Abstract
The mechanisms involved in coiling phagocytosis are not yet known, and it is not even clear whether this phenomenon is either an incidental event or a specific response. Therefore, the phagocytic uptake of Borrelia burgdorferi and other spirochetes by human monocytes in vitro was used to investigate the involvement of both sides—microbes and phagocytes—in coiling phagocytosis. As seen with electron microscopy, morphologically similar Borrelia, Leptospira and Treponema strains induced markedly different frequencies of coiling phagocytosis. The monocytes used coiling phagocytosis for both live (motile) and killed (nonmotile) B. burgdorferi, but pseudopod coils were observed neither with fragmented B. burgdorferi nor with cell-free supernatant from B. burgdorferi cultures. Investigation of the relationship of coiling phagocytosis with other pseudopod-based cellular mechanisms revealed that the use of bioreagents that inhibit conventional phagocytosis also inhibited coiling phagocytis but did not affect membrane ruffling. Bioreagents that increase membrane ruffling did not affect phagocytosis of B. burgdorferi, except for granulocyte-macrophage colony-stimulating factor and phorbol myristate acetate, which increased coiling phagocytosis selectively. These results demonstrate that coiling phagocytosis is not induced by microbial motility, viability, or a certain morphology and that it is not a random event. Rather, it is a selective uptake mechanism actively driven by the phagocytes. However, whether coiling phagocytosis represents an independent alternative to conventional phagocytosis or, alternatively, a fault in conventional phagocytosis remains to be determined.
The characteristic of coiling phagocytosis (23) is the appearance of pseudopod whorls, which are based on single folds of the phagocyte plasma membrane wrapping around microbes in multiple turns. Coiling phagocytosis shares some features with macropinocytosis and conventional phagocytosis (Fig. 1), such as the use of single membrane folds (which is typical of macropinocytosis) or the uptake of particulate material (which is the function of phagocytosis); however, there also are differences. In macropinocytosis, the single pseudopods eventually bend back toward the cell surface, thereby randomly trapping large droplets of pericellular fluid which may accidentally include particles as well. In conventional phagocytosis, particles are specifically engulfed by pseudopods which are strictly microbe apposed, since for this process receptors on the surface of the pseudopod have to interact directly with ligands on the surface of the particle. In coiling phagocytosis, the single pseudopods do not trap fluid droplets but enclose microbes; however, the multiple pseudopod whorls have largely self-apposed instead of microbe-apposed surfaces.
FIG. 1.
Schematic drawing of the morphological characteristics of macropinocytosis and of conventional and coiling phagocytosis. Single membrane folds which bend back toward the cell surface constitutes macropinocytosis; large droplets of pericellular fluid (which may contain some particles) are randomly trapped during this process. In conventional phagocytosis, particles are specifically engulfed by circumferential pseudopods which are guided by direct receptor-ligand interactions with the particle. Characteristic of coiling phagocytosis are unilateral pseudopods repetitively rotating around particles, giving rise to largely self-apposed pseudopod whorls. Depending on the microbes studied, pseudopod coils will transform either to ribosome-studded replicative vacuoles (23) or to organelle exclusion zones (34). The microbe-apposed membranes of the engulfing pseudopods are drawn bold.
Coiled pseudopods were randomly observed with a variety of microbes (for a review, see reference 37), but further studies of coiling phagocytosis have concentrated on Legionella pneumophila and Borrelia burgdorferi. Our group could demonstrate that coiling phagocytosis of B. burgdorferi comprises several unique events. In sequence, these are the participation of an unilateral pseudopod, the nonappearing membrane fusion following the first completed rotation of this pseudopod, the ongoing rotation leading to pseudopod whorls with largely self-apposed plasma membranes, the fusion and subsequent dissipation of the apposed membranes, and finally the nonlysosomal degradation of the engulfed Borrelia within the cytosol (34–36, 38). These unique features not only are related to several basic aspects of phagocyte biology such as microbe recognition, membrane turnover, and particle uptake and processing but also may contribute to the pathogenesis of Lyme disease. Therefore, insights into the mechanisms of coiling phagocytosis may shed light on unsolved problems in both these fields.
However, progress in the work on coiling phagocytosis was hampered by two major difficulties. First, the sequence of events involved in this process has been described phenotypically, but the factors which promote coiling phagocytosis are not yet known. It is not even clear whether coiling phagocytosis reflects either a microbial strategy, a specific response of the phagocyte, or a random event. Second, phagocytes use coiling and conventional phagocytosis as well as macropinocytosis simultaneously for the uptake of B. burgdorferi, which makes the interpretation of experimental effects difficult and requires electron microscopy to distinguish between the different mechanisms. The present study addressed these problems by trying to classify coiling phagocytosis.
In this study, the possible role of microbial shape, motility, and viability in the onset of coiling phagocytosis was determined by comparing the frequencies of coiling phagocytosis for different spirochetes with similar morphologies and for viable (motile) versus killed (nonmotile) Borrelia. Bioreagents which either inhibit conventional phagocytosis or stimulate membrane ruffling were tested for their possible effects on the frequencies of coiling versus conventional phagocytosis. The results obtained thereby indicate that coiling phagocytosis of B. burgdorferi is initiated by heat- and aldehyde-insensitive moieties on the surfaces of the microbes and further regulated by signals on the side of the monocytes. With granulocyte-macrophage colony-stimulating factor (GM-CSF) and phorbol 12-myristate 13-acetate (PMA), we found two substances which increase the frequency of coiling phagocytosis selectively. Apart from these results, the formation of surplus pseudopods was noticed. The surplus pseudopods represent a hitherto unrecognized feature of coiling phagocytosis possibly caused by the lateral spreading of intracellular signals which control pseudopod extension.
MATERIALS AND METHODS
Reagents.
The separation media Nycoprep, Polymorphprep, and dextran 500 were obtained from Life Technologies, Eggenstein, Germany; PMA, N-acetyl-S-geranylgeranyl-l-cysteine (AGGC), genistein, staurosporine, bisindolylmaleimide I-HCl (BIM), okadaic acid, and wortmannin were from Calbiochem, Bad Soden, Germany; 4-bromophenacyl bromide (4BPB) was from ICN Biomedicals, Eschwege, Germany; recombinant human GM-CSF and basic fibroblast growth factor (bFGF) were from R&D Systems, Bad Nauheim, Germany; endothelial cell growth supplement (ECGS) prepared from bovine brain, lipopolysaccharide (LPS) prepared by phenol extraction from Escherichia coli serotype O111:B4, N-formyl-Met-Leu-Phe (fMLP), 5-N,N-dimethyl-amiloride (DMA), bafilomycin A1 and sodium azide (NaN3) were from Sigma, Deisenhofen, Germany. The remaining chemicals were from Merck, Darmstadt, Germany, and all cell culture reagents were from BioConcept, Umkirch, Germany.
Phagocytes and platelets.
Peripheral blood leukocytes and platelets were isolated from leukocyte-rich plasma (generously provided by the blood bank at the University Hospital of Erlangen) of healthy human blood donors. All preparative steps were performed in polyethylene tubes at room temperature; Ca/Mg-free Dulbecco’s phosphate-buffered saline supplemented with 0.13% (wt/vol) sodium EDTA and 1% (vol/vol) heat-inactivated fetal calf serum (FCS) was used for washings and dilutions. Peripheral blood mononuclear cells (PBMC) and polymorphonuclear cells (PMNC) were isolated by buoyant density gradient centrifugation procedures with the separation media Nycoprep for the PBMC and Polymorphprep for the PMNC, as specified by the manufacturer’s manual (30). The monocytes were further enriched by removing the CD2+ lymphocytes from the PBMC fraction via a rosetting step on ice with neuraminidase-treated sheep erythrocytes. The eosinophils were negatively enriched by removing the neutrophils from the PMNC fraction by means of magnetic bead-coupled anti-CD16 monoclonal antibodies in a magnetic cell sorter. All phagocytes were checked for viability by means of trypan blue exclusion, counted, resuspended in RPMI 1640 culture medium containing 10% (vol/vol) heat-inactivated FCS (RPMI-FCS), and allowed to recover for 1 h at room temperature prior to the phagocytosis assay. Platelets were enriched from the leukocyte-rich plasma by removing contaminating erythrocytes via sedimentation with dextran 500 and contaminating leukocytes via centrifugation at low speed and were allowed to recover in RPMI-FCS at 37°C until use.
Spirochetes.
Strains from six different Leptospira interrogans serovars (copenhageni, icterohaemorrhagiae Bücker, grippotyphosa Mallersdorf, pomona, hardjo, and sejroe) from our own strain collections were grown in Ellinghausen-McCullough-Johnson-Harris leptospire culture medium. Treponema phagedenis (7) was cultured in thioglycolate broth. The eight B. burgdorferi sensu lato strains (B. burgdorferi sensu stricto, B. afzelii, and B. garinii; for details see Table 1 and Fig. 2) and two strains of relapsing fever Borrelia (B. turicatae and B. parkeri [50]) were cultured in BSK-H culture medium supplemented with 6% (vol/vol) heat-inactivated rabbit serum and 2 mM l-glutamine. Only highly motile spirochetes growing in log phase were used for the experiments. Prior to incubation, the spirochetes were washed in Hanks’ balanced salt solution, counted, and aliquoted in RPMI-FCS.
TABLE 1.
Characterization of B. burgdorferi sensu lato strains used in this study
| Genospecies | Strain | Passage no. | Biological origina | Geographical origin | Reference or source |
|---|---|---|---|---|---|
| B. burgdorferi sensu stricto | LW2 | >200 | Human ligament | Germany | 21 |
| B31 | >200 | Tick | United States | ATCC 35210 | |
| ZS7 | 8 | Tick | Germany | 42 | |
| B. afzelii | PLe | 10 | Human EM | Germany | 51 |
| B29 | >100 | Tick | Germany | 43 | |
| B. garinii | BITS | >100 | Tick | Italy | 11 |
| PBi | 8 | Human CSF | Germany | 51 | |
| 387 | >100 | Human CSF | Germany | 51 |
EM, erythema migrans; CSF, cerebrospinal fluid.
FIG. 2.
Dendrogram showing the phylogenetic relationship of the different spirochetes used in this study.
Pretreatment of the spirochetes.
Aliquots of B. burgdorferi sensu stricto LW2 were killed by various physical and chemical methods (for details, see Table 2), the effectiveness of which was checked by light microscopy. Portions of the aliquots were either directly heated, illuminated with UV light, or treated with a sonifier, or others were centrifuged first, and the B. burgdorferi pellets were resuspended in either ethanol, glutaraldehyde, or p-formaldehyde. The aldehyde-treated spirochetes were subsequently incubated with sodium borohydride to block reactive aldehyde groups (27). The chemically killed microbes were washed carefully and resuspended in fresh RPMI-FCS before being added to the monocytes, whereas this was not necessary for the physically killed microbes.
TABLE 2.
Methods used to kill the Borrelia and the effect on frequency of coiling phagocytosis by human monocytes
| Tool | Application | Time | Subsequent washing | Coiling phagocytosis |
|---|---|---|---|---|
| Ethanol | 70% (vol/vol) | 15 min | Yes | Unaffected |
| Glutaraldehyde | 2.5% (vol/vol) | 15 min | Yesa | Unaffected |
| p-Formaldehyde | 4% (wt/vol) | 15 min | Yesa | Unaffected |
| Laboratory oven | 80°C | 5 min | No | Unaffected |
| 32-W UV bulb | 1-cm distance | 30 min | No | Unaffected |
| 30-W sonifier | 20 kHz | 15 s (twice) | No | Abolished |
Additional treatment with 1% (wt/vol) sodium borohydride for 10 min.
Pretreatment of the phagocytes.
Aliquots of monocytes were stimulated by adding either GM-CSF (41), bFGF (32), ECGS as a crude extract of acidic fibroblast growth factor (28), PMA (22), fMLP (8), LPS (8), or autologous platelets to the incubation medium. Enzymes were inhibited as follows: protein tyrosine kinases by addition of genistein (19), protein serine/threonine kinases by staurosporine (19), protein phosphatases 2A and 1 by okadaic acid (20), and protein kinase C by BIM (48). Other approaches were the inhibition of phosphatidylinositol 3-kinase by wortmannin (2), phospholipase A2 by 4BPB (26), geranylgeranyltransferase I by AGGC (9), the Na+/H+ antiport by DMA (22), and the V-type H+-ATPases by bafilomycin A1 (22). In addition, monocyte aliquots were killed by poisoning the cells with NaN3. All of these bioreagents were administered 10 min prior to the incubation with B. burgdorferi sensu stricto LW2. Except for the monocyte aliquots treated with NaN3, which were carefully washed before incubation, the substances remained in the incubation medium, and the concentration of each (for details, see Fig. 6) was readjusted to the final volume when the spirochetes were added.
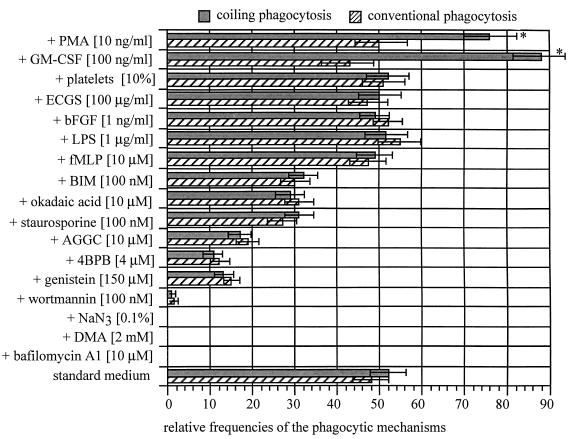
FIG. 6.
Effects of various stimulatory and inhibitory bioreagents on the frequency of coiling and conventional phagocytosis by human monocytes. Human monocytes (2 × 106) were pretreated with substances which either inhibit conventional phagocytosis or stimulate membrane ruffling. After 10 min, B. burgdorferi cells (2 × 107) were added for another 45 min; only the incubation medium containing NaN3 was replaced by normal medium. The frequencies of phagocytosis were determined by electron microscopy as described in Materials and Methods; untreated monocytes gave the spontaneous phagocytic activity, set at 100%. The normalized results are expressed as means ± standard errors of the means of results for usually three (five for control, PMA, and GM-CSF) separate experiments using monocytes from different donors. All inhibitors of phagocytosis reduced coiling and conventional phagocytosis equally, regardless how strong their inhibitory effect was. The ruffling-stimulating or activating substances did not affect the uptake of Borrelia except for PMA and particularly GM-CSF, which increased the frequency of coiling phagocytosis selectively (*, P = 0.05 by the χ2 test) compared with the spontaneous uptake.
Phagocytosis assay.
For each experiment, 2 × 106 monocytes and 2 × 107 spirochetes, each in 0.5 ml of RPMI-FCS, were mixed in polypropylene tubes, giving a total incubation volume of 1.0 ml. In one experiment, pelleted monocyte aliquots were resuspended in 1.0 ml of cell-free culture supernatant from B. burgdorferi sensu stricto LW2. Incubation generally took place at 37°C under 7% CO2 for 45 min and was stopped by adding cold Ito’s fixative (24) to the cell suspensions. Usually, each experiment was performed in triplicate (some in quintuplicate) with monocytes from different individuals. The frequency of phagocytosis observed with untreated monocytes for untreated spirochetes, reflecting the spontaneous frequency of phagocytosis, was used as a control.
Electron microscopy.
Following fixation for 4 h at 4°C, the specimens were prepared for electron microscopy according to standard protocols (18). Briefly, they were postfixed with reduced osmium, encapsulated in agar, stained en bloc with uranyl acetate and phosphotungstic acid, dehydrated in a series of graded ethanolic solutions ending with pure acetone, and then embedded in Epon 812. Ultrathin sections were cut and placed onto 200-mesh standard square copper grids, contrasted with uranyl acetate and lead citrate, and examined with a Zeiss type 906 transmission electron microscope.
Evaluation of the results.
First, all experiments performed with the aliquoted monocytes from the same individual donor were analyzed. Therefore, two different ultrathin sections from each experiment and the control were scored in a blind fashion by counting the incidences of coiling and conventional phagocytosis for at least 100 randomly chosen monocytes. Proportionally expressed, this gave the frequencies of coiling and conventional phagocytosis. Then, the frequencies of the control (reflecting the spontaneous phagocytic activity of the monocytes) was set at 100%, and the frequencies of the experiments were calculated proportionally to that of the control. This normalization of the values from the different individuals allowed, in a second step, the comparison between the triplicate or quintuplicate performances. The differences were analyzed by the χ2 test, and a P of <0.05 was considered to be significant.
RESULTS
Morphologically similar spirochetes induce different frequencies of coiling phagocytosis.
If coiling phagocytosis is triggered by a certain microbial morphology, then microbes with similar shapes should induce similar frequencies of coiling phagocytosis. Therefore, different strains of B. burgdorferi sensu lato as well as relapsing fever Borrelia, Treponema and Leptospira strains, which all have an elongated, helical form, were compared for the frequency of coiling phagocytosis.
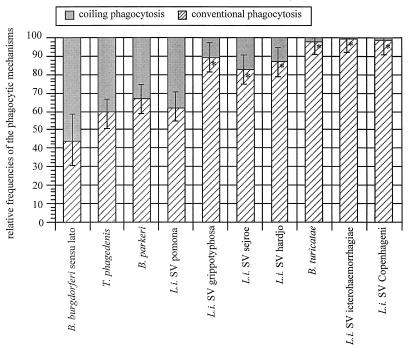
For all different strains of B. burgdorferi sensu stricto, B. garinii, and B. afzelii, the frequency of coiling phagocytosis was in the range of 40 to 60% of total phagocytosis, regardless of their genospecies, geographic or biological origin, or how often they had been subcultured (Table 1). Therefore, the mean of these frequencies was used for B. burgdorferi sensu lato (Fig. 3). In contrast, considerable differences were seen in the rate of coiling phagocytosis among the other spirochetes investigated (Fig. 3). With regard to their frequencies of coiling phagocytosis, a pattern which placed the spirochetes in different groups emerged. T. phagedenis was the only other spirochete which induced coiling phagocytosis almost as frequently as B. burgdorferi (30 to 40%). B. parkeri and L. interrogans SV pomona reached about half the frequency of B. burgdorferi (10 to 30%). The next group, consisting of the L. interrogans SVs grippotyphosa Mallersdorf, sejroe, and hardjo, showed a low frequency of coiling phagocytosis (5 to 20%), whereas this uptake mechanism was very occasionally found in the final group consisting of B. turicatae and the L. interrogans SVs icterohaemorrhagiae Bücker and copenhageni (1% or less). Obviously, the frequencies of coiling phagocytosis observed with the different spirochetes were not correlated to their evolutionary relationship (Fig. 2).
FIG. 3.
Frequencies of coiling and conventional phagocytosis observed with different spirochetes. Human monocytes (2 × 106) were incubated with different spirochetes (2 × 107) for 45 min, and the frequencies of phagocytosis were determined by electron microscopy as described in Materials and Methods. The normalized results are expressed as means ± standard errors of the means of results from three separate experiments using monocytes from different donors, but the value given for B. burgdorferi sensu lato summarizes the mean values for the eight different species tested (Table 1). For the differences between B. burgdorferi sensu lato and the other spirochetes, a P of <0.05 was considered to be significant (*). Although they are quite similar in morphology, the different spirochetes induced totally different frequencies of coiling phagocytosis which show no correlation with their phylogenetic relationship. L.i., L. interrogans.
Coiling phagocytosis of spirochetes involves surplus pseudopods.
The pseudopod coils were more pronounced with the B. burgdorferi sensu lato species than with the other spirochetes. However, no differences between the different spirochetes were observed regarding the further fate of the pseudopod coils (Fig. 4E). Briefly, the pseudopod-microbe complexes were moved into the monocytes, where the granular cytoplasms of the pseudopods gave rise to large organelle exclusion zones. The apposing membranes of the coiled pseudopods fused to electron-dense lines which subsequently disintegrated. Although host cell vesicles, especially lysosomes, were not present in the vicinity of the spirochetes within the organelle exclusion zones, the microbes were degraded.
FIG. 4.
Electron micrographs showing the uptake of various spirochetes by human monocytes. Human monocytes (2 × 106) were incubated with various spirochetes (2 × 107; arrows) for 45 min. Pseudopod coils are shown for B. afzelii (A; bar = 0.1 μm), T. phagedenis (B; bar = 0.4 μm), L. interrogans SV icterohaemorrhagiae Bücker (C; bar = 0.5 μm), and B. parkeri (D; bar = 0.5 μm); in panels A, C, and D, the pseudopod coils are partly covered by contrarotating surplus pseudopods (arrowheads). In panel E, a pseudopod coil enwrapping an L. interrogans SV pomona organism has already been transformed to an organelle exclusion zone (asterisk) within the cytoplasm of the monocyte. The remnants of the fused pseudopod membranes are clearly visible (bar = 2.0 μm). The pseudopod whorls are easily distinguished from the funnel-like, symmetrical surface extensions of conventional phagocytosis, shown in panel F for a B. burgdorferi sensu stricto organism (bar = 0.2 μm). The asterisk in panel D indicates a spacious phagosome.
A hitherto unrecognized feature, which was frequently observed with coiling phagocytosis of all spirochetes, was the formation of surplus pseudopods (Fig. 4A, C, and D). In as much as 20% of the pseudopod whorls, the most peripheral rotation of the coiled pseudopod was covered by an additional pseudopod pointing in the opposite direction. These contrarotating pseudopods did not contact the enwrapped spirochetes but were apposing the pseudopod whorls. Both the initial coiling pseudopods and the surplus pseudopods were clearly distinct from the funnel-like surface extensions which engulfed the spirochetes in conventional phagocytosis (Fig. 4F).
Both viable (motile) and killed (nonmotile) microbes but neither fragmented B. burgdorferi cells nor culture supernatant induce coiling phagocytosis.
If highly motile microbes eventually rolled themselves into phagocyte pseudopods, then only live (motile) but not killed (nonmotile) microbes should induce coiling phagocytosis. Therefore, monocytes were incubated with B. burgdorferi sensu stricto LW2 cells killed by different chemical and physical methods (Table 2).
It was found that live (motile) and killed (nonmotile) B. burgdorferi cells were engulfed with similar frequencies of coiling phagocytosis. Furthermore, as long as the overall morphology remained intact, there was no obvious difference between the different methods used to kill the microbes. In contrast, neither conventional nor coiling phagocytosis was observed when the microbes were disintegrated to small fragments by use of a sonifier. In this case, the monocytes contained numerous spacious vacuoles filled with small particles of variable size, but it was not possible to unequivocally identify this material as spirochetal fragments. Phagocytic structures were also absent when monocytes were incubated with cell-free supernatant from B. burgdorferi sensu stricto LW2 cultures instead of the microbes (data not shown).
To reaffirm that coiling phagocytosis was actively driven by the phagocytes, monocytes were killed by blocking their oxidative metabolism with NaN3 before being incubated with viable microbes. In this experiment, B. burgdorferi cells still attached to the cell surfaces of the monocytes, but no spirochetes were detected inside the killed phagocytes (Fig. 5A).
FIG. 5.
Electron micrographs showing the effects of various bioreagents on the phagocytosis of B. burgdorferi by human monocytes. Human monocytes (2 × 106) were pretreated for 10 min and then incubated with B. burgdorferi cells (2 × 107) for another 45 min. (A) Borrelia (arrows) are not found inside monocytes which had been poisoned with 0.1% NaN3 (bar = 1.0 μm). (B) Upon treatment with 10 μM bafilomycin A1, monocytes still extend membrane ruffles (asterisks) but do not phagocytose Borrelia (bar = 2.0 μm). (C) Although 100 nM wortmannin almost completely inhibits phagocytosis, Borrelia (arrows) are occasionally internalized in spacious vacuoles (asterisk) reminiscent of macropinosomes (bar = 0.4 μm). (D) GM-CSF at 100 ng/ml induces a dramatic increase in the rate of coiling phagocytosis (bar = 0.3 μm). (E) Even abundant membrane ruffles of monocytes, as seen in the presence of 10% serum enriched with activated platelets, do not spontaneously form whorls (bar = 0.6 μm). (F) Empty pseudopod coils possibly indicating spontaneously coiled pseudopods, such as this one (asterisk) displayed by an untreated monocyte, are an extremely rare occurrence (bar = 0.6 μm).
Surface membrane folds are necessary but alone not sufficient for coiling phagocytosis.
If pseudopod whorls were formed spontaneously by surface folds, then the frequency of coiling phagocytosis should parallel the frequency of membrane ruffles, and coiling phagocytosis should not be restricted to a certain object. Therefore, the sections were screened for the spontaneous formation of pseudopod whorls, and monocytes were pretreated with bioreagents known to enhance membrane ruffling before being incubated with B. burgdorferi sensu stricto LW2.
When looking for spontaneous coiling, we observed a total of three empty pseudopod whorls in the several hundreds of sections investigated (Fig. 5F). The growth factors bFGF and ECGS, the chemotaxins fMLP and LPS, and particularly platelet-rich serum enhanced membrane ruffling without affecting the uptake of B. burgdorferi (Fig. 5E and 6). No matter how pronounced membrane ruffling was, no increase in the frequency of coiling phagocytosis was noticed with these substances. In contrast, both the growth factor GM-CSF and the phorbol ester PMA not only stimulated membrane ruffling but enhanced the frequency of coiling phagocytosis between 1.5- and 2-fold, whereas conventional phagocytosis was not increased (Fig. 5D and 6).
To see whether the effect of GM-CSF was restricted to the monocytic lineage, neutrophils and eosinophils were tested in addition (Fig. 7). With these two polymorphonuclear phagocytes, the increase in the frequency of coiling phagocytosis was 2.5-fold for the neutrophils and, most pronounced, 5-fold for the eosinophils. Since the spontaneous frequency of coiling phagocytosis in untreated neutrophils and eosinophils was much lower than for untreated monocytes (32 and 17% versus 43%), in the end the frequencies following stimulation by GM-CSF were about the same for the three phagocyte populations (80 to 86%).
FIG. 7.
Effects of GM-CSF on the frequency of coiling phagocytosis by different human phagocytes. Human monocytes, neutrophils, and eosinophils (2 × 106) isolated from the same individual (different from those represented in Fig. 6), were incubated with B. burgdorferi cells (2 × 107) for 45 min in the presence or absence of GM-CSF (100 ng/ml) added 10 min before. The bars represent the relative frequencies of coiling and conventional phagocytosis with GM-CSF and without, determined by electron microscopy as described in Materials and Methods, given as means ± standard errors of the means of triplicate determinations. Essentially the same results were observed in two additional identically performed experiments with phagocytes from different donors, and the results shown are therefore considered to be representative. PMA and especially GM-CSF increase the frequency of coiling phagocytosis selectively. The stimulated frequencies of the different phagocyte populations peak at about the same level, although their spontaneous frequencies are quite different.
Coiling phagocytosis is closely related to conventional phagocytosis but not to macropinocytosis.
Coiling and conventional phagocytosis of B. burgdorferi are always observed simultaneously with almost the same frequencies and can be distinguished only at the electron microscopic level. This not only makes the evaluation of experimental effects laborious but also suggests a close relationship between these two uptake mechanisms. To see whether coiling and conventional phagocytosis can be separated, monocytes were pretreated with various bioreagents which are known to inhibit phagocytosis before incubation with B. burgdorferi sensu stricto LW2.
The results of these experiments are shown in Fig. 5B and C and 6. It was found that all of the phagocytosis inhibitors equally decreased the frequencies of both coiling and conventional phagocytosis. With respect to the differences in efficacy, the substances can be classified in three groups. Blockade of ATP-requiring processes with bafilomycin A1, acidification of the submembranous cytosol with DMA, or inhibition of phosphatidylinositol 3-kinase by wortmannin virtually abolished phagocytosis; inhibition of protein tyrosine kinases by genistein and blockade of geranylgeranyltransferase I by AGGC and phospholipase A2 by 4BPB reduced the frequency of phagocytosis by approximately 50%; whereas inhibition of protein serine/threonine kinases by staurosporine, protein phosphatases 2A and 1 by okadaic acid, and protein kinase by BIM resulted in only about 25% reduction of phagocytosis. In general, these inhibitors did not affect the occurrence of membrane folds, except for the most effective substances, of which bafilomycin A1 and DMA almost completely and wortmannin also considerably inhibited macropinocytosis.
DISCUSSION
The aim of this electron microscopic study was to classify coiling phagocytosis of B. burgdorferi by human monocytes, first by determining the role of the microbes and the phagocytes in this process and second by characterizing its relationship with conventional phagocytosis and macropinocytosis, the two major pseudopod-based uptake mechanisms of the monocytes. The results obtained demonstrate that coiling phagocytosis is an active and selective process of the phagocytes, initially triggered by heat- and aldehyde-insensitive moieties of the microbial surface. As far as the phagocytes are concerned, these results suggest that coiling and conventional phagocytosis are very closely related, most likely starting from the same phagocytosis-promoting receptor(s) which remain to be identified. Apart from the insights into its mechanisms, this study revealed an additional feature of coiling phagocytosis which has not been recognized so far, namely, the involvement of contrarotating surplus pseudopods.
It has for long been known that various particles, even when opsonized equally, are phagocytosed with different frequencies, which has been attributed to their different morphologies (5). Possibly, differences in microbial morphology may account not only for different frequencies of one phagocytic mechanism but also for the use of different uptake mechanisms such as conventional and coiling phagocytosis. In the present study, however, totally different frequencies of coiling phagocytosis were noticed for morphologically similar spirochetes under standardized experimental settings, which rules out unequivocally that coiling phagocytosis is triggered by a certain microbial shape. It appears that morphologically alike Legionella strains too induce different frequencies of coiling phagocytosis (reviewed in reference 13), and the importance of a certain microbial shape had previously been questioned by the morphological variety of the microbes which were randomly found to be enwrapped by pseudopod coils (for a review, see reference 37). However, the disparity of methods applied to these former studies limits comparative conclusions and may give rise to some debate.
Although not dependent on a certain morphology, coiling phagocytosis nevertheless is restricted to a minimum particle size and is not triggered by soluble factors, as neither supernatant from B. burgdorferi cultures nor spirochetal fragments induced this mechanism. Comparable results were obtained in the Legionella model where whole bacteria but not liposomes containing the major outer membrane protein of L. pneumophila induced coiling phagocytosis (3).
For trypanosomatides, it has been suggested that coiling phagocytosis results from the movements of the flagellum, rolling these protozoa into the membrane veils to which they are attached (10, 45). This hypothesis was supported by the finding that coiling phagocytosis was no longer observed with glutaraldehyde-killed Leishmania (10). In contrast, both in the present study and in the Legionella model (6, 23, 33), the use of either motile (live) or nonmotile (killed) microbes did not alter the frequency of coiling phagocytosis. A causative role of microbial motility is also questioned by the fact that pseudopod coils were randomly observed with motile as well as nonmotile microbes. However, this random nature of the previous observations here again limits their use for comparative conclusions. A likely explanation for the contradictionary results for the glutaraldehyde-killed Leishmania (10) is the subsequent handling; we found it mandatory to treat aldehyde-killed Borrelia cells with sodium borohydride before using them in the phagocytosis assays; otherwise, the monocytes would not engulf them.
The lack of difference between viable and killed microbes indicates that coiling phagocytosis is actively driven by the phagocytes and not by the microbes. This distinguishes coiling phagocytosis from nonclassical uptake mechanisms such as the forced endocytosis typical of apicomplexan protozoa (4, 44), the directed phagocytosis of some enteroinvasive bacteria (40), or the triggered macropinocytosis of Salmonella (16), which all represent microbial invasion strategies. On the side of the phagocytes, coiling phagocytosis obviously is a regulated mechanism, because the monocytes used it selectively for certain spirochetes, which is inconsistent with an accidental trapping of pericellular microbes. Accordingly, stimulation of membrane ruffling by bFGF, ECGS, or platelets was not accompanied by an increase in the frequency of coiling phagocytosis, and also the consistent observation of very few empty pseudopod whorls does not support the view that coiling phagocytosis is a spontaneous event. The mechanisms by which PMA and especially GM-CSF increase the frequency of coiling phagocytosis remain to be determined, but considering the rather prompt onset of this effect, the upregulation of receptors and/or the activation of signal transduction pathways may be involved.
Although eukaryotic cells definitely display different types of membrane folds, it seems unlikely that coiling phagocytosis is the result of a committed coiling pseudopod. Video monitoring revealed that the engulfing pseudopods are not preexistent, as one would expect in this case, but are formed in reaction to the attachment of the Borrelia (39), which suggests a role of attachment-induced intracellular signals. Also, recent work indicates that different pseudopod-based activities of the phagocytes involve the differential regulation of one pool of pseudopods rather than the existence of different populations of pseudopods (2), and in conventional phagocytosis itself, morphological differences between phagocytic cups depend on the different receptors involved rather than on different pseudopods (1, 46). Therefore, it is more likely that either a certain coiling-promoting receptor or distinct intracellular coiling-promoting signals mediate pseudopod coils.
The phagocytosis-promoting receptor(s) for B. burgdorferi has yet to be determined. In the Legionella model, antibody-mediated blockade of the complement-receptors (CR) of types 1 and 3 (CR1 and CR3) almost completely inhibited the adherence and subsequent uptake of L. pneumophila Philadelphia 1 (29, 31). Since this particular microbe is exclusively internalized via coiling phagocytosis (23), it would be natural to assume that CR1 and/or CR3 is the putative coiling-promoting receptor if CR-mediated phagocytosis were not a classical model of zipper-type phagocytosis. Moreover, the complement-fixing protein of L. pneumophila, the major outer membrane protein, induces conventional but not coiling phagocytosis when incorporated into liposomes (3), and other Legionella strains induce conventional phagocytosis as well (12, 15, 33). Obviously, the appearance of pseudopod whorls in CR-mediated phagocytosis is not the rule but the exception, suggesting the superimposing effect of a coiling-promoting factor.
It has long been known that most spirochetes, including B. burgdorferi (35), adhere in a polar orientation with their free end in motion, which has been attributed to a polar clustering of spirochetal adhesins upon contact with the host cells (14). An asymmetrical capping of microbial adhesins could result in an asymmetrical ligand-receptor interaction with an unilateral pseudopod. Alternatively, an asymmetrical clustering of phagocytosis-promoting host cell receptors could have the same effect. The view of coiling phagocytosis as an irregularity in conventional phagocytosis easily explains why all bioreagents which inhibited conventional phagocytosis also inhibited coiling phagocytosis. For B. burgdorferi, only plasminogen and urokinase-type plasminogen activator (uPA) have been shown to cluster at either pole of this organism (25). Although both the plasminogen and uPA receptors on the host cell do not mediate phagocytosis, the uPAR forms multimeric complexes with other membrane receptors, including CR3 (49), which do so. In such receptor complexes, these phagocytosis-promoting receptors may not be capable of guiding the enclosing pseudopods symmetrically.
Whereas this asymmetry hypothesis implies that pseudopod coils result from the incapability of the phagocyte to form conventional cups, there is at least one example where the coiling of pseudopods is a physiological event. In the nonendocytic process of myelination, single membrane folds are used by glial cells to enwrap axons, and the resulting pseudopod whorls have axon-apposed as well as self-apposed membrane faces. Thus, the formation of pseudopod whorls may result from a basic coiling-promoting program of metazoan cells, regularly used by glial cells to myelinate axons but occasionally by phagocytes to engulf microbes.
In this respect, the observation that part of the pseudopod whorls in coiling phagocytosis are covered by an additional membrane fold is interesting. In triggered macropinocytosis, the local outburst of overshooting pseudopods has been explained by a lateral spreading of intracellular signals upon attachment of the Salmonella (17). The surplus pseudopods in coiling phagocytosis as well are never directly apposed to the engulfed spirochetes and therefore result from a lateral spreading of signals involved in the generation of unilateral pseudopods, rather than from a classical receptor-ligand interaction. Although this may be the first time that surplus pseudopods have been remarked upon, these structures can be retrospectively seen in several previous publications not only with B. burgdorferi (34, 47), but also with T. brucei (45) and L. pneumophila (23), suggesting that they are a general characteristic of coiling phagocytosis.
In conclusion, the results of this study demonstrate that coiling phagocytosis is neither a random feature of membrane folds in general nor concomitant to other pseudopod-based activities of the phagocytes but rather a selective uptake mechanism obviously triggered by microbial surface moieties. Based on these results, the identification of the phagocytosis-promoting receptors for B. burgdorferi will be most helpful to determine whether coiling phagocytosis reflects an intrinsic coiling-promoting program which diverts the pathway of conventional phagocytosis, or whether pseudopod coils display a fault in the receptor-ligand interaction during the onset of phagocytosis. In this respect, the identification of GM-CSF and PMA as coiling-promoting substances may be a clue as to the regulatory mechanisms involved in coiling phagocytosis.
ACKNOWLEDGMENTS
This work was supported by the Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie (grants 01 KI 9104 and 9503) and by the Deutsche Forschungsgemeinschaft (grant Sp 395/2-1).
We are indebted to Andrea Hilpert for skillful technical assistance and to Tony Simpson and especially Helen Robey, London, England, for critically reading the manuscript and for helpful discussions.
REFERENCES
- 1.Allen L A H, Aderem A. Mechanisms of phagocytosis. Curr Opin Immunol. 1996;8:36–40. doi: 10.1016/s0952-7915(96)80102-6. [DOI] [PubMed] [Google Scholar]
- 2.Araki N, Johnson M T, Swanson J A. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J Cell Biol. 1996;135:1249–1260. doi: 10.1083/jcb.135.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellinger-Kawahara C, Horwitz M A. Complement component C3 fixes selectively to the major outer membrane protein (MOMP) of Legionella pneumophila and mediates phagocytosis of liposome-MOMP complexes by human monocytes. J Exp Med. 1990;172:1201–1210. doi: 10.1084/jem.172.4.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benson L A, Kar S, McLaughlin G, Ihler G M. Entry of Bartonella bacilliformis into erythrocytes. Infect Immun. 1986;54:347–353. doi: 10.1128/iai.54.2.347-353.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Besterman J M, Low R B. Endocytosis: a review of mechanisms and plasma membrane dynamics. Biochem J. 1983;210:1–13. doi: 10.1042/bj2100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bozue J A, Johnson W. Interaction of Legionella pneumophila with Acanthamoeba castellanii: uptake by coiling phagocytosis and inhibition of phagosome-lysosome fusion. Infect Immun. 1996;64:668–673. doi: 10.1128/iai.64.2.668-673.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruckbauer H, Preac-Mursic V, Fuchs R, Wilske B. Cross-reactive proteins of Borrelia burgdorferi. Eur J Clin Microbiol Infect Dis. 1992;11:1–9. doi: 10.1007/BF02098084. [DOI] [PubMed] [Google Scholar]
- 8.Carpentier J L, Lew S P, Paccaud J P, Gil R, Iacopetta B, Kazatchkine M, Stendahl O, Pozzan T. Internalization pathway of C3b receptors in human neutrophils and its transmodulation by chemoattractant receptors stimulation. Cell Regul. 1991;2:41–55. doi: 10.1091/mbc.2.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casey P J. Protein lipidation in cell signaling. Science. 1995;268:221–225. doi: 10.1126/science.7716512. [DOI] [PubMed] [Google Scholar]
- 10.Chang K P. Leishmania donovani: promastigote-macrophage surface interactions in vitro. Exp Parasitol. 1979;48:175–189. doi: 10.1016/0014-4894(79)90097-3. [DOI] [PubMed] [Google Scholar]
- 11.Cinco M, Costantini C, Wilske B, Graziosi G, Trevisan G, Florian F. Use of PCR and specific monoclonal antibodies as rapid method to recognize B. burgdorferi sensu stricto, B. garinii and B. afzelii among Italian isolates of B. burgdorferi. Med Microbiol Immunol. 1994;183:307–313. doi: 10.1007/BF00196681. [DOI] [PubMed] [Google Scholar]
- 12.Cirillo J D, Falkow S, Tompkins L S. Growth of Legionella pneumophila in Acanthamoeba castellanii enhances invasion. Infect Immun. 1994;62:3254–3261. doi: 10.1128/iai.62.8.3254-3261.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dowling J N, Saha A K, Glew R H. Virulence factors of the family Legionellaceae. Microbiol Rev. 1992;56:32–60. doi: 10.1128/mr.56.1.32-60.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellen R P, Dawson J R, Yang P F. Treponema denticola as a model for polar adhesion and cytopathogenicity of spirochetes. Trends Microbiol. 1994;2:114–119. doi: 10.1016/0966-842x(94)90597-5. [DOI] [PubMed] [Google Scholar]
- 15.Elliott J A, Winn W C. Treatment of alveolar macrophages with Cytochalasin D inhibits uptake and subsequent growth of Legionella pneumophila. Infect Immun. 1986;51:31–36. doi: 10.1128/iai.51.1.31-36.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Francis C L, Ryan T A, Jones B D, Smith S J, Falkow S. Ruffles induced by Salmonella and other stimuli direct macropinocytosis of bacteria. Nature. 1993;364:639–642. doi: 10.1038/364639a0. [DOI] [PubMed] [Google Scholar]
- 17.Galán J E. Molecular and cellular bases of Salmonella entry into host cells. Curr Top Microbiol Immunol. 1996;209:43–60. doi: 10.1007/978-3-642-85216-9_3. [DOI] [PubMed] [Google Scholar]
- 18.Glauert A M. Practical methods in electron microscopy. 3/I. Fixation, dehydration and embedding of biological specimens. Amsterdam, The Netherlands: North-Holland Publishing Company; 1975. [Google Scholar]
- 19.Greenberg S, Chang P, Silverstein S C. Tyrosine phosphorylation is required for Fc receptor-mediated phagocytosis in mouse macrophages. J Exp Med. 1993;177:529–534. doi: 10.1084/jem.177.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harbecke O, Lundqvist H, Dahlgren C. Okadaic acid inhibits the signal responsible for activation of the NADPH-oxidase in neutrophils stimulated with serum-opsonized yeast. J Leukocyte Biol. 1996;59:754–762. doi: 10.1002/jlb.59.5.754. [DOI] [PubMed] [Google Scholar]
- 21.Häupl T, Hahn G, Rittig M, Krause A, Schoerner C, Schönherr U, Kalden J R, Burmester G R. Persistence of Borrelia burgdorferi in ligamentous tissue from a patient with chronic Lyme borreliosis. Arthritis Rheum. 1993;36:1621–1626. doi: 10.1002/art.1780361118. [DOI] [PubMed] [Google Scholar]
- 22.Heming T A, Bidani A. Effects of myristate phorbol ester on V-ATPase activity and Na+-H+ exchange in alveolar macrophages. J Leukocyte Biol. 1995;57:600–608. doi: 10.1002/jlb.57.4.600. [DOI] [PubMed] [Google Scholar]
- 23.Horwitz M A. Phagocytosis of the Legionnaires’ disease bacterium (Legionella pneumophila) occurs by a novel mechanism: engulfment within a pseudopod coil. Cell. 1984;36:27–33. doi: 10.1016/0092-8674(84)90070-9. [DOI] [PubMed] [Google Scholar]
- 24.Ito S, Karnovsky M J. Formaldehyde-glutaraldehyde fixatives containing trinitro compounds. J Cell Biol. 1968;39:168a–169a. [Google Scholar]
- 25.Klempner M S, Noring R, Epstein M P, McCloud B, Hu R, Limentani S A, Rogers R A. Binding of human plasminogen and urokinase-type plasminogen activator to the Lyme disease spirochete, Borrelia burgdorferi. J Infect Dis. 1995;171:1258–1265. doi: 10.1093/infdis/171.5.1258. [DOI] [PubMed] [Google Scholar]
- 26.Lennartz M R, Brown E J. Arachidonic acid is essential for IgG Fc receptor-mediated phagocytosis by human monocytes. J Immunol. 1991;147:621–626. [PubMed] [Google Scholar]
- 27.Lillie R D, Pizzolato P. Histochemical use of borohydrides as aldehyde blocking reagents. Stain Technol. 1972;47:13–16. doi: 10.3109/10520297209116528. [DOI] [PubMed] [Google Scholar]
- 28.Maciag T, Cerundolo J, Ilsley S, Kelley P R, Forand R. An endothelial cell growth factor from bovine hypothalamus: identification and partial characterization. Proc Natl Acad Sci USA. 1979;76:5674–5678. doi: 10.1073/pnas.76.11.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marra A, Horwitz M A, Shuman H A. The HL-60 model for the interaction of human macrophages with the Legionnaires’ disease bacterium. J Immunol. 1990;144:2738–2744. [PubMed] [Google Scholar]
- 30.Nycomed Pharma AS. Isolation of blood cells. 4th ed. Oslo, Norway: Nycomed Pharma AS; 1993. [Google Scholar]
- 31.Payne N R, Horwitz M A. Phagocytosis of Legionella pneumophila is mediated by human monocyte complement receptor. J Exp Med. 1987;166:1377–1389. doi: 10.1084/jem.166.5.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pepper M S, Sappino A P, Stücklin R, Montesamo R, Orci L, Vassalli J D. Upregulation of urokinase receptor expression on migrating endothelial cells. J Cell Biol. 1993;122:673–684. doi: 10.1083/jcb.122.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rechnitzer C, Blom J. Engulfment of the Philadelphia strain of Legionella pneumophila within pseudopod coils in human phagocytes. Comparison with other Legionella strains and species. APMIS. 1989;97:105–114. [PubMed] [Google Scholar]
- 34.Rittig M, Krause A, Häupl T, Schaible U E, Modolell M, Kramer M D, Lütjen-Drecoll E, Simon M M, Burmester G R. Coiling phagocytosis is the preferential phagocytic mechanism for Borrelia burgdorferi. Infect Immun. 1992;60:4205–4212. doi: 10.1128/iai.60.10.4205-4212.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rittig M, Kressel M, Häupl T, Burmester G R. Sectional uptake and cytosolic processing of Borrelia burgdorferi by human phagocytes. In: Axford J S, Rees D H E, editors. Lyme borreliosis. NATO ASI Series in Life Sciences. Vol. 260. New York, N.Y: Plenum Press; 1994. pp. 241–247. [Google Scholar]
- 36.Rittig M G, Häupl T, Krause A, Kressel M, Groscurth P, Burmester G R. Borrelia burgdorferi-induced ultrastructural alterations in human phagocytes: a clue to pathogenicity? J Pathol. 1994;173:269–282. doi: 10.1002/path.1711730311. [DOI] [PubMed] [Google Scholar]
- 37.Rittig M, Häupl T, Burmester G R. Coiling phagocytosis—a way for MHC class I presentation of bacterial antigens? Int Arch Allergy Immunol. 1994;103:4–10. doi: 10.1159/000236598. [DOI] [PubMed] [Google Scholar]
- 38.Rittig M G, Kuhn K H, Dechant C A, Gauckler A, Modolell M, Ricciardi-Castagnoli P, Krause A, Burmester G R. Phagocytes from both vertebrate and invertebrate species use “coiling” phagocytosis. Dev Comp Immunol. 1996;20:393–406. doi: 10.1016/s0145-305x(96)00023-7. [DOI] [PubMed] [Google Scholar]
- 39.Rittig M G, Seack K H, von Briesen H, Kreutz M, Sander U, Andreesen R. Macrophages: the function of phagocytosis. Videotape C1922. Göttingen, Germany: Institut für Wissenschaftlichen Film; 1996. [Google Scholar]
- 40.Rosenshine I, Finlay B B. Exploitation of host signal transduction pathways and cytoskeletal function by invasive bacteria. Bioessays. 1993;15:17–24. doi: 10.1002/bies.950150104. [DOI] [PubMed] [Google Scholar]
- 41.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schaible U, Kramer M D, Justus C W E, Museteanu C, Simon M M. Demonstration of antigen-specific T cells and histopathological alterations in mice experimentally inoculated with Borrelia burgdorferi. Infect Immun. 1989;57:41–47. doi: 10.1128/iai.57.1.41-47.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schönberg A, Camey C, Kahl O, Wilske B, Preac-Mursic V, Hovind-Hougen K. First isolation of Borrelia burgdorferi, the agent of Lyme borreliosis, from Ixodes ricinus (Acari: Ixodidae) in Berlin (West) Zentralbl Bakteriol Hyg Reihe A. 1988;268:487–494. doi: 10.1016/s0176-6724(88)80128-7. [DOI] [PubMed] [Google Scholar]
- 44.Sibley D. Invasion of vertebrate cells by Toxoplasma gondii. Trends Cell Biol. 1995;5:129–132. doi: 10.1016/s0962-8924(00)88964-3. [DOI] [PubMed] [Google Scholar]
- 45.Stevens D R, Moulton J E. Ultrastructural and immunological aspects of the phagocytosis of Trypanosoma brucei by mouse peritoneal macrophages. Infect Immun. 1978;19:972–982. doi: 10.1128/iai.19.3.972-982.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Swanson J A, Baer S C. Phagocytosis by zippers and triggers. Trends Cell Biol. 1995;5:89–93. doi: 10.1016/s0962-8924(00)88956-4. [DOI] [PubMed] [Google Scholar]
- 47.Szczepanski A, Fleit H B. Interaction between Borrelia burgdorferi and polymorphonuclear leukocytes. Phagocytosis and the induction of the respiratory burst. Ann N Y Acad Sci. 1988;539:425–428. [Google Scholar]
- 48.Toullec D, Pianetti P, Coste H, Bellevergue P, Grand-Perret T, Ajakanes M, Baudet V, Boissin P, Boursier E, Loriolle F, et al. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem. 1991;266:15771–15781. [PubMed] [Google Scholar]
- 49.Wei Y, Lukashev M, Simon D I, Bodory S C, Rosenberg S, Doyle M V, Chapman H A. Regulation of integrin function by the urokinase receptor. Science. 1996;273:1551–1555. doi: 10.1126/science.273.5281.1551. [DOI] [PubMed] [Google Scholar]
- 50.Wilske B, Preac-Mursic V, Schierz G, Kühbeck R, Barbour A G, Kramer M. Antigenic variability of Borrelia burgdorferi. Ann N Y Acad Sci. 1988;539:126–143. doi: 10.1111/j.1749-6632.1988.tb31846.x. [DOI] [PubMed] [Google Scholar]
- 51.Wilske B, Preac-Mursic V, Göbel U B, Graf B, Jauris-Heipke S, Soutschek E, Schwab E, Zumstein G. An OspA serotyping system for Borrelia burgdorferi based on reactivity with monoclonal antibodies and OspA sequence analysis. J Clin Microbiol. 1993;31:340–350. doi: 10.1128/jcm.31.2.340-350.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]