Abstract
The results of phase 3 efficacy trials have shown that acellular and whole-cell pertussis vaccines can confer protection against whooping cough. However, despite the advances in vaccine development, clinical trials have not provided significant new information on the mechanism of protective immunity against Bordetella pertussis. Classical approaches based on measurement of antibody responses to individual antigens failed to define an immunological correlate of protection. A reliable animal model, predictive of acellular and whole-cell pertussis vaccine potency in children, would facilitate an elucidation of the mechanism of immune protection against B. pertussis and would assist in the regulatory control and future development of pertussis vaccines. In this study, we have shown that the rate of B. pertussis clearance following respiratory challenge of immunized mice correlated with vaccine efficacy in children. Using this model together with mice with targeted disruptions of the gamma interferon (IFN-γ) receptor, interleukin-4 or immunoglobulin heavy-chain genes, we have demonstrated an absolute requirement for B cells or their products in bacterial clearance and a role for IFN-γ in immunity generated by previous infection or immunization with the whole-cell pertussis vaccine. The results of passive immunization experiments suggested that protection early after immunization with acellular pertussis vaccines is mediated by antibody against multiple protective antigens. In contrast, more complete protection conferred by previous infection or immunization with whole-cell pertussis vaccines reflected the induction of Th1 cells. Our findings suggest that the mechanism of immunity against B. pertussis involves humoral and cellular immune responses which are not directed against a single protective antigen and thus provide an explanation for previous failures to define an immunological correlate of protection.
Recent clinical trials have demonstrated that acellular pertussis vaccines (Pa) comprising different combinations of the putative protective antigens, pertussis toxin (PT), filamentous hemagglutinin (FHA), pertactin (PRN), and fimbriae from Bordetella pertussis, can confer protection against World Health Organization-defined whooping cough (1, 6, 11, 12, 37, 39). The safety profile of Pa from the clinical trials revealed a considerable reduction in the number of mild or severe adverse events in vaccinated children compared with conventional whole-cell vaccines (Pw) (6, 11, 12, 21, 37, 39). However, the mechanism of protection conferred by natural or vaccine-induced immunity remains to be defined. Assessment of antibody responses in the clinical trials did not reveal a clear correlation between serum antibody responses against a single B. pertussis antigen and protection (10–12, 21, 37). Investigations of cell-mediated immunity against B. pertussis in children demonstrated that recovery from whooping cough or immunization with Pw is associated with selective induction of Th1 cells (30), whereas immunization with Pa induces T cells with a mixed Th1-Th2 cytokine profile (2, 31, 32). These studies suggest that distinct arms of the immune response may make different contributions to protection induced by different types of immunization or natural infection.
Animal models for infectious diseases of humans have made significant contributions to our understanding of the immunological mechanisms that determine resolution of infection or progression to disease and provide the most realistic possibility of defining the mechanism of immune protection against B. pertussis. Although the murine intracerebral challenge model has been used to assess the potency of Pw (14, 20), this test fails to predict the efficacy of Pa in humans. In contrast, aerosol inoculation results in a respiratory infection in adult mice displaying many of the characteristics seen in infected children and has been used by a number of laboratories to examine mechanisms of immunity to B. pertussis (18, 19, 22, 26, 33, 34, 36).
Studies in our laboratory using the murine respiratory infection model have provided evidence that T cells may play a significant role in protection against B. pertussis (3, 18, 19, 22, 28). We have demonstrated that adoptive transfer of CD4+ T cells from convalescent mice can confer protection against B. pertussis respiratory challenge in irradiated or T-cell-deficient athymic mice (22). Respiratory infection or immunization with Pw selectively primed Th1 cells, whereas immunization with Pa induced high antibody levels and Th2 cells (3, 28). Furthermore, addition of interleukin (IL-12) to an acellular vaccine polarized the T-cell response to the Th1 subtype and improved its protective efficacy (18). These studies point to a role for Th1 cells in protection against B. pertussis but do not exclude a role for antibody.
In the present study, the availability of samples of the Pw and Pa vaccines that had been tested in the National Institute of Allergy and Infectious Diseases-sponsored phase 3 clinical trials in Italy (11) and Sweden (12) allowed us to demonstrate that protection in the murine respiratory challenge model correlates with vaccine efficacy in children. It also allowed us to compare these results with those obtained with Pw and Pa that were tested in the Senegal efficacy trial (37). We have used this model in the context of active and passive immunization of normal mice and mice with disruptions in genes encoding either the membrane axon of the μ-chain constant region of the immunoglobulin (Ig) molecule (Ig−/−), IL-4 (IL-4−/−), or the gamma interferon (IFN-γ) receptor (IFN-γR−/−) to examine the mechanisms of natural and vaccine-induced immunity against B. pertussis. Taken together with the results of our previous studies (3, 18, 19, 22, 28), the findings demonstrate complementary roles for cellular and humoral immunity in protection against B. pertussis.
MATERIALS AND METHODS
Mice.
All mice used were commercially obtained (B&K Universal Ltd., Hull, United Kingdom) and were bred and maintained according to the guidelines of the Irish Department of Health. The IFN-γR−/− mice, in which IFN-γ is nonfunctional (13), were used with the kind permission of M. Aguet (Molecular Biology Department, University of Zurich, Zurich, Switzerland). These mice were generated from the wild-type 129Sv/Ev (H-2b) strain. The IL-4−/− mice (16) were used with the kind permission of Werner Muller (Institute for Genetics, University of Cologne, Cologne, Germany), and the Ig−/− (μMT) mice (15) were used with the kind permission of Klaus Rajewsky (Institute for Genetics, University of Cologne). The Ig−/− and IL-4−/− mice were generated from wild-type C57BL/6 (H-2b) mice. Unless otherwise stated, all mice were 8 to 12 weeks old at the initiation of experiments.
Bacterial antigens.
Heat-killed B. pertussis was prepared by incubation of cells at 80°C for 30 min. Genetically detoxified recombinant PT (rPT) mutant (PT-9K/129G) (27) and native FHA and PRN prepared from B. pertussis were kindly provided by Rino Rappuoli (Chiron SpA, Siena, Italy).
Immunization.
Groups of 20 BALB/c mice were immunized intraperitoneally at 0 and 4 weeks with 0.2 human dose of either Wellcome (W), Pasteur Mérieux (PM), or U.S. Connaught Laboratories Inc. (CLI) Pw, SmithKline Beecham (SB) Pa2, SB Pa3, PM Pa2, Canadian Connaught Laboratories Ltd. (CLL) Pa5, and Chiron Biocine (CB) Pa3. The antigenic compositions of the vaccines and their estimated efficacies in children are shown in Table 1. With the exception of the W Pw, which was the third British Reference Preparation (88/522) from the National Institute for Biological Standards and Control, Potters Bar, Hertfordshire, United Kingdom, all vaccines were provided by the manufacturers as combined diphtheria-tetanus-pertussis formulations adsorbed to alum. Mice were challenged 2 weeks after the second immunization. In experiments comparing active and passive immunization and assessing the contribution of individual antigens to protection, BALB/c mice were immunized with W Pw (0.2 human dose) or 5.0 μg each of different combinations of Pa components (rPT, FHA, and PRN). In active immunization experiments, mice were challenged 2 weeks after two immunizations (0 and 4 weeks). In passive immunization experiments, mice were immunized three times (0, 3, and 6 weeks) and were bled at week 8. Recipient BALB/c mice were injected intravenously with 0.1 ml serum from mice immunized with W Pw or Pa (rPT, FHA, and PRN) or with 0.1 ml of each serum (in different combinations) from mice immunized with rPT, FHA, or PRN alone. Mice were challenged 4 h after passive transfer of antibody.
TABLE 1.
Vaccine composition and estimated efficacy in children
| Vaccine | Antigen compositiona (μg) | Trial/studyb | Estimated efficacy (%) |
|---|---|---|---|
| CLI Pw | Whole cell | Italy/Sweden | 36/48 |
| PM Pw | Whole cell | Senegal | 96 |
| W Pw | Whole cell | United Kingdomc | 93–94 |
| SB Pa2 | PTd (25), FHA (25) | Sweden | 59 |
| SB Pa3 | PTd (25), FHA (25), PRN (8) | Italy | 84 |
| CLL Pa5 | PTd (10), FHA (5), PRN (3), Fim2,3 (5) | Sweden | 85 |
| PM Pa2 | PTd (25), FHA (25) | Senegal | 85 |
| CB Pa3 | rPT (5), FHA (2.5), PRN (2.5) | Italy | 84 |
B. pertussis respiratory challenge model.
Respiratory infection of mice was initiated by aerosol challenge by a modification of the method described by Sato et al. (33). B. pertussis W28 phase I was grown under agitation conditions at 37°C in Stainer-Scholte liquid medium. Bacteria from a 48-h culture were resuspended at a concentration of approximately 2 × 1010 CFU/ml in physiological saline containing 1% casein. The challenge inoculum was administered to mice as an aerosol over a period of 15 min by means of a nebulizer as described previously (18). Groups of four mice were sacrificed at various times after aerosol challenge to assess the number of viable B. pertussis in the lungs. Lungs were removed aseptically from infected mice and homogenized in 1 ml of sterile physiological saline with 1% casein on ice. Aliquots of 100 μl of undiluted homogenate or of serially diluted homogenate from individual lungs were spotted in triplicate onto Bordet-Gengou agar plates, and the number of CFU was estimated after 5 days of incubation at 37°C. Results are reported as the mean viable B. pertussis for individual lungs from at least four mice per time point per experimental group. The limit of detection was approximately log10 0.5 CFU per lung.
Anti-B. pertussis antibodies.
The levels of B. pertussis-specific antibodies in serum and lung lavage (22) were determined by enzyme-linked immunosorbent assay using purified B. pertussis antigens (2 μg/ml) to coat plates. Bound antibodies were detected using alkaline phosphatase-conjugated anti-mouse IgG or IgG subclasses (PharMingen, San Diego, Calif.).
Cytokine assays.
Spleen cells (2 × 106/ml) from naive and immune mice were cultured with heat-killed B. pertussis (105, 106, or 107/ml), heat-inactivated PT (0.2 to 5.0 μg/ml), FHA (0.2 to 5.0 μg/ml), PRN (0.2 to 5.0 μg/ml), or medium alone. Supernatants were removed after 72 h, and IFN-γ and IL-5 concentrations were determined by immunoassay as previously described (18). Results are given as mean cytokine concentrations against the optimum antigen concentration for groups of four mice tested individually in triplicate.
Statistical analysis.
Calculation of the areas under bacterial clearance curves and the regression analysis were performed by using the Fig. P graphics and statistical software.
RESULTS
Protection in a murine respiratory challenge model correlates with vaccine efficacy in children.
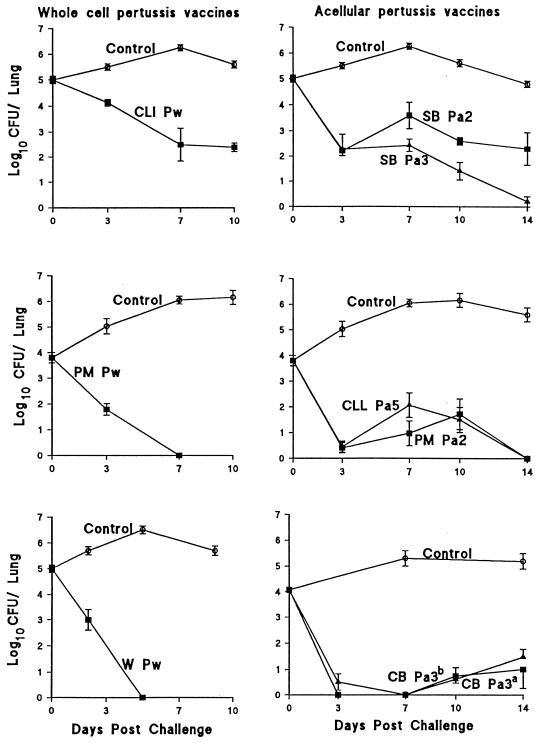
We have evaluated three Pw and five Pa, including clinical lots evaluated in different efficacy trials (Table 1). The number of bacteria in naive control mice increased to a peak 7 to 10 days after challenge and gradually declined to undetectable levels after 35 to 42 days (Fig. 1). Immunization with the W Pw or PM Pw, which like other European Pw have estimated efficacy in the region of 95% (6, 8, 25, 37), conferred the highest level of protection in mice, with complete bacterial clearance 5 to 7 days after challenge. In contrast, CLI Pw, which protected only 36 and 48% of children against whooping cough in the National Institute of Allergy and Infectious Diseases-sponsored Italian and Swedish trials, respectively (11, 12), showed significantly poorer clearance in immunized mice, with significant levels of bacteria still detectable in the lungs 10 days after challenge. Mice immunized with different Pa showed an early decline in the bacterial load in the lungs by day 3 after challenge but failed to completely eliminate the bacteria until 11 to 14 days later. The rate of bacterial clearance was lower in mice immunized with SB Pa2 than in those immunized with the SB Pa3. The same lots of vaccine had 59 and 84% efficacy, respectively, in the Swedish and Italian trials (11, 12). In contrast, another two-component vaccine manufactured by Pasteur Mérieux (PM Pa2) generated an equivalent level of protection as the CLL Pa5 in the mouse model. The estimated absolute efficacy of these vaccines was 85% in the Senegal (37) and Swedish (12) trials. Finally, the clinical lot of the CB Pa3, which protected 84% of children in the Italian trial (11), conferred a high level of protection in the mouse model. The numbers of CFU in lungs were close to or below the level of detection 3, 7, 10, and 14 days after challenge. The almost identical clearance curves observed in mice with a recent clinical lot of the CB Pa3 provides convincing evidence of the reproducibility of the test within experiments and is indicative of the long-term stability of this vaccine (Fig. 1). Further evidence of the reproducibility of the aerosol challenge model was evident from comparisons of the vaccines from the same manufacturer tested on different occasions (data not shown).
FIG. 1.
Kinetics of B. pertussis clearance from the lungs after respiratory challenge of mice immunized with Pw or Pa. Groups of 20 BALB/c mice were immunized intraperitoneally at 0 and 4 weeks with 0.2 human dose of either W Pw, PM Pw, CLI Pw, SB Pa2, SB Pa3, PM Pa2, CLL Pa5, or CB Pa3 (a, lot used in Italian trial manufactured in 1992; b, clinical lot manufactured in 1996) or with alum alone (control). Two weeks after the second immunization, mice were challenged with B. pertussis W28 and CFU counts were performed on individual lung homogenate at intervals after challenge. Results are mean (±standard error) viable B. pertussis counts from four mice per group at each time point.
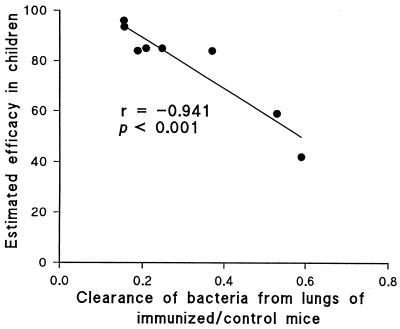
We performed statistical analysis to test the significance of the correlation between protection in children and in the murine respiratory model. Regression analysis revealed a highly significant (P < 0.001) correlation between the bacterial clearance in mice and the estimated efficacy in children (Fig. 2). Expression of the area under the clearance curves (days 0 to 14 after challenge) of immunized mice as a ratio of the area under the curve for unimmunized control mice allows compensation for any experiment-to-experiment variation in the challenge, and this ratio gave a negative correlation (r = −0.941) with the estimated vaccine efficacy in children (Fig. 2). It should be noted that the data on efficacy in children are derived from a number of trials and studies carried out under different conditions.
FIG. 2.
Correlation between bacterial clearance from the lungs following aerosol challenge of immunized mice and vaccine efficacy in children. The areas under the curves of bacterial clearance 0 to 14 days after challenge of mice immunized with a vaccine, expressed as a ratio of area under the curves for control unimmunized mice in the same experiment (as shown in Fig. 1, except area was calculated up to 14 days in all cases), were plotted against the estimated efficacy of the vaccine shown in Table 1. The efficacies of the CLI Pw and W Pw were taken as the means of the values given in Table 1.
Gene knockout mice reveal distinct mechanisms of natural and vaccine-induced immunity.
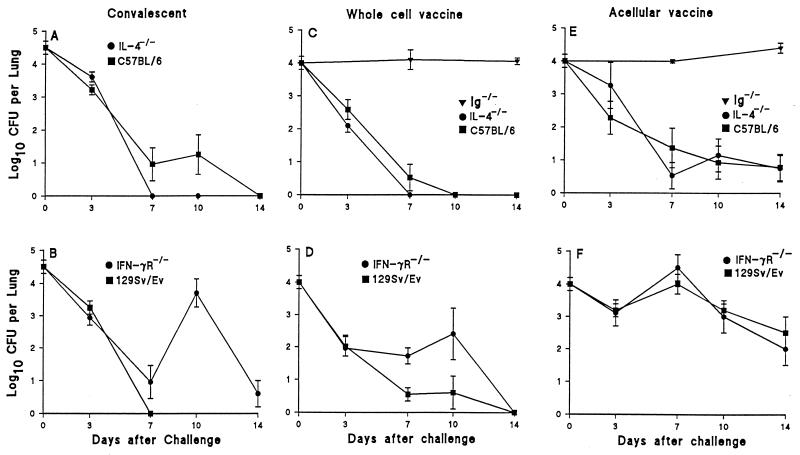
To examine the roles of B cells and of two key type 1 and type 2 cytokines, IFN-γ and IL-4, in natural and vaccine-induced immunity against B. pertussis, we examined the course of infection in Ig−/−, IL-4−/−, IFN-γR−/−, and wild-type mice that had previously been infected or immunized with Pw or Pa. Since Ig−/− mice develop a chronic infection after primary challenge (19), we could not examine the role of B cells in a secondary infection. Nevertheless, although IFN-γR−/− mice developed an atypical disseminated infection, they did clear the bacteria from the lungs 80 to 100 days after challenge (19). However, following reinfection, the rate of bacterial clearance was lower in IFN-γR−/− mice than in the wild-type strain (Fig. 3). Bacteria were undetectable in the lungs of wild-type 129Sv/Ev mice 7 days after challenge, whereas the CFU counts in the lungs of IFN-γR−/− mice declined to low levels on day 7 but rebounded on day 10 and were still at detectable levels 14 days after challenge. In contrast, the rate of bacterial clearance was higher in IL-4−/− mice than in the wild-type C57BL/6 mice (Fig. 3).
FIG. 3.
Bacterial clearance following B. pertussis respiratory challenge of immunized or convalescent gene-disrupted mice. IL-4−/− (A) and IFN-γR−/− (B) and the wild-type C57BL/6 and 129Sv/Ev mice were infected with B. pertussis, allowed to clear the bacteria (12 to 14 weeks), and challenged 2 weeks later. Ig−/−, IL-4−/− (C and E), and IFN-γR−/− (D and F) mice and the wild-type C57BL/6 and 129Sv/Ev mice were immunized twice (weeks 0 and 4) with the W Pw (C and D) or Pa prepared from rPT, FHA, and PRN adsorbed to alum (E and F) and were challenged 2 weeks after the second immunization. Results from four experiments are mean (±standard error) CFU per lung for four to nine mice per time point.
Immunization of Ig−/− mice with pertussis vaccines did not confer protection against infection with B. pertussis; the levels of bacteria in the lung remained at high levels 7 and 14 days after respiratory challenge (Fig. 3). In contrast, the levels of bacteria were low or undetectable 7 to 14 days after challenge of wild-type mice following two immunizations with Pw (Fig. 3). However, the numbers of viable bacteria were higher 7 and 10 days after challenge in IFN-γR−/− mice than in 129Sv/Ev mice but were almost identical in IL-4−/− and the wild-type C57BL/6 mice immunized with Pw (Fig. 3). Disruption of the IL-4 or IFN-γR gene had little effect on clearance of B. pertussis from the lungs of mice immunized with Pa (Fig. 3).
Immune responses in normal and gene knockout mice.
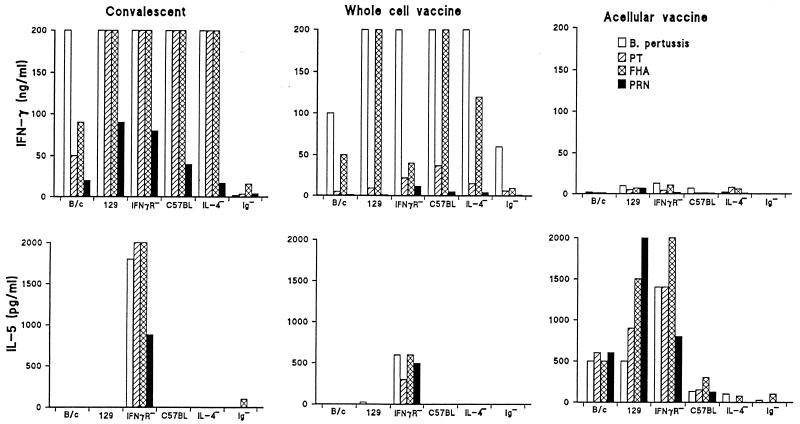
An examination of the systemic T-cell responses in infected and immunized mice revealed that the earliest bacterial clearance was observed in mice with the most polarized Th1 responses. Respiratory infection or immunization with Pw selectively stimulated systemic Th1 cells specific for PT, FHA, and PRN in all strains examined except the IFN-γR−/− mice, where IL-5 was detected in addition to IFN-γ and IL-2 (Fig. 4 and data not shown). In contrast, immunization with Pa induced T cells that secreted high levels of IL-5 in BALB/c and 129Sv/Ev mice and lower levels in C57BL/6 mice but almost undetectable levels of IFN-γ in all strains except the IL-4−/− mice (Fig. 4). B-cell-deficient mice were found to have defective T-cell responses. In comparison with the wild-type C57BL/6 mice, B. pertussis-specific T-cell proliferation and cytokine production were significantly reduced in Ig−/− mice following infection or immunization with Pw, and responses were almost undetectable following immunization with Pa (Fig. 4 and data not shown).
FIG. 4.
T-cell responses in immune wild-type and gene-disrupted mice. BALB/c (B/c), 129Sv/Ev (129), IFN-γR−/−, C57BL/6 (C57BL), IL-4−/−, or Ig−/− mice were immunized or infected as described in the legend to Fig. 3, and responses were assessed on the day of challenge. Spleen cells were stimulated in vitro with killed B. pertussis, inactivated PT, FHA, or PRN, and supernatants were assessed for IL-5 and IFN-γ by immunoassay. Results are mean responses for four mice assessed individually in triplicate.
Apart from the B-cell-deficient mice, which failed to mount an antibody response, immunization with Pa generated high antibody titers against PT and PRN and modest responses against FHA, whereas Pw induced high antibody titers against PRN and modest levels against PT and FHA (Table 2 and data not shown). Although the IgG antibody response to individual antigens were lower following infection, significant levels of antibodies against whole bacteria were detected (not shown). We failed to find a significant correlation between IgG titers against individual antigens and protection against B. pertussis challenge.
TABLE 2.
Anti-PT IgG levels in serum and lungs at time of challenge
| Immunizationa | Antibody titerb (mean ± SE)
|
|
|---|---|---|
| Serum | Lungs | |
| Pw active | 1,600 ± 20 | NTc |
| Pw passive | 1,587 ± 50 | 40 ± 20 |
| Pa active | 12,800 ± 410 | NT |
| Pa passive | 10,413 ± 1,400 | 320 ± 50 |
Mice were immunized as described in the legend to Fig. 6, and serum and lung lavage samples were recovered from groups of four mice 2 weeks after active immunization or 4 h after passive immunization.
Mean reciprocal endpoint titer (based on 1.0 ml of lavage fluid for lungs) for four mice in each experimental group.
NT, not tested.
Analysis of the subclass of the anti-B. pertussis IgG response suggested that protection was associated with IgG2a antibody responses. The responses were highly polarized to IgG2a in infected wild-type mice and to a lesser extent in mice immunized with Pw (Fig. 5). In contrast, IgG1 was the dominant subclass in mice immunized with the Pa. Deletion of functional IFN-γR−/− resulted in an increase in the ratio of IgG2a to IgG1, whereas deletion of IL-4 had the opposite effect (Fig. 5).
FIG. 5.
Anti-PT IgG subclasses in immune wild-type and gene-disrupted mice. Mice were immunized or infected as described in the legends to Fig. 3 and 4, and antibody responses were tested by enzyme-linked immunosorbent assay on the day of challenge. Results are reciprocal endpoint titers.
Passive transfer of antibodies confers protection against B. pertussis.
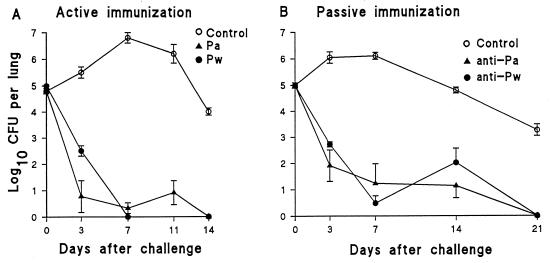
Since we had demonstrated defective T-cell responses in Ig−/− mice and since these mice do not make mature B cells, we could not conclude that their failure to confer resistance against B. pertussis challenge following immunization was solely due to defective antibody production. We had previously demonstrated that antibody from convalescent mice had only a marginal effect on the rate of bacterial clearance when passively transferred prior to challenge (22). Here we show that antibody may play a more significant role in protection induced by immunization, especially with Pa. Passive transfer of serum from BALB/c mice immunized with Pw or Pa resulted in a 3- to 5-log reduction in the bacterial load early after challenge (Fig. 6). However, compared with active immunization with Pw, where bacteria are undetectable on day 7, passive immunization with anti-Pw serum was associated with a rebound in the counts on day 14 and a delay in complete bacterial elimination until day 21 (Fig. 6). In contrast, active and passive immunization with Pa resulted in similar kinetics of bacterial clearance after challenge. Transfer of serum (0.1 ml) from animals that had received three immunizations with the Pw or Pa resulted in serum antibody titers against PT, FHA, and PRN at the time of challenge similar to those observed in mice that received two active immunizations with the same vaccines (Table 2 and data not shown). Analysis of bronchoalveolar lavage samples from recipient mice at the time of challenge demonstrated that significant levels of B. pertussis-specific IgG had transudated into the lung (Table 2).
FIG. 6.
Clearance of B. pertussis following active or passive immunization with Pw or Pa. (A) Active immunization. BALB/c mice were immunized twice (0 and 4 weeks) with W Pw or Pa (rPT, FHA, and PRN). (B) Passive immunization. Serum was prepared from BALB/c mice immunized three times (0, 3, and 6 weeks) with Pa or Pw, and recipient mice were injected with 0.1 ml 4 h before respiratory challenge. Results are mean (±standard error) CFU per lung for four mice per time point.
Multiple antigen protective mechanism.
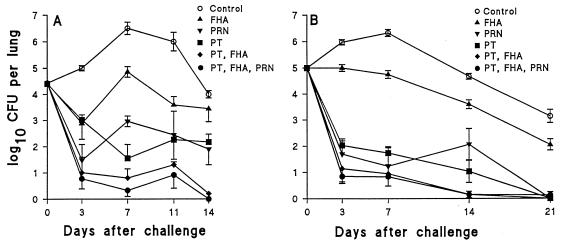
Since the exact contribution of distinct B. pertussis antigens to protection has not been established, we used the murine model to examine the protective effect of active or passive immunization with individual antigens and antigen combinations found in a number of Pa. Two active immunizations (two doses at 4-week intervals) with rPT resulted in an almost 5-log drop in the bacterial counts 7 days after challenge (Fig. 7). Immunization with PRN also had a significant protective effect, whereas FHA alone was the least protective of the three antigens tested. However, the combination of FHA and PT was more protective than PT alone, and the addition of PRN resulted in a further modest enhancement of the rate of bacterial clearance in immunized mice.
FIG. 7.
Multiple protective antigens of B. pertussis. (A) Active immunization. Mice were immunized twice (0 and 4 weeks) with 5 μg of either FHA, PRN, rPT, rPT and FHA, or rPT, FHA, and PRN adsorbed to alum or with alum only (control) and were challenged 2 weeks later. (B) Passive immunization. Sera were recovered from mice 2 weeks after three immunizations (0, 3, and 6 weeks) with rPT, FHA, or PRN only and 0.1 ml of each antiserum, in the combinations described for active immunization, injected (intravenously) into naive BALB/c mice, which were challenged 4 h later. Results are mean (±standard error) CFU per lung for four mice per time point.
Passive transfer of serum from mice immunized with PT alone resulted in an approximately 4-log reduction of the CFU counts in the lungs compared with serum from naive mice (Fig. 7). Anti-PRN antibodies also conferred a high level of protection, but anti-FHA antibodies alone only had only a modest effect on the rate of bacterial clearance. However, the addition of anti-FHA antibodies enhanced the protective effect of anti-PT, whereas the addition of anti-PRN serum to this combination had no further protective effect.
DISCUSSION
The murine intracerebral challenge test (14) has been used to assess the potency of Pw, but until now there has been no animal model in which protection correlates with Pa efficacy in children. In this study, we provide evidence that a murine model of respiratory challenge with live B. pertussis may fulfill this role. Although certain of the features of the human disease, notably the persistent coughing and neurological manifestations, are not evident in the murine model, the course of respiratory infection has many of the characteristics of that seen in infants (7, 26, 33). Furthermore, we have preliminary evidence that the systemic effects of B. pertussis infection in mice include elevated levels of proinflammatory cytokines in the brain (17). The highly significant correlation between bacterial clearance in immunized mice and vaccine efficacy in children suggests that the murine respiratory challenge model will have considerable applications in the regulatory control and future development of pertussis vaccines. Our data for the mouse model are consistent with the failure to identify a single immunological correlate of protection in clinical trials and suggest that immunogenicity studies against individual B. pertussis antigens in mice are unlikely to predict vaccine efficacy in children. In contrast, assessment of the lung bacterial clearance following aerosol challenge of immunized mice, relative to naive mice or to mice immunized with a standard vaccine preparation, may provide a reproducible and more definitive method of evaluating pertussis vaccines prior to licensure.
The murine respiratory challenge model has also provided an experimental basis for an elucidation of the mechanisms of natural and vaccine-induced immunity against B. pertussis. We have previously demonstrated that B. pertussis-infected IFN-γR−/− mice develop atypical disseminating disease, whereas both Ig−/− and athymic mice develop a chronic infection following challenge with B. pertussis (19, 22). In the present study, we observed a delayed clearance of bacteria from the lungs of IFN-γR−/− mice primed by previous infection or immunization with Pw and enhanced clearance following rechallenge of IL-4−/− mice. These observations may be explained on the basis of defective and enhanced Th1 responses through removal of positive and negative regulators of Th1 cells, respectively. The rebound effect observed on day 10 postchallenge in IFN-γR−/− mice previously infected or immunized with Pw, and to a lesser extent in conventional mice immunized with Pa, may reflect lack of effective cell-mediated immunity against intracellular B. pertussis. The early decline in bacterial counts may be mediated through the effector function of antibody, whereas complete clearance may be dependent on IFN-γ-mediated activation of macrophages and neutrophils (38). In conventional mice, priming by infection or immunization with Pw induced the most polarized Th1 response and conferred the highest level of protection. This finding is consistent with the observation in children of selective induction of Th1 cells following infection (30) or immunization with Pw (32) and with our previous studies in mice that Th1 cells can clear the infection after adoptive transfer into irradiated recipients (22). The outcome of challenge of IFN-γR−/− and IL-4−/− mice immunized with Pa and Pw also suggests a degree of redundancy in the cytokines involved in vaccine-induced immunity against B. pertussis. Although there was some evidence of broadening of the Th1 and Th2 responses and the profile of IgG subclasses induced with Pa in IFN-γR−/− and IL-4−/− mice and with Pw in IL-4−/− mice, the rate of bacterial clearance was not significantly different in knockout compared with wild-type strains. It appears that either Th1 or Th2 cytokines may provide helper function for antibody production but that Th1 cytokines may play an additional role, perhaps mediating direct cellular immunity against intracellular B. pertussis (4, 19, 35, 38) in the lungs.
The demonstration that protection against B. pertussis can be conferred by passive transfer of anti-B. pertussis serum and the failure to prime protective immune responses in Ig−/− mice are in agreement with previous suggestions that antibodies can make a major contribution to vaccine-induced protection against B. pertussis (7, 23, 29, 34, 36, 40). Although we have previously reported that passive transfer of convalescent serum has little protective effect against respiratory infection in mice, the present study demonstrated that targeted disruption of the Ig μ-chain gene did compromise the capacity of the immune system to clear the infection, suggesting that antibody may also be involved in natural immunity. However, as these mice do not have mature B cells, the defect may not be confined to antibody production. Indeed, we observed weak or undetectable antigen-specific T-cell responses in Ig−/− mice primed by infection or immunization with Pw or Pa. This finding is consistent with some, but not all, studies that have reported defective T-cell priming in Ig−/− mice and suggests a role for B cells in antigen presentation (9, 19, 24). Furthermore, the observation that active immunization of mice with Pw leads to complete bacterial clearance as early as 5 days after challenge, whereas immunization with Pa or passive transfer of anti-Pw serum was associated with a delay in bacterial elimination and occasional rebound in bacterial load, suggests that direct cell-mediated immunity is also required for optimum protection conferred by immunization. It is also noteworthy that in our studies animals were challenged at the peak of the antibody response, and it has been reported that antibody levels decline rapidly after immunization in children, especially with Pa (5). In contrast, studies from clinical trials and from the mouse model have demonstrated that T-cell responses are maintained for more prolonged periods after immunization (3, 5), emphasizing the requirement for both arms of the adaptive immune response in vaccine induced protection.
The contribution of different B. pertussis antigens to protection has been extensively debated (7, 23, 29, 34, 36). The results of active and passive immunization experiments in our study demonstrate that either PT or PRN can confer relatively high levels of protection. Although FHA alone was comparatively less protective, it did augment the protective efficacy of PT. In addition, we have preliminary evidence that fimbriae augment the protective efficacy of PT and that PT-FHA-fimbria types 2 and 3 and PT-FHA-fimbria types 2 and 3-PRN are highly protective combinations in the murine respiratory challenge model (unpublished observations). These findings are consistent with the results of clinical trials that have demonstrated that five-, three-, and most two-component vaccines have higher estimated efficacious than a monocomponent chemically detoxified PT (PTd) vaccine, albeit in different clinical trials, with different antigen doses and inactivation procedures, and that vaccine efficacy may increase with increasing number of antigens in the formulation (1, 6, 11, 12, 37, 39). The poorer protection induced by SB Pa2 compared with SB Pa3 in clinical trials and in our mouse model suggests that PRN does make a significant contribution to the protection afforded by these vaccines. However, the results with other two-component vaccines (6, 37) suggest that, in addition to the combination of components, the formulation of the antigens may affect the potency of Pa. We cannot exclude the contribution of other antigens, including adenylate cyclase or heat shock proteins, to protection. Indeed, the superior efficacy of Pw may reflect a contribution of additional antigens or bacterial components that contribute to adjuvanticity, such as lipopolysaccharide, which is known to contribute to pertussis vaccine efficacy by enhancing Th1 responses through IL-12 production (18).
Our findings also provide an explanation for the failure to find an immunological correlate of protection induced by pertussis vaccines in children. Clinical trials have until recently focused exclusively on humoral immunity and have shown that monospecific antibody responses against either FHA, PT, PRN, or fimbriae do not correlate with protection (10–12, 21, 37). However, the results of our study demonstrate that B. pertussis has multiple protective antigens and antibody and T-cell responses against several antigens may have an additive effect for optimum protection. Antibody appears to be important in limiting infection and disease by preventing initial bacterial adherence to ciliated epithelial cells in the respiratory tract (40), through the neutralization of bacterial toxins, and may also be required for the removal of bacteria through opsonization. Our data suggested that early bacterial clearance was associated with high levels of complement-fixing IgG2a antibodies, which were influenced by the levels of functional IFN-γ induced by immunization. Although the induction of T cells with mixed Th1-Th2 cytokine profiles appear to mediate the same level of protection as Th1 cells, a polarized Th2 response was associated with a delay in complete bacterial clearance from the lungs. It is now accepted that B. pertussis can occupy an intracellular niche in the lungs, and we and others have demonstrated that IFN-γ is required to contain infection to the respiratory mucosae by abolishing a reservoir of B. pertussis within macrophages (4, 19, 35, 38). Therefore, the mechanisms that prevent replication and dissemination and eventually succeed in eliminating B. pertussis from the body appear to reflect the dual extra- and intracellular location of the bacteria in the host and require the distinct but coordinated functions of the cellular and humoral arms of the immune response for optimal protection.
ACKNOWLEDGMENTS
This work was supported by grant 039583 from The Wellcome Trust. Mark Ryan is supported by a grant from The Health Research Board of Ireland.
We are grateful to Fiona Griffin and Geraldine Murphy for technical assistance and to SmithKline Beecham, Chiron Biocine, and Pasteur Mérieux Connaught for providing vaccines.
REFERENCES
- 1.Ad Hoc Group for the Study of Pertussis Vaccines. A randomized controlled trial of a two-component, a three component and a five-component acellular pertussis vaccine and a British whole-cell pertussis vaccine. Lancet. 1997;350:1569–1577. doi: 10.1016/s0140-6736(97)06508-2. [DOI] [PubMed] [Google Scholar]
- 2.Ausiello C M, Urbani F, La Sala A, Lande R, Cassone A. Vaccine- and antigen-dependent type 1 and type 2 cytokine induction after primary vaccination of infants with whole-cell or acellular pertussis vaccines. Infect Immun. 1997;65:2168–2174. doi: 10.1128/iai.65.6.2168-2174.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnard A, Mahon B P, Watkins J, Redhead K, Mills K H G. Th1/Th2 cell dichotomy in acquired immunity to Bordetella pertussis: variables in the in vivo priming and in vitro cytokine detection techniques affect the classification of T cell subsets as Th1, Th2 or Th0. Immunology. 1996;87:372–380. doi: 10.1046/j.1365-2567.1996.497560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bromberg K, Tannis G, Steiner P. Detection of Bordetella pertussis associated with the alveolar macrophages of children with human immunodeficiency virus infection. Infect Immun. 1991;59:4715–4719. doi: 10.1128/iai.59.12.4715-4719.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cassone A, Ausiello C M, Urbani F, Lande R, Giuliano S for the Progetto Pertosse-CMI Working Group. Cell-mediated and antibody responses to Bordetella pertussis antigens in children vaccinated with acellular or whole-cell pertussis vaccines. Arch Pediatr Adolesc Med. 1997;151:283–289. doi: 10.1001/archpedi.1997.02170400069013. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Pertussis vaccination: use of acellular pertussis vaccines among infants and young children—recommendations of the advisory committee on immunization practices (ACIP) Morbid Mortal Weekly Rep. 1997;46:(RR)1–(RR)25. [PubMed] [Google Scholar]
- 7.Cherry, J. D., P. A. Brunnell, G. S. Golden, and D. T. Karzon. 1988. Report of the task force on pertussis and pertussis immunization—1988. Pediatrics 81(Suppl.):939–984.
- 8.Church M A. Evidence of whooping-cough-vaccine efficacy from 1978 whooping-cough epidemic in Hertfordshire. Lancet. 1978;ii:188–190. doi: 10.1016/s0140-6736(79)91447-8. [DOI] [PubMed] [Google Scholar]
- 9.Constant S, Schweitzer N, West J, Ranney P, Bottomly K. B lymphocytes can be competent antigen presenting cells for priming CD4+ T cells in vivo. J Immunol. 1995;155:3734–3741. [PubMed] [Google Scholar]
- 10.Edwards K M, Meade B D, Decker M D, Reed G F, Rennels M B, Steinhoff M C, Anderson E L, Enguland J A, Pichichero M E, Deloria M A. Comparison of thirteen acellular pertussis vaccines: overview and serological response. Pediatrics. 1995;96:548–557. [PubMed] [Google Scholar]
- 11.Greco D, Salmaso S, Mastrantonio P, Guiliano M, Tozzi A G, Anemona A, Giori Delgi Atti M L, Giammanco A, Panel P, Blackwelder W C, Klein D L, Wassilak S G F the Progetto Pertosase Working Group. A controlled trial of two acellular vaccines and one whole-cell vaccine against pertussis. N Engl J Med. 1996;334:341–348. doi: 10.1056/NEJM199602083340601. [DOI] [PubMed] [Google Scholar]
- 12.Gustafsson L, Hallander H O, Olin P, Reizenstein E, Storsaeter J. A controlled trial of a two-component acellular, a five component acellular, and a whole cell pertussis vaccine. N Engl J Med. 1996;334:349–355. doi: 10.1056/NEJM199602083340602. [DOI] [PubMed] [Google Scholar]
- 13.Huang S, Hendriks W, Althage A, Hemmi S, Bleuthmann H, Kamijo R, Vilcek J, Zinkernagel R M, Aguet M. Immune response in mice that lack the IFN-γ receptor. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 14.Kendrick P L. Mouse protection tests in the study of pertussis vaccine. Am J Public Health. 1947;37:803–810. [PubMed] [Google Scholar]
- 15.Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin μ chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 16.Kuhn R, Rajewsky K, Muller W. Generation and analysis of interleukin-4 deficient mice. Science. 1991;254:707–710. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- 17.Loscher, C. E., S. Donnelly, M. A. Lynch, and K. H. G. Mills. 1997. Pro-inflammatory cytokines and fever: involvement in the neurological sequelae of Bordetella pertussis infection and immunization. Immunology 92(Suppl. 1):66.
- 18.Mahon B P, Ryan M, Griffin F, Mills K H G. Interleukin-12 is produced by macrophages in response to live or killed Bordetella pertussis and enhances the efficacy of an acellular pertussis vaccine by promoting induction of Th1 cells. Infect Immun. 1996;64:5295–5301. doi: 10.1128/iai.64.12.5295-5301.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahon B P, Griffin F, Sheahan B, Mills K H G. Atypical disease following Bordetella pertussis respiratory infection of mice with targeted disruptions in IFN-γ receptor or immunoglobulin μ chain genes. J Exp Med. 1997;186:1843–1851. doi: 10.1084/jem.186.11.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Medical Research Council, Whooping Cough Immunisation Committee. Vaccination against whooping-cough: relation between protection in children and results of laboratory tests. Br Med J. 1956;2:454–462. [PMC free article] [PubMed] [Google Scholar]
- 21.Miller E, Ashworth L A E, Redhead K, Thornton C, Waight P A, Coleman T. Effect of schedule on reactogenicity and antibody persistence of acellular and whole-cell pertussis vaccines: value of laboratory tests as predictors of clinical performance. Vaccine. 1997;15:51–60. doi: 10.1016/s0264-410x(96)00112-0. [DOI] [PubMed] [Google Scholar]
- 22.Mills K H G, Barnard A, Watkins J, Redhead K. Cell-mediated immunity to Bordetella pertussis: role of Th1 cells in bacterial clearance in a murine respiratory infection model. Infect Immun. 1993;61:399–410. doi: 10.1128/iai.61.2.399-410.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oda M, Cowell J L, Burstyn D G, Thaib S, Manclark C R. Antibodies to Bordetella pertussis in human colostrum and their protective activity against aerosol infection of mice. Infect Immun. 1985;47:441–445. doi: 10.1128/iai.47.2.441-445.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phillips J A, Romball C G, Hobbs M V, Ernst D N, Schultz L, Weigle W O. CD4+ T cell activation and tolerance induction in B cell knockout mice. J Exp Med. 1996;183:1339–1344. doi: 10.1084/jem.183.4.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.PHLS Epidemiological Research Laboratory and 21 area health authorities. Efficacy of pertussis vaccination in England. Br Med J. 1982;285:357–359. doi: 10.1136/bmj.285.6338.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pittman M, Furman B L, Wardlaw A C. Bordetella pertussis respiratory tract infection in the mouse: pathophysiological responses. J Infect Dis. 1980;142:56–66. doi: 10.1093/infdis/142.1.56. [DOI] [PubMed] [Google Scholar]
- 27.Pizza M G, Covacci A, Bartoloni A, Pergini M, Nencioni L, De Magistris M T, Villa L, Nucci D, Manetti R, Bugnoli M, Giovannoni F, Oliveri R, Barbieri J, Sato H, Rappuoli R. Mutants of pertussis toxin suitable for vaccine development. Science. 1989;246:497–500. doi: 10.1126/science.2683073. [DOI] [PubMed] [Google Scholar]
- 28.Redhead K, Barnard A, Watkins J, Mills K H G. Effective immunisation against Bordetella pertussis respiratory infection in mice is dependent on the induction of cell mediated immunity. Infect Immun. 1993;61:3190–3198. doi: 10.1128/iai.61.8.3190-3198.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robinson A, Irons L I, Ashworth L A E. Pertussis vaccine: present status and future prospects. Vaccine. 1985;3:11–22. doi: 10.1016/0264-410x(85)90004-0. [DOI] [PubMed] [Google Scholar]
- 30.Ryan M, Murphy G, Gothefors L, Nilsson L, Storsaeter J, Mills K H G. Bordetella pertussis respiratory infection in children is associated with preferential activation of type 1 Th cells. J Infect Dis. 1997;175:1246–1250. doi: 10.1086/593682. [DOI] [PubMed] [Google Scholar]
- 31.Ryan M, Gothefors L, Storsaeter J, Mills K H G. B. pertussis-specific Th1/Th2 cells generated following respiratory infection or immunization with an acellular vaccine: comparison of the T cell cytokine profiles in infants and mice. Dev Biol Stand. 1997;89:251–259. [PubMed] [Google Scholar]
- 32.Ryan M, Murphy G, Nilsson L, Shackley F, Gothefors L, Ømar K, Miller E, Storsaeter J, Mills K H G. Distinct Th cell subtypes induced with whole cell and acellular pertussis vaccines in children. Immunology. 1998;93:1–10. doi: 10.1046/j.1365-2567.1998.00401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sato Y, Izumiya K, Sato H, Cowell J L, Manclark C R. Aerosol infection of mice with Bordetella pertussis. Infect Immun. 1980;29:261–266. doi: 10.1128/iai.29.1.261-266.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sato H, Sato Y. Bordetella pertussis infection in mice: correlation of specific antibodies against two antigens, pertussis toxin, and filamentous hemagglutinin with mouse protectivity in an intracerebral or aerosol challenge system. Infect Immun. 1984;46:415–421. doi: 10.1128/iai.46.2.415-421.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saukkonen K, Cabellos C, Burroughs M, Prasad S, Tuomanen E. Integrin-mediated localization of Bordetella pertussis within macrophages: role in pulmonary colonization. J Exp Med. 1991;173:1143–1149. doi: 10.1084/jem.173.5.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shahin R D, Brennan M J, Li Z M, Meade B D, Manclark C R. Characterization of the protective capacity and immunogenicity of the 69-kD outer membrane protein of Bordetella pertussis. J Exp Med. 1990;171:63–73. doi: 10.1084/jem.171.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simondon F, Preziosi M-P, Yam A, Kane C T, Chabirand L, Iteman I, Sanden G, Mboup S, Hoffenbach A, Knudsen K, Guiso N, Wassilak S, Cadoz M. A randomized double blind trial comparing a two-component acellular to a whole-cell pertussis vaccine in Senegal. Vaccine. 1997;15:1606–1612. doi: 10.1016/s0264-410x(97)00100-x. [DOI] [PubMed] [Google Scholar]
- 38.Torre D, Ferrario G, Bonetta G, Perversi L, Tambini R, Speranza F. Effects of recombinant human gamma interferon on intracellular survival of Bordetella pertussis in human phagocytic cells. FEMS Immunol Med Microbiol. 1994;9:183–188. doi: 10.1111/j.1574-695X.1994.tb00492.x. [DOI] [PubMed] [Google Scholar]
- 39.Trollofors B, Taranger J, Lagergard T, Lind L, Sundh V, Zackrisson G, Lowe C U, Blackwelder W, Robbins J B. A placebo-controlled trial of a pertussis-toxoid vaccine. N Engl J Med. 1995;333:1045–1050. doi: 10.1056/NEJM199510193331604. [DOI] [PubMed] [Google Scholar]
- 40.Tuomanen E I, Zapiain L A, Galvan P, Hewlett E L. Characterization of antibody inhibiting adherence of Bordetella pertussis to human respiratory epithelial cells. J Clin Microbiol. 1984;20:167–170. doi: 10.1128/jcm.20.2.167-170.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]