Abstract
Anal squamous cell carcinoma (SCC) is not a common disease in the general population, although its incidence is higher in people living with human immunodeficiency virus (PLWH). Anal SCC is caused by human papillomavirus (HPV) infection and arises from premalignant lesions termed squamous intraepithelial lesions (SILs). SIL surveillance programs are based on the early detection and treatment of SILs, especially those with a higher risk of transforming into cancer. An anal surveillance program has been under development in our institution since 2011. In this context, we performed a retrospective cohort study at the anal dysplasia unit of Álvaro-Cunqueiro Hospital (Spain). Epidemiological and clinical data were gathered from our Infectious Diseases Sample Collection (an open sample cohort including PLWH) from January 2011 to January 2022. A total of 493 PLWH were considered, 122 (24.7%) of whom were diagnosed with anal dysplasia at baseline, including 2 cases of anal SCC. Briefly, most of individuals were young men (median age, 38 years old) born in Spain (76%), whose vaccination rate before their inclusion in the program was scarce (<3%). Throughout the study period, 81 (16.4%) cases were diagnosed with high-grade squamous-intraepithelial lesions (HSILs) and 3 with anal SCC. At the baseline, severe immunosuppression (i.e., nadir CD4+ lymphocyte count below 200 cell/μL), and prior diagnosis of condyloma acuminata were more frequent within the group with SILs. Conversely, the baseline CD4+ lymphocyte count was similar among both groups. HPV-16 was related to a higher risk of HSILs (odds ratio: 2.76). At the end of the follow-up, 385 PLWH had been retained in care; one patient had died of anal cancer. Anal dysplasia was common (25% of cases), especially among patients infected by HPV-16, diagnosed with condyloma acuminata, and who were severely immunosuppressed. HPV-16 was the main risk factor for the presentation of HSILs.
Keywords: human immunodeficiency virus (HIV), human papillomavirus (HPV), anal dysplasia, squamous anal cancer, screening
Introduction
With a prevalence of 1.2–2 cases per 100,000 inhabitants, anal squamous cell carcinoma (SCC) is not a common disease in the general population.1,2 However, it is more common among people living with human immunodeficiency virus (PLWH), reaching an incidence of 90–130 cases per 100,000 inhabitants.2,3 Anal SCC arises from premalignant lesions, termed squamous intraepithelial lesions (SILs) and shares a similar mechanism of action to cervical SSC. Human papillomavirus (HPV) infection is the main cause of both cervix and anal SSCs, although most of cases of HPV spontaneously resolve without consequence.4 However, several conditions, especially those compromising the immune system [e.g., human immunodeficiency virus (HIV) infection], increase the risk of anogenital dysplasia.5
Persistent HPV infection induces several changes in the anal canal (i.e., basal epithelial stratum invasion, persistent low-grade replication, and impaired cell cycle regulation) especially in the transformation zone. In the long term, these changes may drive to the formation of SILs, classified based on the severity of the histological changes present. Mild alterations are referred to as low-grade squamous-intraepithelial lesions (LSILs), while high-grade squamous-intraepithelial lesions (HSILs) are characterized by more intense histological changes such as severe keratinization and loss of normal cell architecture. A higher risk of malignization has been reported for the latter form, although a significant proportion may regress into LSILs or remain stable over the time.6 In addition, severe immunosuppression [i.e., a nadir CD4+ lymphocyte count under 200 cells/μL or a prior acquired immunodeficiency syndrome (AIDS) diagnosis] is related to a higher risk of transforming into an anal SSC.7,8
SSCs usually require several years of progressive histological changes and cumulative DNA mutations to develop and so SIL surveillance programs are based on the early diagnosis of premalignant lesions and the treatment of those with a higher risk of transforming into invasive carcinomas (i.e., HSILs). The first successful surveillance systems were developed for cervix SCC and served as a model for the anal counterpart. The main components of anal SCC surveillance programs are anal cytology, HPV DNA detection, anal examinations, and high-resolution anoscopy (HRA). Most HRA instruments are adaptations of colposcopes which allow direct visualization of the anal canal with the aid of tissue impregnations with acetic acid and aqueous iodine. In addition to its diagnostic capability, HRA can also be employed to carry out local treatments such as electroablation. Of note, the recent ANCHOR clinical trial (NCT02135419), “Treatment in Preventing Anal Cancer in Patients with HIV and Anal High-Grade Lesions,” which included more than 4400 patients, reported a lower progression rate to cancer in patients with HSILs that were treated early compared to those that were actively monitored (173 anal cancer cases per 100,000 person-years vs. 402 per 100,000 person-years, respectively).9 Thus, in this context, the aim of this current study was to evaluate the results of an anal screening programme implemented since 2011 in a real-life cohort of PLWH.
Methods
Study design and participants
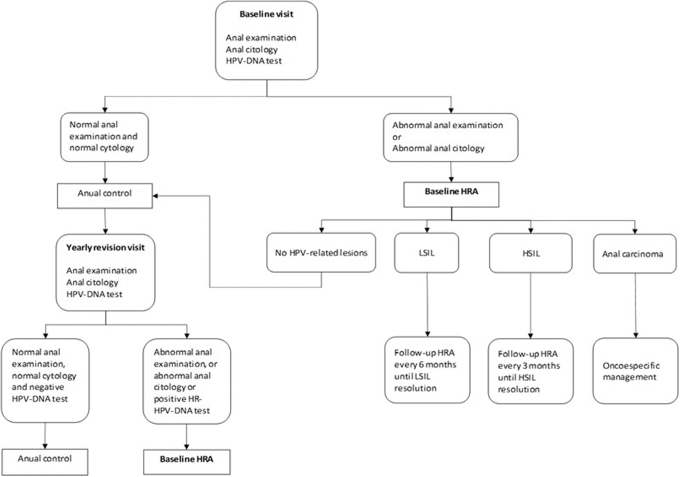
A retrospective cohort study was undertaken at the Álvaro Cunqueiro Hospital in Vigo (Spain), a tertiary hospital serving a population of around 500,000 inhabitants. An anal dysplasia-screening program was started at this center in January 2011 as a multidisciplinary unit formed by the General Surgery Department and the Infectious Diseases Unit. Patients included in the anal dysplasia screening were invited to contribute to the Infectious Diseases Sample Collection (reference No. C.0004320), an open cohort of biological samples and clinical data obtained from corresponding medical records. This collection belongs to the Galicia Sur Research Institute Biobank, and each patient signed their informed consent when their tissue samples were included. Baseline patient visits consisted of an anal examination, anal cytology, and an anal smear HPV test. HRA was then performed according to the protocol described in Fig. 1. The study period considered in this work started in January 2011, with data censoring on January 31, 2022. Only PLWH who had attended the baseline visit were included in this current study analysis (Fig. 2).
FIG. 1.
Algorithm for the surveillance of squamous intraepithelial lesions.
FIG. 2.
Flowchart for the inclusion and exclusion of patients in this study.
Sampling and HPV detection
During the baseline and follow-up visits, a liquid-based biopsy was obtained for cytology (Thin Prep® PreservCyt® Solution; Hologic) which was later processed in the Pathology Department. Samples were examined by a pathologist and were classified according to the standard cytology criteria as normal, atypical squamous cells of undetermined significance (ASCUS), LSILs, HSILs, or anal SCC. Anal samples were then tested for high-risk HPV (HR-HPV) using the Cobas 4800 system (Roche, Basel, Switzerland) and classified based on their genotype: HPV-16, HPV-18, or a another high-oncogenic risk genotype in a pooled analysis [pooled high-oncogenic risk human papillomavirus (pHR-HPV): HPV-31, HPV-33, HPV-35, HPV-39, HPV-45, HPV-51, HPV-52, HPV-56, HPV-58, HPV-59, HPV-66, or HPV-68]. All samples were obtained in the hospital facilities by trained personnel (i.e., nurses or physicians), and self-administered pap was not considered. Anal biopsies obtained during HRAs were also analyzed by an expert pathologist and classified according to the current recommendations.
High-resolution anoscopy
HRA was performed in the Anal Dysplasia Unit by a specialized surgeon using an adapted Carl Zeiss 150 FC (Jena, Germany) colposcope. Acetic acid and aqueous iodine (Lugol) impregnations were used to reveal suspected SILs (Fig. 3) which were then biopsied, with follow-up HRAs being scheduled according to the SIL grade (Fig. 1). Cases of anal SCC were referred to a cancer committee that prescribed specific treatment for the disease, with follow-up HRAs performed every 3 months.
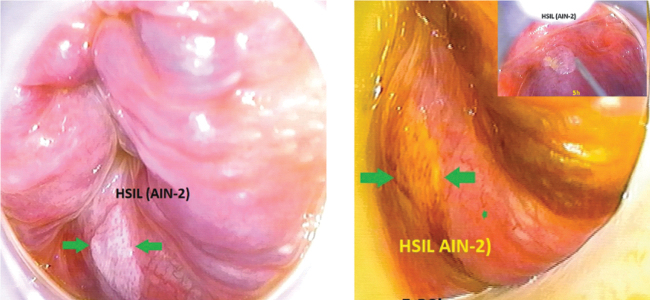
FIG. 3.
Images of anal tissue suspected of anal intraepithelial neoplasia taken during the high-resolution anoscopy examinations. Images provided courtesy of Dr. Pilar Fernández in the Álvaro Cunqueiro Hospital General Surgery Department. AIN-2, type-2 anal intraepithelial neoplasia; HSIL, high-grade squamous-intraepithelial lesion.
Definition of variables
Baseline dysplasia was defined as a SIL diagnosed at the time a patient joined the program, while incident dysplasia was defined as a new SIL diagnosed during a follow-up, regardless of whether the patient had been affected by a SIL at baseline. According to the current recommendations, type-1 anal intraepithelial neoplasia (AIN-1) lesions were classified as LSILs, and type-2 and 3 AIN-2 and AIN-3 lesions were classified as HSILs.
Statistical analysis
The baseline patient characteristics were analyzed using descriptive statistics. Quantitative variables were expressed as medians and interquartile ranges; qualitative variables were shown as absolute values and percentages. Categorical variables were compared using chi-squared or Fischer exact tests, as appropriate; quantitative variables were compared using Mann–Whitney U tests. The statistical analyses were performed using SPSS software (version 22; IBM Corp., Armonk, NY) and probabilities exceeding 95% (alpha p values <0.05) were considered significant.
Ethics
All the procedures performed in studies involving human participants were conducted in accordance with the ethical standards of the institutional research committee and the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was approved by the Ethics Committee of Pontevedra-Vigo-Ourense on February 16, 2023 (reference No. 2023/066). All the patients included in the Infectious Diseases Sample Collection signed an informed consent for their inclusion.
Results
A total of 493 patients were included in our analysis (Fig. 2). The baseline characteristics of the study population are summarized in Table 1. Briefly, most of the patients were young men born in Spain; 70% had had a sexually transmitted infection (STI) other than HIV in the past, with syphilis accounting most cases. Around 15.0% (74) of the study population had had a prior diagnosis of an AIDS-defining illness, while 23.7% (117) had a nadir CD4+ lymphocyte count bellow 200 cells/μL.
Table 1.
Baseline Characteristics of the Study Population
| Sex | |
| Men | 473 (95.9%) |
| Transgender women | 20 (4.1%) |
| Age, in years | 38 (17) |
| Ethnicity | |
| Spanish | 372 (75.5%) |
| Latin-American | 108 (21.9%) |
| Other | 13 (2.6%) |
| Tobacco consumption | |
| Active smoker | 28 (44.2%) |
| Former smoker | 30 (6.1%) |
| Never smoker | 238 (48.3%) |
| Unknown | 7 (1.4%) |
| HCV positive antibodies | 18 (3.7%) |
| Previous STI | 357 (70.4%) |
| Condyloma acuminate | 151 (30.6%) |
| Syphilis | 230 (46.7%) |
| Gonorrhoea | 79 (16.0%) |
| Chlamydia trachomatis infection | 36 (7.3%) |
| Received HPV vaccine before inclusion | 14 (2.8%) |
| Prior AIDS-defining illness | 74 (15.0% |
| Nadir CD4+ | 344.5 (268) |
| Nadir CD4+ below 200 cells/μL | 117 (23.7%) |
| CD4+ count at the time of HIV diagnosis | 667 (440) |
| Baseline HPV | |
| Any HR-HPV | 394 (79.9%) |
| HPV-16 | 170 (34.5%) |
| HPV-18 | 72 (14.6%) |
| pHR-HPV | 363 (73.6%) |
| Baseline cytology | |
| Normal cytology | 286 (58.0%) |
| LSIL cytology | 95 (19.3%) |
| HSIL cytology | 19 (3.9%) |
| ASCUS | 93 (18.9%) |
| Dysplasia at baseline | 122 (24.7%) |
| LSIL (AIN-1) | 70 (14.2%) |
| HSIL (AIN-2/3) | 50 (10.1%) |
| Anal SCC | 2 (0.4%) |
Qualitative variables are expressed as total numbers and percentages; quantitative variables are presented as medians and interquartile ranges.
AIDS, acquired immunodeficiency syndrome; AIN, anal intraepithelial neoplasia; ASCUS, atypical squamous cells of undetermined significance; HCV, hepatitis C virus; HIV, human immunodeficiency virus; HPV, human papillomavirus; HR-HPV, high-risk HPV; HSIL, high-grade squamous-intraepithelial lesion; LSIL, low-grade squamous-intraepithelial lesion; pHR-HPV, pooled high-oncogenic risk human papillomavirus; SCC, squamous cell carcinoma; STI, sexually transmitted infection.
The baseline prevalence of cytological abnormalities and anal dysplasia
At baseline, a probable abnormal cytology result was detected in 207 (42.0%) participants: ASCUS in 93 individuals (18.9%), LSIL in 95 (19.3%), and HSIL in 19 (3.5%). A HR-HPV genotype was detected in 394 (79.9%) patients, while HPV-16 and HPV-18 were identified in 170 (34.5%) and 72 (14.6%) cases, respectively. A HRA was recommend to every individual in which an abnormal cytology was detected, although nine patients declined this offer. From among those who underwent an HRA (198 patients), 122 were diagnosed with anal dysplasia of any grade. Thus, the prevalence of dysplasia detected at baseline in the overall study population was around 25%. Most of these patients were diagnosed with mild dysplasia (n = 70, 14.2%), while HSIL was detected in 50 (10.1%) and an anal SSC was diagnosed in 2 (0.4%).
The sample was grouped into two groups according to the presence of dysplasia (Table 2). The variables of age, tobacco consumption, time living with HIV, and a prior diagnosis of a STI were similar between both groups (72.1% vs. 69.9% in the latter case). Nonetheless, a prior diagnosis of an AIDS-defining illness was more common among participants diagnosed with dysplasia (23.0% vs. 12.7%, p = 0.007). Moreover, the group with dysplasia exhibited a slightly lower nadir CD4+ lymphocyte count (339 cells/μL vs. 345 cells/μL, p = 0.047), and a higher prevalence of HPV infection (94.3% vs. 74.9%, p < 0.001). The presence of anogenital condylomas was also more common among the group with dysplasia (41.0% vs. 26.8%, p = 0.003).
Table 2.
Characteristics of the Study Participants With and Without Squamous Intraepithelial Lesions at Baseline
| SIL at baseline, n = 122 (24.7%) | No SIL at baseline, n = 362 (74.4%) | p | |
|---|---|---|---|
| Age, in years | 37 (17) | 39 (17) | 0.188 |
| Current smoker | 58 (47.9%) | 155 (43.4%) | 0.388 |
| Time since HIV diagnosis, in years | 9 (9.25) | 9 (9.5) | 0.612 |
| Any history of an AIDS-defining illness | 28 (23.0%) | 46 (12.7%) | 0.007 |
| Baseline CD4+ lymphocyte count, cells/μL | 700 (542) | 650 (374) | 0.316 |
| Nadir CD4+ lymphocyte count, cells/μL | 339 (321) | 345 (249) | 0.047 |
| Nadir CD4+ lymphocyte count <200 cells/μL | 38 (31.7%) | 75 (21.2%) | 0.021 |
| HCV positive antibodies | 8 (6.6%) | 10 (2.8%) | 0.091 |
| Previous diagnosis of any STI | 88 (72.1%) | 253 (69.9%) | 0.639 |
| Syphilis | 51 (41.8%) | 176 (48.5%) | 0.192 |
| Gonorrhea | 19 (15.6%) | 60 (16.6%) | 0.796 |
| Chlamydia trachomatis infection | 10 (8.2%) | 25 (6.9%) | 0.639 |
| Anogenital condyloma acuminate | 50 (41.0%) | 97 (26.8%) | 0.003 |
| Baseline HR-HPV | 115 (94.3%) | 271 (74.9%) | <0.001 |
| Baseline HPV-16 | 63 (51.6%) | 103 (28.5%) | <0.001 |
| Baseline HPV-18 | 29 (23.8%) | 42 (11.6%) | 0.002 |
| Baseline pHR-HPV | 106 (86.9%) | 249 (68.8%) | <0.001 |
Qualitative variables are expressed as total numbers and percentages; quantitative variables are expressed as medians and interquartile ranges.
HCV, hepatitis C virus; HIV, human immunodeficiency virus; HPV, human papillomavirus; HR-HPV, high-risk HPV; pHR-HPV, pooled high-oncogenic risk human papillomavirus; SIL, squamous intraepithelial lesion; STI, sexually transmitted infection.
Retention in care during the study period
Among the patients included in the screening programme, 411 (83.4%) completed both the baseline visit and at least one follow-up visit, while 53 (16.6%) attended the baseline visit but did not schedule a follow-up appointment. Throughout the study period, a total of 385 (78.1%) individuals were retained in care and 108 (21.9%) left the screening program: 62 (12.6%) abandoned the program without giving a reason, 42 (8.5%) moved to another city, and 4 (0.8%) died. A total of 112 (22.7%) patients were diagnosed with anal dysplasia throughout the study period and presented HSILs and LSILs in a similar proportion (51 and 58, respectively); anal SSC was detected in 3 individuals.
The clinical characteristics of patients diagnosed with a HSIL
During the study period, a total of 81 patients (16.4%) received a diagnosis of a HSIL, 50 at baseline and 31 during the follow-up. The main characteristics of both these groups are shown in Table 3. Briefly, age, tobacco consumption, time living with HIV, and immune status (i.e., prior diagnosis with an AIDS-defining illness and nadir CD4+ lymphocyte count) were similar among them both. On the contrary, the prevalence of condyloma acuminata (42.0% vs. 26.8%, p = 0.015) and HPV infection (96.3% vs. 76.7%, p < 0.001) were both more common among the cohort with HSILs.
Table 3.
Characteristics of the Study Participants With and Without High-Grade Squamous Intraepithelial Lesions at Baseline or During Follow-Up
| HSIL, n = 81 (16.4%) | No HSIL, n = 412 (83.6%) | Total, n = 493 (100%) | p | |
|---|---|---|---|---|
| Age, in years | 38 (14) | 38 (17) | 38 (16.8) | 0.807 |
| Active smoker | 37 (46.7%) | 181 (44.6%) | 218 (44.9%) | 0.784 |
| Time since HIV diagnosis, in years | 11 (7.5) | 9 (9) | 9 (9) | 0.065 |
| Any history of AIDS-defining illness | 14 (17.3%) | 60 (14.6%) | 74 (15.0%) | 0.501 |
| Baseline CD4+ lymphocyte count, cells/μL | 445 (427) | 429 (402) | 438 (408) | 0.761 |
| Nadir CD4+ lymphocyte count, cells/μL | 340 (337) | 363 (276) | 354 (281) | 0.138 |
| Nadir CD4+ lymphocyte count <200 cells/μL | 24 (30.0%) | 93 (23.1%) | 117 (24.3%) | 0.200 |
| HCV positive antibodies | 5 (6.2%) | 13 (3.2%) | 18 (3.7%) | 0.194 |
| Previous diagnosis of any STI | 61 (75.3%) | 286 (69.4%) | 347 (70.4%) | 0.351 |
| Syphilis | 40 (49.4%) | 190 (46.1%) | 230 (46.7%) | 0.590 |
| Gonorrhea | 16 (19.8%) | 63 (15.3%) | 79 (16.0%) | 0.322 |
| Chlamydia trachomatis infection | 6 (7.4%) | 30 (7.3%) | 36 (7.3%) | 1.000 |
| Anogenital condyloma acuminata | 34 (42.0%) | 117 (28.4%) | 151 (30.6%) | 0.015 |
| Baseline HR-HPV | 78 (96.3%) | 316 (76.7%) | 394 (79.9%) | <0.001 |
| Baseline HPV-16 | 48 (59.3%) | 122 (29.6%) | 170 (34.5%) | <0.001 |
| Baseline HPV-18 | 22 (27.2%) | 50 (12.1%) | 72 (14.6%) | 0.001 |
| Baseline pHR-HPV | 73 (90.1%) | 290 (70.4%) | 363 (73.6%) | <0.001 |
Qualitative variables are expressed as total numbers and percentages; quantitative variables are expressed as medians and interquartile ranges.
HCV, hepatitis C virus; HIV, human immunodeficiency virus; HPV, human papillomavirus; HR-HPV, high-risk HPV; HSIL, high-grade squamous-intraepithelial lesion; pHR-HPV, pooled high-oncogenic risk human papillomavirus; STI, sexually transmitted infection.
Risk factors for HSILs during the follow-up of PLWH without SILs at baseline
At the baseline visit, 362 patients did not have anal dysplasia, of whom 296 completed at least one follow-up visit 12 months later. In this subgroup, 18 patients (6.1%) were later diagnosed with an incident HSIL (Table 4). Of note, poorer immune status was not associated with a higher incidence of HSIL. Condyloma acuminata was more common in patients with HSIL but this factor did not reach statistical significance (38.9% vs. 27.0%). Nonetheless, a diagnosis of HPV-16 or pHR-HPV at baseline was related to an increased incidence of HSIL (odds ratio = 2.76 and 7.87, respectively). Detection of HPV-18 at baseline was more common in the group diagnosed with incident HSIL, although this did not reach statistical significance.
Table 4.
Risk Factors for High-Grade Squamous Intraepithelial Lesions in the Study Participants with No Squamous Intraepithelial Lesions at Baseline
| Incident HSIL, n = 18 (6.1%) | No incident HSIL, n = 278 (93.1%) | Total, n = 296 (100%) | p | OR (95% CI) | |
|---|---|---|---|---|---|
| Age, in years | 41.5 (15.3) | 40 (17.5) | 40 (17) | 0.394 | |
| Baseline CD4+ lymphocyte count, cells/μL | 428 (378) | 403 (426) | 406 (426) | 0.728 | |
| Nadir CD4+ lymphocyte count, cells/μL | 345 (274) | 346.5 (268) | 345 (261) | 0.716 | |
| Nadir CD4+ lymphocyte count <200 cells/μL | 2 (11.1%) | 57 (21.0%) | 59 (19.9%) | 0.544 | |
| HCV positive antibodies | 0 | 8 (2.9%) | 8 (2.7%) | 1.000 | |
| Previous diagnosis of any STI | 15 (83.3%) | 195 (70.1%) | 210 (70.9%) | 0.232 | |
| Syphilis | 12 (66.7%) | 137 (49.3%) | 149 (50.3% | 0.223 | |
| Gonorrhea | 3 (16.7%) | 46 (16.5%) | 49 (16.6%) | 1.000 | |
| Chlamydia trachomatis infection | 2 (11.1%) | 13 (4.7%) | 15 (5.1%) | 0.229 | |
| Anogenital condyloma acuminata | 7 (38.9%) | 75 (27.0%) | 82 (27.7%) | 0.284 | |
| Baseline HR-HPV | 17 (94.4%) | 204 (73.4%) | 221 (74.4%) | 0.046 | 6.17 (0.81–47.15) |
| Baseline HPV-16 | 9 (50%) | 74 (26.6%) | 83 (28.0%) | 0.032 | 2.76 (1.05–7.21) |
| Baseline HPV-18 | 4 (22.4%) | 28 (10.1%) | 32 (10.8%) | 0.108 | |
| Baseline pHR-HPV | 17 (94.4%) | 190 (68.3%) | 207 (69.9%) | 0.019 | 7.87 (1.03–60.11) |
Qualitative variables are expressed as total numbers and percentages; quantitative variables are expressed as medians and interquartile ranges.
CI, confidence interval; HCV, hepatitis C virus; HPV, human papillomavirus; HR-HPV, high-risk HPV; HSIL, high-grade squamous-intraepithelial lesion; OR, odds ratio; pHR-HPV, pooled high-oncogenic risk human papillomavirus; STI, sexually transmitted infection.
The clinical characteristics of patients diagnosed with anal squamous cell carcinoma
During the study period, a total of five patients were diagnosed with an anal SCC, of whom two were detected at baseline and three were incident cases; the latter had all been previously diagnosed with an HSIL at baseline and HPV-16 had been detected at baseline in all of them. The median age of patients diagnosed with anal SSC was 45 years and three of them had previously suffered an AIDS-defining illness. All five of these patients received chemoradiotherapy according to the usual standards of care. One case, who had been diagnosed at baseline, died 2 months later as a result of disease progression, while the other four patients survived to the end of the study period.
Discussion
HPV infection is the most common STI around the world and is a major cause of anogenital cancer, especially among PLWH.5 Indeed, the higher incidence of anal SSC among PLWH compared to the HIV-uninfected population fuelled the development of several anal cancer screening strategies, including ours10 (Fig. 1). Among our study population, the prevalence of HPV infection (including HPV-16 and HPV-18) was high (79.9%), in line with previously reported data.11–13 HPV-16 and HPV-18 are the main causes of several cancers, especially anal SSC, in which one of these two virus strains can be detected in 87% of cases.14 In recent years, three HPV vaccines have been developed which promptly became the mainstay for HPV prevention in the general population, regardless of HIV status.15 A clinical trial comparing the quadrivalent vaccine (which offers coverage against HPV-6, 11, 16, and 18) to a placebo, reported a lower incidence of anal intraepithelial neoplasia or anal cancer related to these HPV genotypes (20.5 vs. 906.2 cases per 10,000 person-years).15 The baseline vaccination rate in our study population was low because most of these patients were included before the approval of these vaccines for PLWH. However, universal vaccination is now recommended, with a special focus on key populations such as PLWH.16
The overall prevalence of SILs in our study cohort was high, both at baseline and during the follow-up (33.5%), although HSILs were less common (16.4%). An accurate comparison with other screening program is difficult because of the heterogenicity of the research carried out to date and the design of anal screening programs. Nevertheless, Sanger et al. reported a prevalence of advanced anal disease (HSIL or anal SCC) of around 33%,17 while Jongen et al., reported a slightly lower rate of 23.4%.18 Wells et al. carried out a cross-sectional study analyzing a total of 150 individuals for whom an abnormal anal cytology was detected during the follow-up (LSIL 43.5%; ASCUS 40.8%, HSIL 5.9%). The authors reported a lower likelihood of receiving HRA among those patients affected by HIV-stigma and those with a low health motivation.19
Of note, severe immunodepression (i.e., a CD4+ lymphocyte count below 200 cells/μL or a prior AIDS diagnosis) has been reported as a risk factor for anal dysplasia8,20,21 and progression to HSIL or SCC.18 In contrast, in our study population, a poor immune status was related to an increased frequency of overall SILs but not specifically with HSILs and baseline immune status was not associated with a higher risk of incident HSILs.
In turn, the presence of condyloma acuminata was frequent in our population and was related to a higher prevalence of SILs, including HSILs. Other groups have also found that perianal or intraanal warts are a risk factor for HSILs22 and HPV-16 infection.23 Moreover, in PLWH, condyloma acuminata usually coexists with SILs, HR-HPVs are often detected,24 and those diagnosed with anal warts have an increased risk of anal SCC.25 Nonetheless, other factors such as sexual behavior might also be involved given that a high number of sexual partners increases the risk of condyloma acuminata26,27 and HR-HPV infection.28,29
Over the last decade, for various reasons, there has been a debate about whether treating HSILs can reduce the risk of anal SCC. First, spontaneous regression of HSILs has been reported,6 although severe immunodepression and nonvirological suppression might reduce the chance clearing them. Second, there is no standard treatment for anal lesions, and several approaches have been proposed, including intra-anal imiquimod, electroablation, and surgical excision. Our anal dysplasia unit chose electroablation as the standard treatment for SILs and previously reported successful results with this method.30 Electroablation has been proven to be safe and effective in this context,31,32 although high recurrence rates have been reported.33,34 Nonetheless, high recurrence rates have also been described for other treatment modalities.35 Importantly, the ANCHOR clinical trial was stopped early after an interim analysis showed a higher incidence of anal SCC when an observational approach was taken to HSILs rather than an interventional attitude.9 Thus, an interventional attitude to HSILs will likely become the mainstay for anal cancer prevention in the future. Nonetheless, several barriers to these screening programs have emerged, such as lack of material (i.e., access to HRA instruments), human resources (i.e., skilled, and trained personnel), and a low level of knowledge or awareness among patients and care providers.36,37
Finally, it is important to note that this study had some limitations. First, the retrospective study design meant that some data may have been lost or absent from patient medical records. Second, the HPV detection method only reported individual data for HPV-16 and HPV-18, with other HR-HPVs reported as a pooled group. Third, we only considered the diagnosis of HPV at baseline and so the dynamics of HPV (i.e., clearance and reinfection rates) were not evaluated. Lastly, the HPV vaccination rates before screening for inclusion were extremely low, thereby increasing the chances of HPV-16 or HPV-18 infection in these patients.
Conclusions
In conclusion, HPV infection and anal dysplasia, including HSIL, were common in the PLWH. A poorer immune status (i.e., a nadir lymphocyte CD4+ count below 200 cells/μL) was related to an increased overall frequency of SILs, although this was not true for the HSIL subgroup. Condyloma acuminata and HPV-16 were both related to an increased prevalence of HSILs and so PLWH diagnosed with anogenital condylomas or HPV-16 infection should be screened and followed-up in more detail. Diagnosis of anal SSC was unusual in this cohort but was strongly related to the detection of HPV-16 in these patients.
Data Availability
The datasets generated during and/or analyzed in this current study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors would like to thank all the patients, clinicians, and nurses at the Álvaro Cunqueiro Infectious Disease Unit without whom this work would not have been possible.
Authors' Contributions
A.P.-G.: conceptualization, methodology, formal analysis, investigation, writing—original draft. S.R.-R.: conceptualization, methodology, investigation, data curation. P.F.-V.: investigation, methodology, data curation. E.F.: investigation, methodology, data curation. E.P.: validation, writing—review and editing. J.G.-C.: investigation. S.P.-C.: investigation, writing—review and editing. L.L.-L.: investigation, data curation. C.M.: investigation. A.O.: conceptualization, investigation, resources, supervision.
Author Disclosure Statement
The authors declare no competing interests.
Funding Information
This research did not receive any specific funding grants from any agencies in the public, commercial, or not-for-profit sectors. A.P.-G. was employed on a Rio Hortega contract financed by the Instituto de Salud Carlos III, file number CM20/00243.
References
- 1. Centers for Disease Control and Prevention (CDC). Human papillomavirus-associated cancers—United States, 2004–2008. MMWR Morb Mortal Wkly Rep 2012;61:258–261. [PubMed] [Google Scholar]
- 2. Silverberg MJ, Lau B, Justice AC, et al. Risk of anal cancer in HIV-infected and HIV-uninfected individuals in North America. Clin Infect Dis 2012;54(7):1026–1034; doi: 10.1093/cid/cir1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Colón-López V, Shiels MS, Machin M, et al. Anal cancer risk among people with HIV infection in the United States. J Clin Oncol 2018;36(1):68–75; doi: 10.1200/JCO.2017.74.9291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gravitt PE, Winer RL. Natural history of HPV infection across the lifespan: Role of viral latency. Viruses 2017;9(10):267; doi: 10.3390/v9100267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pérez-González A, Cachay E, Ocampo A, et al. Update on the epidemiological features and clinical implications of human papillomavirus infection (HPV) and human immunodeficiency virus (HIV) coinfection. Microorganisms 2022;10(5):1047; doi: 10.3390/microorganisms10051047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mathews WC, Agmas W, Cachay ER, et al. Natural history of anal dysplasia in an HIV-infected clinical care cohort: Estimates using multi-state Markov modeling. PLoS One 2014;9(8):e104116; doi: 10.1371/journal.pone.0104116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Milanés Guisado Y, Sotomayor C, Fontillón M, et al. Incidence rate and risk factors for anal squamous cell carcinoma in a cohort of people living with HIV from 2004 to 2017: Implementation of a screening program. Dis Colon Rectum 2022;65(1):28–39; doi: 10.1097/DCR.0000000000002218. [DOI] [PubMed] [Google Scholar]
- 8. Ye Y, Burkholder GA, Mukherjee A, et al. A 12-year retrospective evaluation of anal pre-cancerous lesions and cancer in people living with HIV-1 infection in the Southeastern U.S. Infect Agent Cancer 2021;16(1):14; doi: 10.1186/s13027-021-00354-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Palefsky JM, Lee JY, Jay N, et al. Treatment of anal high-grade squamous intraepithelial lesions to prevent anal cancer. N Engl J Med 2022;386(24):2273–2282; doi: 10.1056/NEJMoa2201048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Iribarren Díaz M, Ocampo Hermida A, González-Carreró Fojón J, et al. Preliminary results of a screening program for anal cancer and its precursors for HIV-infected men who have sex with men in Vigo-Spain. Rev Esp Enferm Dig 2017;109(4):242–249; doi: 10.17235/reed.2017.4274/2016. [DOI] [PubMed] [Google Scholar]
- 11. Wei F, Gaisa MM, D'Souza G, et al. Epidemiology of anal human papillomavirus infection and high-grade squamous intraepithelial lesions in 29 900 men according to HIV status, sexuality, and age: A collaborative pooled analysis of 64 studies. Lancet HIV 2021;8(9):e531–e543; doi: 10.1016/S2352-3018(21)00108-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bruzzesi E, Galli L, Poli A, et al. Prevalence and risk factors of anal HPV infection in MSM living with HIV: Identifying the target groups to prioritize for immunization. J Acquir Immune Defic Syndr 2022;91(2):226–231; doi: 10.1097/QAI.0000000000003057. [DOI] [PubMed] [Google Scholar]
- 13. Squillace N, Bernasconi DP, Lapadula G, et al. HPV 16 and 18 contribute to development of anal dysplasia in HIV infection irrespective of gender and sexual orientation. HIV Med 2021;22(9):860–866; doi: 10.1111/hiv.13143. [DOI] [PubMed] [Google Scholar]
- 14. de Martel C, Plummer M, Vignat J, et al. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer 2017;141(4):664–670; doi: 10.1002/ijc.30716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goldstone SE, Giuliano AR, Palefsky JM, et al. Efficacy, immunogenicity, and safety of a quadrivalent HPV vaccine in men: Results of an open-label, long-term extension of a randomised, placebo-controlled, phase 3 trial. Lancet Infect Dis 2022;22(3):413–425; doi: 10.1016/S1473-3099(21)00327-3. [DOI] [PubMed] [Google Scholar]
- 16. Petrosky E, Bocchini JA, Hariri S, et al. Use of 9-valent human papillomavirus (HPV) vaccine: Updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep 2015;64(11):300–304. [PMC free article] [PubMed] [Google Scholar]
- 17. Sanger CB, Xu Y, Carchman E, et al. Prevalence of high-grade anal dysplasia and anal cancer in veterans living with HIV and CD4/CD8 ratio as a marker for increased risk: A regional retrospective cohort study. Dis Colon Rectum 2021;64(7):805–811; doi: 10.1097/DCR.0000000000002009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jongen VW, Richel O, Marra E, et al. Anal squamous intraepithelial lesions (SILs) in human immunodeficiency virus–positive men who have sex with men: Incidence and risk factors of SIL and of progression and clearance of low-grade SILs. J Infect Dis 2020;222(1):62–73; doi: 10.1093/infdis/jiz614. [DOI] [PubMed] [Google Scholar]
- 19. Wells J, Flowers L, Mehta CC, et al. Follow-up to high-resolution anoscopy after abnormal anal cytology in people living with HIV. AIDS Patient Care STDS 2022;36(7):263–271; doi: 10.1089/apc.2022.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hidalgo-Tenorio C, García-Martínez CM, Pasquau J, et al. Risk factors for ≥high-grade anal intraepithelial lesions in MSM living with HIV and the response to topical and surgical treatments. PLoS One 2021;16(2):1–13; doi: 10.1371/journal.pone.0245870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ye Y, Burkholder GA, Wiener HW, et al. CD4 trajectory models and onset of non-AIDS-defining anal genital warts, precancer, and cancer in people living with HIV infection-1. Sex Transm Dis 2020;47(9):628–633; doi: 10.1097/OLQ.0000000000001215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goddard SL, Templeton DJ, Petoumenos K, et al. Prevalence and association of perianal and intra-anal warts with composite high-grade squamous intraepithelial lesions among gay and bisexual men: Baseline data from the study of the prevention of anal cancer. AIDS Patient Care STDS 2020;34(10):436–443; doi: 10.1089/apc.2020.0067. [DOI] [PubMed] [Google Scholar]
- 23. Cerejeira A, Cunha S, Coelho R, et al. Perianal warts as a risk marker for anal high-risk-human papillomavirus (HPV) detection and HPV-associated diseases. J Eur Acad Dermatol Venereol 2020;34(11):2613–2619; doi: 10.1111/jdv.16834. [DOI] [PubMed] [Google Scholar]
- 24. Kreuter A, Siorokos C, Oellig F, et al. High-grade dysplasia in anogenital warts of HIV-positive men. JAMA Dermatol 2016;152(11):1225–1230; doi: 10.1001/jamadermatol.2016.2503. [DOI] [PubMed] [Google Scholar]
- 25. Arnold JD, Byrne ME, Monroe AK, et al. The risk of anal carcinoma after anogenital warts in adults living with HIV. JAMA Dermatol 2021;157(3):283–289; doi: 10.1001/jamadermatol.2020.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tyros G, Mastraftsi S, Gregoriou S, et al. Incidence of anogenital warts: Epidemiological risk factors and real-life impact of human papillomavirus vaccination. Int J STD AIDS 2021;32(1):4–13; doi: 10.1177/0956462420958577. [DOI] [PubMed] [Google Scholar]
- 27. Daugherty M, Byler T. Genital wart and human papillomavirus prevalence in men in the United States from penile swabs: Results from National Health and Nutrition Examination Surveys. Sex Transm Dis 2018;45(6):412–416; doi: 10.1097/OLQ.0000000000000761. [DOI] [PubMed] [Google Scholar]
- 28. Pedroza-Gonzalez A, Reyes-Reali J, Campos-Solorzano M, et al. Human papillomavirus infection and seroprevalence among female university students in Mexico. Hum Vaccin Immunother 2022;18(1):2028514; doi: 10.1080/21645515.2022.2028514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tuan LA, Prem K, Pham QD, et al. Anal human papillomavirus prevalence and risk factors among men who have sex with men in Vietnam. Int J Infect Dis 2021;112:136–143; doi: 10.1016/j.ijid.2021.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Iribarren-Díaz M, Ocampo Hermida A, González-Carreró Fojón J, et al. Practical considerations for high resolution anoscopy in patients infected with human immunodeficiency virus. Enferm Infecc Microbiol Clin 2014;32(10):676–680; doi: 10.1016/j.eimc.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 31. Willems N, Libois A, Nkuize M, et al. Treatment of anal dysplasia in HIV-positive men who have sex with men in a large AIDS reference centre. Acta Clin Belg 2017;72(1):29–35; doi: 10.1080/17843286.2015.1116725. [DOI] [PubMed] [Google Scholar]
- 32. Goldstone SE, Johnstone AA, Moshier EL. Long-term outcome of ablation of anal high-grade squamous intraepithelial lesions: Recurrence and incidence of cancer. Dis Colon Rectum 2014;57(3):316–323; doi: 10.1097/DCR.0000000000000058. [DOI] [PubMed] [Google Scholar]
- 33. Burgos J, Curran A, Landolfi S, et al. The effectiveness of electrocautery ablation for the treatment of high-grade anal intraepithelial neoplasia in HIV-infected men who have sex with men. HIV Med 2016;17(7):524–531; doi: 10.1111/hiv.12352. [DOI] [PubMed] [Google Scholar]
- 34. Gaisa MM, Liu Y, Deshmukh AA, et al. Electrocautery ablation of anal high-grade squamous intraepithelial lesions: Effectiveness and key factors associated with outcomes. Cancer 2020;126(7):1470–1479; doi: 10.1002/cncr.32581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. de Pokomandy A, Rouleau D, Lalonde R, et al. Argon plasma coagulation treatment of anal high-grade squamous intraepithelial lesions in men who have sex with men living with HIV: Results of a 2-year prospective pilot study. HIV Med 2018;19(2):81–89; doi: 10.1111/hiv.12544. [DOI] [PubMed] [Google Scholar]
- 36. Cruz G, Ramos-Cartagena JM, Torres-Russe JL, et al. Barriers and facilitators to anal cancer screening among people living with HIV in Puerto Rico. BMC Public Health 2023;23(1):1940; doi: 10.1186/s12889-023-16847-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sanger CB, Kalbfell E, Cherney-Stafford L, et al. A qualitative study of barriers to anal cancer screenings in US veterans living with HIV. AIDS Patient Care STDS 2023;37(9):436–446; doi: 10.1089/apc.2023.0144. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed in this current study are available from the corresponding author upon reasonable request.