Abstract
Unplanned human population shifts in urban areas are expected to increase the prevalence of vector-borne diseases. This study aimed to investigate mosquito species composition, blood meal sources, and malaria vectors in an urban area. Indoor-resting adult mosquitoes were collected using Prokopack and host-seeking mosquitoes using Centers for Disease Control and Prevention light traps in Arba Minch town. Larval collection from artificial containers was done in those houses selected for adult mosquito collection. Anopheles adults collected and emerged from larvae were identified morphologically using a taxonomic key. ELISA was used to identify blood meal sources in freshly fed Anopheles and Culex mosquitoes, and CSP of Anopheles mosquitoes. A total of 16,756 female mosquitoes were collected. Of these, 93% (15,571) were Culex, 6% (1016) were Anopheles, and 1% (169) were Aedes mosquitoes. Out of the 130 adult mosquitoes that were raised from larvae collected from the containers, 20% were An. rhodesiensis, while the remaining 80% were Aedes mosquitoes. Out of 823 mosquitoes tested for blood meal origins, 86.3% (710/823) tested positive for human blood, 2.2% (18/823) tested positive for bovine blood, and 11.5% (95/823) were negative for human and bovine antibodies. Anopheles gambiae complex had a human blood meal index (HBI) of 50% (90/180; CI 42.3–57.5%) and a bovine blood meal index (BBI) of only 0.5% (95% CI 0.01–3.1%). Culex HBI was 96.7% (620/641), and its BBI index was 2.4% (15/641). While it was low (0.8%) in Culex, the proportion of An. gambiae complex with unidentified blood meal sources was 49.5% (95 CI% 41.9–56.9%). Among the 1016 Anopheles mosquitoes tested, a single An. gambiae complex (0.1%; 1/1016) was positive for P. vivax CSP. The high HBI indicates frequent contact between humans and vectors. To reduce human exposure, personal protection tools should be implemented.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00436-024-08121-4.
Keywords: Bovine blood meal index, Circum-sporozoite protein, Human blood meal index, Urban mosquitoes
Introduction
Over half of the world’s population is at risk of vector-borne diseases (World Health Organization 2014). Urban malaria cases account for between 6 and 28% of annual malaria cases worldwide (Keiser et al. 2004). This number could increase because of population migration to urban settings, and it remains a concern in the context of malaria elimination efforts (De Silva and Marshall 2012).
Rapid population growth in urban areas may change the epidemiology of malaria in African cities (Hay et al. 2005; World Health Organization 2022). In the past, population movement has contributed to the spread of the disease and the campaign’s failure to eliminate malaria. Cities may suffer from “ruralisation,” which occurs when migrants bring their rural habits and practices to urban areas, such as livestock husbandry, traditional water usage and storage methods (wells), agriculture, and food production, which may offer breeding grounds for mosquitoes (Keiser et al. 2004; Hay et al. 2005; World Health Organization 2022). Irrigated urban agriculture, which has helped to alleviate poverty and increase food security in rapidly urbanizing sub-Saharan Africa, may inadvertently support malaria vectors (Klinkenberg et al. 2008). In addition, rural malaria control strategies cannot be transferred directly to urban areas (World Health Organization 2022). Designing appropriate interventions requires a better understanding of the vectors’ biology and behaviour in towns.
The three most common malaria vectors in Africa are An. gambiae, An. arabiensis, and An. funestus (Doumbe-Belisse et al. 2021). In Ethiopia, An. arabiensis is the primary vector for malaria in both rural and urban areas, while An. pharoensis is a secondary vector (Animut et al. 2013; Abraham et al. 2017; Esayas et al. 2020). In urban Ethiopia, notably in Eastern parts, the new species An. stephensi has been identified (Carter et al. 2018; Balkew et al. 2020). It is a potent vector for P. vivax and P. falciparum transmission in Asia and the primary malaria vector in India and the Persian Gulf (Sinka et al. 2011). This mosquito is highly adapted to typical urban conditions, and its presence can pose a threat to malaria control efforts, especially in urban areas (Balkew et al. 2020; Tadesse et al. 2021). Additionally, this species has developed resistance to various insecticides such as DDT, pirimiphos-methyl, propoxur, bendiocarb, and deltamethrin (Yared et al. 2020), which makes it challenging to prevent its spread to new locations.
Although several studies have described the entomological indicators of malaria-transmitting mosquitoes in rural areas (Massebo et al. 2013a; Abraham et al. 2017; Esayas et al. 2020), there is a need to investigate urban mosquito species, blood-feeding patterns, and malaria transmission vectors. The blood meal sources of mosquitoes can indicate their potential to transmit pathogens. Mosquitoes that frequently bite humans are more likely to transmit pathogens than those that infrequently visit human dwellings and bite humans (Sherrard-Smith et al. 2019). To effectively design and implement interventions, it is crucial to understand blood-feeding patterns, species composition, and primary vectors.
Urban areas may see increased malaria and other vector-borne diseases due to poor housing, sanitation, water management, healthcare, and economic disparities. Those who inhabit the outskirts of towns may be at the greatest risk due to the aforementioned factors. Arba Minch is a malaria-endemic town in Ethiopia, where both Plasmodium falciparum and P. vivax are common parasites and An. arabiensis is the principal vector (Getawen et al. 2018). Various mosquito breeding sites may contribute to the year-round transmission of malaria. A better understanding of species composition, blood-feeding behaviour, and breeding habitats may be necessary to adapt strategies against present and emerging urban vectors. Thus, this study provides information about the key entomological indicators, including species composition, feeding and resting densities, blood meal indexes, CSP infection rate, and entomological inoculation rates. The purpose of this study was to get a better understanding of urban malaria vector species, their feeding behaviour, and their role in malaria transmission.
Materials and methods
Description of the study area
Arba Minch town is located in Ethiopia’s Southern Nations, Nationalities, and Peoples Regional State (SNNPRs) (Fig. 1). It is located 505 km to the southwest of the capital of Ethiopia, Addis Ababa. It comprises four administrative sub-cities: Secha, Sikella, Abaya, and Nechsar. The town has 16 Kebeles (the smallest administrative unit), which are all malarious. The average altitude of the study area was 1281 m above sea level. The average annual temperature is 29.7 °C, and the yearly rainfall is 900 mm.
Fig. 1.
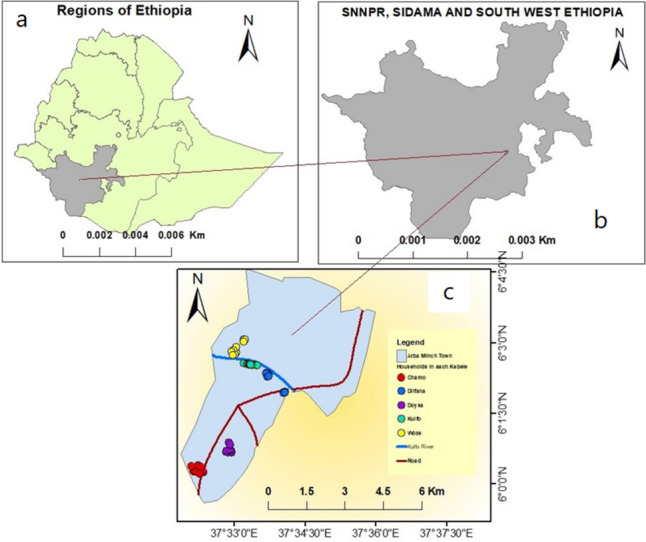
Map of the study area: a map of Ethiopia, b former Southern Nation and nationalities and people regional state, c Arba Minch town and study Kebeles
The river Kulfo crosses the town on the northwest side and is being used for irrigation. There are many mosquito breeding sites at the edge of the river. The climate is favourable to the cultivation of different fruits and vegetables.
Study design
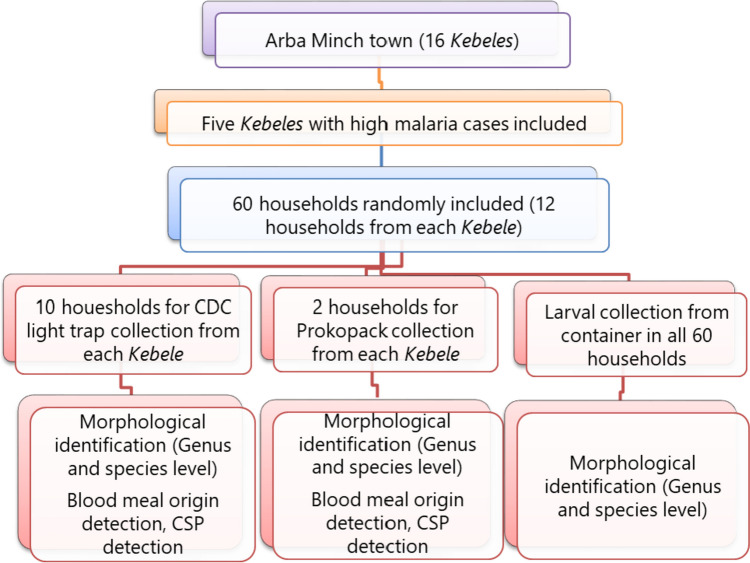
An entomological study was conducted in Arba Minch town from April to November 2022, except October. Five Kebeles (Dilfana, Kulfo, Woze, Chamo, and Doysa) were selected from 16 Kebeles based on the number of malaria cases. The three entomological sampling techniques employed were CDC light traps, Prokopacks, and container searching for larvae (Fig. 2).
Fig. 2.
The study design flow chart
Fifty houses were selected for the CDC light trap collection, with ten houses included from each of the five Kebeles. The first house was chosen randomly from one corridor, and the following houses were selected systematically. For the Prokopack aspirator collection, two houses were selected randomly from each Kebele. Additionally, Anopheles larvae and pupae were collected from artificial containers in the 60 houses selected for CDC light traps and Prokopack aspirator collection. All larval and pupal samples were reared in the laboratory at Arba Minch University until they emerged into adult stages and then identified using a morphological key (Coetzee 2020).
Mosquito collection and processing
Larval collection
All artificial containers, including waste tyres, plastic storage for domestic and construction usage, and water storage, were inspected, and those that tested positive for mosquito larvae were sampled by dipping. Pupae and the last two instar larvae were collected and raised into adults in the Medical Entomology and Vector Control laboratory at 25 to 27 °C and 70–80% relative humidity. No additional food was provided to the larvae as they were collected from nutrient-rich habitats. Pupae were placed in cages for adult development and adults were provided with 10% sugar solution.
CDC light trap collection
Adult female Anopheles mosquito sampling was conducted using CDC light traps (Model 512; John W. Hock Company, Gainesville, FL, USA) once per month in 50 randomly selected houses. The traps were installed near the foot of the bed where the individual slept, about 1.5 m above the floor. Light traps were switched on at 6:00 pm and switched off at 6:00 am (WHO 1975).
Prokopack aspirator collection
Prokopack aspirator collection was done once a month in two homes in each Kebele. All selected houses conducted aspirations indoors, beginning at 6:00 am and ending at about 8:00 am. Mosquitoes were systematically aspirated on the walls and ceilings for 20–30 min per house once per month (Vazquez-Prokopec et al. 2009).
Morphological identification of mosquitoes
Alive mosquito killing was done by freezing. Anopheline and culicine were first identified. Speciation of Anopheles was done using a morphological key (Coetzee 2020). The abdominal classification was done under the dissecting microscope into four categories: unfed, freshly fed, half-gravid, and gravid. Female Anopheles mosquitoes were preserved individually in vials with silica gel for blood meal source assay and circum-sporozoite protein (CSPs) test. Each month, ten Culex mosquitos were collected from each Kebele and stored for blood meal analysis to obtain a representative sample.
Blood meal sources and CSP detection assays
The enzyme-linked immunosorbent assay (ELISA) was used to detect for human and bovine blood antigens following the procedure of Beier et al. (1988) and P. falciparum and P. vivax_210 CSPs following the protocol of Beier et al. (1987). The head and thorax of female Anopheles mosquitoes were used to test for P. falciparum and P. vivax_210 CSPs, while the abdomen of freshly fed Anopheles and Culex mosquitoes was tested for blood meals using anti-human and anti-bovine antibodies. The detailed description was indicated in previous publications (Massebo et al. 2013b, a).
Statistical analysis
IBM SPSS 20 statistical software (SPSS International, Chicago, USA) was used to enter and analyse all data. The human blood index (HBI) and bovine blood index (BBI) were calculated as follows.
The percentage of mosquitoes that tested positive for P. falciparum and P. vivax out of all mosquitoes was used to determine the sporozoite rate. The Plasmodium EIRs were computed from CDC trap collections of mosquitoes using the following formula (Lines et al.1991):
where n is the number of CSP positive; N is the number of mosquitoes tested; ni is the number of mosquitoes collected; and Ni is the number of CDC light trap catches. An analysis of variance (ANOVA) was used to compare the monthly Anopheles mosquito population density. The months with the highest mean mosquito density were identified using Tukey’s honestly significant difference (HSD) test. Every test was run using a 0.05 threshold of significance.
Results
Mosquito species composition
Sixteen thousand seven hundred fifty-six adult female mosquitoes were collected using CDC light traps and Prokopack collection techniques. During the study period, the Culex mosquito predominated, accounting for 93% of the collected mosquitoes (15,571/16,756). The remaining mosquitoes were of the genera Aedes (1%, 169 mosquitoes) and Anopheles (6%, 1016 mosquitoes). Most of the mosquito larvae that developed into adults (80%, 104 out of 130) were of the Aedes species, while the remaining 20% (26 out of 130) were An. rhodesiensis, which is the only Anopheles species that was identified in the containers. Out of the 1016 adult Anopheles mosquitoes collected, 97% (986 out of 1016) were of the An. gambiae complex, 2.6% (27 out of 1016) were An. rhodesiensis, and 0.3% (3 out of 1016) were An. pharoensis.
Monthly distribution of mosquitoes
Table 1 shows the seasonal abundance of mosquito species over 7 months and varied between months (F = 136.1, DF = 6, P < 0.001). Anopheles mosquitoes were found in higher numbers in September, with 2.4 Anopheles per Prokopack per night and 13.5 Anopheles per CDC light trap per night.
Table 1.
Monthly distribution of Anopheles and Culex mosquitoes collected indoors from Arba Minch town, southwest Ethiopia: April to November 2022, except October
| Month | CDC collection | Prokopack collection | Total (%) | ||||
|---|---|---|---|---|---|---|---|
| Anopheles | Aedes | Culex | Anopheles | Aedes | Culex | ||
| Apr | 5 | 2 | 1516 | 2 | 2 | 538 | 2065 |
| May | 2 | 9 | 1629 | 1 | 2 | 397 | 2040 |
| June | 13 | 14 | 1956 | 1 | 1 | 569 | 2554 |
| July | 20 | 2 | 2459 | 1 | 0 | 407 | 2889 |
| Aug | 58 | 7 | 1706 | 9 | 0 | 833 | 2613 |
| Sep | 679 | 98 | 1494 | 24 | 0 | 365 | 2660 |
| Nov | 195 | 32 | 1305 | 6 | 0 | 397 | 1935 |
| Total | 972 | 164 | 12,065 | 44 | 5 | 3506 | 16,756 |
Distribution of mosquitoes in Kebeles
Sixteen thousand seven hundred fifty-six female mosquitoes were collected in five Kebeles of Arba Minch Town during the study period. The majority of them (35.6%; 5711 out of 16,756) were collected from Doysa Kebele, while the least (10.6%; 1769 out of 16,756) were found in Woze (Table 2). The difference in mosquito population density among the Kebeles was statistically significant (DF = 4, F = 4.1, and P = 0.003). The highest density of Anopheles mosquitoes was also found in Doysa Kebele (23.5%; 239 out of 1016).
Table 2.
Distribution of Anopheles and Culex mosquitoes collected indoors from Arba Minch town, southwest Ethiopia
| Kebeles | Mosquitoes collected | |||
|---|---|---|---|---|
| Anopheles, n (%) | Culex, n (%) | Aedes, n (%) | Overall | |
| Dilfana | 219 (21.6) | 1537 (9.9) | 39 (23.1) | 1795 (10.7) |
| Kulfo | 166 (16.3) | 2893 (18.6) | 45 (26.6) | 3104 (18.5) |
| Woze | 168 (16.5) | 1571 (10.1) | 30 (17.8) | 1769 (10.6) |
| Chamo | 224 (22.0) | 3859 (24.8) | 41 (24.3) | 4124 (24.6) |
| Doysa | 239 (23.5) | 5711 (36.7) | 14 (8.3) | 5964 (35.6) |
| Overall | 1016 (6.1) | 15,571 (92.9) | 169 (1.0) | 16,756 (100) |
Blood meal sources of mosquitoes
The overall human blood meal index of mosquitoes was 86.3% (710/823, 95% CI 83.7–88.5), while the bovine blood meal index was 2.1% (17/823, 95% CI 1.2–3.23). There were many unidentified blood meal origins (11.4%; 94/823, 95% CI 9.3–13.8). The mixed blood origin of human and bovine was low and accounted for only 0.1% (Table 3).
Table 3.
Blood meal origins of Anopheles and Culex mosquitoes collected indoors from Arba Minch town, southwest Ethiopia
| Mosquitoes tested | Number tested | Blood meal sources | |||
|---|---|---|---|---|---|
| Human, n (%) | Bovine, n (%) | Mixed, n (%) | Unidentified, n (%) | ||
| An. gambiae s.l | 180 | 90 (50) | 1 (0.5) | - | 89 (49.5) |
| An. pharoensis | 2 | 1 (50) | 1 (50) | - | - |
| Culex species | 641 | 620 (96.7) | 15 (2.4) | 1 (0.17) | 5 (0.8) |
| Total | 823 | 711 (86.4) | 17 (2.1) | 1 (0.1) | 94 (11.4) |
The human blood meal index of An. gambiae complex was 50% (90/180, 95% CI 42.3–57.5), but the bovine blood meal index was only 0.5% (95% CI 0.01–3.1). In Culex mosquitoes, the human blood index was 96.7% (620/641), while the bovine blood meal index was only 2.4% (15/641). The study also found that the proportion of An. gambiae complex with unidentified blood meal sources was 49.5% (95% CI 41.9–56.9), which was quite high. The Culex mosquitoes had a low percentage (0.8%) of unidentified blood meal sources (Table 3).
Blood meal indices of mosquitoes in different Kebeles
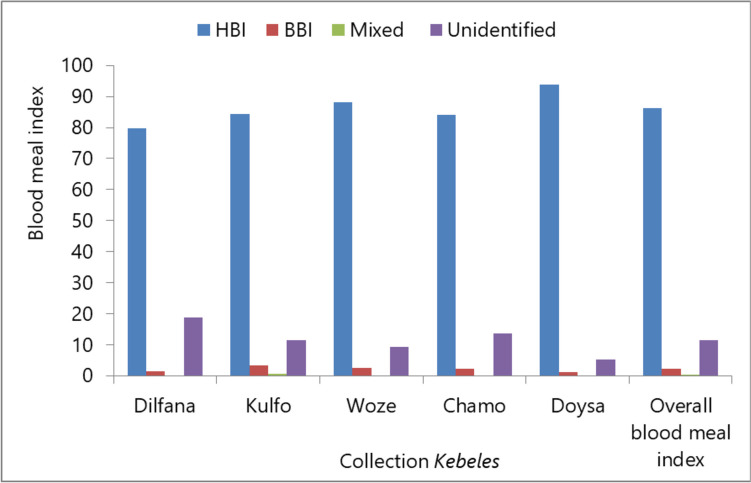
The human blood meal index of mosquitoes ranged from the minimum of 79.7% in Dilfana to 93.7% in Doysa. The bovine blood meal index varied from 1.1% in Doysa to 3.4% in Kulfo. Mixed blood meal sources (human and bovine) were extremely rare, ranging from 0 in most Kebeles to 0.7% in Kulfo. In addition, the percentage of mosquitoes with an unidentified blood meal origin varied from 5.1% in Doysa to 18.8% in Dilfana (Fig. 3).
Fig. 3.
Overall blood meal index of mosquitoes collected by CDC light traps and Prokopack in Arba Minch town, southwest Ethiopia. HBI, human blood meal index; BBI, bovine blood meal index
Blood meal index of Anopheles species
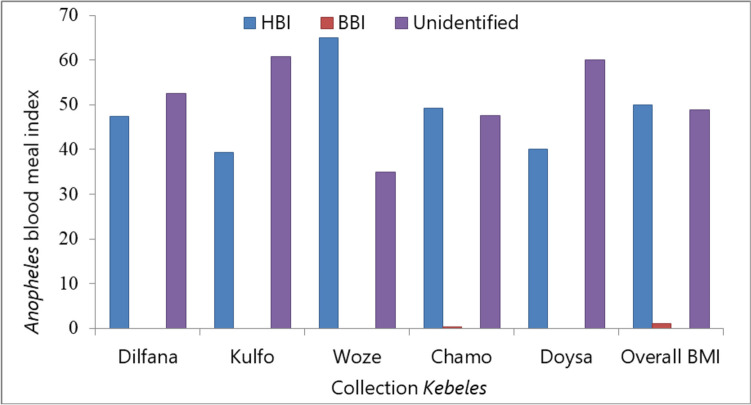
Anopheles species obtained blood meals from humans with indexes ranging from 39.3% in Kulfo to 65% in Woze. On the other hand, bovine blood meal indexes varied from 0% in most Kebeles to 0.3% in Chamo. The Anopheles species did not have any mixed blood origin. However, the unidentified blood meal index ranged from 35% in Woze to 60.7% in Kulfo (Fig. 4).
Fig. 4.
Blood meal index of Anopheles mosquitoes collected by CDC light traps and Prokopack in Arba Minch town, southwest Ethiopia. HBI, human blood meal index; BBI, bovine blood meal index
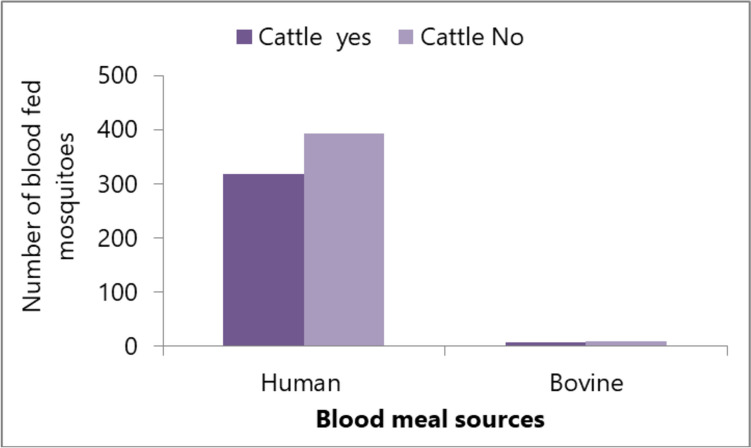
Although the presence or absence of cattle in houses did not influence the blood meal index of mosquitoes, more mosquitoes with human blood meal origins were collected from houses without cattle (Fig. 5).
Fig. 5.
The number of human and bovine blood-fed mosquitoes collected using CDC light traps and Prokopack in households with and without cattle in Arba Minch town, southwest Ethiopia
CSP rate and EIR
A total of 1016 Anopheles mosquitoes were tested for Plasmodium circum-sporozoite proteins (CSPs). Of these, 97% (986/1016) were An. gambiae complex, 2.7% (27/1016) were An. rhodesiensis, and 0.3% (3/1016) were An. pharoensis. The only P. vivax sporozoite was detected in the An. gambiae complex. Overall, the P. vivax CSP rate was 0.1% (95% CI 0.003–0.5%), and the sporozoite rate of An. gambiae s.l. was 0.1% (95% CI 0.003–0.6). The monthly P. vivax sporozoite rate among the An. gambiae s.l. caught by the CDC trap was 0.15% (1/679) in September 2022 and zero for other months. The entomological inoculation rate (EIR) estimates varied from 0 (in most months) to 0.13% in September 2022.
Discussion
Our research findings indicate that the majority of Anopheles and Culex mosquitoes fed on human blood, which suggests a higher risk of mosquito bites for humans. Several species of mosquitoes, including An. gambiae complex, An. pharoensis, An. rhodesiensis, Aedes, and Culex, were found in the study area. The Anopheles gambiae complex was identified as the primary vector for malaria in urban areas.
Anopheles gambiae complex was the primary mosquito responsible for carrying malaria in Arba Minch town. Both P. falciparum and P. vivax parasites have been detected in the An. gambiae complex in Arba Minch Getawen et al. 2018) and neighbouring areas (Massebo et al. 2013a; Abraham et al. 2017; Esayas et al. 2020). Unplanned urbanization, characterized by the development of slums, construction activities, and a lack of drainage systems, has led to the creation of numerous breeding habitats for An. gambiae complex in the urban environment (De Silva and Marshall 2012; Doumbe-Belisse et al. 2021). Besides the water bodies formed at construction sites in the town, the Kulfo River that passes through the town and flows into Lake Chamo may also provide breeding habitats for the mosquitoes. Thus, much work remains to be done in urban settings to mitigate malaria-related problems. Despite being a secondary malaria vector in Ethiopia, An. pharoensis is rarely sampled at the current study site (Abraham et al. 2017). This species was collected near the Kulfo River, where permanent water sources exist. Anopheles rhodesiensis could breed in containers like discarded plastics and barrels. At one of the study sites in Bahir Dar City, An. rhodesiensis was the most frequently captured species by CDC light traps, accounting for 90% of all collections (Getaneh et al. 2021). In contrast to the Bahir Dar study, where adult mosquitoes were caught using CDC light traps, we collected the larval stage of the species from containers at our study location.
Culex mosquitoes were the most abundant throughout the study Kebeles and periods. The mosquito density was similar during the dry and rainy seasons and thus less likely to be related to rainfall. In most cities in Cameroon, Culex mosquitoes were the main species causing the highest nuisance to the population (Nchoutpouen et al. 2019). The dominant presence of Culex mosquitoes in this study area may be due to poor management of stagnant water sources. The rise in car washing stations, construction sites, and poor drainage systems might have contributed to this issue. To address this, a comprehensive strategy is needed.
The human blood meal index of An. gambiae complex was higher than the bovine meal index, indicating its higher opportunity to feed humans than animals. However, studies conducted in rural settings have reported different results. For instance, a study conducted in south-central Ethiopia reported similar proportions of human-fed and bovine-fed An. arabiensis (Animut et al. 2013). Another study conducted in southwest Ethiopia showed that An. arabiensis preferred bovine blood meal over human blood meal (Massebo et al. 2015). Although Culex mosquitoes feed on various blood sources worldwide (Zinser et al. 2004; Greenberg et al. 2013), in the study site, they have greater access to human blood than bovine. These mosquitoes often enter houses and feed on human blood, increasing the risk of transmitting diseases such as West Nile virus, lymphatic filariasis, and Rift Valley fever in urban areas (Zinser et al. 2004). Despite being a vector for several diseases, Culex mosquitoes have not received as much attention as Anopheles species in disease transmission. It is believed that this species contributes to the rapid growth and spread of many viral diseases (Riccetti et al. 2022).
In the current study, a significant number of An. gambiae complex mosquitoes had unidentified blood sources, indicating that they may have fed on different animals (Kent and Norris 2005). In southwest Ethiopia, 22.5% of An. arabiensis mosquitoes had unidentified blood sources (Massebo et al. 2013b), while in northern Ethiopia, the An. gambiae complex had unidentified blood sources comprising 42.8% of their total blood meals (Kindu et al. 2018). The limited number of antibodies used in the blood meal sources testing could be a contributing factor to this issue. Our study aimed to determine whether mosquitoes feed on humans or cattle by analysing their blood meals. We considered the results of a previous study conducted in nearby rural villages, which indicated that mosquitoes have a preference for feeding on cattle (Massebo et al. 2015). Additionally, animal composition in urban areas may differ from rural villages, contributing to unidentified blood meal sources. To address this issue, it is important to use a variety of antibodies for ELISA testing and PCR, along with diverse primers, to detect all blood meal sources of Anopheles mosquitoes accurately.
Out of all the Anopheles species that were tested for CSP, only one An. gambiae (probably An. arabiensis) complex tested positive for P. vivax CSP. However, the CSP rate was found to be lower than in a previous study conducted in the same town, where P. falciparum was the dominant parasite (Getawen et al. 2018). Another study conducted in a nearby rural village reported that both An. gambiae complex and An. pharoensis tested positive for CSP (Abraham et al. 2017). Despite the low CSP rate, An. gambiae complex, likely An. arabiensis, adapted to urban settings and persistently plays a role in malaria transmission. Although the EIR was calculated for P. vivax, which may not indicate active transmission, it is clear that An. gambiae complex plays a role in malaria transmission in urban areas. In a previous study conducted in the same town near Kulfo River, where malaria transmission is more intense compared to other areas, An. arabiensis was found to be carrying both P. falciparum and P. vivax parasites. The study also reported an P. falciparum EIR of up to 6.45 (Getawen et al. 2018). This implies that An. arabiensis is a well-adapted malaria vector in urban settings.
Anopheles gambiae complex was not molecularly identified. However, a previous study identified it as An. arabiensis (Massebo et al. 2013a), which is predominantly responsible for malaria transmission (Getawen et al. 2018). Moreover, the study was unable to identify other animal sources of blood meals for malaria vectors other than humans and cattle. Lastly, the study did not screen Aedes and Culex mosquitoes for filarial and arboviral infections.
Conclusions
The study findings indicate that the An. gambiae complex is an efficient malaria vector dominant in Arba Minch town. These mosquitoes have a high tendency to feed on human blood, indicating frequent contact between humans and mosquitoes. Personal protection tools like bed nets and screening of houses could be implemented to minimize the human-vector contacts. The Anopheles mosquito identified as a container breeder was An. rhodesiensis, while An. stephensi was not found.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The Arba Minch Town Health Department and participating community members deserve special acknowledgement for their contribution to the study.
Author contributions
A.A. designed the study, preformed species identification and conducted fieldwork; F.M. designed the study, preformed species identification, conducted data interpretation and wrote the first draft of manuscript; G.T. and N.E. processed laboratory samples; B.L. performed data analysis and revised the manuscript. All authors read and approved the final manuscript.
Funding
The Norwegian Programme for Capacity Development in Higher Education and Research for Development (QZA-21/0162) funded this study. The funding agency played no role in any of the research activities.
Data availability
The data used to draw a conclusion was presented in the article.
Declarations
Ethical consideration
Arba Minch University’s Institutional Review Board reviewed and approved this study (IRB/12452022). Verbal and written consents were obtained from all household heads before mosquito collection. The field collection was not done in a protected area or with endangered species. There is no environmental or animal health risk while using research materials.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abraham M, Massebo F, Lindtjørn B. High entomological inoculation rate of malaria vectors in area of high coverage of interventions in southwest Ethiopia: implication for residual malaria transmission. Parasite Epidemiol Control. 2017;2:61–69. doi: 10.1016/j.parepi.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Animut A, Balkew M, Gebre-Michael T, Lindtjørn B. Blood meal sources and entomological inoculation rates of anophelines along a highland altitudinal transect in south-central Ethiopia. Malar J. 2013;12:76. doi: 10.1186/1475-2875-12-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkew M, Mumba P, Dengela D, Yohannes G, Getachew D, Yared S, Chibsa S, Murphy M, George K, Lopez K, Janies D, Choi SH, Spear J, Irish SR, Carter TE. Geographical distribution of Anopheles stephensi in eastern Ethiopia. Parasit Vectors. 2020;13:35. doi: 10.1186/s13071-020-3904-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier JC, Perkins PV, Wirtz RA, Whitmire RE, Mugambi M, Hockmeyer WT. Field evaluation of an enzyme-linked immunosorbent assay (ELISA) for Plasmodium falciparum sporozoite detection in anopheline mosquitoes from Kenya. Am J Trop Med Hyg. 1987;36:459–468. doi: 10.4269/ajtmh.1987.36.459. [DOI] [PubMed] [Google Scholar]
- Beier JC, Perkins PV, Wirtz RA, et al. Bloodmeal identification by direct enzyme-linked immunosorbent assay (ELISA), tested on Anopheles (Diptera : Culicidae) in Kenya. J Med Entomol. 1988;25:9–16. doi: 10.1093/jmedent/25.1.9. [DOI] [PubMed] [Google Scholar]
- Carter TE, Yared S, Gebresilassie A, Bonnell V, Damodaran L, Lopez K, Ibrahim M, Mohammed S, Janies D. First detection of Anopheles stephensi Liston, 1901 (Diptera : Culicidae) in Ethiopia using molecular and morphological approaches. Acta Trop. 2018;188:180–186. doi: 10.1016/j.actatropica.2018.09.001. [DOI] [PubMed] [Google Scholar]
- Coetzee M. Key to the females of Afrotropical Anopheles mosquitoes (Diptera : Culicidae) Malar J. 2020;19:70. doi: 10.1186/s12936-020-3144-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Silva PM, Marshall JM (2012) Factors contributing to urban malaria transmission in sub-saharan Africa: a systematic review. J Trop Med 2012:819563. 10.1155/2012/819563 [DOI] [PMC free article] [PubMed]
- Doumbe-Belisse P, Kopya E, Ngadjeu CS, Sonhafouo-Chiana N, Talipouo A, Djamouko-Djonkam L, Awono-Ambene HP, Wondji CS, Njiokou F, Antonio-Nkondjio C. Urban malaria in sub-Saharan Africa: dynamic of the vectorial system and the entomological inoculation rate. Malar J. 2021;20:364. doi: 10.1186/s12936-021-03891-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esayas E, Woyessa A, Massebo F. Malaria infection clustered into small residential areas in lowlands of southern Ethiopia. Parasite Epidemiol Control. 2020;10:e00149. doi: 10.1016/j.parepi.2020.e00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getaneh A, Yimer M, Alemu M, Dejazmach Z, Alehegn M, Tegegne B. Species composition, parous rate, and infection rate of Anopheles mosquitoes (Diptera: Culicidae) in Bahir Dar City administration, Northwest Ethiopia. J Med Entomol. 2021;58:1874–1879. doi: 10.1093/jme/tjab034. [DOI] [PubMed] [Google Scholar]
- Getawen SK, Ashine T, Massebo F, Woldeyes D, Lindtjørn B. Exploring the impact of house screening intervention on entomological indices and incidence of malaria in Arba Minch town, southwest Ethiopia: a randomized control trial. Acta Trop. 2018;181:84–94. doi: 10.1016/j.actatropica.2018.02.009. [DOI] [PubMed] [Google Scholar]
- Greenberg JA, Lujan DA, DiMenna MA, Wearing HJ, Hofkin BV. Identification of blood meal sources in Aedes vexans and Culex quinquefasciatus in Bernalillo County. New Mex J Insect Sci. 2013;13:75. doi: 10.1673/031.013.7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay SI, Guerra CA, Tatem AJ, Atkinson PM, Snow RW. Urbanization, malaria transmission and disease burden in Africa. Nat Rev Microbiol. 2005;3:81–90. doi: 10.1038/nrmicro1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiser J, Utzinger J, Caldas de Castro M, Smith TA, Tanner M, Singer BH. Urbanization in sub-Saharan Africa and implication for malaria control. Am J Trop Med Hyg. 2004;71:118–127. doi: 10.4269/ajtmh.2004.71.118. [DOI] [PubMed] [Google Scholar]
- Kent RJ, Norris DE. Identification of mammalian blood meals in mosquitoes by a multiplexed polymerase chain reaction targeting cytochrome B. Am J Trop Med Hyg. 2005;73:336–342. doi: 10.4269/ajtmh.2005.73.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindu M, Aklilu E, Balkew M, Gebre-Michael T. Study on the species composition and ecology of anophelines in Addis Zemen, South Gondar. Ethiop Parasites Vectors. 2018;11:215. doi: 10.1186/s13071-018-2701-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinkenberg E, McCall P, Wilson MD, Amerasinghe FP, Donnelly MJ. Impact of urban agriculture on malaria vectors in Accra. Ghana Malar J. 2008;7:151. doi: 10.1186/1475-2875-7-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lines JD, Curtis CF, Wilkes T, Njunwa K. Monitoring human-biting mosquitoes (Diptera : Culicidae) in Tanzania with light-traps hung beside mosquito nets. Bull Entom Res. 1991;81:77–84. doi: 10.1017/S0007485300053268. [DOI] [Google Scholar]
- Massebo F, Balkew M, Gebre-Michael T, Lindtjorn B. Entomologic inoculation rates of Anopheles arabiensis in southwestern Ethiopia. Am J Trop Med Hyg. 2013;89:466–473. doi: 10.4269/ajtmh.12-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massebo F, Balkew M, Gebre-michael T, Lindtjørn B. Blood meal origins and insecticide susceptibility of Anopheles arabiensis from Chano in South-West. Parasites Vectors. 2013;6:44. doi: 10.1186/1756-3305-6-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massebo F, Balkew M, Gebre-Michael T, Lindtjørn B. Zoophagic behaviour of anopheline mosquitoes in southwest Ethiopia: opportunity for malaria vector control. Parasites Vectors. 2015;8:645. doi: 10.1186/s13071-015-1264-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nchoutpouen E, Talipouo A, Djiappi-Tchamen B, Djamouko-Djonkam L, Kopya E, Ngadjeu CS, Doumbe-Belisse P, Awono-Ambene P, Kekeunou S, Wondji CS, Antonio-Nkondjio C. Culex species diversity, susceptibility to insecticides and role as potential vector of Lymphatic filariasis in the city of Yaoundé. Cameroon Plos Negl Trop Dis. 2019;13:e0007229. doi: 10.1371/journal.pntd.0007229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccetti N, Fasano A, Ferraccioli F, Gomez-Ramirez J, Stilianakis NI. Host selection and forage ratio in West Nile virus-transmitting Culex mosquitoes: challenges and knowledge gaps. PLoS Negl Trop Dis. 2022;16:e0010819. doi: 10.1371/journal.pntd.0010819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrard-Smith E, Skarp JE, Beale AD, Fornadel C, Norris LC, Moore SJ, Mihreteab S, Charlwood JD, Bhatt S, Winskill P, Griffin JT, Churcher TS. Mosquito feeding behavior and how it influences residual malaria transmission across Africa. Proc Natl Acad Sci USA. 2019;116:15086–15095. doi: 10.1073/pnas.1820646116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinka ME, Bangs MJ, Manguin S, Chareonviriyaphap T, Patil AP, Temperley WH, Gething PW, Elyazar IR, Kabaria CW, Harbach RE, Hay SI. The dominant Anopheles vectors of human malaria in the Asia-Pacific region: occurrence data, distribution maps and bionomic précis. Parasit Vectors. 2011;4:89. doi: 10.1186/1756-3305-4-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadesse FG, Ashine T, Teka H, Esayas E, Messenger LA, Chali W, Meerstein-Kessel L, Walker T, Wolde Behaksra S, Lanke K, Heutink R, Jeffries CL, Mekonnen DA, Hailemeskel E, Tebeje SK, Tafesse T, Gashaw A, Tsegaye T, Emiru T, Simon K, Bogale EA, Yohannes G, Kedir S, Shumie G, Sabir SA, Mumba P, Dengela D, Kolaczinski JH, Wilson A, Churcher TS, Chibsa S, Murphy M, Balkew M, Irish S, Drakeley C, Gadisa E, Bousema T. Anopheles stephensi mosquitoes as vectors of Plasmodium vivax and falciparum, Horn of Africa, 2019. Emerg Infect Dis. 2021;27:603–607. doi: 10.3201/eid2702.200019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Prokopec GM, Galvin WA, Kelly R, Kitron U. A new, cost-effective, battery-powered aspirator for adult mosquito collections. J Med Entomol. 2009;46:1256–1259. doi: 10.1603/033.046.0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . Manual on practical entomology in malaria: methods and techniques. Division of Malaria and Other Parasitic Diseases: World Health Organization; 1975. [Google Scholar]
- World Health Organization . A global brief on vector-borne diseases. Geneva: World Health Organization; 2014. p. 2014. [Google Scholar]
- World Health Organization . Global framework for the response to malaria in urban areas. Geneva: World Health Organization, Geneva; 2022. [Google Scholar]
- Yared S, Gebressielasie A, Damodaran L, Bonnell V, Lopez K, Janies D, Carter TE. Insecticide resistance in Anopheles stephensi in Somali Region, eastern Ethiopia. Malar J. 2020;19:180. doi: 10.1186/s12936-020-03252-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinser M, Ramberg F, Willott E. Culex quinquefasciatus (Diptera: Culicidae) as a potential West Nile virus vector in Tucson, Arizona: blood meal analysis indicates feeding on both humans and birds. J Insect Sci. 2004;4:20. doi: 10.1093/jis/4.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used to draw a conclusion was presented in the article.