Abstract
Closed incision negative pressure therapy (ciNPT) system use compared with standard of care dressings (SOC) on surgical site infection (SSI) in cardiac surgery was assessed. A systematic literature review was conducted. Risk ratios (RR) and random effects models were used to assess ciNPT with foam dressing (ciNPT‐F) or multilayer absorbent dressing (ciNPT‐MLA) versus SOC. Health economic models were developed to assess potential per patient cost savings. Eight studies were included in the ciNPT‐F analysis and four studies were included in the ciNPT‐MLA analysis. For ciNPT‐F, a significant reduction in SSI incidence was observed (RR: 0.507, 95% confidence interval [CI]: 0.362, 0.709; p < 0.001). High‐risk study analysis reported significant SSI reduction with ciNPT‐F use (RR: 0.390, 95% CI: 0.205, 0.741; p = 0.004). For ciNPT‐MLA, no significant difference in SSI rates were reported (RR: 0.672, 95% CI: 0.276, 1.635; p = 0.381). Health economic modelling estimated a per patient cost savings of $554 for all patients and $3242 for the high‐risk population with ciNPT‐F use. Health economic modelling suggests ciNPT‐F may provide a cost‐effective solution for sternotomy incision management. However, limited high‐quality literature exists. More high‐quality evidence is needed to fully assess the impact of ciNPT use following cardiac surgery.
Keywords: cardiac surgery, meta‐analysis, negative pressure, surgical site infection
1. INTRODUCTION
Cardiac surgery is associated with a high risk of developing postoperative infections. Reported rates of surgical site infection (SSI) incidence in cardiac surgery have ranged between 0.5% and 25% with mortality rates due to SSIs reaching 25%. 1 , 2 , 3 , 4 More recently, overall rates of SSI development after cardiac surgery have been estimated at 1.7% to 1.9% in the United States based on a retrospective review of the Medicare Limited Data Set and the Premier Healthcare database. 5
Risk factors for SSI development in cardiac surgery include high European System for Cardiac Operative Risk Evaluation (EuroSCORE), high Society of Thoracic Surgeons (STS) score, obesity, diabetes, tobacco use, advanced age (>70 years), chronic obstructive pulmonary disease, heart failure with left ventricular dysfunction, renal failure, female sex, peripheral vascular disease and prolonged length of hospital stay prior to surgery. 1 , 6 , 7 , 8 , 9 Additionally, emergency surgery, use of bilateral internal mammary arteries, multiple surgery procedures, prolonged surgery duration, duration of cardiopulmonary bypass, aortic clamping, postoperative respiratory failure, transfusion, prolonged intensive care unit stay and use of inotropic support can also increase the risk of SSI development. 1 , 8
Standard SSI prevention measures (such as blood glucose optimization, tobacco use cessation, prophylactic antibiotic use, nasal and total body bacterial decolonization and sterile surgical field) are commonly utilized before and during surgery 10 ; however, closed incision negative pressure therapy (ciNPT) can offer clinicians a post‐surgical tool to help manage the closed surgical incision. ciNPT helps hold the incision edges together, reduces lateral tension and provides a barrier against external contamination over an extended time period. ciNPT use has been reported to improve patient outcomes following abdominal, cardiac, orthopaedic, vascular and thoracic procedures. 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 Multiple ciNPT systems are available; though not all devices are the same (differences in negative pressure amount, dressing type, power source, dysfunction alarms and use of a canister for exudate collection). This systematic review and meta‐analysis compared the impact of ciNPT use on SSI development following cardiac surgery between two different ciNPT systems (ciNPT‐F [3M™ Prevena™ Incision Management System, 3M Health Care, St. Paul, MN, USA] and ciNPT‐MLA [PICO™ Single Use Negative Pressure Wound Therapy System, Smith + Nephew, London, UK]) versus standard of care (SOC) dressings.
2. METHODS
This work conformed to the statement and reporting checklist of the preferred reporting items for systematic reviews and meta‐analysis. 20 The systematic review and meta‐analyses were conducted to compare the potential to mitigate SSI in cardiac surgery between two ciNPT systems (Prevena™ Incision Management System, 3M Health Care; and PICO™ Single Use Negative Pressure Wound Therapy System, Smith + Nephew) and SOC dressings, which included gauze dressings, waterproof dressings with absorbent pad and bacterial barrier and sterile post‐surgical dressings. Overall SSI outcomes were assessed due to limited reporting on deep or superficial SSI rates. The systematic literature search was performed according to valid standardized protocols and was not registered. Data utilized in this meta‐analysis were extracted from previously published articles and are publicly available through PubMed, EMBASE, QUOSA or Medline.
2.1. Literature search
A systematic literature search using PubMed, EMBASE, QUOSA and Medline was performed. Literature published between 1 January 2005 and 15 June 2022 were examined. Two separate literature searches were conducted, one for ciNPT‐F and one for ciNPT‐MLA. The ciNPT‐F literature search utilized the following search terms: ‘negative pressure wound therapy’ OR ‘negative pressure’ OR ‘negative pressure therapy’ OR ‘NPWT’) AND (‘Prevena’ OR ‘ciNPT’ OR ‘prophylactic NPWT’ OR ‘preventive NPWT’ OR ‘incision management’ OR ‘incisional management’ OR ‘closed incision negative pressure wound therapy’ OR ‘closed incision negative pressure therapy’). The ciNPT‐MLA literature search utilized the following search terms: ‘negative pressure wound therapy’ OR NPWT, ‘negative pressure’ OR ‘negative therapy’ OR ‘topical negative pressure’ AND ‘PICO’ OR ‘ciNPT’ OR ‘Prophylactic npwt’ OR ‘Prophylactic negative pressure’ OR ‘preventative npwt’ OR ‘preventative negative pressure’ OR ‘incisional npwt’, ‘incisional negative pressure’ AND ‘surgical’ OR ‘surgery’.
2.2. Meta‐analysis inclusion/exclusion criteria
Study inclusion criteria consisted of English language peer‐reviewed manuscripts or published abstracts reporting on SSI in patients >18 years with closed surgical incisions following cardiac surgery with ciNPT‐F or ciNPT‐MLA applied post‐operatively compared to SOC dressings or any non‐NPWT dressing. Study types considered for analysis were randomized controlled trials (RCTs), prospective observational studies, prospective cohort compared to a historical cohort and retrospective comparative studies.
Study exclusion criteria consisted of unpublished, non‐peer‐reviewed studies or posters in a non‐English language, reporting on patients with open surgical incisions, non‐surgical wounds, or patient populations <18 years of age. Studies with non‐ciNPT negative pressure therapy, no mention of ciNPT product type or mixed used of negative pressure wound therapy were also excluded. Studies that did not report on SSI outcomes were excluded. Pre‐clinical studies, case studies, case series, reviews and meta‐analyses were also excluded.
2.3. Study selection
Titles and abstracts of publications identified in the databases were logged. After removing all duplicates, titles and abstracts of each paper were read to assess eligibility. Remaining publications were read to ensure all inclusion criteria and no exclusion criteria were met. References from identified publications were also reviewed for relevant studies that fit the inclusion criteria.
2.4. Data collection
One reviewer completed data extraction from all eligible studies and a second reviewer validated the findings. Disagreements were resolved by discussion between the two reviewers or by the addition of a third reviewer. The following data were extracted from each study: funding source, bias assessments, study design, publication status, study date range, number of study sites, study location, surgical procedures, high‐risk enrolment criteria (if applicable), study objectives, control type, number of treatment days, follow‐up period, number of patients/incisions, number of patients/incisions analysed and definition of SSI. Overall population health status reported as health scorings (such as EuroSCORE, American Society of Anaesthesiologists [ASA] score and Society of Thoracic Surgeons [STS] score) were also collected. The study endpoint evaluated was overall SSI. Due to the mix of prospective, retrospective and randomized controlled trials (RCTs) included in the meta‐analysis, the risk of bias assessment was not considered necessary.
2.4.1. High‐risk population
Risk factors identified by the included studies for high‐risk patients included advanced age, sex, body mass index (BMI) ≥ 30 kg/m3, BMI ≤ 18 kg/m2, diabetes, chronic obstructive pulmonary disease, tobacco use, chest radiation therapy, chronic renal failure, peripheral arterial disease and bilateral internal mammary artery grafting.
The high‐risk study population for the sub‐group analysis was defined by the published studies. Three studies identified high‐risk patients as individuals with two or more risk factors for SSI development. 15 , 21 , 22 One study identified patients with a body mass index (BMI) ≥ 30 kg/m2 as high‐risk for SSI development. 18 The remaining study defined the high‐risk patient population as patients having a BMI ≥ 30 kg/m2 or a presence of diabetes. 23 Potential bias related to baseline population risk factors was collected during data extraction. For the meta‐analysis, only the high‐risk statement from the published article was utilized with no further risk factor standardization.
2.5. Statistical analysis
Meta‐analyses were performed using risk ratios and random effects models. Weighted risk ratios and 95% confidence intervals (CI) were calculated to pool study and control groups in each publication for analysis. I 2 was utilized to assess data heterogeneity. Treatment effects were combined, and a random effects model was used for each analysis performed, regardless of the heterogeneity assessment. When the analyses of ciNPT‐F and ciNPT‐MLA had similar statistical outcomes, prediction intervals were used to assess the levels of difference in the true effects. If it was assumed that the true effects were normally distributed, tau‐squared (an estimate of the between study variance in random effects models) was used to determine the prediction interval. If tau‐squared was estimated as zero, the assumption was that all studies shared a common effect size, and no separate prediction interval was reported. A subgroup analysis for patients at high‐risk for SSI was also conducted. Sensitivity analyses using the one study removed method were utilized. All analyses were performed using Comprehensive Meta‐Analysis Version 3 (Borenstein M, Hedges L, Higgins J, & Rothstein H. Biostat Englewood, MJ, 2013).
2.6. Health economic modelling
Health economic models were created to examine the potential impact of ciNPT usage on cost of care for both the all‐patient and high‐risk patient subgroups. The overall baseline SSI rate was calculated as the number of SSI divided by the total number of incisions from the SOC dressing group across all ciNPT‐F studies included in the meta‐analysis multiplied by 100. The SSI reduction rate for ciNPT‐F from the meta‐analyses were utilized for the relative SSI risk reduction rate. The SSI rate was calculated by multiplying the base SSI rate with the reduction rates from the meta‐analysis and subtracting the results from the base SSI rate. The average cost per SSI was obtained from the Premier Healthcare Database of deidentified clinical and cost data published by Hou et al. 5 Here, the average overall SSI cost was utilized as the health economic benchmark to assess the potential cost‐effectiveness of ciNPT use on overall incidence of SSI. Total cost per SSI was calculated as the SSI rate with ciNPT multiplied by the average mean cost of SSI. ciNPT device cost was obtained from the list price of the device. Total cost per patient was calculated as total cost for SSI per patient plus the device cost. Mean per patient savings was calculated as the total cost per patients for SOC minus total cost per patient for ciNPT‐F.
3. RESULTS
3.1. Literature search
Two separate literature searches were conducted. For ciNPT‐F, a total of 1377 articles were identified (Figure 1). After duplicates and excluded studies were removed, eight studies were included in the systematic review (2 prospective studies, 1 prospective study with retrospective control and 5 retrospective comparative studies, Table 1). 15 , 18 , 19 , 24 , 26 , 27 , 28 , 29 Two of the included studies identified the patient population as high risk for development of SSI (1 prospective study and 1 retrospective study). 15 , 18 A total of 1451 patients received ciNPT‐F and 4712 received SOC. The baseline overall SSI risk for the SOC dressing group ranged from 3% to 16% for the total ciNPT‐F study population and between 12% and 16% for the high‐risk population.
FIGURE 1.

PRISMA flow diagram of closed incision negative pressure therapy with foam dressing (ciNPT‐F) article selection for inclusion into the meta‐analysis.
TABLE 1.
Summary of ciNPT studies.
| Study | Study design | Study location | Surgery type | SSI definition | Number of patients | SSI incidence | SSSI incidence | DSSI incidence | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ciNPT | SOC | ciNPT | SOC | ciNPT | SOC | ciNPT | SOC | |||||
| Fauvel et al. 24 | R | US | Placement of cardiac left ventricular assist device | INTERMACS 2016 definition for drive line infection | 18 | 22 | 1 | 3 | NR | NR | NR | NR |
| Grauhan et al. 18 | P | Germany | CABG with LITA; valve repair; other | US CDC and Oakley and Wright 25 classification for sternotomy wound healing abnormalities | 75 | 75 | 3 | 12 | 2 | 8 | 1 | 4 |
| Grauhan et al. 19 | P, RC | Germany | Cardiac surgery patients undergoing median sternotomy | Wound infections requiring surgical revision | 237 | 3508 | 3 | 119 | 1 | NR | NR | NR |
| Nguyen et al. 26 | R | US | CABG, aortic or mitral valve repair/replacement; lung transplant; ventricular assist device; Ross procedure | NR | 706 | 493 | 21 | 31 | NR | NR | NR | NR |
| Richter et a 27 , a | R | Germany | Median sternotomy, majority CABG, followed by combined and double valve procedure | NR | 108 | 216 | NR | NR | NR | NR | 3 | 9 |
| Ruggieri et al. 28 , a | P | France | CABG with BIMA grafts | US CDC | 128 | 128 | 13 | 14 | 6 | 1 | 2 | 3 |
| Santarpino et al. 29 | R | Germany | Myocardial revascularization with BITA or ITA with vein grafts | NR | 21 | 108 | 0 | 6 | 0 | 0 | 0 | 6 |
| Suelo‐Calanao et al. 15 , b | R | UK | CABG; valve repair/replacement; CABD with valve repair/replacement; other | NR | 158 | 162 | 9 | 20 | NR | NR | NR | NR |
| ciNP‐F total | 1451 | 4712 | 53 | 214 | ||||||||
| Elmistekawy et al. 21 | P, RC | Canada | CABG | NR | 31 | 31 | 5 | 5 | NR | NR | NR | NR |
| Myllykangas et al. 23 , a | P, RC | Finland | CABG | US CDC | 174 | 174 | 18 | 12 | NR | NR | 7 | 6 |
| Tabley et al. 22 | R | France | Valve repair/replacement; IMA bypass; valve repair/replacement with bypass; Ross procedure; transplant; Bentall procedure | NR | 142 | 91 | 8 | 13 | 3 | 3 | 5 | 10 |
| Witt‐Majchrzak et al. 30 | RCT | Poland | CABG with IMA | European CDC and El Oakley and Wright 25 classification to categorize sternotomy wound healing abnormalities | 40 | 40 | 1 | 7 | 1 | 7 | 0 | 0 |
| ciNPT‐MLA total | 387 | 336 | 32 | 37 | ||||||||
Abbreviations: BIMA, bilateral internal mammary artery graft; BITA, bilateral internal thoracic artery; BMI, body mass index; CABG, coronary artery bypass grafting; CDC, center for disease control; ciNPT‐F, closed incision negative pressure therapy with foam dressing; ciNPT‐MLA, closed incision negative pressure therapy with multilayer absorbent dressing; COPD, chronic obstructive pulmonary disease; DSSI, deep surgical site infection; IMA, internal mammary artery; ITA, internal thoracic artery; LITA, left internal thoracic artery; NA, not applicable; NR, not reported; P, prospective study; PAD, peripheral arterial disease; R, retrospective comparative study; RC, retrospective control; SOC, standard of care; SSI, surgical site infection; SSSI, superficial surgical site infection; SWI, sternal wound infection; UK, United Kingdom; US, United States.
Propensity matched cohort used of assessment.
High‐risk cohort.
For ciNPT‐MLA, a total of 1229 articles were identified (Figure 2). After duplicates and excluded studies were removed, four studies were included in the meta‐analysis (1 RCT, 2 prospective with historical controls and 1 retrospective comparative study, Table 1). 21 , 22 , 23 , 30 Three studies identified the population as high‐risk for SSI development (2 prospective studies with retrospective control and 1 retrospective study). 21 , 22 , 23 A total of 387 patients received ciNPT‐MLA and 336 patients received SOC dressings. The baseline overall SSI risk for the SOC dressing group ranged from 7% to 18% throughout all four studies for the total patient population, while the baseline SSI risk ranged from 7% to 16% for the high‐risk study population.
FIGURE 2.

PRISMA flow diagram of ciNPT‐MLA article selection for inclusion into the meta‐analysis. ciNPT‐F, closed incision negative pressure therapy with foam dressing.
3.2. Study population profile
3.2.1. ciNPT‐F study population
Potential bias in ciNPT‐F study population risk factors was assessed for all studies (Table 2). Two studies reported significant differences in baseline patient demographics between the ciNPT‐F and SOC dressing groups. 26 , 29 Nguyen et al reported a significantly older ciNPT‐F patient population with high rates of hyperlipidaemia and antihypertensive medication and statin use. 26 Additionally, the ciNPT‐F population reported reduced use of steroids and rates of emergency procedures. 26 Conversely, Santarpino et al reported a significantly younger ciNPT‐F group compared to the SOC dressing group (55.9 ± 7.6 years vs. 60.8 ± 10.8 years). 29 Baseline population bias in risk factors was not reported in three studies. 19 , 24 , 27 The remaining three studies reported no differences between the ciNPT‐F and SOC dressing group populations. 15 , 18 , 28 For two studies with a high‐risk patient population, no differences were reported between the ciNPT‐F and SOC dressing populations at baseline. 15 , 18 The remaining study did not report baseline population characteristics. 27
TABLE 2.
Baseline patient profile for ciNPT studies.
| Study | Surgery type | High risk population | ASA risk score | EuroScore | STS score | |||
|---|---|---|---|---|---|---|---|---|
| ciNPT | SOC | ciNPT | SOC | ciNPT | SOC | |||
| Fauvel D et al. 24 | Placement of cardiac left ventricular assist device | NR | NR | NR | NR | NR | NR | NR |
| Grauhan et al. 18 | CABG with LITA; valve repair; other | BMI > 30 kg/m2 | NR | NR | NR | NR | NR | NR |
| Grauhan et al. 19 | Cardiac surgery patients undergoing median sternotomy | NA | NR | NR | NR | NR | NR | NR |
| Nguyen et al. 26 | CABG, aortic or mitral valve repair/replacement; lung transplant; ventricular assist device; Ross procedure | NA |
2: 0.6% 3: 18.8% 4:77.6% 5: 1.6% |
2: 0.4 3: 14.8 4: 82.2% 5: 0.8 |
NR | NR | NR | NR |
| Richter et al. 27 , a | Sternotomy with CABG surgery in 77% of ciNPT population | Defined by surgeon | NR | NR | NR | NR | NR | NR |
| Ruggieri et al. 28 | CABG with BIMA grafts | NA | NR | NR | 3.1 ± 1.4 | 3.2 ± 1.4 | 1.4 ± 1.2 | 1.5 ± 1.2 |
| Santarpino et al. 29 | Myocardial revascularization with BITA or ITA with vein grafts | NA | NR | NR | 2.0 ± 2.3 | 3.8 ± 2.8 | NR | NR |
| Suelo‐Calanao et al. 15 | CABG; valve repair/replacement; CABD with valve repair/replacement; other | Risk factors ≥2: BMI > 32 kg/m2; age >80 years; COPD; diabetes | NR | NR | 10.9 ± 12.8 | 9.1 ± 12.4 | NR | NR |
| Elmistekawy et al. 21 | CABG | Risk factors ≥2 for SWI | NR | NR | NR | NR | NR | NR |
| Myllykangas et al. 23 , a | CABG | BMI ≥ 31 kg/m2; diabetes | NR | NR | 3.2 ± 4.3 | 3.8 ± 5.7 | NR | NR |
| Tabley et al. 22 | Valve repair/replacement; IMA bypass; valve repair/replacement with bypass; Ross procedure; transplant; Bentall procedure | Risk factors ≥2 for SWI: BIMA; COPD; chest radiation therapy; chronic renal failure; diabetes; ejection fraction <40%; BMI < 18.5 kg/m2; BMI > 35 kg/m2; PAD; tobacco use | NR | NR | 6.10 | 5.53 | NR | NR |
| Witt‐Majchrzak et al. 30 | CABG with IMA | NA | NR | NR | 1.4 ± 0.9 | 1.4 ± 1.3 | NR | NR |
Abbreviations: ASA score, American society of Anaesthesiologists score; BIMA, bilateral internal mammary artery graft; BMI, body mass index; BITA, bilateral internal thoracic artery; CABG, coronary artery bypass graft; CDC, center for disease control; ciNPT‐F, closed incision negative pressure therapy with foam dressing; ciNPT‐MLA, closed incision negative pressure therapy with multilayer absorbent dressing; COPD, chronic obstructive pulmonary disease; IMA, internal mammary artery; ITA, internal thoracic artery; LITA, left internal thoracic artery; NA, not applicable; NR, not reported; SOC, standard of care; SSI, surgical site infection; STS score, Society of Thoracic Surgeons score; SWI, sternal wound infection.
Propensity matched groups used for assessment.
Patient risk scores were reported in four studies (Table 2). 15 , 26 , 28 , 29 EuroScores ranged between 2.0 and 10.9 for ciNPT‐F and 2.0 and 9.1 for the SOC populations. 15 , 28 , 29 ASA scores ≥3 were reported for 99% of the study population, with an ASA ≥ 4 reported in more than 80% of the study population in the Nguyen et al study. 26 Ruggieri et al., the only study to report an STS score, reported similar scores between the ciNPT‐F and SOC dressing group (Table 2). 28 Only one ciNPT‐F high‐risk population study reported a EuroScore (10.9 for the ciNPT‐F group and 9.1 for the SOC dressing group). 15
3.2.2. ciNPT‐MLA study population
ciNPT‐MLA study population risk factors were also assessed (Table 2). Two studies reported significant differences between ciNPT‐MLA and SOC dressing groups. 22 , 30 One study reported a higher number of risk factors for SSI development including BMI ≥ 35 kg/m2 and a higher rate of patients undergoing a bilateral internal mammary artery graft in the ciNPT‐MLA group compared to SOC. 22 In the remaining study, the ciNPT‐MLA population was older than the SOC group. 30 No significant differences between ciNPT‐MLA and SOC populations were reported after propensity score matching in two studies. 21 , 23 For the three studies with a high‐risk patient population, one reported a significant difference between the ciNPT‐MLA and SOC dressing groups. 22 Tabley et al reported a higher number of risk factors including BMI ≥ 35 kg/m2 and a higher rate of patients undergoing a bilateral internal mammary artery graft procedures in the ciNPT‐MLA group. 22
Patient risk scores were reported in three studies, ranging from 1.4 to 6.1 in the ciNPT‐MLA group and 1.4 to 5.53 in the SOC dressing group (Table 2). 22 , 23 , 30 The EuroScore, reported in two studies for the high‐risk population, ranged from 3.2 to 6.1 in the ciNPT‐MLA group and 2.8 to 5.53 in the SOC dressing group. 22 , 23
3.3. SSI risk outcome assessment
3.3.1. All patient subgroup
SSI risk was assessed in seven studies for ciNPT‐F, resulting in a weighted risk ratio of 0.507 (95% CI: 0.362, 0.709; p < 0.001), indicating a significant reduction in the SSI rates with ciNPT‐F compared to SOC (Figure 3A, Table 3). A low level of heterogeneity was observed for the ciNPT‐F studies (I 2 = 0). Thus, the tau‐squared was also estimated as zero indicating no dispersion of true effects.
FIGURE 3.
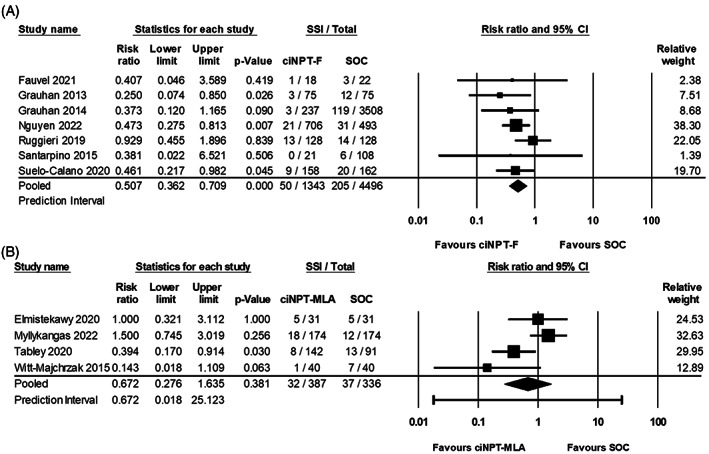
Forest plot of surgical site infections. (A) ciNPT‐F forest plot; (B) ciNPT‐MLA forest plot; Risk ratio, lower limit, upper limit, surgical site infection total, relative weight and prediction interval are shown.
TABLE 3.
Summary of overall SSI meta‐analyses.
| Subgroup analysis | Studies | Risk ratio (LL, UL) | I 2 | p‐Value | Relative risk reduction (%) |
|---|---|---|---|---|---|
| All ciNPT‐F | 7 | 0.507 (0.362, 0.709) | 0.000 | <0.001 | 49.3 |
| High‐risk ciNPT‐F | 2 | 0.390 (0.205, 0.741) | 0.000 | <0.004 | 61.0 |
| All ciNPT‐MLA | 4 | 0.672 (0.276, 1.635) | 65.457 | 0.381 | 32.8 |
| High‐risk ciNPT‐MLA | 3 | 0.848 (0.359, 2.002) | 65.410 | 0.706 | 15.2 |
Note: p‐Values in bold represent statistically significant values.
Abbreviations: ciNPT‐F, closed incision negative pressure therapy with foam dressing; ciNPT‐MLA, closed incision negative pressure therapy with multilayer absorbent dressing; LL, lower limit; UL, upper limit.
For ciNPT‐MLA, four studies were assessed, resulting in a weighted risk ratio of 0.672 (95% CI: 0.276, 1.635; p = 0.381), indicating similar SSI rates between ciNPT‐MLA and SOC (Figure 3B, Table 3). A high level of heterogeneity was observed for the ciNPT‐MLA studies (I 2 > 65). With a tau‐squared of 0.503, the predication interval was 0.018–25.123, where the true effect size in 95% of all comparable populations would fall within this interval. A sensitivity analysis was performed; however, the meta‐analysis results remained non‐significant.
The relative risk reductions for SSI were compared with ciNPT‐F use associated with a 49.3% reduced risk of SSI compared to SOC, while no significant differences were observed between ciNPT‐MLA and SOC (Table 3).
3.4. High‐risk patient subgroup
SSI risk was assessed for patients at high‐risk of developing SSI in two studies for ciNPT‐F, resulting in a weighted risk ratio of 0.390 (95% CI: 0.205, 0.741; p = 0.004), indicating a significant reduction in the SSI rates with ciNPT‐F compared to SOC (Figure 4A, Table 3). A low level of heterogeneity was observed between these studies (I 2 = 0). The tau‐squared was also estimated as zero indicating that there was no dispersion of true effects.
FIGURE 4.

Forest plot of surgical site infections in patient population at high‐risk for surgical site infection. (A) ciNPT‐F forest plot; (B) ciNPT‐MLA forest plot; Risk ratio, lower limit, upper limit, surgical site infection total, relative weight and prediction interval are shown.
For ciNPT‐MLA, SSI risk was assessed for patients at high‐risk of developing SSI in three studies, resulting in a weighted risk ratio of 0.848 (95% CI: 0.359, 2.002; p = 0.706), indicating similar SSI rates between ciNPT‐MLA and SOC (Figure 4B, Table 3). There was a high level of heterogeneity between the ciNPT‐MLA studies (I 2 > 65). A 0.374 tau‐squared resulted in a predication interval of 0.000 and 12036.617. Thus, the true effect size in 95% of all comparable populations would fall within this interval. The meta‐analysis results remained non‐significant after the sensitivity analysis.
The relative risk reductions for SSI in the high‐risk subgroup analysis were compared, ciNPT‐F use was associated with a 61.0% reduced risk of SSI compared to SOC, while no significant differences were observed between ciNPT‐MLA and SOC SSI rates.
3.4.1. Health economic model
A health economic model was created to estimate the potential per patient cost savings with use of ciNPT‐F following cardiac surgery (Table 4). A baseline overall SSI rate of 4.6% from the SOC dressing groups across all seven ciNPT‐F studies was used. Relative reduction rate was taken from the all‐patient subgroup meta‐analysis (49.3%, Table 3). Overall cost per SSI was estimated at $45578. 5 Total cost per patient was estimated to be $1543 for ciNPT‐F and $2097 for SOC, resulting in a $554 potential savings per patient with ciNPT‐F use.
TABLE 4.
Health economic model of potential overall surgical site infection (SSI) cost savings with closed incision negative pressure therapy with foam dressing (ciNPT‐F) use.
| SOC | ciNPT‐F | |
|---|---|---|
| Baseline overall SSI rate | 4.6% | |
| Relative overall SSI risk reduction rate | NA | 49.3% |
| SSI rate with ciNPT | 4.6% | 2.3% |
| Cost per SSI (USD) | $45578 | |
| Total cost of SSI per patient (USD) | $2097 | $1048 |
| Device cost (USD) | NA | $495 |
| Total cost per patient (USD) | $2097 | $1543 |
| Mean per patient cost savings (USD) | NA | $554 (26.4%) |
Abbreviations: NA, not applicable; USD, United States Dollar.
A second health economic model was created to estimate the potential per patient cost savings with use of ciNPT‐F following cardiac surgery in patients at high risk for developing SSI (Table 5). A baseline rate of 13.5% across the SOC dressing groups from the two ciNPT‐F studies with a defined high‐risk population was used. Relative reduction rate was taken from the high‐risk subgroup meta‐analysis (61.0%, Table 3). Overall cost per SSI was estimated at $45578. 5 Total cost per patient was estimated to be $2911 for ciNPT‐F and $6153 for SOC, resulting in at $3242 potential savings per patient with ciNPT‐F use in the high‐risk population.
TABLE 5.
Health economic model of potential overall surgical site infection (SSI) cost savings with closed incision negative pressure therapy with foam dressing (ciNPT‐F) use in patients at high risk of SSI development.
| SOC | ciNPT‐F | |
|---|---|---|
| Baseline overall SSI rate | 13.5% | |
| Relative overall SSI risk reduction rate | NA | 61.0% |
| SSI rate with ciNPT | 13.5% | 5.3% |
| Cost per SSI (USD) | $45578 | |
| Total cost of SSI per patient (USD) | $6153 | $2416 |
| Device cost (USD) | NA | $495 |
| Total cost per patient (USD) | $6153 | $2911 |
| Mean per patient cost savings (USD) | NA | $3242 (52.7%) |
Abbreviations: NA, not applicable; USD, United States Dollar.
4. DISCUSSION
SSI development following cardiac surgery can be a serious complication resulting in increased morbidity and mortality rates and cost of care. 1 , 2 , 3 , 4 , 5 ciNPT is available as a post‐surgical tool to help manage the closed incision and has been reported to reduce the incidence of SSI across other surgical specialties. A systematic literature review and meta‐analysis were conducted to assess the effect of ciNPT use on overall SSI rates between two different ciNPT systems as compared with SOC. Overall SSI outcomes were assessed due to limited reporting of deep or superficial SSI incidence. A total of 12 articles were included in the meta‐analyses resulting in a statistically significant reduction in overall SSI incidence with ciNPT‐F use compared to SOC in the all‐patient and high‐risk patient subgroup analyses. No overall SSI rate differences were observed between ciNPT‐MLA and SOC in any analysis.
Cardiac surgery involves patient populations with increased risk of SSI development. These populations often have multiple comorbidities such as diabetes, advanced age, chronic obstructive pulmonary disease (COPD), heart failure and chronic kidney disease resulting in an increased risk for SSI development. 1 , 6 , 7 , 8 The patient population included in this meta‐analysis had a similar patient profile (presence of obesity, diabetes, COPD, chronic kidney disease, patient age range 30–89 years). Additionally, seven studies reported a patient health assessment score (EuroScore, ASA Score or STS score), though a majority of the studies reported EuroScores in the low risk range (EuroScore 0–5). 23 , 28 , 29 , 30 Only one ciNPT‐F study with a patient population deemed high‐risk reported a EuroScore in the moderate risk range (score = 10–15). 15 Similarly, one ciNPT‐MLA high‐risk population was reported to have a EuroScore in the average risk range (score = 5–10). 22 Only one study reported an ASA score. Here, the majority of the patient population had a reported ASA of three or four corresponding to patients with severe systemic disease. 26 Ruggieri et al was the only study to report an STS score. 28 However, both the ciNPT and SOC dressing groups were reported to have low risk (1.4–1.5) of mortality and morbidity after cardiac surgery. Thus, the patient populations assessed in this meta‐analysis may have had a lower risk for SSI development prior to any post‐surgical intervention resulting in the low baseline SSI rate observed in the SOC dressing groups.
Use of ciNPT‐F was associated with reduced SSI compared to SOC dressings. Similarly, a previously published meta‐analysis on ciNPT use following cardiac surgery reported an association with a significantly lower risk of any sternal wound infection (SWI) and deep SWI with ciNPT use compared to SOC. 31 The recently published Tao et al meta‐analysis demonstrated a significant reduction in rates of both overall SSI and deep SSI in patients receiving ciNPT compared to patients receiving standard wound dressings. 32 However, a mix of ciNPT systems were compared to SOC dressings in the Biancari et al. and Tao et al. meta‐analyses, whereas the results reported in this work limited and differentiated ciNPT to two systems (ciNPT‐F and ciNPT‐MLA). While no other cardiac surgery specific meta‐analyses have been published, other published articles have assessed rates of SSI following ciNPT use. 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 The Singh et al meta‐analysis compared the use of ciNPT‐F and ciNPT‐MLA against SOC dressings to assess clinical outcomes after surgery, including a mix of surgical specialty types within the analysis. 33 Singh et al reported reduced rates of SSI following use of ciNPT‐F compared to SOC dressings, while no differences were observed between ciNPT‐MLA and SOC dressings. 33 Five meta‐analyses reported reduced SSI rates with ciNPT use compared to SOC dressings across a mix of surgical specialties and ciNPT systems. 34 , 35 , 36 , 37 , 38 , 39 Two ciNPT‐F‐specific meta‐analyses have reported reduced SSI with ciNPT use across all surgical specialties. 40 , 41 When Singh et al assessed cardiac surgery separately, ciNPT‐F also showed reduced rates of SSI when compared to SOC dressings. 41 Similarly, an examination of STS registry data indicated a significant reduction in deep SSIs following the implementation of ciNPT‐F. 44 In contrast, two studies examining SSI rates following use of ciNPT‐MLA across a mix of surgical specialties reported reduction of SSI compared to SOC dressings, though cardiac surgery was either not assessed separately or not able to be further examined due to only one cardiac surgery study included in the analysis. 42 , 43
In this meta‐analysis, only two ciNPT systems were examined against SOC dressings. Both systems utilize a battery‐powered device to provide negative pressure. However, the negative pressure delivered, the method of exudate collection, dressing materials utilized and device costs differ between the two systems. Use of ciNPT‐F has previously demonstrated a superior ability to medialize incision edges compared to ciNPT with non‐foam dressings in a tissue model assessing ability of incisional space closure. 45 Similarly, Grauhan et al. 19 reported no complications after the removal of ciNPT‐F from cardiac surgery incisions as the incisions were fully closed after 6–7 days of ciNPT‐F use compared to SOC dressings. Thus, the ability of ciNPT with foam dressings to draw the incision edges together and reduce the incisional space may be a key component for the ciNPT‐F ability to help reduce post‐surgical complications including infection.
Development of SSIs can increase patient cost of care, though there is limited evidence reporting cost analysis of ciNPT use in cardiac surgery. Cost saving potential is highly dependent on the SSI baseline rate, the type of health insurance used, the methods for care and the negotiated price rate between the patient, the health insurance and the healthcare facility. Large variations can exist between health insurance rates for similar care between healthcare facilities. Additionally, the use of ciNPT is often determined by clinicians and based on a patient's SSI risk. Therefore, the baseline SSI rate and population risk profile are critical in cost analysis approaches and are utilized to develop an estimate of potential cost‐savings in patient care. For example, a recent retrospective study examined the use of ciNPT versus SOC dressings in patients undergoing cardiac surgery. 46 The patient population was limited to patients with recorded predicted risk of deep sternal wound infection (a risk score comprised of 14 demographic and comorbidity risk factors). Hawkins et al reported no reduction of SWI or cost‐savings associated with ciNPT use in this population. 46 However, rates of SWI were low, ranging from 0.9% to 2.25%. When a hypothetical model was evaluated to determine when ciNPT use might be cost‐effective, Hawkins and colleagues found that use of ciNPT in a high‐risk population with a >2.4% overall SWI rate could result in cost savings. 46 In our health economic model, a potential 26.4% cost savings was found with ciNPT‐F use in the total cardiac surgery population with a baseline overall SSI rate of 4.6%. For the high‐risk patient subgroup, with a baseline overall SSI rate of 13.5%, a potential 52.7% cost savings was observed with ciNPT‐F use. Similarly, Grauhan et al. 19 reported clinical and potential cost benefits with ciNPT‐F use in a population of patients undergoing cardiac surgery compared to a retrospective control group with a 3.4% baseline SSI rate.
5. LIMITATIONS
Limitations for this meta‐analysis include assessment of limited high‐quality studies available for analysis, differences in data reporting, unknown variation in healthcare expenditures and evaluation of only two ciNPT systems. Limited high‐quality evidence exists for the use of ciNPT following cardiac surgery. As such, a mix of retrospective studies, prospective studies with retrospective controls and one RCT were assessed in this meta‐analysis. Additionally, variations in data reporting across the studies exist. The included studies had differences in study populations, high‐risk population classification, use of health status scoring, type of cardiac surgery, SSI definition and SSI reporting. This resulted in a potential for bias in the analysis. However, a random‐effects model was utilized to help mitigate potential data heterogeneity.
As not all studies reported incidence of deep or superficial SSIs, overall SSI rates were utilized. While use of overall SSI rates is limiting, it can provide information on the effect of ciNPT systems on patient outcomes. Future assessments for deep and superficial SSIs are required as more clinical evidence becomes available for ciNPT use in cardiac surgery. Additionally, the small number of studies assessed in the high‐risk subgroup analysis also limits the strength of the results.
US healthcare pricing is a complex system that utilizes price negotiation between insurance providers and the healthcare facilities along with cost‐sharing within the insured population. As such, the increase in cost of care due to an SSI may affect patients and healthcare systems differently depending on the health insurance used. Thus, the potential cost‐savings identified with ciNPT‐F use are only estimates with limited precision as generic health insurance economic data was used. However, regardless of the health insurance used, development of an SSI does increase patient costs. The SSI rate reduction observed with ciNPT‐F use compared to SOC dressings can help reduce cost of care by reducing the need for SSI care. Further, only two ciNPT systems were assessed. As ciNPT system differences, such as negative pressure settings and type of dressing utilized may impact patient outcomes, the results of this meta‐analysis may not be applicable to other ciNPT systems. Similarly, an indirect comparison between two ciNPT systems and SOC dressings was used as no evidence exists that directly compares the two systems, limiting the strength of the analysis.
6. CONCLUSIONS
For patients undergoing cardiac surgery, developing an SSI following cardiac surgery often results in increased patient mortality, patient morbidity and cost of care. ciNPT use has been associated with reduced rates of SSI across other surgical specialties; however, limited evidence exists for ciNPT use in cardiac surgery. This systematic literature review and meta‐analysis compared the effect of ciNPT use from two systems (ciNPT‐F and ciNPT‐MLA) against SOC dressings on SSI rates following cardiac surgery. A statistically significant reduction in SSI incidence with ciNPT‐F use compared to SOC dressings in the all‐patient and high‐risk patient subgroup analyses was observed. No SSI rate differences were observed between ciNPT‐MLA and SOC dressings in any analysis. Health economic modelling estimated a modest (26.4%) per patient cost savings in the all patient group and substantial (52.7%) per patient cost savings in the high‐risk patent population with ciNPT‐F use. Thus, implementing the use of ciNPT‐F may be considered a cost‐effective solution in the management of sternotomy incisions. ciNPT system‐specific assessments including large, randomized controlled studies are recommended to further identify effects on clinical and health economic outcomes as there is a high potential for differences in clinical effectiveness among all ciNPT systems.
CONFLICT OF INTEREST STATEMENT
M. Cooper, R. Silverman, C. Bongards and L. Griffin are employees of 3M Health Care. M. Loubani is a consultant for 3M Health Care.
ACKNOWLEDGEMENT
The authors thank Julie M. Robertson, PhD (3M Health Care) for assistance with manuscript preparation and editing.
Loubani M, Cooper M, Silverman R, Bongards C, Griffin L. Surgical site infection outcomes of two different closed incision negative pressure therapy systems in cardiac surgery: Systematic review and meta‐analysis. Int Wound J. 2024;21(1):e14599. doi: 10.1111/iwj.14599
DATA AVAILABILITY STATEMENT
The data that support the findings of this study were derived from previously published literature and are openly available from PubMed, EMBASE, QUOSA, and Medline.
REFERENCES
- 1. Zukowska A, Zukowski M. Surgical site infection in cardiac surgery. J Clin Med. 2022;11(23):6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Berg TC, Kjorstad KE, Akselsen PE, et al. National surveillance of surgical site infections after coronary artery bypass grafting in Norway: incidence and risk factors. Eur J Cardiothorac Surg. 2011;40(6):1291‐1297. [DOI] [PubMed] [Google Scholar]
- 3. European Centre for Disease Prevention and Control . Surveillance of Surgical Site Infections in Europe 2010–2011. ECDC; 2013. [Google Scholar]
- 4. Le Guillou V, Tavolacci MP, Baste JM, et al. Surgical site infection after central venous catheter‐related infection in cardiac surgery. Analysis of a cohort of 7557 patients. J Hosp Infect. 2011;79(3):236‐241. [DOI] [PubMed] [Google Scholar]
- 5. Hou Y, Collinsworth A, Hasa F, Griffin L. Incidence and impact of surgical site infections on length of stay and cost of care for patients undergoing open procedures. Surg Open Sci. 2023;11:1‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Andrade LS, Siliprandi EM, Karsburg LL, et al. Surgical site infection prevention bundle in cardiac surgery. Arq Bras Cardiol. 2019;112(6):769‐774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jayakumar S, Khoynezhad A, Jahangiri M. Surgical site infections in cardiac surgery. Crit Care Clin. 2020;36(4):581‐592. [DOI] [PubMed] [Google Scholar]
- 8. Lemaignen A, Birgand G, Ghodhbane W, et al. Sternal wound infection after cardiac surgery: incidence and risk factors according to clinical presentation. Clin Microbiol Infect. 2015;21(7):674.e11‐e18. [DOI] [PubMed] [Google Scholar]
- 9. Paul M, Raz A, Leibovici L, Madar H, Holinger R, Rubinovitch B. Sternal wound infection after coronary artery bypass graft surgery: validation of existing risk scores. J Thorac Cardiovasc Surg. 2007;133(2):397‐403. [DOI] [PubMed] [Google Scholar]
- 10. Lazar HL, Salm TV, Engelman R, Orgill D, Gordon S. Prevention and management of sternal wound infections. J Thorac Cardiovasc Surg. 2016;152(4):962‐972. [DOI] [PubMed] [Google Scholar]
- 11. Kim JH, Kim HJ, Lee DH. Comparison of the efficacy between closed incisional negative‐pressure wound therapy and conventional wound management after total hip and knee arthroplasties: a systematic review and meta‐analysis. J Arthroplasty. 2019;34:2804‐2814. [DOI] [PubMed] [Google Scholar]
- 12. Meyer J, Roos E, Abbassi Z, Toso C, Ris F, Buchs NC. The role of perineal application of prophylactic negative‐pressure wound therapy for prevention of wound‐related complications after abdomino‐perineal resection: a systematic review. Int J Colorectal Dis. 2021;36(1):19‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gombert A, Dillavou E, D'Agostino R Jr, Griffin L, Robertson JM, Eells M. A systematic review and meta‐analysis of randomized controlled trials for the reduction of surgical site infection in closed incision management versus standard of care dressings over closed vascular groin incisions. Vascular. 2020;28(3):274‐284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wells CI, Ratnayake CB, Perrin J, Pandanaboyana S. Prophylactic negative pressure wound therapy in closed abdominal incisions: a meta‐analysis of randomised controlled trials. World J Surg. 2019;43(11):2779‐2788. [DOI] [PubMed] [Google Scholar]
- 15. Suelo‐Calanao RL, Thomson R, Read M, et al. The impact of closed incision negative pressure therapy on prevention of median sternotomy infection for high risk cases: a single centre retrospective study. J Cardiothorac Surg. 2020;15(1):222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gabriel A, Sigalove S, Sigalove N, et al. The impact of closed incision negative pressure therapy on postoperative breast reconstruction outcomes. Plast Reconstr Surg Glob Open. 2018;6(8):e1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Colli A. First experience with a new negative pressure incision management system on surgical incisions after cardiac surgery in high risk patients. J Cardiothorac Surg. 2011;6(1):160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grauhan O, Navasardyan A, Hofmann M, Muller P, Stein J, Hetzer R. Prevention of poststernotomy wound infections in obese patients by negative pressure wound therapy. J Thorac Cardiovasc Surg. 2013;145(5):1387‐1392. [DOI] [PubMed] [Google Scholar]
- 19. Grauhan O, Navasardyan A, Tutkun B, et al. Effect of surgical incision management on wound infections in a poststernotomy patient population. Int Wound J. 2014;11(Suppl 1):6‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Elmistekawy E, Vo TX, Chaudry S, Sun LS, Lam BK. Prophylactic negative‐pressure wound dressing does not reduce the incidence of sternal wound infection after coronary artery bypass grafting: a propensity‐matched analysis. Innov Technol Tech Cardiothorac Vas Surg. 2020;15(S1):50s‐51s. [Google Scholar]
- 22. Tabley A, Aludaat C, Le G, et al. A survey of cardiac surgery infections with PICO negative pressure therapy in high‐risk patients. Ann Thorac Surg. 2020;110:2034‐2040. [DOI] [PubMed] [Google Scholar]
- 23. Myllykangas HM, Halonen J, Husso A, Vaananen H, Berg LT. Does incisional negative pressure wound therapy prevent sternal wound infections? Thorac Cardiovasc Surg. 2022;70(1):65‐71. [DOI] [PubMed] [Google Scholar]
- 24. Fauvel D, Taveras M, Skendelas JP, et al. The pressure is on: single center's experience with negative pressure wound therapy and driveline infection. J Heart Lung Transplant. 2021;40(4S):S417‐S418. [Google Scholar]
- 25. El Oakley RM, Wright JE. Postoperative mediastinitis: classification and management. Ann Thorac Surg. 1996;61(3):1030‐1036. [DOI] [PubMed] [Google Scholar]
- 26. Nguyen KA, Taylor GA, Webster TK, et al. Incisional negative pressure wound therapy is protective against postoperative cardiothoracic wound infection. Ann Plast Surg. 2022;88(3 Suppl 3):S197‐S200. [DOI] [PubMed] [Google Scholar]
- 27. Richter M, Moschovas A, Bargenda S, Abdyvasiev K, Kirov H, Doenst T. Negative pressure wound therapy for closed incisions and deep sternal wound infection after median sternotomy: single‐center experience. Thorac Cardiovasc Surg. 2022;70(Supp1):S1‐S61. [Google Scholar]
- 28. Ruggieri VG, Olivier ME, Aludaat C, et al. Negative pressure versus conventional sternal wound dressing in coronary surgery using bilateral internal mammary artery grafts. Heart Surg Forum. 2019;22(2):E092‐E096. [DOI] [PubMed] [Google Scholar]
- 29. Santarpino G, Gazdag L, Sirch J, et al. A retrospective study to evaluate use of negative pressure wound therapy in patients undergoing bilateral internal thoracic artery grafting. Ostomy Wound Manage. 2015;61(12):26‐30. [PubMed] [Google Scholar]
- 30. Witt‐Majchrzak A, Zelazny P, Snarska J. Preliminary outcome of treatment of postoperative primarily closed sternotomy wounds treated using negative pressure wound therapy. Pol Przegl Chir. 2015;86(10):456‐465. [DOI] [PubMed] [Google Scholar]
- 31. Biancari F, Santoro G, Provenzano F, et al. Negative‐pressure wound therapy for prevention of sternal wound infection after adult cardiac surgery: systematic review and meta‐analysis. J Clin Med. 2022;11(15):4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tao Y, Zhang Y, Liu Y, Tang S. Effects of negative pressure wound therapy on surgical site wound infections after cardiac surgery: a meta‐analysis. Int Wound J. 2023; 1‐8. ePub ahead of print. doi: 10.1111/iwj.14398 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33. Singh DP, Gabriel A, Silverman RP, Griffin LP, D'Agostino McGowan L, D'Agostino RB Jr. Meta‐analysis comparing outcomes of two different negative pressure therapy systems in closed incision management. Plast Reconstr Surg Glob Open. 2019;7(6):e2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Norman G, Shi C, Goh EL, et al. Negative pressure wound therapy for surgical wounds healing by primary closure. Cochrane Database Syst Rev. 2022;4(4):CD009261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hyldig N, Birke‐Sorensen H, Kruse M, et al. Meta‐analysis of negative‐pressure wound therapy for closed surgical incisions. Br J Surg. 2016;103(5):477‐486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shiroky J, Lillie E, Muaddi H, Sevigny M, Choi WJ, Karanicolas PJ. The impact of negative pressure wound therapy for closed surgical incisions on surgical site infection: a systematic review and meta‐analysis. Surgery. 2020;169(5):1257‐1259. [DOI] [PubMed] [Google Scholar]
- 37. Sandy‐Hodgetts K, Watts R. Effectiveness of negative pressure wound therapy/closed incision management in the prevention of post‐surgical wound complications: a systematic review and meta‐analysis. JBI Database System Rev Implement Rep. 2015;13(1):253‐303. [DOI] [PubMed] [Google Scholar]
- 38. De Vries FE, Wallert ED, Solomkin JS, et al. A systematic review and meta‐analysis including GRADE qualification of the risk of surgical site infections after prophylactic negative pressure wound therapy compared with conventional dressings in clean and contaminated surgery. Medicine. 2016;95(36):e4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Semsarzadeh NN, Tadisina KK, Maddox J, Chopra K, Singh DP. Closed incision negative‐pressure therapy is associated with decreased surgical‐site infections: a meta‐analysis. Plast Reconstr Surg. 2015;136(3):592‐602. [DOI] [PubMed] [Google Scholar]
- 40. Cooper HJ, Singh DP, Gabriel A, Mantyh C, Silverman R, Griffin L. Closed incision negative pressure therapy versus standard of care in reduction of surgical site complications: a systematic review and meta‐analysis. Plast Reconstr Surg Glob Open. 2023;11(3):e4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Singh DP, Gabriel A, Parvizi J, Gardner MJ, D'Agostino R Jr. Meta‐analysis of comparative trials evaluating a single‐use closed‐incision negative‐pressure therapy system. Plast Reconstr Surg. 2019;143(Suppl 1):41S‐46S. [DOI] [PubMed] [Google Scholar]
- 42. Saunders C, Nherera LM, Horner A, Trueman P. Single‐use negative‐pressure wound therapy versus conventional dressings for closed surgical incisions: systematic literature review and meta‐analysis. BJS Open. 2021;5(1):zraa003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Strugala V, Martin R. Meta‐analysis of comparative trials evaluating a prophylactic single‐use negative pressure wound therapy system for the prevention of surgical site complications. Surg Infect (Larchmt). 2017;18(7):810‐819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Traylor LB, Bhatia G, Blackhurst D, Wallenborn G, Ewing A, Bolton W. Efficacy of incisional negative pressure therapy in preventing post‐sternotomy wound complications. Am J Surg. 2023;226:762‐767. doi: 10.1016/j.amjsurg.2023.07.016 [DOI] [PubMed] [Google Scholar]
- 45. Kilpadi DV, Olivie M. Evaluation of closed incision negative pressure therapy systems on the closure of incisional space model. J Wound Care. 2019;28(12):850‐860. [DOI] [PubMed] [Google Scholar]
- 46. Hawkins RB, Mehaffey JH, Charles EJ, et al. Cost‐effectiveness of negative pressure incision management system in cardiac surgery. J Surg Res. 2019;240:227‐235. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study were derived from previously published literature and are openly available from PubMed, EMBASE, QUOSA, and Medline.


