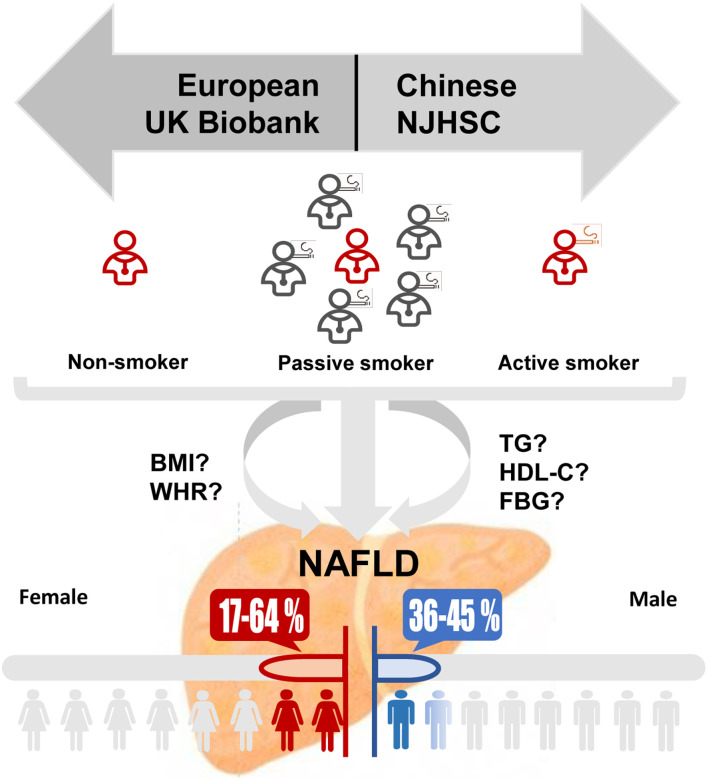
Graphical abstract
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease with an estimated worldwide prevalence of 32.4%.1 The multisystem condition is related to an increased risk of liver-related and cardiovascular extrahepatic diseases.2 Smoking is the leading preventable risk factor for premature disability and mortality. As NAFLD and smoking are both associated with the development of metabolic features, there has been increasing interest in testing the relationship between smoking and NAFLD. The causal relevance of smoking to NAFLD incidence have been implicated in cross-sectional and prospective cohort studies.3,4 However, previous studies mainly focused on the effect of active smoking, discussion on the influence of passive smoking on NAFLD will facilitate illustrating the association between smoking and NAFLD. We conducted a national two-center cross-section study of active, passive smoking and NAFLD risk in Chinese and European population. This design allowed us to (1) test for associations between active, passive smoking and NAFLD risk by sex and consolidate evidence for causality by estimating dose-response relationship, (2) identify mediated factors, and (3) resolve their possible interaction with smoking.
In this study, we reported results from the UK Biobank study (application number: 85248), a large-scale, prospective study which recruited more than 500,000 participants from 40 to 70 years of age,5 and independently validated the association in a Chinese population, the Nanjing Health Examination Cohort (NJHE Cohort). All participants provided written informed consent. The UK Biobank obtained ethical approval from the NHS National Research Ethics Service. After excluded those with missing data on NAFLD diagnosis and smoking status, high alcohol consumption or baseline liver diseases (Supplementary Table 1), 14,348 and 11,211 participants were included in further analysis, respectively (Supplementary Fig. 1). Smoking status was classified as non-, current, former, and passive smokers. NAFLD diagnosis was based on the presence of three findings, (1) evidence of hepatic steatosis by either histology or imaging, (2) without heavy alcohol consumption, (3) without history of specific diseases that could lead to steatosis.
Log-binomial logistic regression was used to examine the association of active, passive smoking with NAFLD risk by sex, in which age, ethnicity, education level, physical activity, and drinking status were adjusted. To assess the influence of potential mediating factors, we further adjusted for body mass index (BMI), triglycerides, fasting blood glucose, high-density lipoprotein cholesterol (HDL-C) and waist-hip ratio (UK Biobank only) and performed mediation analysis using the above variables as potential mediators. Stratified analyses were conducted to assess interaction effects of aforementioned variants. Generalized additive models (GAMs) were used to evaluate nonlinear relations between exposure to secondhand smoke and the value of liver proton density fat fraction (PDFF) among nonsmokers in UK Biobank. Mendelian randomization (MR) analyses between smoking initiation and NAFLD were performed to consolidate the association. We then calculated category-specific population attributable fraction (PAF), a fraction of total NAFLD risk in the population that would be eliminated if persons in the specific exposure category shifted to the low-risk group.6 We performed a series of sensitivity analyses to test the stability of the results. First, we restricted the analysis to participants with complete covariates. Second, in order to consist with the definition in UK Biobank, we redefined passive smoker in the NJHE Cohort. Third, we restricted the analysis to those 40 years of age or older in the NJHE Cohort to coincide with the age distribution in UK Biobank. Fourth, we adopted a more rigorous exclusion criteria to ensure the specificity of smoking with NAFLD. Detailed information on methods and statistical analyses are provided in the Supplementary File 1.
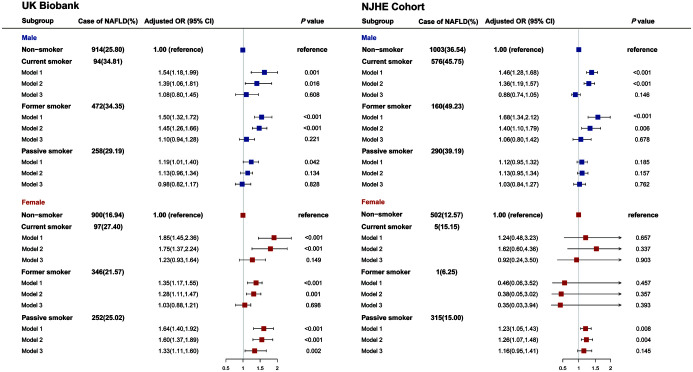
The baseline characteristics of study participants from the UK Biobank and NJHE Cohort are shown in Table 1. We observed a higher prevalence of NAFLD in Chinese men (40.03%), but a lower rate in Chinese women (13.40%). Compared with nonsmokers, current smokers had a significantly higher NAFLD risk in both sexes in the UK Biobank (adjusted OR, 1.39 [1.06–1.81] and 1.75 [1.37–2.24] for men and women, respectively; Fig. 1). An equivalent effect for men was observed in the NJHE Cohort, with an adjusted OR of 1.36 [1.19–1.57]. Female passive smoking in women was found prominently associated with an increased risk of NAFLD (adjusted OR, 1.60 [1.37–1.89] and 1.26 [1.07–1.48] in the UK Biobank and NJHE Cohort, respectively), not in men. Strength of the aforementioned association was weakened after adjusted for potential mediators. The results were substantially unchanged in sensitivity analyses (Supplementary Tables 2–5). What is more, greater cumulative cigarettes consumptions, shorter durations of smoking cessation and longer duration of secondhand smoke exposure showed stronger associations with NAFLD risk (Supplementary Table 6). The dose-response relationship was also found through the GAM (Supplementary Fig. 2), where substantially rising curves were observed when we assessed the association between absolute exposure of secondhand smoke and value of liver PDFF among female nonsmokers in the UK Biobank (p=0.001 for exposure at home and p<0.001 for exposure outside home). Ensured the absence of pleiotropy (MR Egger’s intercept p-value=0.086) and homogeneity (the p-value for heterogeneity was 0.218) of instrumental variables, we used the results from inverse-variance weighted (IVW) in MR analysis (Supplementary Table 7), and found that genetic liability to smoking initiation was positively associated with NAFLD (OR, 1.76 [1.29–2.41]; p=3.65×10−4).
Table 1. Baseline characteristics of participants according to NAFLD by sex†.
| Characteristic | UK Biobank, n=14,348 |
NJHE Cohort, n=11,211 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men |
Women |
Men |
Women |
|||||||||
| NAFLD, n=1,738 | No NAFLD, n=4,332 | p-value | NAFLD, n=1,595 | No NAFLD, n=6,683 | p-value | NAFLD, n=2,029 | No NAFLD, n=3,040 | p-value | NAFLD, n=823 | No NAFLD, n=5,319 | p-value | |
| Age, year | 55.05 (7.42) | 55.25 (7.80) | 0.363 | 54.41 (7.03) | 53.66 (7.36) | <0.001 | 39.09 (10.91) | 36.55 (10.79) | <0.001 | 44.89 (11.84) | 37.46 (9.40) | <0.001 |
| Ethnicity | ||||||||||||
| White ethnicity/Han nationality | 1,685 (96.95) | 4,200 (96.95) | 0.657 | 1,551 (97.24) | 6,536 (97.80) | 0.220 | 1,984 (97.78) | 2,982 (98.09) | 0.443 | 811 (98.54) | 5,226 (98.25) | 0.550 |
| Other | 50 (2.88) | 119 (2.75) | 42 (2.63) | 133 (1.99) | 45 (2.22) | 58 (1.91) | 12 (1.46) | 93 (1.75) | ||||
| Unknown | 3 (0.17) | 13 (0.30) | 2 (0.13) | 14 (0.21) | 0 | 0 | 0 | 0 | ||||
| Education level | ||||||||||||
| College or University degree | 702 (40.39) | 2,192 (50.60) | <0.001 | 576 (36.11) | 3,083 (46.13) | <0.001 | 1,899 (93.59) | 2,907 (95.63) | 0.001 | 712 (86.51) | 5,023 (94.44) | <0.001 |
| Other level | 875 (50.35) | 1,810 (41.78) | 836 (52.41) | 3,109 (46.52) | 130 (6.41) | 133 (4.38) | 110 (13.37) | 295 (5.55) | ||||
| Unknown | 161 (9.26) | 330 (7.62) | 183 (11.47) | 491 (7.35) | 0 | 0 | 1 (0.12) | 1 (0.02) | ||||
| Body mass index, kg/m2 | 29.16 (4.10) | 26.19 (3.34) | <0.001 | 29.56 (5.09) | 25.25 (4.04) | <0.001 | 26.71 (3.02) | 23.44 (2.50) | <0.001 | 25.62 (3.33) | 21.49 (2.33) | <0.001 |
| Physical activity | ||||||||||||
| Yes | 744 (42.81) | 2,181 (50.35) | <0.001 | 600 (37.62) | 2,965 (44.37) | <0.001 | 416 (20.50) | 489 (16.09) | <0.001 | 242 (29.40) | 1,559 (29.31) | 0.393 |
| No | 773 (44.48) | 1,632 (37.67) | 706 (44.26) | 2,554 (38.22) | 1,613 (79.50) | 2,547 (83.78) | 577 (70.11) | 3,748 (70.46) | ||||
| Unknown | 221 (12.72) | 519 (11.98) | 289 (18.12) | 1,164 (17.42) | 0 | 4 (0.13) | 4 (0.49) | 12 (0.23) | ||||
| Drinking status | ||||||||||||
| Never | 44 (2.53) | 104 (2.40) | 0.985 | 64 (4.01) | 191 (2.86) | 0.107 | 1,520 (74.91) | 2,312 (76.05) | 0.488 | 791 (96.11) | 5,155 (96.92) | 0.003 |
| Former | 47 (2.70) | 114 (2.63) | 33 (2.07) | 149 (2.23) | 9 (0.44) | 11 (0.36) | 0 | 1 (0.02) | ||||
| Current | 1,646 (94.71) | 4,112 (94.92) | 1,498 (93.92) | 6,342 (94.90) | 499 (24.59) | 717 (23.59) | 30 (3.65) | 163 (3.06) | ||||
| Unknown | 1 (0.06) | 2 (0.05) | 0 | 1 (0.01) | 1 (0.05) | 0 | 2 (0.24) | 0 | ||||
| Smoking status | ||||||||||||
| Nonsmoker | 914 (52.59) | 2,628 (60.66) | <0.001 | 900 (56.43) | 4,413 (66.03) | <0.001 | 1,003 (49.43) | 1,742 (57.30) | <0.001 | 502 (61.00) | 3,491 (65.63) | 0.051 |
| Passive smoker | 258 (14.84) | 626 (14.45) | 252 (15.80) | 755 (11.30) | 290 (14.29) | 450 (14.80) | 315 (38.27) | 1,785 (33.56) | ||||
| Former smoker | 472 (27.16) | 902 (20.82) | 346 (21.69) | 1,258 (18.82) | 160 (7.89) | 165 (5.43) | 1 (0.12) | 15 (0.28) | ||||
| Current smoker | 94 (5.41) | 176 (4.06) | 97 (6.08) | 257 (3.85) | 576 (28.39) | 683 (22.47) | 5 (0.61) | 28 (0.53) | ||||
| Pack years of smoking | 24.32 (18.70) | 19.37 (15.80) | <0.001 | 20.26 (15.54) | 15.66 (12.14) | <0.001 | 12.11(12.97) | 10.24(12.79) | 0.004 | 5.95(6.78) | 2.40(3.31) | 0.042 |
| Duration of smoking cessation, year | 18.51 (11.49) | 22.04 (11.57) | <0.001 | 17.62 (11.26) | 19.41 (11.12) | 0.005 | 6.91(8.30) | 5.96(7.24) | 0.366 | 1.13(1.24) | 4.58(3.62) | 0.012 |
| Exposure of SHS at home, hour per week‡ | 0.50 (4.16) | 0.21 (2.59) | 0.007 | 0.41 (3.56) | 0.27 (2.88) | 0.168 | 19.07 (13.06) | 16.01 (12.38) | 0.007 | 21.17 (11.03) | 16.72 (10.13) | <0.001 |
| Exposure of SHS outside home, hour per week§ | 0.48 (2.58) | 0.32 (1.62) | 0.013 | 0.38 (1.89) | 0.21 (0.86) | <0.001 | 2.01 (2.65) | 1.86 (2.58) | 0.216 | 1.58 (2.54) | 1.58 (2.39) | 0.999 |
| Fasting blood glucose, mmol/L | 5.16 (1.17) | 4.95 (0.83) | <0.001 | 5.07 (1.05) | 4.91 (0.71) | <0.001 | 5.34 (1.18) | 4.99 (0.65) | <0.001 | 5.47 (1.35) | 4.87 (0.50) | <0.001 |
| Triglycerides, mmol/L | 2.27 (1.17) | 1.76 (0.91) | <0.001 | 1.84 (0.92) | 1.31 (0.65) | <0.001 | 2.18 (1.53) | 1.33 (0.82) | <0.001 | 1.84 (1.10) | 1.01 (0.49) | <0.001 |
| HDL-C, mmol/L | 1.19 (0.24) | 1.31 (0.27) | <0.001 | 1.48 (0.31) | 1.66 (0.34) | <0.001 | 1.14 (0.20) | 1.29 (0.25) | <0.001 | 1.29 (0.25) | 1.54 (0.29) | <0.001 |
| Waist-hip Ratio | 0.95 (0.06) | 0.91 (0.06) | <0.001 | 0.85 (0.06) | 0.79 (0.06) | <0.001 | – | – | – | – | – | – |
†p-values were calculated using Student’s t-test for equal variances, Kruskal-Wallis test for unequal variances and χ2 tests for categorical variables. Values are mean (SD) or n (%).‡The unit of this variable in NJHE Cohort is year. §The unit of this variable in NJHE Cohort is hour per day. HDL-C, high-density lipoprotein cholesterol; NAFLD, nonalcoholic fatty liver disease; SHS, secondhand smoke.
Fig. 1. Association between risk of NAFLD and smoking status by sex.
Odds ratios (ORs) were derived from logistic regression models. Model 1: unadjusted. Model 2: adjusted for age, ethnicity, physical activity, education level, drinking status. Model 3: model2+BMI, triglycerides, fasting blood glucose, HDL-C, waist-hip ratio (UK Biobank only). BMI, body mass index; HDL-C, high-density lipoprotein cholesterol.
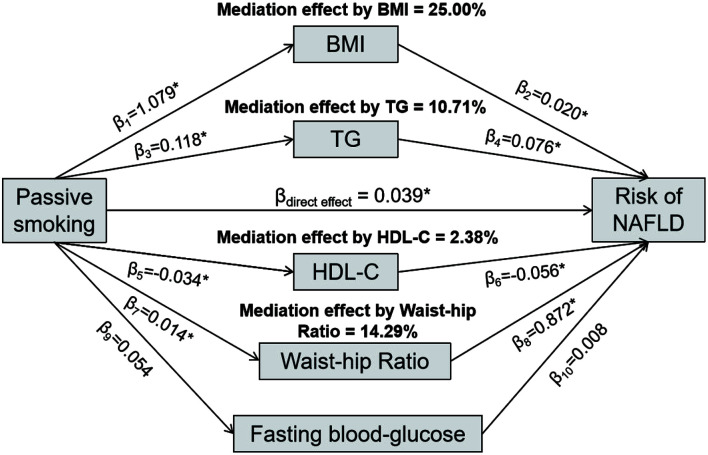
In stratified analysis (Supplementary Figs. 3–4), the associations between smoking status and NAFLD risk were stronger among those with abnormal metabolic condition. On this basis, we found that the percentages of the effect mediated by BMI, triglycerides, HDL-C, and waist-hip ratio were estimated as 25.00%, 10.71%, 2.38% and 14.29% in the association between female passive smoking and NAFLD risk in the UK Biobank (Fig. 2), and the relations between active smoking and NAFLD risks were significantly mediated by aforementioned factors in both sexes (Supplementary Fig. 5). PAFs for population counterfactuals were reported in Supplementary Table 8. In the UK Biobank, if passive smokers avoided exposure to secondhand smoke, 5.70% [3.40–7.95%] of observed NAFLD cases could have been averted in the whole population, while an absolute higher PAF (8.25% [5.19–11.22%]) was calculated for women. More detailed description of results is provided in Supplementary File 2.
Fig. 2. Mediation effects of BMI, TG, HDL-C, FBG and WHR on the association between female passive smoking and risk of nonalcoholic fatty liver disease in UK Biobank.
Data are regression coefficients adjusted for age, ethnicity, physical activity, education level, and drinking status; *p<0.05 for coefficients different from 0. BMI, body mass index; HDL-C, high-density lipoprotein cholesterol; NAFLD, nonalcoholic fatty liver disease; TG, triglycerides.
In our study, patterns of association between smoking status and NAFLD varied among the multi-ethnic populations. Both male and female active smokers were related to increased risk of NAFLD in the UK Biobank, while the positive association was only observed among men in the NJHE Cohort. Similarly, a cross-sectional analysis also reported a positive association between current smoking and NAFLD risk among Korean men but not among women.7 Low exposure rate of smoking in Asian women may account for the sex heterogeneity.8 Additionally, dose-response relationship results further reinforced the causality correlation. In the present study, accumulated pack-year was strongly associated with the severity of NAFLD. Among former smokers who have quit smoking for more than 15 years, the association with the risk of NAFLD was weakened. This variation trend might be regulated by a greater decrease in insulin resistance, which was verified in a Korean study.9 Previous studies on a potential association between passive smoking and the NAFLD risk have shown conflicting results. A Finnish longitudinal cohort revealed that passive smoking in both child and adult lives were associated with increased risk of adult fatty liver.3 Data from the National Health and Nutrition Examination Survey failed finding the positive association between NAFLD and serum cotinine level.10 Our study demonstrated that a longer duration of secondhand smoke exposure was significantly associated with the risk of NAFLD in both sexes.
Smoking is proved to be associated with an increase in low-density lipoprotein cholesterol (LDL-C), plasma triglycerides, and insulin resistance as well as a decrease in plasma HDL-C levels,11,12 which are also relevant to the occurrence of NAFLD.13 Emerging evidence now suggests that nicotine in the blood exacerbates hepatic steatosis through increased oxidative stress, hepatocellular apoptosis, and decreased phosphorylation (inactivation) of adenosine-5-monophosphate-activated protein kinase, leading to increased hepatic lipogenesis.14 Given these findings, we explored the potential pathways and affirmed that the associations of smoking with NAFLD were mediated through above factors, consistent with previous findings that cigarette smoking is a cofactor of lipid profiles in hepatic steatosis,15 highlighting the necessity of smoking cessation, especially among those of abnormal metabolic markers who were more vulnerable to fatty liver. To the best of our knowledge, this is the first study to reveal the public health implications of cigarette control on the incidence of NAFLD using the calculation of PAFs. Among women about 7% of NAFLD cases could be attributed to secondhand smoke exposure, suggesting that effective strategies should be implemented on preventing secondhand smoke exposure.
The present study has several limitations. The cross-sectional study limited our ability to establish a temporal relationship between smoking and NAFLD, and internal exposure such as serum cotinine level requires evidence of the association of smoking exposure and NAFLD. In summary, our study extends the range of adverse health outcomes positively associated with cigarette exposure, lending robust support to smoking intervention on the reduction of NAFLD in multi-ethnic populations.
Supporting information
PDFF, proton density fat fraction; SHS, secondhand smoke. Generalized additive models were used to estimate degree of freedom and p-values.
BMI, body mass index; FBG, fasting blood glucose; HDL-C, high-density lipoprotein cholesterol; NAFLD, nonalcoholic fatty liver disease; TG, triglycerides; WHR, waist-hip ratio. Risk estimates were adjusted for age, ethnicity, physical activity, education level, and drinking status.
BMI, body mass index; FBG, fasting blood glucose; HDL-C, high-density lipoprotein cholesterol; NAFLD, nonalcoholic fatty liver disease; TG, triglycerides. Risk estimates were adjusted for age, ethnicity, physical activity, education level, and drinking status.
*p<0.05 for coefficients different from 0. Data are regression coefficients with adjustment for age, ethnicity, physical activity, education level, and drinking status. BMI, body mass index; HDL-C, high-density lipoprotein cholesterol; NAFLD, nonalcoholic fatty liver disease; TG, triglycerides.
Acknowledgments
This research was conducted using the UK Biobank Resource (Application Number: 85248). We thank the investigators and participants in the UK Biobank and Nanjing Health Examination Cohort for their contributions to this study.
Abbreviations
- BMI
body mass index
- CI
confidence interval
- GAM
generalized additive model
- HDL-C
high-density lipoprotein cholesterol
- IVW
inverse-variance weighted
- LDL-C
low-density lipoprotein cholesterol
- MR
mendelian randomization
- MRI
magnetic resonance imaging
- NAFLD
nonalcoholic fatty liver disease
- NJHE Cohort
Nanjing Health Examination Cohort
- OR
odds ratio
- PAF
population attributable fraction
- PDFF
proton density fat fraction
Ethical statement
UK Biobank has full ethical approval from the NHS National Research Ethics Service (21/NW/0157).
Data sharing statement
The Nanjing Health Examination Cohort data used to support the findings of this study have not been made available; The UK Biobank data are available from https://www.ukbiobank.ac.uk/. Restrictions apply to the availability of these data, which were used under license for the current study (Project ID: 85248). Data are available for bona fide researchers upon application to the UK Biobank.
References
- 1.Riazi K, Azhari H, Charette JH, Underwood FE, King JA, Afshar EE, et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2022;7(9):851–861. doi: 10.1016/S2468-1253(22)00165-0. [DOI] [PubMed] [Google Scholar]
- 2.Patel AH, Peddu D, Amin S, Elsaid MI, Minacapelli CD, Chandler TM, et al. Nonalcoholic Fatty Liver Disease in Lean/Nonobese and Obese Individuals: A Comprehensive Review on Prevalence, Pathogenesis, Clinical Outcomes, and Treatment. J Clin Transl Hepatol. 2023;11(2):502–515. doi: 10.14218/JCTH.2022.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu F, Pahkala K, Juonala M, Jaakkola J, Rovio SP, Lehtimäki T, et al. Childhood and Adulthood Passive Smoking and Nonalcoholic Fatty Liver in Midlife: A 31-year Cohort Study. Am J Gastroenterol. 2021;116(6):1256–1263. doi: 10.14309/ajg.0000000000001141. [DOI] [PubMed] [Google Scholar]
- 4.Liu Y, Dai M, Bi Y, Xu M, Xu Y, Li M, et al. Active smoking, passive smoking, and risk of nonalcoholic fatty liver disease (NAFLD): a population-based study in China. J Epidemiol. 2013;23(2):115–121. doi: 10.2188/jea.je20120067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caleyachetty R, Littlejohns T, Lacey B, Bešević J, Conroy M, Collins R, et al. United Kingdom Biobank (UK Biobank): JACC Focus Seminar 6/8. J Am Coll Cardiol. 2021;78(1):56–65. doi: 10.1016/j.jacc.2021.03.342. [DOI] [PubMed] [Google Scholar]
- 6.Lee M, Whitsel E, Avery C, Hughes TM, Griswold ME, Sedaghat S, et al. Variation in Population Attributable Fraction of Dementia Associated With Potentially Modifiable Risk Factors by Race and Ethnicity in the US. JAMA Netw Open. 2022;5(7):e2219672. doi: 10.1001/jamanetworkopen.2022.19672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jung HS, Chang Y, Kwon MJ, Sung E, Yun KE, Cho YK, et al. Smoking and the Risk of Non-Alcoholic Fatty Liver Disease: A Cohort Study. Am J Gastroenterol. 2019;114(3):453–463. doi: 10.1038/s41395-018-0283-5. [DOI] [PubMed] [Google Scholar]
- 8.Giovino GA, Mirza SA, Samet JM, Gupta PC, Jarvis MJ, Bhala N, et al. Tobacco use in 3 billion individuals from 16 countries: an analysis of nationally representative cross-sectional household surveys. Lancet. 2012;380(9842):668–679. doi: 10.1016/S0140-6736(12)61085-X. [DOI] [PubMed] [Google Scholar]
- 9.Jang YS, Joo HJ, Park YS, Park EC, Jang SI. Association between smoking cessation and non-alcoholic fatty liver disease using NAFLD liver fat score. Front Public Health. 2023;11:1015919. doi: 10.3389/fpubh.2023.1015919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen H, Peng JL, Tayarachakul S, Liangpunsakul S. Association between serum cotinine level and prevalence of non-alcoholic fatty liver disease: a cross-sectional study from the Third National Health and Nutrition Examination Survey. J Investig Med. 2017;65(1):43–48. doi: 10.1136/jim-2016-000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeong SH, Joo HJ, Kwon J, Park EC. Association Between Smoking Behavior and Insulin Resistance Using Triglyceride-Glucose Index Among South Korean Adults. J Clin Endocrinol Metab. 2021;106(11):e4531–e4541. doi: 10.1210/clinem/dgab399. [DOI] [PubMed] [Google Scholar]
- 12.Herath P, Wimalasekera S, Amarasekara T, Fernando M, Turale S. Effect of cigarette smoking on smoking biomarkers, blood pressure and blood lipid levels among Sri Lankan male smokers. Postgrad Med J. 2022;98(1165):848–854. doi: 10.1136/postgradmedj-2021-141016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sripongpun P, Churuangsuk C, Bunchorntavakul C. Current Evidence Concerning Effects of Ketogenic Diet and Intermittent Fasting in Patients with Nonalcoholic Fatty Liver. J Clin Transl Hepatol. 2022;10(4):730–739. doi: 10.14218/JCTH.2021.00494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sinha-Hikim AP, Sinha-Hikim I, Friedman TC. Connection of Nicotine to Diet-Induced Obesity and Non-Alcoholic Fatty Liver Disease: Cellular and Mechanistic Insights. Front Endocrinol (Lausanne) 2017;8:23. doi: 10.3389/fendo.2017.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ciardullo S, Oltolini A, Cannistraci R, Muraca E, Perseghin G. Sex-related association of nonalcoholic fatty liver disease and liver fibrosis with body fat distribution in the general US population. Am J Clin Nutr. 2022;115(6):1528–1534. doi: 10.1093/ajcn/nqac059. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PDFF, proton density fat fraction; SHS, secondhand smoke. Generalized additive models were used to estimate degree of freedom and p-values.
BMI, body mass index; FBG, fasting blood glucose; HDL-C, high-density lipoprotein cholesterol; NAFLD, nonalcoholic fatty liver disease; TG, triglycerides; WHR, waist-hip ratio. Risk estimates were adjusted for age, ethnicity, physical activity, education level, and drinking status.
BMI, body mass index; FBG, fasting blood glucose; HDL-C, high-density lipoprotein cholesterol; NAFLD, nonalcoholic fatty liver disease; TG, triglycerides. Risk estimates were adjusted for age, ethnicity, physical activity, education level, and drinking status.
*p<0.05 for coefficients different from 0. Data are regression coefficients with adjustment for age, ethnicity, physical activity, education level, and drinking status. BMI, body mass index; HDL-C, high-density lipoprotein cholesterol; NAFLD, nonalcoholic fatty liver disease; TG, triglycerides.