Abstract
Objectives:
Calcific aortic stenosis (AS) is the most common valvular disease in older adults, yet its risk factors remain insufficiently studied in this population. Such studies are necessary to enhance understanding of mechanisms, disease management, and therapeutics.
Methods:
The Cardiovascular Health Study is a population-based investigation of older adults that completed adjudication of incident AS over long-term follow-up. We evaluated traditional cardiovascular risk factors or disease, as well as novel risk factors from lipid, inflammatory and mineral-metabolism pathways, in relation to incident moderate or severe AS (including AS procedures) and clinically significant AS (severe AS, including procedures).
Results:
Of 5,390 participants (age 72.9±5.6 years, 57.6% female, 12.5% Black), 287 developed moderate or severe AS, and 175 clinically significant AS, during median follow-up of 13.1 years. After full adjustment, age (HR=1.66 per SD [95% CI=1.45,1.91]), male sex (HR=1.41 [1.06,1.87]), diabetes (HR=1.53 [1.10,2.13]), coronary heart disease (CHD, HR=1.36, [1.01,1.84]), lipoprotein-associated phospholipase-A2 (LpPLA2) activity (HR=1.21 [1.07,1.37]) and sCD14 (HR=1.16 [1.01,1.34]) were associated with incident moderate/severe AS, while Black race demonstrated an inverse association (HR=0.40 [0.24, 0.65]), and creatinine-based estimated glomerular filtration rate (eGFRcr) showed a U-shaped relationship. Findings were similar for clinically significant AS, although CHD and sCD14 fell short of significance, but interleukin-(IL) 6 showed a positive association.
Conclusion:
This comprehensive evaluation of risk factors for long-term incidence of AS identified associations for diabetes and prevalent CHD, LpPLA2 activity, sCD14 and IL-6, and eGFRcr. These factors may hold clues to biology, preventive efforts, and potential therapeutics for those at highest risk.
Introduction
Calcific aortic stenosis (AS) is the most common valvular condition requiring hospitalization.1 Calcific aortic valve disease (CAVD) chiefly affects older adults, whose cumulative frequency of moderate or severe AS reaches 6.0–7.0%.2 Once symptomatic, untreated AS has a high mortality.1 The only effective treatment is aortic valve replacement, which carries substantial risks and costs.1
Development of preventive medical therapies for AS rests on characterizing its determinants and mechanisms. Clinical and population studies have identified various risk factors for CAVD, including hypertension, dysglycemia, obesity, and smoking, as well as circulating LDL cholesterol (LDLc), lipoprotein (Lp)(a), creatinine, and high-sensitivity C-reactive protein (hsCRP).3–8 Among these, blood pressure, body mass index (BMI), LDLc and Lp(a) have a potentially causal role.9–12 Clinical trials of LDL-lowering therapies have been null,3 however. Furthermore, based on documented links between mineral metabolism and CAVD, randomized trials have tested osteoporosis therapies13 for aortic valve calcification (AVC), but results have been disappointing.
Available epidemiological evidence of CAVD determinants has partly come from imaging studies of AVC, which have often been cross-sectional.3 Large-scale longitudinal studies have examined incident AS, but these have relied on administrative data for outcomes and, in some cases, risk factors, which can lead to misclassification.3,7,8,11 Epidemiologic investigations have identified risk factors mostly in middle-aged cohorts, but determinants of AS incidence in elders remain insufficiently studied.
We recently adjudicated clinically meaningful AS over long-term follow-up in the Cardiovascular Health Study (CHS).2 Here, we undertook a broad-based investigation of traditional and novel risk factors for incident AS in this older cohort in order to define their relevance late in life and inform strategies for potential intervention.
Methods
Population
CHS is a prospective cohort study of community-dwelling adults ≥65. Eligible participants were sampled from Medicare-eligibility lists at four U.S. sites.14 An initial cohort of 5,201 participants was enrolled in 1989–90, followed by an additional cohort of 687 Black participants in 1992–93. Follow-up involved semi-annual contacts through in-person examinations and/or telephone interviews.14 All research centers received institutional review board approval. All participants gave informed consent.
Traditional Risk Factors
Traditional determinants included CVD or its risk factors. For the first cohort, these variables were taken from the 1989–90 visit, at which echocardiography was performed. For the second cohort, which did not undergo echocardiography until 1994–95, risk factors from the latter exam were used or, when missing, from previous years. History, physical, and laboratory assessments were standardized.14 Hypertension was defined by blood pressure ≥140 mmHg (systolic), ≥90 mmHg (diastolic) or antihypertensive therapy; diabetes by glucose ≥126 mg/dL (fasting), ≥200 mg/dL (non-fasting) or hypoglycemic treatment. Estimated glomerular filtration rate was calculated using creatinine (eGFRcr) or cystatin C (eGFRcys) equations.15 Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as fasting glucose (mmol/L)×fasting insulin (mU/L)/22.5. Coronary heart disease (CHD), stroke or transient ischemic attack (TIA), heart failure (HF), and peripheral arterial disease (PAD) were ascertained using reported methods.14
Novel Risk Factors
Novel determinants comprised biochemical markers from lipid, inflammatory, and mineral pathways previously measured in CHS for evaluation of other outcomes. Fasting blood was stored at −70–80°C at a core laboratory. All biomarkers were measured from 1989–90 specimens for the first cohort and 1992–93 specimens for the second cohort, with a few exceptions. Lp(a) was only available for the first cohort, while fetuin-A and klotho were measured in 1992–93 specimens for both cohorts. Lp(a) and lipoprotein-associated phospholipase-A2 (LpPLA2) mass and activity were determined as previously reported.16 HsCRP, interleukin (IL)-6, soluble cluster of differentiation (sCD)14, sCD163, fetuin-A and klotho were measured by ELISA, as described elsewhere.16–19
Echocardiography
Standardized echocardiograms were performed in 1989–90 (first cohort) and 1994–95 (first and second cohorts), with interpretations completed at core reading centers. Methods for AS grading in CHS echocardiograms are detailed in the Supplemental Methods.
Incident AS
Procedures for adjudication of incident AS are described in the Supplemental Methods.2 The primary outcome for the current analysis was probable/definite moderate or severe AS. The secondary outcome was probable/definite “clinically significant AS,” defined as severe AS or procedural intervention for AS. Secondary analyses were also performed for possible/probable/definite moderate or severe AS, and clinically significant AS.
Statistical Analysis
Cox models were used to evaluate the relationship of risk factors with AS outcomes. Because the focus was on association rather than prediction, we applied cause-specific survival methods appropriate for the high competing risk of death in CHS.2 Testing of the proportionality assumption with Schoenfeld residuals revealed no violations. Model 1 included age, sex and race. Model 2 (main model) additionally included education, BMI, smoking, hypertension, diabetes, LDLc, HDLc, triglycerides, prevalent CHD, stroke/TIA, HF, PAD and eGFRcr. Secondary analyses examined waist circumference instead of BMI, and HOMA-IR in those without diabetes. Among novel risk factors, we evaluated individual markers in Models 1 and 2 above. In Model 3, we assessed the impact of concurrent adjustment for each novel biomarker class in the main model. We evaluated the functional form of associations using penalized cubic splines. These were approximately linear for all risk factors except eGFRcr, which showed a U-shaped association that was modeled with linear and quadratic terms.
In exploratory analyses, we evaluated eGFRcys; additionally adjusted for statins, oral glucocorticoids, and warfarin; censored follow-up at 10 years; and assessed interactions with age, sex, and race.
All analyses used STATA 12. Two-tailed p<0.05 defined statistical significance for main analyses, and p<0.01 for interaction analyses.
Participant and Public Involvement
Participants were not involved in the design of CHS.
Results
Descriptive Summary
Selection of the two study samples is described in Figure 1, and their characteristics given in Table 1. For the 1989–90/1994–95 baseline, 287 participants experienced incident probable/definite moderate or severe AS (345 participants possible/probable/definite moderate or severe AS) during median follow-up of 13.1 (maximum 25.0) years. There were 175 participants with incident probable/definite clinically significant AS (including possible, 234) during this period. For the 1992–93/1994–95 baseline, the corresponding numbers of probable/definite moderate or severe AS and clinically significant AS were 235 (including possible, 282) and 146 (including possible, 193), respectively, over median follow-up of 11.6 (maximum 22.1) years.
Figure 1.
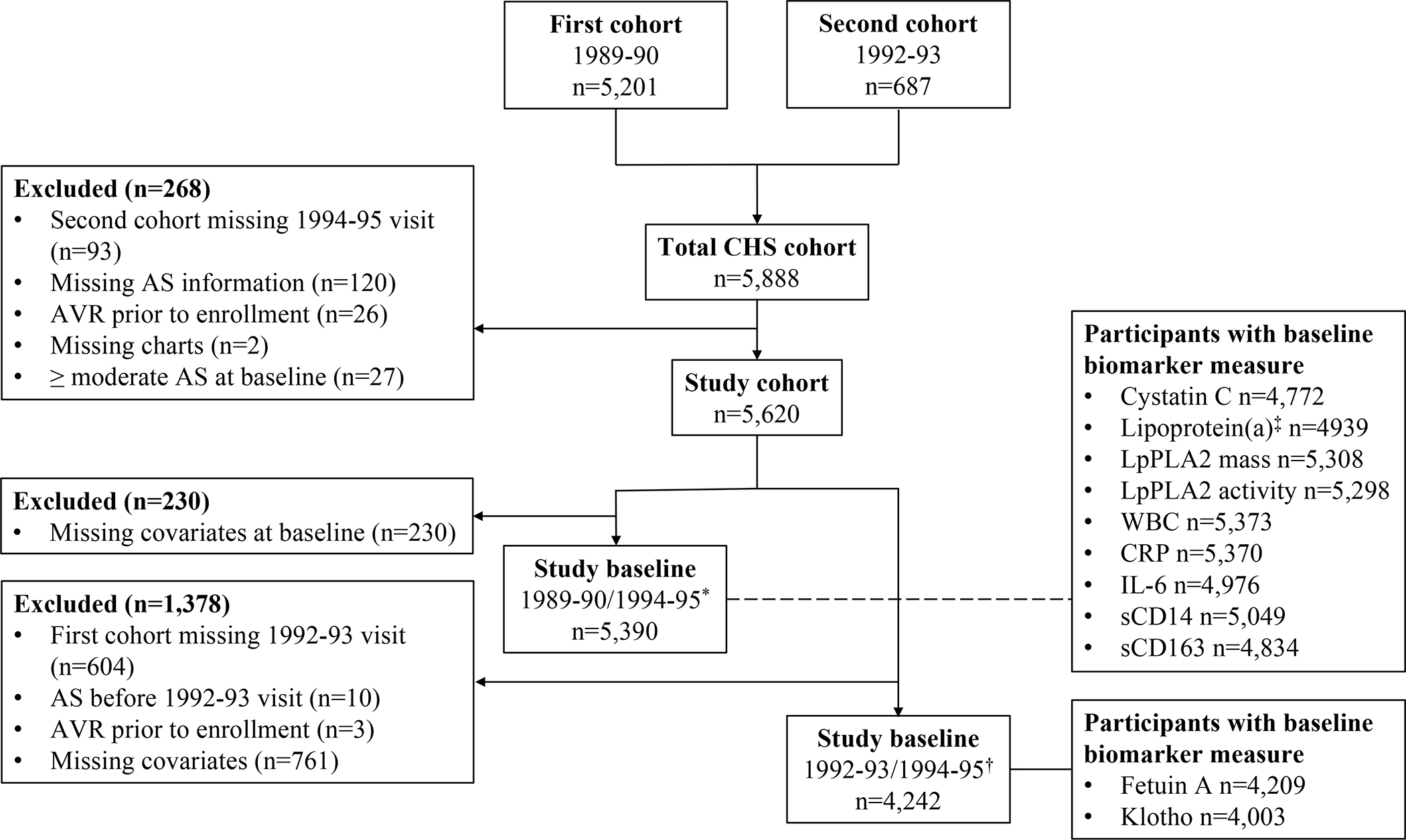
Flow chart of the study.
* 1989–90 for the first cohort, 1994–95 for the second cohort.
† 1992–93 for the first cohort, 1994–95 for the second cohort.
‡ For the first cohort only.
Table 1.
Baseline Characteristics
| Variable | 1989–90/1994–95 Baseline (n=5390) |
1992–93/1994–95 Baseline (n=4242) |
|---|---|---|
| Age, years | 72.9 (5.6) | 75.1 (5.2) |
| Male sex, n (%) | 2288 (42.4) | 1784 (42.1) |
| Black race, n (%) | 675 (12.5) | 610 (14.4) |
| BMI, kg/m2 | 26.6 (4.6) | 26.7 (4.6) |
| Waist circumference, cm | 94.17 (12.98) | N/A |
| Education, < high school, n (%) | 1531 (28.4) | 1080 (25.5) |
| HOMA-IR* | 3.43 (2.37) | N/A |
| Current smoker, n (%) | 631 (11.7) | 406 (9.6) |
| Ever smoker, n (%) | 2916 (54.1) | 2332 (55.0) |
| LDLc, mg/dL | 130 (36) | 127 (34) |
| HDLc, mg/dL | 54 (16) | 53 (14) |
| Triglycerides, mg/dL | 135 (60) | 138 (65) |
| eGFRcr, ml/min/1.73 m2 | 72 (18) | 71 (17) |
| eGFRcys, ml/min/1.73 m2 | 72 (19) | 66 (18) |
| Hypertension, n (%) | 3123 (57.9) | 2370 (55.9) |
| Diabetes, n (%) | 824 (15.3) | 704 (16.6) |
| Coronary heart disease, n (%) | 1037 (19.2) | 900 (21.2) |
| Stroke/TIA, n (%) | 307 (5.7) | 304 (7.2) |
| Heart failure, n (%) | 236 (4.4) | 236 (5.6) |
| Peripheral arterial disease, n (%) | 138 (2.6) | 125 (2.9) |
| Statin use, n (%) | 116 (2.2) | 199 (4.7) |
| Oral steroid use, n (%) | 111 (2.1) | 84 (2.0) |
| Warfarin use n (%) | 72 (1.3) | 95 (2.2) |
| Lp(a), μg/mL | 54.3 (56.0) | NA |
| LpPLA2 Mass, ng/mL | 343.6 (117.3) | NA |
| LpPLA2 activity, nmol/min/mL | 39.6 (13.0) | NA |
| Fetuin-A, g/L | NA | 0.5 (0.1) |
| Klotho, pg/mL | NA | 900.0 (529.3) |
| CRP, g/L | 4.7 (8.1) | NA |
| IL-6, pg/mL | 2.2 (1.9) | NA |
| WBC, ×103/μL | 6.3 (2.1) | NA |
| sCD14, ng/mL | 1641 (356) | NA |
| sCD163, ng/mL | 788 (220) | NA |
Among participants free of diabetes.
Abbreviations: BMI = body mass index; CRP = C-reactive protein; eGFRcr = creatinine-based estimated glomerular filtration rate; eGFRcys = cystatin C-based estimated glomerular filtration rate; HDLc = high density lipoprotein cholesterol; HOMA-IR = homeostasis model assessment of insulin resistance; IL-6 = interleukin-6; LDLc = low density lipoprotein cholesterol; Lp(a) = lipoprotein(a); LpPLA2 = lipoprotein-associated phospholipase-A2; sCD14 = soluble CD14; sCD163 = soluble CD163; TIA = transient ischemic attack; WBC = white blood cell count.
Traditional Risk Factors and AS
After minimal adjustment, several traditional risk factors were associated with onset of probable/definite moderate or severe AS, including age, male sex, adiposity, hypertension, dysglycemia, triglycerides, CHD, and HF (all positively), as well as Black race, HDLc (both inversely) and eGFRcr (U-shaped) (Table 2). Upon full adjustment (Model 2), relationships were generally attenuated. A few factors remained significantly associated with increased risk of moderate or severe AS, namely, age, male sex, diabetes and CHD, while Black race remained significantly associated with decreased risk (Table 2). The U-shaped association of eGFRcr with moderate or severe AS was also statistically significant (p=0.01), with a risk nadir at ~65.2 ml/min/1.73 m2 (Figure 2). Using the nadir as reference, the HR for 30 ml/min/1.73 m2 was 1.73 (95% CI=1.00, 3.00); for 60 ml/min/1.73 m2, 1.01 (0.97, 1.06); and for 90 ml/min/1.73 m2, 1.31 (1.05, 1.64).
Table 2.
Associations of Traditional Risk Factors with Incidence of Probable/Definite Moderate or Severe Aortic Stenosis, as well as Probable/Definite Clinically Significant Aortic Stenosis
| Variable | Moderate/severe AS | Clinically significant AS | ||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |||||
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| Age (per SD) | 1.63 (1.44,1.85) | <0.01 | 1.66 (1.45,1.91) | <0.01 | 1.45 (1.23,1.72) | <0.01 | 1.48 (1.23,1.78) | <0.01 |
| Black race | 0.50 (0.31,0.82) | 0.01 | 0.40 (0.24,0.65) | <0.01 | 0.43 (0.22,0.84) | 0.01 | 0.31 (0.16,0.63) | <0.01 |
| Male sex | 1.52 (1.20,1.92) | <0.01 | 1.41 (1.06,1.87) | 0.02 | 1.63 (1.21,2.20) | <0.01 | 1.67 (1.17,2.39) | <0.01 |
| Education <HS | 1.24 (0.96,1.61) | 0.11 | 1.15 (0.89,1.50) | 0.29 | 1.23 (0.88,1.72) | 0.23 | 1.13 (0.80,1.58) | 0.49 |
| BMI (per SD) | 1.20 (1.06,1.35) | <0.01 | 1.10 (0.97,1.26) | 0.14 | 1.19 (1.02,1.39) | 0.03 | 1.09 (0.92,1.29) | 0.34 |
| Waist circumference (per SD)* | 1.19 (1.05,1.35) | 0.01 | 1.10 (0.96,1.25) | 0.18 | 1.16 (0.99,1.36) | 0.07 | 1.05 (0.88,1.25) | 0.60 |
| Hypertension | 1.31 (1.03,1.66) | 0.03 | 1.17 (0.92,1.50) | 0.20 | 1.47 (1.08,1.99) | 0.01 | 1.31 (0.95,1.79) | 0.10 |
| HOMA-IR (per SD)† | 1.11 (1.02,1.21) | 0.01 | 1.07 (0.94,1.22) | 0.28 | 1.10 (0.97,1.24) | 0.15 | 1.04 (0.86,1.27) | 0.67 |
| Diabetes | 1.82 (1.33,2.50) | <0.01 | 1.53 (1.10,2.13) | 0.01 | 2.11 (1.44,3.10) | <0.01 | 1.79 (1.19,2.69) | <0.01 |
| Current smoking | 0.75 (0.46,1.22) | 0.24 | 0.69 (0.41,1.15) | 0.15 | 1.10 (0.64,1.87) | 0.74 | 1.08 (0.61,1.91) | 0.79 |
| Ever smoking | 1.18 (0.93,1.50) | 0.18 | 1.26 (0.98,1.62) | 0.08 | 1.12 (0.82,1.52) | 0.49 | 1.10 (0.80,1.53) | 0.56 |
| LDLc (per SD) | 1.05 (0.93,1.18) | 0.40 | 1.06 (0.94,1.19) | 0.35 | 1.12 (0.96,1.30) | 0.15 | 1.13 (0.97,1.31) | 0.11 |
| HDLc (per SD) | 0.84 (0.73,0.96) | 0.01 | 0.93 (0.79,1.09) | 0.37 | 0.83 (0.70,1.00) | 0.04 | 0.98 (0.80,1.20) | 0.85 |
| Triglycerides (per SD) | 1.14 (1.02,1.27) | 0.02 | 1.03 (0.90,1.17) | 0.65 | 1.19 (1.03,1.36) | 0.01 | 1.08 (0.92,1.27) | 0.35 |
| Coronary heart disease | 1.55 (1.16,2.07) | <0.01 | 1.36 (1.01,1.84) | 0.04 | 1.62 (1.13,2.34) | 0.01 | 1.39 (0.95,2.04) | 0.09 |
| Stroke/TIA | 1.37 (0.78,2.40) | 0.27 | 1.14 (0.64,2.01) | 0.66 | 1.06 (0.47,2.40) | 0.89 | 0.83 (0.36,1.89) | 0.65 |
| Heart failure | 2.17 (1.21,3.90) | 0.01 | 1.54 (0.84,2.84) | 0.16 | 2.54 (1.24,5.21) | 0.01 | 1.83 (0.86,3.86) | 0.11 |
| Peripheral arterial disease | 1.67 (0.74,3.78) | 0.21 | 1.32 (0.58,3.01) | 0.51 | 2.29 (0.93,5.63) | 0.07 | 1.72 (0.69,4.30) | 0.24 |
| eGFRcr (per SD) | 0.29 (0.13,0.62) | <0.01 | 0.36 (0.16,0.78) | 0.01 | 0.21 (0.08,0.56) | <0.01 | 0.29 (0.11,0.78) | 0.03 |
| eGFRcr (per SD) squared | 1.18 (1.07,1.29) | 1.15 (1.04,1.27) | 1.22 (1.08,1.37) | 1.17 (1.04,1.33) | ||||
Included in Model 2 in place of BMI.
Among participants free of diabetes.
Model 1. Includes age, sex and race and other traditional risk factors individually.
Model 2 (Main Model). Includes age, sex, race, education, BMI, hypertension, diabetes, smoking, LDLc, HDLc, triglycerides, coronary heart disease, stroke/TIA, heart failure, peripheral arterial disease and eGFRcr.
Abbreviations: BMI = body mass index; eGFRcr = creatinine-based estimated glomerular filtration rate; HDLc = high density lipoprotein cholesterol; HOMA-IR = homeostasis model assessment of insulin resistance; HS = high school; LDLc = low density lipoprotein cholesterol; TIA = transient ischemic attack.
Figure 2.
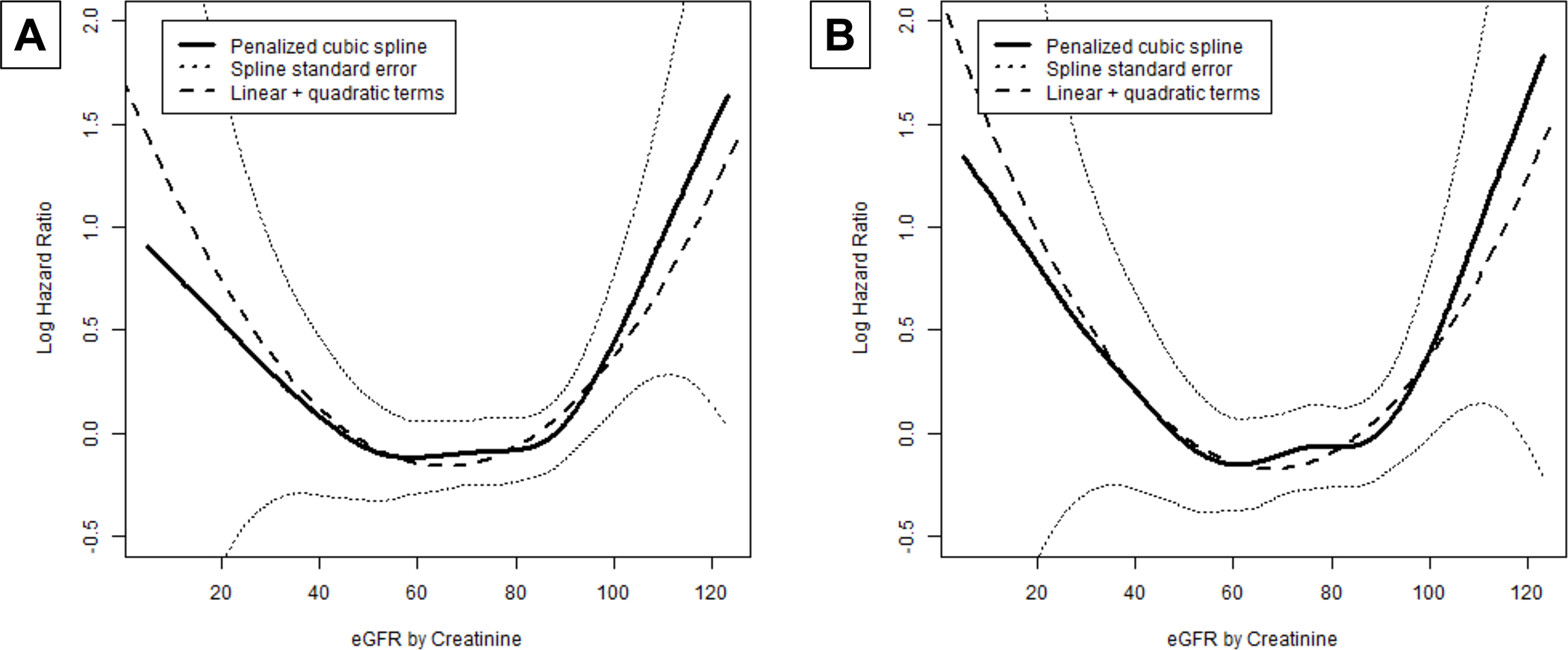
Penalized cubic spline plots of estimated glomerular filtration rate (eGFR) by creatinine and (A) incident probable/definite moderate/severe aortic stenosis, (B) probable/definite clinically significant aortic stenosis.
For the secondary outcome, probable/definite clinically significant AS, findings were broadly consistent, except that the fully adjusted association for CHD was no longer significant (Table 2).
In contrast to eGFRcr, the relationship of eGFRcys with incident probable/definite moderate or severe AS showed no departure from linearity. eGFRcys was significantly associated with lower incidence of the primary outcome in the minimally adjusted model (HR=0.83 per SD [95% CI=0.72, 0.95], p=0.01). After full adjustment, however, the association became non-significant (HR=0.88 per SD [0.75, 1.02], p=0.09).
In exploratory analyses assessing possible/probable/definite AS outcomes (Table S1), similar associations were seen for the same traditional risk factors as for probable/definite AS outcomes. There was also a significant relationship between hypertension and possible/probable/definite moderate or severe AS.
Novel Risk Factors and AS
In the minimally adjusted models examining novel lipid and mineral biomarkers, only LpPLA2 activity was associated with incident probable/definite moderate or severe AS (Table 3). This relationship persisted after main-model adjustment, as well as simultaneous adjustment for LpPLA2 mass and Lp(a). For inflammatory markers, IL-6, sCD14 and sCD163 showed significant positive associations with the primary outcome with minimal adjustment. These associations persisted for sCD14 and marginally for IL-6 after full adjustment for traditional risk factors but ceased to be significant for both after concurrent adjustment (Table 3). For the secondary outcome, LpPLA2 activity and IL-6 were significantly associated with probable/definite clinically significant AS, but sCD14 was not (Table 3).
Table 3.
Associations of Novel Risk Factors with Incidence of Probable/Definite Moderate or Severe Aortic Stenosis and Probable/Definite Clinically Significant Aortic Stenosis
| Variables | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| HR* (95% CI) | p | HR* (95% CI) | p | HR* (95% CI) | p | |
| Probable/Definite Moderate or Severe AS | ||||||
| Lipid Markers | ||||||
| Lp(a) | 1.02 (0.89,1.17) | 0.78 | 1.03 (0.90,1.18) | 0.70 | 1.04 (0.90,1.20) | 0.59 |
| LpPLA2 Mass | 1.10 (0.99,1.23) | 0.09 | 1.08 (0.96,1.21) | 0.19 | 1.00 (0.87,1.14) | 0.95 |
| LpPLA2 Activity | 1.22 (1.10,1.35) | <0.01 | 1.21 (1.07,1.37) | <0.01 | 1.21 (1.06,1.39) | 0.01 |
| Mineral Markers | ||||||
| Fetuin-A | 1.04 (0.91,1.18) | 0.61 | 0.99 (0.86,1.13) | 0.89 | 0.99 (0.86,1.13) | 0.84 |
| Klotho | 1.00 (0.87,1.15) | 0.99 | 1.00 (0.87,1.15) | 1.00 | 1.00 (0.87,1.15) | 1.00 |
| Inflammatory Markers | ||||||
| hsCRP | 1.06 (0.95,1.19) | 0.30 | 1.00 (0.87,1.14) | 1.00 | 0.96 (0.80,1.14) | 0.62 |
| IL-6 | 1.11 (1.03,1.20) | 0.01 | 1.09 (1.00,1.20) | 0.06 | 1.08 (0.98,1.20) | 0.13 |
| WBC | 1.11 (1.00,1.23) | 0.05 | 1.06 (0.93,1.20) | 0.37 | 1.06 (0.92,1.22) | 0.42 |
| sCD14 | 1.18 (1.04,1.34) | 0.01 | 1.16 (1.01,1.34) | 0.03 | 1.15 (0.98,1.34) | 0.08 |
| sCD163 | 1.20 (1.06,1.36) | <0.01 | 1.10 (0.97,1.26) | 0.15 | 1.07 (0.93,1.22) | 0.36 |
| Probable/Definite Clinically Significant AS | ||||||
| Lipid Markers | ||||||
| Lp(a) | 1.00 (0.84,1.20) | 0.99 | 1.00 (0.84,1.20) | 0.98 | 1.00 (0.83,1.21) | >0.99 |
| LpPLA2 Mass | 1.11 (0.97,1.28) | 0.14 | 1.06 (0.91,1.23) | 0.43 | 0.98 (0.83,1.15) | 0.79 |
| LpPLA2 Activity | 1.26 (1.11,1.43) | <0.01 | 1.20 (1.03,1.40) | 0.02 | 1.21 (1.02,1.44) | 0.03 |
| Mineral Markers | ||||||
| Fetuin-A | 1.11 (0.94,1.32) | 0.22 | 1.02 (0.86,1.22) | 0.78 | 1.02 (0.85,1.21) | 0.84 |
| Klotho | 1.05 (0.91,1.21) | 0.49 | 1.04 (0.90,1.20) | 0.58 | 1.04 (0.90,1.20) | 0.58 |
| Inflammatory Markers | ||||||
| hsCRP | 1.11 (0.98,1.24) | 0.09 | 1.06 (0.92,1.21) | 0.44 | 1.07 (0.92,1.25) | 0.37 |
| IL-6 | 1.14 (1.04,1.24) | <0.01 | 1.12 (1.01,1.25) | 0.04 | 1.10 (0.97,1.25) | 0.13 |
| WBC | 1.16 (1.04,1.30) | 0.01 | 1.11 (0.96,1.29) | 0.15 | 1.11 (0.94,1.31) | 0.20 |
| sCD14 | 1.10 (0.92,1.31) | 0.28 | 1.05 (0.87,1.26) | 0.64 | 0.98 (0.80,1.21) | 0.88 |
| sCD163 | 1.24 (1.06,1.46) | 0.01 | 1.14 (0.96,1.35) | 0.13 | 1.11 (0.94,1.33) | 0.22 |
Per SD increment in each biomarker.
Model 1. Adjusted for age, sex and race and individual novel risk factors.
Model 2 (Main Model). Adjusted for age, sex, race, education, BMI, hypertension, diabetes, smoking, LDLc, HDLc, triglycerides, coronary heart disease, stroke/TIA, heart failure, peripheral arterial disease and eGFRcr.
Model 3. Lipids: Model 2 variables, with concurrent inclusion of LpPLA2 mass, LpPLA2 activity and Lp(a) (in first cohort only). Mineral Markers: Model 2 variables, with concurrent inclusion of fetuin-A and klotho. Inflammatory Markers: Model 2 variables, with concurrent inclusion of hsCRP, IL-6, WBC, sCD14, and sCD163.
Abbreviations: hsCRP = high-sensitivity C-reactive protein; IL-6 = interleukin-6; Lp(a) = lipoprotein(a); LpPLA2 = lipoprotein- associated phospholipase A2; sCD14 = soluble CD14; sCD163 = soluble CD163; WBC = white blood cell count.
In additional analyses, LpPLA2 activity and sCD14, though not IL-6, remained significantly associated with possible/probable/definite moderate or severe AS, but a significant association was also seen for sCD163 (Table S2). In the case of possible/probable/definite clinically significant AS, only LpPLA2 activity and IL-6 again showed significant associations (Table S2).
Exploratory Analyses
Additional adjustment for statins, glucocorticoids and warfarin did not alter the findings. Censoring follow-up at 10 years yielded <½ the number of AS events (primary outcome, n=115; secondary outcome, n=69), but mostly showed a similar pattern of associations for traditional risk factors (Table S3). Yet, for both the primary and secondary outcome, there was attenuation of the risk estimates for diabetes, which became non-significant, but significant associations were detected for BMI. For novel risk factors, associations were not significant, with the exception of sCD14 and moderate or severe AS (Table S4).
There was no significant effect modification of risk factor associations with probable/definite moderate or severe AS by age, sex, or race.
Discussion
Main Results
This investigation of risk factors for advanced AS in elders yielded the following findings: (i) older age, male sex, diabetes and prevalent CHD were positively associated with incident moderate or severe, as well as clinically significant, AS, while eGFRcr showed a U-shaped association, and Black race an inverse relationship with these outcomes after adjustment for demographic and clinical covariates; and (ii) the circulating lipid marker LpPLA2 activity, and the inflammatory markers IL-6 and sCD14, were each independently associated with higher incidence of one or both of these AS outcomes (Figure 3). Exploratory analyses that considered possible as well as probable and definite cases of AS showed consistent findings, although they also identified positive associations for hypertension and sCD163 with moderate or severe AS.
Figure 3.
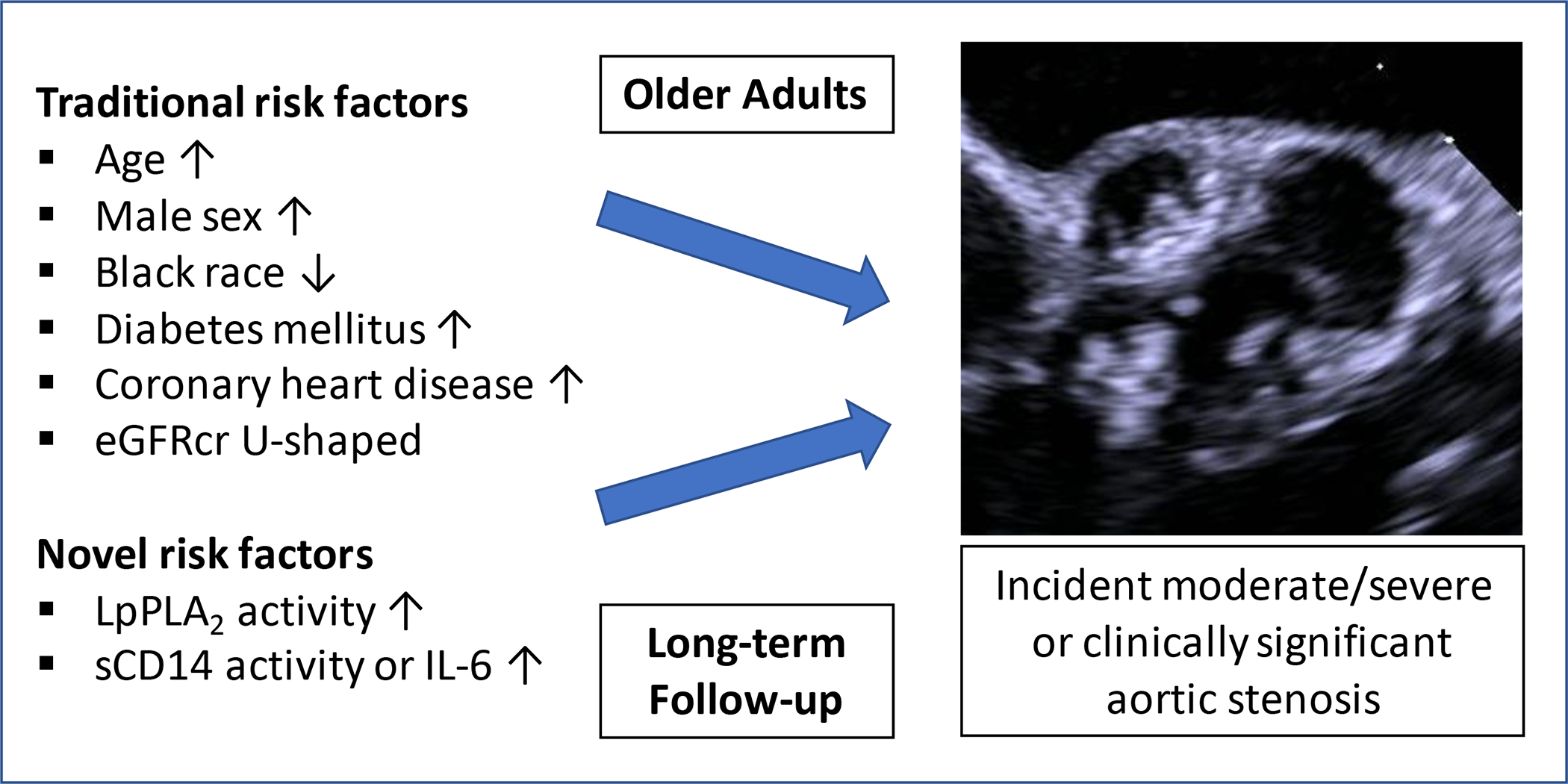
Graphic figure summarizing the key findings of the study.
Previous Studies
Multiple studies have evaluated the relationship of traditional or emerging CVD risk factors with AVC and incident AS outcomes.3–11 These investigations have permitted identification of clinical and biological determinants of CAVD and leveraged Mendelian randomization analyses for causal inference, including in a large, predominantly male, multi-ethnic population.7,10–12 Such genetic analyses have supported causal roles not just for obesity and hypertension, but also for LDLc, Lp(a), IL-6 and polyunsaturated fatty acids, identifying these as potential targets for AS therapeutics.7,10–12 Despite important contributions, however, longitudinal studies of AS outcomes3,5–9,11,12 have been subject to limitations, namely, uniform reliance on administrative diagnostic codes for AS, and, of relevance to observational studies, lack of baseline echocardiography for exclusion of prevalent disease and frequently incomplete consideration of risk factors.
To our knowledge, this is the first study to undertake a wide-ranging evaluation of traditional and novel risk factors for clinically adjudicated AS in elders. As we previously documented, age and male sex demonstrated positive, and Black race inverse, associations with incident AS, findings that align with prior reports.2 Among traditional risk factors, only diabetes, CHD (both positively) and eGFRcr (U-shaped relationship) exhibited significant associations after main-model adjustment.
That diabetes emerged as a prominent risk factor for advanced AS is consistent with results from some,5,8 though not all,4,6 prior longitudinal studies. We did not detect a corresponding association for HOMA-IR, in contrast with an earlier report linking metabolic syndrome and AVC incidence in a younger population.20 Our findings suggest that among elders the contribution resides with more severe metabolic dysregulation and hyperglycemia. Moreover, censoring follow-up at 10 years rendered the diabetes association with AS weaker and non-significant, suggesting that the disorder’s consequences take time to become manifest. Contrariwise, BMI surfaced as a significant risk factor within this shortened follow-up, and waist circumference nearly so. Adiposity has been consistently implicated as a causal risk factor for AS,7,11,12 but the observed weakening of the relationship during long-term follow-up could signify parallel contributions of weight loss and related inflammation to CAVD late in life.14
The findings on eGFRcr illuminate an important feature of CAVD risk factor assessment particular to older populations. Prior work in a middle-aged population associated serum creatinine with a monotonically increased risk of incident AS.7 We now document that this association in elders is U-shaped instead. The inverse relationship observed in the lower range of eGFRcr is likely a true reflection of increased AS risk with worsening kidney function and its attendant oxidative stress, inflammation and mineral dysregulation.21 But the positive relationship at the higher eGFRcr range could well reflect lower creatinine production in the setting of sarcopenia and its adverse implications for aging-related outcomes.22
The observed association of prevalent CHD with moderate or severe AS accords with well-recognized commonalities in atherosclerosis and CAVD pathogenesis.1 That hypertension, LDLc and smoking did not exhibit significant associations with the primary outcome runs counter to findings in younger populations.3 For hypertension, this could relate to a weakening of associations with aging-related weight loss or onset of HF. In the case of LDLc, a diminished role in the propagation phase, as contrasted with the initiation phase of CAVD, could account for the null association, as might aging-associated weight loss and LDLc’s negative acute phase reactant properties.14 For smoking, health factors prompting cessation versus continuation late in life might explain the lack of association in this older population.
Novel Factors
Histopathologic studies have implicated lipoprotein infiltration, inflammatory cell accumulation, heightened oxidative stress and fibrotic calcification as central to CAVD,1 findings that have been bolstered by Mendelian randomization analyses.12 We investigated several circulating markers from relevant lipid, mineral, and inflammatory pathways, of which only LpPLA2 activity exhibited consistent associations with AS outcomes, while sCD14 and IL-6 were significantly or near-significantly related to the primary outcome.
LpPLA2 is elevated in plasma from patients with CAVD,23 as well as AS histologic specimens.24 The enzyme contributes to fibrocalcific remodeling and mineralization by generating lysophosphatidylcholine, a powerful promoter of inflammation and apoptosis of valve interstitial cells.23 LpPLA2 is primarily bound to circulating lipoproteins but also synthesized by aortic valve macrophages, possibly in response to oxidized LDL.24 The current findings extend earlier analyses linking LpPLA2 activity, though not mass, with faster echocardiographic progression of stenosis in mild, but not advanced, AS.25 Mendelian randomization analyses did not support a causal role for LpPLA2 in CAVD, however, much as was seen in CVD,23 making it unlikely that LpPLA2 could be directly targeted for CAVD therapeutics. But the present results indicate that the molecule’s role as marker and pathogenic contributor deserves further investigation.
Unlike LpPLA2 activity, Lp(a) was not associated with AS incidence. There is strong evidence supporting a causal role for Lp(a) in CAVD through its oxidized phospholipid cargo.12 Yet a recent study associated Lp(a) to incidence of AVC, but not progression, raising the possibility that Lp(a) is more relevant to initiation than propagation of CAVD.26 The implications for therapeutics will await completion of clinical trials evaluating Lp(a) lowering. It is also possible that Lp(a) may be buffered by other protective factors for survival to older age. Hence, causal associations reported for Lp(a) – as for other factors like LDLc – based on lifelong genetic exposure12 may not hold for circulating levels of these biomarkers late in life. Whether resulting from survival bias or biology, in the case of Lp(a), or aging-related weight loss and comorbidities, in the case of LDLc or IL-6, our findings underscore the importance of defining risk factor associations specifically in older populations.
While inflammation has been implicated in CAVD, precise mechanisms remain unclear.1 The association documented here for sCD14 and advanced AS may shed new light on the pathobiology. sCD14, the soluble form of a membrane glycoprotein expressed by myeloid and other cells, has been implicated in chronic lipopolysaccharide endotoxemia due to reduced intestinal barrier function.27 Lipopolysaccharide upregulates sCD14, leading to immune activation,28 which may explain sCD14’s association with cardiovascular events.18 Prior work from our group has linked sCD14 to echocardiographic AVC,16 and the current association with advanced AS bolsters its potential role in CAVD.
IL-6, which showed a significant association with clinically significant AS, has been causally implicated in CAVD.12 IL-6 levels, which are associated with risk of CVD, are also upregulated in AS specimens, where they promote calcification.29 By contrast, hsCRP showed no relationship here, indicating its status as a weaker marker for AS in this older population.
Although bone and mineral dysregulation can contribute to CAVD, this study did not uncover associations of two corresponding markers, fetuin-A and klotho, with incident AS. While previous population-level data on klotho and CAVD are lacking, similar results were shown for fetuin-A and AVC in CHS16 and MESA.30 No or limited availability of additional mineral markers, including phosphate and fibroblast growth factor 23,30 precluded their evaluation here.
Limitations
This observational study cannot demonstrate causality. Mendelian randomization analyses could be undertaken in consortia with more AS events. Given that our case-capture methods relied on review of hospitalization records over a long follow-up period, cases of asymptomatic severe AS or those without access to care may not have been detected in our cohort. Yet participants’ Medicare eligibility combined with their high rates of hospitalization and the natural history of CAVD likely rendered those cases rare. Similarly, pre-hospital death, clinical misattribution of symptoms or incomplete information may have led to missed cases or misclassification. We evaluated a large number of biomarkers, but selection was limited by prior availability in CHS. Because of strong prior probabilities based on prior literature, we did not correct for multiple comparisons; the present findings will require independent replication.
Conclusions
In this older cohort, assessment of traditional risk factors revealed diabetes and prevalent CHD to be positively, and eGFRcr bimodally, associated with incident AS. Evaluation of novel risk factors linked LpPLA2 activity, sCD14 and IL-6 with increased risk of AS, implicating potentially specific lipid and inflammatory pathways in CAVD later in life. These findings underscore differences in risk profiles for elders, and the need to study older cohorts for identifying proper biomarkers for this disease in those at greatest risk.
Supplementary Material
Key messages.
What is already known about this subject?
Clinical and population studies have identified various risk factors for calcific aortic stenosis, including hypertension, dysglycemia, obesity, and smoking, as well as LDL cholesterol, lipoprotein (a), creatinine, and high-sensitivity C-reactive protein. Among these, blood pressure, body mass index, LDL cholesterol and lipoprotein (a) have a potentially causal role.
What does this study add?
In older adults, assessment of traditional risk factors revealed diabetes and prevalent CHD to be positively, and eGFRcr bimodally, associated with incidence of adjudicated, clinically meaningful aortic stenosis over 25 years of follow-up. Concurrent evaluation of novel risk factors newly showed that circulating lipoprotein-associated phospholipase-A2 activity, sCD14 and interleukin-6 were prospectively associated with an increased risk of aortic stenosis, implicating potentially specific lipid and inflammatory pathways in the development of CAVD later in life.
How might this impact on clinical practice?
These findings illustrate differences in risk profiles for CAVD in elders, highlighting the need for longitudinal study of older cohorts to properly identify circulating biomarkers and putative therapeutic targets for this disease in those at greatest risk.
Acknowledgements and Sources of Funding
Funding:
This research was supported by contracts HHSN268201200036C, HHSN268200800007C, HHSN268201800001C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, 75N92021D00006, and grants U01HL080295 and U01HL130114 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org. Dr. Massera was supported by the Glorney-Raisbeck Fellowship Program, Corlette Glorney Foundation, and the New York Academy of Medicine. Dr. Owens was supported by a Mentored Clinical and Population Research Award from the American Heart Association’s Western States Affiliate. Dr. Kizer was supported by K24HL135413 from the NHLBI.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Dr. Massera has received consulting fees from Bristol Meyers Squibb, Sanofi Genzyme and Tenaya Therapeutics. Dr. Kizer reports stock ownership in Abbott, Bristol Myers Squibb, Johnson & Johnson, Medtronic, Merck and Pfizer. Dr Psaty serves on the steering committee of the Yale Open Data Access Project, funded by Johnson & Johnson. All other authors declare that they have no conflicts of interest.
Footnotes
Ethics Statement
This study complied with the Declaration of Helsinki. All participants provided written informed consent. CHS was approved by the Institutional Review Boards of the Coordinating Center (University of Washington CR00004872) and field centers (University of Pittsburgh CR19040035-004, Johns Hopkins University 11007/CR811, Wake Forest University BG00-497, and University of California, Davis 300401-16).
References
- 1.Lindman BR, Clavel MA, Mathieu P, et al. Calcific aortic stenosis. Nat Rev Dis Primers 2016;2:16006. doi: 10.1038/nrdp.2016.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Owens DS, Bartz TM, Buzkova P, et al. Cumulative burden of clinically significant aortic stenosis in community-dwelling older adults. Heart 2021;107(18):1493–502. doi: 10.1136/heartjnl-2021-319025 [published Online First: 2021/06/05] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen HY, Engert JC, Thanassoulis G. Risk factors for valvular calcification. Curr Opin Endocrinol Diabetes Obes 2019;26(2):96–102. doi: 10.1097/med.0000000000000471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eveborn GW, Schirmer H, Lunde P, et al. Assessment of risk factors for developing incident aortic stenosis: the Tromsø Study. Eur J Epidemiol 2014;29(8):567–75. doi: 10.1007/s10654-014-9936-x [published Online First: 2014/07/16] [DOI] [PubMed] [Google Scholar]
- 5.Martinsson A, Östling G, Persson M, et al. Carotid plaque, intima-media thickness, and incident aortic stenosis: a prospective cohort study. Arterioscler Thromb Vasc Biol 2014;34(10):2343–8. doi: 10.1161/atvbaha.114.304015 [published Online First: 2014/09/19] [DOI] [PubMed] [Google Scholar]
- 6.Yan AT, Koh M, Chan KK, et al. Association Between Cardiovascular Risk Factors and Aortic Stenosis: The CANHEART Aortic Stenosis Study. J Am Coll Cardiol 2017;69(12):1523–32. doi: 10.1016/j.jacc.2017.01.025 [published Online First: 2017/03/25] [DOI] [PubMed] [Google Scholar]
- 7.Huang N, Zhuang Z, Liu Z, et al. Observational and Genetic Associations of Modifiable Risk Factors with Aortic Valve Stenosis: A Prospective Cohort Study of 0.5 Million Participants. Nutrients 2022;14(11) doi: 10.3390/nu14112273 [published Online First: 2022/06/11] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rawshani A, Sattar N, McGuire DK, et al. Left-Sided Degenerative Valvular Heart Disease in Type 1 and Type 2 Diabetes. Circulation 2022;146(5):398–411. doi: 10.1161/circulationaha.121.058072 [published Online First: 2022/06/10] [DOI] [PubMed] [Google Scholar]
- 9.Smith JG, Luk K, Schulz CA, et al. Association of low-density lipoprotein cholesterol-related genetic variants with aortic valve calcium and incident aortic stenosis. JAMA 2014;312(17):1764–71. doi: 10.1001/jama.2014.13959 [published Online First: 2014/10/27] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nazarzadeh M, Pinho-Gomes AC, Smith Byrne K, et al. Systolic Blood Pressure and Risk of Valvular Heart Disease: A Mendelian Randomization Study. JAMA Cardiol 2019;4(8):788–95. doi: 10.1001/jamacardio.2019.2202 [published Online First: 2019/07/11] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaltoft M, Langsted A, Nordestgaard BG. Obesity as a Causal Risk Factor for Aortic Valve Stenosis. J Am Coll Cardiol 2020;75(2):163–76. doi: 10.1016/j.jacc.2019.10.050 [published Online First: 2020/01/18] [DOI] [PubMed] [Google Scholar]
- 12.Small AM, Peloso GM, Linefsky J, et al. Multiancestry Genome-Wide Association Study of Aortic Stenosis Identifies Multiple Novel Loci in the Million Veteran Program. Circulation 2023;147(12):942–55. doi: 10.1161/circulationaha.122.061451 [published Online First: 20230220] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pawade TA, Doris MK, Bing R, et al. Effect of Denosumab or Alendronic Acid on the Progression of Aortic Stenosis: A Double-Blind Randomized Controlled Trial. Circulation 2021;143(25):2418–27. doi: 10.1161/CIRCULATIONAHA.121.053708 [published Online First: 2021/04/30] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fried LP, Kronmal RA, Newman AB, et al. Risk factors for 5-year mortality in older adults: the Cardiovascular Health Study. Jama 1998;279(8):585–92. doi: 10.1001/jama.279.8.585 [published Online First: 1998/03/05] [DOI] [PubMed] [Google Scholar]
- 15.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012;367(1):20–9. doi: 10.1056/NEJMoa1114248 [published Online First: 2012/07/06] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bortnick AE, Bartz TM, Ix JH, et al. Association of inflammatory, lipid and mineral markers with cardiac calcification in older adults. Heart 2016;102(22):1826–34. doi: 10.1136/heartjnl-2016-309404 [published Online First: 2016/07/15] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamazaki Y, Imura A, Urakawa I, et al. Establishment of sandwich ELISA for soluble alpha-Klotho measurement: Age-dependent change of soluble alpha-Klotho levels in healthy subjects. Biochem Biophys Res Commun 2010;398(3):513–8. doi: 10.1016/j.bbrc.2010.06.110 [published Online First: 2010/07/06] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Kindi SG, Buzkova P, Shitole SG, et al. Soluble CD14 and Risk of Heart Failure and Its Subtypes in Older Adults. J Card Fail 2020;26(5):410–19. doi: 10.1016/j.cardfail.2020.03.003 [published Online First: 2020/03/14] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Durda P, Raffield LM, Lange EM, et al. Circulating Soluble CD163, Associations With Cardiovascular Outcomes and Mortality, and Identification of Genetic Variants in Older Individuals: The Cardiovascular Health Study. J Am Heart Assoc 2022;11(21):e024374. doi: 10.1161/jaha.121.024374 [published Online First: 2022/11/01] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katz R, Budoff MJ, Takasu J, et al. Relationship of metabolic syndrome with incident aortic valve calcium and aortic valve calcium progression: the Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes 2009;58(4):813–9. doi: 10.2337/db08-1515 [published Online First: 20090109] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarnak MJ, Amann K, Bangalore S, et al. Chronic Kidney Disease and Coronary Artery Disease: JACC State-of-the-Art Review. J Am Coll Cardiol 2019;74(14):1823–38. doi: 10.1016/j.jacc.2019.08.1017 [published Online First: 2019/10/05] [DOI] [PubMed] [Google Scholar]
- 22.Cox HJ, Bhandari S, Rigby AS, et al. Mortality at low and high estimated glomerular filtration rate values: a ‘U’ shaped curve. Nephron Clin Pract 2008;110(2):c67–72. doi: 10.1159/000151720 [published Online First: 2008/09/02] [DOI] [PubMed] [Google Scholar]
- 23.Perrot N, Thériault S, Rigade S, et al. Lipoprotein-associated phospholipase A2 activity, genetics and calcific aortic valve stenosis in humans. Heart 2020;106(18):1407–12. doi: 10.1136/heartjnl-2020-316722 [published Online First: 20200707] [DOI] [PubMed] [Google Scholar]
- 24.Mahmut A, Boulanger MC, El Husseini D, et al. Elevated expression of lipoprotein-associated phospholipase A2 in calcific aortic valve disease: implications for valve mineralization. J Am Coll Cardiol 2014;63(5):460–9. doi: 10.1016/j.jacc.2013.05.105 [published Online First: 2013/10/29] [DOI] [PubMed] [Google Scholar]
- 25.Capoulade R, Mahmut A, Tastet L, et al. Impact of plasma Lp-PLA2 activity on the progression of aortic stenosis: the PROGRESSA study. JACC Cardiovasc Imaging 2015;8(1):26–33. doi: 10.1016/j.jcmg.2014.09.016 [published Online First: 20141101] [DOI] [PubMed] [Google Scholar]
- 26.Kaiser Y, van der Toorn JE, Singh SS, et al. Lipoprotein(a) is associated with the onset but not the progression of aortic valve calcification. Eur Heart J 2022. doi: 10.1093/eurheartj/ehac377 [published Online First: 2022/07/24] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Konig J, Wells J, Cani PD, et al. Human Intestinal Barrier Function in Health and Disease. Clin Transl Gastroenterol 2016;7(10):e196. doi: 10.1038/ctg.2016.54 [published Online First: 2016/10/21] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zanoni I, Granucci F. Role of CD14 in host protection against infections and in metabolism regulation. Front Cell Infect Microbiol 2013;3:32. doi: 10.3389/fcimb.2013.00032 [published Online First: 2013/07/31] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.El Husseini D, Boulanger MC, Mahmut A, et al. P2Y2 receptor represses IL-6 expression by valve interstitial cells through Akt: implication for calcific aortic valve disease. J Mol Cell Cardiol 2014;72:146–56. doi: 10.1016/j.yjmcc.2014.02.014 [published Online First: 2014/03/19] [DOI] [PubMed] [Google Scholar]
- 30.Bortnick AE, Xu S, Kim RS, et al. Biomarkers of mineral metabolism and progression of aortic valve and mitral annular calcification: The Multi-Ethnic Study of Atherosclerosis. Atherosclerosis 2019;285:79–86. doi: 10.1016/j.atherosclerosis.2019.04.215 [published Online First: 2019/05/03] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


