Abstract
Introduction
Usefulness of hysteroscopy before assisted reproductive technique (ART) was considered debatable. However, over the last decade, several new trials have been added to available literature. We aimed to assess the impact of diagnostic and operative hysteroscopy on reproductive outcomes of infertile women with and without intrauterine abnormalities.
Materials and Methods
MEDLINE, Scopus, SciELO, Embase, Cochrane Library at CENTRAL, PROSPERO, CINAHL, grey literature, conference proceedings, and international controlled trials registries were searched without temporal, geographical, or language restrictions. Randomized controlled trials (RCTs) of infertile women comparing hysteroscopy versus no hysteroscopy prior to the first ART or after at least one failed attempt were included. RCTs of infertile women with intrauterine pathology comparing diagnostic versus operative hysteroscopy were included in separate analysis. Random-effect meta-analysis was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Grading of Recommendations, Assessment, Development and Evaluation and Cochrane criteria were used for quality of evidence and risk of bias assessment. Primary outcome was live birth rate (LBR). Secondary outcomes were clinical pregnancy (CPR) and pregnancy loss rate.
Results
Fifteen studies (5,038 women) were included. Compared to no hysteroscopy before first or after failed ART attempts, moderate-quality evidence showed that hysteroscopy increased the LBR (relative risk [RR] 1.24, 95% confidence interval [CI] 1.09–1.43, I2 = 21%), confirmed by subgroup analysis for women with failure after one or more ART cycles (RR 1.43, 95% CI: 1.19–1.72, I2 = 0%) but not before the first ART. Moderate-quality evidence showed that it increased the CPR (RR 1.36, 95% CI: 1.18–1.57; I2 = 51%), confirmed in subgroup analysis for both implantation failure (RR 1.40, 95% CI: 1.12–1.74, I2 = 52%) and before first ART (RR 1.32, 95% CI: 1.11–1.57, I2 = 42%). Low-quality data suggest that operative hysteroscopy increases CPR when used to treat intrauterine pathologies (RR 2.13, 95% CI: 1.56–2.92, I2 = 0%).
Conclusions
Although moderate-quality evidence supports performing hysteroscopy before ART in women with history of implantation failure, hysteroscopic evaluation of uterine cavity should be considered a first-line technique in all infertile women undergoing ART. Additional high-quality RCTs are still needed, particularly to assess yield during couple’s initial evaluation even before ART is considered.
Keywords: Hysteroscopy, In vitro fertilization, Reproductive outcomes, Pregnancy rates
Introduction
Pregnancy rates in women who undergo assisted reproductive technique (ART) are known to be detrimentally affected by intrauterine pathology [1]. It is considered standard of care to evaluate the uterine cavity before initiating ART, to maximize pregnancy rates and minimize failures, given the expense, effort, and emotions invested in obtaining embryos [2]. The standard of care for the assessment of uterine cavity in women seeking pregnancy is changing according to technological advancements. Hysterosalpingography (HSG) used to be utilized to assess uterine anatomy, but due to its low accuracy, with a high rate of false-positive and false-negative results, while not yet outside the standard of care, it is gradually falling out of favor [3]. Regarding the evaluation of the myometrium, magnetic resonance imaging and 3D transvaginal sonography are considered the best imaging modalities for identifying fibroids, adenomyosis, and Müllerian abnormalities [4–6]. However, hysteroscopy is considered the gold standard for the evaluation of intracavitary pathology, where it is superior to sonography in cases of intrauterine adhesions (particularly when lateral), cornual and lower uterine segment pathology, sessile polyps, endometritis, and retained products of conception [2].
Uterine abnormalities are one of the top reasons for recurrent implantation failure, making the hysteroscopic assessment of the uterine cavity increasingly common [7]. Hysteroscopy not only allows reliable visual assessment of the cervical canal and uterine cavity but can also be used for the immediate management of intrauterine pathology when present, “see and treat” [1, 8, 9].
Although the real value of hysteroscopy as part of procreative testing remains subject of debate [10], recent research suggests that hysteroscopic assessment prior to starting ART treatment may improve reproductive outcomes [11]. This is likely secondary to the frequency of intrauterine abnormalities in asymptomatic patients undergoing ART; up to 20–50%, depending on age, risk factors, and a history of recurrent implantation failure [11–14].
Despite several studies demonstrating multiple benefits with the use of hysteroscopy, the National Institute of Health and Care Excellence (NICE) recommendations for its role in fertility evaluation do not support its use [15]. However, this is derived from two randomized controlled trials (RCTs) with important methodological limitations. In neither study did the authors find hysteroscopy to increase fecundity rate; however, it is accepted that the absence of a benefit when the study design is heavily biased toward the null hypothesis does not necessarily mean that the intervention is truly ineffective. The limitations biasing these studies toward the null hypothesis and potentially affecting estimates of yield through hysteroscopic screening include inherently low pregnancy rates, having a partially pretreated population (hysteroscopy prior to enrollment), not intervening when pathology is identified by hysteroscopy, and power limitations given the inherent multifactorial nature of infertility, particularly when embryos of unknown euploidy are used. Additionally, hysteroscopy cost-effectiveness calculations are largely based on operating room evaluation, when modern in-office hysteroscopy can often be performed with low costs that are comparable to saline infusion sonohysterography. Therefore, although limited by the inherent retrospective design for most available studies, laparoscopic and hysteroscopic improvement of fertility chances seems promising [15, 16].
In 2016, Di Spiezio Sardo et al. [17] showed that there is not enough evidence to support the use of hysteroscopy as a primary method for evaluating the uterine cavity of infertile patients. They also reported very low-quality evidence that raises questions about whether hysteroscopy performed prior to ART, regardless of intrauterine abnormalities, improves live birth rate (LBR). Additionally, there was moderate-quality evidence to support the claim that hysteroscopy increases pregnancy rates prior to ART [17]. The authors concluded that more reliable and high-quality RCTs are still needed before hysteroscopy can be considered a first-line treatment for all infertile women, particularly during the first clinical evaluation of a couple, when it may speed up conception and decrease the need for ART [17].
Almost a decade after that publication, the role of hysteroscopy before ART remains debated, emphasizing the need for these high-quality studies [1, 17]. In this context, several new trials have been performed, enlarging the available pool of evidence. Considering the new available data, an updated systematic review and meta-analysis are needed to clarify the impact of hysteroscopic evaluation of the uterine cavity in infertile women. The aim of this systematic review and meta-analysis was to assess whether diagnostic and/or operative hysteroscopy for the evaluation of the uterine cavity before ART could enhance reproductive outcomes in infertile couples compared to alternative methods of uterine cavity evaluation.
Methods
The protocol for this meta-analysis was designed a priori and followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [18]. In-depth consideration was given to the literature search, reporting, article inclusion and exclusion, analysis, data extraction, and statistical analysis in the study protocol that was developed in advance. This study was registered in the PROSPERO database, which houses the International Prospective Registry of Systematic Reviews (CRD42023363344). To accomplish this, we synthesized data from all RCTs evaluating the effects of hysteroscopy (diagnostic and potentially operative) on reproductive outcomes in infertile women without suspected intracavitary pathology (at any stage of the diagnostic workup but prior to the first attempt of standard ART or after one or more failed ART attempts), as well as second attempt of open-label ART.
Search Methods for the Identification of Studies
The following keywords and Medical Subject Heading (MeSH) phrases were used to search a total of eight electronic databases, including MEDLINE (accessible through PubMed), CINAHL, Scopus, Embase, SciELO.br, and PROSPERO: (infertility* and hysteroscopy*, and “pregnancy rate”), or (“Infertility, Female” (Mesh) and (“Hysteroscopy” (Mesh) and “Pregnancy Rate” (Mesh)). These terms for the strategy were modified according to each database search.
We also looked through CINAHL, PsycINFO, and AMED to uncover more relevant articles and reduce publication bias. Further information was also gathered by searching Clinicaltrials.gov, the Cochrane Central Register of Controlled Trials, and the WHO International Clinical Trials Registry Platform (ICTRP). Additionally, a search for conference abstracts from both local and international conferences in the grey literature (NTIS, PsycEXTRA) was performed. The reference lists of all pertinent publications and articles that qualified for inclusion were examined to screen further for studies that were overlooked by automated searches.
There were no restrictions based on geographic area or language. The search did not include editorials, letters to the editor, reviews, or commentary. We included RCTs according to the following populations, interventions, controls and outcomes (PICOs) protocol.
Populations
Infertile women, with or without uterine cavity abnormalities, diagnosed with ultrasonography, HSG, or other imaging modality, who were enrolled during their initial infertility workup before being considered candidates for any ART, or who had undergone one or more unsuccessful ART attempts.
Interventions
The following interventions were separately analyzed: hysteroscopy performed at their initial infertility assessment, before the first ART cycle, or following unsuccessful ART attempts before subsequent interventions.
Controls
Initial infertility evaluation without hysteroscopy, or ART attempts without preceding diagnostic or operative hysteroscopy.
Outcomes
The primary outcome was the LBR, defined as the percentage of live births that occur after completing 20 weeks of gestational age. Single live birth, twin births, or births from a high-order pregnancy were all considered to be one live birth. Secondary outcomes included the clinical pregnancy rate (CPR), which is the proportion of pregnancies that are confirmed by ultrasound visualization of one or more intrauterine gestational sacs or by objective clinical signs of pregnancy; the pregnancy loss rate (PLR), defined as the proportion of clinical pregnancies that spontaneously end before 20 complete weeks of gestation.
This meta-analysis was designed with very specific criteria to be used when abstracting data. Patient descriptions, study duration, setting, hysteroscopic surgery data, infertile cohort, reproductive characteristics, outcomes evaluation, mean follow-up time, findings, and quality elements were among the most important factors documented.
Two authors (G.R. and S.G.V.) independently screened, evaluated, and categorized each abstract. The two writers performed the full-text evaluation of the qualifying studies and separately collected important data regarding the study’s relevant features and outcomes to reach an agreement. In case of disagreement, a third author (J.P.P.) was consulted for resolution. Whenever required, additional unpublished data were acquired through direct communication with the authors of the original studies when greater clarity was needed for the interpretation of the data.
Assessment of Risk of Bias in Included Studies
Two authors (A.D.S.S. and P.D.F.) independently evaluated the risk of bias for RCTs in this review using the Cochrane Handbook’s recommended criteria [19]. The Cochrane Review suggests using a two-part tool to address seven distinct biases, including sequence generation and allocation concealment (selection bias), blinding of participants and providers (performance bias), blinding of outcome assessors (detection bias), incomplete outcome data (attrition bias), selective outcome reporting (reporting bias), and other sources of bias. The events that were alleged to have happened during the research are described in the tool’s first part. The second component of the application allows users to rate each entry’s bias risk as low, high, or unclear.
Grading of Evidence
The Grading of Recommendations, Assessment, Development and Evaluation (GRADE) system (GRADEpro GDT: GRADEpro Guideline Development Tool, McMaster University, 2015; Evidence Prime, Inc. [software]), which grades recommendations, assessments, development, and evaluations was used to assess the overall caliber of the evidence for the main outcome. The directness of the results (i.e., the similarity between the population, the intervention, or the outcomes evaluated in the studies actually identified and those under consideration) are just one example of a validity-related problem that is taken into account by this method.
Data Synthesis and Subgroup Analysis
In order to analyze dichotomous outcomes, the relative risk (RR) for each trial was calculated, and the confidence interval (CI) for each result was used to represent its level of uncertainty. By computing the mean differences or standard mean differences with 95% CI, the continuous outcomes were analyzed.
The funnel plot’s symmetry was examined in order to determine any potential publication bias. Nonetheless, the Egger’s test was utilized to measure the publication bias due to discrepancies among the observation of the same funnel plot by the authors. Two main comparisons were made for each outcome of the meta-analysis.
Comparison 1: infertile women without suspected abnormality of the uterine cavity detected by sonography, HSG, or any other imaging modality were included in the first comparison to determine the impact of hysteroscopy performed before ART on the LBR and CPR. The following subgroup analyses were carried out for this comparison: any step of the infertility evaluation, including IUI, but before the initial in vitro fertilization/intrauterine cytoplasmic sperm injection (IVF/ICSI) attempt or after failed IVF/ICSI.
Comparison 2: women diagnosed with intrauterine cavity abnormalities were evaluated to determine the impact of operative hysteroscopy compared to diagnostic hysteroscopy on LBR and CPR. Statistical analysis was performed using Review Manager Version 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen).
Results
Study Selection
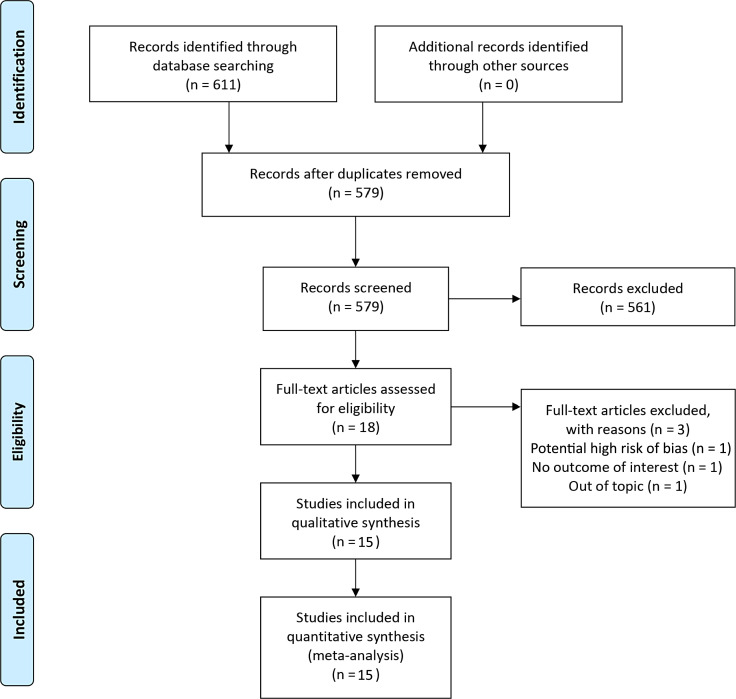
A total of 611 studies were identified through the initial database searches. Of those, 32 were removed as duplicates. After title and abstract screening for applicability, 561 studies were subsequently removed (Fig. 1). Eighteen studies were assessed for full text, of which 2 were removed for being out of topic or not providing the outcome of interest. Sixteen studies were included in qualitative synthesis [11–13, 20–32]. Subsequently, one study was removed due to multiple retractions for the first author of the research [29] (Fig. 1).
Fig. 1.
Flowchart of studies included in systematic review and meta-analysis.
Four trials were conducted in Egypt, three in Iran, two in Turkey, and one in each of the following countries, Spain, Italy, India, Denmark, Tunisia, and the Netherlands. One was a multicenter study conducted in seven hospitals in the UK, Italy, Belgium, and the Czech Republic. Table 1 shows a detailed description of the included trials.
Table 1.
Main characteristics of studies included in quantitative synthesis and meta-analysis
| Author, year | Country | Population | Intervention | Controls | Outcomes |
|---|---|---|---|---|---|
| Aghahosseini et al. [21] (2012) | Iran | Women undergoing IVF who have had two or more implantation failures with unsuspected or no uterine cavity abnormalities, normal HSG, and no history of hysteroscopy in the last 2 months | Hysteroscopy prior to a subsequent IVF attempt | Immediate IVF without prior hysteroscopy | CPR |
| LBR | |||||
| Casini et al. [20] (2006) | Italy | Women affected by uterine fibroids | Hysteroscopic surgery to remove the fibroids | No treatment. Patients were suggested to immediately start having regular fertility-oriented coitus | CPR |
| PLR | |||||
| Demirol et al. [22] (2004) | Turkey | Women with primary infertility and two or more failed IVF cycles with no known intracavitary disease, a normal HSG, and controlled ovarian stimulation for IVF abnormalities | Office hysteroscopic evaluation of the uterine cavity and cervix before commencing treatment | No office hysteroscopic evaluation of the uterine cavity and cervix before commencing controlled ovarian stimulation for IVF treatment | CPR |
| PLR | |||||
| Elsetohy et al. [11] (2015) | Egypt | Women scheduled for first IVF treatment cycle with no known abnormality, apart from intramural myomas without uterine cavity deformity | Hysteroscopic examination in the early-mid-follicular phase of a menstrual cycle. If any intrauterine abnormality was detected, therapeutic hysteroscopy was performed in the same hysteroscopy session or scheduled for an operative procedure later. Subsequent ICSI | IVF without hysteroscopy | CPR |
| El-Nashar. [23] (2011) | Egypt | Women with unexplained infertility after carrying out initial investigations for her and her partner, scheduled to start their first IVF cycle | Hysteroscopy with directed biopsy and correction of any intrauterine abnormalities Exact timing of hysteroscopy before ICSI is not specified | ICSI cycle without undergoing a hysteroscopy | CPR |
| El-Toukhy et al. [24] (2016) | UK, Italy, Belgium, Czech Rep | Infertile women younger than 38, planning IVF | Outpatient hysteroscopy without sedation before starting IVF | IVF without prior hysteroscopy | CPR |
| LBR | |||||
| Perez-Medina et al. [25] (2005) | Spain | Infertile women with at least 24 months of infertility, with a sonographic diagnosis of endometrial polyps and planning IUI | Hysteroscopic polypectomy Women were scheduled to receive four cycles of IUI; the first IUI was planned for three cycles after hysteroscopy | Endometrial polyps were not extracted during diagnostic hysteroscopy and polyp biopsy was performed | CPR |
| Rama Raju et al. [12] (2006) | India | Patients with two or more failed IVF cycles with primary infertility or male factor infertility with no known uterine cavity abnormalities | Office hysteroscopy prior to a subsequent IVF attempt | Immediate IVF without prior hysteroscopy | CPR |
| LBR | |||||
| Shawki et al. [13] (2012) | Egypt | Women suffering from primary infertility, candidate for first IVF or with one or more failed IVF. | IVF after performing office hysteroscopy. Abnormal findings were recorded and treated | IVF without office hysteroscopy | CPR |
| LBR | |||||
| Ghasemi et al. [26] (2022) | Iran | Patients with primary infertility without prior hysteroscopic examination or previous IVF, normal transvaginal sonography in the last month, and a normal HSG (in the past 6–24 months), who were scheduled for the first IVF cycle | Hysteroscopy and irrigation of uterine cavity with a large amount of saline solution (200–300 mL) followed by IVF | IVF without office hysteroscopy | CPR |
| LBR | |||||
| Ben Abid et al. [27] (2021) | Tunisia | Infertile women planning their first IVF cycle. All patients had a normal uterine cavity based on transvaginal sonography and HSG | Diagnostic hysteroscopy followed by IVF | IVF without hysteroscopy | CPR |
| LBR | |||||
| PLR | |||||
| Gurgan et al. [28] (2019) | Turkey | Women <40 years of age with FSH ≤15 IU/mL who met the recurrent implantation failure definition | Endometrial scoring through office hysteroscopy followed by IVF | IVF without hysteroscopy | CPR |
| LBR | |||||
| PLR | |||||
| Smit et al. [30] (2016) | The Netherlands | Infertile women scheduled for IVF with a normal transvaginal ultrasound | Diagnostic hysteroscopy with see and treat | Immediate IVF without hysteroscopy | LBR |
| CPR | |||||
| PLR | |||||
| Moramezi et al. [31] (2012) | Iran | Women planning IUI cycles | Office hysteroscopy | No hysteroscopy | CPR |
| PLR | |||||
| Berntsen et al. [32] (2020) | Denmark | Women planning IVF; one or more previously failed IVF cycle(s) | Endometrial scratching performed by office hysteroscopy in the cycle preceding the IVF/ICSI cycle | Standard fertility treatment in the clinic without office hysteroscopy | CPR |
| LBR |
Types of Patients and Interventions Comparison
Women who had been attempting to spontaneously conceive for at least 1 year without success and were diagnosed with uterine fibroids or unexplained infertility with no previous ART attempt were included in one study [20]. In contrast to the control group, patients in the experimental group underwent hysteroscopic myomectomy. Then both groups were instructed to have timed intercourse for conception. Candidates for their first IUI and infertile women with endometrial polyps were both included in one study [25]. The authors compared women undergoing polyp biopsy and diagnostic hysteroscopy only with patients undergoing hysteroscopic polypectomy. Women who were candidates for their first IVF attempt were included in seven trials [11, 23, 26, 27, 29–31]. Five studies included infertile women with one or more failed IVF cycles and unsuspected or no uterine cavity abnormalities [12, 21, 22, 24, 32]. One study [13] included women who were candidates for their first IVF and women with one or more failed IVF cycles (see Table 1).
In these trials, women who had hysteroscopy with treatment of intrauterine abnormalities, when found, were compared to controls who began their IVF cycles without having hysteroscopy. All studies included used the same definition of failed IVF cycle, which is the lack of implantation (determined by a negative serum HCG test 14 days following oocyte retrieval). Two studies [31, 32] performed endometrial scratching at the time of hysteroscopy, with controls that did not undergo hysteroscopy.
Risk of Bias
The overall risk of bias was considered low in the majority of the included RCTs (online suppl. Fig. S1a–b; for all online suppl. material, see https://doi.org/10.1159/000534794). Due to multiple retractions and expression of concerns for the data acquisition, one paper was considered with overall high risk of bias and was excluded from quantitative synthesis and meta-analysis [29]. Due to an unsatisfactory approach to random sequence generation, three studies [21, 23, 31] exhibited a significant risk of selection bias. As the randomization procedure was not disclosed, the same three trials were determined to have an uncertain risk of bias (online suppl. Fig. S1a-b). One study [20] was deemed to have a significant risk of intervention bias because patients who did not undergo hysteroscopy were told to immediately begin having fertility-focused intercourse, whereas those who had identified pathology did not attempt conception until postoperatively, shortening the relative window for attempted conception.
Because other papers provided insufficient information regarding how similarly other treatments were administered, the other trials were classified as having an uncertain risk of performance bias. All of the included studies were deemed to have a low risk of detection bias since the results were unbiased and unlikely to be influenced by a lack of blinding, despite the fact that none of the studies disclosed whether the outcome assessors were blinded.
There were none or very few dropouts reported in the included studies, the groups were balanced, and the reasons for dropouts were always disclosed. All studies, except three [21, 23, 31], were deemed to be at low risk of attrition bias. Because no data were provided for several outcomes, Moramezi et al., Aghahosseini et al., and El-Nashar and Nasr [21, 23, 31] were classified as having an unclear risk of attrition bias (online suppl. Fig. S1a-b). For the primary outcome of this meta-analysis, neither the funnel plot analysis (online suppl. Fig. S2), nor the Egger test (p = 0.872) detected any publication bias.
Effect of Intervention
Live Birth Rate
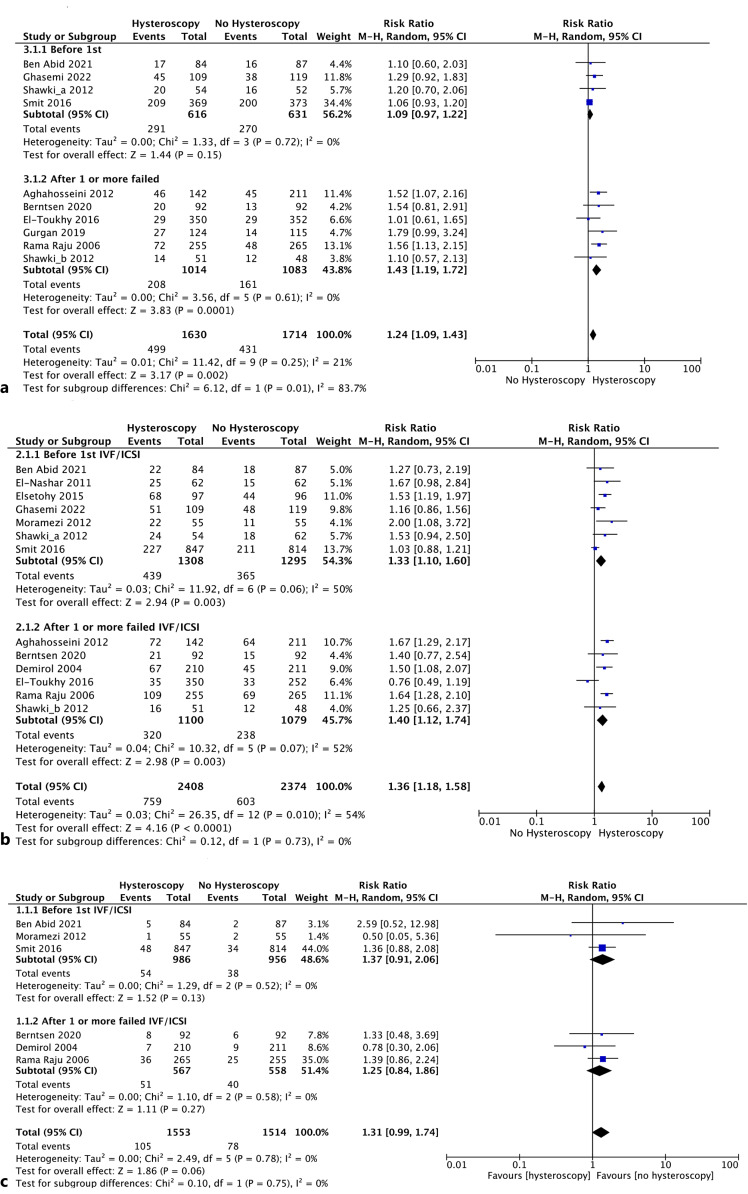
Comparison 1: nine studies [12, 13, 21, 24, 26–28, 30, 32], including 3,344 women, evaluated the LBR between the hysteroscopy versus no-hysteroscopy groups in the infertility workup. Overall, we found a significantly higher LBR in women undergoing hysteroscopy (RR 1.24; 95% CI: 1.09–1.43, I2 = 21%) (Fig. 2a). One study presented different findings for women who had experienced prior implantation failure and those who were conducting their first ART cycle (Fig. 2a–b). [13]. In the subgroup analysis, 4 studies (1,247 women) analyzed the role of hysteroscopy before the first ART attempt, showing a trend, but not a statistically significant difference, in the LBR of patients undergoing hysteroscopic assessment (RR 1.09; 95% CI: 0.97–1.22; I2 = 0%).
Fig. 2.
a Forest plot for LBR and related subgroup analysis (hysteroscopy vs. no hysteroscopy before IVF). b Forest plot for CPR and related subgroup analysis (hysteroscopy vs. no hysteroscopy before IVF). c Forest plot PLR and related subgroup analysis (hysteroscopy vs. no hysteroscopy before IVF).
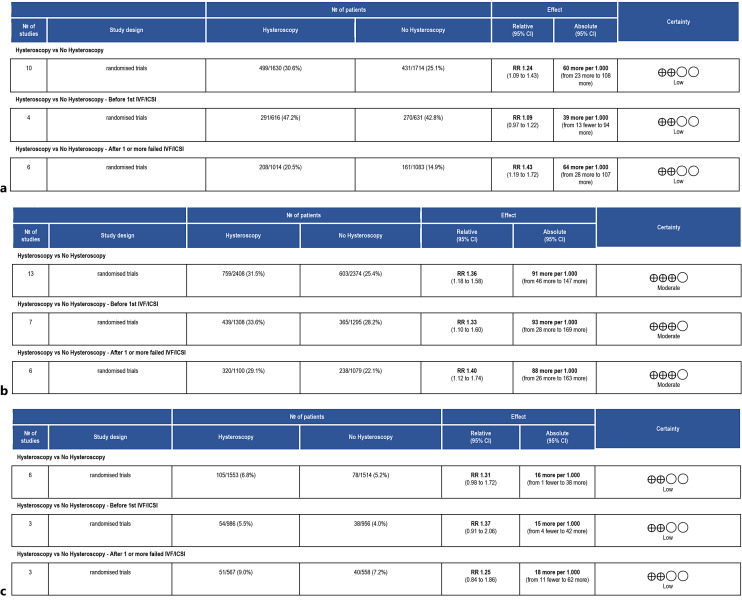
In contrast, we discovered a higher LBR in the hysteroscopy group compared to no hysteroscopy for women who had one or more implantation failures during ART (RR 1.43, 95% CI: 1.19–1.72; I2 = 0%) (Fig. 2a). The quality of evidence was rated as low (Fig. 3a). Comparison 2: none of the studies that were included evaluated this result.
Fig. 3.
Summary of the main findings for the LBR (a), for the CPR (b), for the PLR and ascertainment of the quality of the evidence according to GRADE (hysteroscopy vs. no hysteroscopy before ART) (c). CI, confidence interval; RR, risk ratio.
Clinical Pregnancy Rate
Comparison 1: twelve RCTs with 4,782 women were included [11–13, 21–24, 26, 27, 30–32]. The overall results showed improved CPRs with hysteroscopy (RR 1.36, 95% CI: 1.18–1.58, I2 = 54%). The seven trials with 2,603 individuals that performed hysteroscopy prior to the initial ART attempt confirmed these findings (RR 1.33, 95% CI: 1.10–1.60, I2 = 50%). Similar results were found in the subgroup analysis of six papers with 2,179 participants of patients with implantation failure after ART (RR 1.40, 95% CI: 1.12–1.74, I2 = 52%) (Fig. 2b). The quality of evidence was judged as moderate (Fig. 3b).
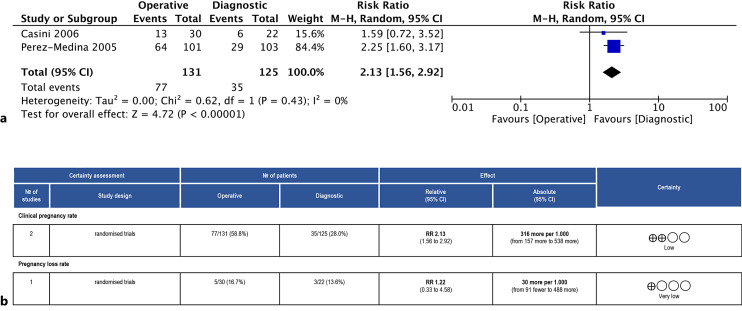
Comparison 2: there were two trials including a total of 256 individuals [20, 25]. Overall, operative hysteroscopy was preferred (RR 2.13, 95% CI: 1.56–2.92, I2 = 0%, Fig. 4a). The quality of evidence was rated as low (Fig. 4b).
Fig. 4.
a Forest plot for CPR (operative vs. diagnostic hysteroscopy). b Summary of the main findings for the CPR and PLR and ascertainment of the quality of the evidence according to GRADE (operative vs. diagnostic hysteroscopy). CI, confidence interval; RR, risk ratio.
Pregnancy Loss Rate
Comparison 1: this outcome was assessed by six trials including 3,067 participants [12, 22, 27, 30–32]. In terms of rates of pregnancy loss, the overall findings did not show a meaningful difference between women who underwent hysteroscopy and those who had no hysteroscopy (RR 1.31; 95% CI: 0.99–1.74, I2 = 0%) (Fig. 2c). The same results were confirmed in the subgroup analysis among patients undergoing hysteroscopy before ART (RR 1.37; 95% CI: 0.91–2.06; I2 = 0%) and in women with two or more failed ART (RR 1.25; 95% CI: 0.84–1.86; I2 = 0%). The quality of evidence was judged as moderate (Fig. 3c).
Comparison 2: there was only one study [20] with 52 individuals. Between the two groups, there was no discernible change in PLR (RR 1.22, 95% CI: 0.33–4.58). The quality of the evidence was deemed to be very low (Fig. 4b).
Discussion
The current systematic review and meta-analysis of RCTs demonstrate that, despite disagreement over the benefit of hysteroscopy in the initial assessment of patients with infertility, there is low-quality evidence showing an increased LBR when hysteroscopy is performed during the initial infertility workup. However, hysteroscopy did not increase the LBRs when performed prior to the first ART cycle, regardless of the presence of intrauterine abnormalities. However, hysteroscopy clearly improves LBR in patients with a history of at least one failed ART cycle. Given that up to two-thirds of initial transfers (depending on patient prognosis and use of preimplantation genetic testing for aneuploidy) will not necessarily implant, this means that a high percentage of patients undergoing IVF are likely to benefit from hysteroscopic evaluation. Moreover, we could argue that the cost of performing a diagnostic in-office hysteroscopic procedure is negligible when considering the financial and the noneconomic devastating consequences of having an unsuccessful embryo transfer.
We also found moderate evidence showing that hysteroscopy improves pregnancy rates before the first and after multiple ART cycles. When submucosal fibroids or endometrial polyps were removed with hysteroscopy, we found low-quality evidence suggesting that operative hysteroscopy would enhance the pregnancy rate. However, no RCTs looking at LBR outcomes of women with submucosal fibroids or endometrial polyps were identified.
Low-quality evidence did not show early PLRs to be improved by performing hysteroscopy in infertile women. However, the CIs for this outcome were wide due to the small sample size. Moreover, although without statistically significant difference, the interval confidence limits barely overlapped with 1 (RR 1.30, 95% CI: 0.98–1.72). This suggests that if additional studies are performed obtaining similar results, the narrowing of the interval confidence would tip this over to where hysteroscopy improves PLRs in a statistically significant manner. Additionally, future studies focusing on outcomes after the transfer of euploid embryos are likely to further amplify the effect of hysteroscopy since reducing aneuploidy-associated pregnancy loss would result in uterine factor sources being a proportionately greater contributor to miscarriage.
Although the quality and quantity of evidence could still be improved, evolving research shows that hysteroscopic evaluation of infertile women can boost reproductive outcomes. However, the mechanism for this improvement remains unknown [1]. Accurate intrauterine evaluation is essential before initiating ART, and hysteroscopy has advantages over other imaging modalities, particularly when it comes to intrauterine adhesions, chronic endometritis, retained products of conception (which can be difficult to visualize on sonography), sessile polyps, and cornual disease.
There have been other proposed advantages to hysteroscopy, such as potentially increasing endometrial receptivity through endometrial scratching (a minor damage of the endometrial layer caused by mechanical traction) [33]. However, recent meta-analyses and well-designed research do not show procreative advantages to endometrial scratching. Equally importantly, a recent RCT suggested this may even worsen fecundity [34].
There is speculation about the potential advantage of performing a diagnostic hysteroscopy with copious irrigation of the uterine cavity before ART [26]. First, irrigation could physically remove damaging anti-adhesive glycoproteins from the surface of the endometrium which enhance endometrial receptivity. Also, some authors argue that uterine flushing may improve fertilization by cleaning out debris from the fallopian tubes and by modifying the release of cytokines [14, 26].
Another explanation stresses the use of hysteroscopy as a diagnostic method in and of itself; by passing the tip of the hysteroscope through the cervical canal, cervical adhesions may be released, and information on the path of the cervical canal can be gathered. Expanding the cervical lumen and overcoming stenosis may facilitate future embryo transfer [11, 26].
Another theory supporting intracavitary assessment as simultaneously being diagnostic and therapeutic is that performing hysteroscopy may trigger alterations in the immune system and gene expression that might enhance endometrial receptivity and implantation [35]. Inagaki et al. [35] demonstrated that the degree of matrix metalloproteinases activity and cytokine concentrations had a distinct pattern in the lavage of patients with repeated implantation failure. Theoretically, irrigation of immunity-related components and cytokines could aid in reversing this pattern [35].
Other than speculated benefits, according to a recent Cochrane analysis published in 2019 [10], there is currently insufficient high-quality evidence to support the routine use of hysteroscopy as a screening tool in the general population of infertile women with a normal HSG or ultrasound in the initial fertility workup. The same Cochrane publication stated that performing a diagnostic screening hysteroscopy before IVF may boost the live birth and CPRs of women undergoing IVF [10]. The inclusion in our systematic review and meta-analysis of more trials than those included in the Cochrane’s meta-analysis (16 vs. 11 RCTs) led to an improvement in the overall quality of the available data, which merits producing our report even if our pooled results concur with the Cochrane’s conclusions.
The routine use of hysteroscopy as a screening technique for enhancing reproductive outcomes of subfertile women with a normal uterine cavity on ultrasonography or HSG in the initial fertility workup is currently not supported by high-quality research. However, this standard is likely to evolve, particularly as the affordability and feasibility of performing in-office hysteroscopy improve. Concurrently, regarding other alternatives for the evaluation of the uterine cavity, the limited accuracy of HSG for the evaluation of the uterine cavity is well recognized, which makes this classic approach likely to be abandoned in the near future. Similarly, hysteroscopic endometrial biopsy performed in the outpatient setting using miniaturized grasping forceps is considered a pivotal tool for diagnosing chronic endometritis and endometrial subtle lesions that cannot be recognized with conventional ultrasound evaluation [36, 37]. This evidence is growing together with the technological breakthroughs in the field of outpatient hysteroscopy. Such innovations could be considered a paradigm-shift reproductive surgery, increasing efficacy and feasibility with reducing invasiveness [1, 38].
While the study populations included in our research varied in many parameters (such as key clinical features, causes for infertility, timing of hysteroscopy, IVF protocol), all studies shared a common trait (the presence or absence of an intrauterine factor). The clinical variability of the studied populations limited our work given its lack of methodological rigor, but the fact that infertile women in our study are similar to those seen in clinical practice suggests that our findings are generalizable. Moreover, infertile couples frequently exhibit various causes of infertility, and numerous confounders at every center typically influence how well the diagnostic and therapeutic workup is performed. Subgroup analysis was effective in reducing the heterogeneity for each evaluated outcome, reducing the intrinsic differences relative to the population, and increasing the robustness of the findings.
We are conscious that combining research on polyps and fibroids in our second comparison analysis might reduce the validity of those findings. Further, independent of any statistical heterogeneity, combining trials with various participants or interventions may impact the clinical plausibility of the findings. Though fibroids and polyps have different pathogenic pathways that lead to infertility, both disorders may be treated with hysteroscopic resection, which is in line with the rationale provided in the previous meta-analysis. The latter information is adequate to answer our review’s question of whether conducting an operative or diagnostic hysteroscopy on infertile women increases the live birth and/or conception rate. Second, larger endometrial polyps and submucosal myomas should be taken into account as possible causes of subfertility.
In summary, evidence regarding the role of hysteroscopy in boosting fertility in women undergoing ART is evolving through new trials. The evidence shows that diagnostic hysteroscopy substantially improves LBR of patients with at least one failed implantation after embryo transfer. When evaluating CPR instead of LBR, there is moderate-quality evidence showing that performing hysteroscopy before ART improves outcomes, even of women without a history of unsuccessful implantation. Although additional studies are needed to determine if hysteroscopic resection of submucosal fibroids and endometrial polyps has an impact on fertility outcomes, we found low-quality evidence showing that the hysteroscopic removal of fibroids and endometrial polyps improves pregnancy rates.
Statement of Ethics
An ethics statement is not applicable because this study is based exclusively on published literature. A consent to participate statement is not applicable because this study is based exclusively on published literature.
Conflict of Interest Statement
The authors declare that they have no conflicts of interest to disclose regarding this publication.
Funding Sources
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author Contributions
S.G.V., A.D.S.S., and G.R. designed the study and reviewed the manuscript; S.G.V. and G.R. searched the literature, extracted data, and revised the manuscript; P.D.F., S.H., and L.A.P. performed statistical analyses; S.H., T.P.M., N.M., P.D.F., and J.C. critically revised the manuscript; and J.P.P., S.A., G.R., S.G.V., and AD.S.S. interpreted data and drafted the manuscript. All authors read and approved the final manuscript.
Funding Statement
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability Statement
All data generated or analyzed during this study are included in this article and its supplementary material. Additional inquiries can be directed to the corresponding author. This work is currently not being submitted to any other journal for consideration for publication and has not been previously presented in any form.
Supplementary Material
References
- 1. Riemma G, Vitale SG, Manchanda R, Rathore A, Török P, De Angelis C, et al. The role of hysteroscopy in reproductive surgery: today and tomorrow. J Gynecol Obstet Hum Reprod. 2022;51(4):102350. [DOI] [PubMed] [Google Scholar]
- 2. Della Corte L, Vitale SG, Foreste V, Riemma G, Ferrari F, Noventa M, et al. Novel diagnostic approaches to intrauterine neoplasm in fertile age: sonography and hysteroscopy. Minim Invasive Ther Allied Technol. 2021;30(5):288–95. [DOI] [PubMed] [Google Scholar]
- 3. Calles-Sastre L, Engels-Calvo V, Ríos-Vallejo M, Serrano-González L, García-Espantaleón M, Royuela A, et al. Prospective study of concordance between hysterosalpingo-contrast sonography and hysteroscopy for evaluation of the uterine cavity in patients undergoing infertility studies. J Ultrasound Med. 2018;37(6):1431–7. [DOI] [PubMed] [Google Scholar]
- 4. van Rijswijk J, van Welie N, Dreyer K, van Hooff MHA, de Bruin JP, Verhoeve HR, et al. The FOAM study: is Hysterosalpingo foam sonography (HyFoSy) a cost-effective alternative for hysterosalpingography (HSG) in assessing tubal patency in subfertile women? Study protocol for a randomized controlled trial. BMC Womens Health. 2018;18(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Martí-Bonmatí L. Hysterosalpingography and fertility: a technical relationship. Fertil Steril. 2018;110(4):642. [DOI] [PubMed] [Google Scholar]
- 6. Wadhwa L, Rani P, Bhatia P. Comparative prospective study of hysterosalpingography and hysteroscopy in infertile women. J Hum Reprod Sci. 2017;10(2):73–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. De Franciscis P, Riemma G, Schiattarella A, Cobellis L, Colacurci N, Vitale SG, et al. Impact of hysteroscopic metroplasty on reproductive outcomes of women with a dysmorphic uterus and recurrent miscarriages: a systematic review and meta-analysis. J Gynecol Obstet Hum Reprod. 2020;49(7):101763. [DOI] [PubMed] [Google Scholar]
- 8. Vitale SG, Bruni S, Chiofalo B, Riemma G, Lasmar RB. Updates in office hysteroscopy: a practical decalogue to perform a correct procedure. Updates Surg. 2020;72(4):967–76. [DOI] [PubMed] [Google Scholar]
- 9. Vitale SG, Carugno J, Riemma G, Török P, Cianci S, De Franciscis P, et al. Hysteroscopy for assessing fallopian tubal obstruction: a systematic review and diagnostic test accuracy meta-analysis. J Minim Invasive Gynecol. 2021;28(4):769–78. [DOI] [PubMed] [Google Scholar]
- 10. Bosteels J, van Wessel S, Weyers S, Broekmans FJ, D’Hooghe TM, Bongers MY, et al. Hysteroscopy for treating subfertility associated with suspected major uterine cavity abnormalities. Cochrane Database Syst Rev. 2018;12(12):CD009461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Elsetohy KA, Askalany AH, Hassan M, Dawood Z. Routine office hysteroscopy prior to ICSI vs. ICSI alone in patients with normal transvaginal ultrasound: a randomized controlled trial. Arch Gynecol Obstet. 2015;291(1):193–9. [DOI] [PubMed] [Google Scholar]
- 12. Rama Raju GA, Shashi Kumari G, Krishna KM, Prakash GJ, Madan K. Assessment of uterine cavity by hysteroscopy in assisted reproduction programme and its influence on pregnancy outcome. Arch Gynecol Obstet. 2006;274(3):160–4. [DOI] [PubMed] [Google Scholar]
- 13. Shawki HE, Elmorsy M, Eissa MK. Routine office hysteroscopy prior to ICSI and its impact on assisted reproduction program outcome: a randomized controlled trial. Middle East Fertil Soc J. 2012;17(1):14–21. [Google Scholar]
- 14. Kilic Y, Bastu E, Ergun B. Validity and efficacy of office hysteroscopy before in vitro fertilization treatment. Arch Gynecol Obstet. 2013;287(3):577–81. [DOI] [PubMed] [Google Scholar]
- 15. O’Flynn N. Assessment and treatment for people with fertility problems: NICE guideline. Br J Gen Pract. 2014;64(618):50–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Naem A, Laganà AS. Editorial: minimally invasive surgery as a mean of improving fertility: what do we know so far? Front Surg. 2023;10:1203816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Di Spiezio Sardo A, Di Carlo C, Minozzi S, Spinelli M, Pistotti V, Alviggi C, et al. Efficacy of hysteroscopy in improving reproductive outcomes of infertile couples: a systematic review and meta-analysis. Hum Reprod Update. 2016;22(4):479–96. [DOI] [PubMed] [Google Scholar]
- 18. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med. 2021;18(3):e1003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Higgins JPT, Cochrane Collaboration . Cochrane handbook for systematic reviews of interventions. 2 edn. Hoboken, NJ: Wiley-Blackwell; 2020. [Google Scholar]
- 20. Casini ML, Rossi F, Agostini R, Unfer V. Effects of the position of fibroids on fertility. Gynecol Endocrinol. 2006;22(2):106–9. [DOI] [PubMed] [Google Scholar]
- 21. Aghahosseini M, Ebrahimi N, Mahdavi A, Aleyasin A, Safdarian L, Sina S. Hysteroscopy prior to assisted reproductive technique in women with recurrent implantation failure improves pregnancy likelihood. Fertil Steril. 2012;98(3 Suppl):S4. https://doi.org/10.1016/j.fertnstert.2012.07.015. [Google Scholar]
- 22. Demirol A, Gurgan T. Effect of treatment of intrauterine pathologies with office hysteroscopy in patients with recurrent IVF failure. Reprod Biomed Online. 2004;8(5):590–4. [DOI] [PubMed] [Google Scholar]
- 23. El-nashar IH, Nasr A. The role of hysteroscopy before intracytoplasmic sperm injection (ICSI): a randomized controlled trial. Fertil Steril. 2011;96(3 Suppl):S266. [Google Scholar]
- 24. El-Toukhy T, Campo R, Khalaf Y, Tabanelli C, Gianaroli L, Gordts SS, et al. Hysteroscopy in recurrent in-vitro fertilisation failure (TROPHY): a multicentre, randomised controlled trial. Lancet. 2016;387(10038):2614–21. [DOI] [PubMed] [Google Scholar]
- 25. Pérez-Medina T, Bajo-Arenas J, Salazar F, Redondo T, Sanfrutos L, Alvarez P, et al. Endometrial polyps and their implication in the pregnancy rates of patients undergoing intrauterine insemination: a prospective, randomized study. Hum Reprod. 2005;20(6):1632–5. [DOI] [PubMed] [Google Scholar]
- 26. Ghasemi M, Aleyasin A, Fatemi HM, Ghaemdoust F, Shahrakipour M. Uterine cavity irrigation with office hysteroscopy during ovarian stimulation for IVF: a randomized controlled trial. Front Endocrinol. 2022;13:778988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ben Abid H, Fekih M, Fathallah K, Chachia S, Bibi M, Khairi H. Office hysteroscopy before first in vitro fertilization. A randomized controlled trial. J Gynecol Obstet Hum Reprod. 2021;50(7):102109. [DOI] [PubMed] [Google Scholar]
- 28. Gürgan T, Kalem Z, Kalem MN, Ruso H, Benkhalifa M, Makrigiannakis A. Systematic and standardized hysteroscopic endometrial injury for treatment of recurrent implantation failure. Reprod Biomed Online. 2019;39(3):477–83. [DOI] [PubMed] [Google Scholar]
- 29. Shokeir T, Ebrahim M, El-Mogy H. Hysteroscopic-guided local endometrial injury does not improve natural cycle pregnancy rate in women with unexplained infertility: randomized controlled trial. J Obstet Gynaecol Res. 2016;42(11):1553–7. [DOI] [PubMed] [Google Scholar]
- 30. Smit JG, Kasius JC, Eijkemans MJC, Koks CAM, van Golde R, Nap AW, et al. Hysteroscopy before in-vitro fertilisation (inSIGHT): a multicentre, randomised controlled trial. Lancet. 2016;387(10038):2622–9. [DOI] [PubMed] [Google Scholar]
- 31. Moramezi F, Barati M, Mohammadjafari R, Barati S, Hemadi M. Effect of hysteroscopy before intra uterine insemination on fertility in infertile couples. Pak J Biol Sci. 2012;15(19):942–6. [DOI] [PubMed] [Google Scholar]
- 32. Berntsen S, Hare KJ, Løssl K, Bogstad J, Palmø J, Prætorius L, et al. Endometrial scratch injury with office hysteroscopy before IVF/ICSI: a randomised controlled trial. Eur J Obstet Gynecol Reprod Biol. 2020;252:112–7. [DOI] [PubMed] [Google Scholar]
- 33. Palomba S, Vitagliano A, Marci R, Caserta D. Endometrial scratching for improving endometrial receptivity: a critical review of old and new clinical evidence. Reprod Sci. 2023;30(6):1701–11. [DOI] [PubMed] [Google Scholar]
- 34. Lensen S, Osavlyuk D, Armstrong S, Stadelmann C, Hennes A, Napier E, et al. A randomized trial of endometrial scratching before in vitro fertilization. N Engl J Med. 2019;380(4):325–34. [DOI] [PubMed] [Google Scholar]
- 35. Inagaki N, Stern C, McBain J, Lopata A, Kornman L, Wilkinson D. Analysis of intra-uterine cytokine concentration and matrix-metalloproteinase activity in women with recurrent failed embryo transfer. Hum Reprod. 2003;18(3):608–15. [DOI] [PubMed] [Google Scholar]
- 36. Vitale SG, Buzzaccarini G, Riemma G, Pacheco LA, Di Spiezio Sardo A, Carugno J, et al. Endometrial biopsy: indications, techniques and recommendations. An evidence-based guideline for clinical practice. J Gynecol Obstet Hum Reprod. 2023;52(6):102588. [DOI] [PubMed] [Google Scholar]
- 37. Vitale SG, Laganà AS, Caruso S, Garzon S, Vecchio GM, La Rosa VL, et al. Comparison of three biopsy forceps for hysteroscopic endometrial biopsy in postmenopausal patients (HYGREB-1): a multicenter, single-blind randomized clinical trial. Int J Gynaecol Obstet. 2021;155(3):425–32. [DOI] [PubMed] [Google Scholar]
- 38. Mahmud A, De Silva P, Smith P, Justin Clark T. Patient experiences of outpatient hysteroscopy. Eur J Obstet Gynecol Reprod Biol. 2023;288:142–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article and its supplementary material. Additional inquiries can be directed to the corresponding author. This work is currently not being submitted to any other journal for consideration for publication and has not been previously presented in any form.