture when compared with sheet calcification on histology, which corresponds to diffuse/dense calcification on radiology [109]. Microcalcification, considered a high-risk plaque feature on histology, still presents a challenge for current non-invasive imaging techniques, but advanced imaging modalities, such as PCCT and DECT, offer promising results. Further studies with these novel technologies will open the door to more accurate visualization of micro-calcified areas within coronary plaques and better risk stratification, identifying those vulnerable patients who will benefit from more aggressive preventive therapy.
Non-standard Abbreviations and Acronyms
- ACS
acute coronary syndrome
- ALP
alkaline phosphatase
- CAC
coronary artery calcification
- CAD
coronary artery disease
- CCTA
coronary CT angiography
- CHD
coronary heart disease
- CKD
chronic kidney disease
- CVD
cardiovascular disease
- DECT
dual-energy CT
- EBCT
electron-beam CT
- EPCs
endothelial progenitor cells
- IVUS
intravascular ultrasound
- Lp (a)
lipoprotein (a)
- MMPs
matrix metalloproteases
- OCT
optical coherence tomography
- PCCT
photon-counting CT
- PET
positron emission tomography
- PIT
pathologic intimal thickening
- SMCs
smooth muscle cells
- TCFA
thin-cap fibroatheroma
Footnotes
Conflict of Interest Disclosures: none
References
- 1.WHO- World Health Organization, “Cardiovascular diseases (CVDs)”, 11th June 2021, who.int, https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) Accessed 12th December 2022
- 2.Nicoll R, Wiklund U, Zhao Y, Diederichsen A, Mickley H, Ovrehus K, Zamorano P, Gueret P, Schmermund A, Maffei E, et al. The coronary calcium score is a more accurate predictor of significant coronary stenosis than conventional risk factors in symptomatic patients: Euro-CCAD study. Int J Cardiol 2016;207:13–19. [DOI] [PubMed] [Google Scholar]
- 3.Osawa K, Nakanishi R, Budoff M. Coronary Artery Calcification. Glob Heart 2016;11(3):287–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med 2008;358(13):1336–1345. [DOI] [PubMed] [Google Scholar]
- 5.Madhavan MV, Tarigopula M, Mintz GS, Maehara A, Stone GW, Généreux P. Coronary artery calcification: pathogenesis and prognostic implications. J Am Coll Cardiol 2014;63(17):1703–1714. [DOI] [PubMed] [Google Scholar]
- 6.Cerci R, Vavere AL, Miller JM, Yoneyama K, Rochitte CE, Dewey M, Niinuma H, Clouse ME, Laham R, Bush DE, et al. Patterns of coronary arterial lesion calcification by a novel, cross-sectional CT angiographic assessment. Int J Cardiovasc Imaging 2013;29(7):1619–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kataoka Y, Wolski K, Uno K, Puri R, Tuzcu EM, Nissen SE, Nicholls SJ. Spotty calcification as a marker of accelerated progression of coronary atherosclerosis: insights from serial intravascular ultrasound. J Am Coll Cardiol 2012;59(18):1592–1597. [DOI] [PubMed] [Google Scholar]
- 8.Nakahara T, Dweck MR, Narula N, Pisapia D, Narula J, Strauss HW. Coronary Artery Calcification: From Mechanism to Molecular Imaging. JACC Cardiovasc Imaging 2017;10(5):582–593. [DOI] [PubMed] [Google Scholar]
- 9.Motoyama S, Kondo T, Sarai M, Sugiura A, Harigaya H, Sato T, Inoue K, Okumura M, Ishii J, Anno H, et al. Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J Am Coll Cardiol 2007;50(4):319–326. [DOI] [PubMed] [Google Scholar]
- 10.Mori H, Torii S, Kutyna M, Sakamoto A, Finn AV, Virmani R. Coronary Artery Calcification and its Progression: What Does it Really Mean?. JACC Cardiovasc Imaging 2018;11(1):127–142. [DOI] [PubMed] [Google Scholar]
- 11.Lanzer P, Hannan FM, Lanzer JD, Janzen J, Raggi P, Furniss D, Schuchardt M, Thakker R, Fok PW, Saez-Rodriguez J, et al. Medial Arterial Calcification: JACC State-of-the-Art Review. J Am Coll Cardiol 2021;78(11):1145–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davaine JM, Quillard T, Chatelais M, Guilbaud F, Brion R, Guyomarch B, Brennan MÁ, Heymann D, Heymann MF, Gouëffic Y. Bone Like Arterial Calcification in Femoral Atherosclerotic Lesions: Prevalence and Role of Osteoprotegerin and Pericytes. Eur J Vasc Endovasc Surg 2016;51(2):259–267. [DOI] [PubMed] [Google Scholar]
- 13.Gambardella J, Wang X, Mone P, Khondkar W, Santulli G. Genetics of adrenergic signaling drives coronary artery calcification. Atherosclerosis 2020;310:88–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.New SE, Goettsch C, Aikawa M, Marchini JF, Shibasaki M, Yabusaki K, Libby P, Shanahan CM, Croce K, Aikawa E. Macrophage-derived matrix vesicles: an alternative novel mechanism for microcalcification in atherosclerotic plaques. Circ Res 2013;113(1):72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sage AP, Tintut Y, Demer LL. Regulatory mechanisms in vascular calcification. Nat Rev Cardiol 2010;7(9):528–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sorini Dini C, Nardi G, Ristalli F, Mattesini A, Hamiti B, Di Mario C. Contemporary Approach to Heavily Calcified Coronary Lesions. Interv Cardiol 2019;14(3):154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jain M, Dev R, Doddapattar P, Kon S, Dhanesha N, Chauhan AK. Integrin α9 regulates smooth muscle cell phenotype switching and vascular remodeling. JCI Insight 2021;6(10):e147134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li T, Yu H, Zhang D, Feng T, Miao M, Li J, Liu X. Matrix Vesicles as a Therapeutic Target for Vascular Calcification. Front Cell Dev Biol 2022;10:825622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Demer LL, Tintut Y. Inflammatory, metabolic, and genetic mechanisms of vascular calcification. Arterioscler Thromb Vasc Biol 2014;34(4):715–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zazzeroni L, Faggioli G, Pasquinelli G. Mechanisms of Arterial Calcification: The Role of Matrix Vesicles. Eur J Vasc Endovasc Surg 2018;55(3):425–432. [DOI] [PubMed] [Google Scholar]
- 21.Kunitomi Y, Hara ES, Okada M, Nagaoka N, Kuboki T, Nakano T, Kamioka H, Matsumoto T. Biomimetic mineralization using matrix vesicle nanofragments. J Biomed Mater Res A 2019;107(5):1021–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blaser MC, Aikawa E. Roles and Regulation of Extracellular Vesicles in Cardiovascular Mineral Metabolism. Front Cardiovasc Med 2018;5:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gössl M, Mödder UI, Atkinson EJ, Lerman A, Khosla S. Osteocalcin expression by circulating endothelial progenitor cells in patients with coronary atherosclerosis. J Am Coll Cardiol 2008;52(16):1314–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gössl M, Khosla S, Zhang X, Higano N, Jordan KL, Loeffler D, Enriquez-Sarano M, Lennon RJ, McGregor U, Lerman LO, et al. Role of circulating osteogenic progenitor cells in calcific aortic stenosis [published correction appears in J Am Coll Cardiol. 2012 Dec 18;60(24):2606. McGregor, Ulrike [added]]. J Am Coll Cardiol 2012;60(19):1945–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohler ER 3rd, Gannon F, Reynolds C, Zimmerman R, Keane MG, Kaplan FS. Bone formation and inflammation in cardiac valves. Circulation 2001;103(11):1522–1528. [DOI] [PubMed] [Google Scholar]
- 26.Gössl M, Mödder UI, Gulati R, Rihal CS, Prasad A, Loeffler D, Lerman LO, Khosla S, Lerman A. Coronary endothelial dysfunction in humans is associated with coronary retention of osteogenic endothelial progenitor cells. Eur Heart J 2010;31(23):2909–2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lavi S, Bae JH, Rihal CS, Prasad A, Barsness GW, Lennon RJ, Holmes DR Jr, Lerman A. Segmental coronary endothelial dysfunction in patients with minimal atherosclerosis is associated with necrotic core plaques. Heart 2009;95(18):1525–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jinnouchi H, Sato Y, Sakamoto A, Cornelissen A, Mori M, Kawakami R, Gadhoke NV, Kolodgie FD, Virmani R, Finn AV. Calcium deposition within coronary atherosclerotic lesion: Implications for plaque stability. Atherosclerosis 2020;306:85–95. [DOI] [PubMed] [Google Scholar]
- 29.Ross R, Glomset JA. The pathogenesis of atherosclerosis (first of two parts). N Engl J Med 1976;295(7):369–377. [DOI] [PubMed] [Google Scholar]
- 30.Ross R The pathogenesis of atherosclerosis--an update. N Engl J Med 1986;314(8):488–500. [DOI] [PubMed] [Google Scholar]
- 31.Ross R The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 1993;362(6423):801–809. [DOI] [PubMed] [Google Scholar]
- 32.Otsuka F, Sakakura K, Yahagi K, Joner M, Virmani R. Has our understanding of calcification in human coronary atherosclerosis progressed? [published correction appears in Arterioscler Thromb Vasc Biol. 2014 Jul;34(7):e17]. Arterioscler Thromb Vasc Biol 2014;34(4):724–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yahagi K, Kolodgie FD, Otsuka F, Finn AV, Davis HR, Joner M, Virmani R. Pathophysiology of native coronary, vein graft, and in-stent atherosclerosis. Nat Rev Cardiol 2016;13(2):79–98. [DOI] [PubMed] [Google Scholar]
- 34.Libby P Mechanisms of acute coronary syndromes. N Engl J Med 2013;369(9):883–884. [DOI] [PubMed] [Google Scholar]
- 35.Stone PH, Libby P, Boden WE. Fundamental Pathobiology of Coronary Atherosclerosis and Clinical Implications for Chronic Ischemic Heart Disease Management-The Plaque Hypothesis: A Narrative Review. JAMA Cardiol 2023;8(2):192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vengrenyuk Y, Carlier S, Xanthos S, Cardoso L, Ganatos P, Virmani R, Einav S, Gilchrist L, Weinbaum S. A hypothesis for vulnerable plaque rupture due to stress-induced debonding around cellular microcalcifications in thin fibrous caps. Proc Natl Acad Sci U S A 2006;103(40):14678–14683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Torii S, Sato Y, Otsuka F, Kolodgie FD, Jinnouchi H, Sakamoto A, Park J, Yahagi K, Sakakura K, Cornelissen A, et al. Eruptive Calcified Nodules as a Potential Mechanism of Acute Coronary Thrombosis and Sudden Death. J Am Coll Cardiol 2021;77(13):1599–1611. [DOI] [PubMed] [Google Scholar]
- 38.Lee T, Mintz GS, Matsumura M, Zhang W, Cao Y, Usui E, Kanaji Y, Murai T, Yonetsu T, Kakuta T, et al. Prevalence, Predictors, and Clinical Presentation of a Calcified Nodule as Assessed by Optical Coherence Tomography. JACC Cardiovasc Imaging 2017;10(8):883–891. [DOI] [PubMed] [Google Scholar]
- 39.Vergallo R, Crea F. Atherosclerotic Plaque Healing. N Engl J Med 2020;383(9):846–857. [DOI] [PubMed] [Google Scholar]
- 40.Self TS, Ginn-Hedman AM, Kaulfus CN, Newell-Fugate AE, Weeks BR, Heaps CL. Iodine-enhanced micro-computed tomography of atherosclerotic plaque morphology complements conventional histology. Atherosclerosis 2020;313:43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hutcheson JD, Goettsch C, Bertazzo S, Maldonado N, Ruiz JL, Goh W, Yabusaki K, Faits T, Bouten C, Franck G, et al. Genesis and growth of extracellular-vesicle-derived microcalcification in atherosclerotic plaques. Nat Mater 2016;15(3):335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kelly-Arnold A, Maldonado N, Laudier D, Aikawa E, Cardoso L, Weinbaum S. Revised microcalcification hypothesis for fibrous cap rupture in human coronary arteries. Proc Natl Acad Sci U S A 2013;110(26):10741–10746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sutton NR, Malhotra R, St Hilaire C, Aikawa E, Blumenthal RS, Gackenbach G, Goyal P, Johnson A, Nigwekar SU, Shanahan CM, et al. Molecular Mechanisms of Vascular Health: Insights From Vascular Aging and Calcification. Arterioscler Thromb Vasc Biol 2023;43(1):15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Drobni ZD, Kolossvary M, Karady J, Jermendy AL, Tarnoki AD, Tarnoki DL, Simon J, Szilveszter B, Littvay L, Voros S, et al. Heritability of Coronary Artery Disease: Insights From a Classical Twin Study. Circ Cardiovasc Imaging 2022;15(3):e013348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miedema MD, Dardari ZA, Nasir K, Blankstein R, Knickelbine T, Oberembt S, Shaw L, Rumberger J, Michos ED, Rozanski A, et al. Association of Coronary Artery Calcium With Long-term, Cause-Specific Mortality Among Young Adults. JAMA Netw Open 2019;2(7):e197440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cassidy-Bushrow AE, Bielak LF, Sheedy PF 2nd, Turner ST, Kullo IJ, Lin X, Peyser PA. Coronary artery calcification progression is heritable. Circulation 2007;116(1):25–31. [DOI] [PubMed] [Google Scholar]
- 47.O'Donnell CJ, Kavousi M, Smith AV, Kardia SL, Feitosa MF, Hwang SJ, Sun YV, Province MA, Aspelund T, Dehghan A, et al. Genome-wide association study for coronary artery calcification with follow-up in myocardial infarction. Circulation 2011;124(25):2855–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pechlivanis S, Moebus S, Lehmann N, Erbel R, Mahabadi AA, Hoffmann P, Jöckel KH, Nöthen MM, Bachmann HS; Heinz Nixdorf Recall Study Investigative Group. Genetic risk scores for coronary artery disease and its traditional risk factors: Their role in the progression of coronary artery calcification-Results of the Heinz Nixdorf Recall study. PLoS One 2020;15(5):e0232735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klenke S, Lehmann N, Erbel R, Jöckel KH, Siffert W, Frey UH, Peters J. Genetic variations in G-protein signal pathways influence progression of coronary artery calcification: Results from the Heinz Nixdorf Recall study. Atherosclerosis 2020;310:102–108. [DOI] [PubMed] [Google Scholar]
- 50.Rutsch F, Buers I, Nitschke Y. Hereditary Disorders of Cardiovascular Calcification. Arterioscler Thromb Vasc Biol 2021;41(1):35–47. [DOI] [PubMed] [Google Scholar]
- 51.Ren Y, Li X, Wang S, Pan W, Lv H, Wang M, Zhou X, Xia Y, Yin D. Serum alkaline phosphatase levels are associated with coronary artery calcification patterns and plaque vulnerability. Catheter Cardiovasc Interv 2021;97 Suppl 2:1055–1062. [DOI] [PubMed] [Google Scholar]
- 52.Li J, Li J, Jian Z, Wu J, Yang J, Guo N, Huang X. Serum marker and CT characteristics of coronary calcified nodule assessed by intravascular ultrasound. BMC Cardiovasc Disord 2022;22(1):475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Campos-Obando N, Bosman A, Kavousi M, Medina-Gomez C, van der Eerden BCJ, Bos D, Franco OH, Uitterlinden AG, Zillikens MC. Genetic Evidence for a Causal Role of Serum Phosphate in Coronary Artery Calcification: The Rotterdam Study. J Am Heart Assoc 2022;11(15):e023024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hisamatsu T, Miura K, Arima H, Fujiyoshi A, Kadota A, Kadowaki S, Zaid M, Miyagawa N, Satoh A, Kunimura A, et al. Relationship of serum irisin levels to prevalence and progression of coronary artery calcification: A prospective, population-based study. Int J Cardiol 2018;267:177–182. [DOI] [PubMed] [Google Scholar]
- 55.Elahirad S, Elieh Ali Komi D, Kiani A, Mohammadi-Noori E, Vaisi-Raygani A, Mozafari H, Bahrehmand F, Saidi M, Toupchi-Khosroshahi V, Salehi N. Association of Matrix Metalloproteinase-2 (MMP-2) and MMP-9 Promoter Polymorphisms, Their Serum Levels, and Activities with Coronary Artery Calcification (CAC) in an Iranian Population. Cardiovasc Toxicol 2022;22(2):118–129. [DOI] [PubMed] [Google Scholar]
- 56.Teng HI, Chen HY, Tsai CT, Huang WC, Chen YY, Hsueh CH, Hau WK, Lu TM. The clinical impact of serum 1,5-anhydro-D-glucitol levels on coronary artery calcification and adverse outcomes assessed by coronary optical coherence tomography in diabetic patients. Front Cardiovasc Med 2022;9:997649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chu X, Lu Y, Mei M, Peng P, Zhao Y, Fu G, Qiu F, Jin C. Correlation Between Serum Uric Acid Levels and Coronary Plaque Characteristics on Optical Coherence Tomography. Int Heart J 2022;63(5):806–813. [DOI] [PubMed] [Google Scholar]
- 58.Collin J, Gössl M, Matsuo Y, Cilluffo RR, Flammer AJ, Loeffler D, Lennon RJ, Simari RD, Spoon DB, Erbel R, et al. Osteogenic monocytes within the coronary circulation and their association with plaque vulnerability in patients with early atherosclerosis. Int J Cardiol 2015;181:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Garg PK, Guan W, Karger AB, Steffen BT, Budoff M, Tsai MY. Lipoprotein (a) and risk for calcification of the coronary arteries, mitral valve, and thoracic aorta: The Multi-Ethnic Study of Atherosclerosis. J Cardiovasc Comput Tomogr 2021;15(2):154–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ong KL, McClelland RL, Allison MA, Cushman M, Garg PK, Tsai MY, Rye KA, Tabet F. Lipoprotein (a) and coronary artery calcification: prospective study assessing interactions with other risk factors. Metabolism 2021;116:154706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Obisesan OH, Kou M, Wang FM, Boakye E, Honda Y, Uddin SMI, Dzaye O, Osei AD, Orimoloye OA, Howard-Claudio CM, et al. Lipoprotein(a) and Subclinical Vascular and Valvular Calcification on Cardiac Computed Tomography: The Atherosclerosis Risk in Communities Study. J Am Heart Assoc 2022;11(11):e024870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kowall B, Lehmann N, Mahabadi AA, Moebus S, Erbel R, Jöckel KH, Stang A. Associations of metabolically healthy obesity with prevalence and progression of coronary artery calcification: Results from the Heinz Nixdorf Recall Cohort Study. Nutr Metab Cardiovasc Dis 2019;29(3):228–235. [DOI] [PubMed] [Google Scholar]
- 63.Fiorino AS. Electron-beam computed tomography, coronary artery calcium, and evaluation of patients with coronary artery disease. Ann Intern Med 1998;128(10):839–847. [DOI] [PubMed] [Google Scholar]
- 64.Kulkarni S, Rumberger JA, Jha S. Electron Beam CT: A Historical Review. AJR Am J Roentgenol 2021;216(5):1222–1228. [DOI] [PubMed] [Google Scholar]
- 65.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990;15(4):827–832. [DOI] [PubMed] [Google Scholar]
- 66.Puchner SB, Liu T, Mayrhofer T, Truong QA, Lee H, Fleg JL, Nagurney JT, Udelson JE, Hoffmann U, Ferencik M. High-risk plaque detected on coronary CT angiography predicts acute coronary syndromes independent of significant stenosis in acute chest pain: results from the ROMICAT-II trial. J Am Coll Cardiol 2014;64(7):684–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nerlekar N, Ha FJ, Cheshire C, Rashid H, Cameron JD, Wong DT, Seneviratne S, Brown AJ. Computed Tomographic Coronary Angiography-Derived Plaque Characteristics Predict Major Adverse Cardiovascular Events: A Systematic Review and Meta-Analysis. Circ Cardiovasc Imaging 2018;11(1):e006973. [DOI] [PubMed] [Google Scholar]
- 68.Williams MC, Moss AJ, Dweck M, Adamson PD, Alam S, Hunter A, Shah ASV, Pawade T, Weir-McCall JR, Roditi G, et al. Coronary Artery Plaque Characteristics Associated With Adverse Outcomes in the SCOT-HEART Study. J Am Coll Cardiol 2019;73(3):291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Rosendael AR, Narula J, Lin FY, van den Hoogen IJ, Gianni U, Al Hussein Alawamlh O, Dunham PC, Peña JM, Lee SE, Andreini D, et al. Association of High-Density Calcified 1K Plaque With Risk of Acute Coronary Syndrome [published correction appears in JAMA Cardiol. 2020 Mar 1;5(3):364]. JAMA Cardiol 2020;5(3):282–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee SE, Chang HJ, Sung JM, Park HB, Heo R, Rizvi A, Lin FY, Kumar A, Hadamitzky M, Kim YJ, et al. Effects of Statins on Coronary Atherosclerotic Plaques: The PARADIGM Study. JACC Cardiovasc Imaging 2018;11(10):1475–1484. [DOI] [PubMed] [Google Scholar]
- 71.Peng AW, Mirbolouk M, Orimoloye OA, Osei AD, Dardari Z, Dzaye O, Budoff MJ, Shaw L, Miedema MD, Rumberger J, et al. Long-Term All-Cause and Cause-Specific Mortality in Asymptomatic Patients With CAC ≥1,000: Results From the CAC Consortium. JACC Cardiovasc Imaging 2020;13(1 Pt 1):83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hecht HS. Coronary Artery Calcium: From the Power of 0 to >1,000. JACC Cardiovasc Imaging 2020;13(1 Pt 1):94–96. [DOI] [PubMed] [Google Scholar]
- 73.Peng AW, Dardari ZA, Blumenthal RS, Dzaye O, Obisesan OH, Iftekhar Uddin SM, Nasir K, Blankstein R, Budoff MJ, Bødtker Mortensen M, et al. Very High Coronary Artery Calcium (≥1000) and Association With Cardiovascular Disease Events, Non-Cardiovascular Disease Outcomes, and Mortality: Results From MESA. Circulation 2021;143(16):1571–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li P, Xu L, Yang L, Wang R, Hsieh J, Sun Z, Fan Z, Leipsic JA. Blooming Artifact Reduction in Coronary Artery Calcification by A New De-blooming Algorithm: Initial Study. Sci Rep 2018;8(1):6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pontone G, Bertella E, Mushtaq S, Loguercio M, Cortinovis S, Baggiano A, Conte E, Annoni A, Formenti A, Beltrama V, et al. Coronary artery disease: diagnostic accuracy of CT coronary angiography--a comparison of high and standard spatial resolution scanning. Radiology 2014;271(3):688–694. [DOI] [PubMed] [Google Scholar]
- 76.Joshi NV, Vesey AT, Williams MC, Shah AS, Calvert PA, Craighead FH, Yeoh SE, Wallace W, Salter D, Fletcher AM, et al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. Lancet 2014;383(9918):705–713. [DOI] [PubMed] [Google Scholar]
- 77.Doris MK, Meah MN, Moss AJ, Andrews JPM, Bing R, Gillen R, Weir N, Syed M, Daghem M, Shah A, et al. Coronary 18F-Fluoride Uptake and Progression of Coronary Artery Calcification. Circ Cardiovasc Imaging 2020;13(12):e011438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tzolos E, Dweck MR. 18F-Sodium Fluoride (18F-NaF) for Imaging Microcalcification Activity in the Cardiovascular System. Arterioscler Thromb Vasc Biol 2020;40(7):1620–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. https://clinicaltrials.gov/ct2/show/NCT02278211 .
- 80.Sandstedt M, Marsh J Jr, Rajendran K, Gong H, Tao S, Persson A, Leng S, McCollough C. Improved coronary calcification quantification using photon-counting-detector CT: an ex vivo study in cadaveric specimens. Eur Radiol 2021;31(9):6621–6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.VanMeter P, Marsh J Jr, Rajendran K, Leng S, McCollough C. Quantification of Coronary Calcification using High-Resolution Photon-Counting-Detector CT and an Image Domain Denoising Algorithm. Proc SPIE Int Soc Opt Eng 2022;12031:120311R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Si-Mohamed SA, Boccalini S, Lacombe H, Diaw A, Varasteh M, Rodesch PA, Dessouky R, Villien M, Tatard-Leitman V, Bochaton T, et al. Coronary CT Angiography with Photon-counting CT: First-In-Human Results. Radiology 2022;303(2):303–313. [DOI] [PubMed] [Google Scholar]
- 83.Danad I Ó Hartaigh B, Min JK. Dual-energy computed tomography for detection of coronary artery disease. Expert Rev Cardiovasc Ther 2015;13(12):1345–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tarkowski P, Czekajska-Chehab E. Dual-Energy Heart CT: Beyond Better Angiography-Review. J Clin Med 2021;10(21):5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.De Santis D, Jin KN, Schoepf UJ, Grant KL, De Cecco CN, Nance JW Jr, Vogl TJ, Laghi A, Albrecht MH. Heavily Calcified Coronary Arteries: Advanced Calcium Subtraction Improves Luminal Visualization and Diagnostic Confidence in Dual-Energy Coronary Computed Tomography Angiography. Invest Radiol 2018;53(2):103–109. [DOI] [PubMed] [Google Scholar]
- 86.Zhang L, Li L, Feng G, Fan T, Jiang H, Wang Z. Advances in CT Techniques in Vascular Calcification. Front Cardiovasc Med 2021;8:716822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Budoff MJ, Kinninger A, Gransar H, Achenbach S, Al-Mallah M, Bax JJ, Berman DS, Cademartiri F, Callister TQ, Chang HJ, et al. When Does a Calcium Score Equates to Secondary Prevention?: Insights From the Multinational CONFIRM Registry [published online ahead of print, 2023 Apr 11]. JACC Cardiovasc Imaging 2023;S1936-878X(23)00151-1. [DOI] [PubMed]
- 88.Hoffmann U, Massaro JM, Fox CS, Manders E, O'Donnell CJ. Defining normal distributions of coronary artery calcium in women and men (from the Framingham Heart Study). Am J Cardiol 2008;102(9):1136–1141.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lessmann N, de Jong PA, Celeng C, Takx RAP, Viergever MA, van Ginneken B, Išgum I. Sex Differences in Coronary Artery and Thoracic Aorta Calcification and Their Association With Cardiovascular Mortality in Heavy Smokers. JACC Cardiovasc Imaging 2019;12(9):1808–1817. [DOI] [PubMed] [Google Scholar]
- 90.Mitchell TL, Pippin JJ, Devers SM, Kimball TE, Cannaday JJ, Gibbons LW, Cooper KH. Age- and sex-based nomograms from coronary artery calcium scores as determined by electron beam computed tomography. Am J Cardiol 2001;87(4):453–A6. [DOI] [PubMed] [Google Scholar]
- 91.Nasir K, Raggi P, Rumberger JA, Braunstein JB, Post WS, Budoff MJ, Blumenthal RS. Coronary artery calcium volume scores on electron beam tomography in 12,936 asymptomatic adults. Am J Cardiol 2004;93(9):1146–1149. [DOI] [PubMed] [Google Scholar]
- 92.Shaw LJ, Min JK, Nasir K, Xie JX, Berman DS, Miedema MD, Whelton SP, Dardari ZA, Rozanski A, Rumberger J, et al. Sex differences in calcified plaque and long-term cardiovascular mortality: observations from the CAC Consortium. Eur Heart J 2018;39(41):3727–3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bigeh A, Shekar C, Gulati M. Sex Differences in Coronary Artery Calcium and Long-term CV Mortality. Curr Cardiol Rep 2020;22(4):21. [DOI] [PubMed] [Google Scholar]
- 94.Budoff MJ, Mayrhofer T, Ferencik M, Bittner D, Lee KL, Lu MT, Coles A, Jang J, Krishnam M, Douglas PS, et al. Prognostic Value of Coronary Artery Calcium in the PROMISE Study (Prospective Multicenter Imaging Study for Evaluation of Chest Pain). Circulation 2017;136(21):1993–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mauriello A, Servadei F, Zoccai GB, Giacobbi E, Anemona L, Bonanno E, Casella S. Coronary calcification identifies the vulnerable patient rather than the vulnerable Plaque. Atherosclerosis 2013;229(1):124–129. [DOI] [PubMed] [Google Scholar]
- 96.Neglia D, Rovai D, Caselli C, Pietila M, Teresinska A, Aguadé-Bruix S, Pizzi MN, Todiere G, Gimelli A, Schroeder S, et al. Detection of significant coronary artery disease by noninvasive anatomical and functional imaging. Circ Cardiovasc Imaging 2015;8(3):e002179. [DOI] [PubMed] [Google Scholar]
- 97.SCOT-HEART investigators. CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial [published correction appears in Lancet. 2015 Jun 13;385(9985):2354]. Lancet 2015;385(9985):2383–2391. doi: 10.1016/S0140-6736(15)60291-4 [DOI] [PubMed] [Google Scholar]
- 98.Hoffmann U, Ferencik M, Udelson JE, Picard MH, Truong QA, Patel MR, Huang M, Pencina M, Mark DB, Heitner JF, et al. Prognostic Value of Noninvasive Cardiovascular Testing in Patients With Stable Chest Pain: Insights From the PROMISE Trial (Prospective Multicenter Imaging Study for Evaluation of Chest Pain). Circulation 2017;135(24):2320–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Arbab-Zadeh A, Fuster V. The myth of the "vulnerable plaque": transitioning from a focus on individual lesions to atherosclerotic disease burden for coronary artery disease risk assessment. J Am Coll Cardiol 2015;65(8):846–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mintz GS. Intravascular imaging of coronary calcification and its clinical implications. JACC Cardiovasc Imaging 2015;8(4):461–471. [DOI] [PubMed] [Google Scholar]
- 101.Fujii K, Carlier SG, Mintz GS, Takebayashi H, Yasuda T, Costa RA, Moussa I, Dangas G, Mehran R, Lansky AJ, et al. Intravascular ultrasound study of patterns of calcium in ruptured coronary plaques. Am J Cardiol 2005;96(3):352–357. [DOI] [PubMed] [Google Scholar]
- 102.Mizukoshi M, Kubo T, Takarada S, Kitabata H, Ino Y, Tanimoto T, Komukai K, Tanaka A, Imanishi T, Akasaka T. Coronary superficial and spotty calcium deposits in culprit coronary lesions of acute coronary syndrome as determined by optical coherence tomography. Am J Cardiol 2013;112(1):34–40. [DOI] [PubMed] [Google Scholar]
- 103.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines [published correction appears in J Am Coll Cardiol. 2019 Jun 25;73(24):3234-3237]. J Am Coll Cardiol 2019;73(24):3168–3209. [DOI] [PubMed] [Google Scholar]
- 104.Janssen EM, Dy SM, Meara AS, Kneuertz PJ, Presley CJ, Bridges JFP. Coronary Artery Calcification, Statin Use and Long-Term Risk of Atherosclerotic Cardiovascular Disease Events (from the Multi-Ethnic Study of Atherosclerosis). Am J Cardiol 2020;125(6):835–839. [DOI] [PubMed] [Google Scholar]
- 105.Osei AD, Mirbolouk M, Berman D, Budoff MJ, Miedema MD, Rozanski A, Rumberger JA, Shaw L, Al Rifai M, Dzaye O, et al. Prognostic value of coronary artery calcium score, area, and density among individuals on statin therapy vs. non-users: The coronary artery calcium consortium. Atherosclerosis 2021;316:79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Khan SS, Post WS, Guo X, Tan J, Zhu F, Bos D, Sedaghati-Khayat B, van Rooij J, Aday A, Allen NB, et al. Coronary Artery Calcium Score and Polygenic Risk Score for the Prediction of Coronary Heart Disease Events. JAMA 2023;329(20):1768–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Blaha MJ, Budoff MJ, DeFilippis AP, Blankstein R, Rivera JJ, Agatston A, OĽeary DH, Lima J, Blumenthal RS, Nasir K. Associations between C-reactive protein, coronary artery calcium, and cardiovascular events: implications for the JUPITER population from MESA, a population-based cohort study. Lancet 2011;378(9792):684–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Figtree GA, Adamson PD, Antoniades C, Blumenthal RS, Blaha M, Budoff M, Celermajer DS, Chan MY, Chow CK, Dey D, et al. Noninvasive Plaque Imaging to Accelerate Coronary Artery Disease Drug Development. Circulation 2022;146(22):1712–1727. [DOI] [PubMed] [Google Scholar]
- 109.Abdelrahman KM, Chen MY, Dey AK, Virmani R, Finn AV, Khamis RY, Choi AD, Min JK, Williams MC, Buckler AJ, et al. Coronary Computed Tomography Angiography From Clinical Uses to Emerging Technologies: JACC State-of-the-Art Review. J Am Coll Cardiol 2020;76(10):1226–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]


