Abstract
A novel class of non-nitrogen-containing heterocycles, the tetrahydrobenzothiophenones, was found to bind to adenosine receptors as antagonists in the micromolar range. Affinity was determined in radioligand-binding assays at rat brain A1 and A2a receptors. A structure–activity analysis indicated that a 3-thioether group is favored and affinity at A2a, but not at A1, receptors is highly dependent on this thioether substituent. A carboxylic acid-derived substituent is required at the 1-position of the thiophene ring, with esters being more potent in binding at A1 receptors than the corresponding carboxyl hydrazide or carboxylic acid derivatives. The methyl (15) and ethyl (16) esters are about equipotent at A1 but not at A2a receptors. A 4-keto group on the saturated ring is favored for receptor affinity. Dimethyl substitution at the 6-position of the saturated ring is allowed. One of the most potent derivatives was the nonselective compound ethyl 3-(benzylthio)-4-oxo-4,5,6,7-tetrahydrobenzo[c]thiophene1-carboxylate (BTH4, 7; Figure 1), which antagonized adenosine agonist-induced inhibition of adenylyl cyclase in rat adipocyte membranes with a KB value of 1.62 ± 0.73 μM and adenosine agonist-induced stimulation of adenylyl cyclase in pheochromocytoma cell membranes with a KB value of 9.19 ± 0.98 μM. Displacement of radioligand binding by BTH4 (7) at cloned human A3 receptors was negligible, but one slightly A3 selective compound (11, 3.9-fold over A1 and >7.5-fold over A2a) was found. A 1-methylpropyl thioether (17) was 29-fold selective for A1 vs A2a receptors. BTH4 (7) alone, at 10 mg/kg, stimulated locomotor activity in mice but paradoxically acted, under certain circumstances, synergistically with an A1 selective agonist to depress locomotor activity. A pharmacophore model relating structural features of xanthine and non-xanthine adenosine antagonists to BTH4 (7) suggests a high degree of similarity in electrostatic surfaces, assuming that the thiophene ring superimposes the region of the uracil ring of xanthines.
Introduction
Adenosine receptors are involved in many peripheral and central regulatory mechanisms, including vasodilatation1 and vasoconstriction in the kidney,2 inhibition of lipolysis3 and insulin release,4 inhibition of neurotransmitter release,5 and moderation of cerebral ischemia.6–8 Four subtypes of adenosine receptors (i.e., A1, A2a, A2b, and A3) have been identified, both pharmacologically and through cloning techniques.6,9 The actions of adenosine on A1 and A2a adenosine receptors are readily antagonized by potent and selective xanthine-based antagonists, but the A3 adenosine receptor subtype, however, seems to be less susceptible to blockade by these compounds.10
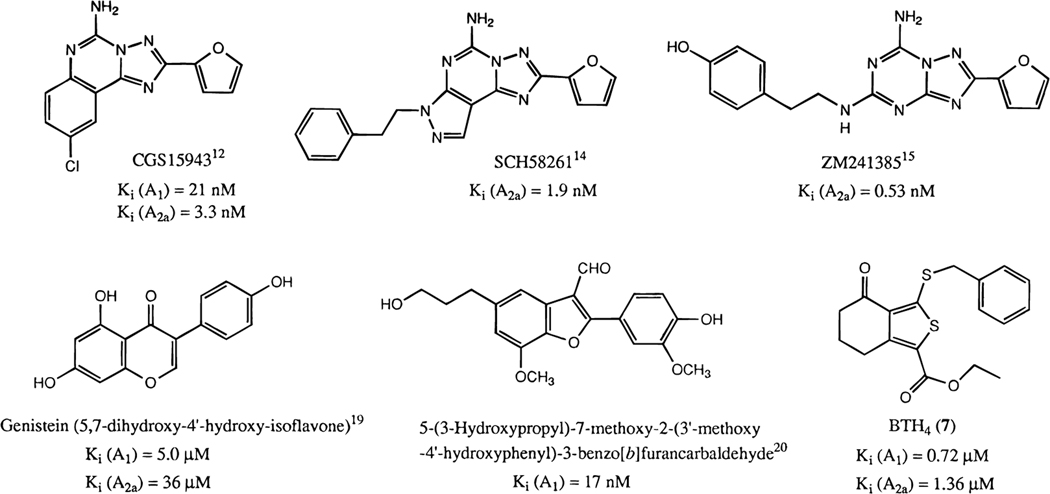
A number of classes of non-xanthine adenosine antagonists (Figure 1),6,11 including triazoloquinazolines (e.g., CGS15943),12 9-methyladenines,13 pyrazolotriazolopyrimidines (e.g., SCH58261),14 and triazolotriazines (e.g., ZM241385),15 have been found. Many of the non-xanthine antagonists are relatively nonselective, although selectivity for A1 receptors13,16–18 or A2 receptors12,14,15 has been achieved. Nearly all of the non-xanthine derivatives previously found to act as adenosine antagonists have been nitrogen-containing heterocycles. There were only two reports of non-nitrogen-containing natural products:19,20 the protein tyrosine kinase inhibitor genistein and a benzofurancarbaldehyde derivative (Figure 1), which bound to A1 receptors with Ki values of 5 μM and 17 nM, respectively. In our ongoing effort to provide selective ligands for adenosine receptors, especially those with affinity at A3 receptors,21,22 we have screened 110 cyclic compounds for affinity at adenosine receptors,23 resulting in the identification of previously unknown classes of adenosine ligands.
Figure 1.
Non-xanthine antagonists of adenosine receptors.
In the present study we have identified a novel class of non-nitrogen-containing derivatives of tetrahydrobenzothiophenone as non-xanthine inhibitors of binding of agonists to adenosine receptors. Moreover, we have investigated the functional effects of one of the more potent analogues (ethyl 3-(benzylthio)-4-oxo-4,5,6,7-tetrahydrobenzo[c]thiophene-1-carboxylate, BTH4, 7) as an adenosine antagonist and have explored its behavioral properties. Although the compounds bear little structural resemblance to xanthines, using molecular modeling we have discovered a possible electronic basis for molecular recognition of these compounds as adenosine receptor antagonists.
Results and Discussion
Affinity at Adenosine Receptors.
The tetrahydrobenzothiophenone 3-thioether derivatives 1–20 bound to rat A1 and A2a adenosine receptors in the micromolar range (Table 1A). BTH4 (7) had one of the highest affinities among the analogues at both A1 and A2a subtypes. Compound 18 had an affinity at A1 receptors that was comparable to that of BTH4 (7) but showed a considerably lower affinity at A2a receptors. BTH4 (7) was nonselective, with Ki values at A1 and A2a receptors in brain membranes (determined with [3H]-(R)-PIA and [3H]CGS21680, respectively, to facilitate comparison with earlier work24,25) of 0.72 and 1.4 μM, respectively. Ki values for compounds 1, 6–8, 11, 14, and 19 at rat or human A3 adenosine receptors were determined by displacement of [125I]AB-MECA from either rat A3 receptors expressed in CHO cells or human A3 receptors expressed in HEK-293 cells (Table 1B).26,39,40 Contrary to earlier findings with xanthine-based antagonists, displaying a higher affinity for human than for rat A3 receptors, BTH4 derivatives were 2–3-fold more potent at rat A3 receptors than at human A3 receptors.39 In general there was a tendency toward A1 selectivity, in particular for compounds 17 and 18, with A1 vs A2a selectivities of 29- and >17-fold (estimated), respectively. Out of 20 compounds determined, there was only one compound that showed any degree of A2a selectivity (14, 3.3-fold over A1 and 2.8-fold over A3) and one compound that showed some degree of A3 selectivity (11, 3.9-fold over A1 and >7.5-fold over A2a). Compound 11 was structurally unique in this series having a double bond between positions 4 and 5 of the benzo ring as shown in Table 1.
Table 1.

| ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| A. Affinities of Tetrahydrobenzothiophenone Derivatives in Radioligand-Binding Assays at A1 and A2a Adenosine Receptors | ||||||
|
| ||||||
|
Ki (μM) or percent inhibitionc |
||||||
| compd | R1 | R2 | X | A1a | A2ab | A2a/A1 |
|
| ||||||
| 1 | CO2CH2CH3 | SCH3 | =O | 1.93 ± 0.21 | 3.66 ± 1.19 | 1.9 |
| 2 | CO2CH2CH3 | S(CH2)2CH3 | =O | 1.26 ± 0.30 | 4.69 ± 1.09 | 3.7 |
| 3 | CO2CH2CH3 | SCH(CH3)2 | =O | 1.79 ± 0.50 | 4.63 ± 0.15 | 2.6 |
| 4 | CO2CH2CH3 | SCH2CH=CH2 | =O | 1.06 ± 0.12 | 6.04 ± 0.44 | 5.7 |
| 5 | CO2CH2CH3 | S(CH2)3CH3 | =O | 1.42 ± 0.27 | 15.6 ± 2.3 | 11.0 |
| 6 | CO2CH2CH3 | SCH2CO2CH2CH3 | =O | 2.22 ± 0.39 | 25.6 ± 6.8 | 11.5 |
| 7 | CO2CH2CH3 | SCH2Ph | =O | 0.715 ± 0.116 | 1.36 ± 0.25 | 1.9 |
| 8 | CO2CH2CH3 | SCH3 | OH, H | 18.3 ± 1.9 | 33.5 ± 7.0 | 1.8 |
| 9 | CO2CH2CH3 | SCH3 | =NOCH3 | 69.8 ± 9.4 | 22 ± 5% (10−4) | >1.4 |
| 10 | CONHNH2 | SCH3 | OH, H | 71.2 ± 7.5 | 28.9% (10−4) | >1.4 |
| 11 | CONHNH2 | SCH3 | Hd | 51.6 ± 6.8 | c (10−4) | >1.9 |
| 12 e | H | SCH3 | =O | 28 ± 7% (10−4) | c (10−4) | >1 |
| 13 e | COOH | SCH3 | =O | 69.4 ± 10.7 | 15 ± 5% (10−4) | >1.4 |
| 14 | COOH | SCH2Ph | =O | 50.0 ± 2.3 | 15.2 ± 5.6 | 0.3 |
| 15 e | CO2CH3 | SCH2CH3 | =O | 3.34 ± 0.70 | 5.64 ± 0.85 | 1.7 |
| 16 e | CO2CH2CH3 | SCH2CH3 | =O | 2.69 ± 0.72 | 23.9 ± 2.6 | 8.9 |
| 17 e | CO2CH2CH3 | SCH(CH3)CH2CH3 | =O | 3.64 ± 0.84 | 106 ± 23 | 29.1 |
| 18 e | CO2CH2CH3 | SCH2Ph | =O | 0.567 ± 0.139 | 34 ± 2% (10−5) | >17.6 |
| 19 e | CO2CH2CH3 | SO2CH3 | =O | 32.7 ± 2.3 | 13% (10−4) | >3.1 |
| 20 e | CO2CH2CH3 | NHNH2 | =O | 17.5 ± 2.2 | 36.1 ± 5.1 | 2.1 |
| B. Affinities of Selected Tetrahydrobenzothiophenone Derivatives in Radioligand-Binding Assays at Human and Rat A3 Adenosine Receptorsf | ||||
|---|---|---|---|---|
|
| ||||
| compd | human A3 | rat A3 | human A3/rat A3 | rat A3/rat A1 |
|
| ||||
| 1 | nd | 15.2 ± 4.9 | 7.88 | |
| 6 | nd | 10.2 ± 1.5 | 4.59 | |
| 7 | 24%g | nd | ||
| 8 | 35.8 ± 0.5 | 15.4 ± 7.0 | 2.32 | 0.84 |
| 11 | nd | 13.1 ± 3.0 | 0.25 | |
| 14 | 117 ± 13 | 42.0 ± 12.5 | 2.79 | 0.84 |
| 19 | nd | 51.8 ± 16.4 | 1.58 | |
Displacement of specific [3H]PIA binding in rat brain membranes, expressed as Ki ± SEM in μM (n = 3–5).
Displacement of specific [3H]CGS21680 binding in rat striatal membranes, expressed as Ki ± SEM in μM (n = 3–6).
Displacement <10% of specific binding at concentration indicated.
C=C double bond at positions 4 and 5.
R3 = CH3.
Displacement of specific [125I]AB-MECA binding in CHO cell membranes transfected with rat A3 cDNA or HEK-293 cell membranes transfected with human A3 cDNA. Values are expressed as Ki ± SEM in μM (n = 3).
Percentage displacement of specific binding at 10−4 M.
Systematic name differs from numbering scheme for compounds 11 and 12. nd = not determined.
The effects of substitutions of the thiophene ring on receptor binding affinity were studied. A 1-carboxyl group appears necessary for affinity, as evidenced by the inactivity in binding of the 1-unsubstituted aryl-H analogue 12. Furthermore, substitution of an alkyl ester group with a 1-carboxylic acid hydrazide decreased affinity at both A1 and A2a receptors (8 vs 10). Among the esters, the methyl ester 15 was nearly equipotent with the ethyl ester 16 in binding at A1 and A2a receptors, whereas the ethyl ester 16 showed a preference for A1 receptors (8.9-fold). At A1 receptors, a nonpolar derivative of the carboxylic acid was preferred, since conversion of the ethyl 1-carboxylate (7) to a 1-carboxylic acid (14) yielded a 70-fold decrease in affinity at A1 receptors but only an 11-fold decrease in affinity at A2a receptors, resulting in the only A2a selective compound in this series (14). The other 1-carboxylic acid in this series (13) had a very low affinity and was slightly A1 selective (vs A2a). Among the derivatives examined, a thioether appeared to be favored at the 3-position, since the 3-sulfone derivative 19 displayed a much lower affinity at A1 receptors and was nearly inactive at A2a receptors. The 3-hydrazino derivative 20 was less potent than the benzylthio compound 18 at A1 receptors (31-fold) but more potent than compound 18 at A2a receptors, resulting in loss of selectivity of the compound. The affinities of some S-alkyl, S-alkenyl, and S-benzyl 3-thioethers were compared. A1 receptor binding affinity was largely insensitive to alkyl (both saturated and unsaturated) or aryl variation at the 3-thio position (1–7). A2a receptor affinity was more dependent on the 3-substituent, and aromatic substitution enhanced the affinity slightly, as in the S-benzyl analogue 7.
The effects of substitutions of the tetrahydrobenzo ring on receptor binding affinity were also studied. Alkyl substitution at the 6-position of the saturated ring was allowed. The 6,6-dimethyl substitution was tolerated and, when combined with the S-benzyl 3-thioether, favored A1 selectivity (18 vs 7) by reducing the affinity for the A2a receptor. For both A1 and A2a receptors, a 4-keto group was necessary for high affinity. When the keto group was reduced to a hydroxyl group (8 and 10) or was absent, as in the case of the unsaturated hydrocarbon (11), the affinity was ca. 10-fold lower at both A1 and A2a receptors. However, affinity for the rat A3 receptor was retained (Table 1B), resulting in the only A3 selective compound of the series. When the keto group was converted to a methoxyimino group (9), affinity at A1 and A2a receptor subtypes was decreased by an even greater degree.
Effects of BTH4 (7) on A1 and A2a Receptor-Coupled Adenylyl Cyclase.
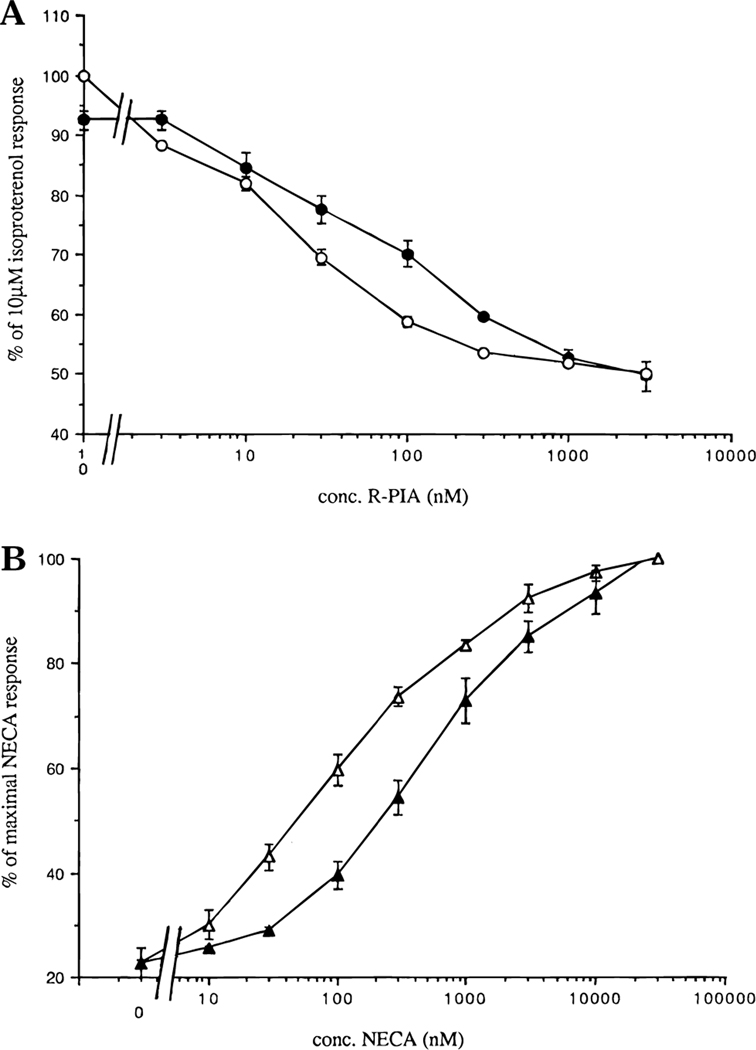
BTH4 (7) antagonized the agonist-elicited ((R)-PIA) inhibition of isoproterenolstimulated adenylyl cyclase assay in rat adipocytes expressing A1 adenosine receptors,11,27 shifting the dose–response curve of (R)-PIA to the right (Figure 2A). A KB value of 1.62 ± 0.73 μM (n = 3) was calculated using the Schild equation KB ) C/(CR – 1), where C denotes the concentration of the competitor BTH4 and CR is the ratio of the EC50 values in the presence and absence of competitor, respectively. BTH4 (7) also attenuated agonist-elicited (NECA) stimulation of adenylyl cyclase activity in pheochromocytoma cells, PC12 cells, expressing A2a adenosine receptors (Figure 2B). The KB value calculated from the Schild equation was 9.19 ± 0.98 μM (n = 3). These experiments demonstrate that the tetrahydrobenzothiophenones are functional antagonists at both A1 and A2a adenosine receptors.
Figure 2.
(A) Effects of BTH4 (7) on agonist-elicited inhibition of adenylyl cyclase via rat A1 receptors in adipocyte membranes (mean ± SEM for n = 3): (O) (R)-PIA alone and (●) (R)-PIA + 3 μM BTH4 (7). (B) Effects of BTH4 (7) on agonist-elicited stimulation of adenylyl cyclase via A2a receptors in PC12 cell membranes (mean ± SEM for n = 3): (▲) NECA alone and (Δ) NECA + 30 μM BTH4 (7).
Effect of BTH4 (7) on Locomotor Activity.
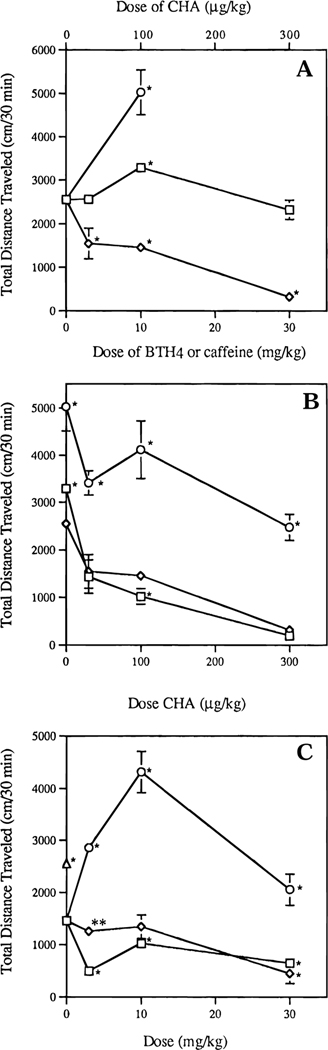
Open field locomotor effects in mice were studied in a computerized monitor by standard methods.28 The effects of the nonselective compound 7 (BTH4) alone or in combination with N6-cyclohexyladenosine (CHA) were examined (Figure 3). The potent and A1 selective adenosine agonist was chosen to maintain consistency throughout a series of publications.27,28 For comparison with BTH4 (7), the weak, nonselective adenosine antagonist caffeine was used.29 The behavioral effects of BTH4 (7) were subtle, with a modest stimulation of locomotor activity observed (Figure 3A). BTH4 (7) affected locomotor activity in mice only when administered intraperitoneally at 10 mg/kg (1.4-fold increase; p < 0.02), indicating a narrow pharmacological bandwidth. At the highest dose tested (30 mg/kg), BTH4 (7) had no effect on locomotor activity. A similar dose–response relationship, although over a broader dose range, is known for caffeine,29 which at high doses (≥50 mg/kg) in mice either does not stimulate or even depresses locomotor activity. At 10 mg/kg, caffeine causes a marked locomotor stimulation (Figure 3A).
Figure 3.
(A) Locomotor effects of BTH4 (7), caffeine, and CHA: (□) BTH4 (7), (O) caffeine, and (◊) CHA (an asterisk indicates significantly different from vehicle at p < 0.02; mean ± SEM of at least 4 and maximally 14 experiments). (B) Locomotor effects of 10 mg/kg BTH4 (7) and 10 mg/kg caffeine administered 10 min prior to varying doses of CHA: (□) BTH4 (7), (O) caffeine, (◊) CHA alone (an asterisk indicates significantly different from CHA alone at p < 0.02; mean ± SEM of at least 4 and maximally 13 experiments). (C) Locomotor effects of BTH4 (7) and caffeine coadministered with 100 μg/kg CHA: (Δ) vehicle, (0) BTH4 (7) 10 min prior to CHA, (◊) CHA 10 min prior to BTH4 (7), and (O) CHA 10 min prior to caffeine (an asterisk indicates significantly different from a single dose of 100 μg/kg CHA at p < 0.02, and double asterisks indicate significantly different from reverse order of administration at p < 0.02; mean ± SEM of at least 3 and maximally 14 experiments).
The stimulation by caffeine was evident even when it was coadministered with the A1 selective agonist CHA (Figure 3B,C). Unexpectedly, a dose of 10 mg/kg BTH4 (7), unlike caffeine or the selective A1 antagonist 1,3-dipropyl-8-cyclopentylxanthine,28 not only failed to block the locomotor depression elicited by 100 μg/kg CHA but rather caused a slight statistically significant potentiation of this locomotor depression (p < 0.02; Figure 3B).
To further investigate this phenomenon, we reversed the order of administration of CHA and BTH4 (7; Figure 3C). When BTH4 (7) was administered 10 min prior to a single dose of 100 μg/kg CHA, the potentiation was observed for all three doses of BTH4 (7; p < 0.02). However, when BTH4 (7) was administered 10 min after CHA, potentiation of locomotor depression by CHA was observed only at 30 mg/kg BTH4 (7; p < 0.02). Furthermore, reversal of the order of administration did not yield a significant change in the locomotor activity of a dose of 10 mg/kg caffeine (p < 0.02; Figure 3B,C).
The differences between the results of the various protocols suggest that rapid metabolism may play a role in the in vivo effects of BTH4 (7). Although the observed potentiation is statistically significant, considering the extent of the potentiation and the possible involvement of metabolic processes therein, the biological significance of this effect is unclear. Apart from the potentiation under specific circumstances, the characteristics of BTH4 (7) are consistent with those of a typical nonselective antagonist and may therefore be mediated by either the A1 or A2a adenosine receptor subtype, or a combination thereof.
Affinity at Other Binding Sites.
Since BTH4 (7) was one of the more potent compounds of the series (both at A1 and A2a receptors) and since it displayed unusual behavioral properties, its affinity at non-adenosine receptor binding sites was examined in a battery of radioligand-binding assays (NovaScreen, Div. of Oceanix Biosciences, Hanover, MD).30 At a concentration of 10−5 M, there was no significant (0 ± 25%) displacement of radioligand from adrenergic (α1, α2, and β), cholinergic (nicotinic and muscarinic M1, M2, M3, M4, and M5), dopaminergic (D1 and D2), serotoninergic (5-HT1 and 5-HT2), central benzodiazepine (RO 151788), GABAA (muscimol), GABAB (baclofen), NMDA, kainate, quisqualate, glycine (strychnine sensitive and insensitive), σ (MK-801), angiotensin (AT-II), substance P (NK1), substance K (NK2), vasopressin V1, neuropeptide Y, cholecystokinin (central and peripheral), neurotensin, somatostatin, ANF1, and EGF receptors. There was also no significant displacement of binding of radioligand from second-messenger sites (forskolin, phorbol ester, and inositol trisphosphate), ion channels (N-, T-, and L-type calcium channels, chloride channels, and low-conductance potassium channels), and uptake sites (dopamine, norepinephrine, serotonin, choline, and adenosine). The observation that significant affinity was not demonstrated at any of these receptors or sites, including the (NBTI sensitive) adenosine transporter, emphasizes the selectivity of BTH4 (7) for adenosine receptors.
Pharmacophore Modeling.
The present series of tetrahydrobenzothiophenones was selected from a commercial library for screening at adenosine receptor binding, based on the occurrence of fused 5:6-membered rings, as are present in xanthines.23 However, in xanthines, as in nearly all non-xanthine adenosine antagonists discovered to date, there are multiple nitrogen atoms present in the heterocyclic rings. The tetrahydrobenzothiophenones bind to A1, A2a, and A3 adenosine receptors, in spite of the lack of nitrogen atoms. Therefore, it was of interest to determine a hypothesis for a common mode of binding of the tetrahydrobenzothiophenones and xanthines in the receptor-binding site based on molecular modeling. Models for the binding of antagonists to A1 adenosine receptors have been proposed earlier by Francis et al.,31 van Galen et al.,32,33 Jacobson et al.,6 and more recently by van der Wenden et al.34 By modeling the electrostatic potential functions of the previously unknown class of tetrahydrobenzothiophenone adenosine antagonists and comparing these with adenosine antagonists previously described in the literature,6,32–34 we were able to offer a rationale for the unexpected antagonistic properties of these compounds.
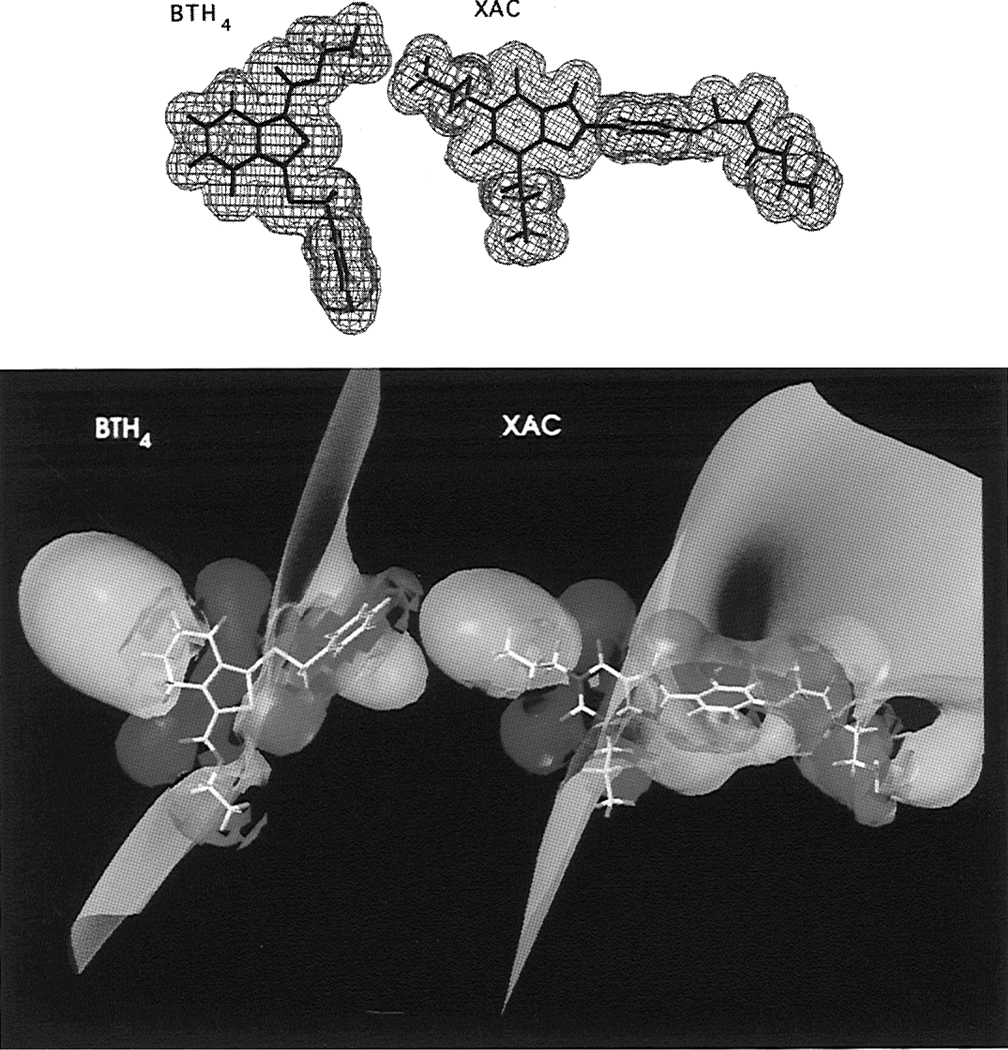
The steric and electronic properties of xanthines, the non-xanthine adenosine antagonists CP66713 and 2-phenyl-1H-imidazo[4,5-c]quinolin-4-amine (PIQA), and BTH4 (7) were calculated using the MOPAC semiempirical program and the MNDO parametrization.6,32,33 Calculations for caffeine, BTH4 (7), CP66713, and PIQA were also performed ab initio with the MC-311G basis set, which was specifically developed for second-row atoms. Although slightly different numerical results were obtained with the MNDO semiempirical and the MC-311G ab initio methods, for the purpose of qualitatively comparing electrostatic surfaces the results were identical. An examination of the van der Waals surfaces of xanthines, such as the A1 selective high-affinity xanthine-based antagonist XAC (xanthine amine congener), and the low-affinity nonselective xanthine-based antagonist caffeine, in comparison with the surfaces of a relatively active member of the tetrahydrobenzothiophenone class (BTH4, 7), failed to reveal any similarity other than the fused 5:6-membered ring system (Figure 4, top). It has been argued earlier that A1 adenosine receptor agonists and antagonists alike bind to the receptor due to a common electrostatic potential profile.6,32–34 We, therefore, calculated the electrostatic contours of the potent and A1 selective xanthine-derived antagonist XAC (Ki(A1) = 1.2 nM; Ki-(A2a) = 60 nM),6 the moderately potent and A2a selective non-xanthine antagonist CP66713 (Ki(A1) = 270 nM; Ki-(A2a) = 21 nM),6 the moderately potent and A1 selective non-xanthine antagonist PIQA (Ki(A1) = 34 nM; Ki(A2a) = 290 nM),33 and the novel nonselective non-xanthine antagonist BTH4 (7; Ki(A1) = 715 nM; Ki(A2a) = 1360 nM). Electrostatic contours were calculated for the 1, 5, and 10 kcal/mol significance levels. Increasing the cutoff potential led to contraction, and a more narrow allocation to the points indicated in Figure 5, of the isoelectric surfaces. To emphasize the overall similarity, rather than exact atomic matches, and to compare our results with those of others,6,32–34 we chose the 5 kcal/mol significance level for this study. The electrostatic contours of all compounds bore a general semblance to each other, as detailed in the next section.
Figure 4.
(Top) van der Waals volumes of BTH4 (7) and XAC. (Bottom) Isopotential surfaces of BTH4 (7) and XAC (red = 5 kcal/mol, yellow = 0, and blue = −5 kcal/mol).
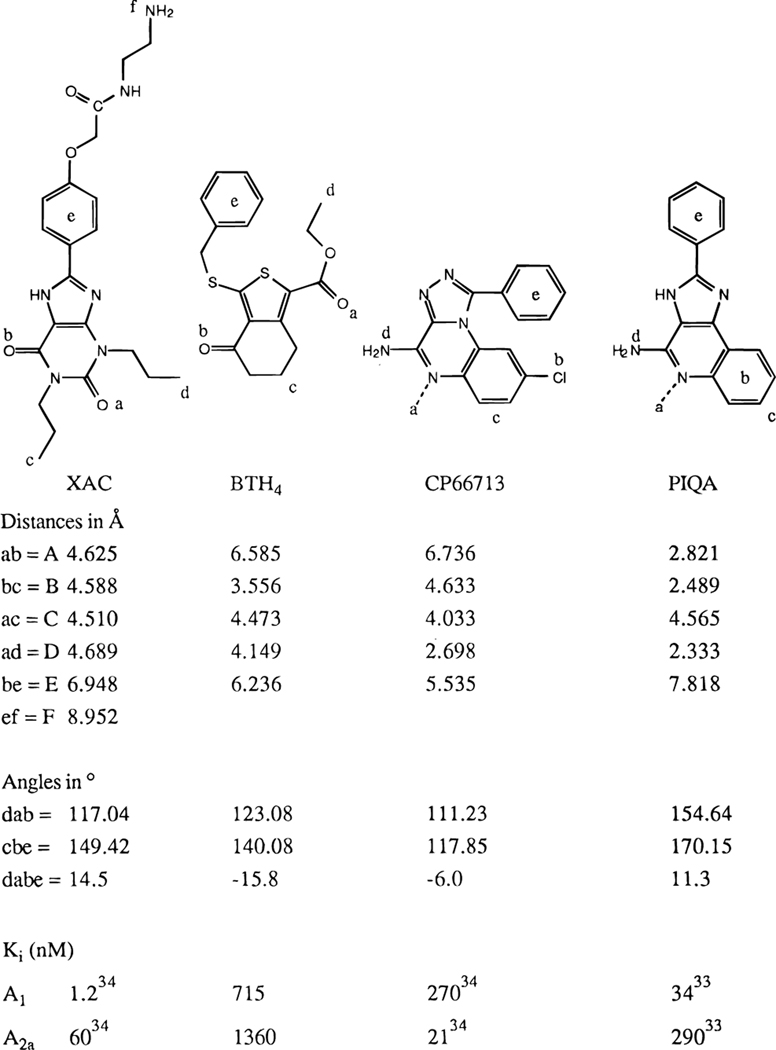
Figure 5.
Pharmacophore models for XAC, BTH4 (7), CP66713, and PIQA, showing distances, in-plane angles, and dihedral angles. Point e is defined as the center of the aromatic ring; all other positions are defined by the atomic coordinates.
The molecular electrostatic potential map (Figure 4, bottom) identifies five points that show a high degree of similarity in all structures, and XAC and BTH4 (7) more specifically. The first two points (marked a and b in Figure 5) are the carbonyl oxygens at positions 2 and 6 in XAC, respectively. The second set of two points (marked c and d) designate the 1- and 3-propyl alkyl regions of XAC, respectively. The fifth point (marked e) identifies the center of the aromatic substituent at the 8-position in XAC. Equivalent positions in BTH4 (7), CP66713, and PIQA are indicated in Figure 5. The sixth point (marked f), which designates a favorable additional substitution site in XAC and is absent in BTH4 (7), its analogues, and the reference compounds CP66713 and PIQA, is the primary amine at the terminal of the 8-substituent. A semblance in electronic properties was demonstrated earlier for various other non-xanthine adenosine antagonists6,32,34 and led to the development of new classes of antagonists.31,33 We propose here that the novel antagonist BTH4 (7) also fits this model.
A high degree of similarity in the electrostatic surfaces of XAC and BTH4 (7) assumes that the thiophene ring roughly superimposes on the region of the uracil ring of xanthines rather than a superpositioning of the 5-membered thiophene and imidazole rings. There are regions of partial negative charge surrounding the two carbonyl groups of BTH4 (7), which correspond spatially to isolated regions of partial negative charge around the two carbonyls of xanthines.6,32–34 Thus, the keto group of the tetrahydrobenzo ring would occupy the same position as the 6-position carbonyl group of xanthines. The carbonyl of the 1-carboxylic acid ester group, although it does not occur in a ring, occupies the same position as the 2-position carbonyl group of xanthines. To accommodate the presence of the tetrahydrobenzo ring, the 6,6-dimethyl groups present in some analogues, and the ester function into a generalized model, we selected the high affinity A1 selective antagonist XAC, which is substituted with 1,3-dipropyl and 8-aryl groups, for further examination. A positively charged region around the 3-propyl group of XAC6,32–34 overlays the 1-ethyl ester group of BTH4 (7); thus, the alkoxy moiety of this ester substituent appears to correspond to the 3-alkyl group of xanthines. The conformationally constrained (CH2)3 portion of the tetrahydrobenzo ring may mimic the flexible 1-propyl group of XAC. In xanthines, a variety of alkyl and aryl substitutions at the 1- and 3-positions are tolerated.6,32–34 The corresponding groups of BTH4 (7) can be similarly substituted without loss of affinity. Consequently, the 1-ester group may consist of either a methyl (15) or an ethyl (16) ester, and the 6-position of the tetrahydrobenzo ring may be branched (18–20).
A small, positively charged region around the imidazole NH of XAC, which is likely a determinant of its high affinity,6,32–34 has an equivalent in BTH4 (7), i.e., a region of partial positive charge around the thioether sulfur atom. Even the 8-aryl group of the high-affinity xanthines, such as XAC, corresponds roughly to the benzyl ring of BTH4 (7). However, the presence of a benzyl group enhances A1 receptor affinity of BTH4 (7) by, at most, only 2-fold, while 8-aryl substituents greatly enhance affinity for A1 receptors.6,32
To compare the binding modes of XAC, BTH4(7), CP66713, and PIQA, we determined distances, in-plane angles, and dihedral angles in the energy-minimized structures (Figure 5). Analysis of the distances A (ab), B (bc), C (ac), D (ad), and E (be), the nonbonded in-plane angles ∠dab and ∠cbe, and the nonbonded dihedral angle ∠dabe reveals that although there is some difference between the values for the various compounds, there is sufficient ground to accept the validity of this pharmacophore model6,32–34 for BTH4 (7). It suggests that a ring structure at the position of the 5-membered ring in xanthines, CP66713, or PIQA is not strictly required and that, inversely, the tetrahydrobenzo ring structure of BTH4 (7) can substitute for the linear propyl group in XAC. The distinct difference in the distances A might be one of the major factors determining the large difference in affinity observed for the compounds. The formation of hydrogen bonds to the xanthine carbonyl groups is limited to a certain range of distances, bond angles, and dihedral angles. If these criteria are not exactly met, a sharply decreased affinity, such as that which occurs in the tetrahydrobenzothiophenones, may be observed. The dependency of the affinity on the distances B and D, both about 4.6 Å, was already shown for XAC6,10 and seems to hold true for BTH4 (7) as well. Also significant is the distance E between the carbonyl oxygen marked b and the center of the aromatic substituent marked e. In BTH4 (7; 715 nM at A1), this distance is 6.3 Å, and in XAC (1.2 nM at A1), it is 6.9 Å. A similar effect is observed for CP66713 (270 nM), which is only about 2.6-fold more potent than BTH4 (7; 715 nM) at A1 adenosine receptors, where the distance E is only 5.5 Å. The distance E in PIQA is 7.8 Å, and this is reflected in a considerably higher affinity at A1 adenosine receptors (34 nM). The rigidity of either XAC, CP66713, or PIQA (one rotatable bond between the heterocycle and the phenyl ring) allows for the occupation of less conformational space than the three corresponding rotatable bonds in BTH4 (7), as reflected in the dihedral angle ∠dabe (14.5°, −6°, 11.3°, and −15.8° for XAC, CP66713, PIQA, and BTH4 (7), respectively). More narrowly defined are the nonbonded in-plane angles ∠dab and ∠cbe (117° and 123° for ∠dab and 149° and 140° for ∠cbe for XAC and BTH4 (7), respectively). All differences between the base structures summed, plus the additional binding enhancing site f in XAC, may account for the difference in affinity (715 nM for BTH4 (7) and 1.2 nM for XAC6) at A1 adenosine receptors. The correspondence of pharmacophores is novel and not immediately obvious without the aid of a computer model.
Conclusions
It was discovered that heterocyclic compounds that do not contain nitrogen can act as antagonists at A1, A2a, and A3 adenosine receptors. The completely new class of ligands described here shows a general selectivity toward A1 adenosine receptors, but minor modifications can readily reverse this selectivity in favor of A2a adenosine receptors. The compound that displayed a relatively high affinity at both A1 and A2a adenosine receptors was shown to be an antagonist in functional studies of inhibition (A1) and stimulation (A2a) of adenylyl cyclase activity and to increase locomotor activity in mice under certain circumstances. The slight, readily reversible A1 selectivity of some BTH4 analogues (1.9-fold A1/A2a selectivity for 7, 3.3-fold A2a/A1 selectivity for 14, and 3.9-fold A3/A1 selectivity for 11) and the apparent absence of affinity for sites other than adenosine receptors, including the (NBTI sensitive) nucleoside transporter, make this novel class of ligands an excellent starting point for the development of new and selective non-xanthine antagonists.
Experimental Section
Materials.
Ethyl 3-(methylthio)-4-oxo-4,5,6,7-tetrahydrobenzo[c]thiophene-1-carboxylate (1), ethyl 3-(propylthio)-4-oxo-4,5,6,7-tetrahydrobenzo[c]thiophene-1-carboxylate (2), ethyl 3-(2-propylthio)-4-oxo-4,5,6,7-tetrahydrobenzo[c]thiophene-1-carboxylate (3), ethyl 3-(2-propargylthio)-4-oxo-4,5,6,7-tetrahydrobenzo[c]thiophene-1-carboxylate (4), ethyl 3-(butylthio)-4-oxo-4,5,6,7-tetrahydrobenzo[c]thiophene-1-carboxylate (5), ethyl 3-[[(ethoxycarbonyl)methyl]thio]-4-oxo-4,5,6,7-tetrahydrobenzo[c]thiophene-1-carboxylate (6), ethyl 3-(benzylthio)-4-oxo-4,5,6,7-tetrahydrobenzo[c]thiophene-1-carboxylate (7), ethyl 4-hydroxy-3-(methylthio)-4,5,6,7-tetrahydrobenzo[c]thiophene-1-carboxylate (8), ethyl 4-(methoxyimino)-3-(methylthio)-4,5,6,7-tetrahydrobenzo[c]thiophene-1-carboxylate (9), 4-hydroxy-3-(methylthio)-4,5,6,7-tetrahydrobenzo[c]thiophene-1-carboxylic acid hydrazide (10), 4,5-dihydro-1-(methylthio)benzo[c]thiophene-3-carboxylic acid hydrazide (11), 5,5-dimethyl-1-(methylthio)-4,5,6,7-tetrahydrobenzo[c]thiophen-7-one (12), 3-(methylthio)-6,6-dimethyl-4-oxo-4,5,6,7-tetrahydrobenzo[c]thiophene-1-carboxylic acid (13), methyl 3-(ethylthio)-6,6-dimethyl-4-oxo-4,5,6,7-tetrahydrobenzo[c]thiophene-1-carboxylate (15), ethyl 3-(ethylthio)-6,6-dimethyl-4-oxo-4,5,6,7-tetrahydrobenzo[c]thiophene-1-carboxylate (16), ethyl 3-(2-butylthio)-6,6-dimethyl-4-oxo-4,5,6,7-tetrahydrobenzo[c]thiophene1-carboxylate (17), ethyl 3-(benzylthio)-6,6-dimethyl-4-oxo-4,5,6,7-tetrahydrobenzo[c]thiophene-1-carboxylate (18), ethyl 3-(methylsulfonyl)-6,6-dimethyl-4-oxo-4,5,6,7-tetrahydrobenzo[c]thiophene-1-carboxylate (19), and ethyl 3-hydrazino-6,6-dimethyl-4-oxo-4,5,6,7-tetrahydrobenzo[c]thiophene-1-carboxylate (20) were purchased from Maybridge (Trevillett, U.K.). HEK-293 cell membranes transfected with human A3 cDNA were obtained from Receptor Biology, Inc. (Baltimore, MD). CHO cell membranes transfected with rat A3 cDNA, provided by Drs. M. Olah and G. Stiles, were prepared as described earlier.26 All other materials were obtained from commercial sources as described previously.10,24–27,35,36
Synthesis.
Proton nuclear magnetic resonance spectroscopy was performed on a Varian GEMINI-300 spectrometer, and spectra were taken in DMSO-d6. Electron-impact mass spectrometry was performed with a VG7070F mass spectrometer at 6 kV. Elemental analysis was performed by Atlantic Microlab Inc. (Norcross, GA).
3-(Benzylthio)-4-oxo-4,5,6,7-tetrahydrobenzo[c]-thiophene-1-carboxylic Acid (14).
Ethyl 3-(benzylthio)-4-oxo-4,5,6,7-tetrahydrobenzo[c]thiophene-1-carboxylate (7; 100 mg, 0.288 mmol) was dissolved in 5 mL of DMF. To the solution was added 500 μL of 4 M NaOH, and the solution was heated at 70 °C for 45 min, at which time the hydrolysis was shown to be complete by TLC (silica gel 60, ethyl acetate: petroleum ether = 10:90). To the reaction mixture was added 15 mL of water. The basic water phase was then extracted three times with 10 mL of ethyl acetate. The water phase was acidified (pH < 3) with concentrated hydrochloric acid until the product precipitated as an amorphous coagulate. The product was twice extracted with 50 mL of ethyl acetate and the ethyl acetate fraction dried over Na2SO4 and evaporated to dryness. The extract was shown to be pure by TLC. The product was dissolved in and crystallized from chloroform. Yield: 79 mg (86%). 1H NMR (ppm relative to TMS): 1.99 (H-6a,b, 2H, q), 2.51 (H-7a,b, coincides partially with DMSO peak), 3.14 (H-5a,b, 2H, t), 4.41 (SCH2, 2H, s), 7.32–7.53 (phenyl, 5H, m), 13.18 (COOH, 1H, br). Molecular mass: calcd, 318.0384; found, m/z = 318.0381. Mp: 226 °C dec. Anal. (C16H14O3S2) C,H,S.
Pharmacology. Radioligand-Binding Studies.
Binding of [125I]-N6-(4-amino-3-iodobenzyl)adenosine-5′-N-methyluronamide ([125I]AB-MECA) to cloned rat A3 receptors expressed on CHO cell membranes or cloned human A3 receptors expressed on HEK-293 cells was performed as described previously.10,26,39,40 Nonspecific binding was determined in the presence of 200 μM N-(ethylcarbamoyladenosine (NECA).
Binding of [3H]-(R)-N6-(phenylisopropyl)adenosine ([3H]-(R)-PIA) to A1 receptors from rat cerebral cortex membranes and of [3H]CGS21680 to A2a receptors from rat striatal membranes was performed as described previously.24,25 Adenosine deaminase (3 U/mL) was present during the preparation of the brain membranes, a preincubation of 30 min at 30 °C, and the incubation with the radioligands.
All nonradioactive compounds were initially dissolved in DMSO and diluted with buffer to the final concentration, where the amount of DMSO never exceeded 1%. At least six different concentrations of antagonist, spanning 3 orders of magnitude adjusted appropriately for the IC50 of each compound, were used. IC50 values, computer-generated using the nonlinear regression method implemented in the InPlot program (Graph-PAD, San Diego, CA), were converted to apparent Ki values using Kd values of 1.0, 14, 1.5, and 0.6 nM for [3H]-(R)-PIA (rat A1), [3H]CGS21680 (rat A2a), [125I]AB-MECA (rat A3), and [125I]AB-MECA (human A3) binding, respectively,24,25,40 applying the Cheng–Prusoff equation.38
The affinity at selected additional receptor- and second-messenger-binding sites was tested by NovaScreen (Div. of Oceanix Biosciences, Hanover, MD), using a variety of binding assays.30
A1 and A2a Receptor-Coupled Adenylyl Cyclase Activity.
Adenylyl cyclase assays were carried out, essentially as described previously, for A1 receptors using rat adipocyte membranes27 and for A2a receptors using rat pheochromocytoma PC12 cell membranes.37
Locomotor Activity.
Adult male mice of the NIH (Swiss) strain weighing 25–30 g were housed in groups of 10 animals/cage with a light–dark cycle of 12:12 h. The animals were given free access to standard pellet food and water and were acclimatized to laboratory conditions for 24 h prior to testing. Each animal was only used once in the activity monitor. Locomotor activity of individual animals was studied in an open field using a Digiscan activity monitor (Omnitech Electronics Inc., Columbus, OH) equipped with an IBM-compatible computer. The computer-tabulated measurements represent multivariate locomotor analysis with specific measures, such as simultaneous measurements of ambulatory, rearing, stereotypical, and rotational behavior. Data were collected in the morning, for three consecutive intervals of 10 min each, and analyzed separately and as a group. Statistical analysis was performed using the Student’s t-test. The results are reported as mean ± standard error for each point. Compound 7 was dissolved initially in DMSO and diluted in at least 20 vol of vehicle, a 20:80 (v/v) mixture of Alkamuls EL-620 (Rhône-Poulenc, Cranbury, NJ) and phosphate-buffered saline. Drugs were administered intraperitoneally in a volume corresponding to 5 mL/kg of body weight.28
Molecular Modeling.
Structures were drawn in the Sybyl molecular modeling package (Tripos Associates Inc., St. Louis, MO; version 6.04) running on an Iris Indigo XZ4000 workstation (Silicon Graphics Inc., Mountain View, CA, MIPS R4000 CPU). Energy minimizations were subsequently performed in the MOPAC program (Quantum Chemistry Program Exchange, version 6.0), using the MNDO Hamiltonian and the keywords PULAY, PRECISE, and MMOK, and in the Gaussian program (Gaussian Inc., Pittsburgh, PA; version 92, revision F4), using the MC-311G basis set with closed shell RHF. Both the MOPAC and the Gaussian-92 program were run on a Convex C3830 system (Convex Computer Corp., Richardson, TX). After completion of the runs the data were converted back into the Sybyl format using the Babel (University of Arizona; version 1.1, babel@mercury.aichem.arizona.edu) conversion program. van der Waals and electrostatic contours were then generated using standard procedures within Sybyl.
Acknowledgment.
A.M.v.R. would like to thank the Cystic Fibrosis Foundation for financial support. We thank Dr. Xiao-duo Ji for measurement of binding at A3 receptors, Gilead Sciences (Foster City, CA) for general support, and NIMH for making the NovaScreen assay available. D.S. was supported by a grant from the International Life Science Institute.
Footnotes
Molecular Recognition Section.
Pharmacodynamics Section.
Abbreviations: [125I]AB-MECA, [125I]-N6-(4-amino-3-iodobenzyl)-adenosine-5′-N-methyluronamide; BTH4, ethyl 3-(benzylthio)-4-oxo-4,5,6,7-tetrahydrobenzo[c]thiophene-1-carboxylate; CGS21680, 2-[[[4-(2-carboxyethyl)phenyl]ethyl]amino]-5′-(N-ethylcarbamoyl)-adenosine; CHA, N6-cyclohexyladenosine; CHO cells, Chinese hamster ovary cells; CP66713, 9-chloro-2-phenyl[1,3,4]triazolo[5,1-c]quinazolin-5-amine; DMF, N,N-dimethylformamide; DMSO, dimethyl sulfoxide; KB, dynamic inhibition constant; Ki equilibrium inhibition constant; NECA, N-(ethylcarbamoyl)adenosine; PIQA 2-phenyl-1H-imidazo[4,5-c]quinolin-4-amine; (R)-PIA, (R)-N6-(phenylisopropyl)adenosine; Tris tris(hydroxymethyl)aminomethane; XAC, 8-[4-[[[[(2-aminoethyl)amino]-carbonyl]methyl]oxy]phenyl]-1,3-dipropylxanthine, xanthine amine congener.
References
- (1).Olsson RA; Pearson JD Cardiovascular purinoceptors. Pharmacol. Rev. 1990, 3, 761–845. [DOI] [PubMed] [Google Scholar]
- (2).Rossi NF; Churchill PC; Jacobson KA; Leahy AE Further characterization of the renovascular effects of N6-cyclohexyladenosine in the isolated perfused rat kidney. J. Pharmacol. Exp. Ther. 1987, 240, 911–915. [PMC free article] [PubMed] [Google Scholar]
- (3).Londos C; Cooper DM; Wolff J. Subclasses of external adenosine receptors. Proc. Natl. Acad. Sci. U.S.A. 1980, 77, 2551–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Hillaire-Buys D; Bertrand G; Gross R; Loubatières-Mariani MM Evidence for an inhibitory A1 subtype adenosine receptor on pancreatic insulin-secreting cells. Eur. J. Pharmacol. 1987, 136, 109–112. [DOI] [PubMed] [Google Scholar]
- (5).Fredholm BB; Dunwiddie TV How does adenosine inhibit transmitter release? Trends Pharmacol. Sci. 1988, 9, 130–134. [DOI] [PubMed] [Google Scholar]
- (6).Jacobson KA; van Galen PJM; Williams M. Adenosine receptors - pharmacology, structure activity relationships, and therapeutic potential. J. Med. Chem. 1992, 35, 407–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).von Lubitz DKJE; Jacobson KA Behavioral effects of adenosine receptor stimulation. In Adenosine and Adenine Nucleotides: From Molecular Biology to Integrative Physiology; Bellardinelli L, Pelleg A, Eds.; Kluwer: Norwell, 1995; pp 489–498. [Google Scholar]
- (8).Stiles GL Adenosine receptors. J. Biol. Chem. 1992, 267, 6451–6454. [PubMed] [Google Scholar]
- (9).Jacobson MA Cloning and expression of human adenosine receptor subtypes. In Adenosine and Adenine Nucleotides: From Molecular Biology to Integrative Physiology; Bellardinelli L, Pelleg A, Eds.; Kluwer: Norwell, 1995; pp 5–13. [Google Scholar]
- (10).Kim HO; Ji XD; Melman N; Olah ME; Stiles GL; Jacobson KA Structure-activity relationships of 1,3-dialkylx-anthine derivatives at rat A3 adenosine receptors. J. Med. Chem. 1994, 37, 3373–3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Daly JW; Hong O; Padgett WL; Shamim MT; Jacobson KA; Ukena D. Non-xanthine heterocycles: activity as antagonists of A1- and A2-adenosine receptors. Biochem. Pharmacol. 1988, 37, 655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Francis JE; Cash WD; Psychoyos S; Ghai G; Wenk P; Friedmann RC; Atkins C; Warren V; Furness P; Hyun JL Structure-activity profile of a series of novel triazoloquinazoline adenosine antagonists. J. Med. Chem. 1988, 31, 1014–1020. [DOI] [PubMed] [Google Scholar]
- (13).Thompson RD; Secunda S; Daly JW; Olsson RA N6,9-Disubstituted Adenines - Potent, Selective Antagonists at the A1-Adenosine Receptor. J. Med. Chem. 1991, 34, 2877–2882. [DOI] [PubMed] [Google Scholar]
- (14).Baraldi PG; Manfredini S; Simoni D; Zappaterra L; Zocchi C; Dionisotti S; Ongini E. Synthesis of new pyrazolo[4,3-e]1,2,4-triazolo[1,5-c]pyrimidine and 1,2,3-triazolo[4,5-e]1,2,4-triazolo[1,5-c] pyrimidine displaying potent and selective activity as A2a adenosine receptor antagonists. Bioorg. Med. Chem. Lett. 1994, 4, 2539–2544. [Google Scholar]
- (15).Poucher SM; Collis MG; Keddie JR; Stoggall SM; Singh P; Caulkett PWR; Jones G. The in vitro pharmacology of ZM241385, a novel non-xanthine, A2a selective adenosine antagonist. Br. J. Pharmacol. 1994, abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Müller CE; Grahner B; Heber D. Amino-substituted 1,8-naphthyridines and pyrido[2,3-d]pyrimidines - new compounds with affinity for A1-adenosine and A2-adenosine receptors. Pharmazie 1994, 49, 878–880. [PubMed] [Google Scholar]
- (17).Sidi A; Wesley R; Barrett R; Rush W; Belardinelli L. Cardiovascular effects of a non-xanthine-selective antagonist of the A1 adenosine receptor in the anaesthetised pig: pharmacological and therapeutic implications. Cardiovasc. Res. 1994, 28, 621–628. [DOI] [PubMed] [Google Scholar]
- (18).Catarzi D; Cecchi L; Colotta V; Filacchioni G; Martini C; Tacchi P; Lucacchini A. Tricyclic heteroaromatic systems. Synthesis and A1 and A2a adenosine binding activities of some 1-aryl-1,4-dihydro-3-methyl-[1]benzopyrano[2,3-c]pyrazol-4-ones, 1-aryl-4,9-dihydro-3-methyl-1H-pyrazolo-[3,4-b]quinolin-4-ones, and 1-aryl-1H-imidazo-[4,5-b]quinoxalines. J. Med. Chem. 1995, 38, 1330–1336. [DOI] [PubMed] [Google Scholar]
- (19).Okajima F; Akbar M; Abdul Majid M; Sho K; Tomura H; Kondo Y. Genistein, an inhibitor of protein tyrosine kinase, is also a competitive antagonist for P1-purinergic (adenosine) receptor in FRTL-5 thyroid cells. Biochem. Biophys. Res. Commun. 1994, 203, 1488–1495. [DOI] [PubMed] [Google Scholar]
- (20).Yang Z; Hon PM; Chui KY; Xu ZL; Chang HM; Lee CM; Cui YX; Wong HNC; Poon CD; Fung BM Naturally occurring benzofuran - isolation, structure elucidation and total synthesis of 5-(3-hydroxypropyl)-7-methoxy-2-(3′-methoxy-4′-hydroxyphenyl)-3-benzo[b]furancarbaldehyde, a novel adenosine-A1 receptor ligand isolated from Salvia Militorrhiza bunge (Danshen). Tetrahedron Lett. 1991, 32, 2061–2064. [Google Scholar]
- (21).Gallo-Rodriguez C; Ji XD; Melman N; Siegman BD; Sanders LH; Orlina J; Pu QL; Olah ME; van Galen PJM; Stiles GL; Jacobson KA Structure-activity relationships at A3-adenosine receptors. J. Med. Chem. 1994, 37, 636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Kim HO; Ji XD; Siddiqi SM; Olah ME; Stiles GL; Jacobson KA 2-Substitution of N6-benzyladenosine-5′-uronamides enhances selectivity for A3 adenosine receptors. J. Med. Chem. 1994, 37, 3614–3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Siddiqi SM; Ji XD; Melman N; Olah ME; Jain R; Evans P; Glashofer M; Padgett WL; Cohen LA; Daly JW; Stiles GL; Jacobson KA A survey of non-xanthine derivatives as adenosine receptor ligands. Nucleosides Nucleotides 1996, 15, 693–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Schwabe U; Trost T. Characterization of adenosine receptors in rat brain by (−) [3H]N6-phenylisopropyladenosine. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1980, 313, 179–187. [DOI] [PubMed] [Google Scholar]
- (25).Jarvis MF; Schutz R; Hutchison AJ; Do E; Sills MA; Williams M. [3H]CGS 21680, an A2 selective adenosine receptor agonist directly labels A2 receptors in rat brain tissue. J. Pharmacol. Exp. Ther. 1989, 251, 888–893. [PubMed] [Google Scholar]
- (26).Olah ME; Gallo-Rodriguez C; Jacobson KA; Stiles GL [125I]AB-MECA, a high affinity radioligand for the rat A3 adenosine receptor. Mol. Pharmacol. 1994, 45, 978–982. [PMC free article] [PubMed] [Google Scholar]
- (27).Daly JW; Padgett WL Agonist activity of 2- and 5′-substituted adenosine analogs and their N6-cycloalkyl derivatives at A1-adenosine and A2-adenosine receptors coupled to adenylate cyclase. Biochem. Pharmacol. 1992, 43, 1089–1093. [DOI] [PubMed] [Google Scholar]
- (28).Jacobson KA; Nikodijevic O; Padgett WL; Gallo-Rodriguez C; Maillard M; Daly JW 8-(3-Chlorostyryl)caffeine (CSC) is a selective A2-adenosine antagonist in vitro and in vivo. FEBS Lett. 1993, 323, 141–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Nikodijevic O; Jacobson KA; Daly JW Locomotor activity in mice during chronic treatment with caffeine and withdrawal. Pharmacol. Biochem. Behav. 1993, 44, 199–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Sweetnam PM; Caldwell L; Lancaster J; Bauer C; Mc-Millan B; Kinnier WJ; Price CH The role of receptor binding in drug discovery. J. Nat. Prod. 1993, 56, 441–455. [DOI] [PubMed] [Google Scholar]
- (31).Francis JE; Cash WD; Psychoyos S; Ghai G; Wenk P; Friedmann RC; Atkins C; Warren V; Furness P; Hyun JL; Stone GA; Desai M; Williams M. Structure-activity profile of a series of novel triazoloquinazoline adenosine antagonists. J. Med. Chem. 1988, 31, 1014–1020. [DOI] [PubMed] [Google Scholar]
- (32).van Galen PJM; van Vlijmen HWT; IJzerman AP; Soudijn W. A model for the antagonist binding site on the adenosine A1 receptor, based on steric, electrostatic, and hydrophobic properties. J. Med. Chem. 1990, 33, 1708–1713. [DOI] [PubMed] [Google Scholar]
- (33).van Galen PJM; Nissen P; van Wijngaarden I; IJzerman AP; Soudijn W. 1H-Imidazo[4,5-c]quinolin-4-amines: novel non-xanthine adenosine antagonists. J. Med. Chem. 1991, 34, 1202–1206. [DOI] [PubMed] [Google Scholar]
- (34).van der Wenden EM; Price SL; Apaya RP; IJzerman AP; Soudijn W. Relative binding orientations of adenosine-A1 receptor ligands - A test-case for Distributed Multipole Analysis in medicinal chemistry. J. Comput.-Aided Mol. Des. 1995, 9, 44–54. [DOI] [PubMed] [Google Scholar]
- (35).Siddiqi SM; Pearlstein RA; Sanders LH; Jacobson KA Comparative Molecular Field Analysis of selective A3 adenosine receptor agonists. Bioorg. Med. Chem. 1995, 3, 1331–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).van Galen PJM; van Bergen AH; Gallo-Rodriguez C; Olah ME; IJzerman AP; Stiles GL; Jacobson KA A binding site model and structure-activity relationships for the rat A3 adenosine receptor. Mol. Pharmacol. 1994, 45, 1101–1111. [PMC free article] [PubMed] [Google Scholar]
- (37).Hide I; Padgett WL; Jacobson KA; Daly JW A2a adenosine receptors from rat striatum and rat pheochromocytoma PC12 cells: characterization with radioligand binding and by activation of adenylate cyclase. Mol. Pharmacol. 1992, 41, 352–359. [PMC free article] [PubMed] [Google Scholar]
- (38).Cheng YC; Prusoff WH Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 percent inhibition (IC50) of an enzyme reaction. Biochem. Pharmacol. 1973, 22, 3099–3108. [DOI] [PubMed] [Google Scholar]
- (39).Ji XD; von Lubitz DKJE; Olah ME; Stiles GL; Jacobson KA Species-differences in ligand affinity at central A3-adenosine receptors. Drug Dev. Res. 1994, 33, 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Ji XD; Melman N; Jacobson KA Interactions of flavonoids and other phytochemicals with adenosine receptors. J. Med. Chem. 1996, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]