Abstract
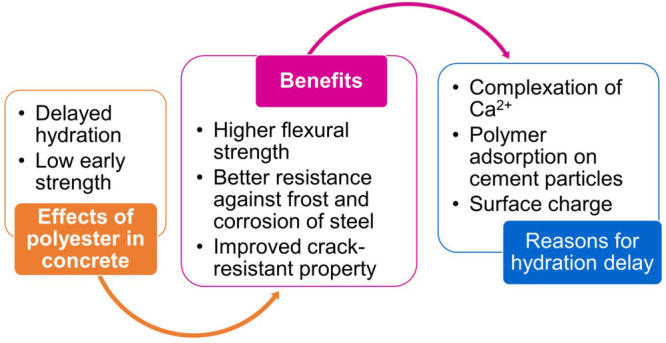
Polymer compounds have become a popular choice for the synthesis of novel products and are being used in cementitious mixtures principally for altering the properties in the fresh state and as repair materials. These polymers are used in various combinations. Their interaction with cement is worth studying because its hydration, followed by setting and hardening, is the primary phenomenon contributing to the strength gain and performance of concrete. This paper summarizes the effects of different polymers on the hydration of cement and the properties of concrete/mortar. Studies have established that the incorporation of polymers as a workability enhancing admixture or for improving strength, durability, and other properties severely affects the early hydration of cement and reduces the overall strength gain in most cases. The hydration retarding effect depends on the charge, architecture, and the amount (wt %) of polymer added. However, owing to the densification of the interfacial transition zone and formation of polymer films/bridges between stacks of calcium hydroxide surfaces and air, the later age properties show beneficial effects such as higher flexural strength, enhanced compressive strength, and modulus of elasticity, better resistance against frost, and corrosion of steel reinforcement. Further, it is seen that the hydration retardation may be mitigated to some extent by the addition of silica fume or zeolite; using a defoaming agent; curing at high temperatures; and following a combination of wet, moist, and dry curing regimes. This review is expected to be helpful to all practicing civil engineers who are the immediate users of these chemicals and are working to achieve quality concrete construction.
1. Introduction
The incorporation of chemical admixtures has revolutionized concrete production by enabling different types of concrete, i.e., pumped concrete, ready mix concrete, self-compacting concrete, polymer concrete, and as the key ingredient in damp proofing and repair materials. With the versatile potential of polymers, their application in the construction industry has become enormous. Interestingly, these polymer compounds are synthesized in various chemical combinations, but to judge the effectiveness of a particular combination is a million-dollar question. These days, various types of polymer compounds are available for preparing concrete with the desired properties. These compounds are mostly used for modifying the properties of concrete in its fresh state, and therefore, their interaction with cement during hydration is obvious. In the chemical fraternity, it is known that the interaction of polymers with cement particles delays hydration and affects the early strength gain. However, the project site engineers who are the immediate users of these chemicals have very little understanding of the interference of polymers on the setting/hardening of cement and other consequences. Even a slight overuse of these chemicals may weaken the construction quality, implicating caution. Therefore, it is necessary to summarize and report the common observations from past works. Additionally, with an increase in the use of industrial wastes in concrete, it becomes even more relevant, especially with the growing production of artificial marble, which contains substantial amounts of unsaturated polymers and is a potential future ingredient as aggregate in concrete from construction demolition sites. Furthermore, the theories of hydration retardation are still not fully understood. This paper presents a review of a few selected studies that have been instrumental in understanding this topic along with the latest advances.
2. Impact of Different Polymers on Cement Hydration and Properties of Concrete
Polymers of different compositions are used in cement and concrete to meet specific requirements such as high flexural strength, impermeability, better adhesion to the old surface in repair works, etc. Broadly, these polymers are categorized as thermoplastics, thermosets, and elastomers. Among these categories, ethylene vinyl acetate,1,2 unsaturated polyester resins,3−5 epoxy resins,6,7 styrene–butadiene latexes,8 and styrene acrylic9 are used to prepare concrete and mortar. Additionally, polymer-based admixtures are used to increase the flow and plasticity of fresh concrete. Polycarboxylate ether (PCE) based admixtures are the most popular and effective plasticizers.
Many researchers have developed polymer-modified concrete either by replacing some portion of cement or by adding it in proportion to cement and have reported higher than control strength and durability.6,10−12 Polymer-modified mortars are widely used as tile adhesives, coatings, waterproofing, and road repair works.8,9 The applications and advantages of concrete prepared with unsaturated polyester resin, fine aggregates, and coarse aggregates have also been presented in various studies.5,12,13 On the contrary, several studies on the interaction of cement with polymers/resins claim that the addition of these materials slows down the hydration process and reduces the hydration peak during the induction and acceleration period1,14−17 resulting in moderate to severe reduction of strength. Additionally, owing to the entrained air, polymer-modified mortar pastes develop a lower compressive strength and increased porosity than the unmodified pastes.2 This effect of hydration retardation and strength reduction is an undesirable outcome. Therefore, the influence of different types of polymers, resins, and latexes used in concrete to increase workability (flow) and strength and for repair works has been summarized in subsequent sections.
2.1. Effect of Ethylene Vinyl Acetate Polymers
The ethylene vinyl acetate copolymers are a type of thermoplastic elastomer polymer that possesses rigidity and flexibility. These polymers are used as additives in concrete and mortar to enhance the modulus of elasticity, bond strength, toughness, and impermeability.18 Silva and Monteiro15 studied the effect of two different polymers viz. a water-soluble polymer (hydroxypropyl methylcellulose) and a latex poly(ethylene-co-vinyl acetate), on the hydration of cement and reported that incorporation of polymer delays the hydration of cement. Hydroxypropyl methylcellulose was seen to form inner products rather than interaction with C3S, whereas the latex prevented the formation of ettringite with the formation of small crystals around C3A.
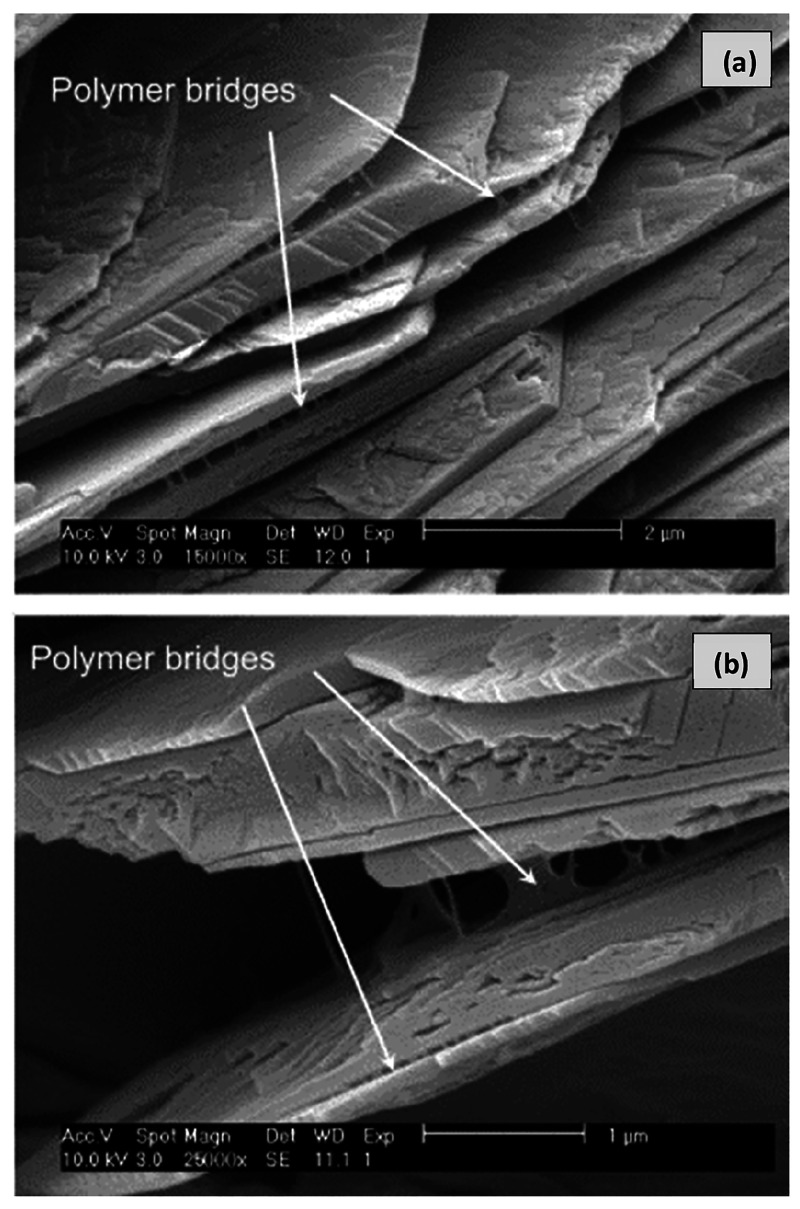
Knapen and Gemert1,2 used water-soluble poly(vinyl alcohol)-acetate, methylcellulose, and hydroxyethylcellulose in a polymer/cement ratio of 1%. This study1 showed evidence of the formation of polymer bridges between the stacks of calcium hydroxide surfaces and air voids (Figure 1). They concluded that polymers adversely affect the hydration of cement by extending the induction period. The delay in hydration was attributed to the possibility of multiple mechanisms in action, such as polymer adsorption on unhydrated and hydrated cement particles, poisoning of C–H and C–S–H, complexation of alkalis, precipitation of insoluble compounds restricting movement of water, formation of protective membrane surrounding cement particles, etc. They further noticed that the polymer possessing the highest viscosity showed the largest delay in hydration, thereby indicating that the increase in viscosity owing to the addition of water-soluble polymers might have limited the dissolution of unhydrated cement. Another study2 by the same authors highlighted the importance of moist and dry curing of mortar samples. They elaborated that cement hydration requires moist curing, whereas the polymer film forms with dry curing. Therefore, the mortar samples were subjected to 2 days of wet and then 5 days of moist followed by 21 days of dry curing. The compressive strength of poly(vinyl alcohol)-acetate modified mortars was comparable to the control; however, the strength of methylcellulose and hydroxyethylcellulose modified mortars was more than 30% lower than the control, which was due to the high amount of entrapped air. The flexural strength was also seen to improve in the poly(vinyl alcohol)-acetate modified mortars.
Figure 1.
Polymer bridges between calcium hydroxide layers.2 Reprinted with permission from ref (18). Copyright 2015 Elsevier Ltd.
2.2. Effect of Unsaturated Polymer Resins
The unsaturated polymer resins (UPRs) are a type of thermosetting polymers, which contain unsaturated and saturated acids, glycols, and cross-linking monomers.3,4 These are low-cost materials and have a wide range of applications. UPRs have been used without cement to prepare polymer concrete.3−5 This composite material has high flexural strength, frost resistance, and corrosion resistance and has applications in building, pavement, and repair materials; electrical insulators and other components; transmission towers, etc.
The studies on the use of UPR for preparing mortar and concrete are mostly accompanied by the hydration retardation effect. Cai et al.13 prepared concrete using cement, fine aggregates, coarse aggregates, and unsaturated polyester resin for road repair applications. The authors reported a low reactivity of cement with unsaturated polyester in the initial stages and complete reaction on a 28-day curing at room temperature. The amount of water used in this study is not specified, thereby making the hydration of the cement unclear. Probably the cement particles worked as fillers in the polyester concrete.
Tawfik et al.19 worked on stabilization of borate waste simulations generated from pressurized water reactors with cement to address environmental issues and explained the possible reasons for hydration retardation. They used different concentrations of borate wastes with cement and emulsion (developed from a water-extended polyester resin containing UPR) to prepare emulsion-cement composite blocks. The UPR was prepared from polyethylene terephthalate wastes. The results showed a considerable decrease in compressive strength with an increase in the concentration of emulsion beyond 3% for pastes containing emulsion and cement. This occurs because first the excess organic polymer forms a thin layer over cement particles and restricts the access of water, thereby retarding hydration and enhancing ettringite formation. Second, the interaction of carboxylate ions (COO–) from the polymer emulsion with Ca2+ cations from cement particles forms a structure that inhibits scission of the polymer chain. Further, even a small quantity of borate, i.e., higher than 1% resulted in a sharp reduction of compressive strength for the borate–emulsion–cement composites. The study concluded the presence of a dual retardation effect of organic polymer and borate salt.
Zhang et al.11 used bisphenol A type UPR mixed with triethanolamine emulsifier as a modifier in concrete for restraining crack propagation and densification of the interfacial transition zone (ITZ). Concrete samples with 3%, 6%, and 9% UPR emulsions were prepared. They used a defoaming agent to cater to the problem of air bubbles in the concrete mixture and inadequate compaction, leading to low compressive strength. In line with previous research, this team also witnessed an increased delay in the hydration of cement with an increase in the UPR content. Concrete with 3%, 6%, and 9% UPR developed approximately 9%, 23%, and 32% lower heats of hydration, respectively. However, the delay in hydration was reduced with an increase in the temperature. The phenomena of delayed hydration were explained as the formation of a polymer film over the cement and hydrated products, which restricts the contact with water; second, the inclusion of polymers increased the viscosity of the mix, which in turn limited the migration rate of Ca2+, SO4–, and OH– for formation of hydration products. The authors proposed 3% UPR as the optimum dosage. Further, they also concluded that 3% UPR improves the microstructure, reduces the pore diameter, narrows the ITZ, densifies the ITZ area, and improves the crack-restraining property. In another study by the same research group,12 the microstructural deterioration of UPR-modified concrete was studied through exposure to freeze–thaw cycles in fresh and salt water. The primary difference from the previous study was that a phase inversion approach was adopted for mixing concrete and preparing specimens. The amount of polymer was limited to 3%. In this approach, deionized water, bisphenol A type UPR, and triethanolamine emulsion were stirred with a magnetic stirrer for 10 min to achieve homogeneous and morphological phase development. The authors observed positive effects with the addition of 3% polymer emulsion in concrete, such as densification of ITZ, pore filling, reduced permeability, increased freeze–thaw resistance, and restraint to the propagation of microdefects. Reasons for these effects were (1) the dehydrated and cured polymer film could have filled the ITZ by reducing the gap between aggregate and hydrated cement gel; (2) the reaction between COO- from UPR and Ca2+ as a product of hydration might have occurred with the formation of calcium-polyester (Ca-UP) compounds, which will result in corresponding effects of depletion of calcium hydroxide, lowering of shrinkage, and finally resulting in enhanced hydration; (3) the formation of an insoluble three-dimensional interpenetrating structure, which increases flexural toughness and relieves internal stresses to resist generation and propagation of cracks; (4) the formation of a three-dimensional reticular structure, which restricts evaporation of free water, reduces pore sizes, and helps in restraining shrinkage.
Li et al.20 examined the properties of mortars prepared with powdered artificial marble as a replacement for cement and fatty acid methyl ester polyoxyethylene ether as an admixture, wherein the artificial marble was prepared using UPR. They found that the addition of both powdered artificial marble and admixture reduces the compressive and flexural strength of mortar, and the reduction in strength increases with increasing the proportion of powdered artificial marble. The possible reasons explained by them are the presence of unsaturated polymers in artificial marble, which might have interfered with the hydration process; and the use of admixture which possessed air-entraining and foaming properties causing a higher quantity of pores in the paste and lower compaction.
2.3. Effect of Epoxy Resins
Epoxy resins are a type of thermosetting polymers. These resins are typically used to improve the rheology of cement-based grouts by increasing the workability and reducing segregation.6 Few studies reported that the addition of epoxy slows the induction and acceleration period of cement hydration.6,7,10,21,22 Anagnostopoulos et al.6 investigated the effect of water-soluble epoxy resin on the strength and durability of cement grouts. They used diglycidyl ether of bisphenol A and an aliphatic amine-based hardener in a ratio of 2.5:1 to synthesize epoxy resin. Cement mortar pastes were prepared with an epoxy resin of up to 30%. It was noticed that the addition of epoxy increased the setting time of pastes. The higher the epoxy content, the more delayed the setting, simultaneously affecting the strength gain for up to 7 days. However, the 28-day and 90-day compressive strength, split tensile strength, and modulus of elasticity of grouts containing 20% and 30% epoxy were significantly improved. It was seen that 5% epoxy grout developed slightly lower than control compressive strength, and the 10% epoxy grout attained comparable to control strength, indicating that higher than 10% epoxy resin provides favorable properties.
Li et al.21 prepared cement mortars by adding 5–20% waterborne epoxy resin in increments of 5%. Mortar specimens were subjected to dry, wet, and wet–dry curing regimes. The compressive strength was seen to decrease with increasing epoxy content; however, the strength of specimens cured under wet curing conditions was the least. Overall, the study concluded that the incorporation of epoxy retards hydration, and the retardation increases with an increase in the epoxy percentage. The mechanism of delay in hydration was explained in another study by the same research group.22 They explained that during initial hydration, the adsorption of epoxy particles on the C3A surface forms a covering layer and hinders the dissolution of C3A and the diffusion of water molecules. Further, the amount of free Ca2+ ions increases due to the dissolution of C3S, and the negatively charged epoxy particles get adsorbed on the negatively charged C3S by bridging through Ca2+ ions to form a stable layer. Epoxy particles adsorbed on C3S act as a barrier and arrest its dissolution further, thereby increasing the concentration of calcium on the surface of C3S. The complexation of Ca2+ delays hydration. Additionally, due to the unavailability of free water molecules, epoxy particles are assumed to form a film with hydrated and unhydrated cement particles. Li et al.7 incorporated 3%, 6%, and 9% waterborne epoxy resin into cement pastes and observed a delay in the acceleration period of hydration. The period of hydration retardation was seen to increase with an increase in the epoxy content. However, this delay was reduced by increasing the curing temperature from 20 to 40 °C. The hydration kinetics were computed, which revealed a reduction in C–H content with an increase in the amount of epoxy. The compressive strength of mortar was observed to decrease by approximately 9%, 18%, and 24% for 3%, 6%, and 9% epoxy contents, respectively, with respect to control. This was attributed to less than 10% polymer addition, which was insufficient in forming a stable interpenetrating polymer network. On the contrary, the flexural strength was seen to increase with a significant improvement of 18% in mortar plates containing 3% polymer.
Contrary to the observations of most of the researchers, El-Hawary and Abdul-Jaleel10 reported the durability of epoxy-modified concrete and observed that the replacement of cement with epoxy in higher percentages gives better corrosion resistance. Concrete mixes with up to 100% replacement of cement with epoxy were prepared, and the corrosion resistance of steel reinforcement was studied in hot marine environments. The results of concrete mixes with 0.45 water-to-cement ratio showed little reduction in compressive strength up to the replacement level of 60%, whereas the strength of 100% epoxy concrete was exceptionally higher than the control, which was obvious. Another concrete mix containing 0.60 water-to-cement ratio showed an increase in compressive strength with increasing the cement replacement level. It was observed that under exposure to seawater, the samples with low epoxy content deteriorated, while those containing higher epoxy exhibited higher compressive strength than initially recorded compressive strength. This was attributed to salt crystallization in the concrete pores. Further, the authors reported an increase in corrosion resistance with an increase in the epoxy percentage. However, a high resistance of 54.5 kΩ was noted for 60% epoxy concrete. The reduction in weight of reinforcement bars was 20%, 37%, 48%, and 100% lower than the control for 10%, 40%, 60%, and 100% epoxy concrete, respectively. Although the authors have stated about the hindrance of cement hydration with the possibility of cement particles being coated with epoxy, the hydration of mixes and delay in setting have not been mentioned.
2.4. Effect of Styrene–Butadiene Latexes
The styrene–butadiene latexes are classified as elastomer polymers. These polymers are used to prepare mortar or dry mix mortar for application as tile adhesives, coatings, and repair works.8,23 Pascal et al.8 studied the mechanical properties of five polymers (latex styrene–butadiene) modified mortars. Earlier this research group witnessed improved flexural strength in styrene–butadiene-modified mortars along with negative effects on porosity and cement hydration.23 Therefore, they used an antifoaming agent with a polymer/cement ratio of 5 to 15% to overcome the negative effects. Although they mentioned that the higher the polymer content, the greater the retardation of hydration, a detailed discussion and period of delay have not been stated. In this study, silica fume was used to lower the retarding effect and increase the strength of the mortars; however, microstructure revealed that silica fume remained as a filler with no signs of degradation due to the pozzolanic reaction. The maximum flexural strength was gained with a 15% polymer-to-cement ratio. The authors suggested that the percolation of the polymer phase into a continuous sample might have increased the strength.
Baueregger et al.17 compared the effect of carboxylated styrene–butadiene copolymer on the hydration of ordinary Portland cement (OPC) and a ternary binder containing OPC (83.08 wt %), calcium alumina cement (10.72 wt % possessing 70% wt. Al2O3), and anhydrite (5.36 wt %). Hydration of OPC and the ternary binder was studied with 5%, 10%, and 20% styrene–butadiene latex dosages. It was observed that the addition of styrene–butadiene latex delayed the induction period in the OPC mixes with a pronounced effect of the polymer content. This was attributed to the chelation of divalent cations (Ca2+) by carboxylate groups. However, the ternary blend showed early hydration of aluminates and late hydration of the silicate groups.
2.5. Effect of Styrene Acrylic Polymers
The styrene acrylic polymers are classified as elastomers. Similar to other types of polymers, these polymers are also used to improve the properties of fresh and hardened concrete and have shown retardation of cement hydration. Wang and Wang9 observed a delay of 360 and 545 min, respectively, on the addition of 10% of styrene acrylic ester to cement. Kong et al.14 studied the interaction between styrene–acrylate polymers and cement. They explained that the hydration is delayed owing to two effects, first, the delaying effect, which includes delay in the acceleration period, and second, the slowing down effect, which shows a reduced hydration peak. It was attributed to the concentration of carboxylic groups in the polymer and the adsorption of the polymer on the surface of cement particles. From the different polymers examined, it was found that the presence of R–COO– groups in polymer shows a pronounced delay in hydration owing to slowing down as well as delaying effect. In further studies by the same researchers, Lu et al.24,25 reported a delay in hydration with the addition of styrene–acrylate copolymers to cement pastes. The study revealed a super-retardation effect of styrene–acrylate latex on hydration of oil well cement at high temperature.24 The hydration retardation at 25 °C was attributed to adsorption of styrene–acrylate polymers on cement particles, while the hydrolysis of polymer was not found to be significant to cause the retardation at this temperature. Further, a super-retardation effect was observed on increasing the temperature to 80 °C. This was explained to have resulted due to the severe alkaline hydrolysis of ester groups in acrylate units. This effect was associated with the acrylate content of the polymer. In another work,25 reasons similar to those mentioned in previous studies have been pointed out, i.e., adsorption of polymer on hydrating cement grains limiting the dissolution of C3A and C3S, and complexation of polymers with Ca2+ and other metal ions. Further, the authors highlighted that the affinity of polymers to get adsorbed on pure C3S is much higher than that observed in OPC. This conclusion was attributed to the fact that, in OPC, there are other mineral phases like C3A, Aft, initially nonreacting C2S and C4AF, which might consume some quantity of polymer as adsorption or in the formation of an embedded layer on these particles.
3. Effect of PCE Admixtures
PCEs are comb-shaped copolymers26,27 and constitute the third-generation of high range water reducing admixture known as superplasticizer. Lei et al.28 presented a state of the art review on the use of PCEs in the past 40 years. These superplasticizers are the key component in fulfilling the requirements of modern-day concrete construction28 and are used in almost every project, from building and infrastructure projects to iconic structures with innovative designs, and yet a complete understanding of the interaction with cement and mechanism hydration is to be established. The PCE superplasticizers are comb-shaped polymers,26 which contain polypropylene glycol groups and carboxylic acid anhydride monomers consisting of acrylic acid, maleic acid, and its anhydride.
Besides the intended use, PCEs are known to delay the induction period of C3S hydration.29 The interaction of cement with PCEs is generally explained with various possibilities of adsorption on the hydrating cement particle; poisoning nucleation and growth of C–H and/or C–S–H; formation of directly bonded layer adhered to silicate and aluminate surfaces; formation of calcium or potassium salt precipitates adhering to the silicate and aluminate surfaces;30−32 increasing the specific area of ettringite;33 and formation of nanohydrates of aluminates and/or aqueous intramolecular complexes.34 Further, the architecture of PCEs are seen to influence the hydration35 with the comb-shaped structure resulting in severe hydration retardation.
Most of the studies have illustrated the retarding effect of comb-shaped PCEs.27,28,35 Zhang et al.35 investigated the effect of PCEs of different architectures on the hydration of cement. They observed that the polymer architecture has a strong relation to the adsorption of PCEs on cement particles. The adsorption was found to decrease with increasing the side chain length and density, resulting in higher early strength of mortar. This work also pointed out that the retardation effect depends on adsorption on cement particles and the complexation of the R–COO– group with Ca2+ has little effect. Ilg and Plank26 developed a noncomb-shaped PCE hyperbranched jellyfish-like structure by preparing PCE having a linear polyetheramine and a hyperbranched polyglycerol which was carboxymethylated in the periphery. They observed much stronger retardation with both hyperbranched polyglycerol skeleton polymers and therefore suggested that these polymer superplasticizers might be beneficial for high-temperature concreting.
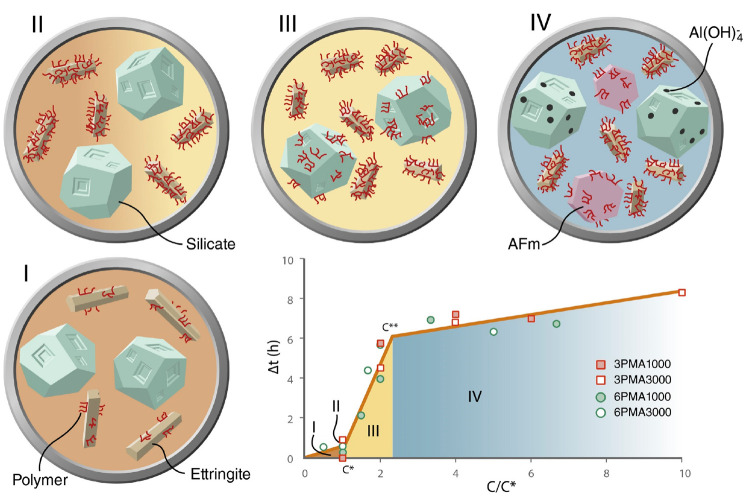
Marcon et al.27 presented the impact of polymer dosage on set retardation through a schematic view (Figure 2). They showed that polymer mobilizes on the precipitated ettringite surface first, and with increasing dosage, the ettringite precipitates are fully covered by polymer molecules. On further increase of the PCE dosage, it adsorbs on the silicate surface, resulting in hydration retardation.
Figure 2.
Hydration delay due to adsorption of polymer on ettringite and silicate surfaces.27 This figure is under a Creative Commons CC BY license.
Caruso et al.34 worked on quantification of elements in the pore solution of hydrating cement paste. They studied pore solutions of cement pastes with PCE and found that the addition of superplasticizer significantly increased the amount of low concentration elements such as aluminum and magnesium. They suggested that the formation of nanohydrates of aluminates could possibly be a nanoettringite/nano-AFm/nano-C–S–H, which could not precipitate on hydrating the clinker; or intramolecular complexes; or miscibility gap causing liquid–liquid separation.
Wang et al.36 studied the hydration of PCEs with cement and observed that with the presence of polymers, a large amount of nanoparticles are generated in the fresh paste solution, which alters the precipitation of ettringite and C–S–H and delays hydration.
4. Effect of Polymer and PCE Admixture Charge on Cement Hydration
Some investigations reveal that the surface charge of polymers influences the hydration of cement.14,17,25,36,37Figure 3 shows a schematic representation of the surface of cement grains covered by the polymer particles.14 Plank and Gretz37 observed that anionic polymers absorb large amounts of Ca2+ from the cement pore solution. The authors explained that besides hydration, Ca2+ was consumed on adsorption on C–S–H. Further, the surface charge of silicate hydrates is negative while that of aluminate hydrates is positive due to which hydrating cement particles provide adsorption sites for both positively and negatively charged ions. Negatively charged polymer particles actively interact with the positively charged cement particles resulting in a hybrid structure of the hardened mortar. Baueregger et al.17 found that anionic polymer (carboxylated styrene–butadiene) retards cement hydration and attributed it to the sequestration of Ca2+ ions from pore solution and adsorption of positively charged clinker phases. Kong et al.14 also stated that polymers with higher anionic charge show a much-increased retardation effect than the ones having a comparatively lower negative charge. Similar to this, Lu et al.25 observed that the hydration of cement and C3S is more delayed by anionic polymers than by cationic polymers. The negatively charged polymers slowed significant delay in the dissolution of C3S and nucleation of C–S–H. Ca2+ in the solution was maintained at a high concentration for a longer period as compared to the reference for the paste containing anionic polymers. The drop in Ca2+ to a specified concentration was delayed by approximately 80% and 15% with anionic and cationic polymers. This is attributed to a strong adsorption affinity of negatively charged polymers, which get adsorbed on the positively charged C3A and AFt phases and also on the negatively charged surface of C3S and C–S–H. This results in greater retardation of hydration when anionic polymers are used as compared to the cationic polymers. Huo et al.16 showed that the adsorption of polymers on cement hydrating particles is strongly related to the surface charge of polymers. They studied paraffin emulsions with three different surface charges, i.e., anionic, cationic, and nonionic. The results showed that the mass of adsorbed anionic and cationic paraffin emulsion was significantly higher than that observed in the nonionic emulsion, indicating both negatively and positively charged paraffin emulsions exhibit stronger hydration retardation effects in comparison to the nonionic paraffin emulsion.
Figure 3.
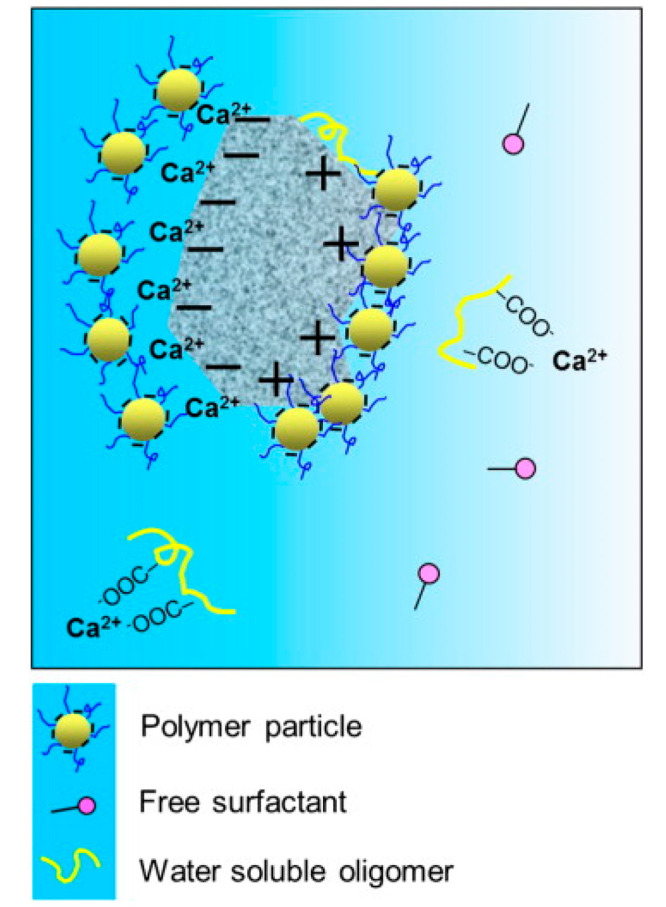
Retardation mechanism of polymer latex on cement hydration.14 Reprinted with permission from ref (13). Copyright 2015 Elsevier Ltd.
Zhang et al.38 investigated the effect of PCE charge on adsorption and hydration retardation. They used different charge species of monomers to synthesize PCEs. It was observed that negatively charged plasticizers with more carboxyl groups have a greater effect on the retardation of cement hydration. Feng et al.39 observed that ester-based slow-release PCEs exhibit greater hydration retardation in comparison to conventional PCEs. Wang et al.36 examined the effect of two PCEs and one sulfonated poly(acrylic acid) linear copolymer with different charge densities. They also concluded that the delay in cement hydration increases with an increase in charge density.
5. Remedial Measures to Mitigate Adverse Effects of Polymers on Cement Hydration
Few researchers have proposed ways to reduce the delay in hydration of cement associated with the use of polymers in concrete and mortar. Knapen and Gemert1,2 proposed a modified curing regime for polymer modified mortars. They suggested that 2 days of wet followed by 5 days of moist and thereafter 21 days of dry curing would overcome the delay in strength gain. Pascal et al.8 proposed that using an antifoaming agent and silica fume reduces the porosity of mortars and helps in reducing the period of hydration delay. Wang and Wang9 observed that the addition of zeolite solved the problem of set retardation in the styrene-acrylic ester-based cement matrix. The addition accelerated hydration and increased the formation of AFt and the C–S–H gel. Upon the addition of a small amount of zeolite, i.e., less than 3%, the compressive strength was less than that of the control; however, with the addition of 5% zeolite, the initial and final setting time was reduced by 68% and 47% in comparison to that for cement pastes without zeolite. Similarly, the compressive strength of cement paste with more than 3% zeolite was higher than that for the control and increased with an increase in the percentage of zeolite. Zhang et al.35 reported that increasing the side chain length of PCE reduces the adsorption on cement particles, thereby increasing the early strength gain of mortar.
6. Concluding Remarks
Application of different polymers such as unsaturated polyester resin, styrene–butadiene latex, ethylene vinyl acetate, styrene acrylic, epoxy resins, and in the form of PCE superplasticizers are accompanied by a drawback, i.e., the interference in normal hydration process of cement. Although, these chemical compounds enhance the later age properties, the early age properties, specifically, the hydration retarding effect, are not desirable.
The heuristic approach supports the retardation of cement hydration by polymer latexes due to the adsorption of the polymer film on the surface of the cement particles, influencing the nucleation and growth of hydration products. This results in slow strength gain; however, the later age compressive and flexural strength was better than that of the control in most of the cases. A few studies showed that the addition of UPR improves the crack restraining property by densifying the microstructure with a pore diameter lower than that of the control and a narrow ITZ. Additionally, the formation of polymer films/bridges between stacks of calcium hydroxide surfaces and air has also been confirmed. The stated evidence is instrumental in understanding that polymer addition could be beneficial for certain applications, provided the early age strength requirements are administered. Furthermore, the use of defoaming agents and mineral admixtures (zeolite and silica fume) have been seen to be effective in reducing the hydration retardation effect of different polymers. It is noteworthy that different combinations of polymer latexes may be used as repair materials and to enhance the performance of concrete.
The possible explanations for hydration retardation are the formation of a thin layer over cement particles, which restricts the access of water, which increases ettringite formation; interaction of carboxylate ions (COO–) from the polymer emulsion with Ca2+ which inhibits scission of polymer chain and results in sequestration of Ca2+ from pore solution; and formation of nanohydrates of aluminates, i.e., nanoettringite/nano-AFm/nano-C–S–H resulting in a hybrid structure of the hardened mortar. The polymer particle surface charge, architecture, and amount wt % added in cement matrix play an important role in the hydration delaying effect. It is seen that anionic polymers show a severe delay in the dissolution of C3S and nucleation of C–S–H resulting in adversely affecting hydration in comparison to the cationic polymers. This retarding effect may be mitigated by the addition of silica fume, zeolite, and defoaming agent and curing at high temperature to some extent.
A closer look at the conclusions made by various studies reveals that mostly the interpretations are similar, but variations exist in examination techniques and conditions. These variations limit a generalized conclusion, reflecting the need to warrant detailed examination prior to adopting a specific polymer combination for field application. By and large, it is demonstrated that the incorporation of polymers shows beneficial results in concrete, such as higher flexural strength, enhanced compressive strength at 90 days of age and modulus of elasticity, and better resistance against frost and corrosion of steel. However, these benefits cannot be attested as a general finding because the results pertain to specific methods of emulsion synthesis, concrete/mortar preparation techniques, and curing regimes, only. As a general observation, the adverse effect of slow hydration is confirmed, which warrants field engineers’ a cautious deployment.
Acknowledgments
The author is thankful to the Ministry of Mines, New Delhi, for funding the project entitled “Development of ready-to-use assorted sand for construction activities from zinc refining wastes and marble powder (Phase 2)” grant no. Met-4 14/3/2021, under which the present review has been conducted.
Biography
Professor Bhavna Tripathi received her graduation from CEPT university, Ahmedabad, India; masters and Ph.D. from MNIT Jaipur, India. She is a Professor of Civil Engineering, and Director of the School of Civil & Chemical Engineering at Manipal University Jaipur, India. She works on circular economy, sustainable materials for concrete, and the durability of concrete. Currently, she is working in the area of durability of concrete containing fine recycled concrete aggregates prepared from construction and demolition waste and artificial fine aggregates prepared using mining wastes.
The author declares no competing financial interest.
References
- Knapen E.; Van Gemert D. Cement Hydration and Microstructure Formation in the Presence of Water-Soluble Polymers. Cem. Concr. Res. 2009, 39 (1), 6–13. 10.1016/j.cemconres.2008.10.003. [DOI] [Google Scholar]
- Knapen E.; Van Gemert D. Polymer Film Formation in Cement Mortars Modified with Water-Soluble Polymers. Cem. Concr. Compos. 2015, 58, 23–28. 10.1016/j.cemconcomp.2014.11.015. [DOI] [Google Scholar]
- Dholakiya B.Unsaturated Polyester Resin for Specialty Applications. In Polyester; Saleh H. E.-D., Ed.; InTech, 2012. 10.5772/48479. [DOI] [Google Scholar]
- Panda S.; Behera D.. Unsaturated Polyester Nanocomposites. In Unsaturated Polyester Resins; Elsevier, 2019; pp 101–124. 10.1016/B978-0-12-816129-6.00004-1. [DOI] [Google Scholar]
- Gao Y.; Romero P.; Zhang H.; Huang M.; Lai F. Unsaturated Polyester Resin Concrete: A Review. Constr. Build. Mater. 2019, 228, 116709 10.1016/j.conbuildmat.2019.116709. [DOI] [Google Scholar]
- Anagnostopoulos C. A.; Sapidis G.; Papastergiadis E. Fundamental Properties of Epoxy Resin-Modified Cement Grouts. Constr. Build. Mater. 2016, 125, 184–195. 10.1016/j.conbuildmat.2016.08.050. [DOI] [Google Scholar]
- Li Y.; Guo Y.; Lyu Z.; Wei X. Investigation of the Effect of Waterborne Epoxy Resins on the Hydration Kinetics and Performance of Cement Blends. Constr. Build. Mater. 2021, 301, 124045 10.1016/j.conbuildmat.2021.124045. [DOI] [Google Scholar]
- Pascal S.; Alliche A.; Pilvin P. Mechanical Behaviour of Polymer Modified Mortars. Mater. Sci. Eng., A 2004, 380 (1–2), 1–8. 10.1016/j.msea.2004.03.049. [DOI] [Google Scholar]
- Wang R.; Wang G. Influence and Mechanism of Zeolite on the Setting and Hardening Process of Styrene-Acrylic Ester/Cement Composite Cementitious Materials. Constr. Build. Mater. 2016, 125, 757–765. 10.1016/j.conbuildmat.2016.08.087. [DOI] [Google Scholar]
- El-Hawary M. M.; Abdul-Jaleel A. Durability Assessment of Epoxy Modified Concrete. Constr. Build. Mater. 2010, 24, 1523–1528. 10.1016/j.conbuildmat.2010.02.004. [DOI] [Google Scholar]
- Zhang Z.; Zhang H.; Liu T.; Lv W. Study on the Micro-Mechanism and Structure of Unsaturated Polyester Resin Modified Concrete for Bridge Deck Pavement. Constr. Build. Mater. 2021, 289, 123174 10.1016/j.conbuildmat.2021.123174. [DOI] [Google Scholar]
- Zhang Z.; Zhang H.; Zhu K.; Tang Z.; Zhang H. Deterioration Mechanism on Micro-Structure of Unsaturated Polyester Resin Modified Concrete for Bridge Deck Pavement under Salty Freeze-Thaw Cycles. Constr. Build. Mater. 2023, 368, 130366 10.1016/j.conbuildmat.2023.130366. [DOI] [Google Scholar]
- Cai H.; Yuan B.; Yang F.; Chen L.; Feng W.; Liang Y. Dynamic Three-Point Flexural Performance of Unsaturated Polyester Polymer Concrete at Different Curing Ages. J. Build. Eng. 2022, 45, 103449 10.1016/j.jobe.2021.103449. [DOI] [Google Scholar]
- Kong X.; Emmerling S.; Pakusch J.; Rueckel M.; Nieberle J. Retardation Effect of Styrene-Acrylate Copolymer Latexes on Cement Hydration. Cem. Concr. Res. 2015, 75, 23–41. 10.1016/j.cemconres.2015.04.014. [DOI] [Google Scholar]
- Silva D. A.; Monteiro P. J. M. The Influence of Polymers on the Hydration of Portland Cement Phases Analyzed by Soft X-Ray Transmission Microscopy. Cem. Concr. Res. 2006, 36 (8), 1501–1507. 10.1016/j.cemconres.2006.05.010. [DOI] [Google Scholar]
- Huo J.; Wang Z.; Zhang T.; He R.; Chen H. Influences of Interaction between Cement and Ionic Paraffin Emulsion on Cement Hydration. Constr. Build. Mater. 2021, 299, 123951 10.1016/j.conbuildmat.2021.123951. [DOI] [Google Scholar]
- Baueregger S.; Perello M.; Plank J. Impact of Carboxylated Styrene–Butadiene Copolymer on the Hydration Kinetics of OPC and OPC/CAC/AH: The Effect of Ca2+ Sequestration from Pore Solution. Cem. Concr. Res. 2015, 73, 184–189. 10.1016/j.cemconres.2015.03.004. [DOI] [Google Scholar]
- Liang G.; Ni D.; Li H.; Dong B.; Yang Z. Synergistic Effect of EVA, TEA and C-S-Hs-PCE on the Hydration Process and Mechanical Properties of Portland Cement Paste at Early Age. Constr. Build. Mater. 2021, 272, 121891 10.1016/j.conbuildmat.2020.121891. [DOI] [Google Scholar]
- Tawfik M. E.; Eskander S. B.; Bayoumi T. A. Immobilization of Borate Waste Simulate in Cement-Water Extended Polyester Composite Based on Polyethylene Terephthalate Waste 1- Mechanical Properties of the Final Waste Forms. Polym.-Plast. Technol. Eng. 2005, 44, 1355–1368. 10.1081/PTE-200065226. [DOI] [Google Scholar]
- Li H.; Ji D.; Chen C.; Wang X.; Tu J.; Zhang K.; Ding Effect of Fatty Acid Methyl Ester Polyoxyethylene Ether on the Rheological Properties of Cement Filled with Artificial Marble Waste Powders. J. Clean. Prod. 2021, 328, 129503 10.1016/j.jclepro.2021.129503. [DOI] [Google Scholar]
- Li P.; Lu W.; An X.; Zhou L.; Du S. Effect of Epoxy Latexes on the Mechanical Behavior and Porosity Property of Cement Mortar with Different Degrees of Hydration and Polymerization. Materials 2021, 14 (3), 517. 10.3390/ma14030517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P.; Jiang Z.; An X.; Maekawa K.; Du S. Time-Dependent Retardation Effect of Epoxy Latexes on Cement Hydration: Experiments and Multi-Component Hydration Model. Constr. Build. Mater. 2022, 320, 126282 10.1016/j.conbuildmat.2021.126282. [DOI] [Google Scholar]
- Bureau L.; Alliche A.; Pilvin P.; Pascal S. Mechanical Characterization of a Styrene–Butadiene Modified Mortar. Mater. Sci. Eng., A 2001, 308 (1–2), 233–240. 10.1016/S0921-5093(00)01980-8. [DOI] [Google Scholar]
- Lu Z.; Kong X.; Zhang Q.; Cai Y.; Zhang Y.; Wang Z.; Dong B.; Xing F. Influences of Styrene-Acrylate Latexes on Cement Hydration in Oil Well Cement System at Different Temperatures. Colloids Surf. Physicochem. Eng. Asp. 2016, 507, 46–57. 10.1016/j.colsurfa.2016.07.082. [DOI] [Google Scholar]
- Lu Z.; Kong X.; Zhang C.; Jansen D.; Neubauer J.; Goetz-Neunhoeffer F. Effects of Two Oppositely Charged Colloidal Polymers on Cement Hydration. Cem. Concr. Compos. 2019, 96, 66–76. 10.1016/j.cemconcomp.2018.11.014. [DOI] [Google Scholar]
- Ilg M.; Plank J. Synthesis and Properties of a Polycarboxylate Superplasticizer with a Jellyfish-Like Structure Comprising Hyperbranched Polyglycerols. Ind. Eng. Chem. Res. 2019, 58 (29), 12913–12926. 10.1021/acs.iecr.9b02077. [DOI] [Google Scholar]
- Marchon D.; Juilland P.; Gallucci E.; Frunz L.; Flatt R. J. Molecular and Submolecular Scale Effects of Comb-Copolymers on Tri-Calcium Silicate Reactivity: Toward Molecular Design. J. Am. Ceram. Soc. 2017, 100 (3), 817–841. 10.1111/jace.14695. [DOI] [Google Scholar]
- Lei L.; Hirata T.; Plank J. 40 Years of PCE Superplasticizers - History, Current State-of-the-Art and an Outlook. Cem. Concr. Res. 2022, 157, 106826 10.1016/j.cemconres.2022.106826. [DOI] [Google Scholar]
- Ridi F.; Dei L.; Fratini E.; Chen S.-H.; Baglioni P. Hydration Kinetics of Tri-Calcium Silicate in the Presence of Superplasticizers. J. Phys. Chem. B 2003, 107, 1056–1061. 10.1021/jp027346b. [DOI] [Google Scholar]
- Mollah M. Y. A.; Adams W. J.; Schennach R.; Cocke D. L. A Review of Cement ± superplasticizer Interactions and Their Models. Adv. Cem. Res. 2000, 12 (4), 153–161. 10.1680/adcr.2000.12.4.153. [DOI] [Google Scholar]
- Cheung J.; Jeknavorian A.; Roberts L.; Silva D. Impact of Admixtures on the Hydration Kinetics of Portland Cement. Cem. Concr. Res. 2011, 41, 1289–1309. 10.1016/j.cemconres.2011.03.005. [DOI] [Google Scholar]
- Marchon D.; Flatt R. J.. Impact of Chemical Admixtures on Cement Hydration. In Science and Technology of Concrete Admixtures; Elsevier, 2016; pp 279–304. 10.1016/B978-0-08-100693-1.00012-6. [DOI] [Google Scholar]
- Dalas F.; Pourchet S.; Rinaldi D.; Nonat A.; Sabio S.; Mosquet M. Modification of the Rate of Formation and Surface Area of Ettringite by Polycarboxylate Ether Superplasticizers during Early C3A–CaSO4 Hydration. Cem. Concr. Res. 2015, 69, 105–113. 10.1016/j.cemconres.2014.12.007. [DOI] [Google Scholar]
- Caruso F.; Mantellato S.; Palacios M.; Flatt R. J. ICP-OES Method for the Characterization of Cement Pore Solutions and Their Modification by Polycarboxylate-Based Superplasticizers. Cem. Concr. Res. 2017, 91, 52–60. 10.1016/j.cemconres.2016.10.007. [DOI] [Google Scholar]
- Zhang L.; Miao X.; Kong X.; Zhou S. Retardation Effect of PCE Superplasticizers with Different Architectures and Their Impacts on Early Strength of Cement Mortar. Cem. Concr. Compos. 2019, 104, 103369 10.1016/j.cemconcomp.2019.103369. [DOI] [Google Scholar]
- Wang J.; Liao J.; Kong X.; Yin J. Characterization of the Nanoparticles Formed in Aqueous Phase of Hydrating Cement Pastes in the Presence of PCEs. Cem. Concr. Res. 2023, 165, 107087 10.1016/j.cemconres.2022.107087. [DOI] [Google Scholar]
- Plank J.; Gretz M. Study on the Interaction between Anionic and Cationic Latex Particles and Portland Cement. Colloids Surf. Physicochem. Eng. Asp. 2008, 330 (2–3), 227–233. 10.1016/j.colsurfa.2008.08.005. [DOI] [Google Scholar]
- Zhang Y.-R.; Kong X.-M.; Lu Z.-B.; Lu Z.-C.; Hou S.-S. Effects of the Charge Characteristics of Polycarboxylate Superplasticizers on the Adsorption and the Retardation in Cement Pastes. Cem. Concr. Res. 2015, 67, 184–196. 10.1016/j.cemconres.2014.10.004. [DOI] [Google Scholar]
- Feng P.; Zhang G.; Zhang W.; Cui H.; Xin T. Comparison of Ester-Based Slow-Release Polycarboxylate Superplasticizers with Their Polycarboxylate Counterparts. Colloids Surf. Physicochem. Eng. Asp. 2022, 633, 127878 10.1016/j.colsurfa.2021.127878. [DOI] [Google Scholar]