Abstract
INTRODUCTION
Mechanical ventilation (MV) is a lifesaving procedure for critically ill patients. Diaphragm activation and stimulation may counteract side effects, such as ventilator-induced diaphragm dysfunction (VIDD). The effects of stimulation on diaphragm atrophy and patient outcomes are reported in this systematic review.
EVIDENCE ACQUISITION
Studies investigating diaphragmatic stimulation versus standard of care in critically ill patients and evaluating clinical outcomes were extracted from a Medline database last on January 23, 2023, after registration in Prospero (CRD42021259353). Selected studies included the investigation of diaphragmatic stimulation versus standard of care in critically ill patients, an evaluation of the clinical outcomes. These included muscle atrophy, VIDD, weaning failure, mortality, quality of life, ventilation time, diaphragmatic function, length of stay in the Intensive Care Unit (ICU), and length of hospital stay. All articles were independently evaluated by two reviewers according to their abstract and title and, secondly, a full texts evaluation by two independent reviewers was performed. To resolve diverging evaluations, a third reviewer was consulted to reach a final decision. Data were extracted by the reviewers following the Oxford 2011 levels of evidence guidelines and summarized accordingly.
EVIDENCE SYNTHESIS
Seven studies were extracted and descriptively synthesized, since a metanalysis was not feasible. Patients undergoing diaphragm stimulation had moderate evidence of higher maximal inspiratory pressure (MIP), less atrophy, less mitochondrial respiratory dysfunction, less oxidative stress, less molecular atrophy, shorter MV time, shorter ICU length of stay, longer survival, and better SF-36 scores than control.
CONCLUSIONS
Evidence of the molecular and histological benefits of diaphragmatic stimulation is limited. The results indicate positive clinical effects of diaphragm activation with a moderate level of evidence for MIP and a low level of evidence for other outcomes. Diaphragm activation could be a therapeutic solution to avoid diaphragm atrophy, accelerate weaning, shorten MV time, and counteract VIDD; however, better-powered studies are needed to increase the level of evidence.
Key words: Critical care; Phrenic nerve; Respiration, artificial; Ventilators, mechanical
Introduction
Mechanical ventilation (MV) is a life-saving intervention for critically ill patients while simultaneously carrying a significant burden regarding its side effects. One side effect is ICU acquired weakness (ICUAW) and ventilator-induced diaphragm dysfunction (VIDD), defined as a reduction in the diaphragm force-generating capacity;1 the latter can overlap with ICUAW or be uncorrelated to it.2 VIDD is a very prevalent condition present in mechanically ventilated patients; 80% of ICU patients develop one or more forms of neuromuscular dysfunction,3 one of which is the diaphragm weakness associated to critical illness.4 VIDD occurs early, within 18-69 hours of inactivity and mechanical ventilation3 and leads to weaning failure in more than 50% of patients with confirmed diaphragm dysfunction,3 prolonged stay in the Intensive Care Unit (ICU),5 and increased ICU mortality.4 The main clinical consequence of VIDD is a weaning failure from the ventilator;3 difficult weaning occurs in about 30% of patients mechanically ventilated for more than 3 days. Ventilator weaning failure results in extended hospital stays and increased patient morbidity and mortality.6
The pathophysiology of VIDD is multifaceted; the atrophy molecular pathway activated by higher oxidative stress was identified as the main pathophysiological mechanism leading to diaphragm muscle tissue disarrangement and diaphragm thickness reduction.7-9 The bioenergetic machinery is also compromised; Martin et al. compared the left and right hemidiaphragm, while one side was inactive, and the other was activated by electric stimulation.10 The inactive hemidiaphragm showed reduced mitochondrial biomass and intrinsic respiratory chain dysfunction within 48-72 hours after diaphragm paralysis during MV in humans.11
VIDD diagnosis and quantification of diaphragm dysfunction have not been standardized. Diagnostic tools for VIDD include lung function testing ultrasound (US) diaphragm assessment to measure diaphragm thickness and thickening fraction according to the most recent ERC guidelines.12 US imaging is an easily available diagnostic tool to assess two classes of parameters: 1) static parameters – the assessment of diaphragm thickness at the end of inspiration (Tdi insp) or expiration (Tdi exp);9, 13 and 2) dynamic parameters – the assessment of diaphragm thickness fraction (ΔTdi%) and diaphragm excursion (DE).14
Currently, there is no therapeutic protocol for VIDD treatment. The aim of the present review was to determine the outcomes of diaphragm muscle stimulation in preventing VIDD and improving outcomes in critically ill patients. Peripheral muscle stimulation prevents muscle atrophy in critically ill patients without evidence of improved muscle strength;15 similar evidence regarding diaphragm atrophy and its function has not been assessed. In parallel to peripheral muscles, we hypothesize that the muscle activation consequent to diaphragm stimulation could decelerate the progression of diaphragm atrophy.
The present report aimed to answer the following research questions systematically:
Does diaphragmatic stimulation of the diaphragm prevent muscle atrophy (diaphragm thickness or reduction of thickness loss) or VIDD (clinical outcomes of diaphragm weakness) in critically ill patients ventilated by active muscle contraction?
Do diaphragmatic stimulation of the diaphragm improve outcomes in critically ill patients?
Evidence acquisition
Study registration
This systematic review is fully compliant with the PRISMA16 (preferred reporting items for systematic reviews and meta-analyses) guidelines and the systematic review has been prospectively registered in an international database of prospectively registered systematic reviews Prospero (CRD42021259353) on June 28, 2021. The review protocol was applied as intended without the need for amendments.
Eligibility criteria
Only systematic reviews with or without meta-analyses, randomized controlled trials, observational studies, and case reports with a substantial number of cases published in English or German were reviewed.
Population
Critically ill adults (≥18 years old) were the population of interest.
Intervention
The reviewed intervention was any type of diaphragmatic stimulation leading to involuntary contraction either directly or via the phrenic nerve, independent of modality (electrically, magnetically, or both).
Comparator
Standard of care and no stimulation (or sham stimulation) were accepted comparators.
Outcomes
Clinical outcomes included muscle atrophy, VIDD (clinical outcomes of diaphragm weakness), weaning failure (reintubation or NIV rescue after extubation), mortality, quality of life (Barthel Index, activities of daily living, SF-36 score), ventilation time, diaphragmatic function, ICU length of stay, and hospital length of stay.
Information sources
We used the Medline database for the systematic literature search and performed the search on June 28, 2021. A search update was performed on January 31, 2023. References were further screened for relevant studies, which were included if they met selection criteria.
Search strategy
The previously listed databases were searched for publications according to the keywords and Medical Subject Headings (MeSH) connected with Boolean logic operators17 defined to cover the relevant PICO questions. The search terms used are presented in the Supplementary Digital Material 1 (Supplementary Text File 1).
Selection process
The identified articles were evaluated in a two-step process using a web-based tool (Rayyan, https://rayyan.ai) that allowed blinded collaboration, and each article was assessed by two reviewers.18 In the first step, all articles were independently evaluated by two reviewers according to their abstract and title. Duplicates were removed at this stage.
In the second step, the full texts of all identified articles were retrieved and evaluated by two independent reviewers to determine their eligibility for inclusion. To resolve diverging evaluations, a third reviewer was consulted to reach a final decision.
Data extraction
Data regarding study design, population characteristics, specifications of the intervention, and relevant outcome parameters, as listed under outcomes, were extracted by the reviewers using the Oxford 2011 Levels of Evidence table.
Level of evidence
The evidence grade for the studies included in the systematic review was assessed according to the Oxford 2011 Levels of Evidence developed by the Oxford Centre for Evidence-Based Medicine (CEBM https://www.cebm.net, University of Oxford, Oxford, UK).19
Risk of bias assessment
Risk of bias assessment was performed according to the Cochrane methods using three different collaboration tools available on Cochrane’s website (https://methods.cochrane.org):
ROBINS-I tool, for non-randomized studies;20
ROB 2 tool, for randomized clinical trials;21
ROBIS tool, for systematic reviews and meta-analyses.22
Three reviewers assessed bias for eligible studies. In the case of incongruence, a further reviewer verified or corrected the bias assessment.
Data synthesis
The extracted data were descriptively synthesized. However, a prospective meta-analysis was not planned.
Evidence synthesis
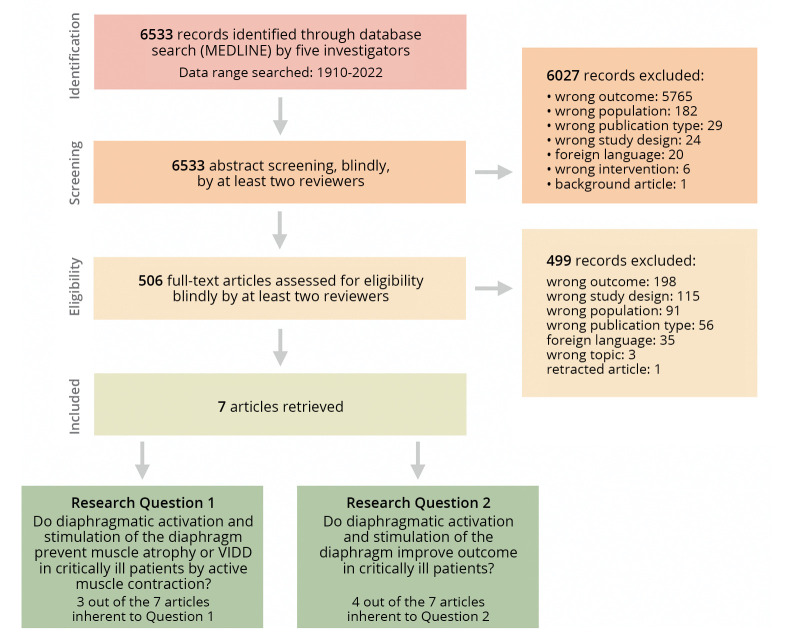
The initial literature search identified 6533 articles. Five hundred six unique titles and abstracts were selected as potentially relevant. Figure 1 depicts the search and evaluation process. Seven articles were included in the systematic review: questions 1 and 2, respectively, yielded three and four articles out of seven extracted. Results are summarized Supplementary Digital Material 2 (Supplementary Table I), while the risk of bias is presented in Figure 2.10, 20-29 A metanalysis, however, was not feasible.
Figure 1.
—Selection process for scientific literature.
Figure 2.
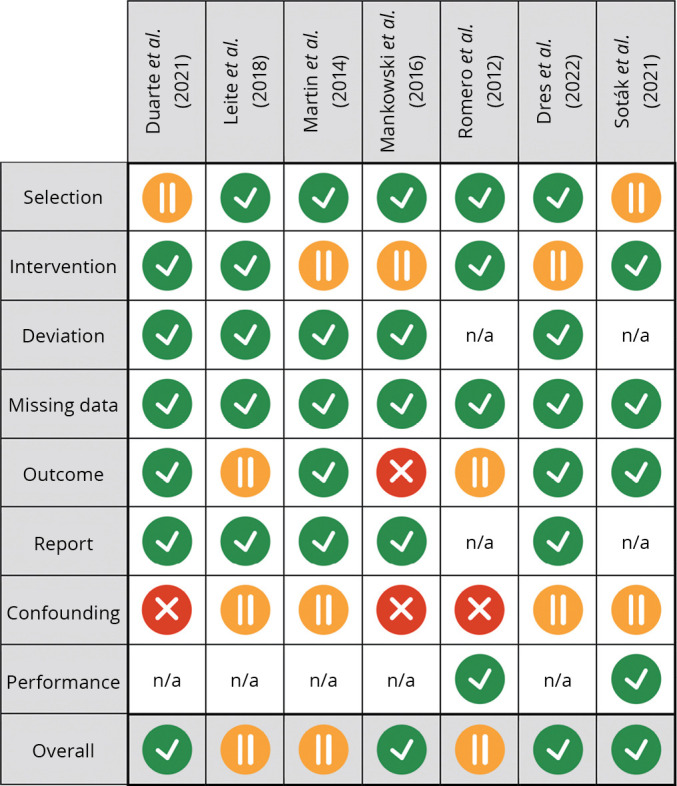
—Risk of bias, “traffic light” plot. Low, medium and high risk of bias are depicted in gray (green in the online version), light gray (yellow in the online version) and dark gray (red in the online version), respectively.10, 23-28 Bias assessment was performed according to Cochrane’s guidelines,29 using ROBINS-I tool (for non-randomized studies),20 ROB 2 tool (for randomized clinical trials)21 and ROBIS tool (for systematic reviews and meta-analyses).22
Classification of diaphragm stimulation techniques
The included studies were performed using different kinds of diaphragm stimulation or activation. These techniques can be classified as invasive and non-invasive.
The invasive diaphragm stimulation techniques are transcutaneous electrical diaphragmatic stimulation23, 30 and percutaneous electrical phrenic nerve stimulation (PEPNS),24 where a needle or stimulation device is inserted close to the phrenic nerves; invasive phrenic nerve pacing, by which the phrenic nerves can be stimulated directly by surgical access to the nerves;10, 25, 26 temporary transvenous phrenic nerve stimulation, by which both phrenic nerves can be stimulated through a central venous line equipped with two electrodes.27
Among the non-invasive techniques, the only study that fulfilled the inclusion criteria was performed with diaphragm external neuromuscular electrical stimulation (NMES), by which a diaphragm contraction is achieved through a thoracic belt equipped with external electrodes.28
Worth noting, even though not fulfilling the inclusion criteria for the present systematic review, was the study using non-invasive magnetic phrenic nerve stimulation, performed with butterfly-shaped transcranial magnetic stimulation (TMS) coils, however too cumbersome to achieve usability in the critical care setting.31
Preventing muscle atrophy or VIDD by diaphragmatic stimulation
Two case-control series studies analyzed biopsies from an electrically stimulated hemidiaphragm. They compared them with biopsies of the contralateral inactive hemidiaphragm, using high-resolution respirometry and western blotting, respectively.10, 26 Both studies showed positive outcomes of diaphragm stimulation:
significantly less mitochondrial respiratory dysfunction was observed in the stimulated hemidiaphragm (better state III and IV respiratory rates, both P<0.05);
the oxidative stress and lipid peroxidation levels (4-HNE) were significantly lower in the stimulated hemidiaphragm (P<0.05);
autophagy biomarker levels (Beclin-1 and LC3II/I) were significantly higher (indicating less oxidative stress and atrophy) in the stimulated hemidiaphragm (P<0.05);
the antioxidant enzyme activities of CuZnSOD and MnSOD (superoxide dismutase) were not statistically different between the stimulated and non-stimulated hemidiaphragm.
A prospective control double-center study compared PEPNS with control (standard of care) using an ultrasound diaphragm assessment of thickness. Both phrenic nerves were identified using ultrasound to steer the percutaneous stimulation. Patients in the intervention group experienced an increase in diaphragm thickness at 48 h (baseline: 1.98±0.52 mm; at 48 h: 2.20±0.45 mm; P=0.001), while a diaphragm thickness reduction occurred in the control patients (baseline: 2.00±0.33 mm; at 48 h: 1.72±0.2 mm; P<0.001). The average diaphragm thickness was 15% thicker in the intervention group and 12% thinner in the control group; this difference was statistically significant (P<0.001).24
Improving outcome by diaphragmatic stimulation
A prospective randomized pilot study by Leite et al. compared three different therapeutic protocols in 67 critically ill ICU patients.28
diaphragm group – one daily session of electrical diaphragm stimulation, in addition to one standard daily physical therapy;
quadriceps group – one daily session of electrical quadriceps stimulation, in addition to one standard daily physical therapy;
the control group underwent two sessions of daily physical therapy, including gross motor therapy and respiratory therapy.
Diaphragm stimulation and quadriceps stimulation had similar outcomes with better maximum inspiratory pressure (MIP) than the control group (P<0.001), which was identified by comparing the last MIP measurement at ICU discharge with the first MIP measurement at MV start. The Rapid Shallow Breathing Index showed no statistical differences between the two intervention groups. Surprisingly, MV duration was shorter in the control group (15.8±5.75 h) than in both intervention groups (diaphragm stimulation: 27.5±12.16 h, quadriceps stimulation: 23.3±10.61 h; P<0.001).
In a retrospective case series performed on mechanically ventilated spinal cord injury patients, Duarte et al. tested the difference in MV time and ICU length of stay of transcutaneous electrical diaphragm stimulation against a standard weaning protocol.23 The intervention group had a significantly shorter MV time (1.77 times shorter) and ICU length of stay (2.54 times shorter) than the control group; however, P values were not published for this study. The authors implicitly assumed that, as known in the scientific literature, spinal cord injury patients develop diaphragm muscle atrophy if the corresponding nerves no longer stimulate the muscle.23
Romero et al. assessed outcomes after phrenic nerve stimulation compared to standard mechanical ventilation in patients with respiratory failure and spinal cord injury (SCI). Survival length, defined as time (days) between SCI and death or end of the study, was higher in the stimulated group (OR=0.98; 95% CI: 0.97-0.99; P=0.04) and better social functioning measured with the SF-36 questionnaire for quality of life (phrenic nerve stimulation group 7.67±1.8 vs. MV group 5.67±1.17; P<0.001) in comparison with the MV group.25
Temporary transvenous diaphragm neurostimulation was used by Dres et al. to achieve diaphragmatic stimulation and contraction.27 Using an invasive transvenous device, the researchers were able to perform phrenic nerve stimulation in the intervention group and compare the results with those of controls, who received a standard weaning protocol. No differences in MV duration or incidence of death were found between the groups. However, the intervention group had a higher MIP at day 8 in comparison with the control (+10.9 cmH2O vs. +3.8 cmH2O, absolute difference 7 [95% CI: 1; 14] cmH2O, P=0.029) as well as on day 15 (+11.9 cmH2O vs. +4.5 cmH2O, absolute difference 7 [95% CI: 1; 14] cmH2O, P=0.024).27
Inclusion criteria, exclusion criteria, type of study, Oxford Evidence Grades, type of intervention, type of control and measurements are presented in Supplementary Table I. Results and characteristics of the included study population are summarized in Table I.10, 23-28 The risk of bias is shown in Figure 2.10, 20-29
Table I. —Results summary of seven articles identified for the systematic review.
| Evidence Grade | Study | Results | Bias | |
|---|---|---|---|---|
| Evidence Grade 3 | Duarte et al.23 | Retrospective case series | MV time 1.77 times shorter for TEDS in comparison with SWP alone. ICU stay 2.54 times shorter for TEDS in comparison with SWP alone. |
|
| Evidence Grade 2 | Leite et al.28 | Prospective randomized pilot study | Diaphragm electrical stimulation (DG) did not produce better results than quadriceps stimulation (QG). Maximum inspiratory pressure (MIP) higher for both DG and QG, in comparison with CG. Difference for all muscle strength indexes (Rapid Shallow Breathing Index and MRC) between DG and QG not significant. QG had shorter ICU stay and better Barthel index than both DG and CG. Duration of mechanical ventilation (MV) shorter for CG in comparison with both DG and QG (CG: 15.8±5.75 hours, DG: 27.5±12.16 hours, QG: 23.3±10.61 hours; P=0.0001). DG and QG had no significant difference in MV duration. |
|
| Evidence Grade 4 | Martin et al.10 | Case control series study | Diaphragm pacing produces positive mitochondrial effects: the stimulated hemidiaphragm has better mitochondrial State III and IV (respiratory rates) than the control, which suffers higher grade of mitochondrial respiratory rate dysfunction. | |
| Evidence Grade 3 | Mankowski et al.26 | Case control series study | 4-HNE (marker of lipid peroxidation levels and oxidative stress) lower in the stimulated hemidiaphragm. Antioxidant enzyme activities of CuZnSOD and MnSOD were not different between the hemidiaphragms. Macroautophagy biomarker levels of Beclin-1 and the LC3II/I ratio were significantly higher in the stimulated side. |
|
| Evidence Grade 3 | Romero et al.25 | Retrospective study of non-randomized, prospectively collected data | Phrenic nerve stimulation group had higher probability of survival than MV group (OR=0.98; 95% CI: 0.97-0.99; P=0.04). Phrenic nerve stimulation group had better social functioning (SF-36) than MV group (PNP group 7.67±1.8 vs. MV group 5.67±1.17; P=0.0002). |
|
| Evidence Grade 2 | Dres et al.27 | Multicenter, open label, randomized, controlled study | No significant difference in MV time and incidence of death between the two groups. Significant difference in MIP changes from baseline to last measurement: +16.6 cmH2O (intervention) and +4.8 cmH2O (control); P=0.001, 95% CI: 11.8 [5;19] cmH2O. Significant change in MIP over time differed significantly between groups by day 8: +10.9 cmH2O (intervention) and +3.8 cmH2O (control); P=0.029, 95% CI: 7 [1;14] cmH2O. Adjusting for baseline differences in MIP and BMI, significant change in MIP reached at day 15: +11.9 cmH2O (intervention) and +4.5 cmH2O (control); P=0.024, 95% CI: 7 [1;14] cmH2O. |
|
| Evidence Grade 3 | Soták et al.24 | Prospective, interventional, controlled, double-center study | PEPNS group (4 patients in PSV, 8 patients in ACV and/or PSV) experienced an increase in diaphragm thickness at 48 h (baseline: 1.98±0.52 mm; 48 h: 2.20±0.45 mm; P=0.001). Control group (9 patients in ACV, 1 patient in PSV) experienced a decrease in diaphragm thickness at 48h of MV (baseline: 2.00±0.33 mm; 48 h: 1.72±0.2 mm; P<0.001). An overall increase shown (right and left together) in diaphragm thickness in the PEPNS group after 48 hours. This increase was statistically significant (P=0.0003). By 48 hours MV, the diaphragm thickness was on average almost 15% thicker than at baseline in the PEPNS group and 12% thinner than at baseline in the control group (P=0.0002). |
|
Discussion
Diaphragm stimulation might be an option to prevent or treat VIDD; nevertheless, evidence of the positive or negative outcomes of diaphragm stimulation on diaphragm muscle atrophy and critically ill patient outcomes has never been assessed by any systematic review.
The crosstalk between the oxidative pathways and atrophy pathways is the key molecular actor for VIDD.32-34 Hints of the positive impact of stimulation on diaphragm atrophy chain reactions at a molecular level, resulting in less oxidative stress and less atrophy, has been shown.10, 26 The causative role of mitochondrial dysfunction and oxidative stress in the pathophysiology of VIDD is, however, controversial, as van der Berg et al. suggested that treatment aimed at mitochondria may not be beneficial for critically ill patients since mitochondrial function in the diaphragm was preserved and oxidative stress levels were not increased, even in severely ill patients.35 Important key biomarkers for muscle atrophy as Titin36 or diaphragm-specific atrophy mRNA markers37, 38 were not assessed in the included studies. Hints of less oxidative stress have been demonstrated, suggesting a positive effect in counteracting VIDD. Nevertheless, a broader spectrum of molecular tests is needed to confirm these results.
Diaphragm atrophy can easily be assessed by ultrasound in the ICU setting to predict weaning outcomes.39 Patients without diaphragm atrophy have a higher rate of liberation from MV,40 shorter ICU length of stay41 and shorter MV time in the ICU setting.7, 9, 13, 42-44 Diaphragm ultrasound can assess diaphragm thickness, a known predictor of MV time. These findings reinforce the results of Soták et al.: diaphragm stimulation by phrenic stimulation maintains or increases diaphragm thickness, avoiding or decelerating diaphragm atrophy.24 No indication of a difference in MV time between intervention and control is available and the study power and optimal population were not calculated; moreover, considering that the patients were enrolled several days after intubation could be a source of confounding, the pathological mechanisms which initiate VIDD are triggered within 72 h after the start of MV.7, 8, 11 The results are promising, nevertheless, some limitations and confounding were found: indicators of a positive effect of diaphragm stimulation on diaphragm thickness have been shown but insufficient to assess effects on key outcomes as MV time.
We found the best available evidence of diaphragmatic stimulation for MIP. In a prospective randomized pilot study by Leite et al., patients who received diaphragm stimulation had higher MIP than control patients.28 These findings were reinforced by assessing MIP using temporary transvenous diaphragmatic neurostimulation in difficult-to-wean patients.27 Higher MIP as an index of “readiness to wean” was established in reviews45 and prospective studies46 nevertheless, some remarks should be made regarding these results. First, MIP and diaphragm thickness combined are better predictors for successful weaning than MIP alone.47 Secondly, although improved muscle strength is an important variable when considering “readiness to wean,”48 MIP is an ancillary clinical parameter, while MV time is an important outcome for critically ill patients;49 in the included studies shorter MV time after diaphragm stimulation was not found, nevertheless no population adjustment was performed and the studies are likely underpowered. Third, the control group in the first study28 received an intensive PT protocol (two sessions per day) and the intervention group received one session of PT per day; two sessions of physical therapy are considered standard care in some clinics, and control patients should receive the kind of physical therapy normally used in loco; muscle or diaphragm stimulation is not meant to substitute for physical therapy; on the contrary, it should be used as a prophylactic therapy to avoid diaphragm atrophy and VIDD. Fourth, many authors have suggested the importance of P0.1 and occlusion pressure, as shown in a recent systematic review and meta-analysis.50 P0.1 and occlusion pressure were never measured after diaphragm stimulation; on the contrary, occlusion was performed with bilateral magnetic phrenic stimulation to diagnose VIDD in critically ill patients.51 These two, however, measure diaphragm drive and effort: P0.1 and occlusion pressure cannot assess muscle function. Since diaphragm stimulation is intended as muscle function enhancement, no change in P0.1 and occlusion pressure (i.e., diaphragm drive and effort) should be expected. While MIP is a good assessment for muscle function, P0.1 and occlusion pressure are not expected to be good parameters to assess the outcome after diaphragm stimulation: no studies to confirm this were performed.
The interesting finding concerning MIP after diaphragm stimulation adds information about the possible therapeutical outcomes in critically ill patients. These results must fit in a wider context since many pathological determinants can influence MV time, and the level of evidence is insufficient to postulate general assumptions concerning positive effects on VIDD.
No scientific literature on long-term survival after diaphragm stimulation in the critical ill was found; similarly aimed randomized controlled studies have never been performed. However, survival was assessed in two studies; the first assessed 30-day survival stating that no difference between diaphragm stimulation and control was identified.27 Romero et al. showed positive outcomes after phrenic nerve stimulation, longer survival rates, and better SF-36 scores in comparison with the standard of care.25 The population considered is only limited to patients with cervical spine injury. The low level of evidence concerning survival after diaphragm activation does not allow any certain assumption on the general ICU population.
The techniques used are heterogeneous and space between highly invasive electric pacing to non-invasive techniques. Phrenic nerve stimulation with invasive techniques is feasible, nevertheless presents risks comparable to the mechanical, infectious, or thrombotic side effects of a central venous line.52 Safety could be increased with magnetic stimulators of the phrenic nerve, which have only been used for diagnostic purposes.53, 54
Limitations of the study
We limited our search to English and German literature. Due to the focus on the critically ill population, we may have missed other important factors not commonly evaluated in studies of critically ill patients yet, like residual autonomy (e.g., better management of the voice like timbre, length of sentences, or autonomy in dialogue). Furthermore, we prospectively decided not to perform a meta-analysis because we assumed that the heterogeneity would be too high. The assumption was confirmed during the execution of the systematic review, and a statistician was involved in the attempt of performing a statistical analysis that could summarize the results of the seven studies included; according to the expert opinion, no statistical analysis was scientifically meaningful in the given circumstances.
Conclusions
In summary, there is some evidence available regarding the molecular benefits and prevention of diaphragmatic atrophy by diaphragmatic stimulation. Hints and indicators of positive outcomes like higher MIP and longer survival are available in the scientific literature. Preventive approaches to VIDD have not been extensively studied, and the few available publications are not adequately powered and insufficient to confirm a clinical benefit. Invasive procedures to achieve diaphragmatic stimulation in critically ill patients present limitations and risks, and further experimental efforts are needed to assess these issues.
Supplementary Digital Material 1
Supplementary Text File 1
Research string for Systematic Review: “Outcomes in Critically Ill Patients after Diaphragmatic Stimulation on Ventilator-induced Diaphragmatic Dysfunction: A Systematic Review”
Supplementary Digital Material 1
Supplementary Table I
Literature extraction summary of seven articles identified for the systematic review. evidence grade, bias assessment. Inclusion criteria, exclusion criteria, type of study, Oxford Evidence Grades, type of intervention, type of control and measurements are given.
Footnotes
Conflicts of interest: Stefan J. Schaller holds stocks in small amount from Alphabet Inc, Bayer AG and Siemens AG; he also received honoraria for lectures from Fresenius Kabi Deutschland GmbH, Springer Verlag GmbH for educational purposes and Advanz Pharma; and received funds not directly related to the manuscript by Fresenius Kabi Deutschland GmbH, ASP GmbH, and Federal Joint Committee (G-BA).
Funding: This work was supported by a project grant and equipment by STIMIT AG, Biel, Switzerland. The funding body had no role in the design, collection, analysis, and interpretation of the data and in writing the manuscript.
References
- 1.Vassilakopoulos T, Petrof BJ. Ventilator-induced diaphragmatic dysfunction. Am J Respir Crit Care Med 2004;169:336–41. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=14739134&dopt=Abstract 10.1164/rccm.200304-489CP [DOI] [PubMed] [Google Scholar]
- 2.Dres M, Goligher EC, Heunks LM, Brochard LJ. Critical illness-associated diaphragm weakness. Intensive Care Med 2017;43:1441–52. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28917004&dopt=Abstract 10.1007/s00134-017-4928-4 [DOI] [PubMed] [Google Scholar]
- 3.Jung B, Moury PH, Mahul M, de Jong A, Galia F, Prades A, et al. Diaphragmatic dysfunction in patients with ICU-acquired weakness and its impact on extubation failure. Intensive Care Med 2016;42:853–61. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26572511&dopt=Abstract 10.1007/s00134-015-4125-2 [DOI] [PubMed] [Google Scholar]
- 4.Medrinal C, Prieur G, Frenoy É, Robledo Quesada A, Poncet A, Bonnevie T, et al. Respiratory weakness after mechanical ventilation is associated with one-year mortality - a prospective study. Crit Care 2016;20:231. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27475524&dopt=Abstract 10.1186/s13054-016-1418-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Supinski GS, Callahan LA. Diaphragm weakness in mechanically ventilated critically ill patients. Crit Care 2013;17:R120. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23786764&dopt=Abstract 10.1186/cc12792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esteban A, Alía I, Ibañez J, Benito S, Tobin MJ, The Spanish Lung Failure Collaborative Group . Modes of mechanical ventilation and weaning. A national survey of Spanish hospitals. Chest 1994;106:1188–93. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=7924494&dopt=Abstract 10.1378/chest.106.4.1188 [DOI] [PubMed] [Google Scholar]
- 7.Kilapong B, Aditianingsih D, Sedono R, Sugiarto A, Salamah T. Diaphragm muscle thinning in mechanically ventilated critically ill patients. J Pak Med Assoc 2021;71:S78–83. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33785947&dopt=Abstract [PubMed] [Google Scholar]
- 8.Levine S, Nguyen T, Taylor N, Friscia ME, Budak MT, Rothenberg P, et al. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med 2008;358:1327–35. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=18367735&dopt=Abstract 10.1056/NEJMoa070447 [DOI] [PubMed] [Google Scholar]
- 9.Goligher EC, Fan E, Herridge MS, Murray A, Vorona S, Brace D, et al. Evolution of Diaphragm Thickness during Mechanical Ventilation. Impact of Inspiratory Effort. Am J Respir Crit Care Med 2015;192:1080–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26167730&dopt=Abstract 10.1164/rccm.201503-0620OC [DOI] [PubMed] [Google Scholar]
- 10.Martin AD, Joseph AM, Beaver TM, Smith BK, Martin TD, Berg K, et al. Effect of intermittent phrenic nerve stimulation during cardiothoracic surgery on mitochondrial respiration in the human diaphragm. Crit Care Med 2014;42:e152–6. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24126442&dopt=Abstract 10.1097/CCM.0b013e3182a63fdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Picard M, Jung B, Liang F, Azuelos I, Hussain S, Goldberg P, et al. Mitochondrial dysfunction and lipid accumulation in the human diaphragm during mechanical ventilation. Am J Respir Crit Care Med 2012;186:1140–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23024021&dopt=Abstract 10.1164/rccm.201206-0982OC [DOI] [PubMed] [Google Scholar]
- 12.Laveneziana P, Albuquerque A, Aliverti A, Babb T, Barreiro E, Dres M, et al. ERS statement on respiratory muscle testing at rest and during exercise. Eur Respir J 2019;53:1801214. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30956204&dopt=Abstract 10.1183/13993003.01214-2018 [DOI] [PubMed] [Google Scholar]
- 13.Goligher EC, Dres M, Fan E, Rubenfeld GD, Scales DC, Herridge MS, et al. Mechanical Ventilation-induced Diaphragm Atrophy Strongly Impacts Clinical Outcomes. Am J Respir Crit Care Med 2018;197:204–13. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28930478&dopt=Abstract 10.1164/rccm.201703-0536OC [DOI] [PubMed] [Google Scholar]
- 14.Huang D, Ma H, Zhong W, Wang X, Wu Y, Qin T, et al. Using M-mode ultrasonography to assess diaphragm dysfunction and predict the success of mechanical ventilation weaning in elderly patients. J Thorac Dis 2017;9:3177–86. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29221294&dopt=Abstract 10.21037/jtd.2017.08.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wollersheim T, Grunow JJ, Carbon NM, Haas K, Malleike J, Ramme SF, et al. Muscle wasting and function after muscle activation and early protocol-based physiotherapy: an explorative trial. J Cachexia Sarcopenia Muscle 2019;10:734–47. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31016887&dopt=Abstract 10.1002/jcsm.12428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19621072&dopt=Abstract 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elston DM. Getting the most out of online literature searches-More on Boolean logic. J Am Acad Dermatol 2020;82:299–300. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29128457&dopt=Abstract 10.1016/j.jaad.2017.11.005 [DOI] [PubMed] [Google Scholar]
- 18.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev 2016;5:210. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27919275&dopt=Abstract 10.1186/s13643-016-0384-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howick J, Glasziou P, Greenhalgh T, Heneghan C, Liberati A, Moschetti I, et al. Explanation of the 2011 OCEBM Levels of Evidence. CEBM; 2011 [Internet]. Available from: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/explanation-of-the-2011-ocebm-levels-of-evidence [cited 2023, Oct 10].
- 20.Schünemann HJ, Cuello C, Akl EA, Mustafa RA, Meerpohl JJ, Thayer K, et al. GRADE Working Group . GRADE guidelines: 18. How ROBINS-I and other tools to assess risk of bias in nonrandomized studies should be used to rate the certainty of a body of evidence. J Clin Epidemiol 2019;111:105–14. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29432858&dopt=Abstract 10.1016/j.jclinepi.2018.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31462531&dopt=Abstract 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 22.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. Cochrane Bias Methods Group ; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22008217&dopt=Abstract 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duarte GL, Bethiol AL, Ratti LD, Franco G, Moreno R, Tonella RM, et al. Transcutaneous electrical diaphragmatic stimulation reduces the duration of invasive mechanical ventilation in patients with cervical spinal cord injury: retrospective case series. Spinal Cord Ser Cases 2021;7:26. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33837183&dopt=Abstract 10.1038/s41394-021-00396-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soták M, Roubík K, Henlín T, Tyll T. Phrenic nerve stimulation prevents diaphragm atrophy in patients with respiratory failure on mechanical ventilation. BMC Pulm Med 2021;21:314. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34625059&dopt=Abstract 10.1186/s12890-021-01677-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romero FJ, Gambarrutta C, Garcia-Forcada A, Marín MA, Diaz de la Lastra E, Paz F, et al. Long-term evaluation of phrenic nerve pacing for respiratory failure due to high cervical spinal cord injury. Spinal Cord 2012;50:895–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22777487&dopt=Abstract 10.1038/sc.2012.74 [DOI] [PubMed] [Google Scholar]
- 26.Mankowski RT, Ahmed S, Beaver T, Dirain M, Han C, Hess P, et al. Intraoperative hemidiaphragm electrical stimulation reduces oxidative stress and upregulates autophagy in surgery patients undergoing mechanical ventilation: exploratory study. J Transl Med 2016;14:305. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27784315&dopt=Abstract 10.1186/s12967-016-1060-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dres M, de Abreu MG, Merdji H, Müller-Redetzky H, Dellweg D, Randerath WJ, et al. RESCUE-2 Study Group Investigators . Randomized Clinical Study of Temporary Transvenous Phrenic Nerve Stimulation in Difficult-to-Wean Patients. Am J Respir Crit Care Med 2022;205:1169–78. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35108175&dopt=Abstract 10.1164/rccm.202107-1709OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leite MA, Osaku EF, Albert J, Costa CR, Garcia AM, Czapiesvski FD, et al. Effects of Neuromuscular Electrical Stimulation of the Quadriceps and Diaphragm in Critically Ill Patients: A Pilot Study. Crit Care Res Pract 2018;2018:4298583. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30123586&dopt=Abstract 10.1155/2018/4298583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cochrane methods. Cochrane; 2023 [Internet]. Available from: https://methods.cochrane.org/ [cited 2023, Oct 10].
- 30.O’Rourke J, Soták M, Curley GF, Doolan A, Henlín T, Mullins G, et al. Initial Assessment of the Percutaneous Electrical Phrenic Nerve Stimulation System in Patients on Mechanical Ventilation. Crit Care Med 2020;48:e362–70. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32191413&dopt=Abstract 10.1097/CCM.0000000000004256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sander BH, Dieck T, Homrighausen F, Tschan CA, Steffens J, Raymondos K. Electromagnetic ventilation: first evaluation of a new method for artificial ventilation in humans. Muscle Nerve 2010;42:305–10. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20544943&dopt=Abstract 10.1002/mus.21698 [DOI] [PubMed] [Google Scholar]
- 32.Smuder AJ, Sollanek KJ, Nelson WB, Min K, Talbert EE, Kavazis AN, et al. Crosstalk between autophagy and oxidative stress regulates proteolysis in the diaphragm during mechanical ventilation. Free Radic Biol Med 2018;115:179–90. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29197632&dopt=Abstract 10.1016/j.freeradbiomed.2017.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hyatt HW, Ozdemir M, Yoshihara T, Nguyen BL, Deminice R, Powers SK. Calpains play an essential role in mechanical ventilation-induced diaphragmatic weakness and mitochondrial dysfunction. Redox Biol 2021;38:101802. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33279868&dopt=Abstract 10.1016/j.redox.2020.101802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dridi H, Yehya M, Barsotti R, Reiken S, Angebault C, Jung B, et al. Mitochondrial oxidative stress induces leaky ryanodine receptor during mechanical ventilation. Free Radic Biol Med 2020;146:383–91. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31756525&dopt=Abstract 10.1016/j.freeradbiomed.2019.11.019 [DOI] [PubMed] [Google Scholar]
- 35.van den Berg M, Hooijman PE, Beishuizen A, de Waard MC, Paul MA, Hartemink KJ, et al. Diaphragm Atrophy and Weakness in the Absence of Mitochondrial Dysfunction in the Critically Ill. Am J Respir Crit Care Med 2017;196:1544–58. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28787181&dopt=Abstract 10.1164/rccm.201703-0501OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van der Pijl R, Strom J, Conijn S, Lindqvist J, Labeit S, Granzier H, et al. Titin-based mechanosensing modulates muscle hypertrophy. J Cachexia Sarcopenia Muscle 2018;9:947–61. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29978560&dopt=Abstract 10.1002/jcsm.12319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu R, Li G, Ma H, Zhou X, Wang P, Zhao Y. Transcriptome profiling of the diaphragm in a controlled mechanical ventilation model reveals key genes involved in ventilator-induced diaphragmatic dysfunction. BMC Genomics 2021;22:472. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34172008&dopt=Abstract 10.1186/s12864-021-07741-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gill LC, Ross HH, Lee KZ, Gonzalez-Rothi EJ, Dougherty BJ, Judge AR, et al. Rapid diaphragm atrophy following cervical spinal cord hemisection. Respir Physiol Neurobiol 2014;192:66–73. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24341999&dopt=Abstract 10.1016/j.resp.2013.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Llamas-Álvarez AM, Tenza-Lozano EM, Latour-Pérez J. Diaphragm and Lung Ultrasound to Predict Weaning Outcome: Systematic Review and Meta-Analysis. Chest 2017;152:1140–50. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28864053&dopt=Abstract 10.1016/j.chest.2017.08.028 [DOI] [PubMed] [Google Scholar]
- 40.Sklar MC, Dres M, Fan E, Rubenfeld GD, Scales DC, Herridge MS, et al. Association of Low Baseline Diaphragm Muscle Mass With Prolonged Mechanical Ventilation and Mortality Among Critically Ill Adults. JAMA Netw Open 2020;3:e1921520. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32074293&dopt=Abstract 10.1001/jamanetworkopen.2019.21520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guzel S, Umay E, Gundogdu I, Bahtiyarca ZT, Cankurtaran D. Effects of diaphragm thickness on rehabilitation outcomes in post-ICU patients with spinal cord and brain injury. Eur J Trauma Emerg Surg 2022;48:559–65. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32601716&dopt=Abstract 10.1007/s00068-020-01426-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakanishi N, Oto J, Ueno Y, Nakataki E, Itagaki T, Nishimura M. Change in diaphragm and intercostal muscle thickness in mechanically ventilated patients: a prospective observational ultrasonography study. J Intensive Care 2019;7:56. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31827804&dopt=Abstract 10.1186/s40560-019-0410-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ni YN, Xu H, Li WJ, Sun JK, Liang BM, Liang ZA. Could the loss of diaphragm thickness measured by computer tomography predict the rate of reintubation? J Thorac Dis 2020;12:581–91. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32274124&dopt=Abstract 10.21037/jtd.2019.12.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schepens T, Verbrugghe W, Dams K, Corthouts B, Parizel PM, Jorens PG. The course of diaphragm atrophy in ventilated patients assessed with ultrasound: a longitudinal cohort study. Crit Care 2015;19:422. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26639081&dopt=Abstract 10.1186/s13054-015-1141-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nemer SN, Barbas CS. Predictive parameters for weaning from mechanical ventilation. J Bras Pneumol 2011;37:669–79. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22042401&dopt=Abstract 10.1590/S1806-37132011000500016 [DOI] [PubMed] [Google Scholar]
- 46.Trivedi S, Davis R, Engoren MC, Lorenzo J, Mentz G, Jewell ES, et al. Use of the Change in Weaning Parameters as a Predictor of Successful Re-Extubation. J Intensive Care Med 2022;37:337–41. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33461374&dopt=Abstract 10.1177/0885066620988675 [DOI] [PubMed] [Google Scholar]
- 47.Zhang P, Jiang HJ, Zhou Q, Ye XM, Li Z, Yuan LP, et al. [The predictive value of ultrasonic measurement of the diaphragmatic thickening fraction combined with the maximal inspiratory pressure in mechanical ventilation patients]. Zhonghua Jie He He Hu Xi Za Zhi 2020;43:778–83. [Chinese]. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32894912&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 48.Martin AD, Smith BK, Davenport PD, Harman E, Gonzalez-Rothi RJ, Baz M, et al. Inspiratory muscle strength training improves weaning outcome in failure to wean patients: a randomized trial. Crit Care 2011;15:R84. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21385346&dopt=Abstract 10.1186/cc10081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Worraphan S, Thammata A, Chittawatanarat K, Saokaew S, Kengkla K, Prasannarong M. Effects of Inspiratory Muscle Training and Early Mobilization on Weaning of Mechanical Ventilation: A Systematic Review and Network Meta-analysis. Arch Phys Med Rehabil 2020;101:2002–14. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32750371&dopt=Abstract 10.1016/j.apmr.2020.07.004 [DOI] [PubMed] [Google Scholar]
- 50.Sato R, Hasegawa D, Hamahata NT, Narala S, Nishida K, Takahashi K, et al. The predictive value of airway occlusion pressure at 100 msec (P0.1) on successful weaning from mechanical ventilation: A systematic review and meta-analysis. J Crit Care 2021;63:124–32. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33012587&dopt=Abstract 10.1016/j.jcrc.2020.09.030 [DOI] [PubMed] [Google Scholar]
- 51.Dres M, Dubé BP, Mayaux J, Delemazure J, Reuter D, Brochard L, et al. Coexistence and Impact of Limb Muscle and Diaphragm Weakness at Time of Liberation from Mechanical Ventilation in Medical Intensive Care Unit Patients. Am J Respir Crit Care Med 2017;195:57–66. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27310484&dopt=Abstract 10.1164/rccm.201602-0367OC [DOI] [PubMed] [Google Scholar]
- 52.Lockwood J, Desai N. Central venous access. Br J Hosp Med (Lond) 2019;80:C114–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31437056&dopt=Abstract 10.12968/hmed.2019.80.8.C114 [DOI] [PubMed] [Google Scholar]
- 53.Demoule A, Molinari N, Jung B, Prodanovic H, Chanques G, Matecki S, et al. Patterns of diaphragm function in critically ill patients receiving prolonged mechanical ventilation: a prospective longitudinal study. Ann Intensive Care 2016;6:75. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27492005&dopt=Abstract 10.1186/s13613-016-0179-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Demoule A, Jung B, Prodanovic H, Molinari N, Chanques G, Coirault C, et al. Diaphragm dysfunction on admission to the intensive care unit. Prevalence, risk factors, and prognostic impact-a prospective study. Am J Respir Crit Care Med 2013;188:213–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23641946&dopt=Abstract 10.1164/rccm.201209-1668OC [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Text File 1
Research string for Systematic Review: “Outcomes in Critically Ill Patients after Diaphragmatic Stimulation on Ventilator-induced Diaphragmatic Dysfunction: A Systematic Review”
Supplementary Table I
Literature extraction summary of seven articles identified for the systematic review. evidence grade, bias assessment. Inclusion criteria, exclusion criteria, type of study, Oxford Evidence Grades, type of intervention, type of control and measurements are given.