Abstract
We reported earlier that a nontoxic form of anthrax toxin was capable of delivering a cytotoxic T-lymphocyte (CTL) epitope in vivo, such that a specific CTL response was primed against the epitope. The epitope, of bacterial origin, was fused to an N-terminal fragment (LFn) from the lethal-factor component of the toxin, and the fusion protein was injected, together with the protective antigen (PA) component, into BALB/c mice. Here we report that PA plus LFn is capable of delivering a different epitope—OVA257–264 from ovalbumin. Delivery was accomplished in a different mouse haplotype, H-2Kb and occurred in vitro as well as in vivo. An OVA257–264-specific CTL clone, GA-4, recognized EL-4 cells treated in vitro with PA plus as little as 30 fmol of the LFn-OVA257–264 fusion protein. PA mutants attenuated in toxin self-assembly or translocation were inactive, implying that the role of PA in epitope delivery is the same as that in toxin action. Also, we showed that OVA257–264-specific CTL could be induced to proliferate by incubation with splenocytes treated with PA plus LFn-OVA257–264. These findings imply that PA-LFn may serve as a general delivery vehicle for CTL epitopes in vivo and as a safe, efficient tool for the ex vivo expansion of patient-derived CTL for use in adoptive immunotherapy.
Cytotoxic T-Lymphocytes (CTL) recognize and clear defective host cells which display nonself peptides on their surface (13, 28). These peptides arise from various sources, such as infectious agents or aberrant expression of self proteins, and mark defective cells for CTL recognition. Proteins within the cytosol are processed by the multicatalytic proteasome to generate small peptides, which are then transported to the lumen of the endoplasmic reticulum (ER) by specific transporters, TAP1 and TAP2 (16). Once in the ER, the peptides complex with newly synthesized class I major histocompatibility molecules (MHC-I), and the peptide–MHC-I complexes egress from the ER and are presented at the cell surface. Upon recognition of foreign peptide–MHC-I complexes, specific CTL are activated to clear the pathogen or defective cell (1, 7). Activated CTL lyse the infected cell, secrete cytokines, proliferate, and differentiate. Lysis of the target cell may deprive an infectious organism of its replicative niche or, in the case of tumor cells, limit the expansion of the defective cells. By secreting cytokines (e.g., interleukin-2 and gamma interferon) CTL recruit other components of the immune response to the site of the defect. Proliferation and differentiation expand the number of specific CTL available for targeting similar defective cells and generate a set of long-lived memory cells available to respond more quickly and effectively to subsequent challenge. Vaccines that prime these memory CTL provide protection for the host upon subsequent exposure to similar defective cells.
The development of vaccines to specifically prime CTL has been hindered by difficulties in developing safe mechanisms to deliver CTL epitopes to the host cell cytosol. Several approaches to this problem have been reported (23, 26, 34, 35), including the use of attenuated viruses, intracellular bacteria, naked DNA, and adjuvants. Each of these approaches presents inherent problems of safety and/or efficiency. The use of attenuated viruses and bacteria raises questions about possible pathogenic effects of these agents, especially in immunocompromised individuals (25). The use of DNA vaccines risks the integration of naked DNA into the host chromosome (10), and most promising adjuvants are not well tolerated in humans (25). Recently we reported the development of a noninfectious, nontoxic vehicle for the delivery of epitopes, involving a modified form of anthrax toxin (4). This technique involves genetically fusing a CTL epitope to a nontoxic component of anthrax toxin that can enter the cytosol of cells.
Anthrax toxin is composed of three proteins that act in binary combinations to elicit two toxic effects, death and edema (18). Lethal factor (LF) and edema factor (EF) are intracellularly acting proteins, which require protective antigen (PA) for translocation to the cytosol of mammalian cells. During cellular entry, PA is proteolytically activated at the cell surface, generating a 63-kDa fragment, PA63, which contains a site at which EF and LF compete for binding. Following EF or LF binding to PA, the protein complex is endocytosed and trafficked to the endosome, where the bound LF or EF is translocated to the cytosol following endosomal acidification. Within the cytosol, EF expresses its adenylate cyclase activity and LF expresses a yet undefined activity inducing the overproduction of cytokines in macrophage target cells (14, 15).
Recently, it was found that the amino-terminal 255-amino-acid domain of LF (LFn) directs interactions with PA (2, 3). LFn contains the information necessary for PA binding and translocation but is devoid of lethal activity. Thus, heterologous proteins genetically fused to LFn can be delivered to the cytosol of cultured mammalian cells in the presence of PA (24). These results suggested that if CTL epitopes were fused to LFn, the resulting fusion proteins might be delivered to the cytosol by PA and generate a CTL response in vivo. In an initial report, we described the use of the PA-LFn system to prime specific CTL in vivo by using a known epitope derived from the intracellular pathogen Listeria monocytogenes (4). Delivery was efficient, with as little as 300 fmol of the fusion priming epitope-specific CTL. Mice vaccinated by this approach were partially protected against an L. monocytogenes challenge.
In the present study, PA-LFn was used to deliver a CTL epitope (OVA257–264) derived from ovalbumin and presented in the context of H-2 Kb (6, 21). LFn was genetically fused to OVA257–264, and the epitope was shown to be presented in complex with MHC-I both in vivo and in vitro. PA mutants attenuated in steps of LFn binding or translocation are unable to deliver LFn-OVA257–264, confirming that cytosolic delivery is an essential step in generating target cells by this approach.
In addition, we show that PA plus LFn-OVA257–264 can be used to generate stimulator cells that expand specific CTL in vitro. Expansion of patient-derived CTL in vitro is an important step for adoptive immunotherapy targeting a variety of infectious diseases and cancers (5, 9, 20, 36).
MATERIALS AND METHODS
Peptide.
The synthetic peptide OVA257–264, with the sequence SIINFEKL, was purchased from Biosynthesis Inc. (Lewisville, Tex.).
Construction, expression, and purification of fusion proteins.
DNA fragments encoding LFn-OVA257–264 were constructed by PCR. LFn-OVA257–264 was amplified with an upstream primer which included an NdeI site and sequence homologous to the 5′ end of the LF gene. The downstream primer was homologous to the sequence encoding the last 6 amino acids of LFn and included (downstream of the homology) sequence encoding the OVA257–264 epitope, stop codons, and a BamHI site. The toxin-encoding plasmid from Bacillus anthracis Sterne, pXO1, was used as the template. The NdeI-BamHI fragment was ligated into compatible sites within the multiple-cloning region of pET15b (Novagen) and used to transform Escherichia coli XL1-Blue (Stratagene). For each clone, the plasmid DNA was amplified, purified, and screened for the appropriate insert by restriction analysis. Clones containing inserts were locally sequenced to confirm that the fusion was correct. These clones were then used to transform E. coli BL21(DE3) (31) for expression of the fusion protein.
Recombinant proteins expressed in pET15b contain a His6 tag at the amino terminus of the protein. This tag allows for a one-step affinity purification of the expressed protein on a Ni2+-charged column. Cultures of BL-21/pET15b(LFn-OVA257–264) were grown in Luria broth containing ampicillin (50 μg/ml) to an optical density at 600 nm of 0.6 to 0.8, and protein expression was induced by the addition of 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for approximately 3 h. The cells were then pelleted and disrupted by sonication. The sonicate was centrifuged, and the supernatant was passed over an equilibrated Ni2+-charged column. The bound fusion protein was removed with 0.5 M imidazole as specified by the manufacturer (Novagen). The eluted protein was then equilibrated in 20 mM Tris-HCl (pH 7.5). The protein concentration was determined, and the sample was frozen at −20°C.
Wild-type PA was isolated from supernatant cultures of an attenuated strain of B. anthracis by an established method (19).
Stimulation of OVA257–264-specific CTL.
All the mice used in this study were female C57BL/6 (Jackson Laboratories), H-2b, between 8 and 12 weeks of age. Mouse splenocytes were harvested and CTL were stimulated as described previously (29), with the following modifications. Spleen cells from immunized and control mice were isolated and washed once in RP-10. Cells used as stimulators were naive, irradiated (2,000 rads), syngeneic splenocytes treated with 10 μM sterile OVA257–264 peptide. The stimulator cells were incubated for 1 h in the presence of peptide and then washed once in RP-10. Cultures contained 3 × 107 stimulator cells and 3 × 107 splenocytes from either immunized or control mice. These were incubated upright in a T-75 flask at 37°C in 7% CO2 in a total volume of 20 ml of RP-10.
CTL assay.
For target cells, mouse thymoma EL-4 (H-2b) cells were incubated with 10 μM OVA257–264 synthetic peptide and labeled with 20 μl of sodium [51Cr]chromate (600 Ci/ml; 1 Ci = 37 GBq) for 1 h. The cells were then washed twice with medium to remove unbound peptide and extracellular radionuclide. Ten thousand radiolabeled cells either treated with peptide or untreated (negative control) were then added to stimulated effector cell dilutions. The total volume in each assay well was 200 μl. Spontaneous and complete lysis of target cells was determined by incubating target cells with RP-10 or 1% Triton X-100, respectively. After 4 h of incubation at 37°C, the 96-well plates were centrifuged at 2,000 × g and 100 μl of the supernatant was counted for release of 51Cr. The percent specific lysis was determined as 100 × (CTL release − spontaneous release)/(maximum release − spontaneous release).
In vitro assay for cytosolic delivery of OVA257–264.
EL-4 cells were aliquoted into 12 15-ml conical tubes at 5 × 105 cells/tube. Six of the tubes contained PA (100 ng) in 1 ml (PA plus), and the other six contained 1 ml of RP-10 medium alone (PA minus). The samples, both PA plus and PA minus, were treated with either 100, 10, or 1 ng of LFn-OVA257–264 for 3.5 h. Duplicate samples were tested at each concentration. The treated cells were then centrifuged, and the total volume was reduced to 50 μl in RP-10. After the cells were resuspended, 20 μl of sodium [51Cr]chromate was added to each sample and the cells were incubated for an additional 1 h. The cells were then washed three times and resuspended in a final volume of 5 ml of RP-10. Dilutions of the CTL clone GA-4 (starting with 105) were made and added to wells of a 96-well round-bottom plate. A 100-μl volume of the treated EL-4 cells (104 cells) was added to each of the GA-4 dilutions. In addition, both peptide-coated and uncoated target cells were mixed with medium alone (spontaneous control) or 1% Triton X-100 (complete lysis control). The assay mixture was incubated for 4 h, the plate was centrifuged, and 100 μl of the supernatant was analyzed in a gamma counter to determine 51Cr release.
Construction, expression, and analysis of PA mutants.
Site mutants of PA defective in receptor binding or translocation of LFn were analyzed by the above system. Both PA mutants have been described previously, and their phenotypes are well established (12, 27). The first mutant, PA --D315, has two amino acid deletions (FFD315 to --D315) within the chymotrypsin loop and is unable to translocate LF to the cytosol but binds LF with similar efficiency to the wild type. The second mutant, PA RSSR167, has three substitutions (RKKR167 to SSSR167) within a furin-sensitive region. This PA mutant is not nicked by furin and is unable to bind LF.
Both mutants were generated by two-step PCR mutagenesis, where the sense-strand primer encodes the appropriate mutations. The mutant PCR products were subcloned into the wild-type PA gene maintained in pET22b (Novagen). The mutant proteins were transformed into E. coli BL-21 and purified from periplasmic extracts by a combination of gel filtration and anion-exchange chromatography.
In vitro expansion of GA-4 CTL with spleen cells treated with PA plus LFn-OVA257–264.
The ability of PA-LFn to be used as a tool for generating specific CTL stimulator cells was evaluated by the following approach. Splenocytes from a C57BL/6 mouse were washed and resuspended in 2 ml of ACK lysis buffer to lyse erythrocytes (8). After 1 min of incubation, 8 ml of RP-10 was added to the cells and the cells were pelleted by centrifugation. The cells were then resuspended to a concentration of 5 × 106 cells/ml in RP-10, and 1 ml was added to each of six tubes. To each of the six tubes was added one of the following: RP-10, 1 μM peptide (SIINFEKL), 10 ng of LFn-OVA257–264 plus 100 ng of PA, 10 ng of LFn-OVA257–264, 100 ng of LFn-OVA257–264 plus 100 ng of PA, or 100 ng of LFn-OVA257–264. The treated cells were incubated for 4 h and then irradiated (2,000 rads), washed, and resuspended in 1 ml of RP-10 supplemented with α-methyl mannoside and rat spleen supernatants treated with concanavalin A (30). A 100-μl volume of each sample was plated in each of triplicate wells of a flat-bottom 96-well plate. GA-4 CTL (104 cells) and irradiated (2,000 rads) syngeneic spleen cells (6 × 105 cells) were added to each well, and the samples were incubated in the presence of 7% CO2 at 37°C for 48 h. Following the incubation, 20 μl of [3H]thymidine (6.7 Ci/mmol) was added to a final concentration of 2.5 μCi/ml. Following 14 h of incubation, the samples were harvested with a model 96 well plate harvester (TomTec Inc., Orange, Conn.) and counted with a model 1205 Beta-Plate counter (Wallac Instruments, Gaithersburg, Md.).
RESULTS
LFn-OVA257–264 plus PA primes an epitope-specific CTL response in vivo.
To determine if LFn-PA could be used to stimulate OVA257–264-specific CTL in vivo, we constructed plasmids encoding the 8-amino-acid OVA257–264 CTL epitope fused downstream of the first 255 amino acids of LF (generating LFn-OVA257–264). Mice (5 per group) were injected intraperitoneally (i.p.) with 30 pmol of the fusion protein mixed with 6 pmol of PA. Control groups of mice were injected with LFn-OVA257–264 without PA. After 2 weeks, the animals were sacrificed and 3 × 107 splenocytes were stimulated on syngeneic spleen cells coated with the OVA257–264 peptide. After 5 days of stimulation in vitro, the cells were assayed for the ability to lyse EL-4 cells coated with OVA257–264.
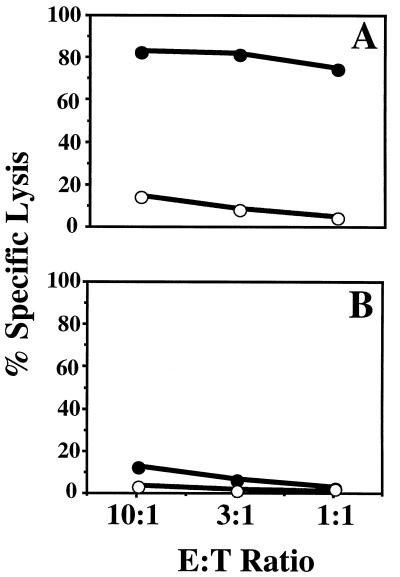
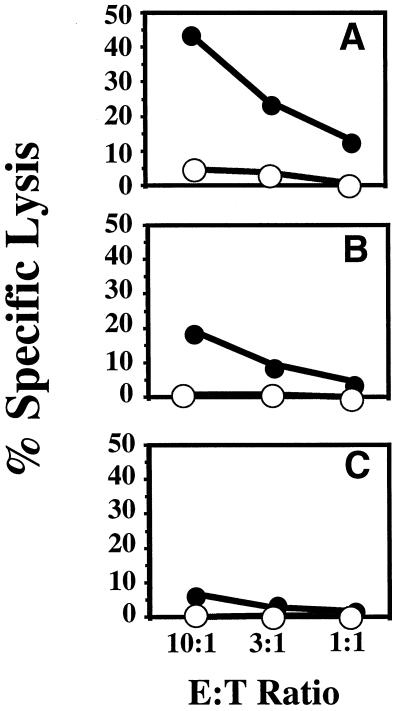
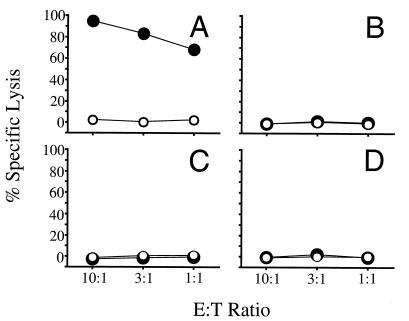
As shown in Fig. 1, lysis of peptide-coated EL-4 was substantially higher than lysis of EL-4 cells alone, indicating that the mice had mounted an OVA257–264-specific CTL response. This result is similar to our initial findings with LLO91–99 epitope fusions, in BALB/c mice. As with the LFn-LLO91–99 fusion protein, LFn-OVA257–264 delivery was dependent on the presence of PA. These results demonstrate the versatility of the system, showing the delivery of a different epitope in vivo in a different murine haplotype.
FIG. 1.
CTL-mediated lysis of OVA257–264 peptide-coated EL-4 cells. Mice were injected i.p. with LFn-OVA257–264 plus PA (A) or LFn-OVA257–264 without PA (B). After in vitro stimulation, samples were assayed for their ability to lyse 51Cr-labeled EL-4 cells coated with OVA257–264 peptide (solid circles) or not coated (open circles). Targeting was evaluated by measuring the amount of 51Cr release. The effector-to-target-cell ratios (E:T ratios) examined were 10:1, 3:1, and 1:1. Similar levels of lysis were observed in each of five replicates.
PA can mediate delivery of an LFn-epitope fusion to the cytosol of cells in vitro.
To study LFn plus PA-mediated delivery of OVA257–264 at the cellular and molecular levels, we designed experiments for in vitro delivery of OVA257–264. EL-4 cells were used as targets and treated with 100 to 1 ng (3 to 0.3 pmol) of the LFn-OVA257–264 fusion protein in the presence or absence of 100 ng of PA (∼1 pmol). The cells were incubated with the fusion proteins for 3.5 h and then loaded with 51Cr. They were then assayed for the presentation of OVA257–264 by incubation with the epitope-specific CTL clone, GA-4. Target cells presenting OVA257–264 are recognized by GA-4 CTL and lysed, releasing 51Cr.
Figure 2 shows that when EL-4 cells were treated with the fusion protein in the presence of PA, the cells presented the OVA257–264 epitope and were lysed by GA-4 CTL. Specific lysis of the treated EL-4 cells decreased when the cells were treated with 10-fold less fusion protein. PA was an essential component of in vitro delivery, since only targets treated with the fusion protein in the presence of PA were recognized by GA-4 (Fig. 2). The amount of fusion protein needed for presentation was minimal. GA-4 recognized target cells treated with 100 and 10 ng of the fusion protein (3 and 0.3 pmol, respectively) and even cells treated with only 1 ng of the fusion protein were recognized, although the specific lysis was low (<10%).
FIG. 2.
GA-4-mediated lysis of EL-4 cells treated with LFn-OVA257–264 plus PA. EL-4 cells were treated with 100 ng of LFn-OVA257–264 (A), 10 ng of LFn-OVA257–264 (B), or 1 ng of LFn-OVA257–264 (C). Each sample was treated in the presence (solid circles) or absence (open circles) of PA. Following treatment with the fusion protein, the target cells were loaded with 51Cr. The target cells were incubated with OVA257–264-specific CTL to give E:T ratios of 10:1, 3:1, and 1:1. Targeting was evaluated by measuring the amount of 51Cr release. Similar levels of lysis was observed in each of three repeat experiments.
Mutations in PA inhibit the delivery of LFn-epitope fusion proteins.
In both the in vitro and in vivo studies, we have found that PA was essential for generating a CTL response with LFn fusions. Several PA-mediated steps are important for the action of anthrax toxin on mammalian cells. To confirm that these steps are also important for the delivery of the CTL epitopes, we used two well-characterized PA mutants. One (SSSR) was altered in the furin-specific cleavage site, which blocks its activation by furin or other cell-associated proteases and thereby prevents toxin self-assembly, including LFn binding. The other (--D) blocks membrane translocation.
EL-4 target cells were treated with the LFn-OVA257–264 fusion (100 ng) mixed with the PA mutants (--D and SSSR) or wild-type PA (100 ng). The cells were then loaded with 51Cr as described above and tested for antigen presentation by incubation with the OVA257–264-specific CTL. As seen in Fig. 3, only EL-4 cells treated with the fusion protein in the presence of wild-type PA were recognized for targeting by GA-4. Both PA mutants showed levels of specific lysis similar to those of the controls without PA and thus were highly attenuated in their ability to mediate epitope delivery.
FIG. 3.
GA-4 targeting of EL-4 cells treated with LFn-OVA257–264 and PA mutants. EL-4 cells were treated with LFn-OVA257–264 plus wild-type PA (solid circles), --D mutant PA (solid squares), SSSR mutant PA (open squares), or no PA (open circles). Following treatment with the fusion protein and PA, the target cells were loaded with 51Cr. The target cells were added to OVA257–264-specific CTL at E:T ratios of 10:1, 3:1, and 1:1. Targeting was evaluated by measuring the amount of 51Cr release. Similar levels of lysis was observed in each of three repeat experiments.
To confirm whether these PA mutations also block the ability of toxin fusions to prime CTL in vivo, C57BL/6 mice (three per group) were injected i.p. with 30 pmol of the LFn-OVA257–264 fusion protein mixed with 30 pmol of the PA mutants (--D and SSSR) or wild-type PA. A control group consisted of mice injected with the LFn-OVA257–264 fusion protein without PA. At 2 weeks after immunization, the animals were sacrificed and splenocytes from each mouse were stimulated in vitro as described above. After 5 days of stimulation, the cultures were tested for CTL activity specific for OVA257–264. As shown in Fig. 4, a specific CTL response was primed only in mice immunized with wild-type PA. CTL specific for OVA257–264 were not primed in mice immunized with either PA mutant. These results further demonstrate the attenuation of these mutants in their ability to deliver epitopes into a compartment that allows for the priming of CTL.
FIG. 4.
CTL-mediated lysis of OVA257–264 peptide-coated EL-4 cells. Mice were injected i.p. with LFn-OVA257–264 plus PA (A), LFn-OVA257–264 without PA (B), LFn-OVA257–264 plus PA mutant SSSR (C), or LFn-OVA257–264 plus PA mutant --D (D). After in vitro stimulation, the samples were assayed for their ability to lyse 51Cr-labeled EL-4 cells coated with OVA257–264 peptide (solid circles) or not coated (open circles). Targeting was evaluated by measuring the amount of 51Cr release. The E:T ratios examined were 10:1, 3:1, and 1:1. Similar levels of lysis were observed in each of three replicates.
Anthrax toxin fusion proteins may be used to expand antigen-specific CTL in vitro.
Having demonstrated the use of anthrax toxin fusion proteins to target cells for lysis by CTL, we performed experiments to determine if an LFn-epitope fusion plus PA might also be used to generate stimulator cells for the in vitro expansion of specific CTL. In this study, splenocytes were incubated with LFn-OVA257–264 (100 or 10 ng) plus PA (100 ng) and control groups were treated with LFn-OVA257–264 without PA, with SIINFEKL peptide, or with RP-10 medium. GA-4 CTL were added to the treated splenocytes, and the proliferation of the CTL clone was measured by [3H]thymidine incorporation.
Splenocytes treated with LFn-OVA257–264 plus PA showed a significantly higher level of proliferation than did the PA-minus and RP-10 controls (Fig. 5). As expected, splenocytes treated with synthetic peptide were also able to stimulate the proliferation of the GA-4 CTL. Additionally, as was the case with the in vitro delivery assay, there was a dose-dependent response to the target cells. Decreasing the amount of fusion protein 10-fold resulted in a lower [3H]thymidine incorporation by CTL.
FIG. 5.
In vitro expansion of OVA257–264 specific CTL. Aliquoted mouse splenocytes were treated for 4 h with medium alone, 1 μM OVA257–264 synthetic peptide, 100 ng of LFn-OVA257–264 fusion plus PA, 100 ng of LFn-OVA257–264 minus PA, 10 ng of LFn-OVA257–264 fusion plus PA, or 10 ng of LFn-OVA257–264 minus PA. OVA257–264-specific CTL were added to each sample. Following 48 h of incubation, [3H]thymidine was added to the samples. At 14 h later, the samples were harvested and thymidine incorporation into the cells was assessed. Statistical analysis was performed by Student’s t test.
DISCUSSION
Novel methods of priming specific CTL may well be important in the development of vaccines against various intracellular pathogens and cancers. Recently, we reported using PA-LFn as a molecular tool to deliver the CTL epitope LLO91–99 in mice (4). Here we report the PA-LFn-mediated delivery of a different epitope in a different mouse haplotype in vivo. OVA257–264-specific CTL were primed in C57BL/6 (H-2b) mice by using the anthrax toxin delivery system (Fig. 1). The results were similar to those obtained with LLO91–99, in which delivery was efficient and PA dependent. Remarkably, only a single injection with a minimal amount of protein was needed to prime specific CTL with either epitope.
Because it is difficult to examine the events at a cellular or molecular level by using the system in vivo, we expanded our studies to determine the capacity of this system to function in vitro. Delivery of the OVA257–264 epitope to EL-4 target cells was demonstrated with a specific CTL clone, GA-4, that recognizes the ovalbumin-derived epitope and targets EL-4 cells for lysis. A dose-dependent response was observed across the range of LFn-OVA257–264 used in the assay.
Epitope delivery with the anthrax toxin system is PA dependent, presumably because PA functions in this system as it does in anthrax toxin action, by mediating receptor binding, LF-EF interaction, and translocation of EF or LF to the cytosol. To test this assumption, we used two PA mutants, known to be attenuated in toxin self-assembly and translocation, in delivery assays in vitro and in vivo. The findings that neither PA mutant allowed targeting of EL-4 by specific CTL and that neither mutant allowed for priming of CTL in vivo serve as strong evidence that PA plays similar functional roles in both LF/EF and epitope delivery.
The in vitro expansion of patient CTL represents a viable and promising approach for therapeutic treatment of a variety of diseases (5, 9, 11, 17, 20, 22, 32, 33). For reasons that remain unclear, patients often mount a CTL response that recognizes cancer or infected cells but are not able to clear the defective cells. This may be due in large part to the number of CTL generated, so that expansion of these CTL might give sufficient numbers to clear the defective cells. CTL expanded in vitro could be transferred back to the patient in sufficient numbers to effectively clear the infected or tumor cells. Patients in this situation are often immunocompromised, which may account in part for their abbreviated immune response. Moreover, the immunocompromised state of the patients makes it important to carefully choose approaches for the in vitro expansion of CTL, avoiding attenuated viruses or other potential infectious agents. As shown in Fig. 4, LFn-OVA257–264 plus PA can be used to stimulate specific CTL. These stimulators can then be used to expand the cultured population of GA-4 CTL. LFn-PA may be well suited for in vitro expansion of CTL since it is both efficient and nontoxic.
REFERENCES
- 1.Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 2.Arora N, Klimpel K R, Singh Y, Leppla S H. Fusions of anthrax toxin lethal factor to the ADP-ribosylation domain of Pseudomonas exotoxin A are potent cytotoxins which are translocated to the cytosol of mammalian cells. J Biol Chem. 1992;267:15542–15548. [PubMed] [Google Scholar]
- 3.Arora N, Leppla S H. Residues 1–254 of anthrax toxin lethal factor are sufficient to cause cellular uptake of fused polypeptides. J Biol Chem. 1993;268:3334–3341. [PubMed] [Google Scholar]
- 4.Ballard J D, Collier R J, Starnbach M N. Anthrax toxin-mediated delivery of a cytotoxic T-cell epitope in vivo. Proc Nat Acad Sci USA. 1996;93:12531–12534. doi: 10.1073/pnas.93.22.12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Celis E, Tsai V, Crimi C, DeMars R, Wentworth P A, Chesnut R W, Grey H M, Sette A, Serra H M. Induction of anti-tumor cytotoxic T lymphocytes in normal humans using primary cultures and synthetic peptide epitopes. Proc Natl Acad Sci USA. 1994;91:2105–2109. doi: 10.1073/pnas.91.6.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Celluzzi C M, Falo L D., Jr Epidermal dendritic cells induce potent antigen-specific CTL-mediated immunity. J Invest Dermatol. 1997;108:716–720. doi: 10.1111/1523-1747.ep12292095. [DOI] [PubMed] [Google Scholar]
- 7.Clark W R, Walsh C M, Glass A A, Hayashi F, Matloubian M, Ahmed R. Molecular pathways of CTL-mediated cytotoxicity. Immunol Rev. 1995;146:33–44. doi: 10.1111/j.1600-065x.1995.tb00682.x. [DOI] [PubMed] [Google Scholar]
- 8.Coligan J E, Kruisbeek A M, Margulies D H, Shevach E M, Strober W. Current protocols in immunology. Vol. 1. New York, N.Y: Green Publishing Co.; 1994. [Google Scholar]
- 9.DiMaio J M, Van Trigt P, Gaynor J W, Davis R D, Coveney E, Clary B M, Lyerly H K. Generation of tumor-specific T lymphocytes for the treatment of posttransplant lymphoma. Circulation. 1995;92(9 Suppl.):II202–II205. doi: 10.1161/01.cir.92.9.202. [DOI] [PubMed] [Google Scholar]
- 10.Ertl H C J, Xiang Z. Novel vaccine approaches. J Immunol. 1996;156:3579–3582. [PubMed] [Google Scholar]
- 11.Ferrari G, King K, Rathbun K, Place C A, Packard M V, Bartlett J A, Bolognesi D P, Weinhold K J. IL-7 enhancement of antigen-driven activation/expansion of HIV-1-specific cytotoxic T lymphocyte precursors (CTLp) Clin Exp Immunol. 1995;101:239–248. doi: 10.1111/j.1365-2249.1995.tb08345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gordon V M, Klimpel K R, Arora N, Henderson M A, Leppla S H. Proteolytic activation of bacterial toxins by eukaryotic cells is performed by furin and by additional cellular proteases. Infect Immun. 1995;63:82–87. doi: 10.1128/iai.63.1.82-87.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gotch F, Gallimore A, McMichael A. Cytotoxic T cells--protection from disease progression--protection from infection. Immunol Lett. 1996;51:125–128. doi: 10.1016/0165-2478(96)02566-7. [DOI] [PubMed] [Google Scholar]
- 14.Hanna P, Kruskal B, Ezekowitz R A, Bloom B, Collier R J. Role of macrophage oxidative burst in the action of anthrax lethal toxin. Mol Med. 1994;1:7–18. [PMC free article] [PubMed] [Google Scholar]
- 15.Hanna P C, Acosta D, Collier R J. On the role of macrophages in anthrax. Proc Natl Acad Sci USA. 1993;90:10198–10201. doi: 10.1073/pnas.90.21.10198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heemels M T, Ploegh H. Generation, translocation, and presentation of MHC class I-restricted peptides. Annu Rev Biochem. 1995;64:463–491. doi: 10.1146/annurev.bi.64.070195.002335. [DOI] [PubMed] [Google Scholar]
- 17.Heslop H E, Ng C Y, Li C, Smith C A, Loftin S K, Krance R A, Brenner M K, Rooney C M. Long-term restoration of immunity against Epstein-Barr virus infection by adoptive transfer of gene-modified virus-specific T lymphocytes. Nat Med. 1996;2:551–555. doi: 10.1038/nm0596-551. [DOI] [PubMed] [Google Scholar]
- 18.Leppla S H. The anthrax toxin complex. In: Alouf J, editor. Sourcebook of bacterial protein toxins. New York, N.Y: Academic Press, Inc.; 1991. pp. 277–302. [Google Scholar]
- 19.Leppla S H. Bacillus anthracis calmodulin-dependent adenylate cyclase: chemical and enzymatic properties and interactions with eucaryotic cells. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:189–198. [PubMed] [Google Scholar]
- 20.Lieberman J, Fabry J A, Shankar P, Beckett L, Skolnik P R. Ex vivo expansion of HIV type 1-specific cytolytic T cells from HIV type 1-seropositive subjects. AIDS Res Hum Retroviruses. 1995;11:257–271. doi: 10.1089/aid.1995.11.257. [DOI] [PubMed] [Google Scholar]
- 21.Lipford G B, Bauer S, Wagner H, Heeg K. In vivo CTL induction with point-substituted ovalbumin peptides: immunogenicity correlates with peptide-induced MHC class I stability. Vaccine. 1995;13:313–320. doi: 10.1016/0264-410x(95)93320-9. [DOI] [PubMed] [Google Scholar]
- 22.Lubaki M N, Egan M A, Siliciano R F, Weinhold K J, Bollinger R C. A novel method for detection and ex vivo expansion of HIV type 1-specific cytolytic T lymphocytes. AIDS Res Hum Retroviruses. 1994;10:1427–1431. doi: 10.1089/aid.1994.10.1427. [DOI] [PubMed] [Google Scholar]
- 23.Melief C J M, Kast M. T-cell immunotherapy of tumors by adoptive transfer of cytotoxic T lymphocytes and by vaccination with minimal essential epitopes. Immunol Rev. 1995;146:167–177. doi: 10.1111/j.1600-065x.1995.tb00081.x. [DOI] [PubMed] [Google Scholar]
- 24.Milne J C, Blanke S R, Hanna P C, Collier R J. Protective antigen-binding domain of anthrax lethal factor mediates translocation of a heterologous protein fused to its amino- or carboxy-terminus. Mol Microbiol. 1995;15:661–666. doi: 10.1111/j.1365-2958.1995.tb02375.x. [DOI] [PubMed] [Google Scholar]
- 25.Mims C A, Dimmock N J, Nash A, Stephen J. Mims’ pathogenesis of infectious disease. San Diego, Calif: Academic Press, Inc.; 1995. [Google Scholar]
- 26.Shen H, Slifka M K, Matloubian M, Jensen E R, Ahmed R, Miller J F. Recombinant Listeria monocytogenes as a live vaccine vehicle for the induction of protective anti-viral cell-mediated immunity. Proc Natl Acad Sci USA. 1995;92:3987–3991. doi: 10.1073/pnas.92.9.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh Y, Klimpel K R, Arora N, Sharma M, Leppla S H. The chymotrypsin-sensitive site, FFD315, in anthrax toxin protective antigen is required for translocation of lethal factor. J Biol Chem. 1994;269:29039–29046. [PubMed] [Google Scholar]
- 28.Splitter G, Oliveira S, Carey M, Miller C, Ko J, Covert J. T lymphocyte mediated protection against facultative intracellular bacteria. Vet Immunol Immunopathol. 1996;54:309–319. doi: 10.1016/s0165-2427(96)05703-0. [DOI] [PubMed] [Google Scholar]
- 29.Starnbach M N, Bevan M J. Cells infected with Yersinia present an epitope to class I MHC-restricted CTL. J Immunol. 1994;153:1603–1612. [PMC free article] [PubMed] [Google Scholar]
- 30.Starnbach M N, Bevan M J, Lampe M F. Protective cytotoxic T lymphocytes are induced during murine infection with Chlamydia trachomatis. J Immunol. 1994;153:5183–5189. [PubMed] [Google Scholar]
- 31.Studier F W, Moffatt B A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986;189:113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- 32.Thibault C, Nelson H, Chapoval A I. Tumor-infiltrating lymphocytes can be activated in situ by using in vivo activants plus F(ab′)2 bispecific antibodies. Int J Cancer. 1996;67:232–237. doi: 10.1002/(SICI)1097-0215(19960717)67:2<232::AID-IJC14>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 33.Torpey D D, Huang X L, Armstrong J, Ho M, Whiteside T, McMahon D, Pazin G, Heberman R, Gupta P, Tripoli C, et al. Effects of adoptive immunotherapy with autologous CD8+ T lymphocytes on immunologic parameters: lymphocyte subsets and cytotoxic activity. Clin Immunol Immunopathol. 1993;68:263–272. doi: 10.1006/clin.1993.1127. [DOI] [PubMed] [Google Scholar]
- 34.Traversari C, van der Bruggen P, Van den Eynde B, Hainaut P, Lemoine C, Ohta N, Old L, Boon T. Transfection and expression of a gene coding for a human melanoma antigen recognized by autologous cytolytic T lymphocytes. Immunogenetics. 1992;35:145–152. doi: 10.1007/BF00185107. [DOI] [PubMed] [Google Scholar]
- 35.White W I, Cassatt D R, Madsen J, Burket S J, Woods R M, Wassef N M, Alvings C R, Koenig S. Antibody and cytotoxic T-lymphocyte responses to a single liposome-associated peptide antigen. Vaccine. 1995;13:1111–1122. doi: 10.1016/0264-410x(94)00058-u. [DOI] [PubMed] [Google Scholar]
- 36.Whiteside T L, Elder E M, Moody D, Armstrong J, Ho M, Rinaldo C, Huang X, Torpey D, Gupta P, McMahon D, et al. Generation and characterization of ex vivo propagated autologous CD8+ cells used for adoptive immunotherapy of patients infected with human immunodeficiency virus. Blood. 1993;81:2085–2092. [PubMed] [Google Scholar]