Abstract
Staphylococcus aureus is an important pathogen of humans and other animals, causing bacteremia, abscesses, endocarditis, and other infectious syndromes. A signature-tagged mutagenesis (STM) system was adapted for use in studying the genes required for in vivo survival of S. aureus. An STM library was ultimately created in S. aureus RN6390, with Tn917 being used to create the transposon mutations. Pools of S. aureus RN6390 mutants were screened in mouse abscess, bacteremia, and wound infection models for growth attenuation after in vivo passage. One of the mutants that was identified displayed marked attenuation following large-pool screening in all three animal models, which was confirmed in bacteremia and endocarditis models of infection with a smaller pool of mutants. Sequence analysis of the entire open reading frame showed a 99% identity to the high-affinity proline permease (putP) gene characterized in another strain of S. aureus. In wound and murine abscess infection models, the putP mutant was approximately 10-fold more attenuated than was wild-type strain RN6390. Another S. aureus strain transduced with the putP mutation also displayed an attenuated phenotype after passage in the wound model. A [3H]proline uptake assay showed that less proline was specifically transported into the putP mutant than into strain RN6390. The reduced viability of the bacteria possessing the mutation in the S. aureus high-affinity proline permease suggests that proline scavenging by the bacteria is important for in vivo growth and proliferation and that analogs of proline may serve as potential antistaphylococcal therapeutic agents.
Staphylococcus aureus is one of the leading causes of both community-acquired and hospital-acquired infections (22, 39), and it is thought to cause up to one-third of all food-borne illness in the United States (3). This organism is responsible for a variety of distinct and divergent diseases, including osteomyelitis, endocarditis, bacteremia, wound and skin infections, abscess formation, and a host of other afflictions, in humans and other animals (14). Current antibiotic therapies against S. aureus are losing their effectiveness as methicillin resistance continues to increase (23, 26), and vancomycin resistance in clinical isolates has emerged (7, 8). The identification by molecular biological techniques of new anti-staphylococcal drug targets and therapeutics with novel mechanisms of action is a critical goal.
One approach to the identification of genes necessary for in vivo growth is signature-tagged mutagenesis (STM), originally described by Hensel et al. (19). In this system, unique oligonucleotide signature tag (OST) sequences are inserted into a transposon between the flanking terminal repeat regions. When the transposon inserts into the genome of the bacteria, a tagged mutation is created that can be screened in animal models to identify genes required for growth in the in vivo environment.
Although this basic STM approach was effective at identifying attenuated Salmonella mutants (19), a number of adaptations were necessary to accomplish the same task for the more genetically intractable S. aureus cells (10). In this report, we describe the identification and characterization of a mutant with an OST mutation in the putP gene that demonstrated marked attenuation in initial in vivo screening and follow-up analyses. The putP gene encodes a high-affinity proline permease that scavenges proline for use as a carbon and nitrogen source (31, 43, 44). We show that disruption of this proline permease by transposon insertion has a deleterious effect on proline uptake, which in turn affects the in vivo survival of the S. aureus cells.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth media.
S. aureus RN4220 is a mutant of strain 8325-4 that accepts foreign DNA via transformation or transduction (27). Strain RN6390 is a virulent, hemolytic laboratory strain that was used for final genetic transfers (27), and strain RN6911 is an agr::TetM deletion mutant of RN6390 missing all of the accessory global regulator (agr) gene plus additional DNA downstream of RNAIII (32). The strains described above were provided by B. Kreiswirth and A. Cheung. S. aureus S6C, a hyperhemolytic variant provided by S. Projan (21), was used as a recipient strain for transductions of the putP mutation into a different genetic background. MAX efficiency Escherichia coli DH10B (Gibco/BRL, Gaithersburg, Md.) cells were used for marker rescue experiments of the flanking S. aureus DNA, E. coli XL1-Blue MRF′ (Stratagene) was exploited for lambda library expression, and E. coli SOLR (Stratagene) was used for phagemid excision. The vector developed for use in S. aureus, pMOD-1, was modified from the gram-positive shuttle vector pLTV1 (6) (supplied by G. Muller and D. Portnoy), which contains Tn917; its construction is described elsewhere (10). Phage φ80α, given to us by B. Kreiswirth, was used as the transducing phage for S. aureus strains. S. aureus cells were grown in brain heart infusion medium (BHI; Gibco/BRL), while Luria-Bertani medium (Gibco/BRL) was used for growing E. coli. The media were supplemented with antibiotics (Sigma Chemical Co., St. Louis, Mo.) at the following concentrations: ampicillin, 100 μg/ml; tetracycline, 12.5 μg/ml; lincomycin, 5 μg/ml; chloramphenicol, 10 μg/ml (plasmid) or 5 μg/ml (integrated mutants); and erythromycin, 1 μg/ml (construction of ST mutants) or 5 μg/ml (transductions).
Construction of OST mutants.
The construction of the OST Tn917 mutant library in S. aureus RN6390 is described in detail elsewhere (10). OSTs were ligated into a pMOD-1 vector, and the ligated products were transformed into E. coli cells (35) to establish the library. From the transformed E. coli cells, plasmid isolation of the pMOD-1 vectors containing individual signature tags was performed as specified by the manufacturer (Qiagen).
Because S. aureus RN6390 cannot be easily transformed directly with foreign DNA, transformations of the pMOD-1 constructs were initially performed in S. aureus RN4220 by electroporation (20). Transformants were selected at 30°C on BHI agar plates containing chloramphenicol. To move the pMOD-1 constructs into the virulent strain RN6390, transductions were performed with the donor φ80α phage by the method of Proctor and Kloos (30). Transductants were selected on BHI agar plates containing chloramphenicol and 2 mM sodium citrate. Cells grown at 43°C to cure the plasmid were selected on BHI medium containing erythromycin followed by screening on BHI agar containing chloramphenicol or tetracycline. Mutants that were Emr, Cmr, and Tcs were arrayed into pools of 95 unique OSTs in 96-well microtiter plates. Southern blot hybridizations were performed as described below to verify single insertions of Tn917 into the OST mutants.
Screening of OST mutants.
Pools of 95 individual Tn917 mutants were grown to mid-logarithmetic phase in BHI broth, washed once with BHI broth, and suspended to a final concentration of 1.0 × 108 to 4.5 × 108 CFU/ml in BHI broth containing 15% (vol/vol) glycerol for storage at −80°C. Following in vivo selection, agar plates containing 1,000 to 5,000 bacterial colonies were flooded with 3 ml of sterile distilled H2O and the bacteria were suspended to homogeneity. An aliquot from each sample was lysed with lysostaphin at 37°C for 10 to 15 min. Aliquots of the supernatant were PCR amplified with 50 pmol of the SIG-BGL-2 (5′ ATCTTACAACCTCAAGCTT 3′) and SIG-BGL-4 (5′ ATCTCATTCTAACCAAGC 3′) primers to generate an 89-bp double-stranded DNA fragment corresponding to the variable tags. PCR amplifications were set up as follows: initial denaturation at 95°C for 90 s, followed by 30 cycles of 95°C for 30 s, 48°C for 60 s, and 72°C for 30 s. The DNA was digested with HindIII to remove the flanking invariant arms, and the variable OST region was then treated with HK phosphatase (Epicentre) as specified by the manufacturer. OST region DNA probes made by 32P-end labelling with [γ-32P]ATP (DuPont/NEN, Wilmington, Del.) (35) were denatured, mixed with hybridization buffer (4× SSPE [1× SSPE is 0.18 M NaCl, 10 mM NaH2PO4, and 1 mM EDTA; pH 7.7], 10% dextran sulfate, 0.5% sodium dodecyl sulfate [SDS], 5× Denhardt’s solution, 0.1 mg of salmon sperm DNA [Digene, Beltsville, Md.]), and hybridized overnight at 65°C in a hybridization oven (37). The filters were washed with 1× SSC (0.15 M NaCl plus 0.015 M sodium citrate)–0.1% SDS for 15 min at room temperature, 2× SSC for 20 min at 65°C, and 0.2 × SSC–0.1% SDS for 20 min at 65°C. The hybridization patterns were visualized with a phosphorimager (Bio-Rad Molecular Imager System model GS263).
To verify the Tn917 insertions via Southern blot hybridization, genomic bacterial DNAs were prepared from overnight cultures of OST mutants with the AGTC kit (Advanced Genetic Technologies Corp.). The manufacturer’s instructions were modified with the addition of a lysostaphin (AMBI) treatment (32 μl of 2-mg/ml lysostaphin in 800 μl of spheroplast buffer) at 37°C for 30 min to facilitate breakdown of the cell wall. Purified genomic DNA was resuspended in 10 mM Tris (pH 8.0) and digested with HindIII (Boehringer Mannheim, Indianapolis, Ind.), which cut adjacent to the 3′-terminal genomic insertion site within Tn917. These fragments of DNA were transferred to Hybond N (Amersham) membranes (35) and probed with either a random-prime labeled PCR amplified DNA fragment to open reading frame (ORF) 5 of Tn917 (Redi-Prime; Amersham) or a random-primed labeled PCR product spanning part of the putP gene. The 600-bp Tn917 probe fragment was PCR amplified with primers (5′ TCAGGTGTTTGGAATGAC 3′ and 5′ CTTCGGGATCTATTTTGAC 3′) under the following conditions: an initial denaturation at 94°C for 90 s followed by 30 cycles of 94°C for 60 s, 57°C for 30 s, and 72°C for 30 s. A putP probe fragment was PCR amplified with putP-specific primers (5′ TTCTCTAACGATGTCACGAAC and 5′ CGAAAGCGCTTTCTATATTGGT 3′) under the following conditions: an initial denaturation at 94°C for 2 min followed by 30 cycles of 94°C for 1 min, 56°C for 1 min, and 72°C for 2 min. Hybridizations were performed as described above.
For Southern dot and slot blot hybridizations of large-pool (95 mutants) and small-pool (11 mutants) screening of mutants, individual OST regions in Tn917 were PCR amplified from the pMOD-1 constructs to generate a 600-bp fragment from each chosen clone. Amplifications were performed with 100 pmol of primer SIG-BLOT-F (5′ AGTCATAAGATTAGTCACTGGTAG 3′) and primer SIG-BLOT-R (5′ CACGGAAATGTTGAATACTCATAC 3′) under the following conditions: an initial denaturation at 95°C for 90 s followed by 30 cycles of 95°C for 30 s, 56°C for 30 s, and 72°C for 30 s. The PCR products were normalized to 10 ng/ml in 10 mM Tris (pH 8.0). OST substrate DNAs (10 ng/ml) were denatured by the addition of 0.4 N NaOH and spotted onto precut nylon membranes (Pall Biodyne B) in a 96-well format with a 96-pin replicator (Nunc). The DNAs were triple loaded onto each spot, and three adjacent spots per clone were pinned. Small-pool membranes for Southern blot hybridizations were prepared with a 24-well slot blot apparatus (Gibco/BRL). Probes were prepared by labeling 6 to 25 pmol of the 52-bp HindIII-digested DNAs, prepared as described above.
Animal models.
Three murine animal models were used for the primary screening of the large pools of Tn917 mutants. The mouse abscess model and the mouse burn model involved Crl:SKH-1-hr BR (outbred) mice (Charles River, Hollister, Calif.). Beige (C57BL/6J-bgj+) mice (Jackson Laboratory, Bar Harbor, Maine) were used for a disseminated systemic model of infection. For the mouse abscess model, SKH-1 mice (two to four per time point) were injected subcutaneously (106 to 107 CFU/ml) (45). At 2 to 4 days postinoculation, the animals were sacrificed by cervical dislocation and the abscesses were collected in disposable tissue grinders. Abscess suspensions were 10-fold serially diluted in sterile saline and plated onto BHI agar and blood agar (Remel). For long-term abscess formation (5 to 7 days), a mouse-to-mouse bacterial transfer procedure was used. Mouse abscesses from 2 to 3 days postinoculation were ground up in 2.5 ml of BHI broth and pelleted by centrifugation at 800 × g for 1 to 2 min to remove large particulate matter. The supernatants were suspended to a 1-ml volume, and a fresh group of uninfected mice were injected as noted above. The abscesses were then harvested at 3 to 5 days postinoculation, and the material was processed as noted above.
The mouse burn wound model was prepared by the method of Vasishta et al. (41), using SKH-1 mice and an inoculum of 104 CFU of S. aureus mutants per ml in a large pool. The analgesic Torbutrol (Fort Dodge Laboratories, Inc., Fort Dodge, Iowa) was adminstered to the mice subcutaneously at a dose of 0.17 mg/kg before the mice were burned. The animals were euthanized 4 days postinoculation, and the pooled wound exudates from two to four mice were processed as noted above for the abscess material. For the murine bacteremia model, Beige mice were injected intravenously with 100 to 200 μl of inoculum at 106 to 107 CFU/ml (18). At 1 or 2 days postinoculation, the mice were sacrificed by cervical dislocation and the spleens and livers were extracted from all mice. Each organ culture was homogenized separately and processed as described above.
Single-mutant screenings of the 16F-157 putP::Tn917 mutant, another unrelated Tn917 mutant, an agr mutant (strain RN6911), and wild-type RN6390 were performed with the mouse abscess and mouse burn wound models characterized above. Eight mice per strain per model were inoculated with 107 CFU/ml for the abscess model and 101 CFU/ml for the wound infection model. This type of screening was also performed with the S6C strain and its putP isogenic mutant, inoculating six mice per strain through the wound model. Abscesses were collected 3 and 7 days postinoculation, homogenized, and plated for viable counts on BHI agar or blood agar. Wound exudates were collected 1, 4, or 7 days postinoculation, homogenized, and plated for viable counts on BHI agar or mannitol salt agar (Difco).
A rabbit model of experimental endocarditis was also used as previously detailed (28). An endocarditis infection was induced in catheterized New Zealand White rabbits (Jackson Laboratory) by the intravenous (i.v.) injection, 24 h postcatheterization, of an S. aureus ST mutant pool of approximately 105 total CFU that contained the putP mutant as well as 10 other ST mutants. The rabbits were euthanized with a rapid i.v. injection of sodium pentobarbitol (Abbott Laboratories, Chicago, Ill.) 24 h after the i.v. challenge with the mutant pool, and several cardiac vegetations were removed and snap-frozen directly within microcentrifuge tubes as described previously (28). Tissue homogenates were plated onto BHI agar and incubated at 37°C overnight. DNAs from staphylococcal cells, harvested from the BHI agar plates, from each rabbit and in vitro-grown cultures were fourfold serially diluted in distilled H2O, starting at 10 ng/μl, and PCR amplified with either the 123 primer (5′ GATCTTGTGTTGGAGCGCTCTGT 3′) or the 157 primer (5′ CATAGACCTCTCCCGACACAC 3′) paired with the SIG-BLOT-R primer (5′ CACGGAAATGTTGAATACTCATAC 3′) under the following conditions: an initial denaturation at 95°C for 2 min followed by 32 cycles of 95°C for 1 min, 60°C for 1 min, and 72°C for 2 min. The PCR products were separated by electrophoresis through 2% agarose gels.
Marker rescue of flanking genomic DNA.
To determine the Tn917 insertion site within the 16F-157 S. aureus chromosome, genomic DNA was prepared from this strain with the AGTC kit and the DNA was digested with KpnI, EcoRI, XbaI, or SphI (Boehringer Mannheim). Digested DNA was recircularized by ligation of 100 ng of DNA per ligation reaction and transformed into E. coli DH10B MAX efficiency competent cells. Transformants were selected on Luria agar plates containing ampicillin.
Phagemid screening and excision.
A commercially prepared (Stratagene) lambda ZAP II bacterial genomic library from S. aureus RN6390 was used for phagemid screening and excision. The phage library was grown on E. coli XL1-Blue MRF′ as specified by the manufacturer, and the propagated phage were aligned into a 96-well array. Phagemids were excised with the ExAssist interference-resistant helper phage and SOLR cells (Stratagene) as specified by the manufacturer. The DNAs from each XL1-Blue phagemid were extracted, normalized to 10 ng/ml, denatured, and pinned onto nylon filters as noted above. Southern blot hybridization of membranes prepared from the array were performed to identify putP-containing phagemids as described above.
DNA sequencing and homology analysis.
Double-stranded plasmid or PCR-generated DNAs were sequenced by the dideoxy chain termination procedure (36) with an ABI 377 automated sequencer (ABI). The Tn917 5′ primer (5′ CCATACGCAAGACCAATCACT 3′) was used for the initial sequencing to verify the transposon chromosome junction. Additional primers to the putP sequence were synthesized to complete the ORF of the gene as well as flanking DNA sequences. DNA sequencing runs were aligned into a contig with the Sequencher software package (Gene Code Corp., Ann Arbor, Mich.). BLASTX and TBLASTN analyses were performed to identify potential homologies in the GenBank database (1).
Transduction into a new genetic background.
The Tn917 insertion into putP in strain RN6390 was transduced into another S. aureus genetic background, S. aureus S6C, with phage φ80α as described above. Transductions were set up with a multiplicity of infection of 1:1 (phage-to-bacterium ratio) or 0.1:1. Transductants were selected on BHI agar containing erythromycin and 2 mM sodium citrate and incubated at 30°C for 2 to 4 days. Emr colonies were screened for Cmr. The DNAs from transductants that were Emr Cmr were processed for Southern blot hybridization and PCR analyses. To verify the insertion of Tn917 into the putP gene, PCR amplifications were undertaken with a primer in the putP gene (16F-157I, 5′ GTTTAGGTATTAGCTGGATGGC 3′) paired with the Tn917 5′ primer as follows: an initial denaturation at 94°C for 2 min followed by 31 cycles of 94°C for 30 s, 48°C for 1 min, and 72°C for 30 s.
Assay for proline transport.
Transport of proline into the bacterial cell was analyzed by the filtration method described by Bae and Miller (2). Bacteria were suspended to a final concentration of approximately 25 to 40 μg of total cellular protein/ml as measured with the Bio-Rad protein assay kit (5). The S. aureus cells were preincubated at 37°C for 5 min in the transport buffer, and l-[2,3-3H]proline (DuPont/NEN) was added at a final concentration of 5 μM (specific activity, 40 Ci/mmol). The bacterial cells were agitated at 37°C for up to 10 min. Aliquots (1 ml) were removed from the culture after 2, 6, or 10 min and filtered through 0.45-μm-pore-size HA filters (Millipore, Bedford, Mass.) with a sampling manifold (Millipore). The filters were washed twice with unlabeled transport buffer and then dried in scintillation vials. Radioactive samples were counted in 3.5 ml of Ecoscint scintillation solution (National Diagnostics) with a Beckman LS 6000SC scintillation spectrophotometer. When the inhibitors 3,4-dehydro-d,l-proline (DHP) (Sigma) or l-azetidine-carboxylic acid (AZ) (Sigma) were used, they were added at a final concentration of 10 mM at the same time as the [3H]proline. Additions of 100-fold molar excesses of cold l-proline (Sigma) or cold l-valine (Sigma) to the reaction mixture to test the competition with the [3H]proline were also made just before addition of the [3H] proline.
Nucleotide sequence accession number.
The nucleotide sequence data has been submitted to GenBank under accession no. AF024571.
RESULTS
Large- and small-pool screening of the Tn917 SigTag library.
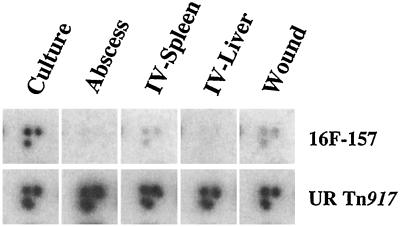
A library of 1,520 Tn917 OST mutants was generated for testing of attenuated virulence. Sixteen pools of 95 individual Tn917 mutants were screened in three animal models (abscess, wound, bacteremia) to determine attenuation for in vivo growth and survival. A total of 237 in vivo attenuated mutants, including mutant 16F-157, were identified (10). This Tn917 mutant demonstrated a diminished hybridization signal in the Southern analyses of the pooled DNA collected from all three animal models tested compared to in vitro-grown cells (Fig. 1). A Tn917 insertion in an unrelated locus showed hybridization patterns from in vivo-passaged bacteria that were similar to those for in vitro-grown cells.
FIG. 1.
Identification of attenuated genes by large-pool DNA dot blot hybridizations. PCR-amplified DNAs from S. aureus cells passaged through culture (in vitro), an abscess model, an i.v. (IV) systemic model (spleen and liver), and a wound model were radiolabelled and used to probe a 96-well array. The hybridization patterns from the array for the putP::Tn917 mutant strain 16F-157 and an unrelated (UR) Tn917 mutant of strain RN6390 are depicted.
To verify that the 16F-157 mutant was attenuated for growth, a smaller pool of 11 mutants that comprised a mixture of large-pool attenuated mutants, including 16F-157, and unattenuated mutants was screened through the bacteremia model of infection. Two days after intravenous injection of the small pool, the spleens and livers of the SKH-1 mice were collected and PCR amplifications of DNA were performed. Hybridization results demonstrated a fainter signal in the in vivo-passaged 16F-157 lane than in the in vitro-passaged cells (data not shown), confirming the attenuation of the 16F-157 mutant.
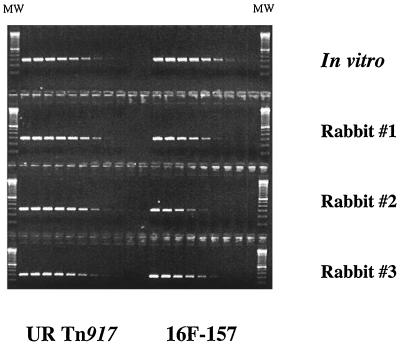
The pool of 11 mutants was also tested in an rabbit endocarditis model of infection. Cardiac vegetations were collected after 24 h and genomic DNA extracted from bacterial colonies arising from the homogenized tissue. PCR amplifications that used primers specific for the 16F-157 OST and an unrelated OST were performed on fourfold serial dilutions of the genomic DNAs, comparing DNAs from in vitro-grown staphylococcal cells to the DNAs from staphylococcal cells obtained after infection of three separate rabbits. The limiting-dilution PCR analyses showed that the amount of strain 16F-157 DNA (the 299-bp PCR product) was reduced 16- to 64-fold in the three rabbits compared to the in vitro preparation whereas the level of the unrelated OST DNA (295-bp PCR product) remained unchanged or even increased in the rabbits that were inoculated (Fig. 2).
FIG. 2.
Limiting-dilution PCR analyses of DNA from S. aureus cells isolated from infected cardiac vegetations compared to in vitro-grown cells. New Zealand White rabbits were infected with a pool of 11 S. aureus ST mutants, and the infected cardiac tissue was collected 1 day postinoculation. DNAs were serially diluted 1/4, starting at a concentration of 10 ng/μl, and each dilution was PCR amplified with either a 157-plus-SIG-BLOT-R primer pair (16F-157) or a 123-plus-SIG-BLOT-R primer pair (unrelated [UR] Tn917 mutant). A 100-bp molecular size marker (MW; Gibco/BRL) was used to measure the sizes of the UR Tn917 PCR product (295 bp) and the 16F-157 PCR product (299 bp).
Localization of the transposon insertion in the 16F-157 mutant.
The transposon and flanking genomic DNA from strain 16F-157 was rescued and transformed into E. coli. The DNA sequence was obtained for the entire ORF and compared to DNA and protein databases for homologies to any known gene(s). The BLAST search indicated that the gene in the 16F-157 strain where Tn917 inserted showed 99% identity to the putP gene previously cloned from S. aureus ATCC 12600 (43). Five nucleotides were changed, but only one predicted amino acid difference was noted at position 374, an Asp-to-His change. The Tn917 insertion was between nucleotides 879 and 880 within the ORF, which is approximately two-thirds of the way through the ORF of putP. On the basis of this marked homology, we concluded that the transposon was inserted into the S. aureus putP gene.
Diminished proline uptake by the putP mutant strain.
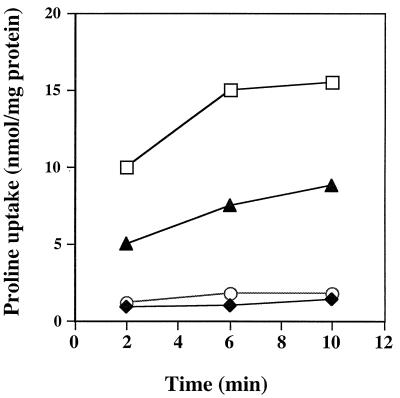
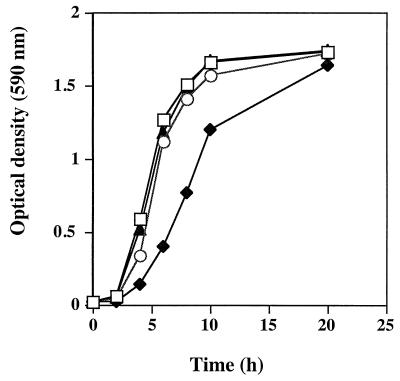
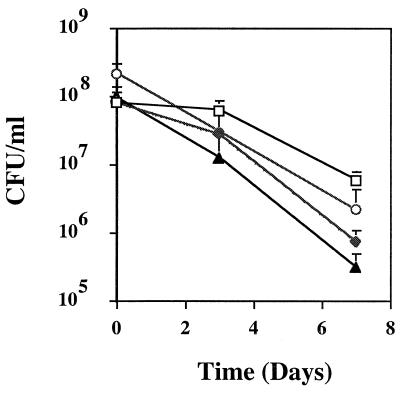
To test whether the putP mutant was functionally impaired for proline transport, proline uptake was measured (2). Compared to wild-type strain RN6390, the putP mutant displayed an approximate 33% overall decline in [3H]proline uptake (Fig. 3). These results demonstrated that the putP mutation affected proline transport into the S. aureus cells. Two analog inhibitors of proline, AZ and DHP (4, 11, 17), showed approximately 95% inhibition of proline uptake when used at a 2,000-fold molar excess. The specificity of the system was ascertained by using either a 100-fold molar excess of cold l-proline or a 100-fold molar excess of l-valine. l-proline inhibited [3H]proline uptake by 95%, whereas a 100-fold molar excess of l-valine did not significantly affect the amount of [3H]proline entering the staphylococcal cells (data not shown). The growth curves of the putP mutant compared to the parent strain RN6390 were the same in BHI broth (Fig. 4) and minimal medium broth (reference 34 and data not shown). Proline analogs that inhibited proline uptake were also tested to determine if they would impede the growth of RN6390 cells. AZ had no effect on the growth of RN6390; however, DHP was able to partially inhibit the growth of RN6390 cells in the first 8 h (Fig. 4).
FIG. 3.
Proline uptake by the high-affinity system of S. aureus. Transport measurements were performed after 2, 6, or 10 min by the filtration method outlined in the text. Proline was present at 5 μM. Cells were suspended in transport buffer at 40 μg of total cellular protein per ml. Symbols: □, RN6390; ⧫, RN6390 plus 10 mM AZ; ○, RN6390 plus 10 mM DHP; ▴, 16F-157 (putP::Tn917). This is a representative graph from three independent experiments.
FIG. 4.
Growth curves of 16F-157 (putP::Tn917) and the parental strain RN6390 with or without proline analogs in BHI broth. Symbols: □, RN6390; ⧫, RN6390 plus 10 mM DHP; ○, RN6390 plus 10 mM AZ; ▴, 16F-157 (putP::Tn917). This is a representative graph from two independent experiments.
Analysis of the putP mutant in animal models.
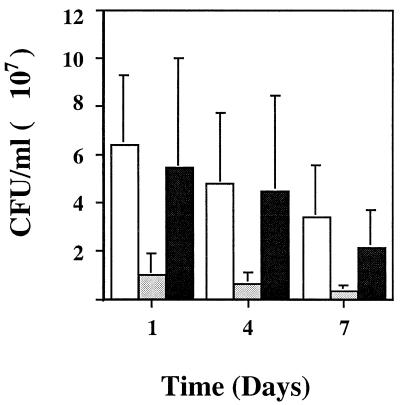
The putP mutant was tested in a wound model and an abscess model to determine the extent of its attenuation compared to wild-type strain RN6390. Viable counts of the putP mutant were significantly lower in both the abscess model (Fig. 5) (P < 0.0088) and the wound model (Fig. 6) (P < 0.0003) of infection than those of the parental strain RN6390. Another unrelated Tn917 mutant showed no significant change in the viable counts from infected abscess (P < 0.1013) or wound (P < 0.2164) tissues. A mutant deficient in the accessory global regulator (agr) that transcriptionally activates a number of S. aureus virulence factors (32) also displayed significantly fewer CFU per milliliter in the abscess model (Fig. 5) (P < 0.0056) but showed no overall difference in the viable counts (3.45 × 107 ± 1.92 × 107 and 3.61 × 107 ± 1.60 × 107 CFU/ml for the mutant and wild-type RN6390, respectively [P < 0.4164]) in the wound model.
FIG. 5.
Comparison of the putP mutant and two other mutants to the wild-type strain RN6390 in the murine abscess model. Viable counts were determined for the initial inocula and at 3 or 7 days postinoculation. Symbols: □, RN6390; ⧫, 16F-157 (putP::Tn917); ○, an unrelated (UR) Tn917 SigTag mutant; ▴, an agr mutant (agr::TetM) of strain RN6390.
FIG. 6.
Comparison of the putP mutant and another Tn917 mutant to the wild-type strain RN6390 in the murine burn wound model. Viable counts were determined at 1, 4, and 7 days postinoculation. Symbols: □, RN6390; ░⃞, 16F-157 (putP::Tn917); ▪, an unrelated (UR) Tn917 SigTag mutant.
Phage φ80α was used to move the putP mutation from strain 16F-157 (RN6390 background) into another S. aureus strain (S6C) containing a different genetic background. The S6C strain was chosen because phage φ80α was able to transduce into this strain and not into other S. aureus strains that we had available. This transfer of the putP mutation into strain S6C was confirmed by PCR and Southern blot hybridization analyses (data not shown). The viable counts of the resulting putP mutant in this genetic background were again significantly less (9.06 × 106 CFU/ml; P < 0.0107) than those of the wild-type strain S6C (3.43 × 107 CFU/ml). This study confirmed that PutP is needed by S. aureus to obtain maximal growth and achieve maximum survival potential when present in murine tissues.
DISCUSSION
Early studies of S. aureus have described the species as being auxotrophic for proline (15). Previous studies have suggested that under high-osmolarity growth conditions, proline accumulates and serves as an osmoprotectant for S. aureus (2, 16, 29, 40, 43) and Bacillus subtilis(42) as well as gram-negative bacteria (12, 44). There appear to be at least two proline transport systems for bringing proline into S. aureus cells: a high-affinity system that is thought to be used primarily for scavenging proline for nitrogen and carbon sources and a low-affinity system that is osmotically regulated (2, 40, 43). Past studies have not examined the role of proline permease in S. aureus survival in vivo and in the overall virulence of the organism.
An STM system was used to generate Tn917 insertions in the S. aureus genome (10) to study the effect of these mutations on growth when the mutants were passaged through animal models. The largest class of mutants affected peptide and amino acid transport functions (10), including two amino acid permease mutants. One of these permease mutants that was identified and characterized from this technique affected the high-affinity proline permease gene, putP. Large-pool and small-pool screening suggested that the putP mutant was attenuated in all of the animal models that were used (abscess, bacteremia, endocarditis, and wound). By using four diverse animal models, the relevance of attenuation of specific genes could be broadly delineated. For example, the burn wound model measures the ability of an organism to initiate rapid growth (41) whereas the abscess model characterizes its ability to grow in a closed space under pressure from host inflammatory responses (45). When the putP mutant was tested as a homogeneous population in two animal models, its virulence was reduced by approximately 1 log unit compared to that of the wild-type parental strain, RN6390 (Fig. 5 and 6). An unrelated Tn917 OST mutant was not significantly attenuated, demonstrating that random Tn917 mutations themselves do not always result in a change in the virulence of the bacteria. Furthermore, an agr mutant of strain RN6390 (32) displayed a 1-log-unit decrease in the viable count from abscess material, but the strain was not significantly changed for its overall survival when growing in wounds. Agr regulates the production of several exoproteins, including α-hemolysin, serine-protease, lipase, and DNase (9, 32). These exoproteins may be needed to establish and sustain an infection in the abscess milieu.
Proline is required by the S. aureus to grow. The in vivo attenuation noted for the putP mutant corresponds to a deficiency in the ability to scavenge proline, and our transport assay results support these findings. Less proline was imported into the S. aureus cells possessing a Tn917 insertion in putP than into the wild-type parental strain. Although the reduction in the uptake of proline was not substantial (approximately 33% overall), it appears to be sufficient to cause less growth of the S. aureus in animal tissues. It has been previously shown that the bacteria have a diminished capacity to import a variety of amino acids, including alanine, lysine, and proline, within the depths of cardiac vegetations infected with gram-positive bacteria in cases of endocarditis (13). Thus, it is quite reasonable to conclude that a limited ability to take up a specific essential amino acid (via a mutation) would result in in vivo attenuation of that organism. l-Proline can be utilized as the sole source of carbon and nitrogen in several species of bacteria (44). In E. coli and Salmonella typhimurium, the putP gene product serves as a major carrier, bringing l-proline across the cytoplasmic membrane (24, 25). By knocking out this high-affinity permease, the bacteria may not be able to procure enough proline in vivo within abscess, cardiac, or wound tissues to allow maximal growth to occur. Gram-negative bacteria have three proline permeases (44), and S. aureus has at least two proline permease systems, a low-affinity system and a high-affinity system (2, 40), which have partially redundant functions. The transport experiments also reflect this redundancy in that a mutation in putP did not totally abolish proline uptake into the staphylococcal cells. Genetic knockouts of all of the proline permease systems in S. aureus may show even greater attenuation of growth in vivo.
The mutation in putP did affect the virulence of the S. aureus cells, suggesting that an inhibitor of proline uptake may function as a bacteriostatic agent. Two proline analogs that are well characterized, AZ and DHP, interrupted 95% of the proline transport into the staphylococcal cells, and DHP also temporarily affected the growth of S. aureus RN6390. Additional retardation of growth by DHP may not have occurred due to catabolism of the inhibitor, rendering it inactive over time. These analogs of proline have different effects on specific proline uptake pathways. It has been demonstrated that AZ and DHP affect the proline transporters differently in E. coli (38). Furthermore, the inhibition of proline uptake was shown to be more effective for DHP than for AZ when the low-affinity proline permease transport systems were tested (33, 40), which could explain why DHP may have impeded the growth of the staphylococcal cells more effectively than AZ did. Both proline analogs are only 77 to 95% effective against inhibiting the uptake of all proline transporters, which could allow enough proline into the bacterial cells to permit growth. Other investigators have shown that these proline analogs can be toxic for E. coli (38) and S. typhimurium (25). On the other hand, both analogs also have deleterious effects on mammalian cells, making both unusable as components of therapeutic drugs. A new proline analog antibiotic that is not toxic to humans but blocks proline uptake in disease-causing bacteria could be useful in treating S. aureus infections.
ACKNOWLEDGMENTS
We thank A. Cheung, B. Kreiswirth, and S. Projan for bacterial strains and phages. We are grateful to D. Portnoy and G. Muller for the pLTV1 plasmid. We appreciate the efforts of L. Goltry and E. Tolentino for the synthesis of oligonucleotides and the ABI DNA sequencing. We thank K. Miller for helpful suggestions about the proline uptake assay. We are also grateful to W. Hufnagle for critiquing the manuscript and S. Earhart for technical assistance. We thank I. Kupferwasser for technical assistance in the animal endocarditis studies.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Meyers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Bae J-H, Miller K J. Identification of two proline transport systems in Staphylococcus aureusand their possible roles in osmoregulation. Appl Environ Microbiol. 1992;58:471–475. doi: 10.1128/aem.58.2.471-475.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergdoll M S. Staphylococcus aureus. In: Doyle M P, editor. Foodborne bacterial pathogens. New York, N.Y: Marcel Dekker, Inc.; 1989. pp. 463–523. [Google Scholar]
- 4.Berman H M, McGandy E L, Burgner II J W, VanEtten R L. The crystal and molecular structure of l-azetidine-2-carboxylic acid. A naturally occurring homolog of proline. J Am Chem Soc. 1969;91:6177–6182. doi: 10.1021/ja01050a044. [DOI] [PubMed] [Google Scholar]
- 5.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 6.Camilli A, Portnoy D A, Youngman P. Insertional mutagenesis of Listeria monocytogenes with a novel Tn917derivative that allows direct cloning of DNA flanking transposon insertions. J Bacteriol. 1990;172:3738–3744. doi: 10.1128/jb.172.7.3738-3744.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Reduced susceptibility of Staphylococcus aureusto vancomycin—Japan, 1996. Morbid Mortal Weekly Rep. 1997;46:624–626. [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Staphylococcus aureuswith reduced susceptibility to vancomycin—United States, 1997. Morbid Mortal Weekly Rep. 1997;46:765–766. [PubMed] [Google Scholar]
- 9.Cheung A L, Koomey J M, Butler C A, Projan S J, Fischetti V A. Regulation of exoprotein expression in Staphylococcus aureus by a locus (sar) distinct from agr. Proc Natl Acad Sci USA. 1992;89:6462–6466. doi: 10.1073/pnas.89.14.6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coulter, S. N., W. R. Schwan, E. Y. W. Ng, M. Langhorne, H. Ritchie, S. Westbrock-Wadman, W. O. Hufnagle, K. Folger, A. S. Bayer, and C. K. Stover.Staphylococcus aureus genetic loci impacting growth and survival in mutliple infection environments. Submitted for publication. [DOI] [PubMed]
- 11.Csonka L N. The role of proline in osmoregulation in Salmonella typhimurium and Escherichia coli. Basic Life Sci. 1981;18:533–542. doi: 10.1007/978-1-4684-3980-9_32. [DOI] [PubMed] [Google Scholar]
- 12.Csonka L N, Hanson A D. Prokaryotic osmoregulation: genetics and physiology. Annu Rev Microbiol. 1991;45:569–606. doi: 10.1146/annurev.mi.45.100191.003033. [DOI] [PubMed] [Google Scholar]
- 13.Durack D T, Beeson P B. Experimental bacterial endocarditis. II. Survival of bacteria in endocardial vegetations. Br J Exp Pathol. 1972;53:50–53. [PMC free article] [PubMed] [Google Scholar]
- 14.Easmon C S F, Adlam C. Staphylococci and staphylococcal infections. New York, N.Y: Academic Press; 1983. [Google Scholar]
- 15.Gladstone G P. The nutrition of Staphylococcus aureus: nitrogen requirements. Br J Exp Pathol. 1937;19:208–226. [Google Scholar]
- 16.Graham J E, Wilkinson B J. Staphylococcus aureusosmoregulation: roles for choline, glycine betaine, proline and taurine. J Bacteriol. 1992;174:2711–2716. doi: 10.1128/jb.174.8.2711-2716.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grant M M, Brown A S, Corwin L M, Troxler R F, Franzblau C. Effect of l-azetidine-2-carboxylic acid on growth and proline metabolism in Escherichia coli. Biochim Biophys Acta. 1875;404:180–187. doi: 10.1016/0304-4165(75)90324-4. [DOI] [PubMed] [Google Scholar]
- 18.Hasegawa N, San Clemente C L. Virulence and immunity of Staphylococcus aureusBB and certain deficient mutants. Infect Immun. 1978;22:473–479. doi: 10.1128/iai.22.2.473-479.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hensel M, Shea J E, Gleeson C, Jones M D, Dalton E, Holden D W. Simultaneous identification of bacterial virulence genes by negative selection. Science. 1995;269:400–403. doi: 10.1126/science.7618105. [DOI] [PubMed] [Google Scholar]
- 20.Iandolo J J, Kraemer G R. High frequency transformation of Staphylococcus aureusby electroporation. Curr Microbiol. 1990;21:373–376. [Google Scholar]
- 21.Iandolo J J, Shafer W M. Regulation of staphylococcal enterotoxin B. Infect Immun. 1977;16:610–616. doi: 10.1128/iai.16.2.610-616.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jarvis, W. R., and W. J. Martone. 1992. Predominant pathogens in hospital infections. J. Antimicrob. Chemother. 29(Suppl A):19–24. [DOI] [PubMed]
- 23.Michel M, Gutmann L. Methicillin-resistant Staphylococcus aureusand vancomycin-resistant enterococci: therapeutic realities and possibilities. Lancet. 1997;349:1901–1904. doi: 10.1016/s0140-6736(96)11192-2. [DOI] [PubMed] [Google Scholar]
- 24.Motojima K, Yamamoto I, Anraku I. Proline transport carrier-defective mutants of Escherichia coliK-12: properties and mapping. J Bacteriol. 1978;136:5–9. doi: 10.1128/jb.136.1.5-9.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Myers R S, Townsend D, Maloy S. Dissecting the molecular mechanism of ion-solute cotransport: substrate specificity mutations in the putPgene affect the kinetics of proline transport. J Membr Biol. 1991;121:201–214. doi: 10.1007/BF01951554. [DOI] [PubMed] [Google Scholar]
- 26.Nicas T I, Zeckel M L, Braun D K. Beyond vancomycin: new therapies to meet the challenge of glycopeptide resistance. Trends Microbiol. 1997;5:240–249. doi: 10.1016/S0966-842X(97)01051-2. [DOI] [PubMed] [Google Scholar]
- 27.Novick R P. The Staphylococcus as a molecular genetic system. In: Novick R P, editor. Molecular biology of the staphylococci. New York, N.Y: VCH Publishers; 1990. pp. 1–40. [Google Scholar]
- 28.Perlman B B, Freedman L R. Experimental endocarditis. II. Staphylococcal infection of the aortic valve following placement of a polyethylene catheter in the left side of the heart. Yale J Biol Med. 1971;42:394–410. [PMC free article] [PubMed] [Google Scholar]
- 29.Pourkomailian B, Booth I R. Glycine betaine transport by Staphylococcus aureus: evidence for feedback regulation of the activity of the two transport systems. Microbiology. 1994;140:3131–3138. doi: 10.1099/13500872-140-11-3131. [DOI] [PubMed] [Google Scholar]
- 30.Proctor A R, Kloos W E. The tryptophan gene cluster of Staphylococcus aureus. J Gen Microbiol. 1970;64:319–327. doi: 10.1099/00221287-64-3-319. [DOI] [PubMed] [Google Scholar]
- 31.Ratzkin B, Grabnar M, Roth J. Regulation of the major proline permease in Salmonella typhimurium. J Bacteriol. 1978;133:737–743. doi: 10.1128/jb.133.2.737-743.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Recsei P, Kreiswirth B, O’Reilly M, Schlievert P, Gruss A, Novick R P. Regulation of exoprotein gene expression in Staphylococcus aureus by agr. Mol Gen Genet. 1986;202:58–61. doi: 10.1007/BF00330517. [DOI] [PubMed] [Google Scholar]
- 33.Rowland I, Tristam H. Specificity of the Escherichia coliproline transport system. J Bacteriol. 1975;123:871–877. doi: 10.1128/jb.123.3.871-877.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rudin L, Sjostrom J E, Lindberg M, Philipson L. Factors affecting competence for transformation in Staphylococcus aureus. J Bacteriol. 1974;118:155–164. doi: 10.1128/jb.118.1.155-164.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 36.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwan W R, Kügler S, Schüller S, Kopecko D J, Goebel W. Detection and characterization by differential PCR of host eukaryotic cell genes differentially transcribed following uptake of intracellular bacteria. Infect Immun. 1996;64:91–99. doi: 10.1128/iai.64.1.91-99.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stalmach M E, Grothe S, Wood J M. Two proline porters in Escherichia coliK-12. J Bacteriol. 1983;156:481–486. doi: 10.1128/jb.156.2.481-486.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thornsberry, C. 1994. Epidemiology of staphylococcal infections—a USA perspective. J. Chemother. 6(Suppl. 2):61–65. [PubMed]
- 40.Townsend D E, Wilkinson B J. Proline transport in Staphylococcus aureus: a high-affinity system and a low affinity system involved in osmoregulation. J Bacteriol. 1992;174:2702–2710. doi: 10.1128/jb.174.8.2702-2710.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vasishta R, Saxena M, Chhibber S. Contribution of silver ion replacement to the pathogenicity of Pseudomonas aeruginosawith special reference to burn sepsis. Folia Microbiol. 1991;36:498–501. doi: 10.1007/BF02884072. [DOI] [PubMed] [Google Scholar]
- 42.von Blohn C, Kempf B, Kappes R M, Bremer E. Osmostress response in Bacillus subtilis: characterization of a proline uptake system (OpuE) regulated by high osmolarity and the alternative transcription factor sigma B. Mol Microbiol. 1997;25:175–187. doi: 10.1046/j.1365-2958.1997.4441809.x. [DOI] [PubMed] [Google Scholar]
- 43.Wengender P A, Miller K J. Identification of a PutP proline permease gene homolog from Staphylococcus aureus by expression cloning of the high-affinity proline transport system in Escherichia coli. Appl Environ Microbiol. 1995;61:252–259. doi: 10.1128/aem.61.1.252-259.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wood J M. Proline porters effect the utilization of proline as nutrient or osmoprotectant for bacteria. J Membr Biol. 1988;106:183–202. doi: 10.1007/BF01872157. [DOI] [PubMed] [Google Scholar]
- 45.Xiong Z, Ge S, Chamberlain N, Kapral F. Growth cycle-induced changes in sensitivity of Staphylococcus aureusto bacterial lipids from abscesses. J Med Microbiol. 1993;39:58–63. doi: 10.1099/00222615-39-1-58. [DOI] [PubMed] [Google Scholar]