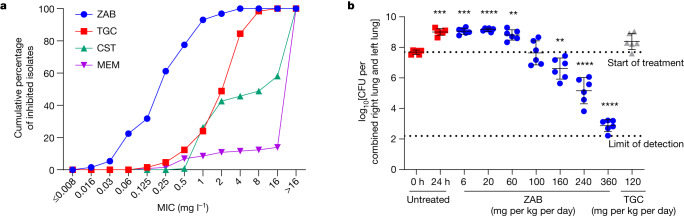
Fig. 4. In vitro activity and in vivo efficacy of zosurabalpin against clinical A. baumannii isolates.
a, In vitro MIC activity of zosurabalpin against 129 A. baumannii clinical isolates shown as the cumulative percentage (MIC90: zosurabalpin (ZAB) = 1 mg l−1; tigecycline (TGC) = 8 mg l−1; colistin (CST) > 16 mg l−1; meropenem (MEM) > 16 mg l−1). Line listing of the data is provided in Supplementary Table 7. b, The in vivo efficacy of zosurabalpin in a mouse model of infection induced by pan-drug-resistant A. baumannii ACC01073 (zosurabalpin MIC = 2 mg l−1 in CAMHB with 20% human serum). Lung infection was induced by bacterial intratracheal inoculation in immunocompromised mice. Treatment, starting 2 h after infection (0 h), was administered subcutaneously (n = 6 mice per treatment group or vehicle) every 6 h over 24 h for zosurabalpin and every 12 h for tigecycline. Dose–response curve of zosurabalpin total daily doses (mg per kg per day) measured as the bacterial burden reduction (CFU) in infected lungs. Results are presented as mean ± s.d. Statistical significance of the difference in bacterial counts between the control and treated mice was calculated using the one-factor ANOVA followed by Dunnett’s multiple-comparison test (P < 0.05 was considered to be significant versus T = 0 h); **P < 0.01, ***P < 0.001.