Abstract
Introduction:
Holmium laser lithotripsy is a standard energy source used for treatment of kidney stones during flexible ureteroscopy. Efficiency of laser surgery may be affected by patient and operator characteristics or perioperative management. Here, we sought to examine intraoperative data from patients undergoing high frequency dusting with high-powered holmium laser lithotripsy to evaluate surgical and demographic factors associated with lasing efficiency (LE).
Methods:
A total of 82 intraoperative reports were analyzed from an ongoing laser lithotripsy clinical trial evaluating the Lumenis Pulse™ 120H holmium laser with renal stones up to 20 mm in diameter with and without Moses 2.0 technology. For each case, the total pause time between lasing activations was corrected to remove lengthy pauses and divided by the total lasing time to calculate an efficiency percentage. This was then compared with patient demographics, anesthesia administration, stone burden, postoperative complications, and stone-free rates using both univariate and multivariate analyses.
Results:
Of the 82 included patients, 36 received endotracheal tube (ETT) intubation and 46 had a laryngeal mask airway (LMA). Patients with ETT had significantly higher LE (78.7%) compared to those with an LMA (73.3%) in our univariate analysis (p < 0.01) as well as in the multivariate model that adjusted for maximum stone size, number of stones, stone density, and patient body mass index (p < 0.05). There was also significantly higher mean LE in patients with no postoperative complications (76.3%) compared to those with any grade (I–V) Clavien-Dindo complication within 30 days after surgery (70.0%) (p < 0.05).
Conclusions:
Flexible ureteroscopy and laser lithotripsy cases with higher LE are associated with lower rates of postoperative complications. The data also support the use of ETT over LMA to improve overall LE; however, this remains one consideration among many for choosing anesthesia administration.
Clinical Trial Registration number: NCT04505956.
Keywords: kidney stones, flexible ureteroscopy, laser lithotripsy, surgical efficiency
Introduction
Flexible ureteroscopy and laser lithotripsy (fURSL) utilizing high-powered holmium laser energy is the standard treatment option for kidney stones of various sizes, locations, and compositions.1 Despite advancements in fURSL, patient and operator characteristics can affect the overall surgical efficiency of laser lithotripsy. This has previously been evaluated through assessing total operative time, ablation speed, and perioperative parameters of stone-free rate and overall complication rates, all of which can be influenced by surgeon experience, patient habitus, and stone characteristics.2–4 Increased operative time has been associated with increased cost and risk of postoperative complications such as ureteral damage, bleeding, and urosepsis.3,5 However, operative time may not be sensitive enough to measure small, yet, potentially modifiable intraoperative differences between patients, such as respiratory motion.
During lithotripsy, patient breathing is known to cause clinically significant movement of the kidneys, reported up to 8.1 ± 4.33 mm in the superior-inferior direction.6 Depth and frequency of respirations may be influenced by factors such as body mass index (BMI) and airway securement by endotracheal tube (ETT) or laryngeal mask airway (LMA).7–9 Repeated movement can defocus laser energy from a visualized stone, requiring the surgeon to halt firing, creating pauses between laser activations. Although these pauses may not significantly affect overall operative time, they can potentially reduce visibility of stone fragments and thus affect stone-free rates and postoperative outcomes. Newer Lumenis Pulse™ 120H holmium laser systems provide access to timestamps and durations of foot pedal laser activations during surgery. These data allow for the calculation of the duration of pauses between laser firings and can be used to calculate a metric of laser surgery efficiency, herein known as “lasing efficiency (LE).”
In this study, we aimed to examine intraoperative data from patients undergoing high frequency dusting during fURSL to evaluate for surgical and demographic factors associated with changes in LE. In addition, we sought to investigate differences in postoperative complications and stone-free rates based on LE. We predicted that anesthesia administration would be one factor to significantly affect LE due to potential effect on patient breathing. We hypothesized that ETT intubation will increase LE due to a greater requirement for paralysis compared to LMA which may be further augmented by increased stone burden or patient BMI.
Methods
Data collection and patient cohort
Patient data were analyzed from an IRB-approved, ongoing clinical trial at Vanderbilt University Medical Center (VUMC) comparing ureteroscopic treatment of kidney stones up to 20 mm in maximum diameter via high frequency dusting fURSL with and without the use of Moses 2.0 technology (NCT04505956). Deidentified study data were provided using REDCap electronic data capture tools hosted at Vanderbilt University10,11 and included metrics such as patient demographics, operative parameters, and postoperative complications. Data also included Excel files of intraoperative laser logs exported directly from the Lumenis Pulse 120H holmium laser system, which records the total lasing time and energy used for each case in addition to timestamps and duration of individual foot pedal laser activations.
Data were collected in December 2022 and only includes patients who had surgery at VUMC between January 2021 and December 2022. Ureteroscopy in each case was solely performed by an attending endourologist utilizing a stone dusting technique. At the time of writing, 88 patients were available but only those with complete intraoperative laser logs and demographic data were included in this study (n = 82).
Calculating the metric of LE
Intraoperative data logs recorded from each case were analyzed. Timestamps indicating the start of each laser activation and the duration of firing were utilized to calculate the pause time leading up to the next laser activation. Adding all pause times between laser activations yielded a “total pause time” and subtracting the end timestamp of the last activation from the first activation yielded the “total lasing time.” In some cases, lengthy pauses reaching up to 10 minutes between laser activations were present due to switching laser fibers, analysis of patient imaging, or movement to a different location within the urinary tract. These outliers were removed to reduce bias, as these may represent false instances of decreased efficiency.
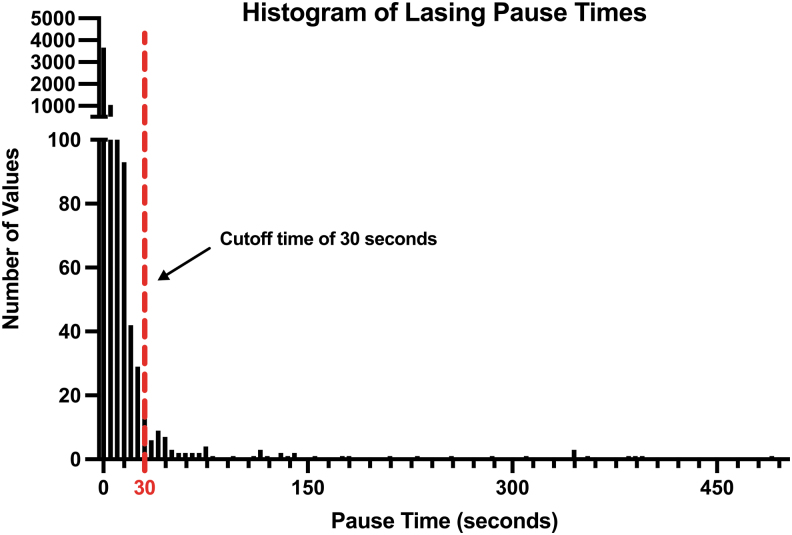
To determine a standardized cutoff point that immediate lasering was not occurring due to a change in instrumentation or location, lasing pause times across all cases were included in a histogram to determine the distribution of data (Fig. 1). Over 10,000 individual data points were included, and a cutoff value of 30 seconds was chosen, which was approximately at the 98.5th percentile. Anecdotally, this cutoff can also capture the time required for anesthesia personnel to deepen the patient or (re)administer paralytic agent. The total pause time for each case log was then modified to exclude any pause times greater than or equal to 30 seconds.
FIG. 1.
Histogram of individual count data of lasing pause times across all cases, including over 10,000 individual data points. Most pause times were recorded as 1–3 seconds; thus, the y-axis was broken at 100 to better show outlier values. Our chosen cutoff of 30 seconds is mapped above, and values greater than or equal to this value were excluded from the final analysis.
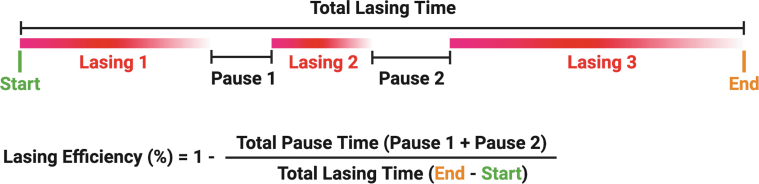
The final metric of LE (%) was calculated by dividing the total pause time (excluding pauses ≥30 seconds) by the total lasing time and subtracting this from 1 (Fig. 2). This metric shows the percentage of time the laser is actively in use, adjusting for total lasing time and pause time outliers.
FIG. 2.
Visual representation of the calculation of LE utilizing total pause time and total lasing time calculated from each laser case log. Of note, most logs contained anywhere from 50 to 200 laser activations. LE = lasing efficiency.
Covariates
The following variables were recorded for each patient: age, sex, race, American Society of Anesthesiologists (ASA) class, BMI, stone density in HU, number of stones, total laser energy (kJ), method of anesthesia administration (LMA or ETT), placement of stent or nephrostomy tube before surgery, stone location within the urinary tract (i.e., renal pelvis, upper pole, lower pole, ureteropelvic junction, etc.), use of a ureteral access sheath (UAS) during the surgery and UAS time, lasing mode (Moses 2.0 or Standard), maximum axial stone size determined by preoperative CT imaging, type of ureteroscope (digital or analog), total lasing time, total operative time, stone-free rate determined by presence of residual stone fragments on postoperative CT imaging, and postoperative complications within 30 days of surgery reported by Clavien-Dindo classification.
Statistical analysis
Data were assumed to follow a Gaussian distribution and parametric tests were used for statistical analysis. Simple linear regression was utilized to predict LE based on continuous variables (e.g., age, BMI, stone density). A two-tailed unpaired t-test was used for comparing mean LE between two categorical variables (e.g., sex, use of UAS, lasing mode), and a one-way analysis of variance was utilized to compare mean LE between three or more categorical variables (e.g., race, ASA classification, stone location). A one-tailed unpaired t-test was utilized to compare mean LE for ETT vs LMA since the original hypothesis predicted ETT to have higher LE. A multivariate linear regression was performed to evaluate five preoperative factors predictive of LE, including patient BMI, stone density, maximum axial stone size, number of stones, and method of anesthesia delivery. All statistical analysis was performed in GraphPad Prism version 10.0.3 (GraphPad Software © 2023. Boston, MA).
Results
Our primary objective was to identify operative and demographic factors associated with our calculated metric of LE. Within this objective, our main study comparison was mean LE between patients who received ETT and LMA during surgery. Of the 82 patients included in the study, 46 received an LMA and 36 received an ETT. Patients who received ETT had significantly higher mean LE compared to LMA (78.7% vs 73.3%, p < 0.01, one-tailed unpaired t-test), shown in Figure 3. There was no significant univariate relationship between mean LE and other demographic and operative variables, including patient BMI, stone density, stone size, and number of stones. Comparison of LE to patient demographics and operative parameters are listed in Table 1.
FIG. 3.
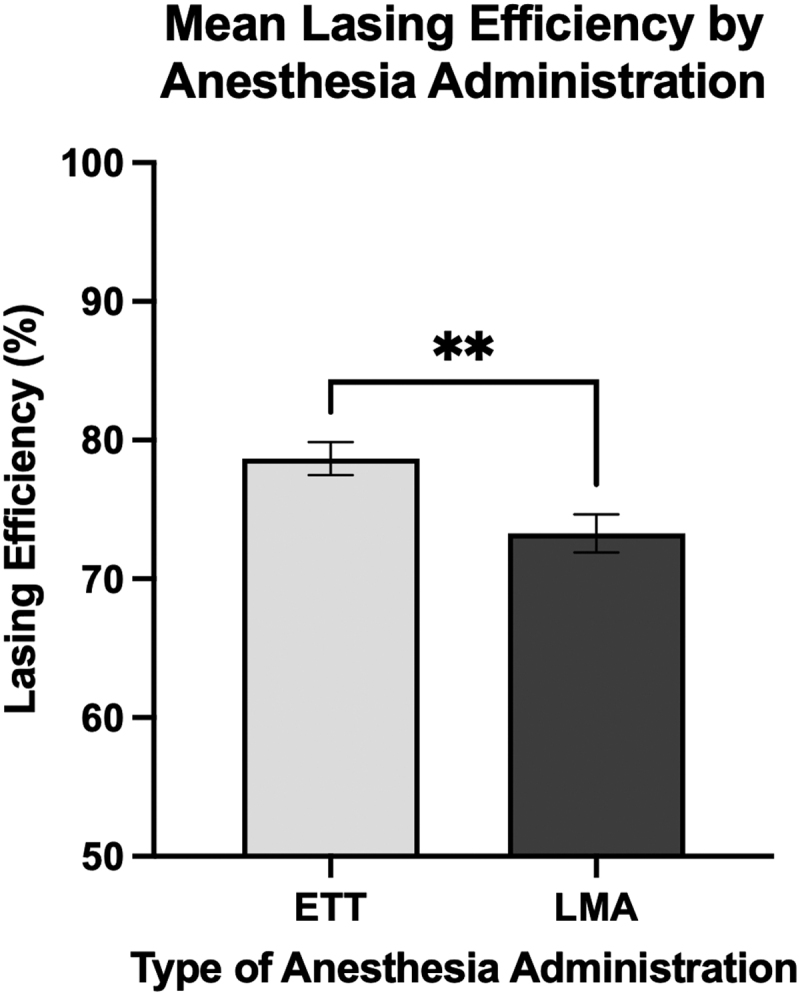
Patients who had an ETT had significantly higher mean LE compared to patients with LMA. There were 36 patients with an ETT and had mean LE of 78.7%, compared to 46 patients with an LMA who had a mean LE of 73.3%. Statistics performed by one-tailed unpaired t-test, comparing mean LE of both groups (**p < 0.01). Error bars show standard error of the mean. The y-axis starts from 50 to better illustrate differences between groups. ETT = endotracheal tube; LMA = laryngeal mask airway.
Table 1.
Comparison of Lasing Efficiency to Demographics, Operative Parameters, and Patient Outcomes
| Variables | R2 (N = 82) | p | |
|---|---|---|---|
| Age | 0.018 | 0.223 | |
| BMI | 0.040 | 0.070 | |
| Stone density (HU) | <0.001 | 0.811 | |
| Maximum axial stone size (mm) | <0.001 | 0.967 | |
| UAS time (minutes) | 0.039 | 0.096 | |
| Operating time (minutes) | 0.037 | 0.082 | |
| Total lasing energy (kJ) | 0.021 | 0.198 | |
| Patients (n) | Mean (SD) | p | |
|---|---|---|---|
| Sex |
|
|
0.423 |
| Female |
41 |
76.4 (7.8) |
|
| Male |
41 |
74.9 (9.6) |
|
| Race |
|
|
0.151 |
| Caucasian |
71 |
75.9 (9.1) |
|
| African American |
7 |
76.2 (5.2) |
|
| Latino |
2 |
71.1 (1.3) |
|
| Asian |
2 |
70.6 (9.6) |
|
| Anesthesia |
|
|
0.003
|
| ETT |
36 |
78.7 (7.1) |
|
| LMA |
46 |
73.3 (9.2) |
|
| ASA classification |
|
|
0.163 |
| 1 |
4 |
76.8 (4.5) |
|
| 2 |
39 |
74.5 (8.9) |
|
| 3 |
37 |
76.7 (8.9) |
|
| 4 |
2 |
76.0 (14.9) |
|
| Pre-op stent or nephrostomy |
|
|
0.801 |
| Stent |
19 |
75.2 (7.8) |
|
| Nephrostomy |
0 |
0 (0) |
|
| None |
63 |
75.8 (9.1) |
|
| Number of stones |
|
|
0.165 |
| 1 |
46 |
24.6 (8.9) |
|
| 2 |
24 |
22.8 (7.2) |
|
| 3 |
6 |
25.3 (8.2) |
|
| 4 |
1 |
34.6 (0) |
|
| 5 |
4 |
31.2 (12.8) |
|
| 6 |
1 |
8.8 (0) |
|
| Stone location |
|
|
0.963 |
| Renal pelvis |
17 |
76.8 (9.4) |
|
| Upper pole |
2 |
76.3 (7.9) |
|
| Lower pole |
22 |
75.6 (8.3) |
|
| Proximal ureter/UPJ |
15 |
74.2 (8.3) |
|
| Multiple locations |
26 |
75.7 (9.5) |
|
| Stone composition |
|
|
0.843 |
| CAP |
21 |
75.8 (8.0) |
|
| COD |
4 |
79.5 (6.5) |
|
| COM |
47 |
74.9 (9.2) |
|
| UA |
5 |
76.8 (7.4) |
|
| Unknown |
5 |
77.6 (12.0) |
|
| Use of UAS |
|
|
0.463 |
| Yes |
75 |
73.3 (10.6) |
|
| No |
7 |
75.9 (8.6) |
|
| Type of ureteroscope |
|
|
0.142 |
| Digital |
59 |
76.5 (7.9) |
|
| Analog |
23 |
73.4 (10.5) |
|
| Lasing mode |
|
|
0.778 |
| Moses |
41 |
75.9 (8.8) |
|
| Standard |
41 |
75.4 (8.8) |
|
| Residual fragments present on postoperative CT imaginga |
|
|
0.588 |
| Yes |
25 |
75.3 (10.1) |
|
| No |
37 |
74.1 (7.8) |
|
| Clavien-Dindo complication within 30 days of surgeryb |
|
|
0.033 |
| Yes (Grade I–V) |
10 |
70.0 (7.0) |
|
| None | 68 | 76.3 (8.7) |
Bold values represent p-values that are less than or equal to the significance level cut-off of 0.05.
Postoperative CT scans were not yet available for 20 cases.
Postoperative complications were not yet available for 4 cases.
ASA = American Society of Anesthesiologists; BMI = body mass index; CAP = calcium phosphate; COD = calcium oxalate dehydrate; COM = calcium oxalate monohydrate; ETT = endotracheal tube; LMA = laryngeal mask airway; SD = standard deviation; UA = uric acid; UAS = ureteral access sheath; UPJ = ureteropelvic junction.
Patients with ETT and LMA were dichotomized, and similar comparisons were made between demographic and operative parameters. Compared to patients with LMA, patients who received ETT had significantly higher BMI (36.1 vs 29.5, p < 0.01, one-tailed unpaired t-test) and lower mean stone density (999.7 vs 1197, p < 0.01, one-tailed unpaired t-test). The rest of these comparisons are shown in Table 2. The multivariate analysis additionally showed no significance between LE and the factors analyzed except for ETT over LMA. There was similarly no significant change in LE predicted by stone burden, density, or BMI. A summary of the multivariate linear regression model is shown in Table 3.
Table 2.
Comparison of Patients with Endotracheal Intubation and Laryngeal Mask Airway to Demographics, Operative Parameters, and Patient Outcomes
| Variables | ETT N = 36 |
LMA N = 46 |
p
|
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Age | 56.5 (13.4) | 53.6 (15.1) | 0.363 |
| BMI | 36.1 (9.9) | 29.5 (5.6) | <0.001 |
| Stone density (HU) | 999.7 (329.0) | 1197 (331.0) | 0.009 |
| Number of stones | 1.8 (1.0) | 1.7 (1.2) | 0.745 |
| Maximum axial stone size (mm) | 11.1 (3.6) | 11.5 (3.7) | 0.639 |
| UAS time (minutes) | 28.5 (10.9) | 28.0 (10.4) | 0.844 |
| Total lasing time (minutes) | 17.0 (11.1) | 18.6 (10.6) | 0.502 |
| Operating time (minutes) | 37.0 (11.2) | 36.0 (13.3) | 0.722 |
| Total lasing energy (kJ) | 12.9 (9.3) | 15.0 (10.3) | 0.335 |
| LE (%) | 78.7 (7.1) | 73.3 (9.2) | 0.003 |
| N (%) | N (%) | ||
|---|---|---|---|
| Sex (female) | 19 (52.7) | 22 (47.8) | 0.824 |
| ASA classification | |||
| 1 | 0 (0) | 4 (8.7) | 0.127 |
| 2 | 15 (41.7) | 24 (52.2) | 0.380 |
| 3 | 20 (55.6) | 17 (37.0) | 0.119 |
| 4 | 1 (2.8) | 1 (2.2) | 1 |
| Stone location | |||
| Renal pelvis | 7 (19.4) | 10 (21.7) | 1 |
| Upper pole | 1 (2.8) | 1 (2.2) | 1 |
| Lower pole | 11 (30.6) | 11 (23.9) | 0.617 |
| Proximal ureter/UPJ | 5 (13.9) | 10 (21.7) | 0.404 |
| Multiple locations | 12 (33.3) | 14 (30.4) | 0.815 |
| Use of UAS | 33 (93.8) | 42 (91.3) | 1 |
| Type of ureteroscope | 1 | ||
| Digital | 26 (72.2) | 33 (71.7) | |
| Analog | 10 (27.8) | 13 (28.3) | |
| Lasing mode | 1 | ||
| Moses | 18 (50.0) | 23 (50.0) | |
| Standard | 18 (50.0) | 23 (50.0) | |
| Residual fragments present on postoperative CT imaginga | 0.791 | ||
| Yes | 10 (43.5) | 15 (38.5) | |
| No | 13 (56.5) | 24 (61.5) | |
| Clavien-Dindo complication within 30 days of surgeryb | |||
| None | 33 (100.0) | 35 (77.8) | 0.004 |
| Grade I | 0 (0) | 5 (11.1) | 0.069 |
| Grade II | 0 (0) | 2 (4.4) | 0.506 |
| Grade IIIa | 0 (0) | 0 (0) | 1 |
| Grade IIIb | 0 (0) | 2 (4.4) | 0.506 |
| Grade Iva | 0 (0) | 0 (0) | 1 |
| Grade IVb | 0 (0) | 1 (2.2) | 1 |
| Grade V | 0 (0) | 0 (0) | 1 |
Bold values represent p-values that are less than or equal to the significance level cut-off of 0.05.
Postoperative CT scans were not yet available 13 ETT cases and 7 LMA cases.
Postoperative complications were not yet available for 3 ETT cases and 1 LMA case.
LE = lasing efficiency.
Table 3.
Multivariate Linear Regression of lasing Efficiency Compared to Anesthesia Type and Stone Burden
| Variables | OR (95% CI) | Standard error | p |
|---|---|---|---|
| BMI | 0.162 (−0.096 to 0.419) | 0.129 | 0.215 |
| Stone density (HU) | 0.005 (−0.002 to 0.011) | 0.003 | 0.161 |
| Maximum axial stone size (mm) | −0.043 (−0.571 to 0.485) | 0.265 | 0.872 |
| Number of stones | −0.137 (−1.850 to 1.577) | 0.861 | 0.874 |
| Anesthesia | |||
| ETT | 5.220 (1.113 to 9.326) | 2.062 | 0.013 |
| LMA | Reference | ||
| Sample size R2 |
82 0.129 |
||
Bold values represent p-values that are less than or equal to the significance level cut-off of 0.05.
Multivariate linear regression includes anesthesia type, maximum axial stone size, number of stones, stone density, and BMI.
CI = confidence interval; OR = odds ratio.
Our secondary objective was to evaluate differences in postoperative complication rates and stone-free rates based on LE. Postoperative complication data within 30 days of surgery by Clavien-Dindo classification grade were available for 78 patients in this study. Of these patients, 68 had no reported postoperative complications within 30 days of surgery and 10 patients had a reported postoperative complication. All 10 of these patients had an LMA, and the distribution by complication grade is listed in Table 2. Grade I complications included return to emergency department due to postoperative pain, nausea, fever, and urinary retention. Grade II complications included readmission for Escherichia coli bacteremia requiring IV antibiotics and readmission for Pseudomonas pyelonephritis. Grade IIIb complications required repeat ureteroscopy for obstructing stone fragments. One grade IVb complication included intensive care unit admission in the setting of sepsis and impacted stone fragments.
There was a significantly higher mean LE in patients with no postoperative complications compared to those with any grade (I–V) Clavien-Dindo complication (76.3% vs 70.0%, p < 0.05, two-tailed unpaired t-test), shown in Figure 4. Stone-free rates determined by residual stone fragments present on postoperative CT imaging were available for 62 patients. Of these patients, 37 had no residual fragments on postoperative imaging, while 25 had residual fragments. There was no significant difference in mean LE between these two groups (74.1% vs 75.3%, p = 0.588, two-tailed unpaired t-test), shown in Figure 5. Postoperative complication rates and stone-free rates are listed at the end of Table 1.
FIG. 4.
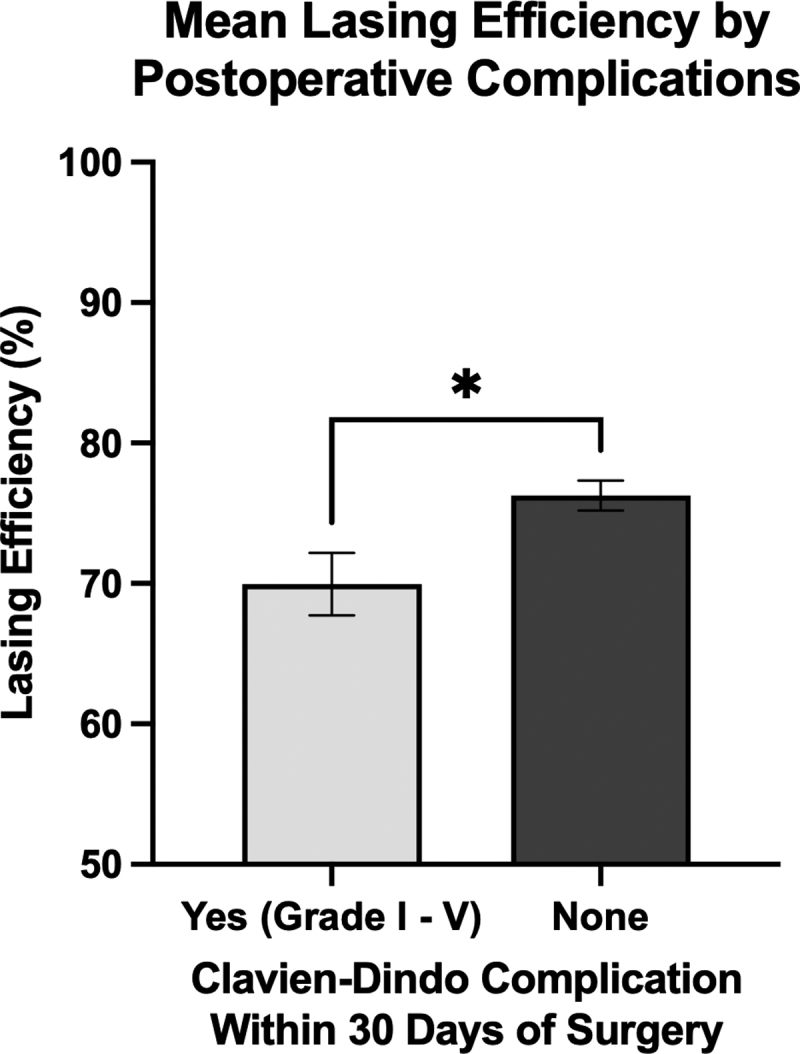
Patients with no reported postoperative complications within 30 days of surgery had significantly higher mean LE compared to those with any grade (I–V) postoperative Clavien-Dindo complication. There were 68 patients with no postoperative complications and had a mean LE of 76.3%, while there were 10 patients with postoperative complications who had a mean LE of 70.0%. Statistics performed by two-tailed unpaired t-test, comparing mean LE of both groups (*p < 0.05). Error bars show standard error of the mean. The y-axis starts from 50 to better illustrate differences between groups.
FIG. 5.
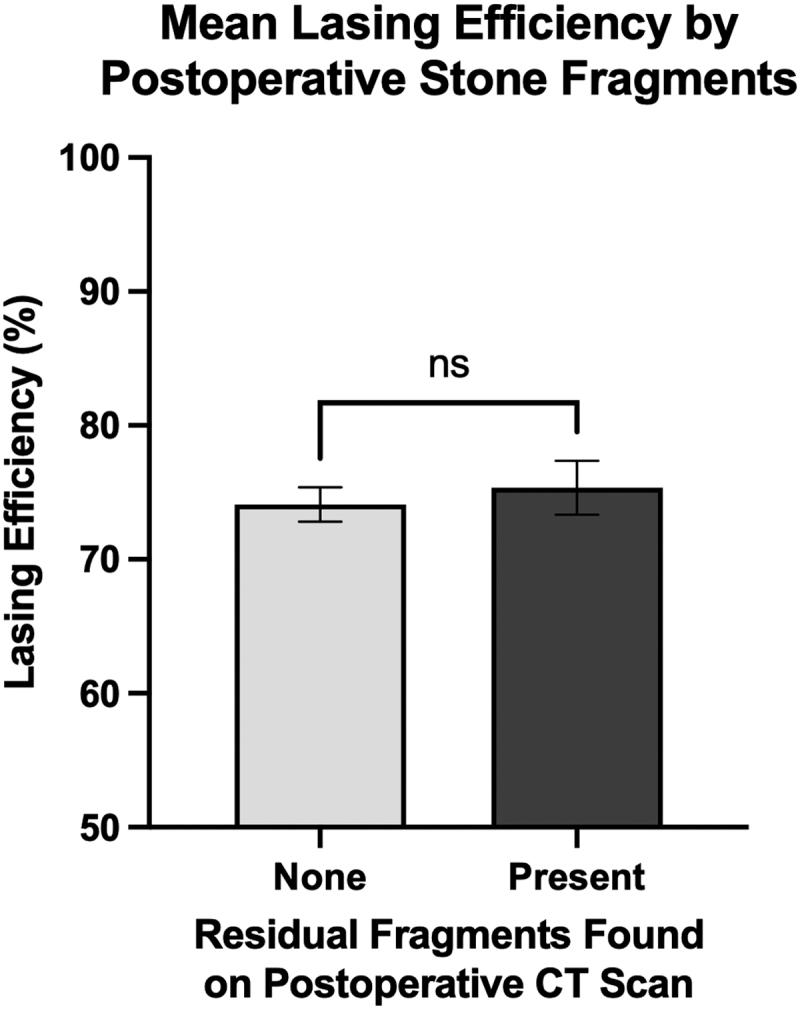
Patients who had no residual stone fragments present on postoperative CT imaging had no significant difference in mean LE compared to patients with residual fragments remaining after surgery. There were 37 patients with no residual stone fragments with a mean LE of 74.1% and there were 25 patients with residual fragments who had a mean LE of 75.3%. Statistics performed by two-tailed unpaired t-test, comparing mean LE of both groups (ns, p = 0.588). Error bars show standard error of the mean. The y-axis starts from 50 to better illustrate differences between groups.
Discussion
In this study, we utilized intraoperative data from Lumenis Pulse 120H holmium laser systems in patients undergoing high frequency dusting fURSL to calculate a new surgical efficiency metric of “LE.” By examining lengths of pauses between laser activations within each case, we established an algorithm determining the percentage of active time that the laser was presumably firing at a stone target, adjusted for total lasing time and removing time required to maneuver to different sites within the kidney, refocus on a stone due to patient breathing, or change laser fibers.
Prior studies have examined similar concepts. Wang et al. evaluated laser lithotripsy efficiency primarily through overall operating time, number of times the laser foot pedal was fired, laser working time, and laser pause time.12 They calculated a “stone fragmentation efficiency” defined as stone volume divided by laser working time, however, they did not use the duration of pauses to calculate their efficiency figure. Ventimiglia et al. estimated a metric of “laser activity” by dividing active laser time by total lithotripsy time to determine the proportion of time during lithotripsy that the laser pedal remains pressed.13 With their ratio, patients who have multiple stones will require longer lithotripsy time to navigate between calculi, which will artificially deflate the laser activity metric.
Majdalany et al. utilized a similar ratio.3 However, their study, they excluded patients with multiple stones, provides a more accurate representation of laser activity due to lack of navigation between stones. We attempted to correct this calculation by examining the lengths of pauses between individual laser activations provided through intraoperative data instead of the provided active lasing time. In certain surgical cases, there were pauses of up to 10 minutes in length between pedal activations. This could be due to a variety of factors, including navigation to a different calculus, change in instrumentation, or a review of patient imaging within the operating room. By setting a cutoff of 30 seconds and disregarding pause times greater than this, we were able to examine instances where there was a break in active lasering presumably caused by patient breathing or other obscuring movement, which we regarded as true instances of decreased efficiency.
We then compared LE to demographic and perioperative factors such as anesthesia administration and postoperative outcomes, including complication rate and stone-free rate. Apart from method of anesthesia administration, no other factors such as patient BMI, stone density, stone size, and number of stones were associated with changes in LE on both univariate and multivariate analysis. Patients who received ETT intubation had significantly higher LE compared to those with an LMA during surgery (78.7% vs 73.3%), shown in Figure 3. This may be due to decreased respiratory movement in patients with an ETT. With less respiratory movement, the surgeon theoretically would not have to halt firing to treat the stone, increasing LE. However, no current research has investigated changes in respiratory movement or overall surgical efficacy between ETT or LMA (with and without use of neuromuscular paralytic agents).
Furthermore, there is no widely accepted guideline for choosing a method of anesthesia administration during fURSL, and the choice is largely dependent on hospital or physician preference, patient BMI, and aspiration risk. LMA use over ETT is associated with benefits, including better patient tolerability and decreased requirement for paralytics.14,15 Although due to its supraglottic positioning, an LMA cannot protect against the risk of aspiration. LMAs are also not the preferred primary airway device in obese individuals.16 In such patients, a higher positive airway pressure is required to adequately inflate the lungs, which can lead to air leaks or risk of hypoventilation.17 These patients are also more likely to have comorbid diabetes which may lead to undetected gastroparesis and lead to retained stomach contents, further increasing aspiration risk.14
Moreover, we noted that patients with no postoperative complications were significantly associated with higher LE compared to those with any grade of Clavien-Dindo complication within 30 days of surgery (76.3% vs 70.0%), shown in Figure 4. In cases with high LE, it is thought that the stone is better visualized, allowing for more thorough dusting, leaving behind less fragments to cause postoperative complications. However, when we compared LE between patients with and without residual fragments on postoperative CT imaging, there was no significant difference, as shown in Figure 5.
This study is not without limitations. Stone volume was not available for most of the cases included in this patient cohort. Maximum axial stone dimension was used to best reflect stone size; however, this is not as accurate of a measurement compared to stone volume. We were also unable to measure renal anatomical differences between patients, including calix size, which may lead to differences in efficiency. Given our sample size, it is difficult to make definitive conclusions regarding use of ETT or LMA in ureteroscopy without controlling for factors such as BMI, but the data do tend to support our hypothesis. An ideal future study would utilize paired comparisons in a larger sample with randomized assignment to ETT or LMA groups regardless of BMI. However, we also recognize that this may not be feasible due to the contraindications of LMA use in some populations.
A large limitation of the study was lack of access to anesthesia data, including use of paralytic agents as well as tidal volumes during surgery which could be used to create more accurate comparisons between patient breathing and use of neuromuscular paralysis. Another major limitation of the study was our calculation of LE. Since the calculation of lasing pauses between firing has never been investigated in previous studies, validation of our cutoff for discarding larger pause times is required. Overall, our approach adds more information to consider but requires further validation to hold true. In the future, this algorithm can also be applied to other surgeries that utilize laser energy such as holmium laser enucleation of the prostate.
Conclusion
This study provides a novel analysis of intraoperative data and calculation of laser surgical efficiency utilizing pause times between laser activations instead of total lasing time. Our data show that cases with higher LE are associated with lower rates of postoperative complications but not overall stone-free rates. These data also support the use of ETT over LMA to improve efficiency when using holmium lasers in fURSL. However, this remains one consideration among many when choosing anesthesia administration.
Acknowledgments
The authors specially thank Zachary J. Friedman for assistance with statistical analysis of data included in this article. We also thank the Endourological Society for their contributions in involving medical students in urologic research through their annual summer scholarship.
Abbreviations Used
- ASA
American Society of Anesthesiologists
- BMI
body mass index
- CAP
calcium phosphate
- COD
calcium oxalate dehydrate
- COM
calcium oxalate monohydrate
- CT
computed tomography
- fURSL
flexible ureteroscopy with laser lithotripsy
- ETT
endotracheal tube
- HU
Hounsfield units
- LE
lasing efficiency
- LMA
laryngeal mask airway
- OR
odds ratio
- SD
standard deviation
- UA
uric acid
- UAS
ureteral access sheath
- UPJ
ureteropelvic junction
- VUMC
Vanderbilt University Medical Center
Authors' Contributions
D.G.G., R.S.H., and N.N. conceptualized and directed the project. D.G.G. performed the algorithmic calculations, statistical analysis, and wrote the initial draft of the article. K.R.I. assisted with statistical analysis and article writing. A.M.R. and N.L.M. performed data acquisition and contributed to the interpretation of results. N.L.M. supervised the findings of this work. All authors provided critical feedback and approved of the final submitted version of the article.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This work was supported by the Endourological Society Summer Scholarship. In addition, the publication described was supported by CTSA award No. UL1 TR002243 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
References
- 1. Dauw CA, Simeon L, Alruwaily AF, et al. Contemporary practice patterns of flexible ureteroscopy for treating renal stones: Results of a worldwide survey. J Endourol 2015;29(11):1221–1230; doi: 10.1089/end.2015.0260 [DOI] [PubMed] [Google Scholar]
- 2. Riveros CA, Chalfant V, Melchart T, et al. Does Moses technology improve the efficiency and outcomes of standard holmium laser lithotripsy? A systematic review and meta-analysis. Cent Eur J Urol 2022;75(4):409–417; doi: 10.5173/ceju.2022.156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Majdalany SE, Levin BA, Ghani KR. The efficiency of moses technology holmium laser for treating renal stones during flexible ureteroscopy: Relationship between stone volume, time, and energy. J Endourol 2021;35(S3):S14–S21; doi: 10.1089/end.2021.0592 [DOI] [PubMed] [Google Scholar]
- 4. Chen J, Chu T, Ghodoussipour S, et al. Effect of surgeon experience and bony pelvic dimensions on surgical performance and patient outcomes in robot-assisted radical prostatectomy. BJU Int 2019;124(5):828–835; doi: 10.1111/bju.14857 [DOI] [PubMed] [Google Scholar]
- 5. Whitehurst L, Pietropaolo A, Geraghty R, et al. Factors affecting operative time during ureteroscopy and stone treatment and its effect on outcomes: Retrospective results over 6.5 years. Ther Adv Urol 2020;12:1756287220934403; doi: 10.1177/1756287220934403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sonier M, Chu W, Lalani N, et al. Evaluation of kidney motion and target localization in abdominal SBRT patients. J Appl Clin Med Phys 2016;17(6):429–433; doi: 10.1120/jacmp.v17i6.6406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zaman B, Noorizad S, Safari S, et al. Efficacy of laryngeal mask airway compared to endotracheal tube: A randomized clinical trial. Anesthesiol Pain Med 2022;12(1):e120478; doi: 10.5812/aapm.120478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hodgson LE, Murphy PB, Hart N. Respiratory management of the obese patient undergoing surgery. J Thorac Dis 2015;7(5):943–952; doi: 10.3978/j.issn.2072-1439.2015.03.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sprung J, Whalley DG, Falcone T, et al. The effects of tidal volume and respiratory rate on oxygenation and respiratory mechanics during laparoscopy in morbidly obese patients. Anesth Analg 2003;97(1):268; doi: 10.1213/01.ANE.0000067409.33495.1F [DOI] [PubMed] [Google Scholar]
- 10. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42(2):377; doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform 2019;95:103208; doi: 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang M, Shao Q, Zhu X, et al. Efficiency and clinical outcomes of moses technology with flexible ureteroscopic laser lithotripsy for treatment of renal calculus. Urol Int 2021;105(7–8):587–593; doi: 10.1159/000512054 [DOI] [PubMed] [Google Scholar]
- 13. Ventimiglia E, Pauchard F, Gorgen ARH, et al. How do we assess the efficacy of Ho:YAG low-power laser lithotripsy for the treatment of upper tract urinary stones? Introducing the Joules/mm3 and laser activity concepts. World J Urol 2021;39(3):891–896; doi: 10.1007/s00345-020-03241-9 [DOI] [PubMed] [Google Scholar]
- 14. Jannu A, Shekar A, Balakrishna R, et al. Advantages, disadvantages, indications, contraindications and surgical technique of laryngeal airway mask. Arch Craniofacial Surg 2017;18(4):223–229; doi: 10.7181/acfs.2017.18.4.223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Danks RR, Danks B. Laryngeal mask airway: review of indications and use. J Emerg Nurs 2004;30(1):30–35; doi: 10.1016/j.jen.2003.11.006 [DOI] [PubMed] [Google Scholar]
- 16. Mason-Nguyen JA, Rodriguez RE. Laryngeal mask airway use in morbidly obese patients undergoing general anesthesia. AANA J 2017;85(2):130–135. [PubMed] [Google Scholar]
- 17. Kim J-N, Lee J-Y, Shin K-J, et al. Haversian system of compact bone and comparison between endosteal and periosteal sides using three-dimensional reconstruction in rat. Anat Cell Biol 2015;48(4):258–261; doi: 10.5115/acb.2015.48.4.258 [DOI] [PMC free article] [PubMed] [Google Scholar]