Abstract
Climate is one of the major factors determining the prevalence and seasonality of vector borne diseases like scrub typhus (ST). We analyzed, the association of the meteorological factors like temperature, rainfall and humidity with scrub typhus using the 15 years scrub typhus data from a tertiary care hospital in Vellore, South India. Demographic data of permanent residents of Vellore, who had IgM ELISA results for scrub typhus for the time period of May 2005 to April 2020 were included. Meteorological data was correlated with the monthly scrub typhus cases; negative binomial regression model was used to predict the relation between scrub typhus occurrence and climate factors. Maximum number of ST cases were reported between the months August and February with October recording the highest number of cases. Elderly people, farmers, agricultural workers and housewives were at higher risk for scrub typhus. For an increase of 1 °C in mean temperature, the monthly ST cases reduced by 18.8% (95% CI − 24.1, − 13.2%). On the contrary, for 1 percent increase in mean relative humidity (RH), there is an increase of 7.6% (95% CI 5.4, 9.9%) of monthly ST cases. Similarly, an increase of 1 mm of rainfall contributed to 0.5 to 0.7% of monthly ST cases (after 2 months) depending on the variables included in the analysis. This study provides information that meteorological factors influence ST occurrence in Vellore. The rise of scrub typhus cases is maximal 2 months post rainfall. Whereas a rise in relative humidity, causes a rise in scrub typhus cases in same month, while rise in temperature has a negative impact on scrub typhus during the same month. These findings based on a retrospective analysis need validation by prospective studies.
Subject terms: Ecology, Microbiology, Climate sciences, Environmental sciences, Risk factors
Introduction
Scrub typhus is caused by the obligate intracellular bacteria Orientia tsutsugamushi in the tsutsugamushi triangle1,2 Orientia chuto in UAE3 and Orientia chiloensis in chile4. The infection is transmitted to humans through the bite of larvae (chiggers) of trombiculid mites which act both as the vector and reservoir of the infection5 and are common ecto-parasites of rodents6 and shrews7. The chiggers get attached to these small mammals, which are the common vertebrate hosts7,8. The chigger infection with Orientia spp. is perpetuated and maintained in nature by transovarial and transstadial transmission9.
Humans are the accidental host for the infection as they can pick up the mite when they walk, sit or lie down in the ground infested by chiggers8. A necrotic lesion, eschar which appears at the site of chigger bite10,11. Once infected, the patients may have symptoms like fever, headache, myalgia, cough, generalized lymphadenopathy, nausea, vomiting and abdominal pain. Of these, fever and head-ache are the most commonly reported symptoms1. Scrub typhus may also lead to severe complications like multi-organ dysfunction (MODS) and the resulting mortality can be very high among untreated cases1,12. Prevalence of eschar among scrub positives ranges from 8 to 58% in Indian studies13–17 and the reason for this less detection of eschar which is a black scab with erythematous halo is due to its painless nature occurring mostly in flexural areas and hence often overlooked18. Diagnosing scrub typhus is mostly by clinical suspicion and serological test19. Without the presence of eschar, the diagnosis of scrub typhus is challenging and often other causes of fever get explored20. Once suspected, oral doxycycline for mild infections1 and a combination of parenteral doxycycline and azithromycin for severe infections have been shown to be effective against scrub typhus21.
The occurrence of ST is seasonal in the endemic areas8 and in Vellore, the cooler months were noticed to have increased number of cases22, secondary to heavy chigger infestation of rodents23. It has also been noted that scrub typhus incidence is associated with relative humidity, temperature and rainfall8,9. Further, there can be country wide variation across seasons and in different geographical condition as observed in China24. Therefore, we analyzed a 15 year dataset of scrub typhus and compared the role of climatic factors like rainfall, temperature and humidity on seasonality of scrub typhus in Vellore.
Materials and methods
Ethical statement
No human subjects were directly involved in the study. The scrub typhus data was obtained from the electronic laboratory register of the Immunology Laboratories, Department of Clinical Microbiology, Christian Medical College, Vellore. All the patient identifying variables in the data were removed before the analysis. The approval to conduct this study was obtained from Institutional Review Board (IRB) and Ethics Committee (EC) of the Christian Medical College (CMC), Vellore vide IRB Min no. 9866 dated 20th January 2016. As the required data had been collected as part of the standard of care for diagnosis of scrub typhus, informed consent waiver was granted by the IRB and EC of CMC, Vellore. The Declaration of Helsinki norms were adhered to while conducting the study.
Study area
Vellore district in Tamil Nadu has an area of 6075 km225. According to the census of India 2011, the population of Vellore was 3,936,331 with a population density of 648/km225. Vellore is bounded by Chittoor district of Andhra Pradesh in the North, Tiruvannamalai district in the South, Kancheepuram and Tiruvallur districts in the East and by Yelagiri hills in the West (Vellore District Statistical Handbook 2016–17: Refer Fig. 1). It has 162,286 hectares of Forest area and the district is mostly surrounded by hills. The majority of the rainfall in this area is from the southwest monsoon. The major occupation of people in Vellore district is agriculture with paddy being the major crop grown. It has a forest cover of 27.94%26 with tropical savannah climate.
Figure 1.
Map of Vellore district
(Source: https://www.researchgate.net/figure/Map-showing-the-study-area-of-Vellore-India_fig1_282975836).
Methodology
The data for this research was obtained from the electronic medical records of a tertiary care hospital (Christian Medical College, Vellore), South India. The data included is for patients with suspected scrub typhus for whom the Scrub typhus IgM ELISA was requested. Data of such patients who underwent scrub typhus testing was obtained for the time period of May 2005 to April 2020. The diagnosis of scrub typhus was done based on the positivity of the serological test for scrub typhus (IgM), on individuals with acute undifferentiated febrile illness (AUFI), who were malarial parasites and blood culture negative. All the assays were performed with appropriate controls in the Immunology Laboratory (an ISO15189:2012 accredited lab) on an automated ELISA workstation (Euroimmun Analyzer I, Euroimmun AG, Lubeck, Germany). The cut-off OD value was set at 1.0 for scrub typhus positivity. Patients with OD values 1.0 and above in the IgM ELISA test were considered positive and those who were negative (OD < 1.0) were used as a comparator group. Patients who are non-residents of Vellore district were excluded from the study.
Variables like age, sex, address, OP (Out-Patient)/IP (In-Patient), occupation, date of testing and results were obtained from the patient medical records of Christian Medical College, Vellore. Climatic factors like monthly mean maximum temperature, monthly mean minimum temperature, monthly mean relative maximum humidity, monthly mean relative minimum humidity and monthly total rainfall of Vellore was obtained from the Regional Meteorological Centre, Chennai.
Statistical analysis
The data was entered in Microsoft excel spreadsheets (excel version 1997) and analyzed using IBM SPSS Statistics for Windows, Version 21.0 (IBM Corp, Armonk, NY, USA). The continuous variables were expressed in mean and standard deviation (SD) for normal distribution and median and inter-quartile range for skewed distribution. The categorical variables were expressed in frequencies and percentages. Spearman’s correlation (for skewed data) and Pearsons correlation (for normally distributed data) was done to correlate two continuous variables and 95% CI was reported. The predictor variables such as mean temperature and mean humidity were calculated as monthly averages, whereas the rainfall, the other predictor variable and the ST cases which is the outcome variable were calculated as monthly aggregates. A negative binomial regression model was used to explore the relationship between monthly scrub typhus cases and meteorological factors. Since the scrub typhus data and rainfall data were overly dispersed we chose a negative binomial regression over the Poisson model. Spearman correlation coefficient matrix was done primarily to check for any existing correlation between the meteorological factors. This revealed a strong negative correlation of − 0.739 (p < 0.001) between mean temperature and mean humidity. To avoid collinearity problems, two negative binomial regression models were done namely model A and model B. Model A was computed using rainfall and mean temperature and model B was computed using rainfall and mean humidity. We calculated the influences (eβ – 1) * 100, which roughly corresponds to the percent increase, to quantify the effects of meteorological variables27. The association between two categorical variables was analyzed using chi-square and logistic regression was done for adjusting the confounders.
Results
From May 2005 to April 2020, 11,001 were tested for scrub typhus, out of which 2784 were positives. Table 1 describes the demographic features of the study population. The overall positivity rate among the people tested for ST is 25.3 percent. The male to female ratio is 1:1.4. The mean age of scrub typhus positive patients is 44.2 with a standard deviation of 19.2 (Median: 45, IQR: 30, 59). The detailed distribution of cases including gender, occupation and climate is given in supplementary data (Figs. S1 to S5).
Table 1.
Demographic characters.
| Demographic characters | Tested | ST IgM ELISA positives | ||
|---|---|---|---|---|
| Numbers | % | Numbers | % | |
| Male | 5570 | 50.6 | 1184 | 42.5 |
| Female | 5431 | 49.4 | 1600 | 57.5 |
| 0–20 years | 2036 | 18.5 | 322 | 11.6 |
| 21–30 years | 2144 | 19.5 | 399 | 14.3 |
| 31–40 years | 1699 | 15.4 | 445 | 16 |
| 41–50 years | 1643 | 14.9 | 499 | 17.9 |
| 51–60 years | 1588 | 14.4 | 510 | 18.3 |
| > 60 years | 1891 | 17.2 | 609 | 21.9 |
| IP | 6902 | 62.7 | 1871 | 67.2 |
| OP | 4020 | 36.5 | 834 | 30 |
| Missing | 79 | 0.7 | 79 | 2.8 |
| Total | 11,001 | 2784 | ||
Spatial and temporal distributions
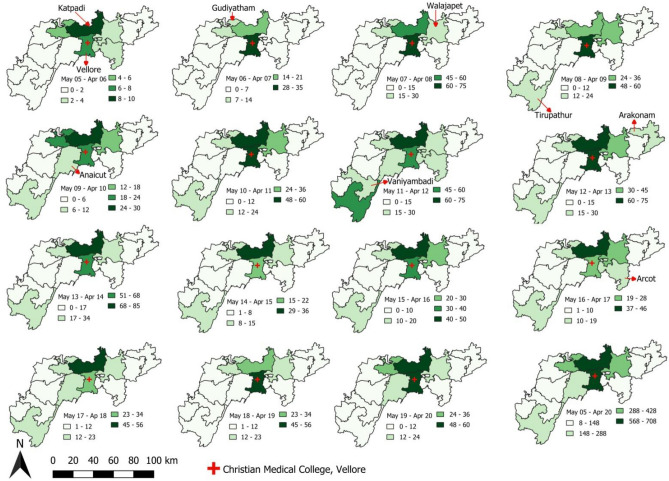
The average number of scrub typhus cases reported per year from the entire Vellore district is 186 with the majority of the cases from Katpadi and Vellore blocks with 45 and 44 cases per year respectively (refer Fig. 2). Except for the year May 05–April 06, Gudiyatham, Katpadi, Vellore and Walajapet blocks have contributed more than 10 scrub typhus cases in all the years.
Figure 2.
Spatiotemporal distribution of scrub typhus cases block wise in Vellore district from May 2005–April 2020.
The mean distance from the patient’s house to CMC hospital ranges from 18.1 km (SD: 19.8 km) in May 19–April 20 to 31.4 km (SD: 26.5 km) in May 11–April 12. The overall mean distance in all 15 years from the hospital to scrub typhus patients is 24.8 km (SD: 22.7 km) (refer Table S3).
Figure 3 (depicted above) shows the time series of scrub typhus positive and the rainfall received, average temperature and humidity of that corresponding month. Increase in cases has occurred only after 2 months of the increased rainfall and it is uniformly observed in all 15 years. The number of ST cases gradually increased with the decrease of the temperature (Max and min) and decreased as the temperature increased (Max and min). This pattern is observed in all 15 years. With relative humidity, the ST cases gradually increased as the maximum and minimum humidity increased and vice versa.
Figure 3.
Month-wise trend (time series) of ST positives vs rainfall, temperature and humidity from May 2005–April 2020.
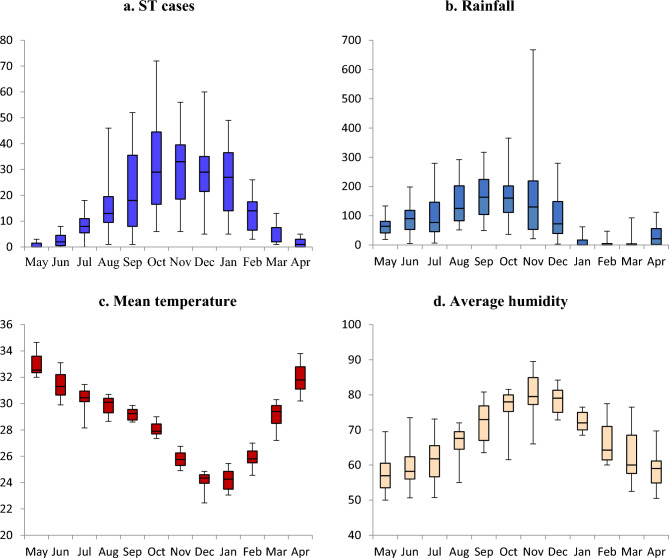
Every year, there is a gradual increase of ST cases from the month of August to February with cases peaking between September and January (refer Fig. 4a). This trend has been consistently observed for the past 15 years. Figure 4b–d demonstrates the consolidated month-wise distribution of rainfall, mean temperature and humidity observed during the 15 year period (2005–2020).
Figure 4.
Consolidated Month-wise distribution of ST cases, rainfall, mean temperature and average humidity distribution of all 15 years.
In Vellore, the average temperature during the cooler months (August to February) is 26.8 °C (Range 22.9–31.9 °C), the average humidity is 73.2% (Range 63.3–83.1%) with a median rainfall of 82.9 mm (IQR: 19.4 mm, 168.5 mm) during this period (Refer Tables S1 and S2). The mean temperature in October and November (when scrub typhus cases peak) is 26.9 °C (Range 22.6 °C–31.3 °C), the average humidity is 78.4% (Range 71.6–85.1%) with a median rainfall of 140.5 mm (IQR: 70.9 mm, 206.3 mm) (refer Tables S1 and S2).
The average temperature during the summer month (March–July) is 31.2 °C (Range 24.9–37.4 °C), the average humidity is 60.1% (Range 48.2–71.9%) with a median rainfall of 50.7 mm (IQR: 5.7 mm, 97.7 mm) (refer Tables S1 and S2).
For better understanding of the association between scrub typhus and climatic condition, Spearman’s correlation was done to find out the association between the time taken for a spurt in ST cases (lag time) and rainfall, temperature and humidity for up to a lag of 5 months (refer Table 2). As observed earlier in the month-wise trend for 15 years, rainfall had the maximum positive correlation of 0.751 (95% CI 0.678, 0.809) with a lag of two months and it is statistically significant (p < 0.001). This positive association, however, reduced in the third and fourth month and became insignificant at the lag time of five months.
Table 2.
Month wise correlation between Scrub typhus cases and climate variables.
| Lag months | Rainfall | Mean temperature | Average humidity | |||
|---|---|---|---|---|---|---|
| r | 95% CI | r | 95% CI | r | 95% CI | |
| L_0 | 0.186* | 0.047, 0.322 | − 0.698** | − 0.773, − 0.608 | 0.733** | 0.663, 0.790 |
| L_1 | 0.586** | 0.492, 0.656 | − 0.452** | − 0.555, − 0.326 | 0.700** | 0.626, 0.755 |
| L_2 | 0.751** | 0.678, 0.809 | − 0.037 | − 0.167, 0.100 | 0.424** | 0.306, 0.527 |
| L_3 | 0.628* | 0.522, 0.717 | 0.383** | 0.269, 0.499 | 0.031 | − 0.109, 0.164 |
| L_4 | 0.363** | 0.237, 0.492 | 0.656** | 0.557, 0.739 | − 0.343** | − 0.449, − 0.225 |
| L_5 | 0.051 | − 0.089, 0.193 | 0.732** | 0.651, 0.792 | − 0.595** | − 0.673, − 0.490 |
**Spearman’s Correlation is significant at the 0.01 level (2-tailed).
*Spearman’s Correlation is significant at the 0.05 level (2-tailed).
Mean temperature showed a negative correlation of − 0.698 initially and reduced in lag of months and became insignificant in three lag months. However, it showed a positive correlation from 4 lag months.
Average maximum humidity showed a positive correlation with no lag and it continued till 2 lag months. Three lag months showed an insignificant correlation with four and five lag months showing a negative correlation.
Table 3 describes the correlation coefficient matrix (Spearman) of the meteorological factors such as rainfall, mean temperature and mean relative humidity. Since the rainfall with 2 months lag has the highest correlation, we considered that for computing the Spearman correlation coefficient matrix and negative binomial regression. Whereas for the mean temperature and mean relative humidity, the same month reading with no lag was used. Mean temperature and mean relative humidity seem to be strongly correlated (− 0.739) with each other.
Table 3.
Spearman correlation coefficient (‘r’) matrix of meteorological variables.
| Rainfall_2monthslag (mm) | Mean temperature (°C) | Mean relative humidity (%) | |
|---|---|---|---|
| Rainfall_2monthslag (mm) | 1 | – | – |
| Mean temperature (°C) | − 0.612** | 1 | – |
| Mean relative humidity (%) | 0.618** | − 0.739** | 1 |
All the p values are significant at 0.001 level.
To address the multicollinearity issues between mean temperatures and mean relative humidity in regression analysis, two different models (A & B) were developed using negative binomial regression for exploring the relation between mean temperature and mean relative humidity among ST cases (refer Table 4). Model A was computed using the independent variables like rainfall_2monthslag and mean temperature. Model B with variables like rainfall_2months lag and mean humidity. For an increase of 1 °C in mean temperature, the monthly ST case reduces by 18.8% (95% CI − 24.1, − 13.1%). On the contrary, for 1 percent increase in mean relative humidity, there is an increase of 7.6% (95% CI 5.4, 9.9%) of monthly ST cases. Similarly, for an increase of 1 mm of rainfall contributes to increase of 0.5 to 0.7% of monthly ST cases after 2 months depending on the variables included in the analysis.
Table 4.
Negative binomial regression analysis of climatic factors associated with scrub typhus in Vellore from May 2005 to April 2020.
| Model | Variables | β | SE | p | Percent increase = (eβ − 1)*100 | 95% CI | |
|---|---|---|---|---|---|---|---|
| Lower | Higher | ||||||
| A | Intercept | 7.756 | 1.0205 | < 0.001 | |||
| Rainfall_2monthslag (mm) | 0.007 | 0.0012 | < 0.001 | 0.7 | 0.4 | 0.9 | |
| Mean temp (°C) | − 0.208 | 0.0343 | < 0.001 | − 18.8 | − 24.1 | − 13.2 | |
| B | Intercept | − 3.059 | 0.675 | < 0.001 | |||
| Rainfall_2monthslag (mm) | 0.005 | 0.0012 | < 0.001 | 0.5 | 0.3 | 0.7 | |
| Mean RH (%) | 0.073 | 0.0104 | < 0.001 | 7.6 | 5.4 | 9.9 | |
*Negative binomial regression model for monthly scrub typhus without mean relative humidity (A) and model with rainfall, mean humidity and without mean temperature (B). CI = confidence interval, S.E. = standard error.
Significant values are in bold.
Univariate and multivariate analysis
Table 5 describes the association of ST with the risk factors analyzed using chi-square and reported with 95% CI of Odds Ratio (OR). In univariate analysis, females, age group of > 20 years, farmers, daily wage workers and house wives had the higher odds to be positive for scrub typhus when compared to males, age group 0–20 years and other occupations. In-patients also had a higher risk of being ST positive when compared to patients tested as out-patient. However, in multivariate analysis, females, age group of 51–60 years and > 60 years, farmers, daily wage workers and house wives were at higher odds for being ST positive and the age group of 21–30 years showed a protective effect against ST when compared with 0–20 years of age.
Table 5.
Factors associated with scrub typhus.
| Variables | ST positive | ST negative | OR (95% CI) | p value | AOR (95% CI) | p value | |
|---|---|---|---|---|---|---|---|
| Gender | Female | 1601 (29.5%) | 3832 (70.5%) | 1.55 (1.42–1.69) | < 0.001 | 1.16 (1.05–1.30) | 0.006 |
| Male | 1183 (21.2%) | 4385 (78.8%) | |||||
| Age category (years) | 0–20 (Ref) | 322 (15.8%) | 1714 (84.2%) | Reference | |||
| 21–30 | 399 (18.6%) | 1745 (81.4%) | 1.22 (1.04–1.43) | 0.017 | 0.67 (0.55–0.80) | < 0.001 | |
| 31–40 | 445 (26.2%) | 1254 (73.8%) | 1.89 (1.61–2.22) | < 0.001 | 0.99 (0.82–1.20) | 0.911 | |
| 41–50 | 499 (30.4%) | 1144 (69.6%) | 2.32 (1.98–2.72) | < 0.001 | 1.13 (0.93–1.37) | 0.224 | |
| 51–60 | 510 (32.1%) | 1078 (67.9%) | 2.52 (2.15–2.95) | < 0.001 | 1.23 (1.02–1.50) | 0.034 | |
| > 60 | 609 (32.2%) | 1282 (67.8%) | 2.53 (2.17–2.95) | < 0.001 | 1.37 (1.13–1.65) | 0.001 | |
| Occupation | Farmers, daily wages and house wife | 1580 (33.8%) | 3100 (66.2%) | 2.74 (2.47–3.04) | < 0.001 | 2.43 (2.13–2.77) | < 0.001 |
| Others | 665 (15.7%) | 3577 (84.3%) | |||||
| OP/IP | In-patient | 1871 (27.1%) | 5031 (72.9%) | 1.42 (1.30–1.56) | < 0.001 | 1.11 (0.99–1.24) | 0.058 |
| Out-patient | 834 (20.7%) | 3186 (79.3%) | |||||
Discussion
In Vellore, scrub typhus is the commonest cause of Acute Undifferentiated Febrile Illness (AUFI), which is a group of acute fevers presenting without localizing signs28. Scrub typhus (ST) is a vector borne disease29, and its prevalence is related to various factors like vector abundance30, climatic factors31 and certain exposures like farming and owning domestic animals32, outdoor activities33 and sanitation34. In this study, we wanted to assess how meteorological factors like temperature, rainfall and humidity and socio-economic factors impact the prevalence of scrub typhus using a 15 year dataset from a tertiary care hospital. The literature suggests that climatic factors like rainfall, temperature and humidity seem to have a positive impact on the occurrence of Scrub Typhus27,35–37.
A clear seasonality of scrub typhus occurrence in Vellore district was noticed with maximum cases occurring from August to February with peak in October and November. The above finding reiterates the observation that scrub typhus cases are maximum in the cooler months of the year in Vellore as reported by Mathai et al. in 200322 and Abhilash et al. in 201628. The primary peak of ST was observed in Jiangxi Province, China in September and October, and a smaller peak from June to August38. Another study from Guangzhou, Southern China in 2021 reported high number of ST cases during the months of May to October with a peak in June39, from June to September in Vientiane, Laos40 and June to November in Taiwan35. A retrospective study from Japan reports that the major epidemic of ST was noticed in October to December in Southern part of Japan, and in May to June in Pacific coast of Northern Japan and Japan sea coast of Northern Japan41. In North-East India cases of scrub typhus occur in July to November in Manipur42, July to October in Sikkim43 and September to November in Darjeeling44.
Rainfall and Scrub Typhus had a positive correlation of 0.751 (p < 0.001) with a lag of 2 months. For an increase of 1 mm of rainfall, 0.5 to 0.7% of ST cases rise after 2 months in our study which is higher than the reported 0.05% to 0.10% ST cases with 1 mm rise in rainfall in Guangzhou, Southern China27. On the contrary, there was an increase of 5.2% of ST cases for every 1 mm rise in the rainfall in Taiwan35. A prospective study conducted in a hospital in Uttar Pradesh reported a positive association of scrub typhus and rainfall as the majority of the cases were observed in the post-monsoon season, but the rainfall associated increase is not mentioned45. The same trend was observed in the study done in Laos40 and in Vellore46 though the associated increase was not documented. The current study documents the associated increase of scrub typhus with variation in meteorological factors.
Mean temperature had a negative correlation with the ST cases in our study which is contradicted by the spatio-temporal analysis in Laos, where the maximum temperature had a positive impact on ST cases40. In our study, the maximum number of cases occurred in the months with a mean temperature is 26.8 °C (implying that lower temperature is more favorable for ST to occur in Vellore, as the summer mean temperature is 31 °C). This is in agreement with the findings reported from previous studies done in this region22,47. In contrast, a multi-centric study in Southern Taiwan reported a positive correlation of ST with increase in temperature. However, the maximum number of cases were reported in summer months where the average temperature ranged from 25 to 30 °C48. A spatial risk analysis done in Jiangsu Province, China reported a suitable temperature during March–November (Summer type) for the ST ranging from 31.7 to 32.8 °C49, which is slightly higher than what we have observed in this study. In spite of having a negative correlation of temperature in our study, the temperature which is favorable for ST is almost similar with the other studies done elsewhere in the tsutsugamushi triangle. In Southern India, scrub typhus occurs in cooler months22, whereas in China it occurs mainly in the summer months50. However, the suitable temperature for the prevalence of scrub typhus is almost similar in all the reported countries leaving the impression that the temperature is more important than comparing the seasons.
For an increase of 1 °C of temperature, the monthly ST case reduces by 18.8% (95% CI − 24.1, − 13.2%) in our study. At initial scrutiny it looks like contradicting to the reported increase of 14.9% of monthly ST cases in Guangzhou, Southern China from 2006 to 201227. A retrospective study done by analyzing the 15 years scrub typhus data in Korea reported that for an increase of 1 °C in temperature, there is an increase of 38 ST cases during the month of May or June36 and an increase of 3.3 cases for every 1 °C temperature increase in Taiwan35. This discrepancy of the result is because of the average temperature during the cooler month’s (August to February) ranges from 7 to 29 °C in Guangzhou, China (https://www.timeanddate.com/weather/@8513624/climate), − 1 to 26 °C in Jeollabuk-do, Korea (https://www.timeanddate.com/weather/@1845789/climate), and in Vellore it is 22.9–31.9 °C. This clearly demonstrates that extreme temperatures are not suitable for the occurrence of scrub typhus. The suitable temperature range for the trombiculid mites to lay eggs is 20 to 30 °C51 and therefore based on temperature, the data from tropical India and temperate East-Asian countries is in agreement.
Humidity, on the other hand had a positive influence on ST cases and the same was observed in Laos40, in Guangzhou, Southern China24,39 and in Thailand by Bhopdhornangkul et al.37. For 1 percent increase in mean relative humidity, there was an increase of 7.6% (95% CI 5.4, 9.9%) of monthly ST cases in our study. This is low when compared to the increase of monthly ST cases by 33% for an increase of 1% of maximum relative humidity in that particular month in Thailand37.
In this study, females were found to have a higher prevalence of scrub typhus (57.5%) than males and it is almost similar to the reported proportion of 58.7% in Vellore46 and 51.46% in Guangzhou, Southern China39. But a surveillance and case–control study done in Vietnam in 2021 reported less proportion of females being positive for scrub typhus (38.6%)52. In multivariate analysis, females had a higher chance of being positive for scrub typhus. This is in agreement with previous studies from Tamil Nadu53–55.
The prevalence of scrub typhus in this retrospective data set shows a steady upward trend from the third decade of life to a maximum prevalence of 21.1% among those older than 60 years. Similar age trend has been reported for scrub typhus in prospective sero-prevalence studies done in Northern Tamil Nadu53,54 and in Bhutan56. Same inferences have been obtained when retrospective data was analyzed in mainland China55, Taiwan35 and in Vientiane City, Lao57. Other studies in Jharkhand58 and North Bengal15 have reported scrub typhus to be common in 1 to 20 years but the number of cases observed were less than 100.
The majority of scrub typhus positives in our study came from the in-patient department, which in our opinion is due to a reference center bias, as more sick patients who need hospitalization are referred to a tertiary care facility28. The occupations at high risk in this study are farmers, daily wage laborers and house wives which is quite similar to the study from Guangzhou, Southern China39. Agricultural work has been identified as a risk factor for acquiring scrub typhus in India from Tamil Nadu34,37,43,53, Darjeeling44. Similar findings have been reported by researchers in Bhutan56, China55, Nepal59, Thailand60, South Korea61 and Japan62.
Conclusion
This study explains the influence of the climate on Scrub Typhus in Vellore, Tamil Nadu. Rainfall is found to be a major influencing factor in the occurrence of Scrub Typhus cases with a lag of 2 months in all fifteen years. Temperature had a negative correlation as the number of scrub typhus cases diagnosed increased in the cooler months and very minimal or nil cases were observed during the hot summer months of Vellore. Humidity on the other hand proved to be positively correlated with the Scrub Typhus and more number of cases was observed in months with mean relative humidity from 55 to 89%. This study suggests the healthcare professionals to be prepared for the early diagnosis and treatment of Scrub Typhus during the rainy season and cooler months with increased humidity. Scrub Typhus prevention measures like health education for high-risk populations such as farmers, farming-related occupations and housewives can be provided.
Supplementary Information
Author contributions
Study concept and design: K.G., and J.A.J.P. Acquisition of data: S.D., K.S., C.L., K.G. and J.A.J.P. Analysis and interpretation of data: S.D., K.S., C.L., K.G. and J.A.J.P. Drafting of the manuscript: S.D., K.S. and C.L. Critical revision of the manuscript: K.G. and J.A.J.P. Statistical analysis: S.D., and K.S. All authors have read the finished manuscript; approve and take primary responsibility for the final content.
Funding
Intramural Research Fund, Christian Medical College, Vellore.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-49333-5.
References
- 1.Xu G, Walker DH, Jupiter D, Melby PC, Arcari CM. A review of the global epidemiology of scrub typhus. PLoS Negl. Trop. Dis. 2017;11(11):e0006062. doi: 10.1371/journal.pntd.0006062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wongsantichon J, Jaiyen Y, Dittrich S, Salje J. Orientia tsutsugamushi . Trends Microbiol. 2020;28(9):780–781. doi: 10.1016/j.tim.2020.02.014. [DOI] [PubMed] [Google Scholar]
- 3.Izzard L, Fuller A, Blacksell SD, Paris DH, Richards AL, Aukkanit N, et al. Isolation of a novel Orientia species (O. chuto sp. nov.) from a patient infected in Dubai. J. Clin. Microbiol. 2010;48(12):4404–4409. doi: 10.1128/JCM.01526-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silva de la Fuente MC, Pérez C, Martínez-Valdebenito C, Pérez R, Vial C, Stekolnikov A, et al. Eco-epidemiology of rodent-associated trombiculid mites and infection with Orientia spp. in Southern Chile. PLoS Negl. Trop. Dis. 2023;17(1):e0011051. doi: 10.1371/journal.pntd.0011051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paris DH, Shelite TR, Day NP, Walker DH. Unresolved problems related to scrub typhus: A seriously neglected life-threatening disease. Am. J. Trop. Med. Hyg. 2013;89(2):301–307. doi: 10.4269/ajtmh.13-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lv Y, Guo X, Jin D, Song W, Peng P, Lin H, et al. Infestation and seasonal fluctuation of chigger mites on the Southeast Asian house rat (Rattus brunneusculus) in southern Yunnan Province, China. Int. J. Parasitol. Parasites Wildl. 2021;1(14):141–149. doi: 10.1016/j.ijppaw.2021.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Candasamy S, Ayyanar E, Devaraju P, Kumar A, Zaman K, Bhaskar Mishra B, et al. Evidence on the prevalence of emerging and re-emerging tick- and flea-borne rickettsial agents in acute encephalitis syndrome endemic areas of northeast Uttar Pradesh, India. Med. Vet. Entomol. 2023 doi: 10.1111/mve.12694. [DOI] [PubMed] [Google Scholar]
- 8.Chakraborty S, Sarma N. Scrub typhus: An emerging threat. Indian J. Dermatol. 2017;62(5):478–485. doi: 10.4103/ijd.IJD_388_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Devaraju P, Arumugam B, Mohan I, Paraman M, Ashokkumar M, Kasinathan G, et al. Evidence of natural infection of Orientia tsutsugamushi in vectors and animal hosts—Risk of scrub typhus transmission to humans in Puducherry, South India. Indian J. Public Health. 2020;64(1):27. doi: 10.4103/ijph.IJPH_130_19. [DOI] [PubMed] [Google Scholar]
- 10.Park J, Woo SH, Lee CS. Evolution of eschar in scrub typhus. Am. J. Trop. Med. Hyg. 2016;95(6):1223–1224. doi: 10.4269/ajtmh.16-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perumalla SK, Paul S, Abhilash KPP, Gunasekaran K, Rose W, Mahasampath G, et al. Eschar and IgM ELISA in the diagnosis of scrub typhus. Indian J. Med. Microbiol. 2019;37(1):113–115. doi: 10.4103/0255-0857.264495. [DOI] [PubMed] [Google Scholar]
- 12.Taylor AJ, Paris DH, Newton PN. A systematic review of mortality from untreated scrub typhus (Orientia tsutsugamushi) PLoS Negl. Trop. Dis. 2015;9(8):e0003971. doi: 10.1371/journal.pntd.0003971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lakshmi RMMVN, Dharma TV, Sudhaharan S, Surya SMV, Emmadi R, Yadati SR, et al. Prevalence of scrub typhus in a tertiary care centre in Telangana, South India. Iran J. Microbiol. 2020;12(3):204–208. [PMC free article] [PubMed] [Google Scholar]
- 14.Varghese GM, Abraham OC, Mathai D, Thomas K, Aaron R, Kavitha ML, et al. Scrub typhus among hospitalised patients with febrile illness in South India: Magnitude and clinical predictors. J. Infect. 2006;52(1):56–60. doi: 10.1016/j.jinf.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Islam A, Saha R, Roy A. Scrub typhus—A threatening scenario in North Bengal. J. Evid. Based Med. Healthc. 2021;8(39):3417–3422. doi: 10.18410/jebmh/2021/620. [DOI] [Google Scholar]
- 16.Premraj SS, Mayilananthi K, Krishnan D, Padmanabhan K, Rajasekaran D. Clinical profile and risk factors associated with severe scrub typhus infection among non-ICU patients in semi-urban south India. J. Vector Borne Dis. 2018;55(1):47–51. doi: 10.4103/0972-9062.234626. [DOI] [PubMed] [Google Scholar]
- 17.Griffith M, Peter JV, Karthik G, Ramakrishna K, Prakash JAJ, Kalki RC, et al. Profile of organ dysfunction and predictors of mortality in severe scrub typhus infection requiring intensive care admission. Indian J. Crit. Care Med. Peer-Rev. Off. Publ. Indian Soc. Crit. Care Med. 2014;18(8):497–502. doi: 10.4103/0972-5229.138145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh T, Kumar HM. Scrub Eschar. IDCases. 2021;27:24. doi: 10.1016/j.idcr.2021.e01086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koh GCKW, Maude RJ, Paris DH, Newton PN, Blacksell SD. Diagnosis of scrub typhus. Am. J. Trop. Med. Hyg. 2010;82(3):368–370. doi: 10.4269/ajtmh.2010.09-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh, O. B. & Panda, P. K. Scrub typhus. In StatPearls. (StatPearls Publishing, 2022) (Accessed 6 Jan 2023) https://www.ncbi.nlm.nih.gov/books/NBK558901/. [PubMed]
- 21.Varghese GM, Dayanand D, Gunasekaran K, Kundu D, Wyawahare M, Sharma N, et al. Intravenous doxycycline, azithromycin, or both for severe scrub typhus. N. Engl. J. Med. 2023;388(9):792–803. doi: 10.1056/NEJMoa2208449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mathai E, Rolain JM, Verghese GM, Abraham OC, Mathai D, Mathai M, et al. Outbreak of scrub typhus in southern India during the cooler months. Ann. N. Y. Acad. Sci. 2003;990(1):359–364. doi: 10.1111/j.1749-6632.2003.tb07391.x. [DOI] [PubMed] [Google Scholar]
- 23.Frances SP, Watcharapichat P, Phulsuksombati D, Tanskul P, Linthicum KJ. Seasonal occurrence of Leptotrombidium deliense (Acari: Trombiculidae) attached to sentinel rodents in an orchard near Bangkok, Thailand. J. Med. Entomol. 1999;36(6):869–874. doi: 10.1093/jmedent/36.6.869. [DOI] [PubMed] [Google Scholar]
- 24.Wei Y, Huang Y, Li X, Ma Y, Tao X, Wu X, et al. Climate variability, animal reservoir and transmission of scrub typhus in Southern China. PLoS Negl. Trop. Dis. 2017;11(3):e0005447. doi: 10.1371/journal.pntd.0005447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DH_2011_3304_PART_B_DCHB_VELLORE.pdf.
- 26.agriculture.pdf. (Accessed 6 Jan 2023) https://www.tn.gov.in/deptst/agriculture.pdf.
- 27.Li T, Yang Z, Dong Z, Wang M. Meteorological factors and risk of scrub typhus in Guangzhou, southern China, 2006–2012. BMC Infect. Dis. 2014;14(1):139. doi: 10.1186/1471-2334-14-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abhilash KP, et al. Acute undifferentiated febrile illness in patients presenting to a Tertiary Care Hospital in South India: Clinical spectrum and outcome. J. Glob. Infect. Dis. 2016;8(4):147. doi: 10.4103/0974-777X.192966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Snowden, J., Ladd, M. & King, K. C. Rickettsial infection. In StatPearls. (StatPearls Publishing, 2022) (Accessed 6 Jan 2023) https://www.ncbi.nlm.nih.gov/books/NBK431127/. [PubMed]
- 30.Lee IY, Kim HC, Lee YS, Seo JH, Lim JW, Yong TS, et al. Geographical distribution and relative abundance of vectors of scrub typhus in the Republic of Korea. Korean J. Parasitol. 2009;47(4):381–386. doi: 10.3347/kjp.2009.47.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olson JG. Forecasting the onset of a scrub typhus epidemic in the Pescadores Islands of Taiwan using daily maximum temperatures. Trop. Geogr. Med. 1979;31(4):519–524. [PubMed] [Google Scholar]
- 32.Ma CJ, Oh GJ, Kang GU, Lee JM, Lee DU, Nam HS, et al. Differences in agricultural activities related to incidence of scrub typhus between Korea and Japan. Epidemiol. Health. 2017;9(39):e2017051. doi: 10.4178/epih.e2017051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh SI, Devi KP, Tilotama R, Ningombam S, Gopalkrishna Y, Singh TB, et al. An outbreak of scrub typhus in Bishnupur district of Manipur, India, 2007. Trop. Doct. 2010;40(3):169–170. doi: 10.1258/td.2010.090468. [DOI] [PubMed] [Google Scholar]
- 34.Park JH, Gill B, Acharya D, Yoo SJ, Lee K, Lee J. Seroprevalence and factors associated with scrub typhus infection among forestry workers in National Park Offices in South Korea. Int. J. Environ. Res. Public Health. 2021;18(6):3131. doi: 10.3390/ijerph18063131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin FH, Chou YC, Chien WC, Chung CH, Hsieh CJ, Yu CP. Epidemiology and risk factors for notifiable scrub typhus in Taiwan during the period 2010–2019. Healthcare. 2021;9(12):1619. doi: 10.3390/healthcare9121619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang GU, Ma CJ, Oh GJ. Association between scrub typhus outbreaks and meteorological factors in Jeollabuk-do Province. J. Environ. Health Sci. 2016;42(1):41–52. doi: 10.5668/JEHS.2016.42.1.41. [DOI] [Google Scholar]
- 37.Bhopdhornangkul B, Meeyai AC, Wongwit W, Limpanont Y, Iamsirithaworn S, Laosiritaworn Y, et al. Non-linear effect of different humidity types on scrub typhus occurrence in endemic provinces, Thailand. Heliyon. 2021;7(2):e06095. doi: 10.1016/j.heliyon.2021.e06095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang S, Liu X, Gao Y, Chen B, Lu L, Zheng W, et al. Spatiotemporal dynamics of scrub typhus in Jiangxi Province, China, from 2006 to 2018. Int. J. Environ. Res. Public Health. 2021;18(9):4599. doi: 10.3390/ijerph18094599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu J, Liu Y, Ma X, Li M, Yang Z. Impact of meteorological factors and southern oscillation index on scrub typhus incidence in Guangzhou Southern China, 2006–2018. Front. Med. 2021 doi: 10.3389/fmed.2021.667549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roberts T, Parker DM, Bulterys PL, Rattanavong S, Elliott I, Phommasone K, et al. A spatio-temporal analysis of scrub typhus and murine typhus in Laos; implications from changing landscapes and climate. PLoS Negl. Trop. Dis. 2021;15(8):e0009685. doi: 10.1371/journal.pntd.0009685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoshikura H. Seasonality and geographical distribution of tsutsugamushi diseases in Japan: Analysis of the trends since 1955 till 2014. Jpn. J. Infect. Dis. 2018;71(1):1–7. doi: 10.7883/yoken.JJID.2017.073. [DOI] [PubMed] [Google Scholar]
- 42.Ks, N., Hussain, S., Kakati, S., Singh, M. U. & Kaguilan, K. Outbreak of Scrub Typhus in Manipur—Experience at a Tertiary Care Hospital.
- 43.Gurung S, Pradhan J, Bhutia PY. Outbreak of scrub typhus in the North East Himalayan region-Sikkim: An emerging threat. Indian J. Med. Microbiol. 2013;31(1):72–74. doi: 10.4103/0255-0857.108729. [DOI] [PubMed] [Google Scholar]
- 44.Sharma PK, Ramakrishnan R, Hutin YJF, Barui AK, Manickam P, Kakkar M, et al. Scrub typhus in Darjeeling, India: Opportunities for simple, practical prevention measures. Trans. R. Soc. Trop. Med. Hyg. 2009;103(11):1153–1158. doi: 10.1016/j.trstmh.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 45.Mittal V, Singh P, Shukla S, Karoli R. Scrub typhus: An under-reported and emerging threat—hospital based study from central and eastern Uttar Pradesh, India. J. Vector Borne Dis. 2021;58(4):323–328. doi: 10.4103/0972-9062.318311. [DOI] [PubMed] [Google Scholar]
- 46.George T, Rajan SJ, Peter JV, Hansdak SG, Prakash JAJ, Iyyadurai R, et al. Risk factors for acquiring scrub typhus among the adults. J. Glob. Infect. Dis. 2018;10(3):147–151. doi: 10.4103/jgid.jgid_63_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stephen S, Sangeetha B, Ambroise S, Sarangapani K, Gunasekaran D, Hanifah M, et al. Outbreak of scrub typhus in Puducherry & Tamil Nadu during cooler months. Indian J. Med. Res. 2015;142(5):591–597. doi: 10.4103/0971-5916.171289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chang YC, Kuo KC, Sun W, Lin JN, Lai CH, Lee CH. Clinicoepidemiologic characteristics of scrub typhus and murine typhus: A multi-center study in southern Taiwan. J. Microbiol. Immunol. Infect. Wei Mian Yu Gan Ran Za Zhi. 2019;52(5):769–778. doi: 10.1016/j.jmii.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 49.Yu H, Sun C, Liu W, Li Z, Tan Z, Wang X, et al. Scrub typhus in Jiangsu Province, China: Epidemiologic features and spatial risk analysis. BMC Infect. Dis. 2018;6(18):372. doi: 10.1186/s12879-018-3271-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Z, Xin H, Sun J, Lai S, Zeng L, Zheng C, et al. Epidemiologic changes of scrub typhus in China, 1952–2016. Emerg. Infect. Dis. 2020;26(6):1091–1101. doi: 10.3201/eid2606.191168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takahashi M, Machida K, Murata M, Misumi H, Hori E, Kawamura A, Jr, et al. Seasonal development of Leptotrombidium pallidum (Acari: Trombiculidae) observed by experimental rearing in the natural environment. J. Med. Entomol. 1993;30(2):320–325. doi: 10.1093/jmedent/30.2.320. [DOI] [PubMed] [Google Scholar]
- 52.Tran HTD, Hattendorf J, Do HM, Hoang TT, Hoang HTH, Lam HN, et al. Ecological and behavioural risk factors of scrub typhus in central Vietnam: A case-control study. Infect. Dis. Poverty. 2021;10(1):110. doi: 10.1186/s40249-021-00893-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.D’Cruz S, Perumalla SK, Yuvaraj J, Prakash JAJ. Geography and prevalence of rickettsial infections in Northern Tamil Nadu, India: A cross-sectional study. Sci. Rep. 2022;12(1):20798. doi: 10.1038/s41598-022-21191-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Devamani CS, Schmidt WP, Ariyoshi K, Anitha A, Kalaimani S, Prakash JAJ. Risk factors for scrub typhus, murine typhus, and spotted fever seropositivity in urban areas, rural plains, and peri-forest hill villages in South India: A cross-sectional study. Am. J. Trop. Med. Hyg. 2020;103(1):238–248. doi: 10.4269/ajtmh.19-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang WY, Wang LY, Ding F, Hu WB, Magalhaes RJS, Sun HL, et al. Scrub typhus in Mainland China, 2006–2012: The need for targeted public health interventions. PLoS Negl. Trop. Dis. 2013;7(12):e2493. doi: 10.1371/journal.pntd.0002493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tshokey T, Stenos J, Durrheim DN, Eastwood K, Nguyen C, Graves SR. Seroprevalence of rickettsial infections and Q fever in Bhutan. PLoS Negl. Trop. Dis. 2017;11(11):e0006107. doi: 10.1371/journal.pntd.0006107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vallée J, Thaojaikong T, Moore CE, Phetsouvanh R, Richards AL, Souris M, et al. Contrasting spatial distribution and risk factors for past infection with scrub typhus and murine typhus in Vientiane City, Lao PDR. PLoS Negl. Trop. Dis. 2010;4(12):e909. doi: 10.1371/journal.pntd.0000909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kamath SD, Kumari S, Sunder A. A study of the profile of scrub typhus in a Tertiary Care Hospital in Jharkhand: An underestimated problem. Cureus. 2022 doi: 10.7759/cureus.26503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thapa S, Hamal P, Chaudhary NK, Sapkota LB, Singh JP. Burden of scrub typhus among patients with acute febrile illness attending tertiary care hospital in Chitwan, Nepal. BMJ Open. 2020;10(9):e034727. doi: 10.1136/bmjopen-2019-034727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chaisiri K, Tanganuchitcharnchai A, Kritiyakan A, Thinphovong C, Tanita M, Morand S, et al. Risk factors analysis for neglected human rickettsioses in rural communities in Nan province, Thailand: A community-based observational study along a landscape gradient. PLoS Negl. Trop. Dis. 2022;16(3):e0010256. doi: 10.1371/journal.pntd.0010256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee HW, Cho PY, Moon SU, Na BK, Kang YJ, Sohn Y, et al. Current situation of scrub typhus in South Korea from 2001–2013. Parasit. Vectors. 2015;8(1):238. doi: 10.1186/s13071-015-0858-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ogawa M, Hagiwara T, Kishimoto T, Shiga S, Yoshida Y, Furuya Y, et al. Scrub typhus in Japan: Epidemiology and clinical features of cases reported in 1998. Am. J. Trop. Med. Hyg. 2002;67(2):162–165. doi: 10.4269/ajtmh.2002.67.162. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.