Abstract
Introduction
Intra-abdominal adhesions are abnormal fibrous attachments between tissues and organs that can be congenital or acquired. Adhesion formation is a critical postoperative complication that may lead to bowel obstruction, chronic abdominal pain, and infertility. Physical barrier agents separate opposing peritoneal surfaces in the critical 5-day period of remesotheliazation. These agents are subdivided into solid or liquid/gel. Liquid agents seem easier to use in laparoscopic procedures than solid agents.
Methods
The search for suitable articles published in English was carried out using the following databases: MEDLINE, Embase, Global Health, the Cochrane Library (Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, Cochrane Methodology Register), Health Technology Assessment Database, Web of Science, and search register (ClinicalTrial.gov). Only studies reporting data about the impact of the use of an antiadhesive agent on adhesion formation after a primary gynecologic laparoscopic surgery were considered eligible.
Results
Twenty-two papers that met the inclusion criteria were included in this systematic review.
Conclusions
Surgeons should consider applying antiadhesive agents after gynecologic surgery to help reduce adhesion formation and its adverse effects. However, further studies are still needed to confirm their impact on reproductive outcome and to implement clear guidelines on their per-operative application.
Keywords: Antiadhesive, Laparoscopy, Adhesion, Minimally invasive surgery, Infertility
Introduction
Intra-abdominal adhesions are abnormal fibrous attachments between tissues and organs that can be congenital or acquired. The majority of acquired adhesions are consequent to surgical trauma, and their formation results from multiple factors. The knowledge and understanding of these factors by surgeons are crucial in order to contribute to the reduction of adhesion formation and its potentially dramatic consequences [1]. The dimension of the problem is substantial, with numbers ranging from 60 to 90% after a gynecological surgery [2]. In infertility surgery, this burden concerns not only the reformation of adhesions but also the formation of de novo adhesions, observed at sites initially adhesions-free, during second-look procedures [3]. Adhesion formation is a critical postoperative complication that may lead to bowel obstruction, chronic abdominal pain, and infertility [4]. This does not lead to an increase in direct and indirect costs [5–7]. Tissue repair after peritoneal surgery involves multiple players in coagulation, inflammation, and fibrinolysis that form a cascade of reactions that control the process [8] and lead to complete reepithelialization 5–7 days after surgical injury [9]. Normal peritoneal healing or adhesion formation depends essentially on the balance between fibrin deposition and degradation [10]. Injuries, such as surgical trauma, can disrupt this balance and lead to irreversible adhesions of the fibrin matrix [11]. Gynecological procedures are at high risk of adhesion formation and, therefore, fertility issues. Since the extent of surgical trauma is a primary factor responsible for inducing the development of adhesions [5], the prophylactic approach starts at the time of the first intervention with a good surgical technique. The European field guidelines [12], the European Society for Gynecological Endoscopy Adhesions Research Working Group [13], and the Practice Committee of the American Society for Reproductive Medicine (ASRM) in collaboration with the Society of Reproductive Surgeons [14] agree that adhering to microsurgical principles and favoring minimally invasive surgery may help decrease postoperative adhesions. Meticulous surgical techniques remain the cornerstone for adhesion prevention, but a significant risk of adhesion formation persists. Current research concepts focus on intraoperative placement of mechanical barriers as an antiadhesion strategy. Physical barrier agents separate opposing peritoneal surfaces in the critical 5-day period of remesotheliazation [15]. These agents are subdivided into solid or liquid/gel. Liquid agents seem easier to use in laparoscopic procedures than solid agents [16]. The studies published so far provide concrete evidence of the effort made to limit adhesion formation and its regretted consequences. Of these consequences, we focus on infertility due to adnexal adhesions that were formed after an initial gynecological procedure [17]. Additionally, the rates of term pregnancy were inversely correlated with adhesion scores at the time of intervention using the ASRM classification system for adnexal adhesions [18]. Unfortunately, surveys showed that the first operating surgeon was unaware of these complications and underestimates the problem [19]. The aim of this systematic review is to evaluate the outcomes of using different physical barrier agents, particularly in laparoscopic gynecologic reproductive surgeries. Describing the impact of using these agents in reducing adhesion scores and, consequently, infertility rates will help increase their use among surgeons when indicated, particularly at the time of the first surgery.
Methods
Data Sources and Searches
This study was carried out according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [20], available through the Enhancing the Quality and Transparency of Health Research (EQUATOR) network and the Cochrane Handbook for Systematic Reviews [21]. MEDLINE, Embase, Global Health, the Cochrane Library (Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, Cochrane Methodology Register), Health Technology Assessment Database, Web of Science, and research register (ClinicalTrial.gov) were searched for studies that described outcomes of using different physical barrier agents in laparoscopic gynecologic reproductive surgeries. The following medical subject heading (MeSH) and key search terms were used: “Adhesion” (MeSH Unique ID: D000267) OR “Infertility” (MeSH Unique ID: D007246) OR “Laparoscopy” (MeSH Unique ID: D010535) OR “minimally invasive surgery” (MeSH Unique ID: D019060) OR “Gynecologic surgery” (MeSH Unique ID: D013509) AND “Anti-adhesive agent.” We selected papers written in English from the inception of each database until December 31, 2022.
Inclusion and Exclusion Criteria
Only original studies (retrospective or prospective) that evaluated, mainly through a second-look laparoscopy (SLL), the impact of the use of an antiadhesive agent on adhesion formation after a primary gynecologic laparoscopic surgery were deemed eligible for inclusion in this systematic review. Case reports and “step-by-step” procedure descriptions were excluded. We excluded other surgical techniques, such as laparotomy or microsurgery, and all non-gynecological surgeries.
Study Selection
Titles and/or abstracts of studies retrieved using the search strategy were screened independently by 2 review authors (A.E. and Z.S.) to identify studies that met the inclusion criteria. The full texts of these potentially eligible articles were retrieved and independently assessed for eligibility by 2 other review team members (A.S.L. and V.C.). Any disagreement between them over the eligibility of articles was resolved through discussion with a third (external) collaborator. All authors approved the final selection.
Data Synthesis and Analysis
Two authors (S.E. and A.K.) independently extracted data from articles about study characteristics and included populations, methods, and results/outcomes using a prepiloted standard form to ensure consistency.
Results
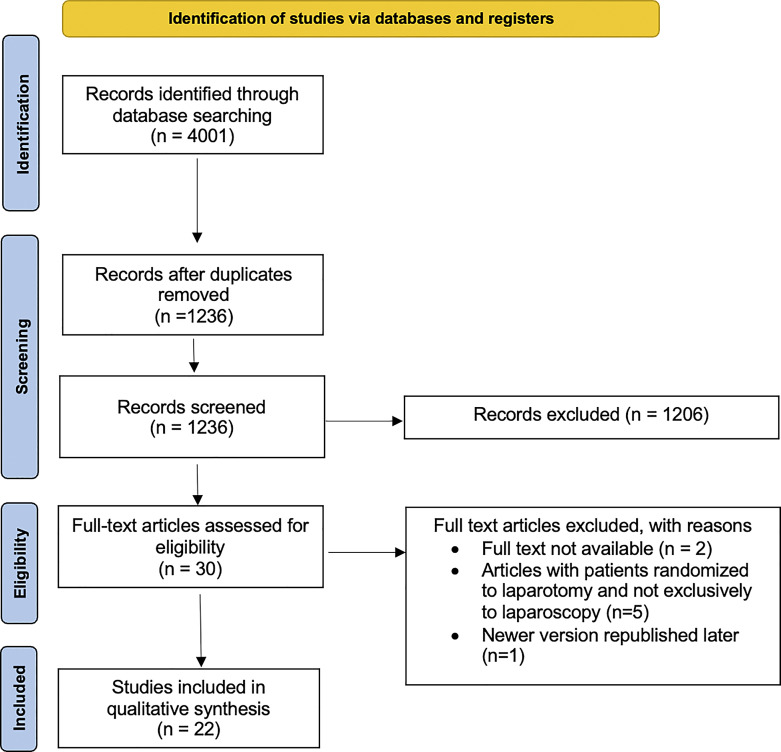
Using the reported search strategy, as shown in Figure 1, we identified 4,001 items. After exclusion of 2,765 duplicates, we screened 1,236 items and further excluded 1,206 of them. The remaining 30 items were selected, and each full text was carefully evaluated to select only relevant information. We excluded two studies because the full text could not be retrieved. An additional five studies were excluded because some of the patients were randomized to laparotomy and not exclusively to laparoscopy. One article was excluded because a newer version was republished later. Ultimately, we included twenty-two studies. The year of publication ranged from 1993 to 2021. Table 1 summarizes the key findings of these studies. Given our inclusion criteria and the aim of this review in demonstrating the effect of these antiadhesive agents on adhesion and, thus, on infertility, all patients evaluated in these studies were premenopausal and nonpregnant. The design of most of these studies is similar: patients were randomized, at the time of a first laparoscopic gynecologic surgery scheduled for a specific therapeutic purpose, to a treatment group with the application of an antiadhesive agent or to a control group. These patients were followed up and scheduled for a SLL, during which the extent, severity, rate of reduction, and adhesion score were evaluated. Only two studies used a partially different scheme at follow-up in which, after randomization to the treatment and control groups and application of an antiadhesive agent, patients did not undergo SLL but were assessed for serum hormone status and follicular monitoring [22] and for quality of life using visual analog scale (VAS), the Endometriosis Health Profile (EHP-5), and the Short Form for Mental and Physical Health (SF-12) questionnaires [23]. Pellicano et al. [24] after conducting a study design similar to the other included studies, also reported, 2 years later, in another paper, reproductive outcome using pregnancy rate [25]. A total of 1,804 patients underwent an initial laparoscopic surgery, and when applicable, a total of 1,506 underwent an SLL. Various antiadhesive agents were tested with this intent. We reported the observed results chronologically by similarity of composition or texture; the main findings are summarized in Table 2.
Fig. 1.
PRISMA flow diagram.
Table 1.
Key findings of the included studies
| Author | Year of publication | Product | Patients, n | SLL time | Rate of adhesion reduction |
|---|---|---|---|---|---|
| Greenblatt and Casper | 1993 | Interceed® | 8 | 3–4 weeks | – |
| Saravelos and Li | 1996 | Interceed® | 21 | 2–11 weeks | – |
| Keckstein et al. | 1996 | Interceed® | 17 | 8–30 weeks | 76% free of adhesions [vs. 35%] |
| Mais et al. | 1995 | Interceed® | 50 | 12–14 weeks | No cohesive adhesions [vs. 23%] |
| diZerega et al. | 2002 | Adept® | 53 | 6–12 weeks | Nonsignificant reduction |
| Brown et al. | 2007 | Adept® | 402 | 4–8 weeks | 49% adhesion reduction [vs. 38%] |
| Trew et al. | 2011 | Adept® | 330 | 4–16 weeks | Nonsignificant |
| Pellicano et al. | 2003 | Hyalobarrier® | 36 | 60–90 days | 27.8% adhesions [vs. 77.8%] |
| Mais et al. | 2006 | Hyalobarrier® | 43 | 12–14 weeks | Nonsignificant |
| Cheong et al. | 2017 | Hyalobarrier® | N/A | N/A | N/A |
| Liu et al. | 2015 | NCH | 196 | 9 weeks | Lower incidence and fewer sites of moderate and severe adhesions |
| Ekin et al. | 2021 | NCH | N/A | N/A | N/A |
| Fossum et al. | 2011 | Sepraspray® | 38 | 4–12 weeks | Nonsignificant |
| Diamond et al. | 2003 | NOCC gel | 32 | 2–10 weeks | Nonsignificant |
| Johns et al. | 2003 | SprayGel® | 14 | 3–16 weeks | Reductions: 71% of frequency, 69% of extent, 43% of severity |
| Tachartchian et al. | 2014 | SprayShield® | 13 | 8–12 weeks | Nonsignificant |
| Trew et al. | 2017 | Actamax | 74 | 4–12 weeks | 41.4% reduction in postoperative adhesion |
| Lundorff et al. | 2005 | Oxiplex/AP gel | 49 | 6–10 weeks | 42% reduction in postoperative adhesion |
| Young et al. | 2005 | Oxiplex/AP gel | 27 | 6–10 weeks | 32% reduction in adhesion formation |
| diZerega et al. | 2007 | Oxiplex/AP gel | 37 | 6–12 weeks | Significant reduction |
| Diamond et al. | 2011 | Adhexil | 16 | 6 weeks | Nonsignificant |
| Kramer et al. | 2021 | 4DryField® PH | 50 | 3–16 weeks | 85% reduction of severity and extent of adhesions, 53% reduction of incidence of adhesion |
SLL, second-look laparoscopy; NCH, new crosslinked hyaluronan; NOCC, N,O-carboxymethylchitosan.
Table 2.
Main findings of the antiadhesive agents considered
| Product | Main findings |
|---|---|
| Interceed® | Effective in preventing reformation of adhesions when the entire ovary was wrapped with the product after thorough hemostasis. Efficacy was not significant when applied only to the treated surface. Proper use of Interceed® optimizes the result |
| Adept® | The data on Adept® are controversial: the fact that it did not show efficacy on new adhesion formation may be due to the characteristics of the studies considered in this systematic review. In addition, in the recruited studies, surgeons performed adhesiolysis during the first surgery, which may have influenced adhesion reformation and contributed to the observed results |
| Hyalobarrier® | Hyalobarrier® has been shown to significantly improve the rate of postoperative adhesions in infertile patients with uterine myomas undergoing laparoscopic myomectomy. A significantly higher pregnancy rate at 12 months was also observed in the Hyalobarrier®-treated patient groups compared with the untreated group |
| NCH | NCH has been shown to significantly reduce the incidence and severity of postoperative adhesions in groups of patients undergoing laparoscopic gynecologic surgery for adhesiolysis, myomectomy, or ovarian cystectomy and subsequently treated with NCH. In addition, the treated groups reported a significant reduction in dysmenorrhea, dyschezia, and dyspareunia |
| Sepraspray® | Differences are not statistically significant between the treated group and the control group |
| NOCC gel | Differences are not statistically significant between the treated group and the control group |
| SprayGel® | Patients treated with SprayGel® showed a 71% reduction in the frequency of new adhesion formation, 69% reduction in the extent of adhesions, and 43% reduction in their severity |
| SprayShield® | Differences are not statistically significant between the treated group and the control group |
| Actamax | Patients treated with Actamax showed a 41.4% reduction in postoperative adhesion formation |
| Oxiplex/AP gel | Patients treated with Actamax showed a 42% reduction in postoperative adhesion formation |
| Adhexil | Differences are not statistically significant between the treated group and the control group |
| 4DryField® PH | Patients treated with 4DryField® PH showed a 53% reduction in the frequency of formation of new adhesions and an 85% reduction in their extent and severity |
NCH, new crosslinked hyaluronan; NOCC, N,O-carboxymethylchitosan.
Interceed®
Interceed® (Ethicon-Inc., Somerville, MA, USA), an oxidized regenerated cellulose barrier, was effective in reducing postoperative adhesion reformation in patients undergoing laparotomy for adhesiolysis, and its use in laparotomy is approved in the USA and in Europe. Its efficacy in laparoscopic surgeries was studied in four trials. The first pilot study [26] published in 1993 applied Interceed® to an ovary after laparoscopic ovarian cautery in eight women with PCOS who had failed to conceive with previous clomiphene citrate therapy. All patients were free of adhesions at the first procedure. At SLL, periovarian adhesion was observed and treated in all patients using the revised American Fertility Score (AFS) [27], with no significant difference between the Interceed® and control sides. In laparoscopic ovarian cautery for PCOS, Interceed® did not protect against adhesion formation and was not related to pregnancy rate in this study; however, seven of these women conceived spontaneously, a finding likely due to the therapeutic role of adhesiolysis during SLL. Three years later, Saravelos and Li [28] restudied Interceed® in 27 women with PCOS and obtained similar results. In a study by Keckstein et al. [29], after bilateral laparoscopic ovarian cystectomy in 25 patients for various indications, including endometrioma, periovarian adhesiolysis, and removal of endometriosis, Interceed® was applied to the whole surface of one of the ovaries, and the other ovary served as a control. At SLL in 17 of these patients, Interceed® proved its safety and effectiveness in reducing the adhesion score, regardless of the size of the cyst at the first procedure and even when sutures were applied to the ovarian surface. Regarding its use in uterine surgery, particularly laparoscopic myomectomy in 50 patients in a study conducted by Mais et al. [30], a significant reduction in de novo adhesion formation was observed. In effect, at SLL in all 50 patients, cohesive adhesion (American Fertility Society – AFS score 3) was noted only in the control group in 23% of the patients. The majority of the adhesion in the treated group (70%) was filmy and avascular (AFS score 1), and none showed an AFS score of 3.
Adept®
Adept® (ML-Laboratories-PLC, Hampshire, UK) is a postsurgical instillate consisting of 4% icodextrin that works by keeping damaged tissues separated at the critical time of postoperative repair and thus preventing adhesion by hydroflotation. This agent is approved in Europe and is the only one approved in the USA for use in laparoscopy. The pilot study by diZerega et al. [31] randomized 62 patients who underwent laparoscopic adnexal surgery to receive either Adept® (n = 34) or Ringer’s Lactate Solution (RLS) (n = 28) for postoperative intraperitoneal lavage. Fifty-three patients underwent SLL to assess the incidence, extent, and severity of adhesions using the modified American Fertility Society score (mAFS). A nonsignificant reduction in the adhesion score and an improvement in more patients were observed in the Adept® group. Brown et al. [32] conducted a larger study to confirm the clinical efficacy and safety of Adept®. A total of 449 patients undergoing a laparoscopic gynecologic procedure that included adhesiolysis, with primary diagnoses such as pelvic pain, endometriosis, and infertility, were randomized to receive either Adept® or RLS as a postoperative instillate. At SLL in 402 patients, the clinical success with adhesion reduction was significantly higher in the treated group (49%) than in the control group (38%), with a particular clinical success in the subgroup of patients with infertility with 55% adhesion reduction in the Adept® group and 33% in the RLS group. A third study by Trew et al. [33] randomized 426 patients to receive either Adept® or RLS at the time of a primary laparoscopic removal of myomas or endometriotic cysts. At SLL in 330 patients, de novo adhesion formation was evaluated using total mAFS and AFS site-specific scores, and no significant difference was observed between the two groups. This study also showed that adhesion outcomes were influenced by the duration of surgery (longer than 2 h), the size of incisions instead of the number of incisions, the number of knots (six or more knots), and blood loss exceeding 200 mL.
Hyaluronic Acid
Hyaluronic acid (HA) is a natural component of the extracellular matrix and peritoneal fluid, and its deposition around surgically treated tissues is approved for the prevention of adhesion formation. Pellicano et al. [24] randomized 36 women with an infertility history of more than 3 years and symptomatic uterine fibroids undergoing laparoscopic myomectomy to either have an autocrosslinked HA Hyalobarrier® gel (Anika-Therapeutics, Abano Terme, Italy) applied on the injured uterine surface (n = 18) or to the control group (n = 18). All patients who underwent SLL showed a significantly lower rate of postoperative adhesions using the ASRM adhesion score system in the treated group (27.8%) than in the control group (77.8%). These same patients were followed-up for 12 months to assess the reproductive outcome with ovulation induction only in the patients who did not conceive after 6 months of follow-up [25]. A significantly higher pregnancy rate at 12 months was observed in the treated group (77.8%) compared to the untreated group (38.8%). In a similar study by Mais et al. [34], 52 patients undergoing laparoscopic myomectomy for single or multiple subserous or intramural myomas ranging from 20 to 50 mm were randomized to either have autocrosslinked HA gel Hyalobarrier® coating all uterine incisions and suture materials (n = 26) or to surgery alone (n = 26). The results obtained were similar to the study conducted by Pellicano et al. [24], with significantly lower mean adhesion scores in the treated group compared to the control group.
A recent trial in 2017 by Cheong et al. [22] randomized 30 patients undergoing laparoscopic salpingo-ovariolysis to reconstruct the tubo-ovarian anatomy to receive Hyalobarrier® (n = 15) or to a control group (n = 15). Hyalobarrier® did not influence follicular development, as shown by an evaluation of serum hormonal status, including day two FSH and LH and day 21 progesterone, performed prior to and after the surgery, in addition to a follicular tracking cycle at 3 months and pregnancy rate at the 2-year follow-up. Liu et al. [35] studied a new crosslinked hyaluronan (NCH) gel characterized by a higher viscosity and a gradual absorption. A total of 216 patients undergoing laparoscopic gynecologic surgery for adhesiolysis, myomectomy, or ovarian cystectomy were randomized to the application of NCH gel or to surgery alone. At SLL in 196 patients, a significantly lower incidence and fewer sites of moderate and severe adhesions were noted in the treated group in addition to lower mAFS scores in the gel group at the studied sites. Recently, in 2021, a trial by Ekin et al. [23] randomized 60 patients with dysmenorrhea, dyspareunia, chronic pelvic pain, and infertility to either a treated group with NCH gel or to a control group after undergoing laparoscopic surgery for deep infiltrating endometriosis. These patients were followed up at the third and sixth postoperative months to evaluate the VAS, EHP-5, and SF-12 questionnaires. The trial showed, in the treated group, a significant reduction in dysmenorrhea, dyschezia, and dyspareunia as proven on the VAS, a significantly lower EHP-5 score, and significantly higher SF-12 mental and physical scores. As an analog to Seprafilm® (Genzyme, Cambridge, MA, USA), a modified HA and carboxymethylcellulose designed and approved for postoperative adhesion reduction after laparotomy, a powder of similar composition, Sepraspray® (Genzyme) was designed for use in laparoscopic surgeries. Fossum et al. [36] randomized 41 patients undergoing laparoscopic myomectomy to a treated group with Sepraspray® (n = 21) or to a control group (n = 20). These groups were similar in terms of patient demographics and surgical modality, including the length of surgery, uterine incisions, number and weight of myomas, adhesiolysis time, and blood loss. At SLL in 38 patients, adhesiolysis was performed, and adhesions were assessed in 14 sites using the mAFS score and showed an increase in adhesion scores in both groups, with larger increases in the control group without any statistical significance.
Gel-Based Agents
In a clinical trial pilot study in 2003 by Diamond et al. [37], 34 patients underwent laparoscopic surgery. These patients were randomized to undergo instillation of RLS (control group) or N,O-carboxymethylchitosan gel (treatment group), which has structural similarities to HA. At SLL, a nonsignificant recurrence of adhesions was noted in 61% of sites in controls and in 38% of sites with a lower extent, severity, and grade of adhesion in the N,O-carboxymethylchitosan group. Two sprayable agents, SprayGel® (Confluent-Surgical Inc., Waltham, MA, USA), approved in Europe, and SprayShield® (Covidien, Waltham, MA, USA), consisting of polyethylene glycol, were studied. They form a biocompatible, absorbable hydrogel when applied and therefore separate damaged surfaces. In a trial in 2003 by Johns et al. [38], after optimal surgical treatment in a laparoscopic ovarian surgery conducted in 14 patients, one adnexa was randomized to the treated group with SprayGel® and the second adnexa to the control group. At SLL in all patients, a statistically significant reduction in the frequency (71% reduction), extent (69% reduction), and severity (43% reduction) of adhesions was observed on the treatment side compared with the control side. The second agent, SprayShield®, was studied in 2014 by Tchartchian et al. [39]. Fifteen patients undergoing laparoscopic myomectomy were randomized to have SprayShield® applied to all uterine suture lines (n = 9) or to the control group (n = 6). At SLL in 13 of these patients, no significant differences were found between the two study groups regarding the incidence, extent, and severity of adhesion formation. In 2017, Trew et al. [40] studied another sprayable degradable hydrogel adhesion barrier, Actamax® (Surgical-Materials LLC, Wilmington, DE, USA). In their trial, a total of 78 patients undergoing laparoscopic gynecologic abdominopelvic surgery were randomized to either have Actamax® sprayed over all sites of surgical trauma (n = 47) or to surgery alone (n = 31). At SLL in 74 patients, there was a 41.4% reduction in postoperative adhesion development in terms of the incidence, severity, extent, and adhesion score, particularly following myomectomy, where a 49.5% reduction was observed. In 2005, Lundorff et al. [41] conducted the first clinical trial evaluating Oxiplex/AP gel, a viscoelastic gel composed of polyethylene oxide and carboxymethylcellulose. Forty-nine patients undergoing laparoscopic surgery for adhesiolysis or removal of endometriosis were randomized to either have Oxiplex/AP gel applied to their adnexa or to a control group. At SLL in all patients, the extent and severity of adhesion involving the fallopian tubes and ovaries were evaluated using the AFS score. There was a significant increase in the mean adnexal adhesion score from 8.8 to 15.8 in the control adnexa and a significant decrease from 11.9 to 9.1 in the treated adnexa with a 42% reduction in second-look AFS scores. Additionally, the majority (93%) of the treated adnexa did not have a worse adhesion score compared to more than half (56%) of the control adnexa that had a worse adhesion score. In the same year, a pilot study by Young et al. [42] randomized 28 patients with pelvic adhesions, tubal occlusion, endometriosis, or dermoid cysts undergoing laparoscopic surgery for at least one of the adnexa to a treatment group (18 patients, 19 adnexa) with Oxiplex/AP gel applied to all areas susceptible to adhesions or to a control group (10 patients, 18 adnexa) with surgery alone. The mean baseline AFS score for each group was 8. At SLL in all except for 1 of the patients, treated adnexa maintained the same mean score (8.1) in opposition to the control group, where the score increased to 11.6. Additionally, 34% of the treated adnexa and 67% of the control adnexa had an increase in their adhesion score, thus implying a 32% reduction in adhesion formation with the use of the Oxiplex/AP gel. Later, in 2007, a trial by diZerega et al. [43] randomized 37 patients undergoing laparoscopic surgical treatment for endometriosis to a treatment group with Oxiplex/AP gel (20 patients, 35 adnexa) or to a control group with surgery alone (17 patients, 30 adnexa). At SLL in all patients, adnexal adhesions were evaluated using the AFS score. Adnexal adhesion formation was significantly reduced in the treated group compared with the control group.
Adhexil
Minimizing bleeding and enhancing the degradation of the fibrinous mass are among the factors that minimize adhesion development. Adhexil is an adhesion prevention kit consisting mainly of thrombin and fibrinogen that, when sprayed or dripped, forms a stable fibrin clot that serves as a hemostatic agent and as a barrier between the treated tissues. In a pilot trial by Diamond et al. in 2011 [44], 17 women with bilateral ovarian disease and adhesions underwent laparoscopic procedure and adhesiolysis. One ovary was treated with Adhexil, and the contralateral ovary served as the untreated control. Sixteen patients underwent SLL to evaluate the incidence, extent, and severity of adhesions. There was a nonsignificant improvement in adhesion incidence (50% adhesion-free ovaries) and in the mean AFS score (from 6.4 to 4.6) in the treated group compared to the control group (31% adhesion-free ovaries and a mean AFS score from 5.6 to 7.1).
4DryField® PH
In 2021, a recent antiadhesive agent was tested in a trial by Kramer et al. [45]. It consists of a starch-based powder that forms a gel after irrigation with saline solution. This gel separates treated surgical sites to prevent adhesion formation. Fifty patients underwent laparoscopic surgical treatment for deep infiltrating endometriosis or extensive peritoneal or ovarian endometriosis and were randomized to a treated group (n = 25) with 4DryField® PH (PlantTec Medical, Lüneburg, Germany) applied on all surgically affected areas or to a control group (n = 25) with only saline solution applied. All patients underwent SLL to evaluate the incidence, extent, and severity of adhesions using the AFS score. A significant reduction of 85% in the severity and extent of adhesions was observed in the treated group (mean total score 2.2) compared to the control group (mean total score 12.2). Additionally, there was a significant reduction of 53% in the incidence of adhesion formation in the treated group (mean 1.1 site) compared to the control group (mean 2.3 sites).
Discussion
The spectrum of symptomatology due to postsurgical peritoneal adhesions can be wide: they can remain silent and cause no symptoms or cause clinically evident complications, such as bowel obstruction, female infertility, chronic pelvic pain, or, in the case of reintervention, can increase the difficulty of performing the surgery. Postsurgical adhesions are well recognized as a cause of female infertility. Adhesions have been found in approximately 20–30% of infertile women, and after surgical adhesiolysis, there has been a marked increase in the cumulative pregnancy rate [46]. The causes contributing to the development of postsurgical adhesions are numerous and seem largely dependent on the peritoneal reaction due to surgical stress and induction of pneumoperitoneum. Locally, pneumoperitoneum, by altering the peritoneal microcirculation [47] and peritoneal fluid, modulates the local immune system and inflammatory response [48] resulting in inhibition of the peritoneal plasma system, leading to peritoneal hypofibrinolysis. Peritoneal damage, whether due to surgical stress, pneumoperitoneum, or other conditions such as infection, initiates an inflammatory reaction that, as a result of activation of the coagulation cascade, increases the number of cells and proteins in the peritoneal fluid, generating a fibrinous exudate that is deposited on its surface [49]. Within the exudate, macrophages, polymorphonucleates, fibroblasts, and mesothelial cells migrate and proliferate. These cells release a number of substances, including cytokines and growth factors, components of the plasminogen system, arachidonic acid metabolites, and reactive oxygen species, which modulate the peritoneal healing process and are proponents of adhesion formation [50]. To allow complete restoration of the surgery-damaged peritoneum, the fibrinous exudate must be degraded [51]. This degradation occurs through the plasminogen system, the main activator of which is tissue-type serine protease, expressed mainly in macrophages but also in mesothelial cells. Therefore, in the presence of fibrin exudate, because a considerable number of cells expressing tissue-type serine protease are found in its context, the rate of plasminogen activation is greatly increased. The balance between fibrin deposition and degradation is key in determining normal peritoneal healing or adhesion formation. When fibrin is completely degraded, normal peritoneal healing is achieved. Conversely, if fibrin is not completely degraded, it will serve as a scaffold for fibroblasts and capillary growth, and therefore adhesions will form. The peritoneal microenvironment, of which the cells of the immune system are major players, is therefore of paramount importance in determining whether or not proper healing occurs.
Evaluation of antiadhesive agents should be accomplished after proper surgical techniques, including complete hemostasis and removal of excess peritoneal fluid. Additionally, careful attention should be given to technical details to apply the agent through the operating channel in accordance with its nature to allow optimal coverage of the surgical sites. To achieve an adequate evaluation of the agent, a trial should be conducted on a proper sample size with an extended clinical follow-up. In the reported studies, this bias was reduced by either using a product similar in appearance or by reviewing recorded surgeries after omitting the application of the agent. Interceed® showed its efficacy on adhesion reformation when the whole ovary was wrapped after careful hemostasis. The efficacy was not significant when it was only applied to the treated surface. This proves that proper use of the substance optimizes the outcome. Adept® showed at first apparent but not significant improvement due to small groups and to more severe baseline condition in the treated group. In further studies, larger populations were allowed to demonstrate clinical success in reducing adhesion reformation. As observed with Interceed®, Adept® did not show efficacy on de novo adhesion formation. This is probably influenced by other factors, such as the duration of surgery, the number of knots, incision characteristics, and blood loss. Additionally, during the first surgery, surgeons performed adhesiolysis that could have impacted adhesion reformation and contributed to the observed results.
HA had various forms of application, such as spray and gel, that were easier to apply in laparoscopic surgeries. Additional clinical endpoints, such as pregnancy rate, serum hormonal status, and quality of life questionnaire, were evaluated, which are important for appreciating the fertility aspects of using antiadhesive agents. Gel agents have the advantage of the facility of application and a better precision in coverage with a better ability to conserve the site of application. This also reduces the operating time, which contributes indirectly to adhesion reduction.
Adhesions are regrettable postoperative complications with major economic and medical impacts, leading to serious consequences. Surgeons, particularly the first operating surgeon, must apprehend the burden of the problem to actively help prevent it by practicing antiadhesive measures.
Gold standard for antiadhesive measures remains meticulous surgical techniques that should be adopted by all surgeons. The laparoscopic approach has been shown to cause less postoperative adhesion formation than laparotomy and should be preferred, particularly in gynecologic surgeries where adhesions contribute largely to infertility. Antiadhesive agents are now available, and surgeons should consider their application to help reduce adhesion formation and thus their undesirable consequences. Further studies are nonetheless still needed to confirm their impact on the reproductive outcome and to implement clear guidelines of their application per-operatively.
Statement of Ethics
An ethics statement is not applicable because this study is based exclusively on published literature.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
The present study did not receive funding.
Author Contributions
S.E. and A.E. were responsible for the acquisition, analysis, and interpretation of the data. A.E. and A.S.L. were responsible for drafting the work. Z.S. and V.C. were responsible for revising the work critically for important intellectual content. H.M.A., A.K., and M.D. gave final approval of the version to be published. S.E. and Z.S. agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors meet the ICMJE criteria for authorship and have read and agreed to the current version of the manuscript.
Funding Statement
The present study did not receive funding.
Data Availability Statement
Data sharing is not applicable to this article, as no new data were created or analyzed in this study. Further inquiries can be directed to the corresponding author.
References
- 1. Menzies D. Peritoneal adhesions. Incidence, cause, and prevention. Surg Annu. 1992;24 Pt 1(Pt 1):27–45. [PubMed] [Google Scholar]
- 2. Menzies D, Ellis H. Intestinal obstruction from adhesions--how big is the problem? Ann R Coll Surg Engl. 1990 Jan;72(1):60–3. [PMC free article] [PubMed] [Google Scholar]
- 3. Operative Laparoscopy Study Group . Postoperative adhesion development after operative laparoscopy: evaluation at early second-look procedures. Fertil Steril. 1991 Apr;55(4):700–4. [PubMed] [Google Scholar]
- 4. Vrijland WW, Jeekel J, van Geldorp HJ, Swank DJ, Bonjer HJ. Abdominal adhesions: intestinal obstruction, pain, and infertility. Surg Endosc. 2003 Jul;17(7):1017–22. 10.1007/s00464-002-9208-9. [DOI] [PubMed] [Google Scholar]
- 5. Schäfer M, Krähenb hl L, Büchler MW. Comparison of adhesion formation in open and laparoscopic surgery. Dig Surg. 1998;15(2):148–52. 10.1159/000018609. [DOI] [PubMed] [Google Scholar]
- 6. Ray NF, Larsen JW, Stillman RJ, Jacobs RJ. Economic impact of hospitalizations for lower abdominal adhesiolysis in the United States in 1988. Surg Gynecol Obstet. 1993 Mar;176(3):271–6. [PubMed] [Google Scholar]
- 7. Ivarsson ML, Holmdahl L, Franzén G, Risberg B. Cost of bowel obstruction resulting from adhesions. Eur J Surg. 1997 Sep;163(9):679–84. [PubMed] [Google Scholar]
- 8. Tang J, Xiang Z, Bernards MT, Chen S. Peritoneal adhesions: occurrence, prevention and experimental models. Acta Biomater. 2020 Oct;116:84–104. 10.1016/j.actbio.2020.08.036. [DOI] [PubMed] [Google Scholar]
- 9. diZerega GS, Campeau JD. Peritoneal repair and post-surgical adhesion formation. Hum Reprod Update. 2001;7(6):547–55. 10.1093/humupd/7.6.547. [DOI] [PubMed] [Google Scholar]
- 10. Molinas CR, Binda MM, Manavella GD, Koninckx PR. Adhesion formation after laparoscopic surgery: what do we know about the role of the peritoneal environment? Facts Views Vis Obgyn. 2010;2(3):149–60. [PMC free article] [PubMed] [Google Scholar]
- 11. van Baal JOAM, Van de Vijver KK, Nieuwland R, van Noorden CJF, van Driel WJ, Sturk A, et al. The histophysiology and pathophysiology of the peritoneum. Tissue Cell. 2017 Feb;49(1):95–105. 10.1016/j.tice.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 12. De Wilde RL, Brölmann H, Koninckx PR, Lundorff P, Lower AM, Wattiez A, et al. Prevention of adhesions in gynaecological surgery: the 2012 European field guideline. Gynecol Surg. 2012 Nov;9(4):365–8. 10.1007/s10397-012-0764-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. De Wilde RL, Bakkum EA, Brölmann H, Crowe A, Koninckx P, Korell M, et al. Consensus recommendations on adhesions (version 2014) for the ESGE adhesions research working group (European Society for Gynecological Endoscopy): an expert opinion. Arch Gynecol Obstet. 2014 Sep;290(3):581–2. 10.1007/s00404-014-3312-7. [DOI] [PubMed] [Google Scholar]
- 14. Practice Committee of the American Society for Reproductive Medicine in collaboration with the Society of Reproductive Surgeons Electronic address asrm@asrmorg; Practice Committee of the American Society for Reproductive Medicine in collaboration with the Society of Reproductive Surgeons . Postoperative adhesions in gynecologic surgery: a committee opinion. Fertil Steril. 2019 Sep;112(3):458–63. 10.1016/j.fertnstert.2019.06.027. [DOI] [PubMed] [Google Scholar]
- 15. Diamond MP. Reduction of postoperative adhesion development. Fertil Steril. 2016 Oct;106(5):994–7.e1. 10.1016/j.fertnstert.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 16. Aref-Adib M, Phan T, Ades A. Preventing adhesions in laparoscopic surgery: the role of anti-adhesion agents. Obstet Gynecol. 2019 Jul;21(3):185–92. 10.1111/tog.12588. [DOI] [Google Scholar]
- 17. Tulandi T, Collins JA, Burrows E, Jarrell JF, McInnes RA, Wrixon W, et al. Treatment-dependent and treatment-independent pregnancy among women with periadnexal adhesions. Am J Obstet Gynecol. 1990 Feb;162(2):354–7. 10.1016/0002-9378(90)90384-j. [DOI] [PubMed] [Google Scholar]
- 18. Marana R, Rizzi M, Muzii L, Catalano GF, Caruana P, Mancuso S. Correlation between the American Fertility Society classifications of adnexal adhesions and distal tubal occlusion, salpingoscopy, and reproductive outcome in tubal surgery. Fertil Steril. 1995 Nov;64(5):924–9. 10.1016/s0015-0282(16)57903-5. [DOI] [PubMed] [Google Scholar]
- 19. Schreinemacher MHF, ten Broek RP, Bakkum EA, van Goor H, Bouvy ND. Adhesion awareness: a national survey of surgeons. World J Surg. 2010 Dec;34(12):2805–12. 10.1007/s00268-010-0778-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009 Jul;6(7):e1000097. 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. 2019 Oct;10:ED000142. 10.1002/14651858.ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cheong Y, Bailey S, Forbes J. Randomized controlled trial of Hyalobarrier® versus No Hyalobarrier® on the ovulatory status of women with periovarian adhesions: a pilot study. Adv Ther. 2017 Jan;34(1):199–206. 10.1007/s12325-016-0453-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ekin M, Kaya C, Erdoğan ŞV, Bahçeci E, Baghaki S, Yaşar L. The effect of new cross linked hyaluronan gel on quality of life of patients after deep infiltrating endometriosis surgery: a randomized controlled pilot study. J Obstet Gynaecol. 2021 Feb;41(2):263–8. 10.1080/01443615.2020.1755628. [DOI] [PubMed] [Google Scholar]
- 24. Pellicano M, Bramante S, Cirillo D, Palomba S, Bifulco G, Zullo F, et al. Effectiveness of autocrosslinked hyaluronic acid gel after laparoscopic myomectomy in infertile patients: a prospective, randomized, controlled study. Fertil Steril. 2003 Aug;80(2):441–4. 10.1016/s0015-0282(03)00597-1. [DOI] [PubMed] [Google Scholar]
- 25. Pellicano M, Guida M, Bramante S, Acunzo G, Di Spiezio Sardo A, Tommaselli GA, et al. Reproductive outcome after autocrosslinked hyaluronic acid gel application in infertile patients who underwent laparoscopic myomectomy. Fertil Steril. 2005 Feb;83(2):498–500. 10.1016/j.fertnstert.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 26. Greenblatt EM, Casper RF. Adhesion formation after laparoscopic ovarian cautery for polycystic ovarian syndrome: lack of correlation with pregnancy rate. Fertil Steril. 1993 Nov;60(5):766–70. 10.1016/s0015-0282(16)56273-6. [DOI] [PubMed] [Google Scholar]
- 27. Peers EM, Brown CB. American fertility society score as a measure of the effectiveness of 4% icodextrin in a pivotal adhesion reduction trial in the USA. Fertility Sterility. 2005 Sep;84:S160. 10.1016/j.fertnstert.2005.07.392. [DOI] [Google Scholar]
- 28. Saravelos H, Li TC. Post-operative adhesions after laparoscopic electrosurgical treatment for polycystic ovarian syndrome with the application of Interceed to one ovary: a prospective randomized controlled study. Hum Reprod. 1996 May;11(5):992–7. 10.1093/oxfordjournals.humrep.a019337. [DOI] [PubMed] [Google Scholar]
- 29. Keckstein J, Ulrich U, Sasse V, Roth A, Tuttlies F, Karageorgieva E. Reduction of postoperative adhesion formation after laparoscopic ovarian cystectomy. Hum Reprod. 1996 Mar;11(3):579–82. 10.1093/humrep/11.3.579. [DOI] [PubMed] [Google Scholar]
- 30. Mais V, Ajossa S, Piras B, Guerriero S, Marongiu D, Melis GB. Prevention of de-novo adhesion formation after laparoscopic myomectomy: a randomized trial to evaluate the effectiveness of an oxidized regenerated cellulose absorbable barrier. Hum Reprod. 1995 Dec;10(12):3133–5. 10.1093/oxfordjournals.humrep.a135873. [DOI] [PubMed] [Google Scholar]
- 31. diZerega GS, Verco SJS, Young P, Kettel M, Kobak W, Martin D, et al. A randomized, controlled pilot study of the safety and efficacy of 4% icodextrin solution in the reduction of adhesions following laparoscopic gynaecological surgery. Hum Reprod. 2002 Apr;17(4):1031–8. 10.1093/humrep/17.4.1031. [DOI] [PubMed] [Google Scholar]
- 32. Brown CB, Luciano AA, Martin D, Peers E, Scrimgeour A, diZerega GS, et al. Adept (icodextrin 4% solution) reduces adhesions after laparoscopic surgery for adhesiolysis: a double-blind, randomized, controlled study. Fertil Steril. 2007 Nov;88(5):1413–26. 10.1016/j.fertnstert.2006.12.084. [DOI] [PubMed] [Google Scholar]
- 33. Trew G, Pistofidis G, Pados G, Lower A, Mettler L, Wallwiener D, et al. Gynaecological endoscopic evaluation of 4% icodextrin solution: a European, multicentre, double-blind, randomized study of the efficacy and safety in the reduction of de novo adhesions after laparoscopic gynaecological surgery. Hum Reprod. 2011 Aug;26(8):2015–27. 10.1093/humrep/der135. [DOI] [PubMed] [Google Scholar]
- 34. Mais V, Bracco GL, Litta P, Gargiulo T, Melis GB. Reduction of postoperative adhesions with an auto-crosslinked hyaluronan gel in gynaecological laparoscopic surgery: a blinded, controlled, randomized, multicentre study. Hum Reprod. 2006 May;21(5):1248–54. 10.1093/humrep/dei488. [DOI] [PubMed] [Google Scholar]
- 35. Liu C, Lu Q, Zhang Z, Xue M, Zhang Y, Zhang Y, et al. A randomized controlled trial on the efficacy and safety of a new crosslinked hyaluronan gel in reducing adhesions after gynecologic laparoscopic surgeries. J Minim Invasive Gynecol. 2015;22(5):853–63. 10.1016/j.jmig.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 36. Fossum GT, Silverberg KM, Miller CE, Diamond MP, Holmdahl L. Gynecologic use of Sepraspray Adhesion Barrier for reduction of adhesion development after laparoscopic myomectomy: a pilot study. Fertil Steril. 2011 Aug;96(2):487–91. 10.1016/j.fertnstert.2011.05.081. [DOI] [PubMed] [Google Scholar]
- 37. Diamond MP, Luciano A, Johns DA, Dunn R, Young P, Bieber E. Reduction of postoperative adhesions by N,O-carboxymethylchitosan: a pilot study. Fertil Steril. 2003 Sep;80(3):631–6. 10.1016/s0015-0282(03)00759-3. [DOI] [PubMed] [Google Scholar]
- 38. Johns DA, Ferland R, Dunn R. Initial feasibility study of a sprayable hydrogel adhesion barrier system in patients undergoing laparoscopic ovarian surgery. J Am Assoc Gynecol Laparosc. 2003 Aug;10(3):334–8. 10.1016/s1074-3804(05)60257-5. [DOI] [PubMed] [Google Scholar]
- 39. Tchartchian G, Hackethal A, Herrmann A, Bojahr B, Wallwiener C, Ohlinger R, et al. Evaluation of SprayShieldTM Adhesion Barrier in a single center: randomized controlled study in 15 women undergoing reconstructive surgery after laparoscopic myomectomy. Arch Gynecol Obstet. 2014 Oct;290(4):697–704. 10.1007/s00404-014-3251-3. [DOI] [PubMed] [Google Scholar]
- 40. Trew GH, Pistofidis GA, Brucker SY, Krämer B, Ziegler NM, Korell M, et al. A first-in-human, randomized, controlled, subject- and reviewer-blinded multicenter study of actamaxTM adhesion barrier. Arch Gynecol Obstet. 2017 Feb;295(2):383–95. 10.1007/s00404-016-4211-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lundorff P, Donnez J, Korell M, Audebert AJM, Block K, diZerega GS. Clinical evaluation of a viscoelastic gel for reduction of adhesions following gynaecological surgery by laparoscopy in Europe. Hum Reprod. 2005 Feb;20(2):514–20. 10.1093/humrep/deh651. [DOI] [PubMed] [Google Scholar]
- 42. Young P, Johns A, Templeman C, Witz C, Webster B, Ferland R, et al. Reduction of postoperative adhesions after laparoscopic gynecological surgery with Oxiplex/AP Gel: a pilot study. Fertil Steril. 2005 Nov;84(5):1450–6. 10.1016/j.fertnstert.2005.04.055. [DOI] [PubMed] [Google Scholar]
- 43. diZerega GS, Coad J, Donnez J. Clinical evaluation of endometriosis and differential response to surgical therapy with and without application of Oxiplex/AP* adhesion barrier gel. Fertil Steril. 2007 Mar;87(3):485–9. 10.1016/j.fertnstert.2006.07.1505. [DOI] [PubMed] [Google Scholar]
- 44. Diamond MP, Korell M, Martinez S, Kurman E, Kamar M; Adhexil Adhesion Study Group . A prospective, controlled, randomized, multicenter, exploratory pilot study evaluating the safety and potential trends in efficacy of Adhexil. Fertil Steril. 2011 Mar;95(3):1086–90. 10.1016/j.fertnstert.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 45. Krämer B, Andress J, Neis F, Hoffmann S, Brucker S, Kommoss S, et al. Adhesion prevention after endometriosis surgery - results of a randomized, controlled clinical trial with second-look laparoscopy. Langenbecks Arch Surg. 2021 Sep;406(6):2133–43. 10.1007/s00423-021-02193-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Diamond MP, Freeman ML. Clinical implications of postsurgical adhesions. Hum Reprod Update. 2001 Nov;7(6):567–76. 10.1093/humupd/7.6.567. [DOI] [PubMed] [Google Scholar]
- 47. Taskin O, Buhur A, Birincioglu M, Burak F, Atmaca R, Yilmaz I, et al. The effects of duration of CO2 insufflation and irrigation on peritoneal microcirculation assessed by free radical scavengers and total glutathion levels during operative laparoscopy. J Am Assoc Gynecol Laparosc. 1998 May;5(2):129–33. 10.1016/s1074-3804(98)80078-9. [DOI] [PubMed] [Google Scholar]
- 48. Brokelman WJA, Lensvelt M, Borel Rinkes IHM, Klinkenbijl JHG, Reijnen MMPJ. Peritoneal changes due to laparoscopic surgery. Surg Endosc. 2011 Jan;25(1):1–9. 10.1007/s00464-010-1139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Holmdahl L. The role of fibrinolysis in adhesion formation. Eur J Surg Suppl. 1997(577):24–31. [PubMed] [Google Scholar]
- 50. Rout UK, Oommen K, Diamond MP. Altered expressions of VEGF mRNA splice variants during progression of uterine-peritoneal adhesions in the rat. Am J Reprod Immunol. 2000 May;43(5):299–304. 10.1111/j.8755-8920.2000.430509.x. [DOI] [PubMed] [Google Scholar]
- 51. Lucas PA. Stem cells for mesothelial repair: an understudied modality. Int J Artif Organs. 2007 Jun;30(6):550–6. 10.1177/039139880703000613. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article, as no new data were created or analyzed in this study. Further inquiries can be directed to the corresponding author.