Abstract
| Strategy, Management and Health Policy | ||||
|---|---|---|---|---|
| Venture Capital Enabling Technology | Preclinical Research | Preclinical Development Toxicology, Formulation Drug Delivery, Pharmacokinetics | Clinical Development Phases I-III Regulatory, Quality, Manufacturing | Postmarketing Phase IV |
The P2Y receptor on turkey erythrocyte membranes was the first P2 receptor to be shown to activate phospholipase C (PLC) in a strictly guanine nucleotide-dependent manner and remains the only G protein-coupled P2 receptor for which G protein-coupling kinetics have been defined. This membrane receptor has provided a model system for detailed pharmacological analyses of a series of chain-extended 2-thioether derivatives of adenine nucleotides that exhibit remarkable selectivity and potency for P2Y receptors. This model system also has led recently to identification of a novel series of P2 receptor antagonists. The turkey erythrocyte receptor is the species homologue of the chick P2Y1 receptor originally cloned by Webb and coworkers [Webb et al., 1993]. We also have cloned the human homologue of the P2Y1 receptor, which exhibits identical pharmacological and second messenger signaling properties to that of the avian P2Y1 receptor.
Keywords: P2Y, receptor, P2Y receptors, phospholipase C, P2 receptor antagonist
INTRODUCTION
Both the importance and the complexity of signal transduction involving extracellular adenine and uridine nucleotides have been brought into focus by the recent cloning of the genes encoding at least a dozen members of the two major classes of receptors that are activated by these extracellular signaling molecules. These include seven ligand-gated ion channels that comprise the P2X receptor class [Surprenant et al., 1995; North, 1996] and four different G protein-coupled receptors that are members of the P2Y receptor class of P2 receptors [Harden et al., 1996]. Pharmacological and second messenger signaling data indicate the existence of additional P2 receptors that are yet to be cloned [Harden et al., 1996].
Although our laboratory has studied the pharmacological and second messenger signaling properties of the P2Y2, P2Y4, and P2Y6 receptors [Nicholas et al., 1996], the most extensive information base has been developed for the P2Y1 receptor. Here, we review our progress in understanding this receptor from its initial detection in a model avian system to the molecular cloning and characterization of the human P2Y1 receptor.
BIOCHEMICAL PROPERTIES OF THE P2Y RECEPTOR OF TURKEY ERYTHROCYTES
Progress in identification of the G protein and phospholipase C (PLC) that mediate the inositol lipid signaling response to a broad range of hormones, neurotransmitters, and growth factors initially was compromised by a number of factors, not the least of which was lack of useful model membrane systems to study this pathway. As such, our laboratory pursued the identification of such a model, and was eventually successful in 1986/1987 in collaboration with Peter Downes and his colleagues in developing the turkey erythrocyte membrane to study regulation of PLC [Harden et al., 1987; Harden et al., 1988]. This model was unique for several reasons. First, the extent of activation of PLC by G protein activators such as AlF−4 or GTPγS was remarkable compared with the response we and others had previously observed with membranes prepared from mammalian tissues or cell lines. Second, the response was stable with time, and detailed kinetic analyses could be carried out for the first time for a G protein-activated PLC. Third, turkey erythrocytes could be obtained in very large quantities and highly purified plasma membranes could be prepared from this homogeneous cell preparation. Thus, a model membrane signaling system was identified in which the kinetics of activation of PLC could be defined in detail and from which the protein components that comprise this signaling cohort could be identified, characterized, purified, and eventually cloned. The turkey erythrocyte remains the only homogeneous cell preparation from which the three proteins of a receptor/G protein/PLC signaling pathway have been unambiguously identified and structurally defined. The properties of one these proteins, the P2Y1 receptor, will be discussed in detail in this chapter. The properties of the G protein (Gα11) and PLC (a novel PLC-β isoenzyme, PLC-βT) of turkey erythrocytes have been described in detail elsewhere [Morris et al., 1990; Waldo et al., 1991; Maurice et al., 1993; Waldo et al., 1996].
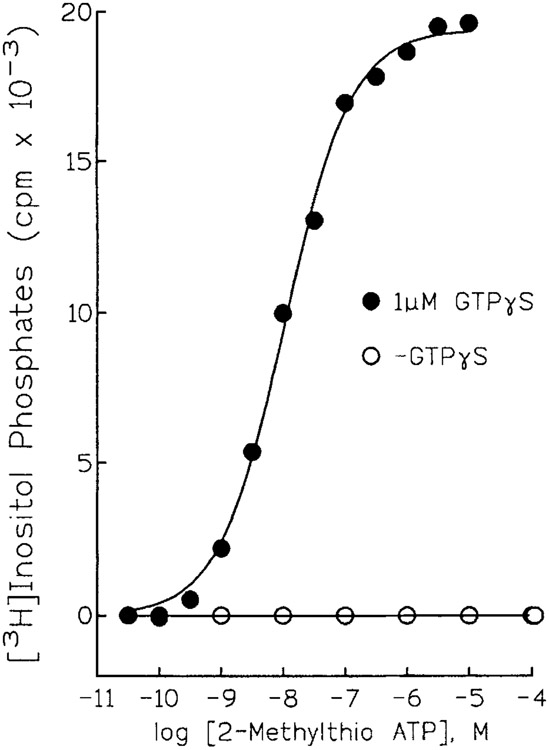
The value of the turkey erythrocyte membrane as a model to study G protein-mediated regulation of PLC was immediately obvious from the fold-stimulation of enzyme activity that was effected by G protein activators [Harden et al, 1987]. What was not evident in these initial studies was the major role played by a P2Y receptor in this response. ATP was included in all of the early assays of PLC activity to support phosphatidylinositol kinase activity. PtdIns(4,5)P2 was thought to be (and later proven to be) the major substrate for the G protein-activated PLC. Because this inositol lipid was present in low mass relative to the levels of PtdIns itself, ATP was included in membrane assays to support conversion of PtdIns to PtdIns 4-P and PtdIns 4-P to PtdIns(4,5)P2. The effects of ATP eventually were shown not to be due to support of PtdIns kinase activity, but rather, were due to receptor activation [Harden et al., 1988]. Moreover, adenine nucleotide analogues such as 2MeSATP which are not substrates for PtdIns kinases, were much more potent than ATP as activators of inositol lipid hydrolysis (Fig. 1). These observations led to pharmacological studies that confirmed the presence of a P2 receptor on turkey erythrocyte membranes [Harden et al., 1988]. The order of potency of a series of seven agonists (2MeSATP > ADPβS > ATPγS > ATP > AppNHp >α,βMeATP > β,γMeATP) closely matched that initially proposed a few years earlier by Burnstock and Kennedy [Burnstock and Kennedy, 1985] for a P2Y receptor. Pharmacological experiments carried out with intact turkey erythrocytes also confirmed the presence of a PLC-activating P2Y receptor [Berrie et al., 1989].
Figure 1.
Guanine nucleotide-dependent activation of PLC by 2MeSATP. Turkey erythrocyte membranes were incubated in the absence (open circles) or presence (filled circles) of 1 μM GTPyS in the presence of the indicated concentrations of 2MeSATP. Inositol phosphate accumulation was quantitated as a measure of PLC activity.
Pharmacological identification of a P2Y receptor that coupled to a highly responsive effector protein allowed us to study for the first time the kinetics of activation of a G protein-regulated PLC [Boyer et al., 1989]. P2Y receptor-mediated activation of PLC was absolutely dependent on the presence of guanine nucleotides (Fig. 1). Activation of PLC by GTPγS occurred with a considerable time lag. The rate of activation followed first order kinetics and was markedly increased by increasing concentrations of a P2Y receptor agonist. In contrast, the rate of activation of PLC at a fixed agonist concentration was independent of guanine nucleotide concentration. P2Y receptor activation had no effect on the off-rate of the guanine nucleotide-activated PLC. These results were consistent with a P2Y receptor-promoted increase in the rate of exchange of GTP for GDP on the involved G protein, and illustrated for the first time that the kinetics of G protein-promoted activation of PLC were essentially identical to those previously described in detail for activation of adenylyl cyclase and cyclic GMP phosphodiesterase.
Although P2Y receptor activation in turkey erythrocytes caused prominent activation of PLC, no effect on adenylyl cyclase activity was observed, suggesting that this receptor selectively coupled to the inositol lipid signaling pathway. It subsequently was shown that the β-adrenergic receptor of turkey erythrocytes not only activated adenylyl cyclase, but also activated PLC [Rooney et al., 1991; Vaziri and Downes, 1992], The molecular basis of this dual G protein selectivity of the β-adrenergic receptor is not completely understood, but could potentially involve activation of G16. G16 is found in cells of hematopoietic origin, activates PLC, and has been shown [Offermanns and Simon, 1995] to exhibit promiscuity of coupling to receptors that otherwise exhibit high selectivity of coupling to a single G protein type, e.g., Gs.
Attempts to radiolabel the P2Y receptors on turkey erythrocyte membranes were made under conditions that were similar to those in which receptor stimulatory activity of nucleotide analogues could be tested [Cooper et al., 1989]. Thus, radioligand-binding assays were carried out in purified plasma membranes and the capacity of P2Y receptor agonists to stimulate phospholipase C was determined in membrane ghosts prepared from erythrocytes. Ki values of a series of ten agonists for inhibition of [35S]ADPβS binding to plasma membranes closely matched the K0.5 values determined for the same agonists for stimulation of phospholipase C activity in the ghost membrane preparation. These pharmacological and biochemical data strongly suggested that the site on turkey erythrocyte membranes that was labeled by [35S]ADPβS was the same site utilized by P2Y receptor agonists to activate phospholipase C. Subsequent work has cast doubt on this initial assertion. The availability of newly synthesized, highly selective P2Y receptor agonists has permitted more detailed pharmacological analyses of the [35S]ADPβS-binding site on turkey erythrocyte membranes. A number of these agonists activated phospholipase C and inhibited [35S]ADPβS binding with very different apparent affinities. Such results could potentially be explained by differential hydrolysis of nucleotides or important differences in conditions necessary for assays of binding versus activity. However, major differences in binding versus biological activity seriously call into question the validity of the [35S]ADPβS-binding assay of P2Y receptors on turkey erythrocyte membranes. For similar reasons we question the utility of [32P]benzoylylbenzoylylATP ([32P]BzATP) to specifically covalently incorporate radiolabel into P2Y receptors as we originally reported [Boyer et al., 1990] for turkey erythrocyte membranes. Although [35S]dATPαS has been introduced recently as a potential radioligand for P2 receptors [Simon et al., 1995; Webb et al., 1996], its activity as an agonist or antagonist at these receptors has not been established and its general usefulness as a P2 receptor radioligand remains suspect.
PHARMACOLOGICAL PROPERTIES OF THE TURKEY ERYTHROCYTE P2Y RECEPTOR
The turkey erythrocyte membrane model has provided a simple in vitro system for detailed pharmacological analysis of a P2Y receptor. We have tested the activities of over 250 molecules, including non-nucleotide compounds and analogs of adenine nucleotides modified in the phosphate chain, in the ribose moiety, and in the purine base. An obvious goal has been to identify key moieties that are important in establishing receptor affinity and subtype selectivity. An underlying goal is to identify P2-R-selective high affinity antagonists. Relative potencies of a series of adenine nucleotide analogues for the P2Y1 receptor are presented in Table 1 and general structure activity relationships for the P2Y1 receptor are illustrated in Table 2.
TABLE 1.
Pharmacological Selectivity of the Turkey Erythrocyte P2Y Receptor*
| Agonist | Ec50 (nM) |
|---|---|
| 2MeSATP | 8 |
| 2MeSADP | 6 |
| ADPβS | 96 |
| ATPγS | 1260 |
| ATP | 6350 |
| ADP | 5550 |
| App(NH)p | 4450 |
| UTP | 143000 |
| α,β-MeATP | > 100,000 |
| β,γ-MeATP | > 100,000 |
| AMP | Inactive |
| Adenosine | Inactive |
EC50 values were obtained in measurements of inositol phosphate accumulation in turkey erythrocyte membranes (Boyer et al., 1989).
TABLE 2.
Structure–Activity Relationships for the P2Y1 Receptor
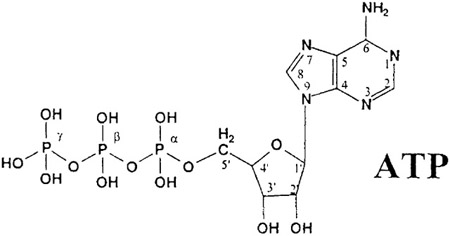
|
||
|---|---|---|
| Modifications | Examples | Effects on potencya |
| Base modifications | ||
| 2-Thioether | 2-MeSATP | ↑ |
| 2-Hexylthio ATP | ↑ | |
| 8-Substitution | 8-Br-ATP | ↓ or no change |
| 8-(6-Aminohexylamino) ATP | ↓ | |
| N6 | N6-methyl ATP | No change |
| Ribose modifications | ||
| Deoxy | 2′-Deoxy ATP | ↓ |
| 3′-Deoxy ATP | ↓ | |
| 2′-,3′-Dideoxy ATP | ↓ | |
| Other substitutions | 3′-Amino, 3′-deoxy ATP | ↑ |
| Adenosine 3′-phosphate 5′-phosphosulfate | ↑, partial agonist | |
| 3′-Benzylamino 3′-deoxy ATP | ↓ | |
| Phosphate substitutions | ||
| Thiophosphate | ATPγS | ↑ |
| Methylene bridge | αβMeATP | ↓ |
| βγMeATP | ↓ | |
| Imino bridge | AppNHp | No change |
| Diphosphates | ||
| ADP | ↑ or similar potency | |
| ADPβS | ↑ | |
| 2-Thioether diphosphates | ↑ | |
| Monophosphates | ||
| AMP | Inactive | |
| 2-(5-Hexenylthio) AMP | ↑ |
Relative to ATP.
We have studied extensively a series of analogs of adenine nucleotides modified on the purine ring as chain-extended 2-thioethers [Fischer et al., 1993; Burnstock et al., 1994; Boyer et al., 1995]. Chemical functionality incorporated in the 2-thioether moiety included cyanoalkyl, nitroaromatic, amino, thiol, cycloalkyl, n-alkyl, and olefinic groups. A group of eleven 2-thioether derivatives of ATP stimulated the production of inositol phosphates by turkey erythrocyte membranes with K0.5 values of 1.5-770 nM. 2-[[2-(p-aminophenyl)ethyl]thio]-ATP was the most potent agonist tested in this initial series. These analogs also displayed pD2 values in the range of 6-8 in smooth muscle assay systems for activity at P2Y receptors. There was a significant correlation for these compounds between pK0.5 values for inositol phosphate production in turkey erythrocyte membranes and pD2 values for relaxation mediated via the P2Y receptor in the guinea pig taenia coli, but not for P2Y receptors on rabbit aorta or rabbit mesenteric artery [Fischer et al., 1993].
ADP is equipotent to ATP for activation of the turkey erythrocyte P2Y receptor. Two chain-extended 2-thioether analogs of ADP were prepared and also were shown to be as potent as the corresponding ATP analogs at this avian P2Y receptor [Fischer et al., 1993].
We also have studied compounds with structural modifications in the C8 and N6 positions of the purine ring, in the 2′ and 3′ positions of the ribose moiety, and in the phosphate side chain. Whereas thioether- or halogen-substitution in the 2-position of the adenine base increased P2Y receptor potency, certain substitutions in the N6-position had no effect and chain-extended amino- or halogen substitution of the 8-position decreased P2Y receptor potency [Fischer et al., 1993; Burnstock et al., 1994]. 2′ - or 3′-deoxyATP were inactive or exhibited low potency for the avian receptor [Burnstock et al., 1994]. Adenine nucleotide analogues with substitutions in the 2′ or 3′-positions generally also exhibited low potency, although exceptions were observed. For example, 3′-NH2 3 ′-deoxyATP and 3′-benzoylbenzoyl ATP were both potent P2Y1 receptor agonists. A number of phosphate chain modifications of ATP and ADP, e.g., ATPγS and ADPβS, produced agonists that are somewhat more potent than ATP or ADP.
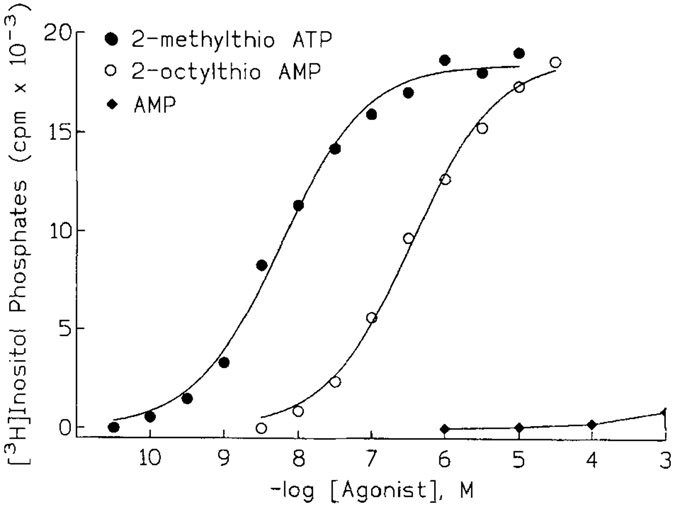
We have attempted to identify P2Y receptor agonists that move away from the nucleotide structure and that would not be substrates for the multitude of ATP-binding proteins, including ecto-nucleotidases. The remarkable enhancement of potency of agonists conferred by chain-extended 2-thioether substitution has provided a powerful approach for generation of novel compounds. For example, AMP itself is not an agonist at the P2Y receptor of turkey erythrocytes (Fig. 2). However, 2-thioether-substituted analogs of AMP are potent full agonists [Boyer et al., 1996]. K0.5 values of 80,300, and 4,000 nM were obtained for 2-thiohexylAMP, 2-thiooctylAMP (Fig. 2), and 2-(p-nitrophenylethyl)thioAMP, respectively, for stimulation of the P2Y receptor in turkey erythrocyte membranes. Thus, AMP-analogues have been identified that are up to 300-fold more potent than the natural receptor agonists ATP and ADP. A single phosphate is apparently the minimal requirement in this series of molecules since 2-thiohexenyl adenosine exhibited no activity. This series of compounds is inactive at the P2Y2 receptor [Boyer et al., 1996] and are unlikely to be active at the uridine nucleotide-selective P2Y4 and P2Y6 receptors.
Figure 2.
P2Y receptor agonist activity of a 2 thioether-derivative of AMP. Turkey erythrocyte membranes were incubated with the indicated concentrations of 2MeSATP (filled circles), 2 octylthioAMP (open circles), or AMP (filled diamonds). Inositol phosphate accumulation was measured as a quantitation of PLC activity.
We also have identified a P2Y receptor on C6 rat glioma cells that couples through Gi to inhibit adenylyl cyclase and that has no effect on inositol lipid hydrolysis or Ca++ mobilization [Boyer et al., 1993; Boyer et al., 1995]. Although the overall pharmacological selectivity of this receptor for agonists is similar to that of the turkey erythrocyte P2Y receptor there are notable differences. Pharmacological differentiation of the C6 cell and turkey erythrocyte P2Y receptors has been effected more convincingly in studies of the relative selectivity of suramin, reactive blue 2, and pyridoxal phosphate 6-azophenyl 2 ′,4 ′-disulphonic acid (PPADS), which have been shown to block P2-R-mediated responses in other tissues [Boyer et al., 1994]. Suramin and reactive blue 2 competitively antagonized (pKB = 5.4 and 7.6, respectively) the inhibitory effect of 2MeSATP on AC in C6 glioma cells, whereas PPADS at concentrations up to 100 μM had no effect [Boyer et al., 1994]. In contrast, PPADS was a competitive antagonist (pKB = 5.9) of the P2Y receptor in turkey erythrocyte membranes. Suramin and reactive blue 2 produced both a shift to the right of the concentration-effect curve of 2MeSATP for activation of PLC in turkey erythrocyte membranes and a significant decrease in the maximal inositol phosphate response. The presence of a PLC-coupled β-adrenergic receptor on turkey erythrocytes allowed us to differentiate competitive versus non-competitive effects of these compounds. The differential effect of PPADS on P2Y receptors of C6 glioma cells and turkey erythrocytes and the 100-fold difference in the pKB values calculated for reactive blue 2 added further support to the idea that different P2Y receptor subtypes mediate coupling to PLC in turkey erythrocytes and adenylyl cyclase in C6 cells.
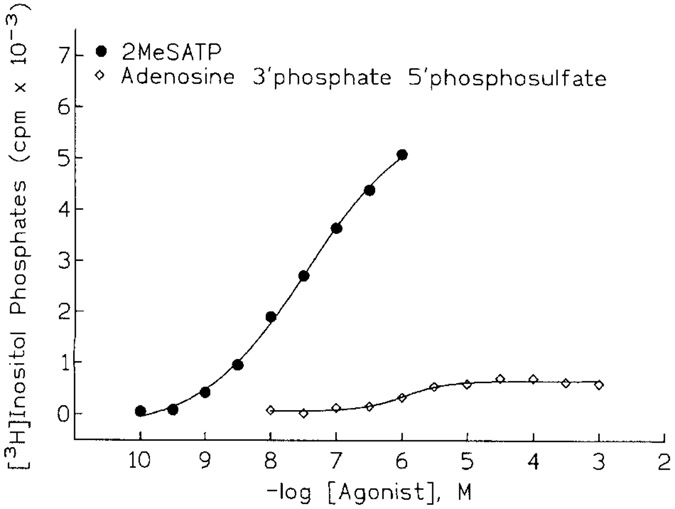
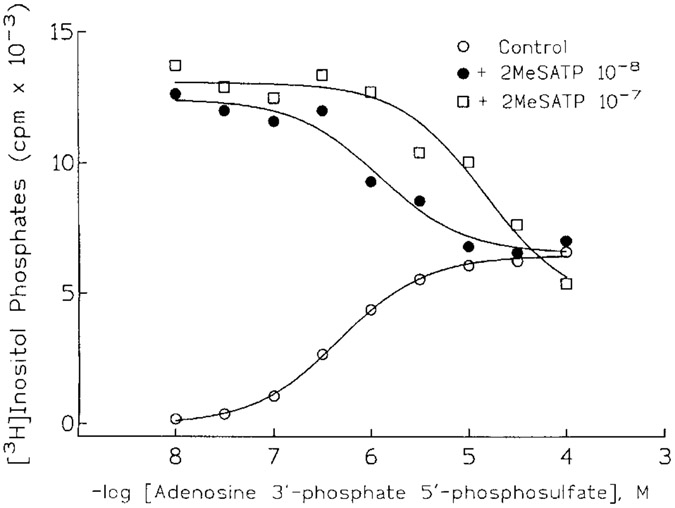
A new direction for antagonist development has evolved from studies examining whether sulfate derivatives of adenine nucleotides might be P2Y receptor agonists that are resistant to enzymatic hydrolysis. A number of sulfate analogs were obtained, and one such compound, adenosine 3′-phosphate 5′-phosphosulfate (A3P5PS) had a surprising and provocative activity [Boyer et al., 1996]. This compound was a partial agonist at the P2Y receptor of turkey erythrocytes (Fig. 3) in that stimulatory effects appeared to saturate at 1–10 μM A3P5PS and at a level that was only 10-20% of that observed with 2MeSATP. That this partial agonist effect occurred solely through occupation of the P2Y receptor was confirmed by demonstration that A3P5PS antagonized the stimulatory effects of 2 MeSATP (Fig. 4). Moreover, the 3′-phosphate derivative is a competitive antagonist of P2Y receptors based on the parallel shift to the right of the concentration effect curve for 2MeSATP The pKB of A3P5PS determined from Schild analyses was 6.3. Our anticipation that antagonist activity was conferred by the sulfate was unfounded, since adenosine 3′-phosphate, 5′-phosphate (A3P5P) also was a competitive antagonist of the effects of 2MeSATP. Phosphate substitution in the 3′-position appears to be more important for antagonism, since an equivalent substitution in the 2′-position, e.g., 2′-phosphate 5′-phosphosulfate, resulted in a less potent partial agonist/antagonist. Other substitutions at the 3′ position of adenosine alone were insufficient for antagonism. For example, 3′-N-benzylamino 3′-deoxyATP was a weak agonist devoid of any antagonist activity and adenosine 3′ monophosphate exhibited neither agonist nor antagonist activity. In contrast, 3′-amino 3′-deoxyATP was a potent full agonist in this system. Adenosine 2′-phosphate 5′-phosphoribose was a low potency partial agonist that exhibited antagonist activity at high μM concentrations.
Figure 3.
Partial agonist activity of adenosine 3′-phosphate 5′-phosphosulfate. Turkey erythrocyte membranes were incubated with the indicated concentrations of 2MeSATP (filled circles) or adenosine 3′-phosphate 5′-phosphosulfate (open diamonds). Inositol phosphate accumulation was measured as a quantitation of PLC activity.
Figure 4.
Competitive antagonist activity of adenosine 3′-phosphate 5′-phosphosulfate. Turkey erythrocyte membranes were incubated in the presence of the indicated concentrations of adenosine 3′-phosphate 5′-phosphosulfate and no additional drug (open circles), 10 nM 2MeSATP (filled circles), or 100 nM 2MeSATP (open squares). Inositol phosphate accumulation was measured as a quantitation of PLC activity.
MOLECULAR CLONING AND EXPRESSION OF THE PLC-COUPLED P2Y RECEPTOR OF TURKEY ERYTHROCYTES
We initially attempted to clone the turkey erythrocyte P2Y receptor using a PCR-based approach and by expression cloning in Xenopus oocytes. During the course of this work, Webb et al. [1993] reported the first molecular cloning of a P2Y receptor. Although expression of this cDNA in frog oocytes conferred an extracellular nucleotide-stimulated current, neither the full pharmacological selectivity (it was suggested to be a P2Y receptor) nor the G-protein/second messenger coupling of this P2-R was resolved. As such we cloned the turkey homologue of this receptor (tP2Y1-R) and stably expressed it in a heterologous cell line (1321N1 human astrocytoma cells) to establish its signaling and pharmacological properties [Filtz et al., 1994]. 2MeSATP promoted a marked activation of PLC in transfected cells. The order of potency of a series of analogs for stimulation of PLC was similar to that observed for P2Y receptors in turkey erythrocyte membranes and other tissues. Stimulation of inositol lipid hydrolysis by P2Y receptor agonists was not affected by preincubation of cells with pertussis toxin, and in contrast to its marked effects on PLC activity, 2MeSATP did not inhibit cyclic AMP accumulation. The tP2Y1 receptor also was stably expressed in Chinese hamster ovary cells where a similar pharmacological and second messenger-coupling selectivity was observed. Ribonuclease protection analyses of RNA from turkey tissues showed that this P2Y receptor is most highly expressed in blood and brain. We believe (based on the presence of tP2Y1 receptor transcripts in adult turkey blood, on its pharmacological selectivity, and on its second messenger coupling selectivity) that the cloned tP2Y1 receptor is the same receptor that we have studied in detail in turkey erythrocyte membranes.
The predicted structure of the cloned avian P2Y1 receptor is that of a typical seven transmembrane-spanning G protein-coupled receptor (Fig. 5). There is little overall homology of this receptor to receptors for adenosine but a surprisingly higher degree of homology to receptors for peptide hormones such as interleukin-8, thrombin, vasoactive intestinal peptide, platelet-activating factor, angiotensin II, and neuropeptide Y. The homology of the P2Y receptor is highest for the recently cloned P2Y2 [Lustig et al., 1993], P2Y4 [Communi et al., 1995; Nguyen et al., 1995], and P2Y6 [Chang et al., 1995] receptors as is discussed below for the human receptor.
Figure 5.
Alignment of the primary amino acid sequences of the tP2Y1 receptor with the hP2Y1, hP2Y2, hP2Y4, hP2Y6, receptors. Identity with amino acid residues in the turkey sequence are shaded.
CLONING OF THE HUMAN P2Y1 RECEPTOR
Since sequences for only avian P2Y1 receptors were available, we also cloned the human homolog of this P2Y receptor from a human genomic library [Schachter et al., 1996]. The overall homology between the human sequence and that of the tP2Y1 receptor is 74% at the nucleotide level and 82% at the amino acid level (Fig. 5). The hP2Y1 receptor gene, as well as the tP2Y1 receptor gene, is intronless. The P2Y1 receptor is 38%, 35%, and 40% identical to the hP2Y2, hP2Y4, and rP2Y6 receptors, respectively (Fig. 5). The human P2Y1 receptor also was stably expressed in 1321N1 cells using a retrovirus vector. 2MeSATP promoted marked stimulation of inositol phosphate accumulation in cells expressing the human P2Y1 receptor. The pharmacological selectivity of eighteen agonists was identical to that observed for the tP2Y1 receptor stably expressed in 1321N1 cells with the retrovirus. In contrast to C6 rat glioma cells, which express an endogenous P2Y receptor that inhibits AC but does not activate PLC, 1321N1 cells expressing the recombinant hP2Y1 receptor exhibited no P2Y receptor agonist-promoted decreases in forskolin- or isoproterenol-stimulated cyclic AMP levels. To confirm that these differences in signaling specificity were due to two distinct P2Y receptors and were not a result of the different cells in which the P2Y receptor were expressed, the hP2Y1 receptor also was stably expressed in C6 glioma cells and was shown to confer 2MeSATP-stimulated inositol lipid hydrolysis to these cells. The PLC-activating hP2Y1 receptor could be distinguished from the endogenous AC-coupled P2Y receptor of C6 glioma cells using the P2 receptor antagonist PPADS, which as described above blocks the P2Y1 receptor but does not block the endogenous P2Y receptor of C6 cells [Boyer et al., 1994]. That is, PPADS shifted the concentration effect curve of 2MeSATP to the right in assays of inositol phosphate accumulation, but had no effect on 2MeSATP-promoted inhibition of cyclic AMP accumulation. P2-R agonists also exhibited differential selectivities for activation of these two P2Y receptors. These results confirm that the Gi/AC-linked P2Y receptor of C6 glioma cells is a different receptor than the P2Y1 receptor.
Not only is the antagonist activity of PPADS similar between the hP2Y1 and tP2Y1 receptors, but the 3’-phosphate derivatives discovered as partial agonists/competitive antagonists at the P2Y receptor of turkey erythrocyte membranes are strictly competitive antagonists with no partial agonist activity at the hP2Y1 receptor [Boyer et al., 1996]. These novel competitive antagonists are highly selective for the P2Y1 receptor since they do not block the P2Y2 receptor of C6 glioma cells or the P2Y2, P2Y4, or P2Y6 receptors expressed in 1321N1 cells. Identification of this antagonist activity for the first time in an adenine nucleotide molecule places us in position to use the large foundation of structure activity information that we have generated to date with P2Y1 receptor agonists to generate potent selective P2Y receptor antagonists.
We have constructed a recombinant baculovirus harboring the hP2Y1 receptor coding sequence with modifications designed to enhance expression levels and to facilitate purification. Nucleotide sequences encoding a cleavable signal sequence from influenza hemagglutinin and the flag epitope (DYKDDDD) have been incorporated at the 5’-end, and sequence encoding a hexahistidine tag has been placed at the 3’ end of the coding sequence of hP2Y1 receptors. Conditions for high level expression in Sf9 insect cells are being established with the goal of producing large amounts of homogeneous recombinant receptor. This epitope-tagged construct of the hP2Y1 receptor has been stably expressed in 1321N1 cells and shown to exhibit pharmacological and second messenger signaling properties indistinguishable from the native P2Y1 receptor. The long-term goal of this research is to obtain large amounts of purified P2Y1 receptor to apply in reconstitution studies with G proteins and effector enzymes and in studies of the regulation of P2Y1 receptor function by phosphorylation by second messenger-activated and G protein receptor protein kinases.
REFERENCES
- Berrie CP, Hawkins PT, Stephens LR, Harden TK, Downes CP (1989): Phosphatidylinositol 4,5-bisphosphate hydrolysis in turkey erythrocytes is regulated by P2Y-purinoceptors. Mol Pharmacol 35:526–532. [PubMed] [Google Scholar]
- Boyer JL, Downes CP, Harden TK (1989) Kinetics of activation of phospholipase C by P2-purinergic receptor agonists and guanine nucleotides. J Biol Chem 264:884–890. [PubMed] [Google Scholar]
- Boyer JL, Cooper CL, Harden TK (1990): Photoaffinity labelling of the P2Y–purinergic receptor by [32P]3′-0-4-benzoyl)benzoyl ATP J Biol Chem 265:13515–13520. [PubMed] [Google Scholar]
- Boyer JL, Lazarowski ER, Chen X-H, Harden TK (1993): Identification of a P2Y-purinergie receptor that inhibits adenylyl cyclase but does not activate phospholipase C. J Pharmacol Exp Ther 267:1140–1146. [PubMed] [Google Scholar]
- Boyer JL, O’Tuel JVV, Fischer B, Jacobson KA, Harden TK (1995): 2–Thioether derivatives of adenine nucleotides are exceptionally potent agonists at adenylyl cyclase-linked P2Y-purinergie receptors. Br J Pharmacol 116:2611–2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer JL, Schachter JL, Romero T, Harden TK (1996): Identification of competitive antagonists of the P2Y1-purinergic receptor. Mol Pharmacol 50:1323–1329. [PubMed] [Google Scholar]
- Boyer JL, Siddiqi SM, Fischer B, Jacobson KA, Harden TK (1996): Analogs of adenosine monophosphate that are potent P2Y-purinergic receptor agonists. Br J Pharmacol 118:1959–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer JL, Zohn I, Jacobson KA, Harden TK (1994): Differential effects of putative P2-purinergic receptor antagonists on adenylyl cyclase- and phospholipase C-coupled P2Y-purinergie receptors. Br J Pharmacol 113:614–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G, Fischer B, Hoyle CHV, Maillard M, Ziganshin AU, Brizzolara AL, von Isakovics A, Boyer JL, Harden TK, Jacobson KA (1994): Structure activity relationships for derivatives of adenosine-5′-triphosphate as agonists at P2-purinoeeptors: heterogeneity within P2X and P2Y subtypes. Drug Dev Res 31:206–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G, Kennedy C (1985): Is there a basis for distinguishing two types of P2-purinoceptors? Gen Pharmacol 16:433–440. [DOI] [PubMed] [Google Scholar]
- Chang K, Hanaoka K, Kumada M, Takuwa Y (1995): Molecular cloning and functional analysis of a novel P2 nucleotide receptor. J Biol Chem 270:26152–26158. [DOI] [PubMed] [Google Scholar]
- Communi D, Pirotton S, Parmentier M, Boeynaems JM (1995): Cloning and functional expression of a human uridine nucleotide receptor. J Biol Chem 270:30849–30852. [DOI] [PubMed] [Google Scholar]
- Cooper CL, Morris AJ, Harden TK (1989): Guanine nucleotide-sensitive interaction of a radiolabeled agonist with a phospholipase C-unked P2-purinergic receptor. J Biol Chem 264:6202–6206. [PubMed] [Google Scholar]
- Filtz TM, Li Q, Boyer JL, Nicholas RA, Harden TK (1994): Expression of a cloned P2Y-purinergie receptor that couples to phospholipase C. Mol Pharmacol 46:8–14. [PubMed] [Google Scholar]
- Fischer B, Boyer JL, Hoyle CHV, Ziganshin AU, Brizzolara AL, Knight GE, Zimmet J, Burnstock G, Harden TK, Jacobson KA (1993): Identification of potent, selective P2Y-purinoceptor agonists: structure activity relationships for 2-thioether derivatives of adenosine-5’-triphosphate. J Med Chem 36:3937–3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden TK, Hawkins PT, Stephens L, Boyer JL, Downes CP (1988): Phosphoinositide hydrolysis by guanosine 5′-0-(3-thiotriphosphate)-activated phospholipase C of turkey erythrocyte membranes. Biochem J 252:583–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden TK, Nicholas RA, Schachter JL, Lazarowski ER, Boyer JL (1997): Pharmacological selectivities of molecularly defined subtypes of the P2Y receptor class of G protein-coupled signaling proteins. (In) The P2-Purinergic Receptors, ed by Weisman CA, Turner J, and Fedan J, Humana Press, in press. [Google Scholar]
- Harden TK, Stephens L, Hawkins PT, Downes CP (1987): Turkey erythrocyte membranes as a model for regulation of phospholipase C by guanine nucleotides. J Biol Chem 262:9057–9061. [PubMed] [Google Scholar]
- Lustig KD, Shiau AK, Brake AJ, Julius D (1993): Expression cloning of an ATP receptor from mouse neuroblastoma cells. Proc Natl Acad Sci USA 90:5113–5117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice DH, Waldo GL, Morris AJ, Nicholas RA, Harden TK (1993): Identification of G 11 as the phospholipase C-activating G-protein of turkey erythrocytes. Bioehem J 290:765–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AJ, Waldo GL, Downes CP, Harden TK (1990): A receptor and G-protein-regulated polyphosphoinositide specific phospholipase C. 2. P2Y-purinergic receptor and G-protein-mediated regulation of the purified enzyme. J Biol Chem 265:13508–13514. [PubMed] [Google Scholar]
- Nguyen T, Erb L, Weismann GA, Marchese A, Heng HHQ, Garrad RC, George SR, Turner JT, O’Dowd BF (1995): Cloning, expression, and chromosomal localization of the human uridine nucleotide receptor gene. J Biol Chem 270:30845–30848. [DOI] [PubMed] [Google Scholar]
- Nicholas RA, Watt WC, Lazarowski ER, Li Q, Harden TK (1996): Uridine nucleotide selectivity of three phospholipase C-activating P2 receptors: Identification of a UDP-selective, a UTP-selective, and an ATP- and UTP-specific receptor. Mol Pharmacol 50:224–229. [PubMed] [Google Scholar]
- North RA (1996): P2X purinoceptor plethora. Sem in Neurosci (In Press). [Google Scholar]
- Offermanns S, Simon MI (1995): G 15 and G 16 couple a wide variety of receptors to phospholipase C. J Biol Chem 270:15175–15180. [DOI] [PubMed] [Google Scholar]
- Rooney TA, Hager R, Thomas AP (1991): β-adrenergic receptor-mediated phospholipase C activation independent of cAMP formation in turkey erythrocyte membranes. J Biol Chem 266:15068–15074. [PubMed] [Google Scholar]
- Schachter J, Nicholas RA, Harden TK (1996): Molecular cloning, pharmacological selectivity, and signalling properties of a human phospholipase C-coupled P2Y-purinergic receptor. Br J Pharmacol 118:167–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon J, Webb TE, King BE Burnstock G, Barnard EA (1995): Characterization of a recombinant P2Y purinoceptor. Eur J Pharmacol 291:281–289. [DOI] [PubMed] [Google Scholar]
- Surprenant A, Buell G, North RA (1995): P2X receptors bring new structure to ligand-gated ion channels. TINS 18:224–229. [DOI] [PubMed] [Google Scholar]
- Vaziri C, Downes CP (1992): G-protein-mediated activation of turkey erythrocyte phospholipase C by β-adrenergic and P2Y-purinergic receptors. Biochem J 284:917–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldo GL, Boyer JL, Morris AJ, Harden TK (1991): Purification of an AIF4- and G-protein βγ-subunit-regulated phospholipase C-activating protein. J Biol Chem 261:14217–14225. [PubMed] [Google Scholar]
- Waldo GL, Nicholas RA, Harden TK (1996): Molecular cloning and expression of a phospholipase β isoenzyme from avian erythrocytes. Biochem J 316:559–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb TE, Kaplan MG, Barnard EA (1996): Identification of 6H1 as a P2Y purinoceptor: P2Y5. Biochem Biophys Res Commun 219:105–110. [DOI] [PubMed] [Google Scholar]
- Webb TE, Simon J, Krishek BJ, Bateson AN, Smart TG, King BF, Burnstock G, Barnard EA (1993): Cloning and functional expression of a brain G-protein-coupled ATP receptor. FEBS Lett 324:219–225. [DOI] [PubMed] [Google Scholar]