Abstract
Objective.
To assess the uptake of the OMERACT-OARSI (Outcome Measures in Rheumatology-Osteoarthritis Research Society International) core outcome set (COS) domains in hip and/or knee osteoarthritis (OA) trials.
Methods.
There were 382 trials of hip and/or knee OA identified from the ClinicalTrial.gov registry from 1997 to 2017. Frequency of COS adoption was assessed by year and per 5-yearly phases.
Results.
COS adoption decreased from 61% between 1997 and 2001 to 38% between 2012 and 2016. Pain (95%) and physical function (86%) were most consistently adopted. Patient’s global assessment (48%) was the principal missing domain.
Conclusion.
Limited adoption of the COS domains indicates that further consideration to improve uptake is required. (First Release March 1 2019; J Rheumatol 2019;46:976–80; doi:10.3899/jrheum.181066)
Keywords: OMERACT, CORE OUTCOME SET, DOMAIN, ADOPTION, TRIAL REGISTRATION
Clinical trials seek to determine whether a treatment is effective and safe for patients by comparing its relative effects on outcomes chosen to identify benefit or harm1. These trials can be used to make decisions on whether the treatment under investigation should be recommended2. It is, therefore, essential that outcomes reported in trials are those that are needed by decision makers, and reflect meaningful outcomes for patients, clinicians, and all those involved in the care of these patients3.
In 1997, OMERACT-OARSI (Outcome Measures in Rheumatology-Osteoarthritis Research Society International) presented the core outcome set (COS) for people involved in trials with hip and knee osteoarthritis (OA). It reported that 4 domains should be measured and reported in all future clinical trials, including patients with hip or knee OA4. These were pain, physical function, patient’s global assessment (PtGA), and an extra conditionally recommended domain for studies with a followup period of a year or longer with putative structure-modifying OA drugs: joint imaging (such as radiographs or magnetic resonance imaging scans). While these recommendations have been in the public domain for 20 years, it remains unknown whether they have changed the selection of outcomes used in trials with this population during this period.
The purpose of our study was to assess the uptake of a COS for hip and knee OA, and to analyze whether specific study characteristics are associated with the failure of COS uptake.
MATERIALS AND METHODS
We adopted Kirkham, et al’s5 recommendations on the assessment of COS uptake. Through this, we searched the trials registry ClinicalTrials.gov on July 6, 2017, to identify all phase III or IV, drug or nondrug trials registered from January 1997 to July 2017, recruiting people with hip or knee OA. The following filters were applied to identify eligible trials: “conditions: osteoarthritis,” “study type: interventional studies,” and “Phase: III and IV.” Only phase III and IV trials were included to reflect the phase III and IV recommendations made in the original OMERACT-OARSI COS4. We excluded trials that did not exclusively recruit people with OA, and did not assess treatment benefit (i.e., effectiveness or efficacy) as endpoints (i.e., medication dosage or safety studies). We also excluded studies assessing outcomes following surgical intervention (principally, joint replacement).
We extracted data on all planned trial outcomes and assessed whether the full OMERACT-OARSI hip and knee OA COS was adopted4. These were the assessment of pain, physical function, PtGA, and with a conditional recommendation for trials with a 12 month or greater followup period and for putative structure-modifying OA drugs, imaging outcomes. We also assessed the uptake of “strongly recommended” domains including health-related quality of life (HRQOL) and physician’s global assessment. We assessed the frequency of use of outcomes that were recommended as “optional,” including stiffness, biologic markers, inflammation, performance-based function, flares, time to surgery, and analgesic count. If a trial had registered a composite outcome, all individual outcomes were considered in the composite, even when not listed separately.
Collected data also included year of trial registration; anatomic location of OA participants presented with hip, knee or hip, and/or knee; country of origin; sample size; duration of followup at endpoint; the intervention type under investigation (drug or non-drug trial); and phase of the trial.
All 382 trial registrations were extracted by 1 reviewer (TOS). An independent reviewer (MM) verified 10% of the data collected to ensure accuracy of extraction from the trial registry, following Kirkham, et al’s5 approach. Disagreement between the reviewers was resolved through discussion. To assess the veracity of the ClinicalTrials.gov registry data, when a trial did not meet the full COS, with any of the core domains missing (n = 230), the published full report was used to verify the data (n = 74). When published reports were not available (n = 156), the chief investigator or named contact on the trial registration was contacted by e-mail to verify the data. Of these, 14% (n = 21) responded and provided additional data.
Data analysis.
We calculated the proportion of trials that reported each OA COS domain and the full domain set, and the percentage of core outcomes reported from the COS per year. These were assessed over the 20-year followup period to determine change over time.
Using a forced entry multivariate logistic regression model, we assessed the relationship between year of registration, sample size, country of origin, duration of followup interval, whether participants presented with isolated hip, isolated knee or hip and/or knee OA, phase of trial (III or IV), whether it was a drug trial or nondrug trial, and full COS domain uptake (yes/no). A forced entry method was adopted to ensure that all variables were included in the model. Data were presented as OR with 95% CI. A 2-sided p value < 0.05 was deemed as indicating statistical significance. Analyses were undertaken using Stata version 14.0 (StataCorp LLC).
RESULTS
In total, 382 phase III or IV trials registered in Clinicaltrials.gov were eligible for analysis. The eligibility assessment and reasons for exclusion of trials are presented in Figure 1. Trial characteristics are presented in Supplementary Table 1 (available from the authors on request).
Figure 1.
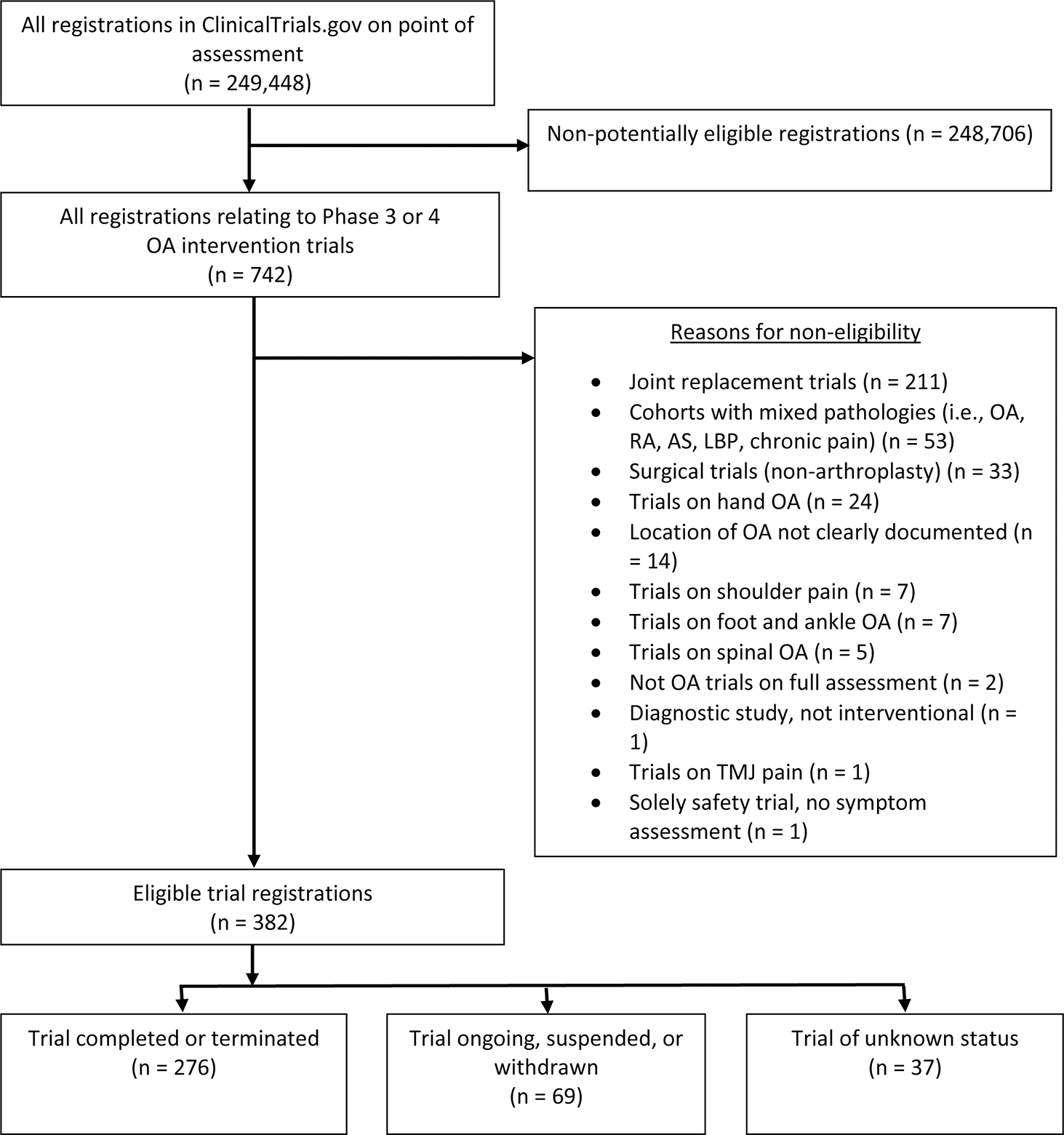
Flowchart of identification of trial registrations from ClinicalTrial.gov database. OA: osteoarthritis; RA: rheumatoid arthritis; AS: ankylosing spondylitis; LBP: low back pain; TMJ: temporomandibular joint.
The assessment of COS uptake is summarized in Table 1 and Figure 2. There was a decrease in the adoption of the full COS from 61% between 1997 and 2001 to 38% between 2012 and 2016. The adoption of the full COS has largely plateaued between 2002 to 2017, within the ranges of 38% to 54% (Table 1; Supplementary Table 2 is available from the authors on request). While trials have consistently assessed pain (over 90%) and physical function (over 80%), there has been greater variability for PtGA (from 67% to 38%). As Figure 2 illustrates, the assessment of PtGA was the principal domain for COS not being fully reported from 1997 to 2017.
Table 1.
Percentage frequency of reported domains and complete adoption of the core outcome set in included trial registrations.
| Domain | Percentage Total | Percentage Frequency by Year | ||||
|---|---|---|---|---|---|---|
| PFrequency, n = 382 | 1997–2001, n = 18 | 2002–2006, n = 94 | 2007–2011, n = 133 | 2012–2016, n = 123 | 2017, n = 14 | |
|
| ||||||
| Core domain | ||||||
| Pain | 94.8 | 100 | 91.5 | 96.9 | 94.4 | 92.9 |
| Physical function | 86.1 | 94.4 | 81.9 | 89.2 | 84.1 | 92.9 |
| Patient’s global assessment | 47.6 | 66.7 | 59.6 | 45.4 | 38.1 | 42.9 |
| Imaging* | 75.0 | 71.4 | 40.0 | 79.2 | 89.5 | 85.7 |
| All core domains measured | 45.3 | 61.1 | 54.3 | 43.1 | 38.1 | 50.0 |
| Recommended domains | ||||||
| HRQOL | 26.2 | 27.8 | 12.8 | 39.1 | 27.6 | 14.3 |
| Clinician global assessment | 23.0 | 44.4 | 36.2 | 16.5 | 20.3 | 14.3 |
| Optional domains | ||||||
| Stiffness | 58.1 | 66.7 | 58.5 | 64.7 | 52.0 | 35.7 |
| Biological markers, i.e., relevant blood tests | 18.8 | 22.2 | 17.0 | 23.3 | 15.4 | 7.1 |
| Swelling | 7.1 | 16.7 | 3.2 | 6.8 | 8.1 | 7.1 |
| Performance assessment | 14.7 | 27.8 | 11.7 | 15.0 | 16.3 | 7.1 |
| Pain flares | 1.6 | 5.6 | 3.2 | 1.5 | 0.8 | 0.0 |
| Time to surgery | 2.6 | 16.7 | 0.0 | 0.0 | 4.1 | 7.1 |
| Analgesic consumption | 27.0 | 50.0 | 29.8 | 24.8 | 22.8 | 21.4 |
Imaging is a required core outcome set domain for trials of 12 months or greater followup in trials of structure-modifying osteoarthritis drugs (total n = 68). HRQOL: health-related quality of life.
Figure 2.
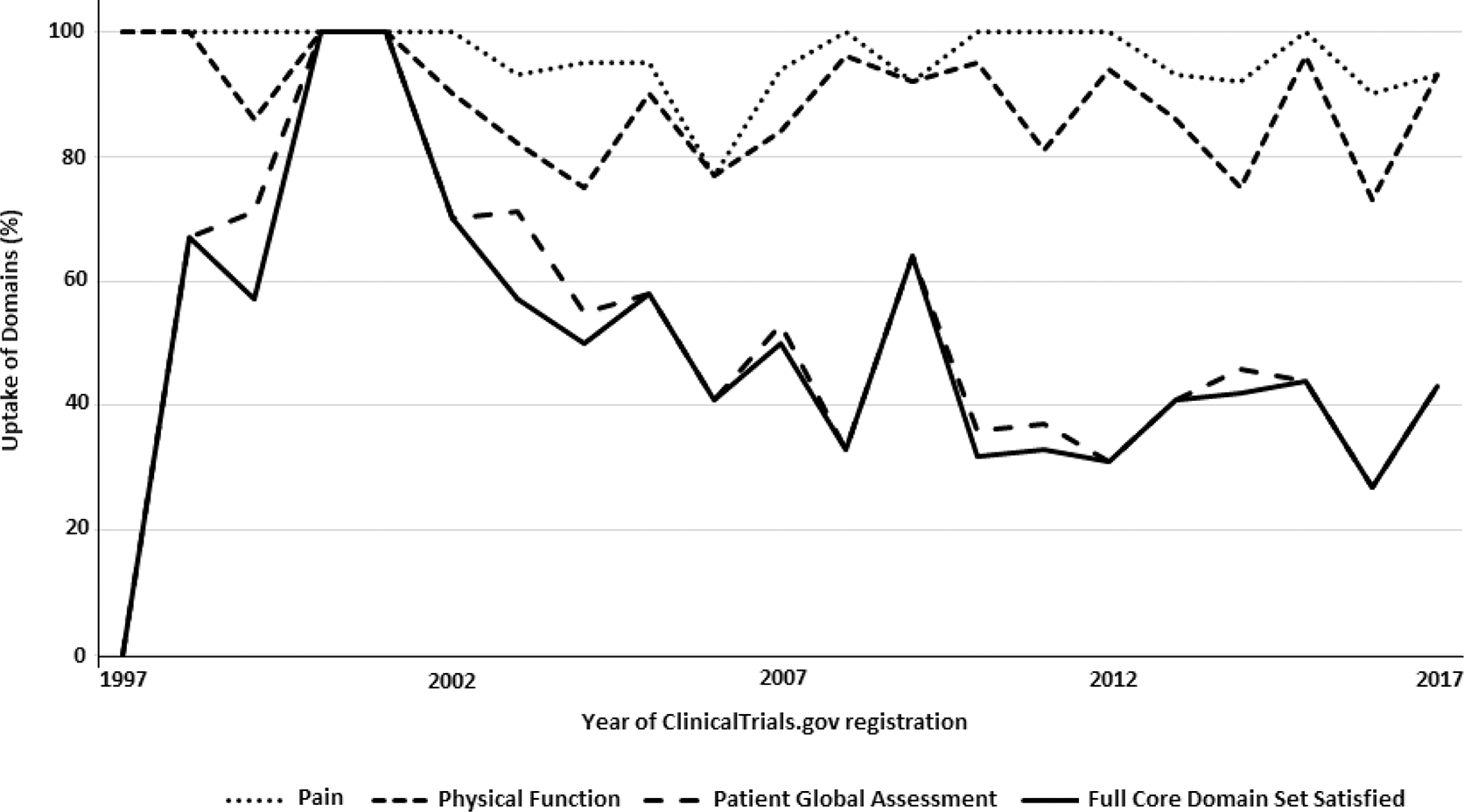
Graph of uptake of core domain and individual domains for the osteoarthritis core outcome set from 1997 to 2017.
On assessment of domains that were “recommended” but not “essential” by the 1997 OMERACT-OARSI COS4, joint stiffness was most commonly assessed (58%) followed by HRQOL (26%) and analgesic consumption (27%). Least frequently assessed included swelling (7%), pain flares (2%), and time to surgery (3%; Table 1).
On analysis of the factors that may be associated with a successful COS uptake, the phase of the trial was significant. Phase III trials were over twice as likely to have reported a full COS, compared to phase IV trials (OR 2.32, 95% CI 1.26–4.26, p = 0.01). Drug trials were over 3 times as likely to have presented the full COS compared to nondrug trials (OR 3.57, 95% CI 1.12–5.37, p = 0.03). The country of trial origin (p = 0.99), year of registration (p = 0.28), duration of the trial (p = 0.07), and whether the trial recruited people with hip, knee, or hip and knee OA (p = 0.53) were not significant. Although statistically significant, there was no important difference in COS adoption based on sample size (OR 1.00, 95% CI 1.00–1.00, p < 0.01).
DISCUSSION
Our study has demonstrated that there has been limited uptake of the full OMERACT-OARSI COS domains in randomized controlled trials of hip and knee OA during the past 20 years. While pain and physical function are consistently assessed (over 90% and over 80%, respectively), PtGA is less frequently evaluated and decreased from 67% to 38%; it is the principal reason for trials not satisfying the full COS uptake.
Of the 3 (conditionally 4) components required to satisfy the COS, PtGA was the principal missing domain for trials not satisfying the full COS. There has been concern that PtGA scores may be influenced by social desirability bias6. This may, therefore, be a reason for the reported lower adoption of PtGA measures. Nonetheless, OMERACT and others have highlighted the importance of patient-reported outcome measures to measure the patient’s overall perceptions of their disease7. Accordingly, the diminishing inclusion of the patient’s global domain warrants an update of the COS to ensure its relevance for OA trials.
The results contrasted with the Kirkham, et al8 analysis of the uptake of the rheumatoid arthritis COS, in which uptake had increased within a 14-year period (from 2002) to 81% of eligible trials. This was attributed to the introduction of consistent guidance provided by regulatory authorities including the US Food and Drug Administration (FDA)9 and the European Medicines Agency (EMA)10. There is less consistency around COS domains in OA11. The OARSI-FDA Disease State Working Group12 recommended the assessment of pain, function, radiological measures, and other wider patient experiences of illness, including fatigue, mood, sleep, and HRQOL12. The EMA guidelines recommend that pain, functional disability, and structural damage should be assessed, but PtGA is recommended rather than mandatory13. Some of this discordance may account for lack of uptake, and therefore future work may be undertaken to standardize recommendations across regulatory authorities.
Trials were evaluated using their ClinicalTrials.gov registration, as recommended by Kirkham, et al5 to provide a more efficient means of assessing COS uptake compared to reviewing final trial reports or publications5,14. However, a disadvantage to the adopted approach was that we did not review additional registries such as the World Health Organization International Clinical Trials Registry Platform or the Netherlands Trial Registry. However, because ClinicalTrials.gov demonstrates international coverage (trial details in Supplementary Table 1, available from the authors on request), the results were representative of trials on this population.
Acknowledgments
Research for this article was supported by the NIHR Oxford BRC (T.O. Smith) and the Leeds BRC (Prof. P.G. Conaghan). The views expressed are those of the authors and not necessarily those of the UK National Health Service, the NIHR, or the UK Department of Health. C.H. Ladel is an employee of Merck Biopharma, Merck KgaA, and Dr. G. Kalsi is an employee of TissueGene Inc.
Contributor Information
Toby O. Smith, Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford, and NIHR Oxford BRC, John Radcliffe Hospital, Oxford.
Michael Mansfield, School of Health and Social Care, London South Bank University.
Gillian A. Hawker, Department of Medicine, Faculty of Medicine, and Institute of Health Policy, Management, and Evaluation, Dalla Lana School of Public Health, University of Toronto.
David J. Hunter, Florance and Cope Chair of Rheumatology Chair of Institute of Bone and Joint Research Professor of Medicine, Institute of Bone and Joint Research, Florance and Cope and Professorial Department of Rheumatology, School of Medicine, University of Sydney and Royal North Shore Hospital
Lyn M. March, Liggins Professor of Rheumatology and Musculosketal Epidemiology Medicine, Institute of Bone and Joint Research, Florance and Cope Professorial Department of Rheumatology, School of Medicine, University of Sydney and Royal North Shore Hospital
Maarten Boers, Department of Epidemiology and Biostatistics, Amsterdam University Medical Centers.
Beverley J. Shea, Ottawa Hospital Research Institute.
Robin Christensen, Musculoskeletal Statistics Unit, the Parker Institute, Bispebjerg and Frederiksberg Hospital and Department of Rheumatology, Odense University Hospital.
Francis Guillemin, Professor of Epidemiology and Public Health, Université de Lorraine, APEMAC.
Caroline B. Terwee, Department of Epidemiology and Biostatistics, Amsterdam University Medical Centers.
Paula R. Williamson, Institute of Translational Medicine, University of Liverpool.
Ewa M. Roos, Center for Muscle and Joint Health, Department of Sports Science and Clinical Biomechanics, University of Southern Denmark.
Richard F. Loeser, Herman and Louise Smith Distinguished Professor of Medicine, Division of Rheumatology, Allergy and Immunology, and Division of Rheumatology, Allergy and Immunology, Thurston Arthritis Research Center, University of North Carolina
Thomas J. Schnitzer, Professor of Physical Medicine and Rehabilitation, Anesthesiology and Medicine (Rheumatology), and Department of Physical Medicine and Rehabilitation, Northwestern University Feinberg School of Medicine
Margreet Kloppenburg, Departments of Rheumatology and Clinical Epidemiology, Leiden University Medical Centre.
Tuhina Neogi, Epidemiology, Sections of Clinical Epidemiology and Rheumatology, Boston University School of Medicine.
Christoph H. Ladel, Merck Biopharma, Merck KGaA.
Gurdyal Kalsi, Chief Medical Officer, Senior Vice President of Clinical Development and Regulatory and Medical Affairs, TissueGene Inc..
Ulrike Kaiser, University Pain Centre, University Hospital Carl Gustav Carus.
Thomas W. Buttel, Psychologist, Inner West Psychology, and Institute of Bone and Joint Research, Florance and Cope Professorial Department of Rheumatology, School of Medicine, University of Sydney and Royal North Shore Hospital
Anne E. Ashford, School of Biological, Earth and Environmental Sciences, University of New South Wales.
Ali Mobasheri, Professor of Musculoskeletal Physiology, Faculty of Health and Medical Sciences, University of Surrey, and Department of Regenerative Medicine, State Research Institute Centre for Innovative Medicine.
Nigel K. Arden, Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford.
Alan Tennant, Faculty of Humanities and Social Sciences, University of Lucerne.
Marc C. Hochberg, Professor of Medicine and Epidemiology and Public Health, Division of Rheumatology and Clinical Immunology, Department of Medicine, and Division of Gerontology, Department of Epidemiology and Public Health, University of Maryland School of Medicine
Maarten de Wit, Amsterdam University Medical Centre, Department of Medical Humanities, Amsterdam Public Health.
Peter Tugwell, Division of Rheumatology, Department of Medicine, and School of Epidemiology and Public Health, Faculty of Medicine, University of Ottawa, and Clinical Epidemiology Program, Ottawa Hospital Research Institute.
Philip G. Conaghan, Leeds Institute of Rheumatic and Musculoskeletal Medicine, University of Leeds, and NIHR Leeds BRC..
REFERENCES
- 1.Gargon E, Gurung B, Medley N, Altman DG, Blazeby JM, Clarke M, et al. Choosing important health outcomes for comparative effectiveness research: a systematic review. PLoS One 2014;9:e99111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boers M, Kirwan JR, Tugwell P, Beaton D, Bingham CO III, Conaghan PG, et al. The OMERACT Handbook. [Internet. Accessed May 17, 2017.] Available from: https://omeract.org/resources
- 3.Boers M, Kirwan JR, Wells G, Beaton D, Gossec L, d’Agostino MA, et al. Developing core outcome measurement sets for clinical trials: OMERACT filter 2.0. J Clin Epidemiol 2014;67:745–53. [DOI] [PubMed] [Google Scholar]
- 4.Bellamy N, Kirwan J, Boers M, Brooks P, Strand V, Tugwell P, et al. Recommendations for a core set of outcome measures for future phase III clinical trials in knee, hip, and hand osteoarthritis. Consensus development at OMERACT III. J Rheumatol 1997;24:799–802. [PubMed] [Google Scholar]
- 5.Kirkham JJ, Clarke M, Williamson PR. A methodological approach for assessing the uptake of core outcome sets using ClinicalTrials.gov: findings from a review of randomised controlled trials of rheumatoid arthritis. BMJ 2017;357:j2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rohekar G, Pope J. Test-retest reliability of patient global assessment and physician global assessment in rheumatoid arthritis. J Rheumatol 2009;36:2178–82. [DOI] [PubMed] [Google Scholar]
- 7.Bijlsma JW. Patient centred outcomes in osteoarthritis. Ann Rheum Dis 2005;64:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirkham JJ, Boers M, Tugwell P, Clarke M, Williamson PR. Outcome measures in rheumatoid arthritis randomised trials over the last 50 years. Trials 2013;14:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.US Department of Health and Human Services: Food and Drug Administration. Guidance for industry: clinical development programs for drugs, devices, and biological products for the treatment of rheumatoid arthritis (RA). 1999. [Internet. Accessed January 3, 2019.] Available from: https://www.fda.gov/downloads/Drugs/Guidances/ucm071579.pdf
- 10.European Medicines Agency. Guideline on clinical investigation of medicinal products other than NSAIDs for treatment of rheumatoid arthritis. [Internet. Accessed January 3, 2019.] Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2015/06/WC500187583.pdf
- 11.US Food and Drug Administration. 1999. Osteoarthritis: structural endpoints for the development of drugs, devices, and biological products for treatment. Guidance for industry. [Internet. Accessed January 3, 2019.] Available from: https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm071577.pdf
- 12.Lane NE, Brandt K, Hawker G, Peeva E, Schreyer E, Tsuji W, et al. OARSI-FDA initiative: defining the disease state of osteoarthritis. Osteoarthritis Cartilage 2011;19:478–82. [DOI] [PubMed] [Google Scholar]
- 13.European Medicines Agency. Committee for Medicinal Products for Human Use (CHMP). Guideline on clinical investigation of medicinal products used in the treatment of osteoarthritis. [Internet. Accessed January 3, 2019.] Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003440.pdf
- 14.Barnes KL, Kirkham JJ, Clarke M, Williamson PR. Citation analysis did not provide a reliable assessment of core outcome set uptake. J Clin Epidemiol 2017;86:153–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


