Abstract
Cytokines are hormone-like proteins which mediate and regulate inflammatory and immune responses. The purpose of this study was to investigate the effect of lipopolysaccharide (LPS) and inflammatory cytokines on regulation of interleukin-6 (IL-6) production by human gingival fibroblasts (HGF). The HGF cell lines used in this study, H-CL and F-CL, were established by the explant technique from healthy gingival tissue. Cultured cells were grown to confluency and incubated with various concentrations of LPS from Escherichia coli or Porphyromonas gingivalis or with the recombinant human cytokine tumor necrosis factor alpha (TNF-α), IL-1α, or IL-1β. Culture supernatants were collected at various times and assessed for IL-6 production by enzyme-linked immunosorbent assay. Total RNA was isolated from the harvested cells and used to assess levels of IL-6 mRNA by the RNase protection assay. Both LPS preparations induced IL-6 production (1 to 4 ng of IL-6 per ml) by both HGF cell lines. Although TNF-α stimulated IL-6 production by HGF, >10-fold-larger amounts were induced with IL-1α and IL-1β. Furthermore, the addition of both IL-1α and TNF-α to cultured cells resulted in approximately 600- to 800-fold-higher levels of IL-6 than seen in control cultures, suggesting that these cytokines synergistically induced IL-6 production by HGF. IL-6 message in cultured cells was upregulated 20-fold by TNF-α, 1,000-fold by IL-1α and IL-1β, and 1,400-fold by IL-1α plus TNF-α. IL-1α and TNF-α alone upregulate IL-6 production in a dose- and time-dependent fashion. The addition of IL-1α and TNF-α to cultured HGF cells resulted in a synergistic induction of IL-6 after 8 h of incubation and when greater than 10 pg of this combination per ml was used. Our studies show that inflammatory cytokines are hundreds of times more potent than LPS in stimulating IL-6 production by HGF.
Both microbial factors and the host immune system have been implicated in the etiology of the chronic oral inflammatory disease periodontitis. The lipopolysaccharide (LPS) from periodontal pathogens has been shown to stimulate host cells, including macrophages and fibroblasts, to produce cytokines. These cytokines can in turn act directly or indirectly on the immune response via activation of host cells involved in inflammatory processes. The inflammatory cytokine interleukin-6 (IL-6) is an endogenous pyrogen and has been shown to regulate T- and B-cell functions and to induce acute-phase response proteins and maturation of megakaryocytes (25). A combination of IL-6 and soluble IL-6 receptor has been found to induce osteoclast formation (17), which could be important in the destruction of the periodontium. IL-6 is produced by numerous cell types, including macrophages, monocytes, fibroblasts, endothelial cells, and smooth muscle cells. In vivo studies have shown low levels of constitutive IL-6 production by fibroblasts of different origins, including dermal (5), liver (myo) (24), duodenal (22), renal (15), foreskin (14), human periodontal ligament (20, 27), oral fibrotic submucosal and buccal mucosal (3), and normal human gingival (3, 11, 12, 19) origins.
Human gingival fibroblasts (HGF) are the predominant cell in periodontal tissue and are responsible for the synthesis and degradation of connective tissue. These cells can be stimulated to produce cytokines and factors which mediate inflammation (21). For example, IL-6 production and IL-6 mRNA expression by fibroblasts can be stimulated by microbial factors such as LPS as well as by cytokines such as IL-1 and tumor necrosis factor alpha (TNF-α) (1, 15, 20, 22, 27). The combined effect of IL-1 and TNF-α on IL-6 production by HGF cells has not been investigated. Furthermore, the difference in the abilities of microbial LPS and host cytokines to stimulate IL-6 production by HGF have not been directly compared. Therefore, the purpose of the present study was to determine if there were differences in the abilities of LPS and the host cytokines IL-1α and TNF-α to stimulate IL-6 production by HGF. The kinetics of the effect of each stimulant on IL-6 production by HGF cell lines was determined. Our findings show that the induction of IL-6 production by each stimulant was dose and time dependent. In addition, IL-1α plus TNF-α synergistically upregulates IL-6 mRNA and protein production. The order of the abilities of the stimulants to elevate IL-6 message and protein levels produced by healthy HGF was IL-1 plus TNF-α > IL-1 > TNF > LPS. Our results show that although LPS induced IL-6 production, cytokine stimulation of the IL-6 response by HGF was much greater. This finding suggests that inflammatory cytokines may be more effective factors than LPS in the HGF response associated with periodontal disease.
(This work was done by Leigh W. Kent in partial fulfillment of the requirements for a Ph.D. from The University of Alabama at Birmingham.)
MATERIALS AND METHODS
Cytokines and LPS.
Recombinant human IL-1α (rhIL-1α), rhIL-1β, rhIL-6, and rhTNF-α were all purchased from R&D Systems, Inc. (Minneapolis, Minn.). Escherichia coli K235 LPS was prepared by phenol-water extraction as previously described (16). Porphyromonas gingivalis ATCC 33277 LPS was obtained by hot-phenol extraction (18). Protein was not detected in the LPS preparations by silver staining of samples electrophoretically resolved in polyacrylamide gels, electroblotted onto a nitrocellulose membrane, and stained with a solution of colloidal gold (Enhanced Colloidal Gold Total Protein Detection Kit; Bio-Rad Laboratories, Hercules, Calif.) or by spectrophotometric methods. Prior to use, LPS diluted in pyrogen-free distilled H2O (1 mg/ml) was boiled for 30 min.
Fibroblast cell lines.
HGF cell lines were established from explant donors undergoing gingival surgery. Clinically, the tissue appeared firm, nonerythematous, nonedematous, and nonbleeding. The method used for culture of gingival fibroblasts has been reported previously (11, 13). Briefly, divided pieces of biopsied tissue were allowed to attach to the walls of plastic culture flasks (Falcon, Oxnard, Calif.) containing Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Grand Island, N.Y.) supplemented with sodium pyruvate (0.1 g/liter), l-glutamine (1.16 g/liter), streptomycin sulfate (100 mg/liter), penicillin (100,000 U/liter), and 10% fetal calf serum (FCS) (complete DMEM). The medium was tested for endotoxin activity with the Limulus amebocyte lysate assay as described by the manufacturer (BioWhittaker, Walkersville, Md.), except half the volume of assay reagents was used.
The spent medium was removed from the primary cultures for subculturing. Fibroblasts were dissociated from the outgrowth by trypsinization and harvested by centrifugation. For each passage, the cells were resuspended in DMEM containing 10% FCS and seeded into plastic dishes at a ratio of 1:4. Stocks of each passage were stored frozen at −70°C. Cells from passage 4 (P4) or P5 were used in the present investigation based on previous studies which showed that increasing passages of the cell line F-CL resulted in decreased production of IL-6 (11).
Culture conditions.
HGF H-CL (P5) or HGF F-CL (P4) cells were grown to confluency in T25 flasks, the supernatants were aspirated, and the cells were washed twice with 5 ml of complete DMEM without FCS. Stimulants diluted in complete DMEM without FCS were added to the flasks, and the cultures were incubated for various periods of time. After incubation, the supernatants were removed, aliquoted, and stored frozen (−20°C) until analyzed for IL-6 levels. The adherent cells in the flasks were washed twice with sterile phosphate-buffered saline (PBS) in diethyl pyrocarbonate-treated water and frozen (−70°C) until RNA was isolated for analysis of IL-6 mRNA.
ELISA.
An enzyme-linked immunosorbent assay (ELISA) was used for the quantification of IL-6 as previously described (11). Two monoclonal antibodies with specificity to different epitopes on each cytokine molecule of interest (Syntex Laboratory, Palo Alto, Calif.) were used. The immunoassay has been shown to be specific (10). The rhIL-6 standard used in the assays was purchased from R&D Systems, Inc. Briefly, 96-well plates were coated with the first anti-IL-6 antibody, 7IL-6-H12A, diluted in PBS. Plates were incubated overnight at 4°C and then were washed twice. Nonspecific binding sites were blocked by incubating the plates with 5% nonfat dry milk and 0.05% thimerosal in PBS (200 μl/well) for 1.5 h at room temperature. The plates were washed three times with ELISA wash buffer, and then twofold serial dilutions of the rhIL-6 standard or sample (100 μl) were added to wells in duplicate. Plates were incubated for 2 h at room temperature and washed, and then biotinylated monoclonal antibody 5IL6-H12 (50 μl) was added. Following a 2-h incubation at room temperature, the plates were washed, and 100 μl of peroxidase-streptavidin (Zymed Laboratories, Inc., San Francisco, Calif. diluted 1:3,000 in bovine serum albumin buffer (1% bovine serum albumin, 0.05% thimerosal, PBS) was added. Plates were incubated for 1 h at room temperature and were washed four times with ELISA wash buffer. The color which developed following the addition of the substrate (100 μl/well) o-phenylenediamine, hydrogen peroxide, and citrate buffer (pH 4.9) was recorded with a Vmax microplate reader (Molecular Devices Corp., Menlo Park, Calif.) at dual wavelengths: 450 nm (sample filter)/630 nm (reference filter). The amount of cytokine in each sample, run simultaneously with the standard, was determined by interpolation from the standard curve with a four-parameter logistic algorithm (Softmax; Molecular Devices Corp.).
RNA isolation.
Fibroblast cultures grown to confluency in T25 flasks from P5 were used for detection of IL-6 mRNA. Total RNA was isolated from fibroblast cultures with the Ultraspec RNA Isolation System (Biotecx Laboratories, Houston, Tex.) according to the manufacturer’s instructions. Briefly, the cells were lysed in the T25 flasks with Ultraspec RNA and transferred to a 1.5-ml Eppendorf tube. The RNA was extracted by chloroform treatment and centrifugation. Following extraction, RNA was precipitated from the aqueous phase with isopropanol. The precipitate was washed with 75% ethanol and vacuum dried in a Speed Vac, and the pellet was dissolved in diethyl pyrocarbonate-treated distilled water.
RPA.
cDNA probes specific for hIL-6 (inserted in a pGEM-4z vector) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (inserted in a pAMP1 vector) were the generous gifts of E. N. Benveniste (Birmingham, Ala.). The hIL-6-pGEM-4z was linearized with EcoRI, and the GAPDH-pAMP1 was linearized with NcoI. Antisense probes, 408 and 230 nucleotides, respectively, labeled with deoxy-[α-32P]CTP were generated by using a Riboprobe II Core System T7 RNA Polymerase kit (Promega, Madison, Wis.) according to the manufacturer’s instructions. The RNase protection assay (RPA) was performed on 10 μg of total RNA with an RPA II kit (Ambion, Austin, Tex.) according to the manufacturer’s instructions as previously described (4). Briefly, 10 μg of total RNA from HGF cell line H-CL (P5) were hybridized with the labeled antisense probes for hIL-6 and β-actin followed by digestion with RNase A/T1. The protected sample fragments were then run on a 5% denaturing (8 N urea) polyacrylamide gel and exposed to X-ray film. The gels were then scanned with a PhosphorImager (Molecular Dynamics, Sunnyvale, Calif.) to quantitate the intensities of the protected RNA fragments. The values for IL-6 were normalized to that for GAPDH, and this ratio was demonstrated graphically.
Quantitative detection of mRNA by competitive RT-PCR.
HGF cytokine mRNA was detected by using a modification of the competitive reverse transcription-PCR (RT-PCR) method previously described (8). Briefly, a general cloning vector, pQPCR1, a modified pBluescript (Statagene), was used to generate the RNA competitor (pQPCR.HMPC1.2). This RNA competitor was constructed to contain the 3′ and 5′ cytokine primers of interest separated by an identifying stuffer region and flanked by a T3 RNA polymerase initiation site, spacer, and poly(A)+ tail. In vitro transcription with the T3 polymerase generated RNA competitors which were quantitated by trace labeling techniques. Different known quantities of RNA competitor contained a unique identifying stuffer, and these were added to the fibroblast guanidine lysates in the same tube. After RNA isolation, the cDNA was reverse transcribed and then amplified by PCR with the desired 3′ and 5′ primers. Therefore, the sample cytokine cDNA (analyte) and the competitor cytokine containing the stuffer insert were amplified. Biotinylation of the 3′ primer yielded PCR products which were captured on avidin-coated enzyme immunoassay microtiter plates and detected by using a digoxigenin-labeled oligonucleotide probe specific for the analyte cytokine or the competitor stuffer insert. The oligonucleotide probe was then detected with an alkaline phosphatase-conjugated antidigoxigenin antibody (Boerhinger Mannheim, Indianapolis, Ind.) and developed with p-nitrophenol phosphate (Sigma, St. Louis, Mo.). The ratio of the optical density (OD) of the competitor to the OD of the analyte was plotted against the known competitor mRNA copy number in a log-log plot, and the number of copies of unknown cytokine mRNA was determined at a 1:1 OD (405 nm) ratio to the competitor RNA. A competitor RNA (pQPCR.GF01.2) for the GAPDH housekeeping gene (9) was used to normalize the results between samples.
RESULTS
LPS induction of IL-6 in the HGF cell line H-CL.
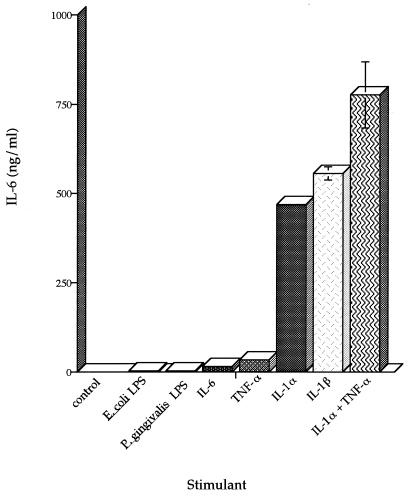
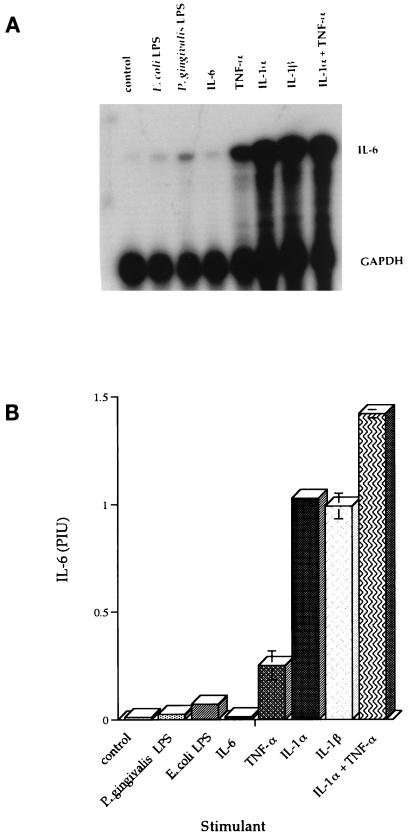
The amount of IL-6 produced by the healthy HGF cell line H-CL (P5) after stimulation with E. coli or P. gingivalis LPS was determined by ELISA. HGF H-CL (P5) fibroblasts were grown to confluency, the cells were washed twice with complete DMEM without FCS, and E. coli or P. gingivalis LPS (1 μg/ml) was added to the cells. Supernatants from cultures incubated with E. coli or P. gingivalis LPS for 24 h contained 3.13 (standard deviation [SD] = 0.859) or 3.04 (SD = 0.133) ng of IL-6 protein per ml, respectively (Fig. 1). These results are in agreement with those of others who showed that P. gingivalis and E. coli LPS stimulated approximately 3 ng of IL-6 secretion per ml from periodontal ligament fibroblasts (27). An increase was also seen in the amount of IL-6 mRNA in cells stimulated with LPS from P. gingivalis (0.026 ± 0.002 PhosphorImager units [PIU]) or from E. coli (0.072 ± 0.013 PIU) compared to nonstimulated control cells (Fig. 2).
FIG. 1.
IL-6 levels in supernatants of stimulated H-CL (P5) fibroblast cultures. E. coli LPS (1 μg/ml), P. gingivalis LPS (1 μg/ml), rhIL-6 (5 ng/ml), rhTNF-α (5 ng/ml), rhIL-1α (100 pg/ml), rhIL-1β (5 ng/ml), or IL-1α (100 pg/ml) plus TNF-α (5 ng/ml) was added to confluent HGF H-CL cultures and incubated for 24 h, and supernatants were assayed for IL-6 levels by ELISA. Bars indicate SDs.
FIG. 2.
IL-6 mRNA levels in stimulated H-CL (P5) fibroblasts after stimulation as determined by RPA. P. gingivalis LPS (1 μg/ml), E. coli LPS (1 μg/ml), rhIL-6 (5 ng/ml), rhTNF-α (5 ng/ml), rhIL-1α (100 pg/ml), rhIL-1β (5 ng/ml), or IL-1α (100 pg/ml) plus TNF-α (5 ng/ml) was added to confluent HGF H-CL cultures and incubated for 24 h. (A) Total RNA was used to assess levels of IL-6 mRNA by RPA. (B) The RPA value was calculated by dividing the PhosphorImager value for IL-6 by the sample’s PhosphoImager value for GAPDH expressed as PIU. Bars indicate SDs.
Cytokine induction of IL-6 in the HGF cell line H-CL.
The amount of IL-6 protein produced by the healthy HGF cell line H-CL (P5) was also determined after stimulation with the inflammatory cytokines rhIL-1β (5 ng/ml), rhIL-6 (5 ng/ml), rhTNF-α (5 ng/ml), rhIL-1α (100 pg/ml), and rhIL-1α (100 pg/ml) plus rhTNF-α (5 ng/ml). Supernatants from cultures incubated with rhTNF-α for 24 h contained 33 ng of IL-6 protein per ml, while 450 to 560 ng of IL-6 per ml was present in supernatants of cultures incubated with rhIL-1α or rhIL-1β (Fig. 1). When rhIL-1α and rhTNF-α were both added to fibroblast cultures and left for 24 h, 775 ng of IL-6 per ml was detected in the supernatants. The response was synergistic, considering the level of IL-6 induced with each cytokine alone. In order to establish that the IL-1α and TNF-α induced the fibroblasts to produce IL-6 message in addition to IL-6 protein, total RNA was isolated from the cells and used to assess levels of IL-6 mRNA by RPA (Fig. 2A). The RPA value was calculated by dividing the PhosphorImager value for IL-6 by the sample’s PhosphorImager value for GADPH expressed as PIU. The IL-6 message was upregulated 20-fold by TNF-α (0.251 ± 0.067 PIU), 1,000-fold by IL-1α and IL-1β (1.027 and 0.992 PIU, respectively), and 1,400-fold by IL-1α plus TNF-α (1.420 ± 0.019 PIU) (Fig. 2B). Thus, costimulation with IL-1 plus TNF-α upregulated IL-6 protein and message in a synergistic manner.
Dose- and time-dependent induction of IL-6.
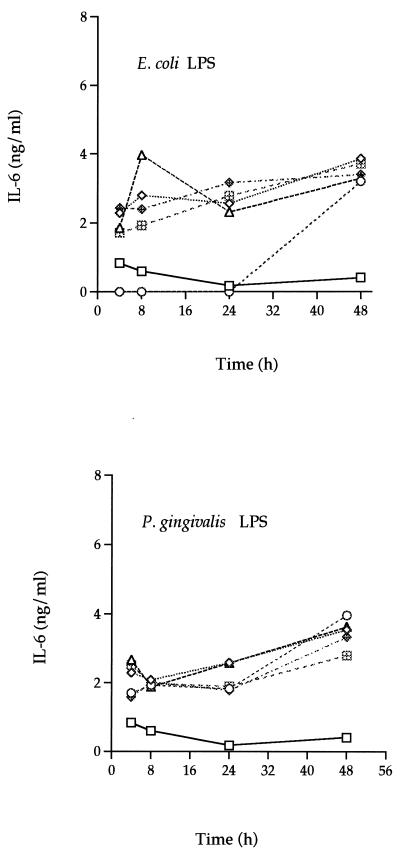
In order to determine the kinetics of IL-6 induction, the healthy HGF cell line F-CL (P4) was grown to confluency, and then various amounts of E. coli or P. gingivalis LPS (10 pg to 10 μg/ml) or of TNF-α or IL-1α (10 pg to 10 ng/ml) were added. At various times (1 to 48 h), culture supernatants were collected and assessed for IL-6 levels by ELISA. The F-CL cells, like the H-CL fibroblasts, produced 1 to 4 ng of IL-6 per ml after incubation with LPS (Fig. 3). A slight increase in IL-6 levels was seen over time after stimulation with either LPS; however, the response to LPS was not dose dependent.
FIG. 3.
Dose-response and time course analysis of IL-6 secretion by E. coli LPS- or P. gingivalis LPS-stimulated F-CL (P4) fibroblasts. Various amounts (10 pg/ml [◊] 1 ng/ml [○], 100 ng/ml [▵], 1 μg/ml [⊞], and 10 μg/ml [◊+]) of E. coli or P. gingivalis LPS were added to confluent cultures of F-CL fibroblasts and incubated for 4, 8, 24, or 48 h. IL-6 protein in the culture supernatant was determined by ELISA. The values are the means of duplicate determinations. The variation associated with each point is within 1 SD of the mean. □, control.
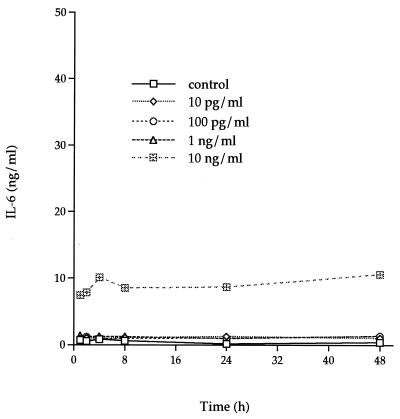
Low concentrations of TNF-α (10 to 100 pg/ml) induced less than a 10-fold increase of IL-6 production throughout a 48-h incubation, whereas 1 ng of TNF-α per ml induced a 20-fold increase (Fig. 4). Supernatants from cultures incubated with 10 ng of TNF-α per ml showed the greatest increase in IL-6 production, starting from a constitutive level of 0.5 ng/ml (with no stimulant) to 30.15 ng/ml (a 60-fold increase) after a 48-h incubation.
FIG. 4.
Dose-response and time course analysis of IL-6 secretion by rhTNF-α-stimulated F-CL fibroblasts. Various amounts of rhTNF-α were added to confluent cultures of F-CL fibroblasts and incubated for 1, 2, 4, 8, 24, or 48 h. IL-6 protein in the culture supernatant was determined by ELISA. The values are the means of duplicate determinations. The variation associated with each point is within 1 SD of the mean.
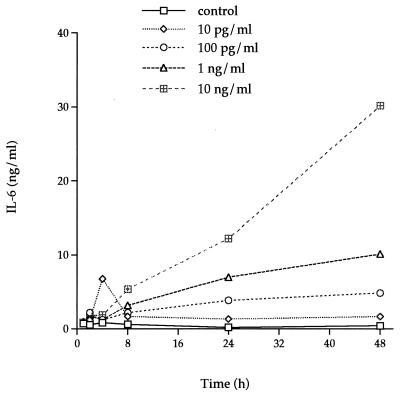
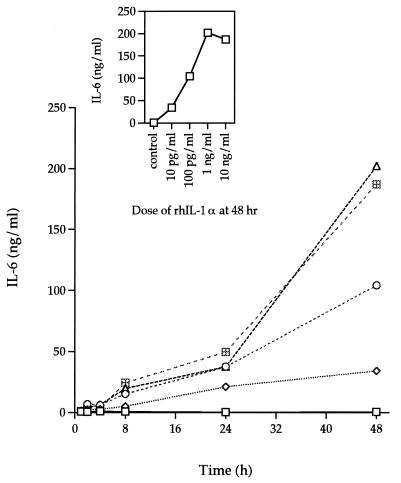
IL-1α induced larger amounts of IL-6 production than TNF-α. A concentration of 10 pg of rhIL-1α per ml induced a level of IL-6 in the supernatant of HGF F-CL cultures (Fig. 5) similar to that induced by 10 ng of TNF-α per ml (Fig. 4). A similar pattern of IL-6 induction was seen during the initial 24 h of stimulation with higher concentrations of rhIL-1α. Furthermore, as with rhTNF-α, stimulation with rhIL-1α resulted in increased levels of IL-6 with time. After 48 h of stimulation with rhIL-1α, the amount of IL-6 in culture supernatants was 200-fold higher with 100 pg of rhIL-1α per ml and 400-fold higher with 1 or 10 ng of rhIL-1α per ml than was seen in control cultures incubated without added cytokines. The increase in IL-6 levels was more rapid from 24 to 48 h than from 0 to 24 h.
FIG. 5.
Dose-response and time course analysis of IL-6 secretion by rhIL-1α-stimulated F-CL fibroblasts. Various amounts (10 pg/ml [◊], 100 pg/ml [○], 1 ng/ml [▵], and 10 ng/ml [⊞]) of rhIL-1α were added to confluent cultures of F-CL fibroblasts and incubated for 1, 2, 4, 8, 24, or 48 h. IL-6 protein in the culture supernatant was determined by ELISA. □, control. The inset graph shows the amount of IL-6 in the supernatants of cultures incubated with various amounts of rhIL-1α for 48 h. The values are the means of duplicate determinations. The variation associated with each point is within 1 SD of the mean.
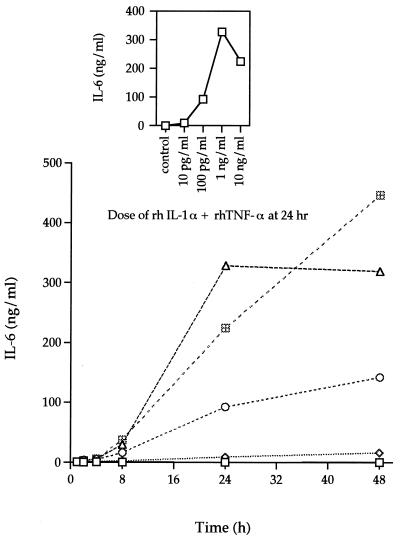
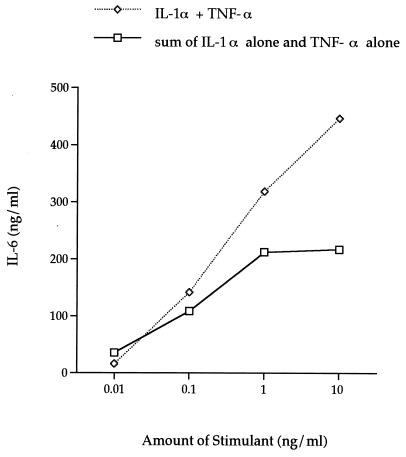
In our initial study with H-CL fibroblasts, it was observed that the addition of IL-1α and TNF-α to the cultures resulted in a synergistic induction of IL-6. Therefore, the next series of experiments were designed to determine the dose of IL-1α plus TNF-α and the time of incubation for optimal IL-6 induction by the HGF cell line F-CL. IL-6 levels increased rapidly after 8 h of incubation with rhIL-1α plus rhTNF-α (Fig. 6). As with rhIL-1α alone, 1 and 10 ng/ml induced comparable levels (300 to 450 ng/ml) of IL-6, which were approximately 600- to 800-fold higher than the control levels. The amount of cytokines inducing peak IL-6 production was 1 ng/ml after a 24-h incubation (Fig. 6, inset). The amount of IL-6 produced by F-CL cells stimulated with both rhIL-1α and rhTNF-α was higher than the sum of the amounts of IL-6 produced by cultures incubated with either rhIL-1α or rhTNF-α (Fig. 7). This synergistic effect occurred when more than 10 pg each of rhIL-1α and rhTNF-α per ml was added to the culture and after at least 8 h of incubation with the stimulant.
FIG. 6.
Dose-response and time course analysis of IL-6 secretion by rhIL-1α plus rhTNF-α-stimulated F-CL fibroblasts. Increasing amounts of rhIL-1α plus rhTNF-α (each at 10 pg/ml [◊], 100 pg/ml [○], 1 ng/ml [▵], or 10 ng/ml [⊞]) was added to confluent cultures of F-CL fibroblasts and incubated for 1, 2, 4, 8, 24, or 48 h. IL-6 protein in the culture supernatant was determined by ELISA. The inset graph is the amount of IL-6 in the supernatant of cultures incubated with various amounts of rhIL-1α for 24 h. The values are the means of duplicate determinations. The variation associated with each point is within 1 SD of the mean.
FIG. 7.
Comparison of the level of induction of IL-6 after the simultaneous addition of IL-1α and TNF-α to cultures with the sum of IL-6 levels induced with IL-1α alone and TNF-α alone after 48 h of incubation with 10 pg, 100 pg, 1 ng, and 10 ng of stimulant per ml.
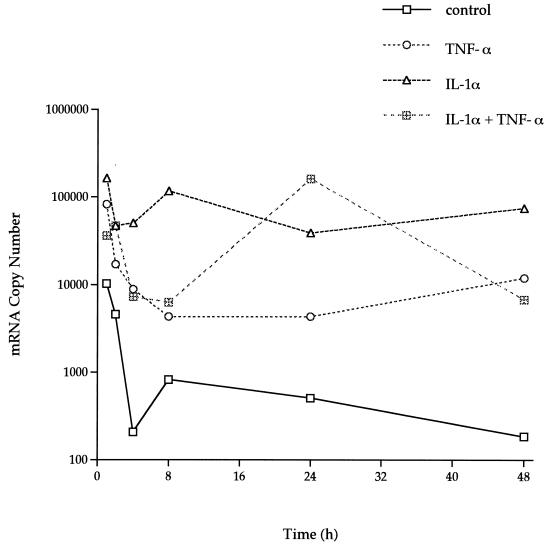
Total RNA was isolated from the F-CL cells at the optimal doses of each stimulant for each time point and was used to compare IL-6 mRNA copy numbers by the quantitative competitive (QC)-RT-PCR. The control cells (no simulation) showed a decreasing trend in the amount of IL-6 message over time (10,228 to 185 mRNA copies) (Fig. 8). As with the other healthy cell line, H-CL, TNF-α, IL-1α, and IL-1α in combination with TNF-α upregulated the levels of IL-6 message by 10- to 1,000-fold in F-CL cells. The IL-6 mRNA levels increased after only 1 h and remained high even after 48 h of stimulation.
FIG. 8.
IL-6 mRNA copy numbers after stimulation as determined by QC-RT-PCR. rhTNF-α (1 ng/ml), rhIL-1α (1 ng/ml), or rhIL-1α (1 ng/ml) plus TNF-α (1 ng/ml) diluted in DMEM without FCS was added to confluent HGF F-CL cells and incubated for 1 to 48 h. Total isolated RNA was used to assess levels of IL-6 mRNA by QC-RT-PCR. The values are means of duplicate determinations. The variation associated with each point is within 1 SD of the mean.
Effect of IL-6 on IL-6 production.
In order to determine if IL-6 was acting in an autocrine manner, IL-6 was measured in the supernatants after cultures of HGF were incubated with rhIL-6. When 5 ng of rhIL-6 per ml was added to H-CL cells, 15.45 ng of IL-6 per ml was detected after 24 h, which represented a threefold increase of IL-6 (Fig. 1). When IL-6 production by the cell line F-CL was assessed after incubation with rhIL-6, the amount of rhIL-6 added to the cells was similar to the amount of IL-6 detected by ELISA in the culture supernatants, even after a 48-h incubation (Fig. 9).
FIG. 9.
Effect of rhIL-6 on IL-6 production by HGF F-CL cells. Various amounts of IL-6 were added to confluent cultures of F-CL fibroblasts and incubated for 1, 2, 4, 8, 24, or 48 h. IL-6 protein in the culture supernatant was determined by ELISA. The values are the means of duplicate determinations. The variation associated with each point is within 1 SD of the mean.
DISCUSSION
HGF play an important role in the production and maintenance of periodontal connective tissue. These resident cells can also participate in periodontal disease by degrading the extracellular matrix and secreting mediators of inflammation such as IL-6. The present study compared the abilities of LPS, IL-1, and TNF-α to regulate the induction of IL-6 mRNA and protein production by two healthy human gingival cell lines. The two HGF cell lines used in this study constitutively produced low levels of IL-6 message and protein. This finding was in agreement with those obtained with fibroblasts of other origins (5, 22, 24, 27). The two healthy HGF cell lines used in the present study produced similar quantities of IL-6 after stimulation with either LPS or inflammatory cytokines, indicating that the findings were reflective of responses by this cell type.
The E. coli and the P. gingivalis LPSs induced similar increases in IL-6 production by the HGF cell lines, which occurred after only 4 h of incubation with LPS. However, we found that this IL-6 induction after LPS stimulation is not dose or time dependent as it is with IL-1 and TNF-α stimulation. This finding could be due to the unique signal transduction mechanisms used for each stimulant. The ability of LPS to stimulate gingival fibroblasts may be mediated through the LPS receptor CD14. Watanabe and coworkers (26) reported the presence of CD14 on HGF as determined by immunohistochemical and Northern and Western blotting techniques. Other studies, by Hayashi et al. (7), showed that soluble CD14 was necessary for stimulation of intercellular adhesion molecule 1 by HGF; however, CD14 mRNA could not be detected by PCR. Although the mechanism by which LPS upregulates IL-6 has not been determined, microbial LPS has been strongly implicated in periodontal disease. In the present study, we provide evidence that cytokines mediate a much greater response than LPS. IL-1 and TNF-α stimulated hundreds of times more IL-6 production than LPS. A recent study has shown that P. gingivalis LPS increases proliferation, upregulates TNF-α and IL-1 mRNA and protein levels, and increases CD14 expression by mononuclear cells to a greater extent than E. coli LPS (23). Thus, we propose a model in which LPS from the periodontal pathogen P. gingivalis may stimulate production of TNF-α and IL-1 by monocytes which in turn synergistically upregulate the production of high levels of IL-6 by HGF.
IL-1α was the strongest single stimulant of IL-6 production by HGF. It was generally 20 to 30 times more effective than TNF-α after 48 h of incubation, whereas previous studies with other fibroblastic cell lines have generally found IL-1α to be about 10-fold more effective than TNF-α (6, 15, 22). Our dose-response and time course studies showed that IL-6 production by healthy HGF cells is time and dose dependent for each stimulant except LPS and IL-6 itself. The dose-response studies showed that 1 ng/ml is an optimal dose of rhIL-1α and rhIL-1α plus rhTNF-α for IL-6 stimulation.
The receptors and signal pathways used to induce IL-6 by inflammatory cytokines and LPS are independent of each other. IL-1 receptor I (IL-1RI) and IL-1RII make up the IL-1 receptor family, with IL-1RI being the important receptor for signal transduction via IL-1. On the other hand, the ability of LPS to stimulate cells is thought to be mainly through CD14, and this has recently been associated with fibroblasts (7, 26). Although the receptors used may be unique to each of these stimulants, both IL-1α and LPS may induce transcription of the IL-6 gene through a common transcription factor, NF-IL-6, which has been shown to be inducible by IL-1, IL-6, TNF-α, and LPS (2). However, since IL-1 and LPS stimulants do not act synergistically but rather additively (unpublished data), the ability of IL-1 to act synergistically with TNF-α and additively with LPS may be regulated at the level of signal transduction.
From the time course studies, we found that IL-1α, TNF-α, and IL-1α in combination with TNF-α upregulate IL-6 mRNA levels 10- to 1,000-fold over control levels. The level of message rapidly increased within only 1 h of stimulation and remained higher than the control level even after 48 h. An explanation for the mechanism of this persistently high level of IL-6 mRNA may relate to the stability of the message. It has been reported that the IL-6 message after stimulation of normal human lung fibroblasts with TNF-α and IL-1 increases due to the IL-6 message becoming more stable rather than to an increase in IL-6 gene transcription (6). The mechanism of IL-6 upregulation by these stimulants is not fully understood, and further studies will be required. Blocking the HGF IL-1 receptors may inhibit this upregulation of IL-6. When these receptors are blocked with IL-1 receptor antagonist in other fibroblast types, they partially inhibit IL-6 production by diseased and normal kidney fibroblasts but less significantly inhibit IL-6 production by foreskin fibroblasts (15).
Recent studies have shown that IL-1 synergizes with TNF-α in stimulating IL-6 production by lung and duodenal fibroblasts (6, 22). However, the kinetics of this response had not been determined. Our study demonstrated that at least 8 h of incubation with greater than 10 pg of rhIL-1α plus rhTNF-α per ml is necessary for synergistic IL-6 production by HGF. Together, these results indicate that IL-1 and TNF-α are more important in the regulation of IL-6 by HGF than the direct effects of bacterial LPS. The action of LPS in mediating inflammatory responses may be via a direct effect on other cells such as monocytes to produce the TNF-α and IL-1 which in turn stimulate gingival fibroblast secretion of high levels of IL-6.
ACKNOWLEDGMENTS
We thank Anthony Allison and Tika Benveniste for generously providing IL-6 ELISA antibodies and RPA probes, respectively. We also thank Vickie Baron for assistance in preparing the manuscript.
The Southern Academy of Periodontology provided financial assistance. This work was supported by USPHS grants DE 00279 and DE 08228.
REFERENCES
- 1.Agarwal S, Baran C, Piesco N P, Quintero J C, Langkamp H H, Johns L P, Chandra C S. Synthesis of proinflammatory cytokines by human gingival fibroblasts in response to lipopolysaccharides and interleukin-1β. J Periodontal Res. 1995;30:382–389. doi: 10.1111/j.1600-0765.1995.tb01291.x. [DOI] [PubMed] [Google Scholar]
- 2.Bankers-Fulbright J L, Kalli K R, McKean D J. Interleukin-1 signal transduction. Life Sci. 1996;59:61–83. doi: 10.1016/0024-3205(96)00135-x. [DOI] [PubMed] [Google Scholar]
- 3.Chen C-C, Huang J-F, Tsai C-C. In vitro production of interleukin-6 by human gingival, normal buccal mucosa, and oral submucous fibrosis fibroblasts treated with betel-nut alkoids. Kaohsiung J Med Sci. 1995;11:604–614. [PubMed] [Google Scholar]
- 4.Chung I Y, Kwon J, Benveniste E N. Role of protein kinase C activity in tumor necrosis factor-α gene expression. J Immunol. 1992;149:3894–3902. [PubMed] [Google Scholar]
- 5.Debets R, Hegmans J P J J, Deleuran M, ’tHooft S, Benner R, Prens E P. Expression of cytokines and their receptors by psoriatic fibroblasts. I. Altered IL-6 synthesis. Cytokine. 1996;8:70–79. doi: 10.1006/cyto.1996.0010. [DOI] [PubMed] [Google Scholar]
- 6.Elias J A, Lentz V. IL-1 and tumor necrosis factor synergistically stimulate fibroblast IL-6 production and stabilize IL-6 messenger RNA. J Immunol. 1990;145:161–166. [PubMed] [Google Scholar]
- 7.Hayashi J, Masaka T, Saito I, Ishikawa I. Soluble CD14 mediates lipopolysaccharide-induced intercellular adhesion molecule 1 expression in cultured human gingival fibroblasts. Infect Immun. 1996;64:4946–4951. doi: 10.1128/iai.64.12.4946-4951.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hockett R, Janowski K, Bucy R. Simultaneous quantitation of multiple cytokine mRNAs by RT-PCR utilizing plate based EIA methodology. J Immunol Methods. 1995;187:273–285. doi: 10.1016/0022-1759(95)00195-5. [DOI] [PubMed] [Google Scholar]
- 9.Hockett, R., M. Roger, and S. Srivastava. Quantitative competitive RT-PCR analysis of biomarkers in the study of neoplasia. In M. Hanausek and A. Walaszek (ed.), Methods in molecular biology, in press. Humana Press, Totowa, N.J.
- 10.Kenney J, Masada M, Allison A. Development of quantitative two-site ELISAs for soluble proteins. In: Zola H, editor. Laboratory methods in immunology. Boca Raton, Fla: CRC Press; 1990. pp. 231–240. [Google Scholar]
- 11.Kent L W, Dyken R A, Rahemtulla F, Allison A C, Michalek S M. Effect of in vitro passage of healthy human gingival fibroblasts on cellular morphology and cytokine expression. Arch Oral Biol. 1996;41:263–270. doi: 10.1016/0003-9969(95)00127-1. [DOI] [PubMed] [Google Scholar]
- 12.Lapp C A, Thomas M E, Lewis J B. Modulation by progesterone of interleukin-6 production by gingival fibroblasts. J Periodontol. 1995;66:279–284. doi: 10.1902/jop.1995.66.4.279. [DOI] [PubMed] [Google Scholar]
- 13.Larjava H, Häkkinen L, Rahemtulla F. A biochemical analysis of human periodontal tissue proteoglycans. Biochem J. 1992;284:267–274. doi: 10.1042/bj2840267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laulederkind S J F, Bielawska A, Raghow R, Hannun Y A, Ballou L R. Ceramide induces interleukin 6 gene expression in human fibroblasts. J Exp Med. 1995;182:599–604. doi: 10.1084/jem.182.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lonnemann G, Engler-Blum G, Müller G A, Koch K M, Dinarello C A. Cytokines in human renal interstitial fibrosis. II. Intrinsic interleukin (IL)-1 synthesis and IL-1-dependent production of IL-6 and IL-8 by cultured kidney fibroblasts. Kidney Int. 1995;47:845–854. doi: 10.1038/ki.1995.127. [DOI] [PubMed] [Google Scholar]
- 16.McIntire F C, Sievert H W, Barlow G H, Finley R A, Lee A Y. Chemical, physical, and biological properties of a lipopolysaccharide from Escherichia coli K-235. Biochemistry. 1967;6:2363–2372. doi: 10.1021/bi00860a011. [DOI] [PubMed] [Google Scholar]
- 17.Mihara M, Moriya Y, Kishimoto T, Ohsugi Y. Interleukin-6 (IL-6) induces the proliferation of synovial fibroblastic cells in the presence of soluble IL-6 receptor. Br J Rheumatol. 1995;34:321–325. doi: 10.1093/rheumatology/34.4.321. [DOI] [PubMed] [Google Scholar]
- 18.Millar S J, Goldstein E G, Levine M J, Hausmann E. Modulation of bone metabolism by two chemically distinct lipopolysaccharide fractions from Bacteroides gingivalis. Infect Immun. 1986;51:302–306. doi: 10.1128/iai.51.1.302-306.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakao K, Yoneda K, Osaki T. Enhanced cytokine production and collagen synthesis of gingival fibroblasts from patients with denture fibromatosis. J Dent Res. 1995;74:1072–1078. doi: 10.1177/00220345950740040701. [DOI] [PubMed] [Google Scholar]
- 20.Ogura N, Shibata Y, Kamino Y, Matsuda U, Hayakawa M, Oikawa T, Takiguchi H, Izumi H, Abiko Y. Stimulation of interleukin-6 production of periodontal ligament cells by Porphyromonas endodontalis lipopolysaccharide. Biochem Med Met Biol. 1994;53:130–136. doi: 10.1006/bmmb.1994.1068. [DOI] [PubMed] [Google Scholar]
- 21.Page R. The role of inflammatory mediators in the pathogenesis of periodontal disease. J Periodontal Res. 1991;26:230–242. doi: 10.1111/j.1600-0765.1991.tb01649.x. [DOI] [PubMed] [Google Scholar]
- 22.Pang G, Couch L, Batey R, Clancy R, Cripps A. GM-CSF, IL-1α, IL-1β, IL-6, IL-8, IL-10, ICAM-1 and VCAM-1 gene expression and cytokine production in human duodenal fibroblasts stimulated with lipopolysaccharide, IL-1α and TNF-α. Clin Exp Immunol. 1994;96:437–443. doi: 10.1111/j.1365-2249.1994.tb06048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberts F A, Richardson G J, Michalek S M. Effects of Porphyromonas gingivalis and Escherichia coli lipopolysaccharide on mononuclear phagocytes. Infect Immun. 1997;65:3248–3254. doi: 10.1128/iai.65.8.3248-3254.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tiggelman A M B C, Boers W, Linthorst C, Brand H, Sala M, Chamuleau R A F M. Interleukin-6 production by human liver (myo)fibroblasts in culture. Evidence for a regulatory role of LPS, IL-1β and TNF-α. J Hepatol. 1995;23:295–306. [PubMed] [Google Scholar]
- 25.VanSnick J. Interleukin-6: an overview. Annu Rev Immunol. 1990;8:253–278. doi: 10.1146/annurev.iy.08.040190.001345. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe A, Takeshita A, Kitano S, Hanazawa S. CD14-mediated signal pathway of Porphyromonas gingivalis lipopolysaccharide in human gingival fibroblasts. Infect Immun. 1996;64:4488–4494. doi: 10.1128/iai.64.11.4488-4494.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamaji Y, Kubota T, Sasaguri K, Sato S, Suzuki Y, Kumada H, Umemoto T. Inflammatory cytokine gene expression in human periodontal ligament fibroblasts stimulated with bacterial lipopolysaccharides. Infect Immun. 1995;63:3576–3581. doi: 10.1128/iai.63.9.3576-3581.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]