Abstract
This study examined apoptotic cell death associated with Shiga-like toxin (Stx)-producing Escherichia coli. Renal cortices from three children with postenteropathic hemolytic-uremic syndrome (HUS) and from mice infected with E. coli O157:H7 and pediatric renal tubular epithelial cells stimulated with Stx and E. coli O157:H7 extracts were examined for apoptotic changes. Apoptotic cells were detected by terminal dUTP nick end labeling of tubuli and glomeruli from HUS patients and from mice inoculated with Stx-2-positive and Stx-negative strains. Apoptosis was more extensive and severe ultramorphological nuclear and cytoplasmic changes were seen in the Stx-2-positive group. Stx caused DNA fragmentation and ultramorphological changes indicating apoptosis in cultured pediatric tubular cells. DNA fragmentation increased when cells were prestimulated with tumor necrosis factor alpha. Polymyxin extracts from Stx-2-positive and Stx-negative strains induced DNA fragmentation, but only extracts from Stx-2-positive strains caused ultramorphological changes and extensive DNA fragmentation. The results indicate that HUS is accompanied by increased apoptosis of kidney cells and that bacterial factors, possibly together with host cytokines in vivo, may activate apoptotic tissue injury.
Hemolytic-uremic syndrome (HUS) is characterized by microangiopathic hemolytic anemia, thrombocytopenia, and acute renal failure. The syndrome has been divided into two forms: typical (or postenteropathic) and atypical (28). The postenteropathic form of HUS occurs due to infection with Shiga-like toxin (Stx)-producing Escherichia coli, which causes a prodrome of diarrhea or hemorrhagic colitis followed by HUS (3, 22). Stx-producing E. coli O157:H7 has been associated with outbreaks of these conditions (15). The atypical form of HUS is not caused by Stx-producing E. coli and is usually not preceded by a diarrheal prodrome (10).
The most extensive tissue damage in HUS occurs in the kidneys. The injury is most prominent in the renal cortex, with pathological changes occurring in the glomerular endothelial cells and also in the tubular epithelial cells (16). The mechanism by which Stx-producing E. coli causes tissue damage is not clear. E. coli O157:H7 has not been found to be invasive (33), and it has therefore been assumed that tissue damage occurs as a result of the spread of bacterial products and/or inflammatory mediators from the intestine to target organs (14). Previous studies found that Stx-producing strains cause systemic symptoms in animals (5, 11, 39, 48, 51, 52) and that these symptoms could be reproduced when the animals were injected with purified Stx (2, 18, 47). In a recent study carried out with mice, we found that E. coli O157:H7 that produced Stx caused glomerular and tubular pathology and a higher frequency of systemic symptoms than E. coli O157:H7 that did not produce Stx, suggesting that this toxin may be important for the HUS-related virulence of the strain (24). Furthermore, in vitro studies have found that Stx is cytotoxic for human endothelial cells (27, 36, 49) and that renal endothelial cells are more susceptible to the cytotoxic effect than umbilical vein endothelial cells (37). After binding to cells, the toxin is endocytosed (44), binds to 60S ribosomes, and inhibits peptide chain elongation and protein synthesis (38, 43), thereby leading to cell death.
Programmed cell death, or apoptosis, is defined by the cell’s ultrastructural morphology (26) and is characterized by cell shrinkage, membrane blebbing, and condensation of nuclear chromatin. The morphological changes are accompanied by DNA fragmentation. This form of cell death is a naturally occurring process by which an organism removes damaged or unnecessary cells and may also be triggered by external stimuli (32). Shigella flexneri (56) has been found to induce apoptosis in host macrophages. This activity was related to the invasive properties of the strain (8, 56) and not the production of toxin. Purified toxins such as diphtheria toxin (6), ricin, and Stx (45) have previously been found to activate apoptosis. Stx induced apoptosis in Burkitt lymphoma cells and Vero cells in vitro (19, 31) and in rabbit intestinal epithelial cells in vivo (25). In addition, plasma samples from patients with atypical HUS, but not from a patient with postenteropathic HUS, were found to induce apoptosis of human microvascular endothelial cells (34).
The aims of this study were to examine kidney tissue from patients with postenteropathic HUS and mice with experimental E. coli O157:H7 infection for signs of apoptosis and to study apoptosis induction in pediatric kidney cultures in vitro.
MATERIALS AND METHODS
Renal tissue specimens from renal biopsies and autopsies of patients and a pediatric control.
Renal cortical tissues from four children were studied. A renal cortical biopsy (n = 1) and postmortem tissues (n = 2) were available from three children with postenteropathic HUS. A renal cortical biopsy specimen from one child with nephrotic syndrome was studied as a control.
A renal biopsy was obtained from a 22-month-old Swedish girl hospitalized in 1990 due to bloody diarrhea and anuria. HUS was suspected due to anemia (hemoglobin, 55 g/liter; normal value, 120 to 160 g/liter), thrombocytopenia (platelet count 65 × 109/liter; normal value, 140 × 109 to 400 × 109/liter), and renal failure (creatinine, 438 μmol/liter; normal value, 27 to 62 μmol/liter). Other coagulation analyses were normal. Hemolysis was evident by the presence of Helmet cells in a blood film and elevated lactic dehydrogenase. Due to persistent anuria and uncertainty regarding the diagnosis, a renal cortical biopsy was performed 5 days after the onset of anuria. The patient was treated with plasma exchange and peritoneal dialysis and made a partial recovery.
Since a bacterial isolate was not available from this patient, EDTA plasma obtained on the day of admission (prior to treatment with plasma exchange) was analyzed for antibodies against the purified lipopolysaccharides (LPSs) of E. coli serogroups O157, O121, O26, O103, and O111 by enzyme-linked immunosorbent assay (4). O121 LPS was purified from a Stx-2-producing E. coli O121:H19 fecal isolate from a 2-year-old Swedish boy with hemorrhagic colitis. The HUS patient was found to have antibodies to O121 but not to the other O antigens. The enzyme-linked immunosorbent assay results showed a value of 0.854 (optical density at 405 nm), which was defined as positive by using a cutoff value of 0.5 for samples diluted 1,000-fold. Serum from a 4-year-old Swedish boy with HUS caused by a Stx-1- and -2-producing E. coli O121:H19 strain (46) was used as a positive control (optical density, 1.042 at 405 nm). These results were confirmed by LPS immunoblotting (7) and indicated infection with E. coli O121. Since the bacterium was not isolated, the HUS-related strain was not identified.
Renal autopsy material was obtained from two American children (a 4-year-old boy and a 5-year-old girl) with postenteropathic HUS, hospitalized in 1989 and 1996. The children had a prodrome of bloody diarrhea, followed by anuria, thrombocytopenia, and microangiopathic hemolytic anemia but had normal coagulation studies. Both children developed neurological symptoms and coma. The boy died of cerebral herniation 4 days after the onset of anuria; the girl died due to severe hypotension 3 days after the onset of anuria. A HUS-related fecal strain was not isolated from the stools of these children. Serum, for analysis of antibodies to the LPSs of HUS-related strains, was not available.
A renal cortical biopsy was taken in 1989 from a 4-year-old Swedish boy with recurrent nephrotic syndrome who was nonresponsive to treatment with steroids and cyclophosphamide. The biopsy material was taken 4 months after treatment with cyclophosphamide, while the patient was taking prednisolone, and used as a control.
Biopsy and autopsy material were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS; pH 7.4), embedded in paraffin, and sectioned. Sections were deparaffinized, rehydrated, stained with hematoxylin-eosin and silver methenamine, and examined for glomerular, tubular, interstitial, and vascular changes. In addition, immunofluorescence was examined in cryosections of the biopsies with fluorescein isothiocyanate-conjugated antibodies to immunoglobulin G (IgG), IgA, IgM, kappa chains, lambda chains, C3, and fibrinogen (all antibodies were from Dako, Stockholm, Sweden). Biopsy sections used for the terminal dUTP nick end labeling (TUNEL) assay were immersed in xylene in order to remove the cover glass, destained with ethanol, and kept in distilled water until they were analyzed. Autopsy sections were deparaffinized and rehydrated prior to processing.
Mouse kidneys.
Kidneys were taken from 159 C3H/HeN mice. Of these mice, 114 were studied previously (24) and 45 were studied as integral parts of this investigation. Mice were inoculated intragastrically with E. coli O157:H7 strains that produce Stx-2 (86-24 and 86BL, n = 77) (15, 24), E. coli O157:H7 strains that do not produce Stx (87-23 and 87BL, n = 42) (15, 24), or the control E. coli strain FN414 (n = 8) (17), or they were treated with PBS (0.06 M, pH 7.2; n = 8). Furthermore, 31 mice inoculated with a Stx-2-producing strain (86BL) were pretreated with a monoclonal antibody (MAb) to the Stx-2 A subunit at 70 μg/ml (n = 16) or 400 μg/ml (n = 15) (24, 40) prior to infection. Mice were examined for systemic symptoms and sacrificed when they were terminally ill or after 10 days if they were asymptomatic, as previously described (24). This study was approved by the animal ethics committee, University of Lund, Lund, Sweden.
Kidneys from 30 of the mice were selected for histology and the TUNEL assay. Eleven kidneys were taken from mice inoculated with Stx-2-positive strains. Nine kidneys were taken from mice inoculated with Stx-2-negative strains, and two were taken from mice inoculated with E. coli FN414. Kidneys from four PBS control mice were studied. In addition, kidneys from four mice pretreated with MAb to the Stx-2 A subunit and inoculated with a Stx-2-positive strain were studied. Two mice were pretreated with 70 μg of MAb per ml, and two were pretreated with 400 μg of MAb per ml.
Transmission electron microscopy (TEM) was carried out with the renal cortices of 18 mice; all but four of these kidneys were also examined by light microscopy and the TUNEL assay. Eight mice were inoculated with E. coli 86-24, and seven mice were inoculated with E. coli 87-23. Renal cortices from three PBS controls were also examined. Tubular epithelial cells were identified by the presence of microvilli and desmosomes. Endothelial cells situated in glomeruli and along blood vessels were identified by their locations and the presence of Weibel-Palade bodies in their cytoplasms (53).
Mouse kidneys were removed and fixed in 4% paraformaldehyde in PBS (pH 7.4) immediately after sacrifice. Tissues were then embedded in paraffin and sectioned. Sections were deparaffinized, rehydrated, stained with hematoxylin-eosin and silver methenamine, and examined for glomerular, tubular, interstitial, and vascular changes. Sections used for TUNEL assay were deparaffinized and rehydrated prior to processing. Four sections from each mouse kidney were studied by the TUNEL assay. Adjacent sections were used for examination by light microscopy and TUNEL analysis. Sections used for TEM were fixed and prepared as described below.
TUNEL assay.
The TUNEL method was based on a modified protocol from Gavrieli et al. (12, 41). TUNEL labeling was defined as positive when cells were highly fluorescent against a background of lightly stained cells. Assay controls were performed by preincubation of sections with 1 μg of DNase (Boehringer, Mannheim, Germany) per ml for 10 min and resulted in strong positive labeling of all visible cells. Omission of the biotin-labeled nucleotide resulted in no detectable signal.
HRTEC.
Human renal tubular epithelial cells (HRTEC) were isolated from the kidney of a 3-year-old boy whose kidney was removed due to hydronephrosis and reduced function. The removal of part of the renal tissue for research purposes was approved by the ethics committee of the University of Lund. The child was not taking medications prior to or at the time of the operation and had a slightly elevated blood pressure (120/80). The renal cortex was macroscopically thinner than normal. The cortex was dissected from the renal medulla. The cortical cells were isolated according to the method described for human renal microvascular endothelial cells (HRMEC) (37, 54). Briefly, the cortical fragments were incubated overnight in a buffer containing 0.1% collagenase type I (Sigma, Stockholm, Sweden) and 0.04% DNase IV (Sigma). The tissue was applied to the top of a two-step Percoll gradient (Pharmacia Biotech, Stockholm, Sweden), and the interface between the two steps was collected, centrifuged, and resuspended in Primaria flasks (Falcon, Stockholm, Sweden) in Dulbecco’s modified Eagle’s medium (GIBCO, Täby, Sweden) supplemented with 15% fetal calf serum (GIBCO), 2 mM l-glutamine (GIBCO), 20 U of heparin (Pharmacia) per ml, 100 U of penicillin per ml, and 100 μg of streptomycin per ml (both from GIBCO). Cells were incubated at 37°C in an atmosphere of 95% air and 5% CO2 and grown to confluence. After trypsinization, the cell suspension was filtered through a 35-μm-pore-size nylon mesh and cells passaged through the net were replated in Primaria flasks and grown to confluence. The cell isolates were trypsinized, concentrated in fetal calf serum containing 5% dimethyl sulfoxide (Merck, Darmstadt, Germany), and stored in liquid nitrogen for subsequent use. Cells used in experiments were passaged three to five times and cultured in Primaria flasks or in 24-well Primaria plates in medium with supplements and 1% endothelial cell growth factor (Sigma).
By light microscopy, more than 90% of the cells had similar morphologies. These cells were characterized and confirmed as epithelial cells by positive staining for cytokeratins (35) with MNF116 (Dako) and CAM 5.2 (Becton Dickinson, San Jose, Calif.) by the alkaline phosphatase anti-alkaline phosphatase technique. By TEM the cells exhibited tight junctions and microvilli, indicating tubular origin. These cells were therefore defined as HRTEC. The presence of leucocytes was ruled out by lack of reactivity with a MAb directed to human leucocyte common antigen. Furthermore, the cells were negative for the following endothelial cell markers: von Willebrand factor, CD31, and CD34 (all antibodies from Dako). With anti-endoglin antibody (Dako), approximately 5% of cells stained weakly. Less than 10% of cells contained Weibel-Palade bodies, as determined by TEM (53).
Cell stimulants. (i) Stx.
Purified Stx was kindly provided by A Lindberg (Department of Microbiology, Karolinska Institute, Huddinge, Sweden). The LPS content of the Stx preparation was 2 endotoxin units (EU)/ml at a Stx concentration of 1 μg/ml as tested by the Limulus amebocyte lysate assay (Coatest; Chromogenix, Gothenburg, Sweden), which has a detection level of 0.04 EU/ml.
(ii) Polymyxin extracts.
E. coli O157:H7 86-24 and 87-23 and the control E. coli strain FN414 were used for cell stimulation. The bacteria were cultured overnight on tryptic soy agar plates, scraped off the plates, and harvested into PBS-A (0.12 M NaCl, 0.03 M phosphate, 0.02% NaN3 [pH 7.2]) with polymyxin B sulfate (2 mg/ml; Dumex, Copenhagen, Denmark) diluted to a weight of 50 mg of bacteria/ml of PBS-A and incubated for 30 min at 37°C. The supernatant was collected after centrifugation at 400 × g for 10 min, and the cytotoxic concentration was determined by a modified Vero cell assay at dilutions of 1/10 to 1/1,280 (13, 21, 42). The polymyxin B sulfate extract of E. coli 86-24 was cytotoxic for Vero cells at dilutions of ≤160. Extracts of E. coli 87-23 and FN414 did not affect the Vero cells. The LPS content of each of the bacterial extracts was over 10,000 EU/ml as tested by the Limulus amebocyte lysate assay. The LPS content of polymyxin B sulfate in PBS-A was 4 to 7 EU/ml.
Cell stimulation assays.
Epithelial cells were grown to confluency in 24-well Primaria plates or in Primaria flasks. At time zero, cells were washed in PBS and the culture medium was removed and replaced with endothelial serum-free medium (GIBCO) supplemented with 2 mM l-glutamine, 20 U of heparin (Pharmacia) per ml, 100 U of penicillin per ml, 100 μg of streptomycin per ml (both from GIBCO), and 1% endothelial cell growth factor (Sigma) with or without 40 ng of tumor necrosis factor alpha (TNF-α; Knoll; BASF, Ludwigshafen, Germany) per ml. At 24 h cells were stimulated with Stx (100 ng/ml to 1 pg/ml) or polymyxin B sulfate extracts at a 1/40 dilution of E. coli strain 86-24, 87-23, or FN414. Dilutions were prepared in endothelial serum-free medium with supplements. Control cells were left unstimulated or exposed to polymyxin B sulfate (2 mg/ml of PBS-A). Supernatants were taken 24 h after addition of stimulant. Cells were removed by centrifugation at 250 × g. The remaining cells were detached with EDTA (0.2 g/liter of PBS) or trypsin. Cells were combined and subjected to DNA analyses or fixed for electron microscopy. For each stimulant, cell experiments were carried out twice.
TEM.
Mouse kidneys and HRTEC were double fixed in 2.5% glutaraldehyde and 1% osmium tetroxide (both diluted in 0.1 M Sörensen buffer), embedded in agar 100 (Link, Stockholm, Sweden), poststained with 4% uranyl acetate and 2% lead citrate (both from Kebo, Stockholm, Sweden), sectioned, and examined by TEM.
DNA fragmentation.
High-molecular-weight DNA fragments were detected by field-inversion gel electrophoresis (FIGE) as described previously (55). DNA size calibration was performed with two sets of pulse markers: chromosomes from Saccharomyces cerevisiae (225 to 2,200 kbp) and a mixture of λ DNA HindIII fragments, λ DNA, and λ DNA concatemers (0.1 to 200 kbp) purchased from Sigma. DNA was visualized under UV light (305 nm) after being stained with ethidium bromide (6 μg/ml) and was photographed with Polaroid 55 positive-negative film.
Detection of apoptosis.
Tissue apoptosis in vivo was detected by the TUNEL assay and TEM. Apoptosis of cultured renal cells in vitro was detected by TEM and FIGE.
Statistics.
Differences in the number of TUNEL-positive cells between mice inoculated with Stx-2-positive strains and mice inoculated with Stx-2-negative strains were evaluated by the Student t test. A P of ≤0.05 was considered significant.
RESULTS
Histopathology of human renal cortical tissue.
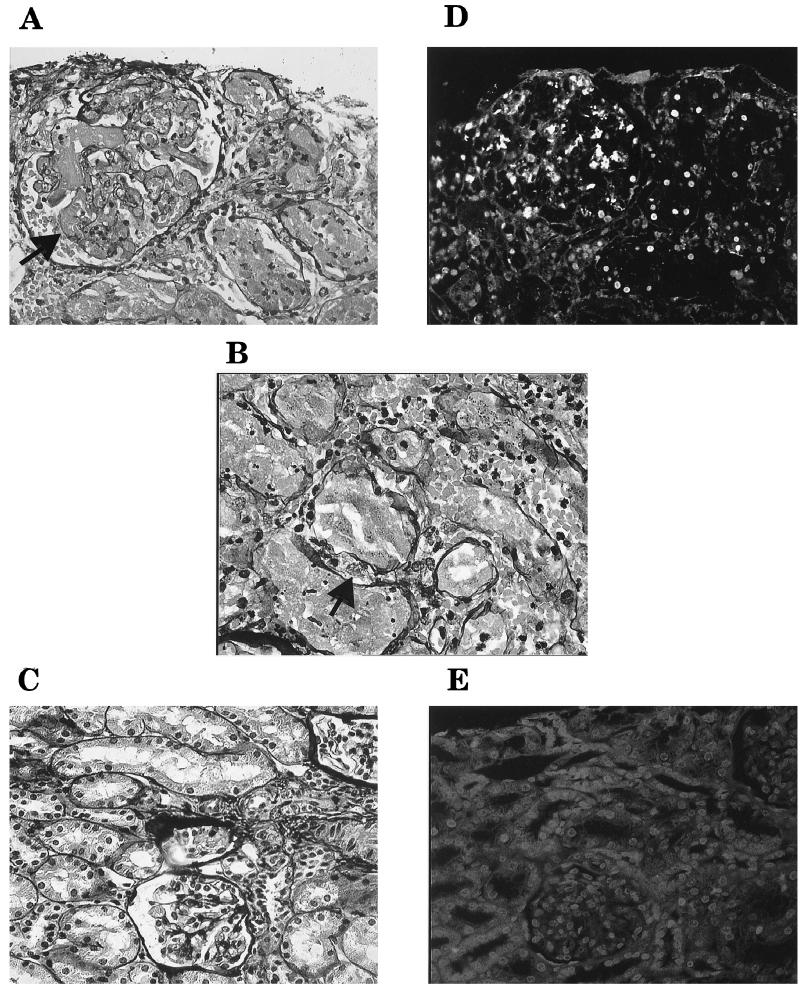
Histopathology of the biopsy from the HUS patient showed extensive renal cortical necrosis (pathological definition). The 10 glomeruli visualized in the biopsy had capillaries filled with thrombi. Cell proliferation was noted in one glomerulus. Blood vessels were dilated and filled with thrombi. Extensive damage to tubular epithelial cells, including pyknotic cell nuclei and fragments of nuclei, was noted. Tubular luminae were filled with these fragments as well as fibrin and erythrocytes (Fig. 1A and B). The interstitium was widened. Immunofluorescence for fibrinogen was strongly positive in glomerular capillaries, blood vessels, and tubuli. Renal cortical necrosis secondary to HUS was diagnosed by a renal pathologist.
FIG. 1.
Light microscopy of renal biopsies from a child with D+ HUS (A and B) and a child with nephrotic syndrome (C). (A) Glomerular thrombi are seen in capillaries (arrow), erythrocytes are seen in the urinary space, and tubular luminae are filled with pyknotic nuclei, fragments of nuclei, and erythrocytes. (B) Larger cells, which may be macrophages, were noted between tubular structures (arrow). (C) Glomerular and tubular structures without histopathological changes. Shown are the results of fluorescent microscopy of renal biopsies from the child with D+ HUS (D) and the child with nephrotic syndrome (E) labeled by the TUNEL assay in sections near those seen in panels A and C, respectively. (D) Numerous fluorescent cells in glomerular and tubular structures. (E) Lack of fluorescent labeling. Magnification, ×392 (all panels).
The autopsy material from the boy with HUS showed extensive renal cortical necrosis. Only a few areas of the renal cortex had viable cells with nuclei. These areas contained glomeruli with capillary thrombi and pyknotic tubular cells sloughing into the luminae. The autopsy material from the girl with HUS showed a majority of glomeruli with capillary fibrin thrombi and pyknotic nuclei. Focal tubular damage with many pyknotic nuclei were seen.
Light microscopy of the control biopsy showed 10 glomeruli with slight proliferation of mesangial cells. Changes were not noted in the blood vessels, tubuli, or interstitium (Fig. 1C). Immunofluorescence was negative for all examined antisera. Minimal-change nephropathy was diagnosed by a renal pathologist.
TUNEL of human renal cortical tissue.
Numerous TUNEL-positive nuclei were identified in the renal cortices from the children with HUS. Labeling was noted in the nuclei of tubular cells (Fig. 1D), and small labeled structures were present in the tubular luminae. Nuclei also stained positively in glomeruli. TUNEL-positive cells were not identified in the renal cortex of the control biopsy (Fig. 1E).
Histologic studies of mouse kidneys.
Histopathology of renal sections from infected mice revealed focal proliferation of glomerular mesangial cells, increased deposition of mesangial matrix, focal interstitial influx of inflammatory cells, necrosis of tubular epithelial cells (disintegration of cytoplasms and absence of nuclei), and an abundance of erythrocytes in the blood vessels. Mice inoculated with E. coli FN414 and PBS control mice sacrificed on day 10 exhibited normal histologies.
Kidneys from mice pretreated with anti-Stx-2 antibodies and inoculated with E. coli 86BL (n = 4) exhibited fewer histopathological changes than mice that were not pretreated. Focal proliferation of glomerular mesangial cells and few inflammatory infiltrates (n = 1), minimal vascular congestion (n = 2), or no changes (n = 1) were noted.
TUNEL analysis of mouse kidneys.
Two patterns of labeling by TUNEL were observed in mouse renal cortices: one pattern was indicative of single fluorescent nuclei of separate cells surrounded by nonlabeled cells (Fig. 2A and B) and the other pattern was patchy fluorescence, indicative of areas of approximately 50 to 150 positively stained nuclei per patch (Fig. 2C). In addition, certain kidneys exhibited slightly positive diffuse fluorescence along their entire outer cortical borders. These border cells did not stain as brightly as cells described above but were more fluorescent than the background staining. The different patterns of labeling by TUNEL could be present in the same kidney. There was no correlation between the duration of infection and the degree of labeling.
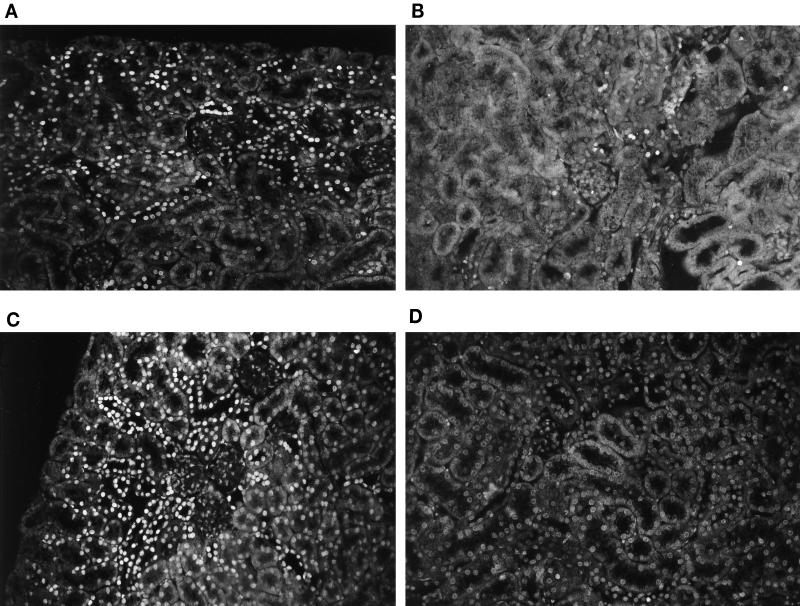
FIG. 2.
TUNEL labeling of mouse renal cortices. (A and C) Mice inoculated with Stx-2-positive strains; (B) mouse inoculated with Stx-2-negative strain; (D) PBS control mouse. (A and B) Single fluorescent nuclei (TUNEL-positive nuclei in tubular structures were not identified as necrotic tubular cells when the nuclei were compared with light microscopy); (C) patchy fluorescence (multiple patches of fluorescent cells seen in the same kidney); (D) lack of fluorescent cells. Magnification, ×134 (all panels).
The different patterns of labeling are shown in relation to the inoculated strain in Table 1. Single tubular fluorescence was detected in nine kidneys and single glomerular fluorescence was seen in three kidneys (one to two nuclei per glomerulus in 1 of 10 glomeruli) of mice inoculated with Stx-2-positive strains (n = 11). Single TUNEL-positive nuclei could also be seen in the tubular lumina. Patchy fluorescence was identified in three kidneys, and diffuse cortical fluorescence was observed in five kidneys. Kidney samples from mice inoculated with E. coli 86BL and pretreated with anti Stx-2 antibody were TUNEL negative regardless of the antibody dose.
TABLE 1.
Patterns of labeling by TUNEL analysis of renal cortices from E. coli O157:H7-infected mice
| Pattern of labeling by TUNEL analysis | No. of mice inoculated with E. coli strain type:
|
|
|---|---|---|
| Stx+ (n = 11) | Stx− (n = 9) | |
| Patchy | 3 | 0 |
| Single cells | 9 | 8 |
| Cortical diffuse | 5 | 4 |
| None | 1 | 1 |
Single tubular fluorescence was detected in eight kidneys and single glomerular fluorescence was detected in one kidney from mice inoculated with Stx-negative strains (n = 9). One mouse exhibited single fluorescence in multiple cells with various morphologies. Most of these cells appeared to be nonepithelial in size and may have been inflammatory. Patchy fluorescence was not observed, but diffuse cortical fluorescence was observed in four kidneys.
A comparison of the degrees of single-cell fluorescence between mice inoculated with Stx-2-positive (n = 11) versus Stx-2-negative (n = 9) E. coli O157:H7 strains provided the following results. Mice inoculated with Stx-2-positive strains showed a range of TUNEL-positive single cells of 0 to >1,000 (median, 29); mice inoculated with Stx-2-negative strains showed a range of 0 to 40 (median, 15) (P < 0.0001). The cells in one kidney from a mouse inoculated with a Stx-negative strain that had 40 fluorescent nuclei were nonepithelial in size and appeared to be inflammatory; all other kidneys from mice inoculated with Stx-negative strains had up to 24 fluorescent nuclei.
The renal cortices from mice inoculated with E. coli FN414 were TUNEL negative. Single fluorescent nuclei were found in the renal cortices of two of the PBS control mice, one of which had five and the other of which had eight positive nuclei per section of renal cortex. The renal cortices of the other two control mice were TUNEL negative (Fig. 2D).
TEM of mouse kidneys.
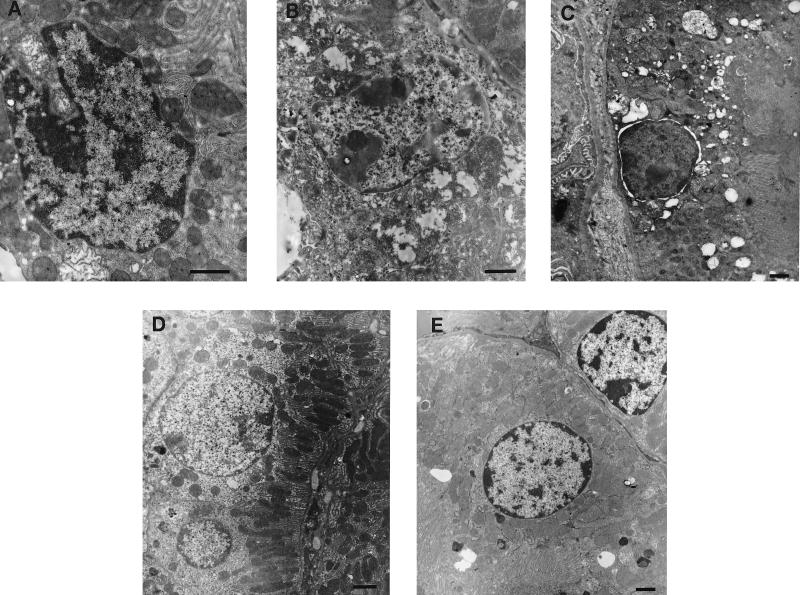
Ultramorphological changes indicating apoptosis or necrosis were evaluated by TEM. Marked nuclear and cytoplasmic changes in tubular epithelial cells were noted for all renal cortices from symptomatic mice inoculated with Stx-2-positive strains (n = 5). Cells (5 of 10 cells) exhibited convoluted nuclei with invaginations in their nuclear membranes (Fig. 3A), chromatin condensation (2 of 10 cells) (Fig. 3B), or separation of the nuclear envelopes (1 of 20 cells) (Fig. 3C). Considerable swelling and disruption of the inner mitochondrial cristae were noted for most cells (Fig. 3B). Cells with severely damaged mitochondriae had a total lack of the brush border, and their luminal spaces were filled with granular deposits. A few endothelial cells exhibited convoluted nuclei with disruption of the nuclear membranes, but intracytoplasmic changes were not noted. A few mesangial cells exhibited convoluted nuclei, but most appeared normal. These changes in tubular epithelial, endothelial, and mesangial cells were noted for all kidneys regardless of when mice were sacrificed.
FIG. 3.
Ultramorphologies of renal tubular cells from the cortices of mice inoculated with E. coli O157:H7 and of controls. (A to C) Mice infected with Stx-2-positive strains; (D) mouse infected with Stx-2-negative strain; (E) PBS control mouse. (A) Convoluted nucleus and swollen mitochondriae; (B) chromatin condensation and swollen mitochondriae with disrupted cristae (changes suggesting that necrosis may also have occurred); (C) separation of the nuclear envelope; (D) round nucleus, normal intracytoplasmic organelles, and brush border; (E) normal tubular cell structure. Bars = 1 μm.
In contrast, asymptomatic mice inoculated with Stx-2-positive strains (n = 3) did not show ultrastructural changes except for one mouse, which had a few tubular cells (1 of 20) with swollen mitochondriae and disrupted cristae.
Nuclear changes in tubular, endothelial, or mesangial cells from symptomatic mice inoculated with Stx-negative strains (n = 5) were not noted (Fig. 3D). One of 20 tubular cells exhibited slight swelling of mitochondriae with few disrupted cristae. One of two asymptomatic mice exhibited a few tubular cells (1 of 30 cells) with swollen mitochondriae. No ultramorphological changes were noticed in the control mice (n = 3) (Fig. 3E).
The effects of Stx and polymyxin extracts on HRTEC. (i) Stx.
Stx had a marked effect on the confluence, adherence, and morphology of HRTEC. At Stx concentrations of 1 ng/ml to 10 pg/ml, considerable dose-related cell detachment was noted by light microscopy after 24 h of stimulation. Cells that did not detach became elongated, with multiple vacuoles and prominent and less centralized nuclei. Stx at concentrations above 1 ng/ml caused detachment of almost all cells. Toxin concentrations below 10 pg/ml did not have a visible effect on the cells. The effect of Stx was enhanced by prestimulation with TNF-α.
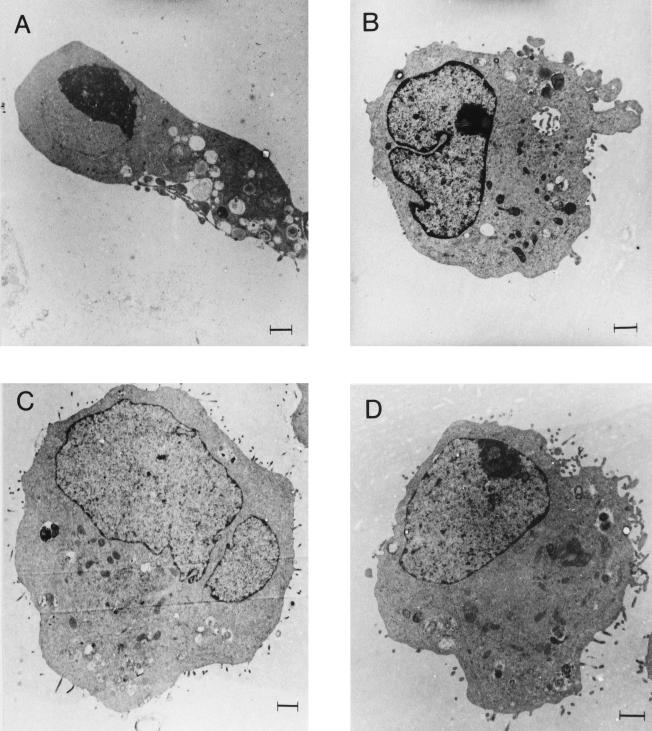
Cell changes indicative of apoptosis or necrosis were examined by TEM. HRTEC stimulated with 100 pg of Stx per ml for 24 h exhibited marked nuclear and cytoplasmic changes, with chromatin condensation and disruption of their nuclear envelopes (Fig. 4A), sparsity of rough endoplasmic reticula, slight mitochondrial swelling, blebbing of their cytoplasmic membranes, and disappearance of microvilli.
FIG. 4.
Ultramorphologies of HRTEC. (A) Cell stimulated with 100 pg of Stx per ml for 24 h showing chromatin condensation, blebbing of the cytoplasmic membrane, and a lack of microvilli; (B) cell stimulated with the polymyxin extract of E. coli 86-24 for 24 h showing convolution of the nuclear membrane and sparsity of microvilli; (C) cell stimulated with polymyxin extract of E. coli 87-23 for 24 h showing no changes; (D) unstimulated cell showing normal morphology. Bars = 1 μm.
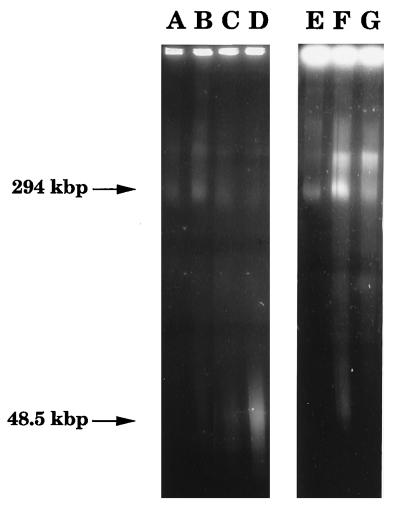
The capacity of Stx to induce DNA fragmentation was examined by FIGE. Stx at concentrations of 1 ng/ml and 100 pg/ml and 10 pg/ml induced the formation of high-molecular-weight DNA fragments, seen as weak bands in the 50-kbp range on agarose gels after 24 h. Prestimulation of cells with TNF-α (40 ng/ml) for 24 h increased the degree of fragmentation, which was reflected in stronger high-molecular-weight bands after 24 h of toxin stimulation (Fig. 5). TNF-α alone did not induce DNA fragmentation.
FIG. 5.
Chromatin cleavage analyzed by FIGE. (A) Unstimulated cells; (B) unstimulated cells pretreated with 40 ng of TNF-α per ml for 24 h; (C) cells stimulated with 100 pg of Stx per ml for 24 h; (D) cells pretreated with 40 ng of TNF-α per ml for 24 h and then stimulated with 100 pg of Stx per ml for 24 h; (E) cells exposed to polymyxin B sulfate (2 mg/ml in PBS-A) for 24 h; (F) cells stimulated with the polymyxin extract of E. coli 86-24 for 24 h; (G) cells stimulated with the polymyxin extract of E. coli 87-23 for 24 h.
(ii) Polymyxin extracts.
E. coli 86-24 extract (Stx-2 positive) caused massive cell detachment after 24 h. TEM revealed convolution of the nuclear envelope (Fig. 4B) and redistribution of chromatin. Mitochondrial swelling and sparsity of microvilli were also apparent. High-molecular-weight DNA fragments (294 and 50 kbp) were formed (Fig. 5). Prestimulation of cells with TNF-α (40 ng/ml) for 24 h increased DNA fragmentation in the 50-kbp range after 24 h of stimulation with the bacterial supernatant. In contrast, E. coli 87-23 extract (Stx negative) did not cause nuclear or intracytoplasmic changes (Fig. 4C) but high-molecular-weight DNA fragments (294 kbp) were formed (Fig. 5) and did not increase after TNF-α prestimulation. E. coli FN414 extract caused the formation of high-molecular-weight DNA fragments in the 294-kbp range. Cells stimulated with this strain were not prestimulated with TNF-α or examined by TEM. Neither morphological changes nor DNA fragmentation was noted in cells exposed to polymyxin B sulfate in PBS-A or in unstimulated cells (Fig. 4D).
DISCUSSION
This study examined apoptosis of renal cortical cells in the HUS. The renal cortical biopsy taken from a child with postenteropathic HUS showed extensive cortical necrosis and thrombotic microangiopathy as defined by pathology. Nuclear fragments were visualized by light microscopy, which indicated that apoptosis was taking place. TUNEL analysis revealed positive staining in the glomerular and tubular structures and in lumina. Labeling was also positive in autopsy material taken from two children with HUS. A recent study showed that processing of tissue postmortem does not, in itself, increase the degree of TUNEL-positive cells (in comparison to the degree of TUNEL-positive cells in biopsy material) (1). Taken together, these results suggest that apoptosis of renal cortical cells takes place in HUS.
Apoptotic cells were also detected in kidneys of mice with experimentally induced E. coli O157:H7 infection. Renal cortices from mice inoculated with Stx-2-positive E. coli O157:H7 exhibited high numbers of TUNEL-positive cells. The presence of patches of fluorescence and high numbers of labeled single cells indicated extensive apoptosis. Ultrastructural changes consisted of severe nuclear and cytoplasmic tubular changes. TUNEL-positive cells were also observed, albeit to a lesser degree, in mice inoculated with toxin-negative strains, and a low frequency of TUNEL-positive cells occurred in normal kidneys. TEM did not show tissue damage in mice inoculated with toxin-negative strains, but the sections represent a small portion of the renal cortex and damaged areas may have been missed. Kidneys from mice inoculated with Stx-2-positive strains showed changes in both epithelial and endothelial cells, whereas kidneys from mice infected with Stx-2-negative strains showed fewer changes and these occurred only in epithelial cells. The kidney apoptosis pattern confirmed the observations regarding human HUS and suggested that infection with Stx-2-producing E. coli was the cause of these changes.
Further studies showed a direct apoptosis-inducing effect of the bacteria and of Stx on pediatric renal cells in culture. Primary kidney cell cultures were susceptible to the cytotoxic effect of Stx-2-positive E. coli O157:H7 and purified Stx. Ultramorphology indicated that the cells were undergoing apoptosis. Furthermore, cells exposed to Stx and polymyxin extracts of Stx-2-positive E. coli O157:H7 were shown to undergo DNA fragmentation. Epithelial cells are less likely to produce typical nucleosome ladders (9), but fragments of 50 kbp represent more extensive DNA digestion. HRTEC stimulated with the polymyxin extract of the toxin-negative strain did not show morphological changes indicative of apoptosis but underwent DNA fragmentation in the higher-molecular-weight range (294 kbp). The same pattern of fragmentation was also noted for cells stimulated with the control strain. These results show that bacteria induced apoptosis in kidney cells and indicate that more than one bacterial virulence factor was involved but that when Stx was present, the damage became more pronounced.
The effect of Stx has been studied in vitro with various types of endothelial cells. A dose-dependent cytotoxic effect was found when human umbilical vein endothelial cells were stimulated with purified Stx (36). These results have since then been confirmed in several studies which found that Stx-1 and Stx-2 had similar effects on endothelial cells (27, 30). The susceptibility of pediatric renal cortical tubular cells to Stx has not been previously studied. We chose to use pediatric renal cortical cells because children are more susceptible to postenteropathic HUS than adults (20). The method employed to isolate HRTEC has previously been used to isolate HRMEC from healthy adult donor kidneys (37, 54), but we obtained tubular cells after passage of cells through a net. The isolation of tubular, instead of endothelial, cells was most probably related to the fact that the pediatric kidney was removed due to disease; the cortex was thinner than normal and contained fewer endothelial cells, which resulted in overgrowth of the tubular cells in culture. Purified Stx was capable of direct cytotoxic action on HRTEC. These results, together with the results of previous studies using cultured endothelial cells (37), suggest that Stx may have separate cytotoxic activities on endothelial and epithelial cells of the renal cortex.
The cytotoxic effect of Stx on endothelial cells has been ascribed to inhibition of protein synthesis (27, 38, 43). In a previous study, Stx was found to induce apoptosis and inhibit protein synthesis in Burkitt lymphoma cells (31). The cells underwent apoptosis not only by stimulation with the holotoxin but also by stimulation with the B subunit which enables binding of the toxin to the globotriaosylceramide (Gb3) receptor but does not inhibit protein synthesis (31). Thus, binding to the cell membrane may transduce an apoptotic signal even before the toxin becomes internalized and inhibits protein synthesis. HRMEC have been found to express more Gb3 receptors than human umbilical vein endothelial cells (37) and are more susceptible to the cytotoxic effects of Stx-2 than to those of Stx-1 (30). A recent study using human glomerular microvascular endothelial cells did not, however, find a difference in the degree of protein synthesis inhibition between these two toxins (50). This may indicate that the cytotoxic effect is initiated prior to the cessation of protein synthesis. The precise mechanism by which Stx induces apoptosis in renal cortical cells needs to be addressed.
The tubular epithelial cell injury in vivo may be a direct effect of circulating bacterial virulence factors and/or host response molecules but may also be secondary to changes in the renal blood flow due to endothelial cell damage. In vitro studies have shown that the cytotoxic effect increased when endothelial cells were stimulated with a combination of Stx and TNF-α (29) or prestimulated with TNF-α (27, 29, 37, 49). Prestimulation of these cells resulted in an increase in Gb3 receptors, rendering the cells more susceptible to the cytotoxic effect of Stx or Stx-1 (27, 37, 49). In this study, prestimulation of cells with TNF-α was shown to enhance the apoptosis-inducing effect of Stx whereas exposure to TNF-α alone did not lead to apoptosis. We have previously shown that TNF-α is present in the urinary tracts of patients with HUS (23). Thus, bacterial factors may act in concert with host cytokines in vivo, increasing the degree of apoptosis and tissue injury.
The mechanisms by which infection with Stx-producing E. coli induces renal cortical injury in vivo have not been elucidated. In this study we have shown that apoptotic cell death occurs in the renal cortex during HUS. Stx and other E. coli O157:H7 factors were found to induce this process in vitro. Thus, induction of apoptosis may contribute to the renal damage in HUS. A better understanding of the factors involved in this form of tissue injury may lead to new perspectives for therapy.
ACKNOWLEDGMENTS
This study was supported by grants from the Swedish Medical Research Council (grant numbers 7934 and 12209); the Swedish Medical Society; the Medical Faculty, University of Lund and the Lund University Hospital; the Samariten Foundation, Sachska Children’s Hospital, Stockholm, Sweden; the Carl J. Michaelsen donation fund; and the Swedish Society for Medical Research.
We thank Per Alm, Department of Pathology, University of Lund, for biopsy material; Bernard Kaplan and David Carpentieri, Children’s Hospital of Philadelphia, for postmortem material; and Lena Sandell, Karin Arnér, Ingela Larsson, Katarzyna Said, Christina Pehrson, and Christina Andersson for excellent technical assistance.
REFERENCES
- 1.Bardales R H, Xie S-S, Hsu S-M. In situ DNA fragmentation assay for detection of apoptosis in paraffin-embedded tissue sections. Am J Clin Pathol. 1997;107:332–336. doi: 10.1093/ajcp/107.3.332. [DOI] [PubMed] [Google Scholar]
- 2.Barrett T J, Potter M E, Wachsmuth I K. Continuous peritoneal infusion of Shiga-like toxin-II (SLT-II) as a model for SLT-II-induced diseases. J Infect Dis. 1989;159:774–777. doi: 10.1093/infdis/159.4.774. [DOI] [PubMed] [Google Scholar]
- 3.Boyce T G, Swerdlow D L, Griffin P M. Escherichia coli O157:H7 and the hemolytic uremic syndrome. N Engl J Med. 1995;333:364–368. doi: 10.1056/NEJM199508103330608. [DOI] [PubMed] [Google Scholar]
- 4.Caprioli A, Luzzi I, Rosmini F, Pasquini P, Cirrincione R, Gianviti A, Matteucci M C, Rizzoni G the HUS Italian Study Group. Hemolytic-uremic syndrome and vero cytotoxin-producing Escherichia coli infection in Italy. J Infect Dis. 1992;166:154–158. doi: 10.1093/infdis/166.1.154. [DOI] [PubMed] [Google Scholar]
- 5.Chae C, Moxley R A, Christopher-Hennings J, Francis D H, Wannemuehler M J. Shiga-like toxin-II-producing Escherichia coli O157:H7 infection in gnotobiotic piglets: protection against brain vascular lesions with SLT-II antiserum. In: Karmali M A, Goglio A G, editors. Recent advances in verocytotoxin producing Escherichia coli infections. Amsterdam, The Netherlands: Elsevier Science; 1994. pp. 241–244. [Google Scholar]
- 6.Chang M P, Bramhall J, Graves S, Bonavida B, Wisnieski B J. Internucleosomal DNA cleavage precedes Diphtheria toxin-induced cytolysis. J Biol Chem. 1989;264:15261–15267. [PubMed] [Google Scholar]
- 7.Chart H, Smith H R, Scotland S M, Rowe B, Milford D V, Taylor C M. Serological identification of Escherichia coli O157:H7 infection in haemolytic uremic syndrome. Lancet. 1991;337:138–140. doi: 10.1016/0140-6736(91)90801-u. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y, Smith M R, Thirumalai K, Zychlinsky A. A bacterial invasin induces macrophage apoptosis by binding directly to ICE. EMBO J. 1996;15:3853–3860. [PMC free article] [PubMed] [Google Scholar]
- 9.Eastman A. Assays for DNA fragmentation, endonucleases, and intracellular pH and Ca2+ associated with apoptosis. In: Schwarz L M, Osborne B A, editors. Cell death. San Diego, Calif: Academic Press; 1995. pp. 41–55. [DOI] [PubMed] [Google Scholar]
- 10.Fitzpatrick M M, Walters M D S, Trompeter R S, Dillon M J, Barratt T M. Atypical (non-diarrhea-associated) hemolytic-uremic syndrome in childhood. J Pediatr. 1993;122:532–537. doi: 10.1016/s0022-3476(05)83531-0. [DOI] [PubMed] [Google Scholar]
- 11.Francis D H, Collins J E, Duimstra J R. Infection of gnotobiotic pigs with an Escherichia coli O157:H7 strain associated with an outbreak of hemorrhagic colitis. Infect Immun. 1986;51:953–956. doi: 10.1128/iai.51.3.953-956.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gavrieli Y, Sherman Y, Ben-Sasson S A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gentry M K, Dalrymple J M. Quantitive microtiter cytotoxicity assay for Shigella toxin. J Clin Microbiol. 1980;12:361–366. doi: 10.1128/jcm.12.3.361-366.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Griffin P M. Escherichia coli O157:H7 and other enterohemorrhagic Escherichia coli. In: Blaser M J, Smith P D, Ravdin J I, Greenberg H B, Cuerrant R L, editors. Infections of the gastrointestinal tract. New York, N.Y: Raven Press Ltd.; 1995. pp. 739–761. [Google Scholar]
- 15.Griffin P M, Ostroff S M, Tauxe R V, Greene K D, Wells J G, Lewis J H, Blake P A. Illnesses associated with Escherichia coli O157:H7 infections. Ann Intern Med. 1988;109:705–712. doi: 10.7326/0003-4819-109-9-705. [DOI] [PubMed] [Google Scholar]
- 16.Habib R. Pathology of the hemolytic uremic syndrome. In: Kaplan B S, Trompeter R S, Moake J L, editors. Hemolytic uremic syndrome and thrombotic thrombocytopenic purpura. New York, N.Y: Marcel Dekker Inc.; 1992. pp. 315–353. [Google Scholar]
- 17.Hagberg L, Engberg I, Freter R, Lam J, Olling S, Svanborg-Edén C. Ascending unobstructed urinary tract infection in mice caused by pyelonephritogenic Escherichia coli of human origin. Infect Immun. 1983;40:273–283. doi: 10.1128/iai.40.1.273-283.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harel Y, Silva M, Giroir B, Weinberg A, Cleary T B, Beutler B. A reporter transgene indicates renal-specific induction of tumor necrosis factor (TNF) by Shiga-like toxin. J Clin Invest. 1993;92:2110–2116. doi: 10.1172/JCI116811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inward C D, Williams J, Chant I, Crocker J, Milford D V, Rose P E, Taylor C M. Verocytotoxin-1 induces apoptosis in vero cells. J Infect. 1995;30:213–218. doi: 10.1016/s0163-4453(95)90693-2. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan B S. Hemolytic-uremic syndrome and thrombotic thrombocytopenic purpura. In: Jacobson H R, Striker G E, Klahr S, editors. The principles and practice of nephrology. B. C. Philadelphia, Pa: Decker; 1991. pp. 326–329. [Google Scholar]
- 21.Karmali M A, Petric M, Lim C, Cheung R, Arbus G S. Sensitive method for detecting low numbers of verotoxin-producing Escherichia coli in mixed cultures by use of colony sweeps and polymyxin extraction of verotoxin. J Clin Microbiol. 1985;22:614–619. doi: 10.1128/jcm.22.4.614-619.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karmali M A, Petric M, Lim C, Fleming P C, Arbus G S, Lior H. The association between idiopathic hemolytic uremic syndrome and infection by verotoxin-producing Escherichia coli. J Infect Dis. 1985;151:775–782. doi: 10.1093/infdis/151.5.775. [DOI] [PubMed] [Google Scholar]
- 23.Karpman D, Andreasson A, Thysell H, Kaplan B S, Svanborg C. Cytokines in childhood hemolytic uremic syndrome and thrombotic thrombocytopenic purpura. Pediatr Nephrol. 1995;9:694–699. doi: 10.1007/BF00868714. [DOI] [PubMed] [Google Scholar]
- 24.Karpman D, Connell H, Svensson M, Scheutz F, Alm P, Svanborg C. The role of lipopolysaccharide and Shiga-like toxin in a mouse model of Escherichia coli O157:H7 infection. J Infect Dis. 1997;175:611–620. doi: 10.1093/infdis/175.3.611. [DOI] [PubMed] [Google Scholar]
- 25.Keenan K P, Sharpnack D D, Collins H, Formal S B, O’Brien A D. Morphologic evaluation of the effects of Shiga toxin and E. coli Shiga-like toxin on the rabbit intestine. Am J Pathol. 1986;125:69–80. [PMC free article] [PubMed] [Google Scholar]
- 26.Kerr J F R, Wyllie A H, Currie A R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keusch G T, Acheson D W K, Aaldering L, Erban J, Jacewicz M S. Comparison of the effects of Shiga-like toxin I on cytokine- and butyrate-treated human umbilical and saphenous vein endothelial cells. J Infect Dis. 1996;173:1164–1170. doi: 10.1093/infdis/173.5.1164. [DOI] [PubMed] [Google Scholar]
- 28.Levin M, Barratt T M. Haemolytic uraemic syndrome. Arch Dis Child. 1984;59:397–400. doi: 10.1136/adc.59.5.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Louise C B, Obrig T G. Shiga toxin-associated hemolytic-uremic syndrome: combined cytotoxic effects of Shiga toxin, interleukin-1β, and tumor necrosis factor alpha on human vascular endothelial cells in vitro. Infect Immun. 1991;59:4173–4179. doi: 10.1128/iai.59.11.4173-4179.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Louise C B, Obrig T G. Specific interaction of Escherichia coli O157:H7-derived Shiga-like toxin II with human renal endothelial cells. J Infect Dis. 1995;172:1397–1401. doi: 10.1093/infdis/172.5.1397. [DOI] [PubMed] [Google Scholar]
- 31.Mangeney M, Lingwood C A, Taga S, Caillou B, Tursz T, Wiels J. Apoptosis induced in Burkitt’s lymphoma cells via Gb3/CD77, a glycolipid antigen. Cancer Res. 1993;53:5314–5319. [PubMed] [Google Scholar]
- 32.McConkey D J, Nicotera P, Orrenius S. Signaling and chromatin fragmentation in thymocyte apoptosis. Immunol Rev. 1994;142:343–363. doi: 10.1111/j.1600-065x.1994.tb00896.x. [DOI] [PubMed] [Google Scholar]
- 33.McKee M L, O’Brien A D. Investigation of enterohemorrhagic Escherichia coli O157:H7 adherence characteristics and invasion potential reveals a new attachment pattern shared by intestinal E. coli. Infect Immun. 1995;63:2070–2074. doi: 10.1128/iai.63.5.2070-2074.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mitra D, Jaffe E A, Weksler B, Hajjar K A, Soderland C, Laurence J. Thrombotic thrombocytopenic purpura and sporadic hemolytic-uremic syndrome plasmas induce apoptosis in restricted lineages of human microvascular endothelial cells. Blood. 1997;89:1224–1234. [PubMed] [Google Scholar]
- 35.Moll R, Franke W W, Schiller D L. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982;31:11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- 36.Obrig T G, Del Vecchio P J, Brown J E, Moran T P, Rowland B M, Judge T K, Rothman S W. Direct cytotoxic action of Shiga toxin on human vascular endothelial cells. Infect Immun. 1988;56:2373–2378. doi: 10.1128/iai.56.9.2373-2378.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Obrig T G, Louise C B, Lingwood C A, Boyd B, Barley-Maloney L, Daniel T O. Endothelial heterogeneity in Shiga toxin receptors and responses. J Biol Chem. 1993;268:15484–15488. [PubMed] [Google Scholar]
- 38.Obrig T G, Moran T P, Brown J E. The mode of action of Shiga toxin on peptide elongation of eukaryotic protein synthesis. Biochem J. 1987;244:287–294. doi: 10.1042/bj2440287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pai C H, Kelly J K, Meyers G L. Experimental infection of infant rabbits with verotoxin-producing Escherichia coli. Infect Immun. 1986;51:16–23. doi: 10.1128/iai.51.1.16-23.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perera L P, Marques L R M, O’Brien A D. Isolation and characterization of monoclonal antibodies to Shiga-like toxin II of enterohemorrhagic Escherichia coli and use of the monoclonal antibodies in a colony enzyme-linked immunosorbent assay. J Clin Microbiol. 1988;26:2127–2131. doi: 10.1128/jcm.26.10.2127-2131.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perez M R, Arnér K, Håkansson A. DNA fragmentation characteristic of apoptosis and cell loss induced by kainic acid in rabbit retinas. Neurochem Int. 1997;31:251–260. doi: 10.1016/s0197-0186(96)00156-8. [DOI] [PubMed] [Google Scholar]
- 42.Petric M, Karmali M A, Arbus G S, Roscoe M, Louie S, Cheung R. Effects of cycloheximide and puromycin on cytotoxic activity of Escherichia coli verocytotoxin (Shiga-like toxin) J Clin Microbiol. 1987;25:1265–1268. doi: 10.1128/jcm.25.7.1265-1268.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reisbig R, Olsnes S, Eiklid K. The cytotoxic activity of Shigella toxin. J Biol Chem. 1981;256:8739–8744. [PubMed] [Google Scholar]
- 44.Sandvig K, Prydz K, Ryd M, van Deurs B. Endocytosis and intracellular transport of the glycolipid-binding ligand Shiga toxin in polarized MDCK cells. J Cell Biol. 1991;113:553–562. doi: 10.1083/jcb.113.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sandvig K, van Deurs B. Toxin-induced cell lysis: protection by 3-methyladenine and cycloheximide. Exp Cell Res. 1992;200:253–262. doi: 10.1016/0014-4827(92)90171-4. [DOI] [PubMed] [Google Scholar]
- 46.Sjöblad S, Larsson B, Kahlmeter G, Wadström T. Pojke fick SLT-associerat hemolytiskt uremiskt syndrom. Läkartidningen. 1989;86:2595–2596. [PubMed] [Google Scholar]
- 47.Tesh V L, Burris J A, Owens J W, Gordon V M, Wadolkowski E A, O’Brien A D, Samuel J E. Comparison of the relative toxicities of Shiga-like toxins type I and type II for mice. Infect Immun. 1993;61:3392–3402. doi: 10.1128/iai.61.8.3392-3402.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tzipori S, Karch H, Wachsmuth K I, Robins-Browne R M, O’Brien A D, Lior H, Cohen M L, Smithers J, Levine M M. Role of a 60-megadalton plasmid and Shiga-like toxins in the pathogenesis of infection caused by enterohemorrhagic Escherichia coli O157:H7 in gnotobiotic piglets. Infect Immun. 1987;55:3117–3125. doi: 10.1128/iai.55.12.3117-3125.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van de Kar N C A J, Monnens L A H, Karmali M A, van Hinsbergh V W M. Tumor necrosis factor and interleukin-1 induce expression of the verocytotoxin receptor globotriaosylceramide on human endothelial cells: implications for the pathogenesis of the hemolytic uremic syndrome. Blood. 1992;80:2755–2764. [PubMed] [Google Scholar]
- 50.van Setten P A, van Hinsbergh V W, van der Velden T J, van de Kar N C, Vermeer M, Mahan J D, Assmann K J, van den Heuvel L P, Monnens L A. Effects of TNF alpha on verocytotoxin cytotoxicity in purified human glomerular microvascular endothelial cells. Kidney Int. 1997;51:1245–1256. doi: 10.1038/ki.1997.170. [DOI] [PubMed] [Google Scholar]
- 51.Wadolkowski E A, Burris J A, O’Brien A D. Mouse model for colonization and disease caused by enterohemorrhagic Escherichia coli O157:H7. Infect Immun. 1990;58:2438–2445. doi: 10.1128/iai.58.8.2438-2445.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wadolkowski E A, Sung L M, Burris J A, Samuel J E, O’Brien A D. Acute renal tubular necrosis and death of mice orally infected with Escherichia coli strains that produce Shiga-like toxin type II. Infect Immun. 1990;58:3959–3965. doi: 10.1128/iai.58.12.3959-3965.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weibel E W, Palade G E. New cytoplasmic components in arterial endothelia. J Cell Biol. 1964;23:101–112. doi: 10.1083/jcb.23.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wojta J, Hoover R L, Daniel T O. Vascular origin determines plasminogen activator expression in human endothelial cells. J Biol Chem. 1989;264:2846–2852. [PubMed] [Google Scholar]
- 55.Zhivotovsky B, Wade D, Gahm A, Orrenius S, Nicotera P. Formation of 50 kbp chromatin fragments in isolated liver nuclei is mediated by protease and endonuclease activation. FEBS Lett. 1994;351:150–154. doi: 10.1016/0014-5793(94)00827-2. [DOI] [PubMed] [Google Scholar]
- 56.Zychlinsky A, Prevost M C, Sansonetti P J. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358:167–169. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]