Abstract
There lacks real‐world study with a large sample size assessing olmesartan medoxomil‐amlodipine besylate (OM‐AML) tablet. Therefore, this study aimed to evaluate the efficacy and safety of OM‐AML tablet in patients with essential hypertension. Totally, 1341 patients from 36 medical centers with essential hypertension who took OM‐AML (20/5 mg) tablet were analyzed in the current prospective, single‐arm, multi‐center, real‐world study (SVK study). Seated systolic blood pressure (SeSBP) and seated diastolic blood pressure (SeDBP) at baseline, week (W)4 and W8 were measured. The mean (±SE) change of SeSBP/SeDBP was ‐10.8 ± 0.4/‐6.6 ± 0.3 mmHg at W4 and ‐12.7 ± 0.5/‐7.6 ± 0.3 mmHg at W8, respectively. At W4, 78.8% and 29.0% patients achieved BP target by China and American Heart Association (AHA) criteria; at W8, 84.7% and 36.5% patients reached blood pressure (BP) target by China and AHA criteria, accordingly. Meanwhile, 80.2% and 86.4% patients achieved BP response at W4 and W8, respectively. Home‐measured SeSBP and SeDBP decreased from W1 to W8 (both p < .001). Besides, patients’ and physicians’ satisfaction were elevated at W8 compared with W0 (both p < .001). The medication possession rate was 94.8% from baseline to W4 and 91.3% from baseline to W8. The most common drug‐related adverse events were nervous system disorders (4.6%), vascular disorders (2.6%), and general disorders and administration site conditions (2.3%) by system organ class, which were generally mild and manageable. In conclusion, OM‐AML tablet is one of the best antihypertensive agents in patients with essential hypertension.
Keywords: adverse events, blood pressure target, essential hypertension, Olmesartan medoxomil‐amlodipine besylate tablet, satisfaction
1. INTRODUCTION
Essential hypertension is one of the main risk factors for cardio‐cerebrovascular diseases and all‐cause mortality, which affects more than 30% of adults worldwide. 1 , 2 , 3 In China, due to the increasing exposure to risk factors such as high sodium intake, obesity, low participation in physical exercise, etc., the prevalence of essential hypertension has increased to about 50% in individuals aging 35−70 years. 4 , 5 The current medical management of essential hypertension mainly includes diuretics, angiotensin receptor blockers, angiotensin converting enzyme inhibitors and long‐acting dihydropyridine calcium channel blockers, which are often administrated in combination. 6 , 7 Although these medical management approaches can effectively control blood pressure (BP), patients’ adherence (or persistence) is still a critical issue to be solved. 8
Olmesartan medoxomil (OM, an angiotensin receptor blocker) plus amlodipine besylate (AML, a calcium channel blocker) is a common therapeutic strategy for the treatment of essential hypertension. 9 By combining the two antihypertensive agents with different acting mechanisms, satisfactory control of BP could be achieved. 10 In recent years, a dose‐fixed single tablet containing both OM and AML (OM‐AML tablet) is offered to patients with essential hypertension, which is able to realize better patients’ adherence and persistence as well as a potential benefit in BP control according to a recent randomized, controlled trial. 11 In China, several studies have been conducted to evaluate the pharmacokinetics, efficacy, safety and cost‐utility of OM‐AML tablet in patients with essential hypertension. 12 , 13 , 14 However, there lack studies conducted in a large sample size to evaluate the efficacy and safety of OM‐AML tablet in Chinese patients with essential hypertension under real‐world setting.
In the current prospective, real‐world study, 1341 patients with essential hypertension who took OM‐AML tablet from 36 Chinese centers were analyzed, which aimed to evaluate the efficacy and safety of OM‐AML tablet for essential hypertension treatment.
2. METHODS
2.1. Study design
This was a prospective, single‐arm, multicenter real‐world study aiming to investigate the efficacy and safety of OM‐AML tablet in patients with essential hypertension in China (SVK study), which was initiated by Zhongshan Hospital, Fudan University with the involvement of the cardiology and/or hypertension departments of 36 hospitals in China. The SVK study was registered in Chinese Clinical Trial Registry (http://www.chictr.org.cn/) with a registration number of ChiCTR1900026574. The present study was approved by the Ethics Committee of Zhongshan Hospital, Fudan University (Approved No. B2019‐174R2), and all other sites. All participants signed the informed consent.
2.2. Study population
A total of 1500 essential hypertension patients were planned to be enrolled in this study from 2019‐10‐30 to 2020‐09‐30. The enrollment criteria were as follows: (1) diagnosed as essential hypertension according to the following criteria: the seated systolic BP (SeSBP) ≥140 mmHg and/or seated diastolic BP (SeDBP) ≥90 mmHg, which were measured three times on different days in the clinic without use of antihypertensive drug (if the patient had a history of hypertension and was currently taking antihypertensive medications, the hypertension was also diagnosed even though the BP was lower than 140/90 mmHg); (2) age 18–80 years; (3) about to receive OM‐AML tablet for antihypertensive therapy prescribed by physicians according to the medication instructions; (4) willing to participate in the study and sign the informed consents. Since this was a real‐world study, no exclusion criteria were set in order to reduce selection bias, and all data related to clinical practice were truthfully recorded.
2.3. Administration of medication
The OM‐AML tablet (Daiichi Sankyo (Shanghai) Holdings Co., Ltd., Shanghai, China) was a compound preparation, and the active ingredients were OM and AML. Each OM‐AML tablet contains 20 mg OM and 5 mg AML. The recommended dose of OM‐AML tablet was one tablet once a day, orally. Based on previous antihypertensive treatment and the handling of previous antihypertensive treatment (if there was) before enrollment, patients were divided into three groups: patients with no history of antihypertensive medications, patients remained previous antihypertensive medications, and patients discontinued previous antihypertensive medications groups. During study, patients were allowed to quit the study and receive salvage antihypertensive medications if their BP was not controlled by OM‐AML tablet.
2.4. Follow‐up and assessment
Patients’ baseline clinical features including demographics, medical histories, vital signs, laboratory tests and medications were documented, then they were followed up at W4 (week 4 ± 7 days) and W8 (week 8 ± 7 days) after initiation of treatment. The SeSBP and SeDBP were measured at baseline (W0), W4, and W8 in the outpatient clinic. After starting the medication, patients were required to record home‐measured BP, medication‐taking as well as adverse events (AEs) every day. The measurement and devices for home‐measured BP were in accordance with the 2019 Chinese Hypertension League guidelines on home BP monitoring; BP was measured three times consecutively with a 1‐min interval, and the average BP was recorded. 15 In addition, the physician's satisfaction with current hypertension treatment and the patient's satisfaction with current hypertension treatment were also assessed using the 10‐cm Visual Analogue Scale (VAS) at W0 and W8.
2.5. Outcomes and definitions
The primary outcome was the mean change in SeDBP from W0 to W8. The secondary outcomes included the mean change in SeSBP from W0 to W8, proportion of patients achieving American Heart Association (AHA) BP target, the proportion of patients achieving China BP target, proportion of patients achieving BP response, change in home‐measured BP from W0 to W8, change in patient's satisfaction with current hypertension treatment (VAS) from W0 to W8, change in physician's satisfaction with current hypertension treatment (VAS) from W0 to W8, medication possession ratio (MPR), and AEs. The AHA BP target was defined as SeSBP < 130 mmHg and SeDBP < 80 mmHg. 16 The China BP target was defined as SeSBP < 140 mmHg and SeDBP < 90 mmHg. 17 The BP response rate was defined as proportion of patients achieving SeSBP < 140 mmHg (or a decrease ≥20 mmHg in SeSBP) and SeDBP < 90 mmHg (or a decrease ≥10 mmHg in SeDBP). The MPR was calculated as the actual days of medication use divided by the total number of days. AEs were described by system organ class and preferred term class.
2.6. Statistical analysis
A total of 1341 patients were included in the full analysis set (FAS) as shown in Figure 1. All analyses were carried out in the FAS. Data were processed by R version 4.0.5 (www.r‐project.org) and analyzed by SPSS version 26.0 (IBM Corp., Armonk, NY). Qualitative data were described as count with percentage (n. (%)) and analyzed using the Chi‐square test or Fisher exact test. Quantitative data were described as mean with standard deviation (SD) or standard error (SE), or median with interquartile range (IQR), as appropriate. Least square means (LSM) of BP was calculated with age, sex, BMI, smoker, and alcohol intake taken into adjustment. Paired comparison of quantitative data was analyzed by Wilcoxon signed‐rank test. Repeated measurements were analyzed by Friedman's test. Subgroup analysis of quantitative data was determined by the Wilcoxon rank‐sum test and Kruskal‐Wallis H rank‐sum test. Factors affecting outcomes were analyzed by logistic regression, and only factors with p‐value <.05 in the univariate logistic regression were further included in the multivariate logistic regression. p‐value <.05 was defined as statistical significance.
FIGURE 1.
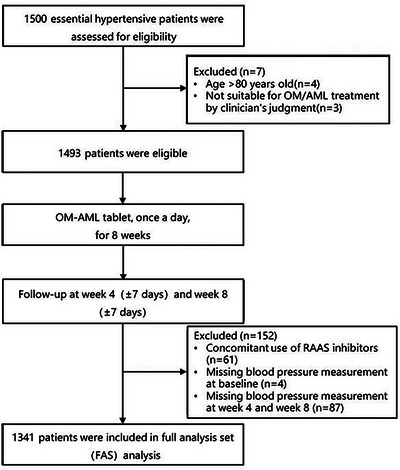
Flow of this study.
3. RESULTS
3.1. Study flow
Initially, 1500 patients with essential hypertension were assessed for eligibility. Then seven patients were excluded, among which four patients were older than 80 years and three patients were not suitable for OM or AML treatment. Subsequently, 1493 eligible patients received OM‐AML tablet once a day for 8 weeks. The patients were followed‐up at W4 and W8, during which 152 patients were excluded from the analysis (including 61 patients concomitant use of renin‐angiotensin‐aldosterone system (RAAS) inhibitors, 4 patients who missed BP measurement at baseline and 87 patients who missed BP measurement at W4 and W8). Finally, 1341 patients were included in the full analysis set analysis (Figure 1).
3.2. Baseline characteristics
The enrolled patients had a mean (± SD) age of 57.2 ± 12.3 years with 564 (42.1%) females and 777 (57.9%) males. The median (IQR) duration of hypertension was 6.1 (2.1‐14.4) years. Regarding the vital signs of hypertension, the mean (± SD) respiratory rate was 17.8 ± 2.3 breaths/min and the mean (± SD) heart rate was 76.4 ± 9.9 beats/min. The mean (± SD) values of SeSBP and SeDBP were 140.1 ± 16.2 and 86.8 ± 11.1 mmHg, respectively; there were 691 (51.5%) patients with abnormal SeSBP, and 544 (40.6%) patients with abnormal SeDBP. Besides, 1341 (100.0%) patients received OM‐AML tablet, among which 1065 (79.4%) patients received no combination with OM‐AML tablet, 117 (8.7%) patients took OM‐AML tablet combined with lipid‐modifying drug, and 159 (11.9%) patients received OM‐AML tablet combined with lipid‐modifying drug and others. More detailed baseline characteristics of the patients were listed in Table 1.
TABLE 1.
Baseline characteristics.
| Characteristics | Total (N = 1341) |
|---|---|
| Demographics | |
| Age (years), mean ± SD | 57.2 ± 12.3 |
| Sex, n (%) | |
| Female | 564 (42.1) |
| Male | 777 (57.9) |
| BMI (kg/m2), mean ± SD | 25.8 ± 3.6 |
| Education level, n (%) | |
| Primary school or less | 134 (10.0) |
| High school | 609 (45.4) |
| Undergraduate or above | 598 (44.6) |
| Smoker, n (%) | |
| No | 923 (68.8) |
| Yes | 418 (31.2) |
| Alcohol intake, n (%) | |
| No | 1101 (82.1) |
| Yes | 240 (17.9) |
| Hypertension duration (years), median (IQR) | 6.1 (2.1‐14.4) |
| Medical history | |
| Family history of hypertension, n (%) | |
| No | 503 (37.5) |
| Yes | 807 (60.2) |
| Unknown | 31 (2.3) |
| History of allergy, n (%) | |
| No | 1189 (88.7) |
| Yes | 137 (10.2) |
| Unknown | 15 (1.1) |
| History of respiratory disease, n (%) | |
| No | 1230 (91.7) |
| Yes | 105 (7.8) |
| Unknown | 6 (0.4) |
| History of kidney disease, n (%) | |
| No | 1267 (94.3) |
| Yes | 71 (5.3) |
| Unknown | 3 (0.2) |
| History of diabetes, n (%) | |
| No | 1121 (83.6) |
| Yes | 214 (16.0) |
| Unknown | 6 (0.4) |
| History of CCVD, n (%) | |
| No | 971 (72.4) |
| Yes | 370 (27.6) |
| History of dyslipidemia, n (%) | |
| No | 788 (58.8) |
| Yes | 523 (39.0) |
| Unknown | 30 (2.2) |
| Vital signs | |
| Respiratory rate (breaths/min), mean ± SD | 17.8 ± 2.3 |
| Heart rate (beats/min), mean ± SD | 76.4 ± 9.9 |
| SeSBP (mmHg), mean ± SD | 140.1 ± 16.2 |
| Abnormal SeSBP, n (%) | 691 (51.5) |
| SeDBP (mmHg), mean ± SD | 86.8 ± 11.1 |
| Abnormal SeDBP, n (%) | 544 (40.6) |
| Hypertension severity class, n (%) | |
| No | 531 (39.6) |
| Mild | 562 (41.9) |
| Moderate | 190 (14.2) |
| Severe | 58 (4.3) |
| History of antihypertensive treatment | |
| History of hypertension treatment, n (%) | |
| Ever treatment | 1221 (91.1) |
| First treatment | 120 (8.9) |
| History of antihypertensive drugs, n (%) | |
| Monotherapy | 735 (54.8) |
| Double combination | 390 (29.1) |
| Triple combination | 78 (5.8) |
| Unknown | 138 (10.3) |
| Current treatment during study | |
| OM‐AML tablet, n (%) | 1341 (100.0) |
| Combinations, n (%) | |
| No combination | 1065 (79.4) |
| Lipid‐modifying drug | 117 (8.7) |
| Lipid‐modifying drug and others | 159 (11.9) |
| Home‐measured BP | |
| Home‐measured SeSBP (mmHg), mean ± SD | 131.5 ± 11.5 |
| Home‐measured SeDBP (mmHg), mean ± SD | 81.7 ± 13.2 |
Abbreviations: BMI, body mass index; BP, blood pressure; CCVD, cardiovascular and cerebrovascular diseases; IQR, interquartile range; SD, standard deviation; SeSBP, seated systolic blood pressure; SeDBP, seated diastolic blood pressure.
3.3. Change in SeSBP and SeDBP
The mean (± SD) SeSBP was 129.3 ± 11.9 mmHg at W4 (p < .001 vs. that at W0) and 127.7 ± 11.1 mmHg at W8 (both p < .001 vs. that at W4 and W0); the mean (± SD) SeDBP was 80.2 ± 8.5 mmHg at W4 (p < .001 vs. that at W0) and 79.2 ± 7.9 mmHg at W8 (both p < .001 vs. that at W4 and W0) (Figure 2A). Meanwhile, the mean (± SE) change of SeSBP was −10.8 ± 0.4 mmHg at W4 and 12.7 ± 0.5 mmHg at W8; the mean (± SE) change of SeDBP was −6.6 ± 0.3 mmHg at W4 and −7.6 ± 0.3 mmHg at W8 (Figure 2B). The LSM (± SE) change of SeSBP was −12.0 ± 1.0 mmHg at W4 and −13.9 ± 1.3 mm Hg at W8; that of SeDBP was −7.9 ± 0.8 mmHg at W4 and −9.0 ± 0.9 at W8 (Supplementary Table 1).
FIGURE 2.
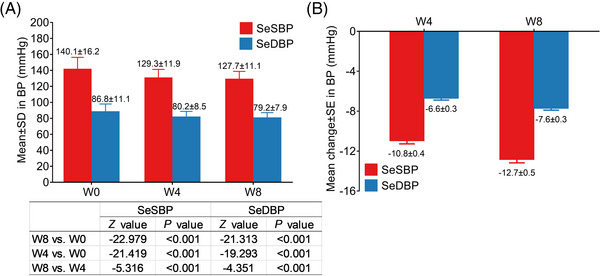
Change of BP after administration of OM‐AML tablet. SeSBP and SeDBP at W0, W4 and W8 (A). Change of SeSBP and SeDBP at W4 and W8 (B).
By comparison analysis, greater decline in SeSBP or SeDBP was found in the following patients including patients with age < 65 years, non‐smokers, patients with shorter hypertension duration, patients without family history of hypertension, patients without history of cardiovascular and cerebrovascular diseases (CCVD), dyslipidemia or diabetes, patients with normal heart rate at baseline, patients with abnormal SeSBP and SeDBP at baseline, patients with moderate or severe hypertension severity class, patients without hypertension treatment history, patients with monotherapy antihypertensive drug history and patients who took OM‐AML tablet plus lipid‐modifying drugs (Supplementary Table 2). Meanwhile, SeSBP and SeDBP change at W4 and W8 was the greatest in patients remained previous antihypertensive medications, followed by patients with no history of antihypertensive medications, and then in patients discontinued previous antihypertensive medications (all p < .001) (Supplementary Table 3).
3.4. Patients achieving BP target
At W4 (n = 1341), 1057 (78.8%) and 389 (29.0%) patients achieved BP target based on China and AHA criteria, respectively; at W8 (n = 1281), 1087 (84.7%) and 469 (36.5%) patients reached BP target based on China and AHA criteria, accordingly (Figure 3A). In detail, regarding patients achieving BP target based on China criteria, 1103 (82.3%) and 1184 (88.3%) patients attained BP target for SeSBP and SeDBP at W4; whilst 1124 (87.5%) and 1177 (91.7%) patients reached BP target for SeSBP and SeDBP at W8 (Figure 3B). Concerning patients achieving BP target based on AHA criteria, 672 (50.1%) and 588 (43.8%) patients achieved BP target for SeSBP and SeDBP at W4; meanwhile, 751 (58.5%) and 642 (40.0%) patients reached BP target for SeSBP and SeDBP at W8 (Figure 3C). Moreover, there were 1076 (80.2%) patients who achieved BP response at W4 and 1109 (86.4%) patients who achieved BP response at W8 (Figure 3D).
FIGURE 3.
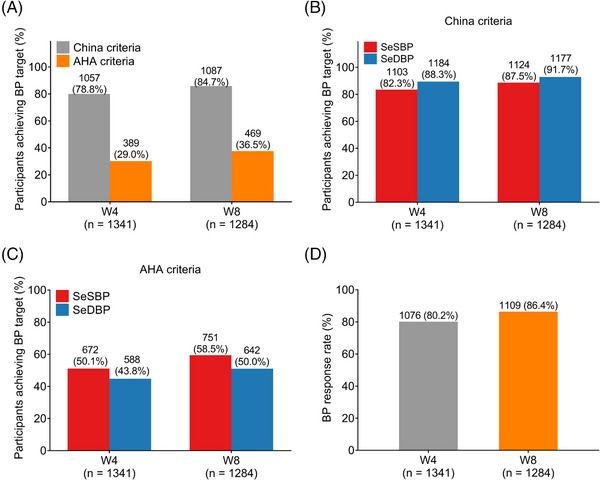
BP target achievement after administration of OM‐AML tablet. The proportion of patients who achieved BP target by China or AHA criteria at W4 and W8 (A). The proportion of patients who achieved SeSBP or SeDBP target by China criteria at W4 and W8 (B). The proportion of patients who achieved SeSBP or SeDBP target by AHA criteria at W4 and W8 (C). The proportion of patients who achieved BP response at W4 and W8 (D).
By subgroup analysis, the following subgroup patients had higher achievement of AHA BP target, China BP target or BP response at W8, including patients with age < 65 years, females, patients with BMI < 30 kg/m2, non‐smokers, non‐alcohol in‐takers, patients with shorter hypertension duration, patients without history of or CCVD, patients with normal SeSBP and SeDBP at baseline, patients with mild hypertension severity class, patients with monotherapy antihypertensive drug history and patients who took monotherapy of OM‐AML tablet (Table 2).
TABLE 2.
Achieving BP target rate and BP response rate by subgroup at W8.
| Subgroup | No. | AHA BP target | p‐value | China BP target | p‐value | BP response | p‐value |
|---|---|---|---|---|---|---|---|
| Age, n (%) | .031 | .062 | .002 | ||||
| <65 years | 847 | 292 (34.5) | 729 (86.1) | 750 (88.5) | |||
| ⩾65 years | 436 | 177 (40.6) | 358 (82.1) | 359 (82.3) | |||
| Sex, n (%) | .000 | .005 | .017 | ||||
| Female | 539 | 243 (45.1) | 474 (87.9) | 480 (89.0) | |||
| Male | 745 | 226 (30.3) | 613 (82.3) | 629 (84.4) | |||
| BMI, n (%) | .024 | .043 | .067 | ||||
| <30 kg/m2 | 1129 | 412 (36.4) | 935 (82.8) | 952 (84.3) | |||
| ⩾30 kg/m2 | 155 | 32 (20.6) | 92 (59.3) | 95 (61.2) | |||
| Education level, n (%) | .234 | .455 | .652 | ||||
| Primary school or less | 126 | 53 (42.1) | 102 (81.0) | 106 (84.1) | |||
| High school | 583 | 201 (34.5) | 494 (84.7) | 502 (86.1) | |||
| Undergraduate or above | 575 | 215 (37.4) | 491 (85.4) | 501 (87.1) | |||
| Smoker, n (%) | .000 | .000 | .001 | ||||
| No | 885 | 353 (39.9) | 771 (87.1) | 784 (88.5) | |||
| Yes | 399 | 116 (29.1) | 316 (79.2) | 325 (81.4) | |||
| Alcohol intake, n (%) | .008 | .005 | .012 | ||||
| No | 1055 | 403 (38.2) | 907 (86.0) | 923 (87.4) | |||
| Yes | 229 | 66 (28.8) | 180 (78.6) | 186 (81.2) | |||
| Hypertension duration, n (%) | .015 | .090 | .001 | ||||
| < 5 years | 539 | 220 (40.8) | 470 (87.2) | 487 (90.3) | |||
| 5−9 years | 227 | 82 (36.1) | 190 (83.7) | 193 (85.0) | |||
| ≥10 years | 518 | 167 (32.2) | 427 (82.4) | 429 (82.8) | |||
| Family history of hypertension, n (%) | .447 | .320 | .588 | ||||
| No | 475 | 178 (37.5) | 408 (85.9) | 413 (86.9) | |||
| Yes | 778 | 275(35.3) | 652 (83.8) | 668 (85.8) | |||
| History of allergy, n (%) | .285 | .466 | .767 | ||||
| No | 1141 | 423 (37.1) | 967 (84.8) | 985 (86.3) | |||
| Yes | 130 | 42 (32.3) | 107 (82.3) | 111 (85.3) | |||
| History of respiratory disease, n (%) | .675 | .277 | .440 | ||||
| No | 1180 | 428 (36.3) | 1002 (84.9) | 1022 (86.6) | |||
| Yes | 99 | 38 (38.4) | 80 (80.8) | 83 (83.8) | |||
| History of kidney disease, n (%) | .976 | .089 | .272 | ||||
| No | 1215 | 444 (36.5) | 1033 (85.0) | 1052 (86.5) | |||
| Yes | 66 | 24 (36.4) | 51 (77.3) | 54 (81.8) | |||
| History of diabetes, n (%) | .261 | .127 | .401 | ||||
| No | 1072 | 398 (37.1) | 914 (85.3) | 929 (86.6) | |||
| Yes | 206 | 68 (33.0) | 167 (81.1) | 174 (84.4) | |||
| History of CCVD, n (%) | .984 | .073 | .039 | ||||
| No | 934 | 341 (36.5) | 801 (85.8) | 818 (87.5) | |||
| Yes | 350 | 128 (36.6) | 286 (81.7) | 291 (83.1) | |||
| History of dyslipidemia, n (%) | .024 | .391 | .560 | ||||
| No | 757 | 259 (34.2) | 649 (85.7) | 658 (86.9) | |||
| Yes | 499 | 202 (40.5) | 419 (84.0) | 428 (85.7) | |||
| Respiratory rate, n (%) | .000 | .620 | .487 | ||||
| Normal | 637 | 285 (44.7) | 912 (43.1) | 931 (46.1) | |||
| Abnormal | 647 | 184 (28.4) | 9 (1.3) | 9 (1.3) | |||
| Heart rate, n (%) | .340 | .401 | .224 | ||||
| Normal | 1199 | 427 (35.6) | 990 (82.5) | 1010 (84.2) | |||
| Abnormal | 85 | 25 (29.4) | 47 (55.2) | 47 (55.2) | |||
| SeSBP, n (%) | .000 | .000 | .000 | ||||
| Normal | 637 | 285 (44.7) | 591 (92.8) | 578 (90.7) | |||
| Abnormal | 647 | 184 (28.4) | 496 (76.7) | 531 (82.0) | |||
| SeDBP, n (%) | .000 | .000 | .126 | ||||
| Normal | 772 | 326 (42.2) | 686 (88.9) | 676 (87.5) | |||
| Abnormal | 512 | 143 (27.9) | 401 (78.3) | 433 (84.5) | |||
| Hypertension severity class, n (%) | .000 | .000 | .000 | ||||
| Mild | 502 | 243 (48.4) | 440 (87.6) | 446 (88.8) | |||
| Moderate or severe | 782 | 214 (27.3) | 616 (78.8) | 616 (78.8) | |||
| History of hypertension treatment, n (%) | .342 | .630 | .324 | ||||
| Ever treatment | 1162 | 422 (36.3) | 958 (82.4) | 978 (84.1) | |||
| First treatment | 122 | 38 (31.1) | 96 (78.6) | 96 (78.6) | |||
| History of antihypertensive drugs, n (%) | .293 | .079 | .001 | ||||
| Monotherapy | 709 | 270 (38.1) | 617 (87.0) | 631 (88.9) | |||
| Double combination | 374 | 134 (35.8) | 309 (82.6) | 312 (83.4) | |||
| Triple combination | 72 | 21 (29.2) | 58 (80.6) | 54 (75.) | |||
| Combinations, n (%) | .113 | <.001 | <.001 | ||||
| No combination | 1024 | 380 (37.1) | 866 (84.5) | 881 (86.0) | |||
| Lipid‐modifying drug | 112 | 39 (34.8) | 85 (75.8) | 87 (77.6) | |||
| Lipid‐modifying drug and others | 148 | 41 (27.7) | 103 (69.5) | 106 (71.6) |
Abbreviations: AHA, American Heart Association; BMI, body mass index; BP, blood pressure; CCVD, cardiovascular and cerebrovascular diseases; SeSBP, seated systolic blood pressure; SeDBP, seated diastolic blood pressure; W8, week 8.
The bold value indicate P‐value of statistical significance.
After adjusted by multivariate logistic regression analysis, male (vs. female), hypertension duration ≥ 5 years (vs. < 5 years), and abnormal SeSBP and SeDBP at baseline (vs. normal at baseline) independently reduced the probability achieving AHA BP target at W8 (Supplementary Table 4). Hypertension duration within 5−9 years (vs. < 5 years), moderate or severe hypertension severity class (vs. mild) and history of triple combination of antihypertensive drugs (vs. monotherapy) independently decreased the probability of achieving China BP target at W8 (Supplementary Table 5). In addition, hypertension duration within 5−9 years (vs. < 5 years) and moderate or severe hypertension severity class (vs. mild) were independent factors that hindered the achievement of BP response rate at W8 (Supplementary Table 6).
3.5. Change in home‐measured BP
Both home‐measured SeSBP and SeDBP reduced from W1 to W8 (both p < .001) (Figure 4A). The mean change values of home‐measured SeSBP from W2 to W8 were −2.0, −2.6, −3.2, −3.9, −4.1, −4.5, and −4.9, respectively; and those of home‐measured SeDBP from W2 to W8 were −1.3, −1.7, −2.2, −2.9, −2.8, −3.1, and −3.1, accordingly (Figure 4B).
FIGURE 4.
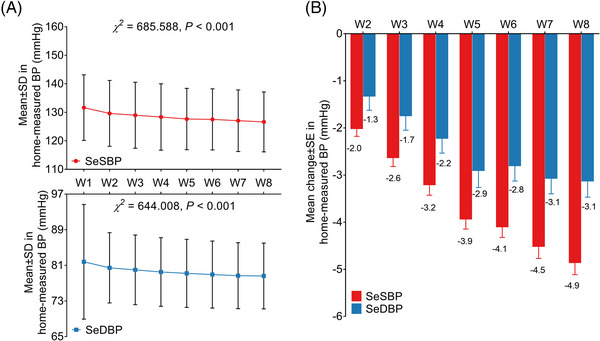
Change of home‐measured BP after administration of OM‐AML tablet. Home measured SeSBP and SeDBP from W1 to W8 (A). Change of home measured SeSBP and SeDBP from W1 to W8 (B).
3.6. Satisfaction
The patients’ satisfaction was increased at W8 compared with W0 (mean ± SD: 9.3 ± 1.2 vs. 8.0 ± 2.1) (p < .001) (Figure 5A). Physicians’ satisfaction was also elevated at W8 compared with W0 (mean ± SD: 9.2 ± 1.1 vs. 7.9 ± 2.1) (p < .001) (Figure 5B). Besides, the medication possession ratio was 94.8% from W0 to W4 and 91.3% from W0 to W8 (Figure 5C).
FIGURE 5.
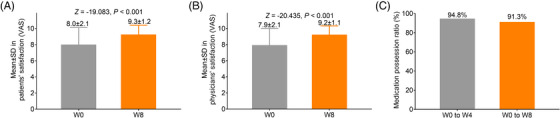
Satisfaction and medication possession. Comparison of patients’ (A) and physicians’ (B) satisfaction between W0 and W8. Medication possession rate from W0 to W4 and to W8 (C).
3.7. AEs
Totally, the main AEs by system organ class were nervous system disorders (12.9%), vascular disorders (8.7%), as well as general disorders and administration site conditions (5.4%). However, these AEs were generally mild. The most common severe AEs were vascular disorders (0.4%). Meanwhile, the most prevalent drug‐related AEs were nervous system disorders (4.6%), vascular disorders (2.6%), and general disorders and administration site conditions (2.3%) (Table 3). More detailed AEs by system organ class and preferred term class were shown in Supplementary Table 7. The most common AEs by preferred term class were headache, dizziness, palpitations, lethargy, fatigue, and hypoesthesia.
TABLE 3.
Number (%) of patients having adverse events (AEs) by system organ class.
| System organ class | Total AEs | Severe AEs | Drug‐related AEs |
|---|---|---|---|
| Nervous system disorders | 174 (12.9) | 0 (0.0) | 62 (4.6) |
| Vascular disorders | 117 (8.7) | 6 (0.4) | 35 (2.6) |
| General disorders and administration site conditions | 73 (5.4) | 1 (0.1) | 31 (2.3) |
| Cardiac disorders | 61 (4.5) | 3 (0.2) | 20 (1.4) |
| Gastrointestinal disorders | 59 (4.3) | 1 (0.1) | 14 (1.0) |
| Respiratory, thoracic and mediastinal disorders | 41 (3.0) | 3 (0.2) | 6 (0.4) |
| Psychiatric disorders | 37 (2.7) | 0 (0.0) | 23 (1.7) |
| Investigations | 20 (1.4) | 0 (0.0) | 2 (0.1) |
| Metabolism and nutrition disorders | 19 (1.4) | 0 (0.0) | 1 (0.1) |
| Skin and subcutaneous tissue disorders | 18 (1.3) | 0 (0.0) | 7 (0.5) |
| Renal and urinary disorders | 15 (1.1) | 2 (0.1) | 1 (0.1) |
| Musculoskeletal and connective tissue disorders | 13 (0.9) | 0 (0.0) | 1 (0.1) |
| Eye disorders | 11 (0.8) | 0 (0.0) | 3 (0.2) |
| Hepatobiliary disorders | 6 (0.4) | 0 (0.0) | 0 (0.0) |
| Reproductive system and breast disorders | 6 (0.4) | 1 (0.1) | 0 (0.0) |
| Neoplasms benign, malignant and unspecified (incl cysts and polyps) | 3 (0.2) | 1 (0.1) | 0 (0.0) |
| Endocrine disorders | 3 (0.2) | 0 (0.0) | 0 (0.0) |
| Ear and labyrinth disorders | 2 (0.1) | 0 (0.0) | 0 (0.0) |
| Surgical and medical procedures | 1 (0.1) | 0 (0.0) | 0 (0.0) |
| Immune system disorders | 1 (0.1) | 0 (0.0) | 1 (0.1) |
| Blood and lymphatic system disorders | 1 (0.1) | 0 (0.0) | 0 (0.0) |
4. DISCUSSION
The OM‐AML tablet is a commonly applied antihypertensive drug with multiple advantages, including effective control of BP, fewer AEs and better patients’ adherence. 9 , 10 , 11 Previously, several studies have reported the efficacy of OM‐AML tablet in patients with essential hypertension. For instance, after administrated with OM‐AML tablet for 12 weeks, the mean change of SeSBP/SeDBP from baseline to week 12 is −11.7 (± 0.8)/−6.1 (± 0.5) mmHg in black and −15.0 (± 0.5)/−8.2 (± 0.3) mmHg in non‐black patients with essential hypertension. 18 Another study conducted in the United States reports that the primary endpoint of SeSBP less than 140 mmHg is achieved in 71.6% of essential hypertensive patients with obesity and 80.2% in those without obesity after taking the OM‐AML tablet. 19 In Hispanic and non‐Hispanic patients with essential hypertension, OM‐AML tablet administration for 12 weeks achieves a mean change of SeSBP/SeDBP of −15.3/−7.3 mmHg and −14.1/−7.8 mmHg, respectively. 20 Whereas in China, where the prevalence of essential hypertension is dramatically increasing during the past few decades, little data about the efficacy and safety of OM‐AML tablet is available. Only one randomized, controlled trial reveals that OM‐AML tablet has a better effect on reducing SeSBP and SeDBP compared with OM or AML monotherapy. 14 In the current prospective, single‐arm, multicenter real‐world study, the data revealed that the mean (± SE) change of SeSBP/SeDBP was −10.8 ± 0.4/−6.6 ± 0.3 mmHg at W4 and −12.7 ± 0.5/−7.6 ± 0.3 mmHg at W8, respectively. Although varied a little, the mean changes of SeSBP and SeDBP were in the range reported by previous studies. 18 , 19 , 20 Meanwhile, the mean change of SeSBP/SeDBP by OM‐AML tablet in the current study is numerically greater than that by OM (−10.5/−9.6 mmHg) or AML (−9.1/−7.7 mmHg) monotherapy reported by the previous study. 14 The explanation might be that OM‐AML tablet combined two antihypertensive drugs with different acting mechanisms, which exerts a superior effect on controlling BP. 21 Elevated DBP is associated with increased risks of cerebral‐cardiovascular diseases, 22 meanwhile, OM‐AML tablet might have a better effect on controlling DBP; thus, the current study set the change in SeDBP as the primary outcome, which was in line with previous studies. 14 , 23 , 24
The primary goal of antihypertensive treatment is to reduce the BP to a certain threshold, thus reducing the risk of cardiovascular diseases. 25 In 2017, the AHA has revised the BP target from 140/90 mmHg to 130/80 mmHg of SeSBP/SeDBP, 16 while 140/90 mmHg of SeSBP/SeDBP is still set as the BP target in China. 17 In the current study, 84.7% of patients achieved BP target by China criteria (SeSBP/SeDBP < 140/90 mmHg) and 36.5% of patients achieved BP target by AHA criteria (SeSBP/SeDBP < 130/80 mmHg) at W8. The proportion of patients who achieved SeSBP/SeDBP < 140/90 mmHg in this study was numerically higher than that reported in black (45.4%), non‐black (50.5%), Hispanic (76.3% after taking OM/AML 10/40 mg) and non‐Hispanic (77.2% after taking OM/AML 10/40 mg) essential hypertensive patients treated by OM‐AML tablet. 18 , 20 A possible explanation might be that the SeSBP and SeDBP at baseline were numerically lower than those reported by previous studies. 18 , 20 Meanwhile, the proportion of patients who achieved SeSBP/SeDBP < 140/90 mmHg in this study was comparable to the previous randomized, controlled trial conducted in China. 14 Regarding the proportion of patients who achieved BP target by AHA criteria after treatment of OM‐AML tablet, it was incomparable since no data is reported by previous studies. Besides, females had a better achievement of BP target, which suggested that OM‐AML tablet might be effective in females.
In the current study, patients’ and physicians’ satisfaction was also assessed, which showed that both of them were elevated at W8 compared to that at W0. Since few previous studies report relevant findings, these data were incomparable to previous studies. The possible explanation for these data might be that: (1) OM‐AML tablet is characterized by high patients’ adherence, which resulted in a low missed dose and medication‐related cost 26 ; thus, patients’ satisfaction was elevated at W8. (2) OM‐AML tablet combines the component of an angiotensin receptor blocker and a calcium channel blocker with supreme efficacy, which has a good effect on controlling BP; therefore, both patients’ and physicians’ satisfaction were higher at W8. In addition, our study also revealed that the medication possession rate was 91.3% at W8, which was numerically similar to the relevant data in a previous study (91.0%). 11
OM‐AML tablet is considered a relatively safe antihypertensive drug since it has good efficacy in controlling BP and a reduced dose could be offered to the patients compared with OM or AML monotherapy. 14 According to previous studies, the incidence of OM‐AML tablet‐related AEs ranges from 2.9% to 8.0%. 14 , 18 , 19 The current study revealed that the incidence of OM‐AML‐related AEs (8.9%) was similar to that reported by previous studies. 14 , 18 , 19 Meanwhile, the main AEs were nervous system disorders, vascular disorders and general disorders and administration site conditions by system organ class, as well as headache, dizziness and edema by preferred term class, which were also in line with previous studies. 14 , 18 , 19 In addition, the incidence of severe AEs was low in the current study. These findings suggested that OM‐AML tablet was a safe option in Chinese patients with essential hypertension.
There were several limitations in this study. Firstly, the follow‐up duration of this study was relatively short, thus the long‐term efficacy and safety of OM‐AML tablet could not be inferred. Secondly, this was a single‐armed study, thus further studies with a control cohort should be conducted to further verify our findings. Thirdly, the efficacy of OM‐AML tablet combined with other antihypertensive drugs could be investigated in the future. Fourthly, the BP‐lowering effect of OM‐AML tablet might be affected by some confounding factors, such as fewer usage of previous antihypertensive medications.
Collectively, OM‐AML tablet is effective and safe in lowering BP, enabling achievement of guideline‐recommended BP target in Chinese patients with essential hypertension, which is one of the best antihypertensive options.
AUTHOR CONTRIBUTIONS
Conception and design of study: Zhaoqiang Cui, Junbo Ge. Acquisition of data: Zhaoqiang Cui, Zhaohui Qiu, Wei Hu, Genshan Ma, Ying Li, Ruxing Wang, Lin Chen, Liuliu Feng, Mei Hong, Yinglong Hou, Mingliang Wang, Jianhong Xie, Yawei Xu, Zhenxing Wang, Xiaojun Ji, Jing Huang, Danhong Fang, Lijiang Tang, Guosheng Fu, Juan Du, Ling Wang, Mengqi Liu, Junbo Ge. Data analysis and/or interpretation: Wenli Cheng, Xiaojun Cai, Yafei Jin, Yi Zhao, Liqun He, Peili Bu, Xiaoping Chen, Peng Dong, Xuebin Han, Minlei Liao, Xiaoyan Wang, Kai Huang, Yongle Li, Dongsheng Li, Jianan Wang, Jun Wang, Yingwu Liu. Drafting of manuscript and/or critical revision: all authors. Approval of final version of manuscript: all authors.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
PATIENT CONSENT STATEMENT
All participants signed the informed consent.
PERMISSION TO REPRODUCE MATERIAL FROM OTHER SOURCES
Not applicable.
The SVK study was registered in Chinese Clinical Trial Registry (http://www.chictr.org.cn/) with a registration number of ChiCTR1900026574.
Supporting information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
The authors thank all the investigating groups of the SVK study (Supplementary Table 8) for their contribution and the participants who contributed their information to this study. This study was funded by Daiichi Sankyo (China) Holdings Co., Ltd, Shanghai, China.
Cui Z, Qiu Z, Cheng W, et al. Efficacy and safety of olmesartan medoxomil‐amlodipine besylate tablet in Chinese patients with essential hypertension: A prospective, single‐arm, multi‐center, real‐world study. J Clin Hypertens. 2024;26:5–16. 10.1111/jch.14700
DATA AVAILABILITY STATEMENT
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Collaborators GBDRF . Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1923‐1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. 2020;16(4):223‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Connelly PJ, Currie G, Delles C. Sex differences in the prevalence, outcomes and management of hypertension. Curr Hypertens Rep. 2022;24(6):185‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yin R, Yin L, Li L, et al. Hypertension in China: burdens, guidelines and policy responses: a state‐of‐the‐art review. J Hum Hypertens. 2022;36(2):126‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li Y, Yang L, Yin L, et al. Trends in obesity and metabolic status in northern and southern China between 2012 and 2020. Front Nutr. 2021;8:811244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Al‐Makki A, DiPette D, Whelton PK, et al. Hypertension pharmacological treatment in adults: a world health organization guideline executive summary. Hypertension. 2022;79(1):293‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rahman F, Muthaiah N, Kumaramanickavel G. Current concepts and molecular mechanisms in pharmacogenetics of essential hypertension. Indian J Pharmacol. 2021;53(4):301‐309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mikulski BS, Bellei EA, Biduski D, De Marchi ACB. Mobile health applications and medication adherence of patients with hypertension: a systematic review and meta‐analysis. Am J Prev Med. 2022;62(4):626‐634. [DOI] [PubMed] [Google Scholar]
- 9. Jo SH, Kang SM, Yoo BS, et al. A prospective randomized, double‐blind, multi‐center, phase iii clinical trial evaluating the efficacy and safety of Olmesartan/amlodipine plus rosuvastatin combination treatment in patients with concomitant hypertension and dyslipidemia: a LEISURE study. J Clin Med. 2022;11(2):350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Volpe M, Santolamazza C, Mastromarino V, Coluccia R, Battistoni A, Tocci G. Monotherapy and dual combination therapies based on Olmesartan: a comprehensive strategy to improve blood pressure control. High Blood Press Cardiovasc Prev. 2017;24(3):243‐253. [DOI] [PubMed] [Google Scholar]
- 11. Sung J, Ahn KT, Cho BR, et al. Adherence to triple‐component antihypertensive regimens is higher with single‐pill than equivalent two‐pill regimens: a randomized controlled trial. Clin Transl Sci. 2021;14(3):1185‐1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ren M, Xuan D, Lu Y, Fu Y, Xuan J. Economic evaluation of olmesartan/amlodipine fixed‐dose combination for hypertension treatment in China. J Med Econ. 2020;23(4):394‐400. [DOI] [PubMed] [Google Scholar]
- 13. Li X, Mo E, Chen L. Pharmacokinetics and bioequivalence evaluation of 2 Olmesartan Medoxomil and amlodipine besylate fixed‐dose combination tablets in healthy Chinese volunteers under fasting and fed conditions. Clin Pharmacol Drug Dev. 2022;11(6):761‐769. [DOI] [PubMed] [Google Scholar]
- 14. Zhu JR, Zhang SY, Gao PJ. Efficacy and safety of olmesartan medoxomil/amlodipine fixed‐dose combination for hypertensive patients uncontrolled with monotherapy. Arch Pharm Res. 2014;37(12):1588‐1598. [DOI] [PubMed] [Google Scholar]
- 15. Wang JG, Bu PL, Chen LY, et al. 2019 Chinese hypertension league guidelines on home blood pressure monitoring. J Clin Hypertens (Greenwich). 2020;22(3):378‐383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Hypertension. 2018;71(6):e13‐e115. [DOI] [PubMed] [Google Scholar]
- 17. Wu S, Xu Y, Zheng R, et al. Hypertension defined by 2017 ACC/AHA guideline, ideal cardiovascular health metrics, and risk of cardiovascular disease: a nationwide prospective cohort study. Lancet Reg Health West Pac. 2022;20:100350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nesbitt S, Shojaee A, Maa JF. Efficacy/safety of a fixed‐dose amlodipine/olmesartan medoxomil‐based treatment regimen in hypertensive blacks and non‐blacks with uncontrolled BP on prior antihypertensive monotherapy. J Clin Hypertens (Greenwich). 2013;15(4):247‐253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hsueh WA, Shojaee A, Maa JF, Neutel JM. Efficacy of amlodipine/olmesartan medoxomil +/‐ HCTZ in obese patients uncontrolled on antihypertensive monotherapy. Curr Med Res Opin. 2012;28(11):1809‐1818. [DOI] [PubMed] [Google Scholar]
- 20. Punzi H, Shojaee A, Maa JF. Investigators B‐C. Efficacy and tolerability of fixed‐dose amlodipine/olmesartan medoxomil with or without hydrochlorothiazide in Hispanic and non‐Hispanic patients whose blood pressure is uncontrolled on antihypertensive monotherapy. Ther Adv Cardiovasc Dis. 2012;6(4):149‐161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee DW, Jung M, Wang HW, Khan Z, Pinton P. Systematic review with network meta‐analysis: comparative efficacy and safety of combination therapy with angiotensin ii receptor blockers and amlodipine in Asian hypertensive patients. Int J Hypertens. 2019;2019:9516279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2019;74(10):e177‐e232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Perez A, Cao C. Azilsartan in patients with mild to moderate hypertension using clinic and ambulatory blood pressure measurements. J Clin Hypertens (Greenwich). 2017;19(1):82‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rump LC, Ammentorp B, Laeis P, Scholze J. Adding hydrochlorothiazide to Olmesartan/amlodipine increases efficacy in patients with inadequate blood pressure control on dual‐combination therapy. J Clin Hypertens (Greenwich). 2016;18(1):60‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tschanz CMP, Cushman WC, Harrell CTE, Berlowitz DR, Sall JL. Synopsis of the 2020 U.S. department of veterans Affairs/U.S. department of defense clinical practice guideline: the diagnosis and management of hypertension in the primary care setting. Ann Intern Med. 2020;173(11):904‐913. [DOI] [PubMed] [Google Scholar]
- 26. Sohn IS, Ihm SH, Kim GH, et al. Real‐world evidence on the strategy of olmesartan‐based triple single‐pill combination in Korean hypertensive patients: a prospective, multicenter, observational study (RESOLVE‐PRO). Clin Hypertens. 2021;27(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.


