Abstract
Introduction
Group B streptococcus(GBS)often causes adverse outcomes such as urinary system infection, intrauterine infection, premature birth, and stillbirth in perinatal women. Perinatal screening of GBS is conducive to guiding clinical scientific intervention and improving delivery outcomes.This study quantitative real-time PCR (RT-qPCR) combined with magnetic separation was used for GBS detection.
Materials and methods
Sample pre-treatment in this study involved the utilization of magnetic separation (MS) technology, aiming to expedite the detection process and enhance detection sensitivity, and the cfb gene of group B streptococcus was used as the target gene to establish quantitative real-time PCR (RT-qPCR) to detect group B streptococcus.
Results
It was found that penicillin-functionalized magnetic beads had a good ability to enrich and capture group B Streptococcus.The findings revealed an exceptional detection sensitivity, with the ability to detect B streptococcus in urine samples at levels as low as 102 CFU/mL.
Conclusions
The utilization of MS technology in conjunction with the RT-qPCR (MS-RT-qPCR) assay, as demonstrated in this study, offers a viable approach for prenatal screening of group B streptococcus among perinatal women.
Keywords: Group B streptococcus, Real-time PCR, Magnetic separation
Highlights
-
•
Group B streptococcus is the main pathogen of infection in perinatal women.
-
•
Penicillin can serve as a recognition molecule to capture the target bacteria.
-
•
Magnetic separation combined with quantitative real-time PCR demonstrates a lower detection limit.
-
•
This method can provide new ideas for the detection of pathogenic bacteria.
1. Introduction
Group B streptococcus (GBS) represents a common gram-positive coccus that usually asymptomatic colonizes the human digestive tract and the female urogenital tract, generally does not cause clinical symptoms, and is a normal part of its microbiome. However, in perinatal women, GBS with a fixed value can cause adverse outcomes such as urinary system infection, intrauterine infection, premature delivery, and stillbirth, or vertical transmission through the birth canal, resulting in early-onset group B Streptococcus infection (EOGBS). The clinical manifestations are sepsis, pneumonia, meningitis, etc., and the morbidity and mortality are high [1,2]. Therefore, there is a need to develop a fast and highly sensitive screening method for GBS in perinatal women.
Currently, methods for GBS detection primarily encompass traditional isolation and culture techniques, immunological approaches, as well as molecular biology methodologies. The traditional separation culture technique does not necessitate sophisticated experimental equipment or specialized facilities, but the positive detection rate is not high, and it is time-consuming and laborious [3]. Immunological methods are prone to cross-contamination and false negatives [4]. In contrast, molecular biological detection has the characteristics of high sensitivity and short time, among which polymerase chain reaction (PCR) is extensively employed for pathogenic bacteria detection. The quantitative real-time PCR (RT-qPCR) used in this study shortened the detection time and improved the detection efficiency compared with the traditional culture approach [[3], [4], [5], [6]].
The target genes detected by GBS include 16sRNA, cfb, scpb, cps and Sip genes et al. [[7], [8], [9]], among which cfb gene is responsible for encoding the specific hemolytic pathogenic substance CAMP factor. The results of Mousavi, S. M. et al. [8] and Sarah Shabayek et al. [10] showed that cfb gene had good specificity and was more suitable for the detection of GBS.
The concentration of GBS in some hospital samples was found to be considerably low, likely influenced by the complex matrix. Consequently, some researchers considered utilizing broth enrichment before conducting real-time PCR to address this issue [11]. The pretreatment of pathogen detection often involves the extensive application of Magnetic separation (MS) technology, which enables an effective detection of bacteria at low concentrations in the samples [12,13]. Penicillin (Pen), a widely utilized β-lactam antibiotic, exhibits an affinity for penicillin-binding proteins (PBPs) located within the cell wall of the majority of Gram-positive bacteria [14]. Compared to antibodies, Penicillin is more cost-effective and simpler to store [15,16]. Wang et al. [17] developed a magnetic nanomaterial, Fe3O4@Ag@Van, which was utilized in conjunction with SERS technology. The integration of enrichment and separation steps permits the efficient capture and detection of the desired bacteria. The enrichment of bacteria from complex samples involved the use of penicillin as the recognition molecule.
MS technology combined with RT-qPCR was employed to detect GBS in the urine of perinatal pregnant women. Initially, the samples were subjected to GBS isolation using Penicillin-modified magnetic nanomaterials. Subsequently, the RT-qPCR system was established using the cfb gene with high specificity. The method from this study could Serve as a sturdy framework for accurately detecting GBS samples containing a scant amount of bacteria in the future.
2. Materials and Methods
2.1. Strains and culture conditions
These bacterial variants are enumerated in Table 2. RT-qPCR system was established with GBS (ATCC 13813) strain. Strains were meticulously introduced into Luria Bertani (LB) medium for culture (37 °C, 144×g). Bacterial concentration was assessed by the Columbia blood agar culture counting method following a series of gradient dilutions of bacterial cultures (PBS, pH 7.4, 0.01 M).
Table 2.
Target and non-target bacteria strains and RT-qPCR results.
| Bacteria strains | ID | Source | mPCR positive for cfb |
|---|---|---|---|
| Bacillus cereus | 14579 | ATCCa | – |
| Cronobacter sakazakii | 45401 | CMCCb | – |
| 45402 | CMCC | – | |
| 21564 | CICCc | – | |
| 21545 | CICC | – | |
| Shigella flexner | 25931 | ATCC | – |
| Salmonella typhimurium | 13311 | ATCC | – |
| Salmonella paratyphi A | 9150 | ATCC | – |
| Salmonella paratyphi B | 40001 | JX-CDCd | – |
| Listeria monocytogenes | 13932 | ATCC | – |
| 54001 | CMCC | – | |
| 54007 | CMCC | – | |
| Pseudomonas aeruginosa | 10104 | CMCC | – |
| Escherichia coli O157:H7 | 43888 | ATCC | – |
| 44102 | CMCC | – | |
| 44828 | CMCC | – | |
| Staphylococcus aureus | 26001 | CMCC | – |
| 26002 | CMCC | – | |
| 26003 | CMCC | – | |
| Streptococcus pneumoniae | 31001 | CMCC | – |
| Group B Streptococcus | 13813 | ATCC | + |
Result (±) indicates positive and negative sig.
ATCC, American Type Culture Collection, USA.
CMCC, China Medical Culture Collection, China.
CICC, China Center of Industrial Culture Collection, China.
JX-CDC, Jiang Xi Province Center for Disease Control and Prevention, China.
2.2. Reagents and materials
Acquisition of Carboxylated magnetic beads (10 mg/mL, 180 nm) was sourced from Shanghai Allrun Nano Science & Technology Co. Ltd. Sigma Aldrich Chemical Co (St. Louis, U.S.A.). served as the supplier of N-Hydroxysulfosuccinimide sodium salt (NHSS) and 1-(3-(dimethylamino) propyl)-3-ethylcarbodiimide hydrochloride (EDC). Penicillin was bought from Shanghai Aladdin Industrial Corporation. Besides, LB medium was obtained from Beijing Land Bridge Technology Co. Ltd. Columbia blood agar plates were supplied by Zhengzhou Autobio Biotechnology Co. Ltd. A solution of 0.01 M PBS (0.137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4, pH 7.4), was provided by Beijing Solarbio Science & Technology Co. Ltd. The Department of Clinical Laboratory, Jiangxi Maternal and Child Health Hospital, Nanchang, China was the provider of urine samples.
2.3. Synthesis of penicillin functionalized magnetic beads (Pen-MBs)
Pen-MBs synthesis followed the methodology outlined by Meng [15]. Initially, 1 mg MB of magnetic beads were washed thrice with PBS (0.01 M, pH = 7.4). Subsequently, the magnetic beads were subjected to the addition of a mixture comprising 0.29 mg EDC and 0.325 mg NHSS in sterilized PBS and activated on an HS-3 vertical mixer (Zhejiang Ningbo Biotechnology Co., Ltd.) for 1 h. After activation, 0.65 mg of Pen was gradually introduced into the MBs solution, and gently mixed with oscillations, followed by coupling for 4 h. The Pen-MBs and GBS@Pen-MBs complexes underwent SEM for characterization (JEOL Ltd., Tokyo, Japan, JSM-6701F).
2.4. Target bacteria separation
The solution (1 mL) was subjected to centrifugation at 9600×g for 5 min, followed by two washes and re-suspension in sterile PBS (1 mL). Subsequently, the GBS concentrations in the target bacterial solution were adjusted by diluting with PBS buffer, from 106 to 101 CFU/mL. Accordingly, Pen-MBs (100 μL) were mixed with sterile PBS (800 μL), with the bacterial solution added (100 μL). The entire mixture was incubated at 37 °C and 144×g for 45 min. After the incubation, an external magnetic field was applied for 6 min to separate the components. The captured bacteria were then counted, with the capture efficiency (CE) determined using the formula:
| CE (%) = [N1/(N1 + N2)] × 100% |
In the context of this study, N1 corresponds to the population of bacteria captured, while N2 denotes the population of bacteria remained uncaptured.
2.5. Extraction of DNA
GBS@Pen-MBs complex was initially re-suspended in 100 μL of sterile PBS, Then the DNA templates were extracted using Smart32 nucleic acid extraction instruments (Guangzhou Daan Gene Co., Ltd., China).
2.6. RT-qPCR assay
In this study, we designed a set of primers (shown in Table 1) and established the RT-qPCR system by optimizing the reaction conditions. In the RT-qPCR system, the overall volume amounted to 25 μL, including 2 μL Taq enzyme and UDG enzyme mixture, 18 μL GBS nucleic acid amplification reaction solution (trimethylol aminomethane, potassium chloride, magnesium chloride, nucleotide primer mixture, probe), and 5 μL target genomic DNA. The RT-qPCR was performed with the following procedures: enzyme treatment at 37 °C for 5 min, pre-denaturation at 95 °C for 5 min, then 40 cycles at 95 °C for 10S and 55 °C for 40S, and fluorescence was detected at 55 °C. The threshold line was 0.08, Positive reactions were defined as a cycle threshold (CT) ≤ 35, CT > 35 was judged to be negative. Plasmids containing the cfb gene fragment were used as positive controls, and sterile water treated with diethyl pyrocarbonate was used as a negative control. RT-qPCR was done on a SLAN-96S PCR system (Shanghai Hongshi Medical Technology Co., Ltd., China) for the amplification.
Table 1.
Primers and probes.
| Primer name | Sequence (5′–3′) |
|---|---|
| Upstream primer | GATTTGGGATAACTAAGCT |
| Downstream primer | TTACATCGTTAACTTGAGCT |
| Probe | CGCATTTTAGATCCATTTGCTTC |
2.7. LOD of MS-RT-qPCR assay
To appraise the efficacy of the MS-RT-qPCR method in detecting the target bacteria, we conducted experiments using selected non-target bacteria (as listed in Table 2). Bacterial solutions, from 106 to 101 CFU/mL, were utilized to ascertain the detection limit in pure culture. Then, 100 μL of Pen-MBS was combined with 800 μL of sterile PBS, followed by adding the bacterial solution (100 μL). Notably, the mixture was then incubated for 45 min at 37 °C, 144×g, after which MS technology was applied for 6 min. Subsequently, GBS@Pen-MBs were accordingly redissolved in sterile PBS (100 μL). RT-qPCR was subjected to DNA extraction.
2.8. GBS detection in urine samples
A total of 100 μL bacterial solution, with concentrations from 107 to 102 CFU/mL was introduced into 900 μL of urine to obtain urine samples containing GBS concentrations from 106 to 101 CFU/mL. Immediately DNA extraction was carried out, and RT-qPCR was performed. For the Pen-MBs functionalization process 100 μL of Pen-MBs and 100 μL of bacterial solution were instantly mixed with 800 μL urine samples, and instantly incubated (37 °C, 144×g) for 45 min, then a 6-min application of the MS technology. Resuspended in 100 μL of sterile PBS after incubation, the GBS@Penn-MBs underwent RT-qPCR as described above.
3. Results
3.1. GBS detection
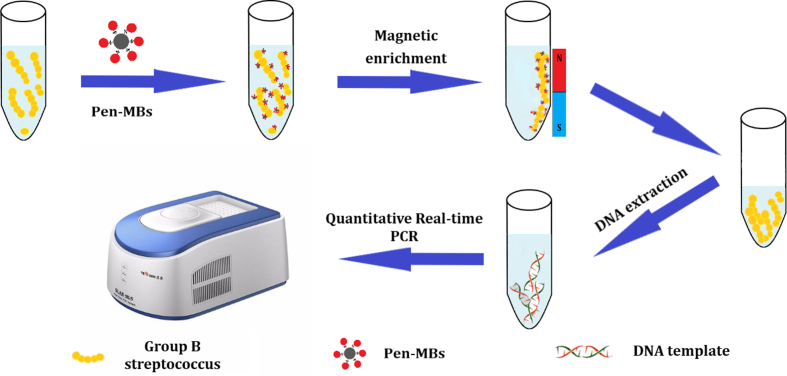
The mechanism is depicted in Fig. S1. To start with, Pen-MBs were synthesized following the aforementioned procedure. Pen is a widely used β-lactam antibiotic that exhibits affinity for penicillin-binding proteins (PBPs) present in the cell walls of numerous Gram-positive bacteria, thereby enabling specific recognition of GBS. Consequently, the application of MS technology allowed for the effective isolation of GBS from the matrix. Subsequently, DNA templates were extracted and RT-qPCR system was established. The resulting RT-qPCR amplification curve was thoroughly analyzed.
3.2. Characterization of Pen-MBS
Scanning electron microscopy (SEM) was employed to validate the morphologies of MBs, Pen-MBs, GBS and GBS@Pen-MBs. The SEM images illustrated in Fig. 1, clearly confirm the efficient binding of Pen-MBs with GBS cell walls. The observed outcomes unequivocally demonstrate the effective capture of GBS by Pen-MBs.
Fig. 1.
SEM micrograph of MBs, Pen-MBs, GBS, and GBS@Pen-MBs complex.
3.3. Pen-MBs potential on GBS
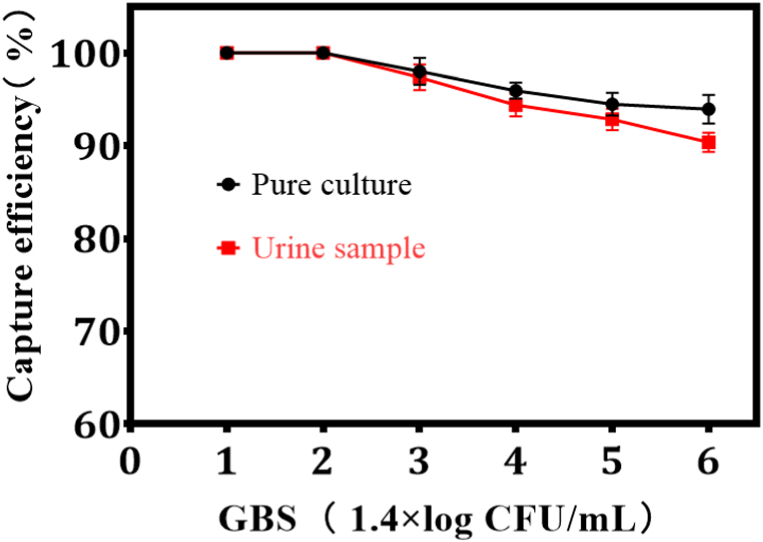
The CE of GBS capture by Pen-MBs was analyzed at different GBS concentrations in both PBS solutions and spiked samples. Fig. 2 illustrates that Pen-MBs demonstrated a remarkable CE of over 90 % for GBS in the PBS concentration spanning from 101 to 106 CFU/mL. To investigate the CE on GBS in spiked samples, various density of GBS were employed to determine the CE. In spiked urine samples, CE values consistently exceeded 90 % across the concentration range of 101–106 CFU/mL. It may be due to the unique chain bacterial morphology of Streptococcus, which makes GBS easily trapped by Pen binding and CE high.
Fig. 2.
The efficiency of Pen-MBs in capturing and binding GBS in urine samples and pure culture.
3.4. LOD of MS-RT-qPCR assay
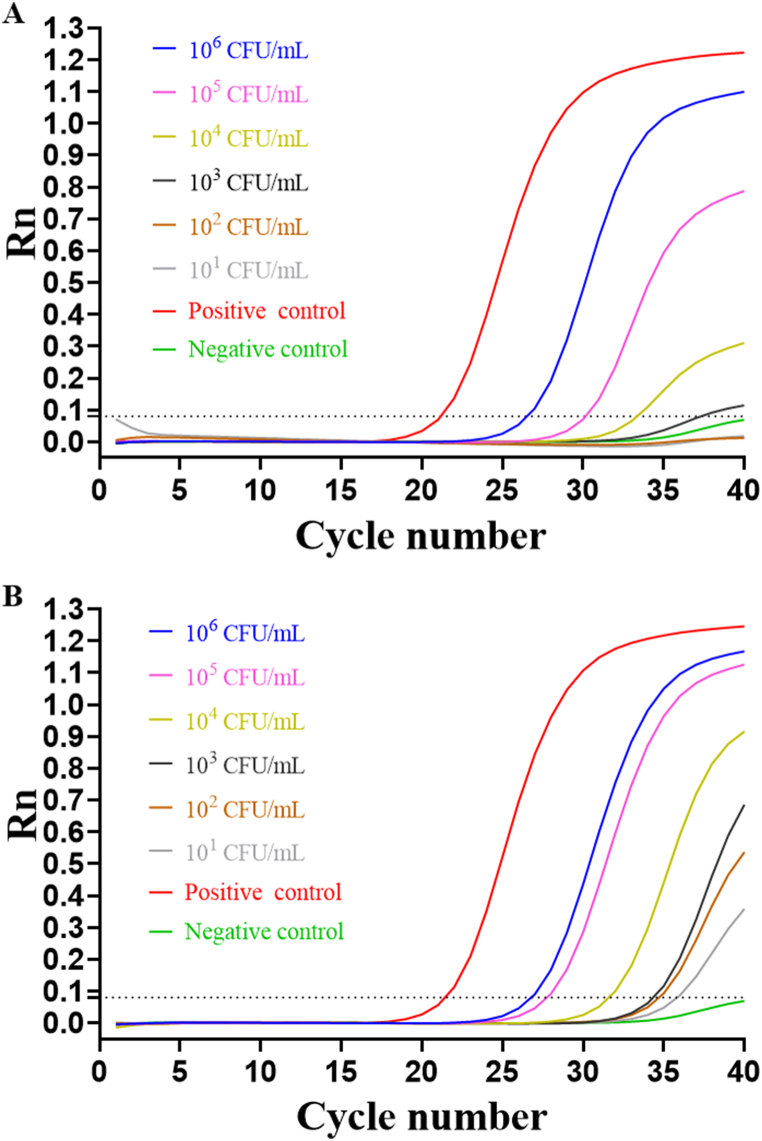
We assessed the limit of detection (LOD) in unmixed bacterial cultures for the MS-RT-qPCR by serially diluting the bacterial solution of concern from 101 to 106 CFU/mL. Based on the results depicted in Fig. 3B, the LOD in pure culture was determined to be 102 CFU/mL. Furthermore, we conducted a comparison of the LOD between MS-RT-qPCR and RT-qPCR. As depicted in Fig. 3A, the LOD for GBS without MS technology was limited to 104 CFU/mL. MS technology results in a more sensitive detection approach as per our proposal.
Fig. 3.
GBS Detection by RT-qPCR and MS-RT-qPCR in pure culture. A, the LOD of GBS by RT-qPCR. B, the LOD of GBS by MS-RT-qPCR. Positive reactions were defined as CT ≤ 35.
3.5. Sensitivity threshold of the MS-RT-qPCR assay for urine sample analysis
The feasibility of the developed MS-RT-qPCR technique was assessed using urine samples inoculated with GBS. As denoted in Fig. 4B, the limit for detecting GBS in urine was 102 CFU/mL. In contrast to the MS-RT-qPCR, the LOD of GBS without MS was only 104 CFU/mL in urine samples. As shown in Fig. 4A.
Fig. 4.
GBS detection by RT-qPCR and MS-RT-qPCR in spiked urine samples. A, the LOD of GBS by RT-qPCR. B, the LOD of GBS by MS-RT-qPCR. Positive reactions were defined as CT ≤ 35.
4. Discussion
GBS, or Streptococcus agalactiae, is a gram-positive coccus that commonly exhibits parasitic characteristics in the vagina and rectal regions. It is a concomitant anaerobic opportunistic pathogen, and the primary pathogen causing invasive infections such as neonatal sepsis and meningitis. It can cause adverse pregnancy outcomes such as late abortion, fetal growth restriction, premature rupture of membranes, and premature delivery during pregnancy [18]. The 2010 CDC guidelines [19] recommend that universal GBS screening for all pregnant women is advocated, irrespective of the presence of early-onset GBS risk factors, which greatly reduces the risk of perinatal GBS infection, especially preterm GBS infection. China's "Guidelines for Pre-Pregnancy and Pregnancy Care" [20] released in 2018 included GBS screening for pregnant women at 35–37 weeks in the third trimester as a reference item for prenatal check-ups. Therefore, the establishment of a rapid and highly sensitive methodology to detect for GBS in perinatal women becomes imperative.
Due to its exceptional sensitivity and specificity, PCR technology has become ubiquitous in the detection of pathogenic bacteria. Quantitative Real-time PCR (RT-qPCR) stands out as a valuable approach that involves monitoring the total product accumulation after each PCR cycle through fluorescence-based chemistry. This technique enables precise quantification of DNA amplification [21]. At present, 16sRNA, cfb, scpb, cps, and Sip genes et al. are used as target genes for GBS detection [[7], [8], [9]]. The 16srRNA gene sequence is known as the "living fossil" of bacteria and is the gold standard for bacterial classification and identification, and there are a large number of homologous conserved regions in its sequence [22]. The positive amplification needs to be confirmed by sequencing, which requires higher primers design [23]. cfb gene encoding CAMP factor and scpb gene encoding C5a peptidase are two target genes that have been Researched more recently. In some reports, the positive rate of 16sRNA, cfb and scpb gene was 19.70 %, 17.24 % and 8.87 %, respectively [8]. Therefore, we have designed primers and probes that target the conserved region of the cfb gene coding sequence in GBS, which can be detected using fluorescently labeled GBS-specific probes.
In previous reports, For PCR analysis, Broth was used to enrich the GBS, which was generally requires more than 18 h [24]. To enhance the detection sensitivity, we have synergistically integrated RT-qPCR with MS technology for GBS detection. MS technology, as an efficient and convenient pretreatment method, facilitates the selective enrichment of the target bacteria, thus effectively reducing the overall reaction time required for experiments [25]. Moreover, MS technology offers the advantage of mitigating sample matrix interference, thus enhancing the experimental accuracy [26]. In this study, GBS was isolated and enriched using a synthetic Pen-MBs magnetic nanomaterial, and prior to DNA extraction, the method exhibited improved detection sensitivity. LOD could reach 102 CFU/mL within 3 h in this study. Based on the experimental findings, the MS-RT-qPCR established in this work demonstrates exceptional proficiency in the detection of GBS.
In conclusion, our study successfully establishes a highly specific and accurate MS-RT-qPCR method for detecting GBS in spiked urine samples. The detection limit achieved for GBS in urine samples using the MS-RT-qPCR is as low as 102 CFU/mL. Therefore, the MS-RT-qPCR method, characterized by its high sensitivity, accuracy and rapidity, holds immense promise for GBS screening in perinatal women.
5. Conclusions
This study introduces a novel MS-RT-qPCR technique for the detection of GBS. The primer design ensures the specificity and MS technology effectively lessens the influence of matrix or non-target bacterial interference in this method. The approach demonstrates robust isolation of GBS from urine samples artificially spiked with the pathogen, the detection sensitivity of RT-qPCR was improved. The refined assay demonstrated remarkable sensitivity, capable of detecting as low as 102 CFU/mL in spiked urine samples. Hence, the developed MS-RT-qPCR method emerges as an invaluable diagnostic tool for GBS screening in perinatal women.
Funding
This research was funded by the National Natural Youth Science Foundation of China (grant number 82102411, 82200194, 82260403); the Project of Science and Technology Innovation Talents in Jiangxi (grant number JSXQ2019201102); the Clinical Research Nurture Project of the First Affiliated Hospital of Nanchang University (grant number YFYLCYJPY202001, YFYPY202201)
Ethical statement
Not applicable.
CRediT authorship contribution statement
Xu Tang: Conceptualization, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. Lin-Ping Fan: Data curation, Formal analysis, Investigation, Software. Yang Liu: Funding acquisition, Project administration, Supervision, Validation, Visualization.
Declaration of competing interest
The authors declared no potential conflicts of interest with respect to the research, author-ship, and/or publication of this article.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.plabm.2023.e00348.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Supplementary Figure 1.
MS technology integrated with RT-qPCR for GBS detection.
Data availability
No data was used for the research described in the article.
References
- 1.Patras K.A., Nizet V. Group B streptococcal maternal colonization and neonatal disease: molecular mechanisms and preventative approaches. Front. Pediatr. 2018;6:27. doi: 10.3389/fped.2018.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu C.J., Lai J.F., Huang I.W., Hsieh L.Y., Wang H.Y., Shiau Y.R., Lauderdale T.L. Multiclonal emergence of levofloxacin-resistant group B Streptococcus, Taiwan. J. Antimicrob. Chemother. 2017;72:3263–3271. doi: 10.1093/jac/dkx297. [DOI] [PubMed] [Google Scholar]
- 3.Overman S.B., Eley D.D., Jacobs B.E., Ribes J.A. Evaluation of methods to increase the sensitivity and timeliness of detection of Streptococcus agalactiae in pregnant women. J. Clin. Microbiol. 2002;40:4329–4331. doi: 10.1128/JCM.40.11.4329-4331.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rallu F., Barriga P., Scrivo C., Martel-Laferriere V., Laferriere C. Sensitivities of antigen detection and PCR assays greatly increased compared to that of the standard culture method for screening for group B streptococcus carriage in pregnant women. J. Clin. Microbiol. 2006;44:725–728. doi: 10.1128/JCM.44.3.725-728.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carrillo-Avila J.A., Gutierrez-Fernandez J., Gonzalez-Espin A.I., Garcia-Trivino E., Gimenez-Lirola L.G. Comparison of qPCR and culture methods for group B Streptococcus colonization detection in pregnant women: evaluation of a new qPCR assay. BMC Infect. Dis. 2018;18:305. doi: 10.1186/s12879-018-3208-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zietek M., Jaroszewicz-Trzaska J., Szczuko M., Mantiuk R., Celewicz Z. Intrapartum PCR assay is a fast and efficient screening method for Group B Streptococcus detection in pregnancy. Ginekol. Pol. 2020;91:549–553. doi: 10.5603/GP.2020.0088. [DOI] [PubMed] [Google Scholar]
- 7.Kannika K., Pisuttharachai D., Srisapoome P., Wongtavatchai J., Kondo H., Hirono I., Unajak S., Areechon N. Molecular serotyping, virulence gene profiling and pathogenicity of Streptococcus agalactiae isolated from tilapia farms in Thailand by multiplex PCR. J. Appl. Microbiol. 2017;122:1497–1507. doi: 10.1111/jam.13447. [DOI] [PubMed] [Google Scholar]
- 8.Mousavi S.M., Hosseini S.M., Mashouf R.Y., Arabestani M.R. Identification of group B streptococci using 16S rRNA, cfb, scpB, and atr genes in pregnant women by PCR. Acta Med. Iran. 2016;54:765–770. [PubMed] [Google Scholar]
- 9.Udo E.E., Boswihi S.S., Al-Sweih N. Genotypes and virulence genes in group B streptococcus isolated in the maternity hospital. Kuwait, Med. Princ. Pract. 2013;22:453–457. doi: 10.1159/000349932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shabayek S., Abdalla S., Abouzeid A.M. Comparison of scpB gene and cfb gene polymerase chain reaction assays with culture on Islam medium to detect Group B Streptococcus in pregnancy. Indian J. Med. Microbiol. 2010;28:320–325. doi: 10.4103/0255-0857.71821. [DOI] [PubMed] [Google Scholar]
- 11.Jordan J.A., Hall G., Davis T. Multicenter study evaluating performance of the Smart Group B Streptococcus (GBS) assay using an enrichment protocol for detecting GBS colonization in patients in the antepartum period. J. Clin. Microbiol. 2010;48:3193–3197. doi: 10.1128/JCM.00106-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim J.P., Chong M.S., Tay L., Yang Y.X., Leung B.P., Yeo A., Yew S., Tan C.H., Lim W.S. Inter-muscular adipose tissue is associated with adipose tissue inflammation and poorer functional performance in central adiposity. Arch. Gerontol. Geriatr. 2019;81:1–7. doi: 10.1016/j.archger.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Lopes A.L.K., Cardoso J., Dos Santos F., Silva A.C.G., Stets M.I., Zanchin N.I.T., Soares M.J., Krieger M.A. Development of a magnetic separation method to capture sepsis associated bacteria in blood. J. Microbiol. Methods. 2016;128:96–101. doi: 10.1016/j.mimet.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 14.Sandanayaka V.P., Prashad A.S. Resistance to beta-lactam antibiotics: structure and mechanism based design of beta-lactamase inhibitors. Curr. Med. Chem. 2002;9:1145–1165. doi: 10.2174/0929867023370031. [DOI] [PubMed] [Google Scholar]
- 15.Meng X., Yang G., Li F., Liang T., Lai W., Xu H. Sensitive detection of Staphylococcus aureus with vancomycin-conjugated magnetic beads as enrichment carriers combined with flow cytometry. ACS Appl. Mater. Interfaces. 2017;9:21464–21472. doi: 10.1021/acsami.7b05479. [DOI] [PubMed] [Google Scholar]
- 16.Tabaraki R., Nazari F. Vancomycin-modified nitrogen and chloride doped carbon dots and their application as a Staphylococcus aureus probe. Anal. Chim. Acta. 2023;1268 doi: 10.1016/j.aca.2023.341311. [DOI] [PubMed] [Google Scholar]
- 17.Wang C., Gu B., Liu Q., Pang Y., Xiao R., Wang S. Combined use of vancomycin-modified Ag-coated magnetic nanoparticles and secondary enhanced nanoparticles for rapid surface-enhanced Raman scattering detection of bacteria. Int. J. Nanomed. 2018;13:1159–1178. doi: 10.2147/IJN.S150336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raabe V.N., Shane A.L. Group B Streptococcus (Streptococcus agalactiae) Microbiol. Spectr. 2019;7 doi: 10.1128/microbiolspec.GPP3-0007-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verani J.R., McGee L., Schrag S.J. N.C.f.I. Division of Bacterial Diseases, C.f.D.C. Respiratory Diseases, Prevention, Prevention of perinatal group B streptococcal disease--revised guidelines from CDC, 2010. MMWR Recomm. Rep. (Morb. Mortal. Wkly. Rep.) 2010;59:1–36. [PubMed] [Google Scholar]
- 20.Association SoGaOotCM., Association Guidelines for pre-pregnancy and pregnancy care(2018) Chin. J. Obstet. Gynecol. 2018;53:7–13. doi: 10.3760/cma.j.issn.0529-567x.2018.01.003. [DOI] [Google Scholar]
- 21.Higuchi R., Fockler C., Dollinger G., Watson R. Kinetic PCR analysis: real-time monitoring of DNA amplification reactions. Biotechnology. 1993;11:1026–1030. doi: 10.1038/nbt0993-1026. [DOI] [PubMed] [Google Scholar]
- 22.Klausegger A., Hell M., Berger A., Zinober K., Baier S., Jones N., Sperl W., Kofler B. Gram type-specific broad-range PCR amplification for rapid detection of 62 pathogenic bacteria. J. Clin. Microbiol. 1999;37:464–466. doi: 10.1128/JCM.37.2.464-466.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang J., Ding X., Guan R., Zhu C., Xu C., Zhu B., Zhang H., Xiong Z., Xue Y., Tu J., Lu Z. Evaluation of different 16S rRNA gene V regions for exploring bacterial diversity in a eutrophic freshwater lake. Sci. Total Environ. 2018;618:1254–1267. doi: 10.1016/j.scitotenv.2017.09.228. [DOI] [PubMed] [Google Scholar]
- 24.Shin J.H., Pride D.T. Comparison of three nucleic acid amplification tests and culture for detection of group B Streptococcus from enrichment broth. J. Clin. Microbiol. 2019;57 doi: 10.1128/JCM.01958-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li C., Li Z., Gan Y., Jiang F., Zhao H., Tan J., Yang Y.Y., Yuan P., Ding X. Selective capture, separation, and photothermal inactivation of methicillin-resistant Staphylococcus aureus (MRSA) using functional magnetic nanoparticles. ACS Appl. Mater. Interfaces. 2022;14:20566–20575. doi: 10.1021/acsami.1c24102. [DOI] [PubMed] [Google Scholar]
- 26.He J., Huang M., Wang D., Zhang Z., Li G. Magnetic separation techniques in sample preparation for biological analysis: a review. J. Pharm. Biomed. Anal. 2014;101:84–101. doi: 10.1016/j.jpba.2014.04.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.