Abstract
Background
Oral submucous fibrosis (OSF) is a persistent oral mucosal condition that carries an elevated risk of undergoing malignant transformation. Our objective was to elucidate the involvement of epithelial-to-mesenchymal transition (EMT) in OSF and its progression to malignancy by studying a panel of EMT markers, thereby understanding the molecular mechanisms.
Methods
An immunohistochemical analysis was done to detect the presence of E-cadherin, N-cadherin, pan-cytokeratin (PanCK), vimentin, α-SMA (alpha-smooth muscle actin), and CD44 in a total of 100 tissue samples. These samples comprised 40 cases of OSF, 20 cases of oral squamous cell carcinoma associated with OSF (OSFSCC), and 40 cases of oral squamous cell carcinoma (OSCC). A whole transcriptomic analysis was performed on a group of seven matched samples encompassing NOM, OSF, OSFSCC, and OSCC.
Results
We observed significantly decreased expression of E-cadherin and PanCK, while N-cadherin, vimentin, α-SMA, and CD44 showed significantly higher expression in OSFSCC and OSCC as compared to OSF, both at protein and RNA levels. CD44 expression was noticeably higher in OSFSCC (p < 0.001) than in OSCC.
Conclusion
Downregulation of epithelial markers with concomitant upregulation of mesenchymal and stem cell markers suggests the potential role of EMT and stemness in accelerating the pathogenesis and malignant transformation of OSF. The high levels of CD44 expression seen in OSFSCC indicate a high propensity for aggressiveness and acquisition of stem-like characteristics by the cells undergoing EMT.
Keywords: Oral submucous fibrosis, Oral squamous cell carcinoma, Epithelial to mesenchymal transition, Malignant transformation, Stemness
Graphical abstract
1. Introduction
Oral submucous fibrosis (OSF) is a chronic oral mucosal condition characterized by epithelial atrophy, submucosal fibrosis and inflammation.1 Persistent chemical irritation of the oral mucosa caused by areca nuts among the betel quid (BQ) chewers is considered to be the main etiological agent in the development of OSF.1 OSF has the potential to undergo malignant transformation, estimated at 7–13 %.2 Several cases of OSCC are reported to be arising in the background of OSF due to the prevalent use of betel quid.3 According to two recent systematic reviews and meta-analyses the proportion of OSF undergoing malignant transformation globally is 5.2 % and 4.6 %, respectively.4,5 However, the wide disparity in the published research adds to the difficulty in determining the actual risk of OSF undergoing malignant change.6 Also, due to the diverse chemical components of BQ, establishing the specific etiological factor involved is often challenging.7 Though OSF is a connective tissue disorder, the epithelial changes observed during malignant transformation require further explanation as to whether the changes are induced by fibrosis in connective tissue or its the cumulative impact of areca nut on the epithelium.
The morphological alterations in OSF in the form of epithelial hyperplasia or dysplasia with subepithelial changes due to matrix thickness may be an impending malignant phenotype of transforming OSF.8 The epithelial cells in such a milieu lose their cellular adhesion and polarity to acquire mesenchymal cell-like characteristics by a process called epithelial-mesenchymal transition (EMT).9
EMT enables polarized epithelial cells to acquire mesenchymal characteristics to promote invasion and metastasis.9 The epithelial markers E-cadherin, cytokeratin (CK), α-catenin, occludins, and claudins are downregulated during EMT, whereas mesenchymal markers including N-cadherin, vimentin, alpha-smooth muscle actin (α-SMA) and fibronectin are upregulated.9
While the alteration in the expression of EMT markers in OSF is evident, its role in oral carcinogenesis needs to be explored.10 It has been demonstrated in in vitro study that oral cell lines exposed to areca nuts induce cancer stemness, EMT, and invasiveness through modulating pluripotent stem cell regulators.11 The cells undergoing EMT acquire stem cell-like characteristics, facilitating the dissemination, initiation, and metastasis of tumors.11 In OSF, the transition from atrophic to hyperplastic or dysplastic epithelium may be a consequence of reactivation of basal cell stemness eventually triggering malignant transformation.12
Some authors indicate that OSCC arising from OSF is clinically more invasive with a greater potential for metastasis and recurrence than conventional OSCC.13 On the contrary, others claim that OSCC in the background of OSF tends to be less aggressive, having fewer neck metastasis, with less extracapsular spread, and histologically well differentiated.14 However, analyzing the OSCC developed in patients previously diagnosed with OSF remains a challenge due to the chronic clinical course of the disease and the poor patient compliance in India resulting in loss of follow-up.3 Hence we studied the expression of EMT, stem cell markers and myofibroblast markers in OSF, OSCC associated with OSF (OSFSCC), and OSCC to decipher the molecular mechanism involved in the malignant potential of OSF.
2. Materials and methods
2.1. Clinical specimen collection
A total of 100 clinical tissue specimens of OSF (n = 40), OSCC (n = 40) and OSFSCC (n = 20) were obtained at the time of surgical resection from the Department of Oral and Maxillofacial Surgery, Manipal College of Dental Sciences, Manipal, India. Normal oral mucosa (NOM) specimens (n = 20) were obtained from healthy individuals who visited the department for minor surgical procedures. The study included patients with clinically diagnosed and histopathologically confirmed cases of OSF, OSCC, and OSCC with the coexistence of OSF (OSFSCC). Histologically diagnosed cases of OSCC with concomitant occurrence of OSF were categorized under the group of OSFSCC, while those without OSF were regarded as OSCC. The patients diagnosed with other oral potentially malignant disorders and OSF coexisting with other oral mucosal pathology and any other type of oral malignancies were excluded from the study. Institutional Ethics Committee approval was obtained (IEC No. 200/2018) before the commencement of the project. Informed consent was obtained from all the patients complying with the rules and regulations of the IEC. Routine tissue processing protocols were employed to formalin-fix and paraffin-embed (FFPE) the tissue biopsies.
2.2. Immunohistochemistry
Immunohistochemistry (IHC) was performed on 4 μm-thick sections of FFPE tissues on APES (3-aminopropyltriethoxysilane)-coated slides. The tissue sections were deparaffinized and rehydrated before antigen retrieval in TRIS-EDTA buffer (pH - 9) at 110 °C for 5 min using a decloaking chamber (NxGen; Biocare Medical, CA, USA). Endogenous peroxidase was blocked with 3 % H2O2 in distilled water. The sections were incubated with primary antibodies against CDH1 (1:1000 dilution), Clone: HECD-1, ThermoFisher Scientific, Rockford, USA), CDH2 (1:1000 dilution, Clone: 5D5, ThermoFisher Scientific, Rockford, USA), VIM (Prediluted, Clone: V9, PathnSitu Biotechnologies, CA, USA), KRK4 (1:1000 dilution, Clone: C11, ThermoFisher Scientific, Rockford, USA), ACTA2 (1:1000 dilution, Clone: 1A4 (asm-1), ThermoFisher Scientific, Rockford, USA) and CD44 (1:1000 dilution, Clone: 156-3C11, ThermoFisher Scientific, Rockford, USA) for 1 h at room temperature. The sections were then incubated with a prediluted primary target binder (PolyExcel Target Binder, PathnSitu Biotechnologies, CA, USA) followed by incubation with the secondary antibody (prediluted PolyExcel Poly HRP, PathnSitu, California, USA). The chromogenic detection was developed with diaminobenzidine (DAB), counterstained with Mayer's hematoxylin, and finally dehydrated, cleared, and mounted with DPX (dibutyl phthalate xylene).
2.3. Evaluation of immunohistochemical staining
The IHC images were captured using a bright-field microscope (Olympus BX21, Tokyo, Japan) equipped with a DP20 camera (Olympus, Tokyo, Japan). The light and camera settings were kept at constant settings using DP2-BSW-1 (Olympus, Tokyo, Japan) software. The images were captured at 10x, 20x, and 40× magnifications.
The evaluation and scoring of the cytoplastic staining of vimentin and PanCK was done using ImageJ software with the IHC Profiler plugin.15 Image J subjects the images to color deconvolution to generate two separate images of DAB and hematoxylin stain. The plugin then generates a histogram profile of the DAB image that corresponds to the number counts of pixel intensity and grades it semi-quantitatively as high positive (+3), positive (+2), low positive (+1), or negative (0).15 The scores were quantified using the formula, IHC optical density score = (percentage of high positive x 4+ percentage of positive x 3 + percentage of low positive x 2 + percentage of negative x 1)/100, as previously described.16
The immunomembrane17 plugin was used to evaluate the membranous staining of E-cadherin, N-cadherin, and CD44. The plugin marks the cell membrane based on completeness of staining as Red for complete and strong membranous staining and Green for incomplete or weak membranous staining. It generates the immune membrane (IM) score by summing the membrane completeness score and intensity score. The IM score was used to quantitatively analyze the membranous staining. The α-SMA expression was analyzed based on the positivity for myofibroblasts and the distribution pattern as previously described.18
2.4. Whole transcriptome sequencing
Additional tissue samples of NOM (n = 2), OSF (n = 2), OSCC (n = 2), and OSFSCC (n = 1) were collected for whole transcriptome sequencing. Total RNA from the clinical specimens for RNA-seq was isolated using the mirVana™ miRNA Isolation Kit (Cat. No. AM1560, Invitrogen, Carlsbad, CA, US) according to the manufacturer's instructions. Library preparation was performed using NEBNext RNA Ultra II (NEB #E7775, Massachusetts, US). Briefly, cytoplasmic and mitochondrial ribosomal RNA was removed using biotinylated, target-specific oligos and rRNA removal beads, followed by purification and first-strand cDNA synthesis using random hexamers. Subsequent second-strand cDNA synthesis was performed and followed up with USER enzyme (NEB #m5508, Massachusetts, US)-based digestion to preserve the functional strand that maps to the DNA strand from which it was transcribed. Enrichment and indexing were carried out in a limited-cycle PCR followed by AMPure bead (Beckman Coulter, Inc.) purification to prepare a cDNA library for sequencing. The prepared libraries were sequenced on an Illumina HiSeq4000/X to generate 60 M, 2x150 bp paired end reads per sample. A quality check (>Q30) and preprocessing of raw data were carried out using Trimmomatic and Bowtie2. Preprocessed data were aligned to the human reference genome (hg19) using HiSAT2, raw read counts mapped to Ensembl IDs were obtained by feature counts, and normalized FPKM (fragments per kilobase of transcripts per million mapped reads) values were calculated.
2.5. Statistical analysis
All statistical analysis were performed using the Statistical Package for Social Sciences (SPSS) version 20.0 (IBM Corp., Armonk, NY, USA). Comparison-of expression between the groups was performed using one-way ANOVA followed by post hoc Tukey's test or Kruskal-Wallis test. A p value of <0.05 was considered statistically significant. Data visualization of the whole transcriptome sequencing was performed to assess the expression pattern of the selected six markers using the ggpubr package in the R program (R Core Team (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/).
3. Results
3.1. Loss of E-cadherin and gain of N-cadherin during the onset of malignancy
E-cadherin and N-cadherin were found in the cell membranes of epithelial and stromal endothelial cells. In NOM, membranous staining was seen for both E-cadherin and N-cadherin in the basal, suprabasal, and spinous layers. In most cases, E-cadherin expression in OSF was found in the basal and suprabasal layers. However, in 20 % of cases, expression in the basal layer was lost. N-cadherin showed similar staining in the basal and suprabasal layers, extending to the spinous layer in OSF. E-cadherin and N-cadherin in OSFSCC and OSCC presented with membranous staining of the central and peripheral cells of the tumor islands, except for the keratinized areas (Fig. 1).
Fig. 1.
Immunohistochemical expression of a panel of EMT markers in NOM, OSF, OSFSCC and OSCC. The expression of E-cadherin, N-cadherin, Vimentin, Pan-cytokeratin, CD44 and α-SMA (Alpha Smooth muscle actin) analyzed in normal oral mucosa (NOM)(x10), oral submucous fibrosis (OSF)(x20), oral squamous cell carcinomas associated with OSF (OSFSCC)(x20) and oral squamous cell carcinoma (OSCC)(x20).
E-cadherin expression was significantly decreased, while N-cadherin exhibited significantly increased expression in OSF as compared to NOM (p < 0.05, p < 0.001). The immunoreactivity of E-cadherin was significantly lower in OSCC than in OSFSCC (p < 0.001) and OSF (p < 0.05). N-cadherin showed significantly higher reactivity in OSCC than in OSF (p < 0.001) but did not show a significant difference with OSFSCC (Fig. 2A and B). On assessing among different histological grades, E-cadherin showed decreased, while N-cadherin exhibited increased expression corresponding to the histological grade in OSF and OSCC (Fig. 3, Fig. 4A, B).
Fig. 2.
Comparison of E-cadherin (A), N-cadherin (B), CD44 (C), PanCK (D), Vimentin (E) and α-SMA (F) expression between normal oral mucosa (NOM), oral submucous fibrosis (OSF), oral squamous cell carcinomas associated with OSF (OSFSCC) and oral squamous cell carcinoma (OSCC) groups. Each bar is presented by mean ± SD, *P < 0.05, **P < 0.001.
Fig. 3.
Illustrative heatmap of case-wise immunohistochemical expression of EMT markers in normal oral mucosa (NOM), oral submucous fibrosis (OSF), oral squamous cell carcinomas associated with OSF (OSFSCC) and oral squamous cell carcinoma (OSCC). Blue color indicates low expression, whereas red color indicates high expression.
Fig. 4.
Comparison of E-cadherin (A), N-cadherin (B), CD44 (C), PanCK (D), Vimentin (E) and α-SMA(F) expression between grades of oral submucous fibrosis (OSF), oral squamous cell carcinomas associated with OSF (OSFSCC) and oral squamous cell carcinoma. Each bar is presented by mean ± SD, *P < 0.05, **P < 0.001.
3.2. Epithelial to mesenchymal shift during the OSF to malignant progression
Vimentin exhibited cytoplasmic staining in a few epithelial cells, while all the fibroblasts in the stroma showed positivity. Homogenous staining of vimentin in the subepithelial and deeper layers of the connective tissue stroma with varying intensity was observed (Fig. 1). NOM did not express vimentin in the epithelial cells. OSF cases (12.5 %) showed staining of vimentin in the basal and suprabasal layers. In OSFSCC (30 %) and OSCC (37.5 %), only the peripheral cells of the tumor islands showed staining for vimentin. Immunostaining for vimentin showed a statistically significant increase in OSFSCC (p < 0.05) and OSCC (p < 0.05) compared to OSF (Fig. 2E). Vimentin expression significantly increased in advanced cases of OSF but did not show a significant difference between histological grades of OSFSCC and OSCC (Fig. 3, Fig. 4E).
The α-SMA showed positivity for myofibroblasts and endothelial cells in the stroma. α-SMA staining for myofibroblasts was categorized into focal, spindle, and network patterns. OSF showed a predominant network (27.5 %) pattern, while OSCC showed a spindle (17.5 %) pattern (Fig. 2F). An increase in α-SMA positivity was noted in OSF (30 %) and OSCC (32.5 %). Different grades of OSF exhibited a similar distribution of myofibroblasts, but there was an increase in positivity for myofibroblasts with higher grades of OSCC (Fig. 3, Fig. 4F).
Pan-cytokeratin (PanCK) stained the cytoplasm of epithelial and stromal endothelial cells. In OSF, the basal layer did not show PanCK positivity. PanCK immunoreactivity was observed in the central cells of tumor islands in OSFSCC and OSCC, whereas the peripheral cells lacked staining (Fig. 1). The immunoreactivity of PanCK decreased in OSFSCC (p < 0.001) and OSCC (p < 0.001) compared to that of OSF (Fig. 2D). Different histological grades of OSCC showed no significant difference in expression for PanCK (Fig. 3, Fig. 4D).
3.3. Immunoexpression pattern of CD44
CD44 exhibited membranous staining in the basal and suprabasal layers, extending up to the spinous layer in both NOM and OSF, but showed a high intensity of staining in OSF. The OSFSCC and OSCC presented with staining of CD44 in the central and peripheral cells of the tumor islands but lacked staining in the keratinized areas (Fig. 1). CD44 showed significantly higher immunoreactivity in OSFCC than in OSCC (p < 0.001) and OSF (p < 0.05) (Fig. 2C). A significantly higher expression of CD44 was observed in higher grades of OSCC (Fig. 3, Fig. 4C).
3.4. Gene expression profile of EMT and stem cell markers
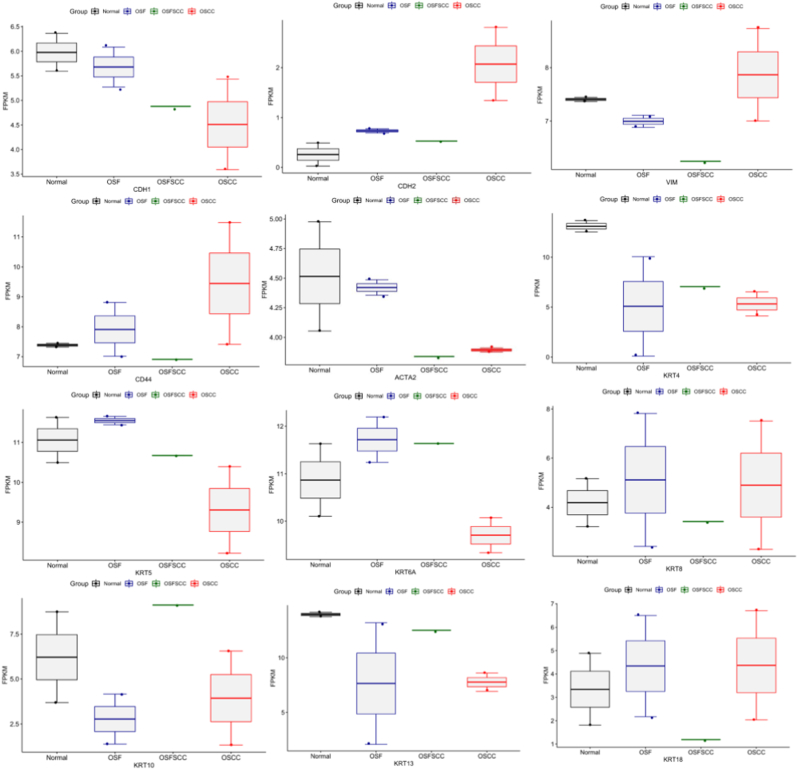
The expression profile of the epithelial marker CDH1 (E-cadherin) was observed to be lower in OSFSCC and OSCC than in OSF and NOM samples. CDH2 (N-cadherin) showed a heterogeneous expression trend in OSF and OSFSCC compared to the NOM samples, but OSCC showed higher expression. VIM (vimentin) and CD44 expression was higher in OSCC than in OSFSCC, OSF, and NOM. PanCK, a cocktail mixture of keratins (KRT4, 5, 6, 8, 10, 13, and 18), was further profiled, where all CK's in the cocktail showed lower expression in OSCC than in OSFSCC, OSF, and NOM except KRT8/18 (Fig. 5).
Fig. 5.
Distribution of FPKM data of EMT markers across normal oral mucosa (NOM), oral submucous fibrosis (OSF), oral squamous cell carcinomas associated with OSF (OSFSCC) and oral squamous cell carcinoma (OSCC) groups derived from whole transcriptome analysis.
4. Discussion
Understanding the interplay between the EMT, stemness, and stromal change can provide a mechanistic perception of the cumulative effect on tumor initiation and metastasis. Studies have proven the role of EMT in the pathophysiology of OSF, with areca nut inducing myofibroblast transdifferentiation of human buccal mucosal fibroblasts (BMFs), thereby upregulating EMT and stemness factors.19,20 It is thus imperative to elucidate how these factors influence the malignant transformation of OSF.
EMT is activated by several signaling pathways, such as TGF-β signaling, PI3 kinase/Akt/mTOR signaling, RTK signaling, hypoxia signaling, Wnt signaling, matrix signaling, and the MAPK/ERK pathway.9 These pathways bring about upregulation of transcription factors such as zinc finger E-box-binding homeobox 1 and 2 (ZEB1 & 2), TWIST1, lymphoid enhancer-binding factor-1 (LEF-1), SNAIL1 and SNAIL2 (also known as Slug) to repress the transcription of E-cadherin to induce EMT.9
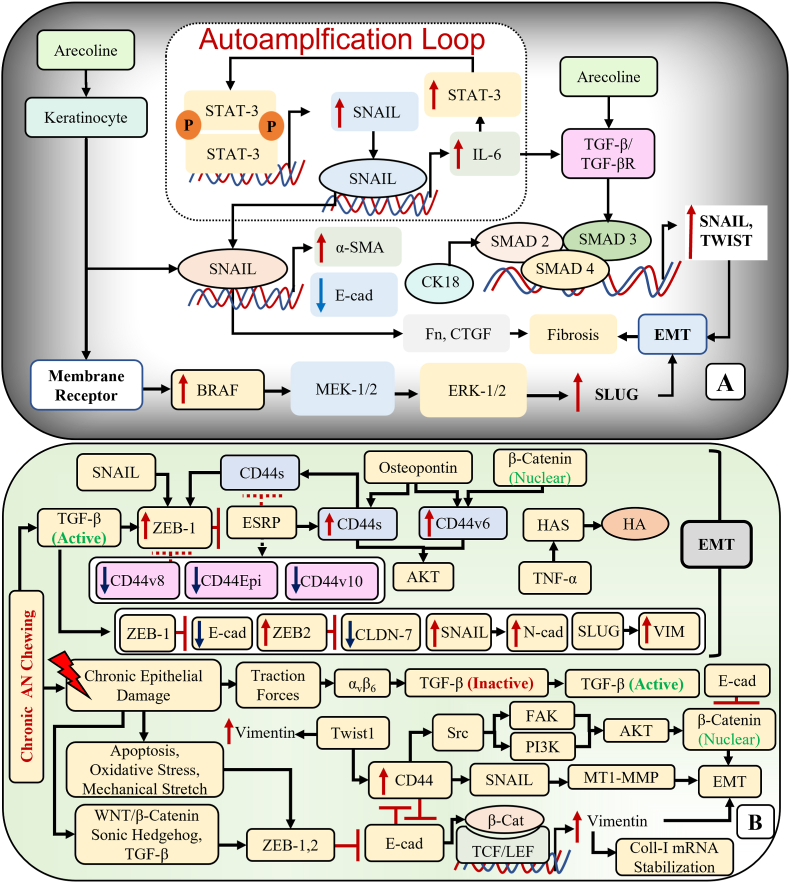
E-cadherin showed a progressive decrease in expression from NOM to OSF and a further decrease in OSFSCC and OSCC both at the protein and RNA levels in the present study. About 20 % of the OSF cases exhibited loss of E-cadherin expression in the basal layer. Hosur et al.,21 and Chakraborti et al.,22 also reported loss of E-cadherin expression in the basal layer in OSF, indicating impaired intercellular junctions and initiation of the carcinogenic process in the basal epithelial layer. Additionally, excessive collagen deposition in OSF can result in decline of E-cadherin integrity.22 The loss of E-cadherin in OSCC was documented by López-Verdín et al.,23 and Hakim et al.,24 proving the acquisition of invasive behavior with the repression of E-cadherin.25 One possible explanation for the suppression of E-cadherin in OSF and OSCC is the transcription factor SNAIL, which is upregulated by TGFβ and IL-6 via the TGFβ-R/SMAD2/SMAD3/SMAD4 and JAK/STAT-3 pathways, respectively.26,27 (Fig. 6A).
Fig. 6.
Signaling pathways regulating the EMT in oral submucous fibrosis (OSF) and oral squamous cell carcinoma (OSCC). A: Arecoline induced upregulation of transcription factors promoting EMT. B: Interplay between the EMT and stem cell regulators in OSF progression.
Non-epithelial N-cadherins are expressed by endothelial cells, mesothelial cells, neurons, lens epithelial cells, muscle, and fibroblasts. However, normal epithelial cells can also express traces of N-cadherin, while tumor cells express higher levels.25 The upregulation of N-cadherin in epithelial cells is an indication of cadherin switching, thereby altering adhesive function, motility, and invasiveness.28 Thus, E-cadherin and N-cadherin expression is reciprocally regulated in tumor cells. OSF exhibited a gain in expression for N-cadherin, which progressively increased in OSFSCC and OSCC reciprocating E-cadherin expression in our cohort. Das et al.,29 reported a similar increase in N-cadherin in OSF, indicating the pro-EMT status of OSF. Our findings concur with those of Angadi et al.,28 suggesting that the elevated N-cadherin expression in OSCC implies its significant role in displacing E-cadherin from the cell membrane, resulting in a change in cellular phenotype and predisposing the cells to EMT. Tumor cells with upregulated N-cadherin expression may exhibit irregular morphology and high motility, invasion, metastasis and also immunity against destruction by natural killer cells.30 In our work, OSFSCC showed higher expression of N-cadherin than OSCC, but the difference was not statistically significant. However, the higher expression may be suggestive of the high aggressiveness or invasive potential of OSCC with OSF in the background. E-cadherin/N-cadherin switching is indicative of EMT, but sometimes the change in E-cadherin expression may be minimal, but the aberrant expression of mesenchymal N-cadherin would be sufficient to have an impact on cancer cell behavior.31
CK and vimentin expression can determine the change in epithelial to mesenchymal phenotype. Vimentin is a cytoskeletal protein found in mesenchymal cells but not in epithelial cells. The expression of vimentin is considered the hallmark of EMT, which enables epithelial cells to adopt a mesenchymal shape and acquire motility.32 Vimentin maintains the mechanical homeostasis of cancer cells by modulating the cytoskeleton organization and focal adhesion stability. Cancer cells undergoing EMT are subjected to mechanical modulation by vimentin, which makes them progressively organized to resist various stresses exhibited by the tumor microenvironment.33 Our study showed aberrant expression of vimentin in basal cells of OSF and in the peripheral cells of tumor islands in OSCC and OSFSCC. The vimentin expression was higher in OSFSCC and OSCC as compared to OSF. Similar abnormal cytoplasmic expression of vimentin was shown by Yao et al.,34 and Sawant et al.,35 in OSF and OSCC, respectively. Although OSF is a connective tissue disorder, keratinocytes play a significant role in the pathogenesis of fibrosis. Hence, aberrant vimentin expression in both epithelial and mesenchymal components is indicative of early molecular changes during oral carcinogenesis.35 An elevated vimentin expression from low to high grade of OSCC suggests its correlation to the more aggressive phenotypes of OSCC.35,36 Vimentin is the downstream effector of various transcription factors, such as SNAIL, TWIST, ZEB1, and C-MYC, in various EMT signaling pathways.37 In addition to being a consequence of EMT, vimentin can further promote EMT via the ERK1/2/SLUG axis.38 (Fig. 6B).
Cytokeratins (CKs), as intermediate filaments in epithelial cells are crucial for cell stability, shape and intercellular signaling. CKs play a significant role in regulating epithelial differentiation both in physiological and pathological conditions. The expression of different CKs depends on stage of differentiation and epithelial type making it an important marker in cancer diagnosis.39 As CK expression can provide a cue on the process of EMT, we studied the expression of PanCK. Higher expression of PanCK was observed in OSF as compared to OSFSCC and OSCC, suggesting that the mesenchyme-induced epithelial alterations in OSF can bring about an alteration in CK expression.40 All the CKs in the PanCK showed decreased expression at RNA level except CK 8/18. Upregulation of CK 8/18 is known to be associated with higher tumor grade and poor prognosis.41 Frohwitter et al.,41 showed higher expression of CK8/18 in transcriptome profile of OSCC, implicating that these low-molecular-weight CKs are often expressed in high-grade malignancies with a higher degree of cell cycle dysregulation.
Epithelial-mesenchymal interaction is exhibited through crosstalk between intrinsic characteristics of the epithelium and the growth factors released by the stromal fibroblasts, which play an essential role in epithelial differentiation and morphogenesis.42 The myofibroblastic differentiation of cancer-associated fibroblasts (CAFs) in the tumor stroma also plays an important role in epithelial-mesenchymal signaling, inducing the malignant transformation and progression of epithelial tumors.43 EMT is considered to be one of the precursors mechanism inducing myofibroblast transdifferentiation.44 Myofibroblastic differentiation is signaled by the biomarker α-SMA. Our research showed high α-SMA expression in OSF, OSFSCC, and OSCC. The elevated expression of α-SMA in OSF also reflects the progression of disease inducing alteration in the keratinocyte phenotype and predisposing it to malignant transformation.45 High expression of α-SMA in OSCC reported by Smitha et al.,18 and Maqsood et al.,46 confirm the role of stromal cells in tumor invasion and progression. Further, high α-SMA positivity of myofibroblasts in poorly differentiated OSCC is indicative of aggressive behavior of tumor cells.46
Activation of EMT involves the dissemination of cancer cells to induce metastasis. The tumor cells exhibit high plasticity by acquiring stem-like properties. A small subpopulation of cancer cells with stem-like characteristics has the potential to drive tumor promotion, invasion and metastasis.47 CD44 is a transmembrane adhesion receptor for hyaluronan (HA) involved in both physiological and pathological processes, including cell adhesion, angiogenesis, inflammation, and tumor development.48 Its critical role in cells undergoing EMT and acquiring stem-like characteristics has been well established. The transcription factors regulating EMT upregulate CD44, while the latter inversely regulates E-cadherin expression.49 Singh et al.,50 demonstrated the correlation between CD44 overexpression and OSCC progression. We found CD44 to be overexpressed in OSF, OSFSCC, and OSCC at both the protein and RNA levels. OSFSCC expressed considerably higher CD44 than OSCC, suggesting the progressive role of underlying fibrosis in inducing pluripotent effect on stem cell regulators to promote stemness and progression to malignancy.51 Additionally, CD44 interacts with MMP9 on the cell surface to bring about the activation of TGFβ, and ECM degradation to promote the invasion, migration and metastasis of cancer cells.52 The overexpression of CD44 in OSFSCC thus demonstrates its aggressiveness and strong propensity for invasion and metastasis. The upregulation of CD44 is regulated by the EMT transcription factors such as TWIST, ZEB1, SNAIL, and SLUG, and mediated through SNAIL/MT-1-MMP pathway, or src pathway that upregulates FAK and PI3K, which in turn upregulates Akt promoting nuclear translocation of β-catenin, resulting in EMT.53 (Fig. 6B).
Given its cross-sectional nature, this study did not entail the collection of follow-up data, which stands as one of its limitations. Yet, exploring the involvement of different EMT markers in the progression of OSF could provide additional insights and establish a cause-and-effect relationship. Follow-up with OSF patients would further strengthen our hypothesis regarding the interplay between EMT and stemness in the malignant transformation of OSF.
5. Conclusion
EMT promotes tumor development and metastasis by facilitating the loss of cell adhesion molecules and promoting the migratory and invasive capabilities of epithelial cells. The decreased expression of E-cadherin and PanCK, as well as the concurrent increase in the expression of Vimentin, N-cadherin, and α-SMA, strongly supports the role of EMT in the etiology of OSF and its malignant transformation. The strong potential of OSF to induce pluripotent effects on stem cell regulators and consequently promote malignant transformation and tumor progression is further supported by the much higher expression of CD44 in OSFSCC than in OSCC. The ability of cells undergoing EMT to resemble stem cells explains how CSCs might enhance tumor heterogeneity. This underscores the importance of developing treatment approaches that target CSCs and EMT to halt the growth and spread of tumors. Implementing anti-EMT therapeutic strategies may serve as a foundation for personalized medicine, effectively managing invasion and metastasis, and overcoming chemotherapeutics resistance by reducing the activity of CSCs.
Funding information
This work was supported by the Science and Engineering Research Board (SERB), Department of Science and Technology, Government of India sanction order No. “EMR/2017/002792” dated 25 September 2018.
Data availability statement
Data is available from the corresponding author upon reasonable request.
Declaration of competing interest
All the authors of the manuscript hereby state that there is no financial implication or personal relationship with other people or organizations that could inappropriately influence the outcome of this work.
References
- 1.Rao N.R., Villa A., More C.B., Jayasinghe R.D., Kerr A.R., Johnson N.W. Oral submucous fibrosis: a contemporary narrative review with a proposed inter-professional approach for an early diagnosis and clinical management. J Otolaryngol - Head Neck Surg. 2020;49(1):3. doi: 10.1186/s40463-020-0399-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bansal S., Pereira T., Desai R.S., Jena A., Bobade P.P., Patil M. Interplay of transforming growth factor-Beta 1 and 3 in the pathogenesis of oral submucous fibrosis and its malignant transformation: an immunohistochemical study. Cureus. 2023;15(7) doi: 10.7759/cureus.42412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Divya B., Vasanthi V., Ramadoss R., Kumar A.R., Rajkumar K. Clinicopathological characteristics of oral squamous cell carcinoma arising from oral submucous fibrosis: a systematic review. J Cancer Res Therapeut. 2023;19(3):537–542. doi: 10.4103/jcrt.jcrt_1467_21. [DOI] [PubMed] [Google Scholar]
- 4.Iocca O., Sollecito T.P., Alawi F., et al. Potentially malignant disorders of the oral cavity and oral dysplasia: a systematic review and meta-analysis of malignant transformation rate by subtype. Head Neck. 2020;42(3):539–555. doi: 10.1002/hed.26006. [DOI] [PubMed] [Google Scholar]
- 5.Kujan O., Mello F.W., Warnakulasuriya S. Malignant transformation of oral submucous fibrosis A systematic review and meta‐analysis. Oral Dis. 2021;27(8):1936–1946. doi: 10.1111/odi.13727. [DOI] [PubMed] [Google Scholar]
- 6.Murthy V., Mylonas P., Carey B., et al. Malignant transformation rate of oral submucous fibrosis: a systematic review and meta-analysis. J Clin Med. 2022;11(7):1793. doi: 10.3390/jcm11071793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang P., Chua N.Q.E., Dang S., et al. Molecular mechanisms of malignant transformation of oral submucous fibrosis by different betel quid constituents—does fibroblast senescence play a role? Int J Mol Sci. 2022;23(3):1637. doi: 10.3390/ijms23031637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ray J.G., Ranganathan K., Chattopadhyay A. Malignant transformation of oral submucous fibrosis: overview of histopathological aspects. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122(2):200. doi: 10.1016/j.oooo.2015.11.024. [Internet] 9. [DOI] [PubMed] [Google Scholar]
- 9.Shetty S.S., Sharma M., Fonseca F.P., et al. Signaling pathways promoting epithelial mesenchymal transition in oral submucous fibrosis and oral squamous cell carcinoma. Jpn Dent Sci Rev. 2020;56(1):97–108. doi: 10.1016/j.jdsr.2020.07.002. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen P.N., Lin C.W., Yang S.F., Chang Y.C. Oral submucous fibrosis stimulates invasion and epithelial-mesenchymal transition in oral squamous cell carcinoma by activating MMP-2 and IGF-IR. J Cell Mol Med. 2021;25(20):9814–9825. doi: 10.1111/jcmm.16929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.chen Li Y., Chang J.T., Chiu C., et al. Areca nut contributes to oral malignancy through facilitating the conversion of cancer stem cells. Mol Carcinog. 2016;55(5):1012–1023. doi: 10.1002/mc.22344. [DOI] [PubMed] [Google Scholar]
- 12.Sharma M., Fonseca F.P., Hunter K.D., Radhakrishnan R. Loss of oral mucosal stem cell markers in oral submucous fibrosis and their reactivation in malignant transformation. Int J Oral Sci. 2020;12(1):1–10. doi: 10.1038/s41368-020-00090-5. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo F., chun Jian X., hui Zhou S., Li N., jia Hu Y., gui Tang Z. [A retrospective study of oral squamous cell carcinomas originated from oral submucous fibrosis] Chinese J Stomatol. 2011 Aug;46(8):494–497. [PubMed] [Google Scholar]
- 14.Chaturvedi P., Vaishampayan S.S., Nair S., et al. Oral squamous cell carcinoma arising in background of oral submucous fibrosis: a clinicopathologically distinct disease. Head Neck. 2013;35(10):1404–1409. doi: 10.1002/hed.23143. [DOI] [PubMed] [Google Scholar]
- 15.Varghese F., Bukhari A.B., Malhotra R., De A. IHC profiler: an open source plugin for the quantitative evaluation and automated scoring of immunohistochemistry images of human tissue samples. PLoS One. 2014;9(5) doi: 10.1371/journal.pone.0096801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seyed Jafari S.M., Hunger R.E. IHC optical density score: a new practical method for quantitative immunohistochemistry image analysis. Appl Immunohistochem Mol Morphol. 2017;25(1):e12–e13. doi: 10.1097/PAI.0000000000000370. [DOI] [PubMed] [Google Scholar]
- 17.Tuominen V.J., Tolonen T.T., ImmunoMembrane Isola J. A publicly available web application for digital image analysis of HER2 immunohistochemistry. Histopathology. 2012;60(5):758–767. doi: 10.1111/j.1365-2559.2011.04142.x. [DOI] [PubMed] [Google Scholar]
- 18.Smitha A., Rao K., Umadevi H.S., Smitha T., Sheethal H.S., Vidya M.A. Immunohistochemical study of α-smooth muscle actin expression in oral leukoplakia and oral squamous cell carcinoma. J Oral Maxillofac Pathol. 2019;23(1):59–64. doi: 10.4103/jomfp.JOMFP_94_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee Y.H., Liao Y.W., Lu M.Y., Hsieh P.L., Yu C.C. LINC00084/miR-204/ZEB1 axis mediates myofibroblastic differentiation activity in fibrotic buccal mucosa fibroblasts: therapeutic target for oral submucous fibrosis. J Pers Med. 2021;11(8):707. doi: 10.3390/jpm11080707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fang C.Y., Yu C.C., Liao Y.W., et al. miR-10b regulated by Twist maintains myofibroblasts activities in oral submucous fibrosis. J Formos Med Assoc. 2020;119(7):1167–1173. doi: 10.1016/j.jfma.2020.03.005. [Internet] [DOI] [PubMed] [Google Scholar]
- 21.Hosur M.B., Puranik R.S., Vanaki S.S., Puranik S.R., M S., Das S. Evaluation of immunohistochemical expression of epithelial– mesenchymal transition markers E-cadherin, Twist and Snail in oral submucous fibrosis and their possible association with malignant transformation Mahadevi. J Oral Maxillofac Pathol. 2021;21(3):244–251. doi: 10.4103/jomfp.jomfp_454_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chakraborti S., Paul R.R., Pal M., Chatterjee J., Das R.K. Collagen deposition correlates with loss of E-cadherin and increased p63 expression in dysplastic conditions of oral submucous fibrosis. Med Mol Morphol. 2022;55(1):20–26. doi: 10.1007/s00795-021-00304-7. [Internet] [DOI] [PubMed] [Google Scholar]
- 23.López-Verdín S., Martínez-Fierro M. de la L., Garza-Veloz I., et al. E-Cadherin gene expression in oral cancer: clinical and prospective data. Med Oral Patol Oral Cir Bucal. 2019;24(4):e444–e451. doi: 10.4317/medoral.23029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hakim S.G., Taubitz C., Hoppe S., et al. Prognostic impact of the loss of E-cadherin and de novo expression of N-cadherin at the invasive front of primary and recurrent oral squamous cell carcinoma. Front Oncol. 2023;13(May) doi: 10.3389/fonc.2023.1151879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loh C.Y., Chai J.Y., Tang T.F., et al. The E-cadherin and N-cadherin switch in epithelial-to-mesenchymal transition: signaling, therapeutic implications, and challenges. Cells. 2019;8(10):1118. doi: 10.3390/cells8101118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peng C.Y., Liao Y.W., Lu M.Y., Yang C.M., Hsieh P.L., chia Yu C. Positive feedback loop of SNAIL-IL-6 mediates myofibroblastic differentiation activity in precancerous oral submucous fibrosis. Cancers. 2020;12(6):1611. doi: 10.3390/cancers12061611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakamura R., Ishii H., Endo K., et al. Reciprocal expression of slug and snail in human oral cancer cells. PLoS One. 2018;13(7) doi: 10.1371/journal.pone.0199442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Angadi P.V., Patil P.V., Angadi V., et al. Immunoexpression of epithelial mesenchymal transition proteins in oral squamous cell carcinoma. Int J Surg Pathol. 2016;24(8):696–703. doi: 10.1177/1066896916654763. [DOI] [PubMed] [Google Scholar]
- 29.Das R.K., Anura A., Pal M., et al. Epithelio-mesenchymal transitional attributes in oral sub-mucous fibrosis. Exp Mol Pathol. 2013;95(3):259–269. doi: 10.1016/j.yexmp.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 30.Lou C., Wu K., Shi J., Dai Z., Xu Q. N-cadherin protects oral cancer cells from NK cell killing in the circulation by inducing NK cell functional exhaustion via the KLRG1 receptor. J Immunother Cancer. 2022;10(9) doi: 10.1136/jitc-2022-005061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang H., Wright S., Zhang J., Brekken R.A. Getting a grip on adhesion: cadherin switching and collagen signaling. Biochim Biophys Acta - Mol Cell Res. 2019;1866(11) doi: 10.1016/j.bbamcr.2019.04.002. [Internet] [DOI] [PubMed] [Google Scholar]
- 32.Mendez M.G., ichiro Kojima S., Goldman R.D. Vimentin induces changes in cell shape , motility , and adhesion during the epithelial to mesenchymal transition. FASEB J. 2016;24(6):1838–1851. doi: 10.1096/fj.09-151639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu C.Y., Lin H.H., Tang M.J., Wang Y.K. Vimentin contributes to epithelial-mesenchymal transition cancer cell mechanics by mediating cytoskeletal organization and focal adhesion maturation. Oncotarget. 2015;6(18):15966–15983. doi: 10.18632/oncotarget.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yao M., Li J., Yuan S., et al. Role of the arecoline/YAP1/BMP4 pathway in promoting endothelial‐mesenchymal transition in oral submucous fibrosis. J Oral Pathol Med. 2019;49:305–310. doi: 10.1111/jop.12945. [DOI] [PubMed] [Google Scholar]
- 35.Sawant S.S., Vaidya M.M., Chaukar D.A., et al. Clinical significance of aberrant vimentin expression in oral premalignant lesions and carcinomas. Oral Dis. 2014;20(5):453–465. doi: 10.1111/odi.12151. [DOI] [PubMed] [Google Scholar]
- 36.Bozgeyik E., Ege B., Koparal M., Ceylan O. 2022. Clinical Significance of Vimentin Antisense RNA 1 and its Correlation with Other Epithelial to Mesenchymal Transition Markers in Oral Cancers; p. 232.https://www.sciencedirect.com/science/article/pii/S0344033822000504 Pathol - Res Pract [Internet] 153807. [DOI] [PubMed] [Google Scholar]
- 37.Ostrowska-Podhorodecka Z., Ding I., Norouzi M., McCulloch C.A. Impact of vimentin on regulation of cell signaling and matrix remodeling. Front Cell Dev Biol. 2022;10(March) doi: 10.3389/fcell.2022.869069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Usman S., Waseem N.H., Nguyen T.K.N., et al. Vimentin is at the heart of epithelial mesenchymal transition (Emt) mediated metastasis. Cancers. 2021;13(19):4985. doi: 10.3390/cancers13194985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanguansin S., Kosanwat T., Juengsomjit R., Poomsawat S. Diagnostic value of cytokeratin 17 during oral carcinogenesis: an immunohistochemical study. Int J Dent. 2021;2021 doi: 10.1155/2021/4089549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nanda K.D.S., Ranganathan K., Devi U., Joshua E. Increased expression of CK8 and CK18 in leukoplakia, oral submucous fibrosis, and oral squamous cell carcinoma: an immunohistochemistry study. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012 Feb;113(2):245–253. doi: 10.1016/j.tripleo.2011.05.034. [DOI] [PubMed] [Google Scholar]
- 41.Frohwitter G., Buerger H., Diest P.J.V.A.N., Korsching E., Kleinheinz J., Fillies T. Cytokeratin and protein expression patterns in squamous cell carcinoma of the oral cavity provide evidence for two distinct pathogenetic pathways. Oncol Lett. 2016;12(1):107–113. doi: 10.3892/ol.2016.4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Katsuno Y., Derynck R. Epithelial plasticity, epithelial-mesenchymal transition, and the TGF-β family. Dev Cell [Internet] 2021;56(6):726–746. doi: 10.1016/j.devcel.2021.02.028. [DOI] [PubMed] [Google Scholar]
- 43.Fonseca F.P., Coletta R Della, Azevedo M.B., et al. Stromal myofibroblasts in squamous cell carcinoma of the tongue in young patients - a multicenter collaborative study. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;118(4):483–489. doi: 10.1016/j.oooo.2014.07.012. [Internet] [DOI] [PubMed] [Google Scholar]
- 44.Wadhwan V., Venkatesh A., Reddy V., Malik S. The role of myofibroblasts in the progression of oral submucous fibrosis: a systematic review. J Oral Maxillofac Pathol. 2019;23:257–266. doi: 10.4103/jomfp.JOMFP_238_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gadbail A.R., Chaudhary M., Sarode S.C., et al. Ki67, CD105, and alpha-SMA expression supports the transformation relevant dysplastic features in the atrophic epithelium of oral submucous fibrosis. PLoS One. 2018;13(7) doi: 10.1371/journal.pone.0200171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maqsood A., Ali A., Zaffar Z., et al. Expression of CD34 and α-SMA markers in oral squamous cell carcinoma differentiation. A histological and histo-chemical study. Int J Environ Res Publ Health. 2021;18(1):192. doi: 10.3390/ijerph18010192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roy S., Sunkara R.R., Parmar M.Y., Shaikh S., Waghmare S.K. EMT imparts cancer stemness and plasticity: new perspectives and therapeutic potential. Front Biosci Landmark. 2021;26(2):238–265. doi: 10.2741/4893. [DOI] [PubMed] [Google Scholar]
- 48.Essa A.A.M., Deraz E.M. Expression of CD44 (NKI-P1) in oral squamous cell carcinoma associated vascular endothelial cells: a relationship to tumor angiogenesis. Saudi Dent J. 2022;34(1):21–26. doi: 10.1016/j.sdentj.2021.09.022. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen C., Zhao S., Karnad A., Freeman J.W. The biology and role of CD44 in cancer progression : therapeutic implications. J Hematol Oncol. 2018;11(1):64. doi: 10.1186/s13045-018-0605-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singh B., Aggarwal S., Das P., Srivastava S.K., Sharma S.C., Das S.N. Over expression of cancer stem cell marker CD44 and its clinical significance in patients with oral squamous cell carcinoma. Indian J Otolaryngol Head Neck Surg. 2023;75(1):109–114. doi: 10.1007/s12070-022-03200-3. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang T.Y., Peng C.Y., Lee S.S., Chou M.Y., Yu C.C., Chang Y.C. Acquisition cancer stemness, mesenchymal transdifferentiation, and chemoresistance properties by chronic exposure of oral epithelial cells to arecoline. Oncotarget. 2016;7(51):84072–84081. doi: 10.18632/oncotarget.11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yosef G., Hayun H., Papo N. Simultaneous targeting of CD44 and MMP9 catalytic and hemopexin domains as a therapeutic strategy. Biochem J. 2021;478(5):1139–1157. doi: 10.1042/BCJ20200628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu H., Niu M., Yuan X., Wu K., Liu A. CD44 as a tumor biomarker and therapeutic target. Exp Hematol Oncol. 2020;9(1):36. doi: 10.1186/s40164-020-00192-0. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is available from the corresponding author upon reasonable request.