Abstract
Staphylococcus aureus is a major mastitis-causing pathogen in cattle. The chronic nature of bovine staphylococcal mastitis suggests that some products or components of S. aureus may interfere with the development of protective immunity. One class of molecules that could be involved are superantigens (SAgs). Although a significant number of mastitis isolates produce SAgs, the effect of these molecules on the bovine immune system is unresolved. To determine if immunosuppression caused by SAgs could play a role in pathogenesis, we monitored bovine lymphocytes exposed to staphylococcal enterotoxin C1 (SEC1). Activation of bovine lymphocytes by either SEC1 or concanavalin A (ConA) was influenced by the γδ/αβ T-cell ratio in the culture. Compared to ConA-induced stimulation, cultures stimulated with SEC1 generated small numbers of CD4+ αβ T cells expressing high levels of interleukin-2 receptor α chain (IL-2Rα) and major histocompatibility complex class II (MHCII), suggesting that SAg exposure does not lead to full activation of these cells. This state of partial activation was most pronounced in cultures with a high γδ/αβ ratio. In contrast, significant numbers of CD8+ αβ T cells expressed high levels of IL-2Rα and MHCII, regardless of the γδ/αβ ratio and the stimulant used. CD8+ blasts in cultures stimulated with SEC1 also expressed another activation marker, ACT3, previously detected predominantly on thymocytes and CD4+ T cells. Although γδ CD2− and CD2+ T cells expressed MHCII and IL-2Rα following stimulation with SEC1, only a few cells increased to blast size, suggesting that they were only partially activated. The results suggest ways in which SAgs might facilitate immunosuppression that promotes the persistence of bacteria in cattle and contributes to chronic intramammary infection.
Staphylococcus aureus is a prominent pathogen in bovine mastitis (24). This organism is frequently isolated from milk (2, 16, 40) and from cows with intramammary infection (IMI) (17). IMI caused by S. aureus tends to become chronic and may resist antibiotic therapy (49). It has been postulated that persistent infection with S. aureus is associated with an impairment of the immune response, mediated by factors produced by S. aureus (34). Thus far, however, no single factor has been clearly implicated.
Bovine isolates of S. aureus frequently produce one or more pyrogenic toxins (PTs), especially types C and D staphylococcal enterotoxins (SEs) and toxic shock syndrome toxin (24). The staphylococcal PTs are prototype microbial superantigens (SAgs), characterized by the ability to bind to major histocompatibility complex class II (MHCII) molecules and to specific Vβ segments of αβ T-cell receptor (TCR) outside the binding groove associated with MHC-restricted immune system recognition of processed peptides. By bypassing antigenic specificity, SAgs stimulate abnormally large numbers of T cells and are able, at nanomolar concentrations, to induce T-cell proliferation (33). Few studies have been performed to investigate the effects of SAgs on the bovine immune system (53); most studies have involved other animals. In several species, SAgs exert wide-ranging and deleterious effects, including induction of shock (4), T-cell unresponsiveness and deletion (23), differential stimulation of CD4+ and CD8+ T-cell subsets (47), and B-cell differentiation (46). Thus, although largely unconfirmed, there is a clear potential for SEs, and other SAgs, to modulate immune responses and contribute to the virulence and persistence of S. aureus in cattle.
The T-cell population consists of cells expressing either the αβ TCR (TCR2) or the γδ TCR (TCR1). While the roles of αβ T cells in immune responses of many species have been well characterized, the function of γδ T cells is less well understood (22). This is especially true in ruminants. Recent investigations have shown that the ruminant γδ T cells comprise two disparate subpopulations, characterized by constitutive expression of cell surface molecules. One subpopulation, similar in composition and tissue distribution to γδ T cells from other species, consists of cells that express CD2, CD5, and CD6 and are positive or negative for CD8 (10). These cells are present in low concentrations (3 to 5%) in peripheral blood and in high concentrations (35 to 40%) in spleen, gut epithelium, and mammary gland secretions (41). The second subpopulation, negative for CD2, CD6, and CD8, is unique and has been identified in only one other member of the Artiodactyla, swine (3, 28). This subpopulation is positive for CD5 and two lineage-restricted molecules, workshop cluster 1 (WC1) (32, 36, 50) and GD3.5 (21). The concentration of WC1+ GD3.5+ CD2− CD6− γδ T cells is high (30 to 50%) in the peripheral blood in young ruminants, decreasing with age, and is low (3 to 8%) in secondary lymphoid tissues and mammary gland secretions (41, 52). Definitive data on the function of either of these major subpopulations of γδ T cells have not been obtained. However, previous studies have suggested that they may be involved in regulating the proliferative response of CD4+ T cells to antigens (7).
Park et al. (42) identified a subset of T cells, positive for CD2 and CD8, in mammary gland secretions of cows infected with S. aureus. These cells had the ability to inhibit the proliferative response of bovine CD4+ cells to staphylococcal antigens (42). Although the mechanisms by which these cells were induced and mediated their effect were not determined, they clearly have the potential to contribute to the pathogenesis of staphylococcal IMI. Although not all bovine staphylococcal isolates produce known SAgs, it is important to determine whether SAg production could induce these or other immunosuppressive subpopulations in cows and promote the development of some infections such as IMI. The objective of this study was to extend these initial observations. We examined the effect of a representative SAg (SEC1) on the major subpopulations of bovine αβ and γδ T cells.
MATERIALS AND METHODS
SEC1 purification.
SEC1 was purified from S. aureus (pMIN121), a recombinant harboring the secMNDON structural gene cloned into a nontoxigenic background (strain RN4220) (4, 19). Cultures were grown with aeration at 37°C in pyrogen-free dialyzable beef heart medium containing erythromycin (50 μg/ml). SEC1 was purified to homogeneity by standard preparative flat-bed isoelectric focusing techniques (44) with broad- and narrow-pH-range ampholytes in succession. Fractions containing purified toxin were identified by immunodiffusion, and the degree of purity was assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (26). Ampholytes were removed from the toxin solution by exhaustive dialysis against pyrogen-free water.
Animals.
Purebred adult healthy Holstein-Frisian cattle were screened as prospective donors and for determination of the relative amounts of γδ and αβ T cells in their systemic circulation. Most experiments were performed with cells from two selected donors: one with a low concentration of γδ T cells (13%) and the other with high concentration (82%) (designated donors A and F, respectively). Donor A (low γδ) was a 5-year-old dairy cow from the University of Idaho dairy farm, Moscow, Idaho. Donor F (high γδ) was a 2.5-year-old steer, housed at Washington State University, Pullman, Wash. The animals were maintained according to Association for the Assessment and Accreditation of Laboratory Animal Care, International, guidelines and regulations established by the Animal Care and Use Committees at Washington State University and the University of Idaho.
Blood collection and cell culture.
Collection, processing, and conditions for culturing of peripheral blood mononuclear cells (PBMC) were described previously (42). PBMC suspensions were adjusted to 2.0 × 106 cells per ml and incubated in plastic petri dishes for 4, 24, or 96 h at 37°C in 5% CO2. The final concentrations of stimulants in cultures (SEC1, 0.1 μg/ml; concanavalin A ConA, 5.0 μg/ml) constituted the doses found to induce optimal T-cell proliferation in dose response assays (results not shown). Concanavalin A (ConA) was purchased from Sigma Chemical Co., St. Louis, Mo.
Flow cytometry.
Before and after stimulation in culture, the cells were processed for single or multiple color flow cytometric analysis by established techniques (11). The monoclonal antibodies used in this study to detect cell surface molecules are listed in Table 1. Polyclonal and isotype-specific anti-mouse immunoglobulins (prepared in goats) conjugated to fluorescein isothiocyanate, phycoerythrin, or Tri-color (Caltag Laboratories, Burlingame, Calif.) were used as second-step reagents. Flow cytometric data were acquired with a FACSort apparatus equipped with a Macintosh computer and CellQuest software (Becton-Dickinson Immunocytometry Systems, San Jose, Calif.). The forward and side scatter gates for bovine leukocytes were set to exclude cell debris and dead cells. Typically, 5,000 events were acquired per sample. When necessary, gates were set on small subpopulations to collect 2,000 events for analysis. Analyses were performed with the CellQuest, PAINT A GATE, or ATTRACTORS analytical program. Specific subpopulations of cells were quantified by using fixed attractors, with a cutoff line at 1.1 log unit. Expression of interleukin-2 receptor α chain (IL-2Rα) was separated into high- and low-intensity categories, i.e., above and below 2.0 log units. Large cells, considered to be blasts, were readily identifiable on plots of linear forward/log right-angle light scatter. A cutoff value of linear forward light scatter, usually >612 channels, was set to distinguish small cells (comprising cells at a low or partial level of activation) from large (blast) cells. The data for these experiments are presented as an index of blastogenesis, which refers to the percentage of large cells in a given subpopulation.
TABLE 1.
Monoclonal antibodies used in this study
| Specificity | Monoclonal antibody | Iso- type | Cell distribution | Reference |
|---|---|---|---|---|
| WC1 | B7A1 | IgM | γδ T CD2− | 51 |
| TCR1 | GB21A | IgG2b | γδ T | 36 |
| BoCD2 | BAQ95A | IgG1 | αβ, some γδ T | 14 |
| BoCD2 | MUC2A | IgG2a | αβ, some γδ T | 27 |
| BoCD3 | MM1A | IgG1 | T | 12 |
| BoCD4 | GC50A1 | IgM | T helper | 27 |
| BoCD4 | CACT138 | IgG1 | T helper | 41 |
| BoCD8α | CACT80C | IgG1 | T cytotoxic | 27 |
| BoCD8 | 7C2B | IgG2a | T cytotoxic | Unpublished |
| CD25 (IL-2Rα) | CACT116A | IgG1 | T, B | 37 |
| CD25 | CACT108 | IgG2a | T, B | 37 |
| ACT2 | CACT77A | IgM | Activated CD8 | 42 |
| CACT26A | IgG1 | 42 | ||
| ACT3 | CACT114A | IgG2b | Activated CD4, CD8 | 41 |
Statistical analysis.
Data on the expression of activation molecules are presented as arithmetic mean ± standard error of the mean (SEM) of triplicate measurements. To ascertain statistical significance of changes in cell numbers, a two-tailed t test (54) was performed on the counts after square root transformation, with P < 0.01 indicating significance.
RESULTS
Stimulation of bovine PBMC by SEC1 is unique and appears to be influenced by γδ T cells.
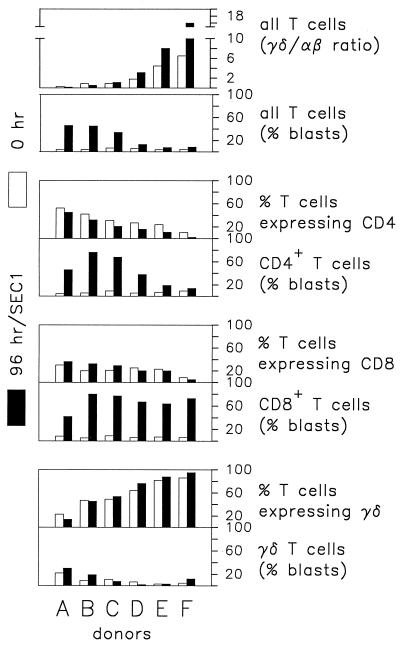
The percentage of γδ T cells expressing WC1 and GD3.5 (referred to here as CD2− γδ T cells) varies in bovine blood from over 80% in young animals to <10% in adults (52). Since this subpopulation of γδ T cells has been reported to modulate αβ T-cell responses (5, 7, 8, 52), the composition and general proliferative responses of PBMC from several prospective donors were assessed. As shown in Fig. 1, cumulative data from six animals revealed a marked difference in the proliferative response of CD4+ T cells to SEC1 in the presence of different concentrations of γδ T cells, consistent with previous observations that indicated that γδ T cells regulate activation of CD4+ T cells. Two initial observations pointed to a potential inhibitory effect mediated by γδ T cells on SEC1-induced stimulation of CD4+ T cells. First, the relative percent of CD4+ T cells in cultures from all six donors declined during 96 h in the presence of SEC1. This decline was most prominent in cultures containing a high concentration of γδ T cells. Second, most of the CD4+ T cells in cultures with a high concentration of γδ T cells did not increase to blast size. A reduction in the percentage of αβ T cells expressing CD8 was also observed with some donors. However, this reduction was smaller than that noted for CD4+ T cells and was evident only in cultures with high concentrations of γδ T cells (donors D, E, and F). In contrast to CD4+, most CD8+ T cells were blast size after 96 h of culture, even in cultures derived from animals with high concentrations of γδ T cells in peripheral blood.
FIG. 1.
Influence of γδ T cells on CD4+ and CD8+ T cells in PBMC cultures following a 96-h incubation with SEC1. Data are percentages of T cells obtained in a representative experiment. Donors A through F were healthy Holstein-Frisian cattle differing in γδ/αβ T-cell ratios as indicated in the figure. Each of the six donors was tested in the following number of replicate experiments: donor A, 7; donor B, 2; donor C, 3; donor D, 4; donor E, 3; and donor F, 6. They consistently yielded similar results.
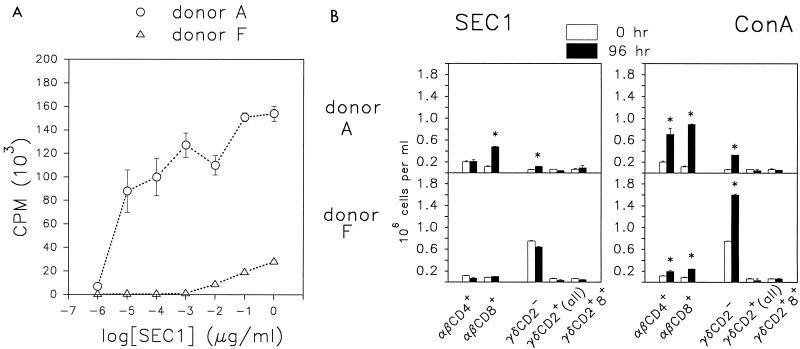
Since these results suggested that γδ T cells modulate the response of αβ T cells to SAgs and to mitogens, donors representing animals from opposite ends of the spectrum of the γδ/αβ T-cell ratio (donors A and F) were compared in subsequent analyses. T-cell subpopulations in cultures from these two donors were quantified in a proliferation assay and by flow cytometry. In a standard 4-day lymphocyte proliferation assay, based on incorporation of tritiated thymidine following stimulation with SEC1, PBMC from donor F (high γδ) were at least 1,000-fold less responsive to SEC1 than were PBMC from donor A (low γδ) (Fig. 2A). Subpopulation analysis by flow cytometry showed that SEC1 induced a statistically significant expansion in the numbers of αβ CD8+ cells and CD2− γδ T cells in cultures of PBMC from donor A (Fig. 2B). However, there was no statistically significant net increase in other subpopulations, including CD4+ cells, after 96 h. The CD4+ cells from donor A were potentially responsive since they proliferated in identical cultures stimulated with ConA. In fact, most major T-cell subpopulations expanded when ConA was used to stimulate cultures derived from donor A. The one exception was the γδ CD2+ subpopulation, which did not expand over the 96-h culture period (Fig. 2B).
FIG. 2.
Proliferative responses of bovine PBMC in cultures stimulated with SEC1. Data are means of triplicate measurements ± SEM. (A) Incorporation of [3H]thymidine in a standard 4-day proliferation assay. (B) Changes in cell numbers within bovine T-cell subpopulations following a 96-h culture of PBMC with SEC1 or ConA. Statistically significant increases (P < 0.01) are indicated (∗).
In contrast, cells from donor F failed to respond to SEC1 or showed a consistently low response following stimulation with SEC1. As with donor A, stimulation of parallel cultures with ConA demonstrated that the donor F cells were capable of proliferating and that multiple subpopulations expanded.
Lack of correlation between SEC1-induced blastogenesis and proliferation.
As shown in Fig. 2B, the net increase in cell number in cultures derived from donor A cells and stimulated with SEC1 was attributed to the expansion of CD8+ cells. Interestingly, though, subsequent experiments suggested that SEC1 partially activates other subpopulations of T cells, presumably through signaling pathways that do not lead to cell division. Analysis of forward and side light scatter patterns of cells from cultures stimulated with SEC1 revealed that many cells had increased in cell size, consistent with the early events of cell activation and blastogenesis. Stimulation with SEC1 caused a significant increase in the number of blast-sized cells within several T-cell subpopulations, especially in cultures with a low concentration of γδ T cells. The majority (76.9%) of CD8+ and a lower but significant (53.9%) percentage of CD4+ cells reached blast size in cultures derived from donor A (Table 2), even though there was no net increase in the number of CD4+ cells at 96 h.
TABLE 2.
Percentages of blast-sized cellsa in T-cell populations after 96 h
| Donor | Cell type | %b of enlarged cells 96 h after:
|
||
|---|---|---|---|---|
| No stimulationc | SEC1 stimulation | ConA stimulation | ||
| A | CD4+ | 8.5 ± 0.8 | 53.9 ± 2.1 | 73.0 ± 2.4 |
| CD8+ | 9.3 ± 0.93 | 76.9 ± 4.2 | 87.3 ± 2.6 | |
| γδ CD2− | 5.0 ± 1.2 | 13.7 ± 0.7 | 63.1 ± 1.1 | |
| γδ CD2+ CD8+ | 18.9 ± 2.5 | 58.3 ± 0.4 | 80.6 ± 1.8 | |
| F | CD4+ | 8.6 ± 0.6 | 21.3 ± 1.4 | 85.4 ± 1.2 |
| CD8+ | 27.0 ± 1.9 | 41.3 ± 2.1 | 88.5 ± 0.6 | |
| γδ CD2− | 3.8 ± 0.1 | 16.8 ± 1.3 | 76.8 ± 0.6 | |
| γδ CD2+ CD8+ | 13.5 ± 2.9 | 20.6 ± 2.9 | 32.9 ± 8.3 | |
Determined as outlined in Materials and Methods.
Mean ± SEM (n = 3).
Background levels measured prior to stimulation (0 h).
SEC1-induced blastogenesis within both CD4+ and CD8+ T-cell subpopulations was low in cultures from donor F (21.3 and 41.3%, respectively) compared to cultures from donor A (Table 2). There was no apparent influence, by γδ T cells from either donor, over the level of blastogenesis induced by ConA.
Activation of CD4+ cells by SEC1.
T-cell activation is typically associated with sequential upregulation of several IL receptors and other cell-surface molecules. For example, following activation, IL-2R expression may be upregulated within hours, followed by upregulation of MHCII molecules on T cells. To further analyze factors affecting CD4+ T-cell activation, we measured the expression of IL-2Rα and MHCII to monitor the progression of T cells through early and late stages of activation. Since the expression of IL-2Rα is affected by both constitutive and inducible factors, the density of this receptor on the surface varies depending on the level of stimulation (1). High-density expression requires IL-2Rα gene transcription as well as mRNA stabilization (6). Flow cytometric dot plots, of SEC1-stimulated cells labeled with anti-IL-2Rα and one additional antibody (for phenotyping the cell population under study), were used to distinguish between low- and high-density expression. Cells with low-density expression were defined as cells with fluorescence staining intensities less than 2.0 log units; cells considered to have high density expression had intensities above 2.0 log units.
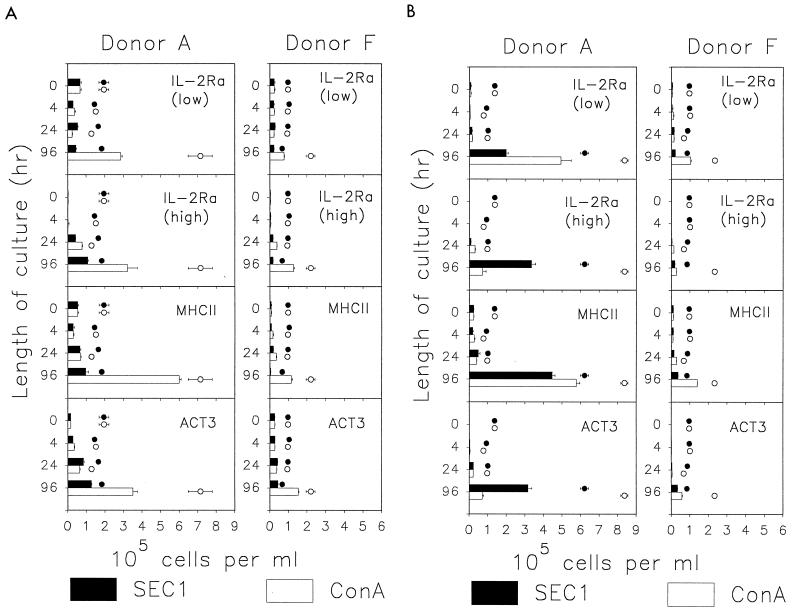
Similar to reports for human and murine peripheral blood (39), a substantial number of CD4+ (30 to 40%) bovine lymphocytes expressed low but significant levels of IL-2Rα (IL-2Rαlow) prior to stimulation (Fig. 3A). The numbers of IL-2Rαlow CD4+ cells did not change substantially in cultures stimulated with SEC1, although they increased severalfold in ConA-stimulated cultures. CD4+ cells expressing high levels of IL-2Rα (IL-2Rαhigh), typical of cells in an early and highly activated state, were rarely detected prior to stimulation. Examination of this subpopulation revealed major differences in the fate of CD4+ cells from both donors A and F and between SEC1-induced and ConA-induced stimulation. A comparison of results from the two donors confirmed observations from the initial experiments which indicated that γδ T cells strongly affect the activation of CD4+ cells by SEC1. For example, an increase in the numbers of IL-2Rαhigh CD4+ cells was evident in SEC1-stimulated cultures from donor A at 24 h and was followed by further increase by 96 h. In contrast, only small numbers of IL-2Rαhigh CD4+ cells were found at 24 h in SEC1-stimulated PBMC from donor F. Moreover, the number of cells with high expression did not increase by 96 h, suggesting a loss of cells or downregulation of IL-2Rα expression.
FIG. 3.
Expression of IL-2Rα, MHCII, and ACT3 by subpopulations of bovine T cells in PBMC cultures stimulated with SEC1 or ConA. Data are numbers of cells expressing a given marker (mean ± SEM of three measurements obtained in a representative experiment). The cells expressing IL-2Rα are separated into nonoverlapping populations characterized by a small or large amount of the receptor on the cell surface. (A) CD4+ cells; (B) CD8+ cells. Data represented by the open and solid circles are means ± SEM of the total number of cells expressing CD4 or CD8 at each time point.
The patterns of MHCII expression on CD4+ cells following stimulation with either SEC1 or ConA were similar to the patterns observed for expression of high levels of IL-2Rα (Fig. 3A). One difference between these markers was that, as expected, the increase in MHCII expression was delayed compared to that of IL-2Rα. A small but significant increase in MHCII expression was observed at 24 h in cultures of PBMC from donor A. The proportion of MHCII+ CD4+ cells increased throughout the 96-h culture period, with the exception of a minor decline during the first 4 h. In contrast, although 20% of CD4+ cells in cultures of SEC1-stimulated PBMC from donor F expressed MHCII at 24 h, the number and proportion of MHCII-positive CD4+ cells declined below baseline levels following 96 h of culture.
SEC1 induces an unique phenotype of activated CD8+ cells.
Activation molecule 3 (ACT3) is a 120-kDa membrane molecule with no identified human or mouse ortholog (13, 42, 45). In ConA-stimulated cultures of PBMC, ACT3 was expressed on at least 50% of CD4+ cells after 96 h (Fig. 3A). Levels of ACT3 expression on CD4+ cells in donor A PBMC cultures, stimulated with either SEC1 or ConA, were similar to the levels of expression of IL-2Rαhigh and MHCII (Fig. 3A). An increase in the expression of ACT3 was clearly evident only in ConA-stimulated cultures of PBMC from donor F. In contrast to CD4+ cells, a much smaller proportion of CD8+ cells expressed ACT3 in ConA-stimulated cultures, but SEC1 induced ACT3 expression on more than 50% of CD8+ cells in culture from donor A and on more than 40% of CD8+ cells in culture from donor F (Fig. 3B). Most of the CD8+ blasts (60 to 80%) were positive for ACT3 in SEC1-stimulated cultures, whereas fewer than 15% were positive in ConA-stimulated cultures (data not shown). Combined, these data suggest that SEC1-induced expression of ACT3 on bovine CD8+ T cells coincides with a highly activated state.
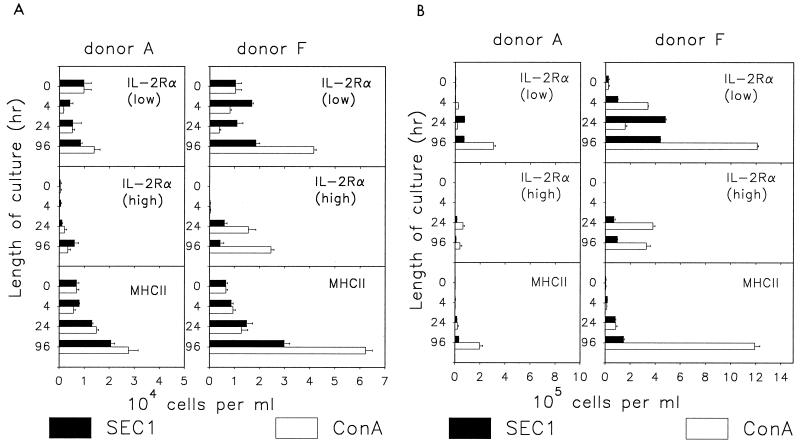
Effect of SEC1 on γδ T cells.
The responses of the CD2− and CD2+ CD8+ subpopulations of γδ T cells differed. γδ CD2+ CD8+ cells failed to expand in cultures stimulated with either ConA or SEC1 (Fig. 2B). The absence of proliferation correlated with minimal activation, evidenced by low blastogenesis and moderate expression of IL-2Rα (Table 2; Fig. 4A). In contrast to other T-cell subpopulations, expression of MHCII was higher than expression of IL-2Rα at 96 h.
FIG. 4.
Expression of IL-2Rα and MHCII on subpopulations of bovine γδ T cells in PBMC cultures stimulated with SEC1 or ConA. Data are numbers of cells positive for a given marker (mean ± SEM of three measurements obtained in a representative experiment). The cells positive for IL-2Rα are separated into nonoverlapping populations characterized by small or large amounts of the receptor on the cell surface. (A) γδ CD2+ CD8+ cells; (B) γδ CD2− cells. Notice the different scale in panels A and B.
γδ CD2− cells in PBMC cultures from either donor were potentially responsive to stimulation. Activation of these cells in cultures stimulated by ConA resulted in proliferation (Fig. 2B), expression of IL-2Rα and MHCII (Fig. 4B), and enlargement to blast size (Table 2). Activation was more pronounced in cultures of cells from donor F (Fig. 4B). In contrast to ConA, SEC1 induced only minimal blastogenesis within the γδ CD2− population (Table 2). This result correlated with a correspondingly low level of IL-2Rα and MHCII expression in this population of cells (Fig. 4B). The patterns obtained with CD2− γδ T cells from donor A were similar except for lower background levels, reflecting the composition of T-cell population in this donor.
DISCUSSION
SAg-induced oligoclonal activation of T cells results from the binding of SAgs to MHCII molecules and TCRs that bear specific Vβ gene products. For PT SAgs such as SEs and toxic shock syndrome toxin type 1, structural data suggest this interaction does not bring the peptide binding groove of the MHCII molecule in juxtaposition with the TCR peptide binding pocket (15, 20, 25). Thus, cross-linking is sufficient to transduce signals of activation. How these signals differ from those that occur following MHC-restricted immune system recognition has not been fully elucidated. However, it is clear that SAg-mediated signaling is unique and that altered signaling leads to a cascade of events associated with toxic shock syndrome and aberrations in the function of lymphocytes (43).
The present study suggests that SAgs have the potential to affect the bovine immune system in a manner that could promote staphylococcal persistence and infections such as IMI. Comparing the response of PBMC to ConA and SEC1 revealed differences in cell activation, proliferation, and expression of activation molecules. Stimulation with SEC1 led to partial activation of CD4+ T cells and γδ T cells as evidenced by the expression of MHCII and IL-2Rα. A significant number of CD4+ T cells also expressed ACT3 and increased in size. However, proliferation was limited or absent. Few CD2− γδ T cells increased in size in 4-day cultures, and there was no increase in the numbers of CD2+ CD8+ γδ T cells in spite of some increase in cell size. In contrast, stimulation with SEC1 led to activation and proliferation of CD8+ T cells and the unique expression of the activation molecule, ACT3. Of interest, the level of activation of CD4+ and CD8+ T cells was affected by the proportion of γδ T cells present in the culture, suggesting that these cells may play a modulatory role in the activation of αβ T cells. The evolution of the immune system in swine and ruminants is unique since the γδ T cell concentration is highly variable in these animals (3, 5, 10, 21, 22, 32, 36, 41, 50, 51, 52). Although γδ T-cell levels are influenced by age, other factors, such as prior disease or exposure, have not been shown to influence the αβ/γδ T cell ratio. Whether animals with high levels of γδ T cells are more susceptible to infection, especially by SAg-producing organisms, is one possibility currently under investigation.
Efforts to elucidate the mechanisms of SAg-driven T-cell responses in other animals have shown that aberrant signaling induced by SAgs leads to the production of multiple regulatory cytokines in vivo (29). This is followed by deletion of cells expressing specific Vβ segments (30, 31) and anergy or hyporesponsiveness to stimuli in the remaining cells (23, 35). Such hyporesponsiveness may be associated with downregulation of the expression of IL-2Rβ and inhibition of JAK-3 kinase, demonstrated for human CD4+ T cells (38). The present study of bovine cells is in agreement with studies performed in other systems, showing that CD4+ T-cell function may be inhibited more than that of CD8+ T cells by prolonged exposure to SAgs (9, 23). Long-term studies in vivo showed that CD8+ T cells predominate among SAg-reactive T cells (i.e., those bearing specific Vβ segments) surviving treatment with SAg (18, 31). The results obtained in the present study are also in line with results of experiments with mice, whereby treatment with SAgs gave rise to CD8+ T cells with an altered phenotype and functional activity. Specifically, CD8+ cells isolated from mice treated with SAg were shown to lack cytotoxic activity, although they were responsive to cytokines and retained the ability to proliferate (48). Further studies are required to determine whether such cells could modulate the activation and function of CD4+ T cells through the production of cytokines that downregulate their capacity to respond to stimuli.
Previous studies with T cells from cows have shown that a subpopulation of activated CD8+ T cells downregulates the response of CD4+ cells to staphylococcal antigens presented by antigen-presenting cells (42). Elevated levels of these CD8+ T cells were demonstrated in mammary secretions from glands infected with S. aureus, indicating that they might play a role in pathogenesis (42). Although the potential role of microbial products in inducing these cells was not established, the present study indicates that SAg-activated CD8+ T cells may play a significant role in the pathogenesis of mastitis caused by SAg-producing S. aureus.
The activation of bovine CD8+ T cells by SEC1 occurs through a mechanism that is associated with unique expression of ACT3, a 120-kDa molecule with no identified ortholog in any other species. Antibodies that recognize this molecule were assigned to workshop cluster 10 in the Second International Workshop on Ruminant Leukocyte Differentiation Antigens (45). High-level expression of ACT3 on activated CD8+ T cells has not been previously observed and could be associated with the expression of cytokines that modulate the capacity of CD4+ T cells to respond to mitogenic stimuli. Previous studies have shown ACT3 is expressed predominantly by CD4+ T cells in ConA-stimulated cultures (13). More recently, investigations have shown that ACT3 is highly expressed on CD4+ and γδ T cells in long-term cultures derived from animals stimulated with Babesia bovis (5). Further studies are now needed to determine specifically whether SEC1-activated CD8+ αβ T cells affect the proliferative and functional activity of CD4+ T cells.
This study also suggests that activation of CD4+ and CD8+ T cells by SEC1 and, to a lesser degree, by ConA is significantly influenced by the proportion of γδ T cells present in cultures. Since the content of γδ T cells is a highly variable characteristic of ruminants, the general susceptibility of animals to SAg-mediated immunosuppression may vary greatly. The mechanism of a putative regulatory influence of γδ T cells remains to be determined. It is not clear whether γδ T cells in ruminants can be activated directly through the binding of SAgs to the γδ TCR. However, expression of activation markers on their surface, following exposure to SEC1, indicates that either direct or indirect activation of γδ T cells does occur.
In conclusion, evidence has been obtained which shows that SAgs could induce immunosuppression in dairy animals and contribute to the pathogenesis of staphylococcal mastitis. The staphylococcal SAg SEC1 induces a unique and aberrant activation of T-cell subpopulations. Further studies are warranted on this basis alone. However, the findings also suggest that the bovine system might serve as a useful model for the investigation of the mechanisms by which SAgs modulate immune system function and cause disease in other animals.
ACKNOWLEDGMENTS
This work was supported by grants from the U.S. Department of Agriculture (project 9402399) and the Public Health Service (grant AI28401).
REFERENCES
- 1.Algarte M, Lecine P, Costello R, Plet A, Olive D, Imbert J. In vivo regulation of interleukin-2 receptor α gene transcription by the coordinated binding of constitutive and inducible factors in human primary T cells. EMBO J. 1995;14:5060–5072. doi: 10.1002/j.1460-2075.1995.tb00188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartlett P C, Miller G Y, Lance S E, Heider L E. Clinical mastitis and intramammary infections on Ohio dairy farms. Prev Vet Med. 1992;12:59–71. [Google Scholar]
- 3.Binns R M. The null/γδ TCR+ T cell family in the pig. Vet Immunol Immunopathol. 1994;43:69–77. doi: 10.1016/0165-2427(94)90122-8. [DOI] [PubMed] [Google Scholar]
- 4.Bohach G A, Schlievert P. Expression of staphylococcal enterotoxin C1 in Escherichia coli. Infect Immun. 1987;55:428–432. doi: 10.1128/iai.55.2.428-432.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown W C, Davis W C, Choi S H, Dobbelaere D A E, Splitter G A. Functional and phenotypic characterization of WC1+ γ/δ T cells isolated from Babesia bovis-stimulated T cell lines. Cell Immunol. 1994;153:9–27. doi: 10.1006/cimm.1994.1002. [DOI] [PubMed] [Google Scholar]
- 6.Cerdan C, Martin Y, Courcoul M, Brailly H, Mawas C, Birg F, Olive D. Prolonged IL-2 receptor α/CD25 expression after T cell activation via the adhesion molecules CD2 and CD28. J Immunol. 1992;149:2255–2261. [PubMed] [Google Scholar]
- 7.Chiodini R J, Davis W C. The cellular immunology of bovine paratuberculosis: the predominant response is mediated by cytotoxic gamma/delta T lymphocytes which prevent CD4+ activity. Microb Pathog. 1992;13:447–463. doi: 10.1016/0882-4010(92)90012-d. [DOI] [PubMed] [Google Scholar]
- 8.Chiodini R J, Davis W C. The cellular immunology of bovine paratuberculosis: immunity may be regulated by CD4+ helper and CD8+ immunoregulatory T lymphocytes which down-regulate gamma/delta+ T cell cytotoxicity. Microb Pathog. 1993;14:355–367. doi: 10.1006/mpat.1993.1035. [DOI] [PubMed] [Google Scholar]
- 9.Damle N K, Leytze G, Klussman K, Ledbetter J A. Activation with superantigens induces programmed death in antigen-primed CD4+ class II+ major histocompatibility complex T lymphocytes via a CD11a/CD18-dependent mechanism. Eur J Immunol. 1993;23:1513–1522. doi: 10.1002/eji.1830230718. [DOI] [PubMed] [Google Scholar]
- 10.Davis W C, Brown W C, Hamilton M J, Wyatt C R, Orden J A, Khalid A M, Naessens J. Analysis of monoclonal antibodies specific for the γδ TcR. Vet Immunol Immunopathol. 1996;52:275–283. doi: 10.1016/0165-2427(96)05578-x. [DOI] [PubMed] [Google Scholar]
- 11.Davis W C, Davis J E, Hamilton M J. Use of monoclonal antibodies and flow cytometry to cluster and analyze leucocyte differentiation molecules. In: Davis W C, editor. Monoclonal antibody protocols. Totowa, N.J: The Humana Press, Inc.; 1995. pp. 149–167. [DOI] [PubMed] [Google Scholar]
- 12.Davis W C, MacHugh N D, Park Y H, Hamilton M J, Wyatt C R. Identification of a monoclonal antibody reactive with the bovine orthologue of CD3 (BoCD3) Vet Immunol Immunopathol. 1993;39:85–91. doi: 10.1016/0165-2427(93)90167-3. [DOI] [PubMed] [Google Scholar]
- 13.Davis W C, Naessens J, Brown W C, Ellis J A, Hamilton M J, Cantor G H, Barbosa J I R, Ferens W A, Bohach G A. Analysis of monoclonal antibodies reactive with molecules upregulated or expressed only on activated lymphocytes. Vet Immunol Immunopathol. 1996;52:301–311. doi: 10.1016/0165-2427(96)05581-x. [DOI] [PubMed] [Google Scholar]
- 14.Davis W C, Splitter G S. Bovine CD2. Vet Immunol Immunopathol. 1991;27:43–50. doi: 10.1016/0165-2427(91)90077-p. [DOI] [PubMed] [Google Scholar]
- 15.Deringer J R, Ely R J, Stauffacher C V, Bohach G A. Subtype-specific interactions of type C staphylococcal enterotoxins with the T-cell receptor. Mol Microbiol. 1997;22:523–534. doi: 10.1046/j.1365-2958.1996.1381506.x. [DOI] [PubMed] [Google Scholar]
- 16.Fox L K, Gershman D D, Hutton C T. Fomites and reservoirs of Staphylococcus aureus causing intramammary infections as determined by phage typing: the effect of milking time hygiene practices. Cornell Vet. 1991;81:183–193. [PubMed] [Google Scholar]
- 17.Gonzales R N, Jasper D E, Farver T B, Bushnell R B, Franti C E. Prevalence of udder infections and mastitis in 50 California dairy herds. J Am Vet Med Assoc. 1988;193:323–328. [PubMed] [Google Scholar]
- 18.Herrmann T, Baschieri S, Lees R K, MacDonald R H. In vivo responses of CD4+ and CD8+ cells to bacterial superantigens. Eur J Immunol. 1992;22:1935–1938. doi: 10.1002/eji.1830220739. [DOI] [PubMed] [Google Scholar]
- 19.Hovde C J, Marr J C, Hoffman M L, Hackett S P, Chi Y-I, Crum K K, Stevens D L, Stauffacher C V, Bohach G A. Investigation of the role of the disulphide bond in the activity and structure of staphylococcal enterotoxin C1. Mol Microbiol. 1994;13:897–909. doi: 10.1111/j.1365-2958.1994.tb00481.x. [DOI] [PubMed] [Google Scholar]
- 20.Jardetzky T S, Brown J H, Gorga J C, Stern L J, Urban R G, Chi Y, Stauffacher C, Strominger J L, Wiley D C. Three-dimensional structure of a human class II histocompatibility molecule complexed with superantigen. Nature. 1994;368:711–718. doi: 10.1038/368711a0. [DOI] [PubMed] [Google Scholar]
- 21.Jones W M, Walcheck B, Jutila M A. Generation of a new γδ T cell-specific monoclonal antibody (GD3.5): biochemical comparisons of GD3.5 antigen with the previously described workshop cluster 1 (WC1) family. J Immunol. 1996;156:3772–3779. [PubMed] [Google Scholar]
- 22.Kaufmann S H. Gamma/delta and other unconventional T lymphocytes: what do they see and what do they do? Proc Natl Acad Sci USA. 1996;93:2272–2279. doi: 10.1073/pnas.93.6.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawabe Y, Ochi A. Selective anergy of Vβ8+, CD4+ T cells in Staphylococcus enterotoxin B-primed mice. J Exp Med. 1990;172:1065–1070. doi: 10.1084/jem.172.4.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kenny K, Reiser R F, Bastida-Corcuera F D, Norcross N L. Production of enterotoxins and toxic shock syndrome toxin by bovine mammary isolates of Staphylococcus aureus. J Clin Microbiol. 1993;31:706–707. doi: 10.1128/jcm.31.3.706-707.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim J, Urban R G, Strominger J L, Wiley D C. Toxic shock syndrome toxin-1 complexed with a class II major histocompatibility molecule HLA-DR1. Science. 1994;266:1870–1874. doi: 10.1126/science.7997880. [DOI] [PubMed] [Google Scholar]
- 26.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 27.Larsen R A, Monaghan M L, Park Y H, Hamilton M J, Ellis J A, Davis W C. Identification and characterization of monoclonal antibodies reactive with bovine, caprine and ovine T-lymphocyte determinants by flow microfluorimetry. Vet Immunol Immunopathol. 1990;25:195–208. doi: 10.1016/0165-2427(90)90035-q. [DOI] [PubMed] [Google Scholar]
- 28.Licence S T, Davis W C, Carr M M, Binns R M. The behaviour of monoclonal antibodies in the First International Pig CD Workshop reacting with γδ/null T lymphocytes in the blood of SLAb/b line pigs. Vet Immunol Immunopathol. 1995;47:253–271. doi: 10.1016/0165-2427(95)05444-b. [DOI] [PubMed] [Google Scholar]
- 29.Litton M J, Sander B, Murphy E, O’Garra A, Abrams J S. Early expression of cytokines in lymph nodes after treatment in vivo with Staphylococcus enterotoxin B. J Immunol Methods. 1994;175:47–58. doi: 10.1016/0022-1759(94)90330-1. [DOI] [PubMed] [Google Scholar]
- 30.MacCormack J E, Callahan J E, Kappler J, Marrack P C. Profound deletion of mature T cells in vivo by chronic exposure to exogenous superantigen. J Immunol. 1993;150:3785–3792. [PubMed] [Google Scholar]
- 31.MacDonald H R, Baschieri S, Lees R K. Clonal expansion precedes anergy and death of Vβ8+ peripheral T cells responding to staphylococcal enterotoxin B in vivo. Eur J Immunol. 1991;21:1963–1966. doi: 10.1002/eji.1830210827. [DOI] [PubMed] [Google Scholar]
- 32.MacHugh N D, Wijngaard P L J, Clevers H C, Davis W C. Clustering of monoclonal antibodies recognizing different members of the WC1 gene family. Vet Immunol Immunopathol. 1993;39:155–160. doi: 10.1016/0165-2427(93)90176-5. [DOI] [PubMed] [Google Scholar]
- 33.Marrack P, Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990;248:705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- 34.Matsunaga T, Kamata S, Kakiichi N, Uchida K. Characteristics of Staphylococcus aureus isolated from peracute, acute, and chronic bovine mastitis. J Vet Med Sci. 1993;55:297–300. doi: 10.1292/jvms.55.297. [DOI] [PubMed] [Google Scholar]
- 35.Migita K, Ochi A. Induction of clonal anergy by oral administration of staphylococcal enterotoxin B. J Immunol. 1994;24:2081–2086. doi: 10.1002/eji.1830240922. [DOI] [PubMed] [Google Scholar]
- 36.Morrison W I, Davis W C. Differentiation antigens expressed predominantly on CD4−CD8− T lymphocytes (WC1, WC2) Vet Immunol Immunopathol. 1991;27:71–76. doi: 10.1016/0165-2427(91)90082-n. [DOI] [PubMed] [Google Scholar]
- 37.Naessens J, Sileghem M, Machugh N, Davis W C, Toye P. Selection of BoCD25 monoclonal antibodies by screening mouse L cells transfected with the bovine p55-interleukin-2 (IL-2) receptor gene. Immunology. 1992;76:305–309. [PMC free article] [PubMed] [Google Scholar]
- 38.Nielsen M, Svejgaard A, Ropke C, Nordahl M, Odum N. Staphylococcal enterotoxins modulate interleukin 2 receptor expression and ligand-induced tyrosine phosphorylation of the Janus protein-tyrosine kinase 3 (Jak3) and signal transducers and activators of transcription (Stat proteins) Proc Natl Acad Sci USA. 1995;92:10995–10999. doi: 10.1073/pnas.92.24.10995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohashi Y, Takeshita T, Nagata K, Mori S, Sugamura K. Differential expression of the IL-2 receptor subunits, p55 and p75 on various populations of primary peripheral blood mononuclear cells. J Immunol. 1989;143:3548–3555. [PubMed] [Google Scholar]
- 40.Oliver S P, Mitchell B A. Prevalence of mastitis pathogens in herds participating in a mastitis control program. J Dairy Sci. 1984;67:2436–2440. doi: 10.3168/jds.S0022-0302(84)81592-1. [DOI] [PubMed] [Google Scholar]
- 41.Park Y H, Fox L K, Hamilton M J, Davis W C. Bovine mononuclear leucocyte subpopulations in peripheral blood and mammary gland secretions during lactation. J Dairy Sci. 1992;75:998–1006. doi: 10.3168/jds.S0022-0302(92)77842-4. [DOI] [PubMed] [Google Scholar]
- 42.Park Y H, Fox L K, Hamilton M J, Davis W C. Suppression of proliferative response of BoCD4+ T lymphocytes by activated BoCD8+ T lymphocytes in the mammary gland of cows with Staphylococcus aureus mastitis. Vet Immunol Immunopathol. 1993;36:137–151. doi: 10.1016/0165-2427(93)90103-b. [DOI] [PubMed] [Google Scholar]
- 43.Schlievert P. Role of superantigens in human diseases. J Infect Dis. 1993;167:997–1002. doi: 10.1093/infdis/167.5.997. [DOI] [PubMed] [Google Scholar]
- 44.Schlievert P M, Schoettie K J, Watson D W. Purification and physicochemical and biological characterization of staphylococcal pyrogenic exotoxin. Infect Immun. 1979;23:609–617. doi: 10.1128/iai.23.3.609-617.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sopp P, Howard C J, Parsons K R. A new non-lineage specific antigen with an Mw of 115 kDa and 39 kDa present on bovine leukocytes identified by monoclonal antibodies within BoWC10. Vet Immunol Immunopathol. 1993;29:209–215. doi: 10.1016/0165-2427(93)90183-5. [DOI] [PubMed] [Google Scholar]
- 46.Stohl W, Elliott J E. Differential human T cell-dependent B cell differentiation induced by staphylococcal superantigens (SAg) J Immunol. 1995;155:1838–1850. [PubMed] [Google Scholar]
- 47.Sundstedt A, Dohlsten M, Hedlund G, Hoiden I, Bjorklund M, Kalland T. Superantigens anergize cytokine production but not cytotoxicity in vivo. Immunology. 1994;82:117–125. [PMC free article] [PubMed] [Google Scholar]
- 48.Sundstedt A, Hoiden I, Hansson J, Hedlund G, Kalland T, Dohlsten M. Superantigen-induced anergy in cytotoxic CD8+ T cells. J Immunol. 1995;154:6306–6313. [PubMed] [Google Scholar]
- 49.Sutra L, Poutrel B. Virulence factors involved in the pathogenesis of bovine intramammary infections due to Staphylococcus aureus. J Med Microbiol. 1994;40:79–89. doi: 10.1099/00222615-40-2-79. [DOI] [PubMed] [Google Scholar]
- 50.Wijngaard P L J, MacHugh N D, Metzelaar M J, Romberg S, Bensaid A, Pepin L, Davis W C, Clevers H C. Members of the novel WC1 gene family are differentially expressed on subsets of bovine CD4−CD8− γδ T-lymphocytes. J Immunol. 1994;152:3476–3482. [PubMed] [Google Scholar]
- 51.Wijngaard P L J, Metzelaar M J, MacHugh N D, Morrison W I, Clevers H C. Molecular characterization of the WC1 antigen expressed specifically on bovine CD4− CD8− γδ T lymphocytes. J Immunol. 1992;149:3273–3277. [PubMed] [Google Scholar]
- 52.Wyatt C, R, Madruga C, Cluff C, Parish S, Hamilton M J, Goff W, Davis W C. Differential distribution of γδ T-cell receptor lymphocyte subpopulations in blood and spleen of young and adult cattle. Vet Immunol Immunopathol. 1994;40:187–199. doi: 10.1016/0165-2427(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 53.Yokomizo Y, Mori Y, Shimoji Y, Shimizu S, Sentsui H, Kodama M, Igarashi H. Proliferative response and cytokine production of bovine peripheral blood mononuclear cells induced by the superantigens staphylococcal enterotoxins and toxic shock syndrome toxin-1. J Vet Med Sci. 1995;2:299–305. doi: 10.1292/jvms.57.299. [DOI] [PubMed] [Google Scholar]
- 54.Zar J H. Biostatistical analysis. 2nd ed. N.J: Prentice Hall, Inc.; 1984. [Google Scholar]