Abstract
Lignans are a group of plant phenolic compounds with various technofunctional and health-promoting properties. They can be found in oilseeds (291.7–2513 mg/100 g), nuts, vegetables, fruits, and alcoholic and nonalcoholic drinks. The most common structural representative feature of lignans’ backbone is a dimeric phenylpropanoid, which consists of two C6–C3 units joined by a central carbon. Compared to other phenolics, such as flavonoids, the literature on lignan stability and bioaccessibility is limited. This Mini-Review aims to present an overview of recent literature, draw connecting lines to the known regarding polyphenols, and suggest the main knowledge gaps. Processing methods and processing conditions influence the stability of lignans with several thermal treatments explored. Roasting, as a major studied processing step, displayed varying effects as a function of the lignan structure and matrix. The content of specific and even total lignans was shown to increase in some cases even after intense thermal treatment. Lignans were also reported to present a stabilizing effect against oxidation to oils when added externally. Different fermentation methods presented inconclusive outcomes on the content of lignans, likely stemming from the various matrices and microorganisms studied in a relatively limited pool of studies. The bioaccessibility of lignans in in vitro studies was usually low (from less than 1% in fermented flaxseed to 30% for microwaved artichokes). Yet, a clear conclusion regarding the digestive fate of lignans as a function of processing and structure cannot be currently suggested, and significant additional effort in this direction is needed.
1. Introduction
Lignans are a large and diverse group of phenolic compounds found in plants and food products with various biological properties like antioxidant and antitumor activities.1 Like polyphenols, lignans are secondary metabolites derived from the shikimic acid pathway involved in the protection of plants from pathogens and ultraviolet radiation. Lignan consumption in the human diet is common and typical, as they are found in everyday foods and beverages, such as oilseed, cereals, nuts, oils, vegetables, fruits, and alcoholic and nonalcoholic drinks (Figure 1).2,3 The produce richest in lignans is seeds, particularly flaxseed and sesame seeds. Other food products contain lignan in lower concentrations.2 For example, the content of total lignans in sesame seed is 834.57 mg/100 g of food, whereas the total lignan of avocado is 0.73 mg/100 g of food. The typical content of lignans in foods can be found in Table 1.2
Figure 1.

Lignan sources in the human diet.
Table 1. Content of Lignans in Different Food Sources.
| source | food | total lignans (mg/100 g food) |
|---|---|---|
| seeds | sesame | 291.7–25131 |
| flaxseed | 301.1294 | |
| cereals | rye grains | 0.26995 |
| buckwheat | 0.8676 | |
| vegetables | broccoli | 1.3254 |
| cucumber | 0.0674 | |
| fruits | grapefruit | 0.1524 |
| pear | 0.1934 | |
| beverages | red wine | 0.0691–0.09134 |
| coffee | 0.0187–0.03134 | |
| oils | olive oil, extra-virgin | 0.02484 |
| sesame seed oil | 275.4–467.67 |
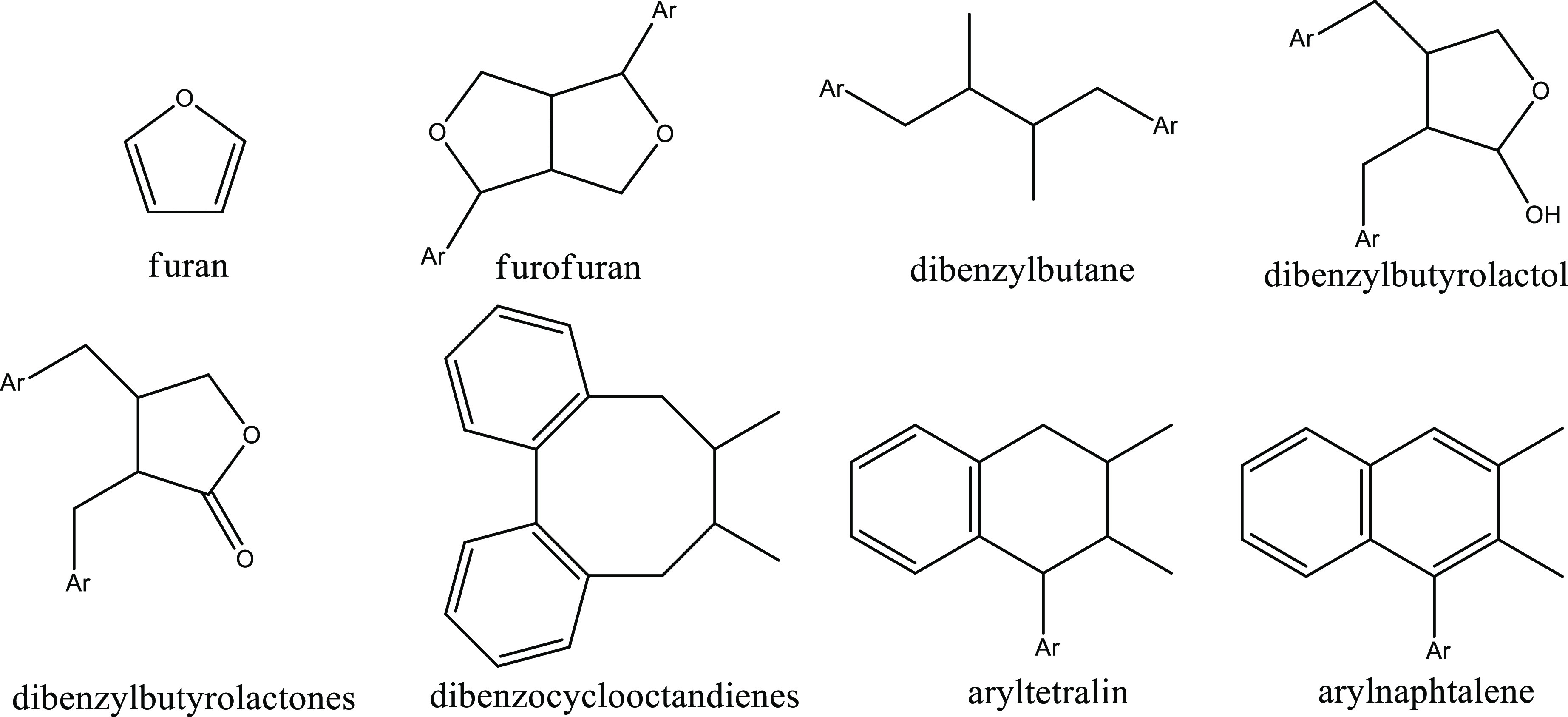
The lignans are a diverse family of compounds, generally composed of two groups of phenylpropanoid. R.D. Haworth established the term “lignan” to describe the structure of a dimeric phenylpropanoid with two C6–C3 units connected by its central carbon (C8).8 A literature survey reveals a slight variation in lignan definition.2,9 Lignans are a subgroup of phenolic compounds with several or one phenolic ring2 and can be classified into several groups: lignan, neolignan, and norlignans. Lignans are phenylpropane dimers linked by a β–β′ (8–8′) bond, whereas neolignans are phenylpropanoid dimers that are not connected by a β–β′ bond (Figure 2).3 The norlignans lack one or more carbon atoms, with some, like norlignans, exhibiting various biological activities such as antifungal, antibacterial, and antioxidant capacity (Figure 2).3,9 The structure of the classical lignans can be classified into eight subgroups, which include furan, furofuran, dibenzylbutane, dibenzylbutyrolactol, dibenzylbutyrolactones, dibenzocyclooctadienes, aryltetralin, and arylnaphtalene (Figure 3).2 The subgroups differ by the presence of oxygen in the backbone and ring formation. Lignans can be found either in free phenolic form or as glucosides. For example, two of the major lignans in the sesame seeds are di- and tri-glucosides of pinoresinol.10 Among other effects, the lignan’s polarity affects the extraction’s efficiency and simplicity. For example, the solubility of glycosylated lignans in organic solvents is restricted, and for the extraction of lignans from sesame oil, 80% ethanol is used, which is suitable for both aglycons and glycosylated lignans. In general, the most commonly used solvent for analytical extraction today is 80% methanol and 20% water.10 In addition to different total contents, foods contain different types, concentrations, and ratios of lignans. Secoisolariciresinol is the major dietary lignan and may be found, for example, in flaxseed (257.6 mg/100 g) and carrots (3.16 mg/100 g).2 Another family of lignans is the mammalian lignans: enterodiol and enterolactone. Plant lignans are metabolized by microorganisms in the human digestive tract into mammalian lignans.2 Nowadays, there is a growing interest in those compounds, as they are suggested to promote health. An example is the lignan secoisolariciresinol, a precursor to enterodiol and enterolactone, proposed to be effective in preventing breast and prostatic cancer in animal models.1
Figure 2.
Chemical structures of phenylpropanoid (A), lignan (B), norlignan (C), and neolignan (D).
Figure 3.
Chemical structures of eight subgroups of lignans (Ar = aryl group).
Various studies reported that lignans lowered blood glucose and cholesterol levels and presented some prevention against cardiovascular diseases in rats when administered orally.2,10 Moreover, lignans were suggested to offer a broad in vitro activity in preventing different types of cancer,2,10 and they may be linked to a lower risk of breast cancer in postmenopausal women.11 Others noted that the consumption of lignans in the diet correlates with a decrease in chronic and degenerative diseases. In addition, in both men and women, an increased long-term lignan diet was linked to a noticeably decreased incidence of total coronary heart disease.12 Generally, phenolic compounds are known to be affected by diverse processing methods, such as different heat treatments, which can lead to their degradation and modify their bioaccessibility.13 Yet for lignans, most studies focused on their potential therapeutic effect and not their stability and bioaccessibility in various food products, which can significantly impact the potential of their health-promoting activity. This Mini-Review focuses on the stability and bioaccessibility of the lignans in food products, especially in correlation with their structure and the impact of processing, aiming at marking important gaps in the research.
2. Stability of Lignans
Thermal treatments, fermentation, bleaching, and drying are among the diverse processing methods affecting lignan-rich matrices5 and, therefore, lignan stability.10 Understanding how these compounds are affected by different environments, especially as a function of their structure, is essential to maintaining their nutritional value during food processing and preparation. In recent years, there has been a growing interest in the utilization of lignans for nutraceuticals, pharmaceuticals, and/or functional foods, increasing the importance of stability understanding for the development of effective, reliable, and nutritional products. Several studies have been conducted to determine the impact of processing and storage on the lignan content of foods; extensive stability research of lignan compounds will ease rational utilization and reveal the potential of these compounds for human health and well-being.1,2
2.1. Thermal Treatment
Thermal treatments are crucial and common processing methods for both food preservation and the modification and development of consumer-accepted sensorial attributes. Such treatments are known to affect phenolic compounds and modify their chemical structure, potentially adversely affecting the nutritional, physicochemical, and antioxidant properties of different food products.11 Therefore, the outcome of thermal processing on lignans and lignan-rich matrixes during the production of foods is of great importance and is summarized in Table 2.
Table 2. Impact of Thermal Processing on Lignan’s Content in Various Foods.
| food source | processing method | content of lignansa | reference |
|---|---|---|---|
| sesame oil | roasting (213/230/247 °C for 21 min) | sesamol ↑ | (14) |
| sesamin ↓ | |||
| sesamolin ↓ | |||
| sesame seed | roasting (240 °C for 20 min) | sesamin ↓ | (15) |
| pinoresinol ↓ | |||
| sesamolin ↓ | |||
| pinoresinol diglucoside ↑ | |||
| defatted-sesame meal | frying (160/200/240 °C for 30 min) | total lignans ↑ (for 240 °C↓) | (16) |
| roasting (160/200/240 °C for 30 min) | total lignans ↑ | ||
| microwaving (160/200/240 °C for 30 min) | total lignans ↑ | ||
| sesame seed | baking (100/200/250 °C for 20–40 min) | lariciresinol ↑ (for 100 °C), ↓ (for 200 and 250 °C) | (5) |
| pinoresinol ↑ (for 100 °C), ↓ (for 200 and 250 °C) | |||
| rye flour | baking (100/200/250 °C for 20–40 min) | syringaresinol ↑ (for 100 °C), ↓ (for 200 and 250 °C) | (5) |
| biscuit | baking (170 °C for 12 min) | total lignans ↑/↓ (depends on the formulation) | (20) |
| virgin olive oil | frying (180 °C for 10–60 min) | total lignans - | (21) |
| boiling (100 °C for 1–4 h) |
↑, increase in content; ↓, decrease in content; -, no change in content.
Roasting is often used for seeds in general and specifically for sesame seeds to improve sensorial properties, for example, in the production of sesame oil or sesame paste (tahini).10 As seeds are major sources of lignans, several studies focused on the effect of roasting on the content of lignans, particularly in sesame seeds. One of the studies found that the amount of sesamol in sesame oil increased after the sesame seeds were roasted. The authors suggested that the higher oxidative stability of sesame oil is due to the continuous production of sesamol by thermal oxidation, formed by sesamolin degradation into sesamol at high temperatures during roasting, leading to the formation of more sesamol in the oil.14 Others examined the effect of roasting on six varieties of sesame seeds and discovered that the contents of the total lignans increased significantly for all six types. However, after examination of specific lignans, it was revealed that sesamin, pinoresinol, and sesamolin content decreased, and pinoresinol diglucoside and sesamol content increased,15 showing that the impact of processing is structure dependent. Such results and others described later suggest that lignans have the potential, as added ingredients, to protect various products that need to be exposed to high temperatures from oxidation.
The effect of other thermal treatments on lignans in addition to roasting was also explored. One of the studies examined the effect of frying, roasting, and microwaving on a defatted sesame meal (a byproduct of sesame oil) at different time/temperature combinations. The study concluded that the content of lignans depends on the treatment type, temperature, and time, with some lignans presenting an increased content at different treatment durations. For example, total lignans increased during roasting at all tested temperatures, reaching 42.38 ± 1.29, 148.36 ± 5.54, and 154.47 ± 3.82 mg/100 g dry weight (DW) after 10 min at 160, 200, and 240 °C, respectively. In addition, in all three times evaluated (10, 20, 30 min), the content also increased for all thermal treatments. On the other hand, during frying (a pan and an induction cooker), total lignan content increased at all three temperatures, but at 240 °C after 30 min, the lignan content decreased to 81.83 ± 1.17 mg/100 g of DW from 218.82 ± 7.12 mg/100 g of DW (quantified after 20 min of frying). The leaching of those compounds may explain the decreasing content of lignan during frying. In a different study, the amount of total phenolic compounds in spinach leaves during frying also decreased, and it was suggested that the phenolic compounds leached to the frying medium. In comparison between the three methods, the highest content of lignans was received by microwave treatment at 800 W after 30 min (359.42 ± 9.02 mg/100 g DW).16,17 The results emphasize that in the planning of commercial lignan-rich products the processing flow should take into account the leaching of those bioactive compounds.
Natural antioxidants present can affect not only the product’s stability during needed thermal processing but also during its shelf life. Research that focused on the antioxidant and oxidative properties of roasted sesame oil during production and storage revealed that sesamin and sesamolin levels were significantly higher in the final product (the oil) that included three filtration steps than in the pressed oil. However, the sesamol content was reduced. The lignan content of the oil decreased during storage; the authors suggested that it likely functioned as a sacrificial agent (antioxidant) in the oil.18 Later, the effect of additive sesame lignans was also examined not only in sesame oil. A study on the impact of sesame lignans on the thermal and storage stabilities of three edible vegetable oils (refined soybean, sunflower, and rice bran) was conducted. The researchers, confirming the protective effect of lignans, discovered that adding sesame lignans to the oils increased the thermal stability. In this regard, adding 1.2% lignans to refined soybean oil increased total free radical scavenging activity (RSA) by 47% after 120 min of heating at frying temperature compared to refined soybean oil without added lignans. Furthermore, after heating to a frying temperature for 120 min, the RSA of the three oils (refined soybean, sunflower, and rice-bran oil) with added lignans increased by 47%, 40%, and 47%, respectively. In addition, the study examined the stability of the added lignans. The findings were that, in all oils, the total lignans content decreased after 120 min of frying, likely due to sesamolin degradation. At the same time, over 60 min, sesamol is formed from nondetectable levels in all of the oils containing lignans. The formation of sesamol can be attributed to conversion from sesamolin, as discussed before. Compared with other lignans, sesamin was more stable and more heat-resistant. From the data, it can be suggested that sesamolin significantly contributes to sesame oil by increasing its stability. The authors concluded that combining sesame oil with other oils has the potential to improve antioxidant capacity.19 It was reported that lignan concentrations moderately increased in heated flaxseed and rye but decreased when rye was steamed before heating. The study also concluded that lignans did not degrade extensively during bread baking and that the matrix influences their thermal stability.5
When discussing lignan stability, the food matrix must be considered as well. A study that monitored the lignans in flaxseed in biscuits before and after baking found that the concentration of lignans in flaxseed biscuits was 30 times higher than in nonenriched whole-wheat biscuits and 170 times higher than in white flour biscuits and that the concentration was unaffected by the baking itself.20 In another study, the content of lignans in virgin olive oil was examined, reporting that the content of lignans was stable during frying (180 °C, 1 h), boiling (100 °C, 4 h), and storage in the dark for 15 months at 20 °C to simulate domestic storage. It was also reported that the stability of lignans during shelf life depended on the type of virgin olive oil.21
It should be emphasized that due to the reported health-promoting effects, the impact during processing (in terms of prevention of oxidation) presented in this section is only a partial attribute of those compounds as their stability suggests they extend the nutritional benefits during shelf life, maintaining the nutritional content and allowing customers to benefit from the suggested health benefits.
2.2. Fermentation
Fermentation is an ancient method used to preserve and extend the shelf life of food products by microorganisms, resulting in diverse compounds, such as organic acids and alcohol, being produced. A common outcome of fermentation is an inhibition of the growth of spoilage organisms. Moreover, fermentation contributes to the food’s unique flavors, textures, and bioactive compounds22 and may affect the content of the phenolic compounds.
Several studies have explored the effect of fermentation on lignans in different foods. A study examined the lignan (secoisolariciresinol diglucoside) content during bread production when using defatted flaxseed flour at three process stages: dough before leavening by yeast fermentation, dough after leavening, and after bread baking. The content of secoisolariciresinol diglucoside decreased after fermentation. The authors explained this observation by the presence of microorganisms and their enzymes metabolizing the lignan to enterodiol and enterolactone.23 The contents of different lignans during the fermentation of triticale dough were also reported. The study discovered that the lignan content was unchanged when acid hydrolysis was used to release the lignans from the sample, but in the case of enzyme hydrolysis, there was an increase of 14% in the total lignans. However, looking at each lignan, one can notice that in acid hydrolysis, where the total lignan did not change, the specific lignan secoisolariciresinol increased after fermentation. On the other hand, the content of lariciresinol did not change significantly, and the content was lower.24
On the other hand, other studies did not observe changes in the content of the lignans after some fermentation steps. A study investigated the stability of added secoisolariciresinol diglucoside during the production and storage of different dairy products, focusing on the effects of various dairy product manufacturing processes such as pasteurization and fermentation. The authors observed that heat treatment and fermentation, which are required for yogurt production, did not affect the content of secoisolariciresinol diglucoside. They also concluded that the fermentation of several hours did not convert secoisolariciresinol diglucoside to the aglycon form. In addition, when looking at the yogurt’s starter, which contained lactic acid bacteria and bifidobacteria, it did not cause degradation of secoisolariciresinol diglucoside during fermentation, suggesting that the lignan is relatively inert to the yogurt’s starter. Moreover, lignan did not appear to interfere with yogurt production from a technological perspective. It was also reported that the secoisolariciresinol diglucoside was stable under acidic conditions of yogurt during storage of 10 and 21 days. As for cheese manufacturing, specifically Edam cheese, it was found that, after different manufacturing steps: pressing, brining, and ripening at 9 °C for 6 weeks, the content of secoisolariciresinol diglucoside did not change as well.25 Furthermore, it is suggested that fermentation may even increase the content of lignans. A study that assessed the content of lignans in cereal bran following fermentation with lactic acid bacteria Pediococcus acidilactici reported increases of up to 34 and 63% in matairesinol and secoisolariciresinol content, respectively. However, different studies found that the increase in the content of matairesinol was lower in barley bran with the same lactic acid bacteria. The explanation for these changes is still lacking, and further studies are needed to understand the mechanisms of the microbial-induced degradation and development of lignans. Furthermore, the increase in the content of lignans may stem from the hydrolysis of the food matrix or deglycosylation of lignan glucosides.22
As expected, different bacteria can have different effects on lignans. For example, bacteria from human gut microbiota are known for their deglycosylation activity. Researchers isolated these bacteria to use for food fermentation. They found a greater amount of secoisolariciresinol in an aglycon form in flaxseed and soy extracts after fermentation with isolated Bifidobacterium strains from adult and child feces. Another fermentation of defatted flaxseed with bacteria from feces, Bacteroides uniformis, has resulted in an 80% increase in the efficiency of converting secoisolariciresinol diglucoside oligomers to free secoisolariciresinol.22 Additional research is needed to explore the effect of the fermentation of various bacteria and their metabolic pathways on products that contain lignans. Such knowledge can assist in the development of specific fermentation processes, focusing on lignan-rich products.
A different study investigated omija beverages made from omija fruit fermented for 12 months with sugar. The study’s results showed that the total content of 7 lignans increased after 12 months. After 9 months, the content increased 2.6 times. It should be noted that six of the lignans increased over time besides angeloylgomisin. The concentration of angeloylgomisin increased up to 7 months and then decreased considerably by 12 months. In addition, the researchers also examined the impact of the type of added sugar used for fermentation. They tested three types of sugars: white, brown, and oligosaccharide/white sugar (1:1). In addition, the sugar samples were also compared to an omija drink prepared by immersing the fruit in alcohol. The highest content of lignans among the samples with the sugar was in the oligosaccharide/white sugar sample. A possible suggested explanation is that the oligosaccharide/white sugar may have acted as the most efficient natural deep eutectic solvent (NADS) to dissolve the lignan compounds in the omija berry. NADS, such as sugars, in the solid form, can improve the solubility of nonwater-soluble bioactive compounds such as lignans. The authors concluded that the type of sugar also plays a part in the fermentation outcome of lignans.26
Pickling is a different form of natural fermentation of fruits and vegetables in acidic or salt solutions. A study focused on the effect of pickling on the lignan content in shallots, celery, green Chinese mustard, and long beans. When compared to the other items, lariciresinol in celery exhibited the most significant decrease, almost 86%, following pickling. Furthermore, the pickled Chinese mustard had roughly 80% less lariciresinol than fresh Chinese mustard. The decline in the content can be explained by endogenous polyphenol oxidase or leaching out to the brine after prolonged storage in acidic and salt solution. The precise mechanism is uncertain, and further research is needed to explain better the mechanism of lignan reduction during pickling. Nonetheless, the discovery that lignan concentration may decrease during pickling is significant for producing lignan-rich pickled products.27 Currently, there are only a few publications on the effect of fermentation on lignans, both as isolated compounds and as a part of a complete food. They are summarized in Table 3. Therefore, much more data are required to understand the impact of fermentation as a function of matrix, microorganism type, and lignan structure.
Table 3. Impact of Fermentation Processing on Lignan’s Content in Various Foods.
| food source | fermentation conditions | content of lignansa | reference |
|---|---|---|---|
| bread dough | 33 °C for 45 min | secoisolariciresinol diglucoside ↓ | (23) |
| yogurt with added lignan | 43 °C for 5 h (pH > 4.6) | secoisolariciresinol diglucoside - | (25) |
| flour-based bun with added lignan | proofing for 0.5 h | secoisolariciresinol diglucoside - | (28) |
| cereal byproducts | 32 C for 72 h | secoisolariciresinol ↑ | (22) |
| matairesinol ↓ | |||
| triticale dough | 30 °C for a total time of 25 h | secoisolariciresinol ↑ | (24) |
| lariciresinol ↓ | |||
| syringaresinol ↑ | |||
| pinoresinol ↑↓ (depends on the type of the hydrolysis) | |||
| matairesinol ↑↓ (depends on the type of the hydrolysis) | |||
| omija beverage | room temperature for 12 months | total lignans ↑ | (26) |
↑, increase in content; ↓, decrease in content; -, no change in content.
2.3. Other Processing Methods
Other reported effects of processing technologies on the levels of lignans are summarized in Table 4. The production of edible oil involves multiple steps that can alter the composition of lignans in the oil. Solvent extraction is common in oil production, which then undergoes refinement to create refined oil. Such oil is significantly cheaper than cold-pressed oil, which does not use solvents. These two oil production processes were shown to differ not only in their yield and cost but also in their impact on lignan content. A previous study found that pressed sesame oil has higher levels of sesamin and sesamolin than refined sesame oil. Moreover, changes occur in lignan content during the refining process itself. Notably, the amount of lignans changes considerably during bleaching. This was suggested to be the outcome of the presence of acid that functions as a catalyst in high temperatures. In this process, sesamin is transformed into asarinin, its epimer, while sesamolin goes through an intermolecular transformation into sesaminol.29
Table 4. Impact of Other Processing Methods on Lignan’s Content in Various Foods.
| food source | processing | content of lignansa | reference |
|---|---|---|---|
| sesame oil | refining | sesamin ↓ | (29) |
| sesamolin ↓ | |||
| sesame oil | bleaching | sesamin ↓ | (29) |
| asarinin ↑ | |||
| sesamolin ↓ | |||
| sesaminol ↑ | |||
| sesame bran | spray-fried | sesamol ↑ | (30) |
| sesamolin ↓ | |||
| flaxseed (brown and golden) | germination | secoisolariciresinol diglucoside ↓ | (31) |
| flaxseed oil (brown and golden) | germination | secoisolariciresinol diglucoside ↑ | (31) |
↑, increase in content; ↓, decrease in content.
Drying is a common technique used in the industry, allowing us to prevent microbial spoilage and extend shelf life. There are several types of drying methods, often involving the application of high temperatures. A recent study focused on the production of plant-based powder from sesame bran and the effectiveness of spray-drying and freeze-drying techniques on the final product. The study revealed that sesame bran contains the lignans sesamin, sesamol, and sesamolin. Sesamin is the main lignan in sesame bran, while sesamol is the main lignan in the protein powder. The study also observed that the spray-dried protein powder had the highest sesamol content and the lowest sesamolin content, which was attributed to the formation of sesamol from sesamolin during the thermal process involved in spray-drying. On the other hand, sesamin was better retained in freeze-drying.30
Germination is a natural and low-cost process widely used to increase the nutritional properties of food. Several studies have been conducted to explore the effect of germination on various foods, such as grains, and the impact of germination on their bioactive components.31 A study investigated the effect of germination on bioactive components, such as lignans, during germination of flaxseed. The authors examined two types of flaxseeds, brown and golden flaxseed, during germination for 5 days. The lignan that was monitored in the flaxseed is secoisolariciresinol diglucoside. The trend in the content of secoisolariciresinol diglucoside in the two types of flaxseed (brown and golden) was identical, showing a decrease in their concentration until day 1, an increase until day 2 (reaching a maximal content), and a later decrease after day 2. In addition, the lignan content in brown flaxseeds was higher than that of golden. After 5 days, the lignan content of flaxseeds was reduced by 32% and 22%, respectively, when compared to that of flaxseeds germinated for 2 days. The authors concluded that long-term germination may negatively affect secoisolariciresinol diglucoside content. The increase in lignan after 2 days is thought to be related to lignan synthesis during germination.31 In addition, the study investigated whether lignan can transfer to the flaxseed oil during germination. They discovered that germination increased the secoisolariciresinol diglucoside content of brown and golden flaxseed oil by 2.4 and 2.3 times, respectively, over nongerminated flaxseed oil.31
3. Bioaccessibility of Lignans
In addition to the stability of the health-promoting components, as a factor potentially affecting their activity, in recent years there has been a growing interest also in the digestive fate of bioactive compounds. Such interests stem from the understanding that stability is not a single factor predicting the potential health-promoting effect, owing to a growing understanding that digestion processes are critical elements in biological activity. Bioaccessibility is the amount of compound released from the food matrix into the gastrointestinal system that becomes available for absorption. It is frequently a prerequisite for the bioavailability and absorption of the compound.11Table 5 summarizes the reported studies regarding the bioaccessibility of lignans.
Table 5. Bioaccessibility of Different Lignans.
| food source | lignan | % bioaccessibility (defined after the intestinal step) | in vitro digestion conditions | reference |
|---|---|---|---|---|
| whole flaxseed | secoisolariciresinol | 0.75 | mouth phase: | (32) |
| enterodiol | 1.56 | pH: 7 | ||
| enterolactone | 1.23 | temperature: 37 °C | ||
| flaxseed flour | secoisolariciresinol | 2.06 | time: 20 s | (32) |
| enterodiol | 2.72 | shaking: + | ||
| enterolactone | 1.04 | gastric phase: | ||
| pH: 2 | ||||
| temperature: 37 °C | ||||
| time: 1 h | ||||
| shaking: + | ||||
| intestinal phase: | ||||
| pH: 6–7.5 | ||||
| temperature: 37 °C | ||||
| time: 4 h | ||||
| shaking: + | ||||
| galician extra-virgin olive oil | acetoxypinoresinol | 59a | mouth phase: | (33) |
| pinoresinol | 25a | pH: 7 | ||
| temperature: 37 °C | ||||
| time: 5 min | ||||
| shaking: + | ||||
| gastric phase: | ||||
| pH: 3 | ||||
| temperature: 37 °C | ||||
| time: 2 h | ||||
| shaking: + | ||||
| intestinal phase: | ||||
| pH: 7 | ||||
| temperature: 37 °C | ||||
| time: 2 h | ||||
| shaking: + | ||||
| virgin olive oil | total lignans | 5–24a (different cultivars) | mouth phase: | (34) |
| temperature: 37 °C | ||||
| time: 5 min | ||||
| shaking: + | ||||
| gastric phase: | ||||
| pH: 2–3 | ||||
| temp: 37 °C | ||||
| time: 2 h | ||||
| shaking: + | ||||
| intestinal phase: | ||||
| pH: 6.5–7 | ||||
| temperature: 37 °C | ||||
| time: 2 h | ||||
| shaking: + | ||||
| flaxseed | secoisolariciresinol | 0.87 (fine) and 0.67 (coarse) | mouth phase: | (36) |
| temperature: 37 °C | ||||
| time: 2 min | ||||
| germinated flaxseed | secoisolariciresinol | 0.55 (fine) and 0.53 (coarse) | shaking: + | (36) |
| fermented flaxseed | secoisolariciresinol | 1.03 (fine) and 1.00 (coarse) | gastric phase: | (36) |
| tea brew | pinoresinol and matairesinol | 24.4 | pH: 3 | (36) |
| temperature: 37 °C | ||||
| time: 2 h | ||||
| shaking: + | ||||
| intestinal phase: | ||||
| pH: 7 | ||||
| temperature: 37 °C | ||||
| time: 2 h | ||||
| shaking: + | ||||
| artichokes | total lignans | raw–13 | mouth phase: | (37) |
| boiled–24 | temperature: 37 °C | |||
| sous vide–25 | time: 2 min | |||
| microwaved–30 | shaking: + | |||
| gastric phase: | ||||
| pH: 3 | ||||
| temperature: 37 °C | ||||
| time: 2 h | ||||
| shaking: + | ||||
| intestinal phase: | ||||
| pH: 7 | ||||
| temperature: 37 °C | ||||
| time: 2 h | ||||
| shaking: + |
Measured in the aqueous phase at the end of the simulated upper intestinal section; +, yes; -, no.
Previous research has shown that flaxseed lignans from whole flaxseed and flaxseed flour are bioaccessible during the in vitro digestive process simulation. The main distinction between their digestion processes was the release of lignans at different stages of digestion.32 Others investigated the digestive stability and the bioaccessibility in an in vitro gastrointestinal method of phenolic compounds, including the lignans, in extra-virgin olive oil. Following the oral step, lignans were mainly identified in the oil phase, likely due to their hydrophobic nature. During gastric digestion, the lignans were relatively stable. Specifically, pinoresinol was the most stable and was mainly found in the oil phase. Regarding the intestinal step, the lignans pinoresinol and acetoxypinoresinol were relatively stable and remained in the oil phase, which was suggested by the authors to be not bioaccessible. The authors concluded that acetoxypinoresinol and pinoresinol had the second highest potential bioaccessibility values, after simple phenols: hydroxytyrosol, tyrosol, and hydroxytyrosol acetate of 59%, 25%, 302%, 172%, and 4186%, respectively. It is important to note that the authors calculated the potential bioaccessibility as the concentration of phenolics in the intestinal water phase divided by the concentration of phenolics in the extra-virgin olive oil, while the bioaccessibility was calculated as the dialyzable fraction (during intestinal digestion) of phenolic compounds divided by the content of phenolic compounds of the extra-virgin olive oil. The bioaccessibility of pinoresinol and acetoxypinoresinol was 9% and 14%, respectively.33 A similar study also reported the bioaccessibility of phenolic compounds in different cultivars of virgin olive oils. The findings were similar: the lignans were in the oil phase and were stable during gastric digestion. In addition, the bioaccessibility of the total lignans was low and distributed differently between the different cultivars of oil, where the highest value belonged to “Habichuelero” virgin olive oil with 24% bioaccessibility and the lowest for the “Chetoui” oil with 5% bioaccessibility. The same lignans were identified in all oil samples, but as can be seen for different olive cultivars, the bioaccessibility was different. The difference in phenolic composition, initial concentration, and stability to gastrointestinal conditions can explain the different bioaccessibility between the cultivars.34 Further studies are needed to comprehensively determine the digestive fate of lignans in different food matrixes, considering potential interactions with the food matrix and extraction from the matrix. The differences between the two studies could be due to differences in the chemical structures of the lignans, the food matrix and structure, or the presence of other affecting compounds.31
Processing has a significant impact on the bioaccessibility of bioactive compounds, and currently, there are relatively few studies on the effect of various processing methods on the bioaccessibility of lignans in lignan-rich food, in comparison to a much richer body of studies reporting the impact of processing on polyphenols.13 A recent study investigated lignan bioaccessibility from fresh, fermented, and germinated flaxseed (focusing on secoisolariciresinol) as a function of two different particle sizes: fine and coarse. In addition, the study used tea leaves as a model for studying the stability of lignans under in vitro simulated gastrointestinal conditions and their interactions with digestive fluids. The main finding was that the bioaccessibility of lignans from flaxseed food matrixes was very low, 1% or less. The lignan’s bioaccessibility was influenced by both processing and particle size. The most bioaccessible sample was fermented flaxseed, which had a bioaccessibility of around 1.03%—significantly different in comparison to fine and coarse particles of fresh flaxseed with a bioaccessibility of 0.87% and 0.67%, respectively. For both particle sizes, germinated flaxseed had the lowest bioaccessibility of secoisolariciresinol. If one looks at the differently sized particles, only the fresh flaxseed showed a significant difference. In addition, the bioaccessibility of lignans in the tea brew sample was approximately 88% after the oral phase, 70% after the gastric phase, and even lower after the intestinal phase. The authors suggested that the presence of bile salt in the intestinal phase may have impacted lignans’ bioaccessibility as it reduces sterols’ solubility.35,36 The researchers concluded that fermentation and germination could improve lignan bioaccessibility.36 A study focused on the effects of roasting and in vitro digestion on six different types of sesame seeds revealed that sesamolin was not released during the in vitro simulated digestion, and sesamol was found to be unstable in the acidic intestinal environment.15 In addition to roasting, other heat treatments were reported to affect lignan bioaccessibility. A previous study examined the effects of boiling, sous vide, and microwaving on the content and bioaccessibility of phenolic compounds of artichokes. The identified lignans in the artichoke are two derivatives of pinoresinol: pinoresinol 4-glucoside and pinoresinol-acetylhexoside. Compared to other phenolic compounds, the bioaccessibility of the lignans in the raw samples was higher than for other phenolic compounds such as phenolic acids (13% and 0.66% after in vitro digestion, respectively). In addition, the lignans were degraded after all of the heat treatments. In contrast, in the studies mentioned before in other experimental systems, the lignans were relatively stable, such as virgin olive oil,21 possibly suggesting a significant matrix effect. Although the lignans decreased after the heat treatment, the bioaccessibility of the heat-treated artichokes was substantially higher than that of the raw sample. The percentage bioaccessibility of boiled, sous vide, and microwaved samples were 24%, 25% and 30%, respectively (yet the values were not compared statistically). The authors suggested that the increase in bioaccessibility (compared to the raw) was due to a protective effect against enzymatic and pH-dependent degradation by heat treatments.37 Furthermore, the pretreatment of the sample greatly influences the bioaccessible fraction. Moreover, changes in phytochemical bioavailability and bioactivity will occur following the processing and digestion of such products. The effect of thermal preparation and digestion on sesame lignans of a defatted sesame meal, a byproduct of sesame oil extraction, was studied. A comparison of the three processing methods (roasting, frying, and microwaving) revealed that the content of lignans was the highest for the microwave process, as discussed before. Besides that, roasted sesame seeds were subjected to in vitro digestion. The content of lignans that were released due to roasting during digestion increased by 19.6%.16 Overall, the existing results suggest that choosing the process is critical not only for the stability of the lignans but also for bioaccessibility. A combination of these two factors and additional factors, such as bioavailability, are essential when approaching the development of a new product. The presence of bioactive compounds capable of counteracting conditions encountered during food processing and the gastrointestinal tract (pH, presence of enzymes, and other nutrients) may be critical. Encapsulation allows a bioactive compound to be protected from destructive environmental conditions, solubilized, and delivered in a controlled manner. The effect of in vitro digestion on the encapsulated and nonencapsulated olive leaf phenolic compounds extract was evaluated. The lignans found in this extract are pinoresinol and syringaresinol, and the extract was microencapsulated using polycaprolactone. The findings indicate that the nonencapsulated form of the extract has a lower stability than the encapsulated extract in the simulated digestive process. The digestion process significantly influenced the nonencapsulated extract composition, and the progress of simulated digestion had a considerable impact on phenolic compound stability. Therefore, the intestinal fluid showed a substantial effect, while the oral and gastric fluids showed a smaller one. When the two lignans were compared, syringaresinol was shown to be more stable than the other compounds. It exhibited significant stability, even more than 100%, in all digestion processes (oral, intestine, and intestinal fluids) compared with the indigested extract. The phenolic compounds, including the lignans, were barely detectable in the encapsulated samples, because the capsules remained intact. The lower amounts released during all stages of digestion indicated that these compounds were held within the capsules and were not exposed to the digestion fluids. This is due to the low solubility of the encapsulation material at low pH, which prevents the phenols release in the acidic environment.38 Thus, the encapsulated samples in this system were less bioaccessible and would most likely pass through the colon without being absorbed in the upper gastric tract. The encapsulated samples can be metabolized by colonic microbiota into metabolites that possibly are absorbed into the bloodstream. The activity of the gut microbiota in the proximal colon metabolizes the lignans to enterodiol and enterolactone, which are known as mammalian lignans. Both enterodiol and enterolactone have been proven to exhibit estrogenic actions in vivo and to inhibit the growth of breast and prostate cancer cells.34
To summarize, despite being a significant group of health-promoting compounds, insufficient data exist regarding the bioaccessibility of lignans, both in isolated systems and in food matrices, as well as their stability and interactions with the food matrix in different parts of the digestive tract.
4. Conclusions and Future Outlook
This Mini-Review provides an overview of recent studies on lignans’ stability and digestive fate in different systems and under various processing methods. These bioactive compounds are common in numerous foods like seeds, grains, vegetables, fruits, and cereals and display promising applicative and health-promoting potential as they exhibit antioxidant, antibacterial, anticancer, and other biological activities such as antifungal, antiviral, and insecticidal properties.
The combination of chemical structure, matrix, and processing strongly affects the lignans’ stability and bioaccessibility. Processing is one of the most studied factors in the literature. Thermal treatments are the most explored among the processing methods. Various heat treatments, such as roasting and frying, affect the lignans differently, as can be expected from their major effects on the matrix and reaction kinetics, although concise data regarding the kinetics of lignan heat-induced transformations cannot be deduced from the existing literature. Additional processing methods were also reported to impact lignans, including fermentation, oil production, drying, and germination, yet the literature is scarce and the relation between the lignan structure and the impact of processing is only sporadically reported.
In terms of bioaccessibility, unlike a large body of research on polyphenols such as flavonoids, only a few studies focused on lignans. They revealed differences during the in vitro digestion based on the compound’s chemical structure, processing, and matrix, similar to the previously observed stability considerations. Overall, the bioaccessibility of the lignans is relatively low, and it was suggested that the lignans, like the polyphenols, proceed to the large intestine and are converted by human fecal microbiota into other compounds. However, the existing literature is still very limited, and more data are needed, particularly on the fundamental mechanisms involved in the stability and bioaccessibility of lignans. Future studies should investigate the stability and bioaccessibility of lignans in the context of processing methods, the food matrix, and the lignan structure more systematically. Furthermore, focus should be placed on the fate of lignans throughout both the upper and lower gastrointestinal tracts to gain information regarding their stability and ability to exert a biological impact, taking into account their bioconversion.
In conclusion, the field of studying lignans holds great promise for improving product quality and as health-promoting components; yet, additional knowledge is required for the optimal utilization of those compounds.
Acknowledgments
The authors acknowledge partial support from the Technion EVPR Fund: Nahum Wilbush Research Fund, ITS.
Biographies
Liora Berenshtein received her BSc in biotechnology and food engineering from Technion, Israel Institute of Technology. She is currently a Ph.D. candidate at the Faculty of Biotechnology and Food Engineering in the laboratory for novel food and bioprocessing. Her Ph.D. work involves studying the stability and bioaccessibility of sesame lignans and their products.
Zoya Okun received her B.Sc. in Chemistry from the Hebrew University of Jerusalem, M.Sc. (Cum Laude) and Ph.D. from the Schulich Faculty of Chemistry, Technion where she has focused on the synthesis and establishment of the structure–activity relationship of catalytic antioxidants in chemical and biological systems.
Avi Shpigelman has completed all his degrees in the faculty of Biotechnology and Food Engineering, and since 2014 he has led the laboratory for novel food and bioprocessing. The research in the group focuses on the processing–structure–function triangle in food systems and especially on the application of novel and nonthermal processing technologies and on health-promoting phenolic compounds.
The authors declare no competing financial interest.
References
- Namiki M. Nutraceutical Functions of Sesame: A Review. Crit Rev. Food Sci. Nutr 2007, 47, 651–73. 10.1080/10408390600919114. [DOI] [PubMed] [Google Scholar]
- Rodríguez-García C.; Sánchez-Quesada C.; Toledo E.; Delgado-Rodríguez M.; Gaforio J. Naturally Lignan-Rich Foods: A Dietary Tool for Health Promotion?. Molecules 2019, 24, 917. 10.3390/molecules24050917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teponno R. B.; Kusari S.; Spiteller M. Recent advances in research on lignans and neolignans. Nat. Prod Rep 2016, 33, 1044–92. 10.1039/C6NP00021E. [DOI] [PubMed] [Google Scholar]
- Milder I. E. J.; Arts I. C. W.; Van De Putte B.; Venema D. P.; Hollman P. C. H. Lignan contents of Dutch plant foods: a database including lariciresinol, pinoresinol, secoisolariciresinol and matairesinol. Br. J. Nutr. 2005, 93, 393. 10.1079/BJN20051371. [DOI] [PubMed] [Google Scholar]
- Gerstenmeyer E.; Reimer S.; Berghofer E.; Schwartz H.; Sontag G. Effect of thermal heating on some lignans in flax seeds, sesame seeds and rye. Food Chem. 2013, 138, 1847–55. 10.1016/j.foodchem.2012.11.117. [DOI] [PubMed] [Google Scholar]
- Yashin A. Y.; Yashunskii D. B.; Vedenin A. N.; Nifant’ev N. E.; Nemzer B. V.; Yashin Y. I. Chromatographic Determination of Lignans (Antioxidants) in Food Products. J. Anal. Chem. 2018, 73, 399–406. 10.1134/S106193481805012X. [DOI] [Google Scholar]
- Kim A. Y.; Yun C. I.; Lee J. G.; Kim Y. J. Determination and Daily Intake Estimation of Lignans in Sesame Seeds and Sesame Oil Products in Korea. Foods 2020, 9, 394. 10.3390/foods9040394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haworth R. D. The chemistry of the lignan group of natural products. Journal of the Chemical Society (Resumed) 1942, 21, 448–56. 10.1039/jr9420000448. [DOI] [Google Scholar]
- Eklund P.; Raitanen J. E. 9-Norlignans: Occurrence, Properties and Their Semisynthetic Preparation from Hydroxymatairesinol. Molecules 2019, 24, 220. 10.3390/molecules24020220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andargie M.; Vinas M.; Rathgeb A.; Möller E.; Karlovsky P. Lignans of Sesame (Sesamum indicum L.): A Comprehensive Review. Molecules 2021, 26, 883. 10.3390/molecules26040883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barba F. J.; Mariutti L. R. B.; Bragagnolo N.; Mercadante A. Z.; Barbosa-Cánovas G. V.; Orlien V. Bioaccessibility of bioactive compounds from fruits and vegetables after thermal and nonthermal processing. Trends Food Sci. Technol. 2017, 67, 195–206. 10.1016/j.tifs.2017.07.006. [DOI] [Google Scholar]
- Hu Y.; Li Y.; Sampson L.; Wang M.; Manson J. A. E.; Rimm E.; et al. Lignan Intake and Risk of Coronary Heart Disease. J. Am. Coll Cardiol 2021, 78, 666–78. 10.1016/j.jacc.2021.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eran Nagar E.; Berenshtein L.; Hanuka Katz I.; Lesmes U.; Okun Z.; Shpigelman A. The impact of chemical structure on polyphenol bioaccessibility, as a function of processing, cell wall material and pH: A model system. J. Food Eng. 2021, 289, 110304 10.1016/j.jfoodeng.2020.110304. [DOI] [Google Scholar]
- Lee S. W.; Jeung M. K.; Park M. H.; Lee S. Y.; Lee J. H. Effects of roasting conditions of sesame seeds on the oxidative stability of pressed oil during thermal oxidation. Food Chem. 2010, 118, 681–5. 10.1016/j.foodchem.2009.05.040. [DOI] [Google Scholar]
- Chen Y.; Lin H.; Lin M.; Zheng Y.; Chen J. Effect of roasting and in vitro digestion on phenolic profiles and antioxidant activity of water-soluble extracts from sesame. Food Chem. Toxicol. 2020, 139, 111239 10.1016/j.fct.2020.111239. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Lin H.; Lin M.; Lin P.; Chen J. Effects of thermal preparation and in vitro digestion on lignan profiles and antioxidant activity in defatted-sesame meal. Food Chem. Toxicol. 2019, 128, 89–96. 10.1016/j.fct.2019.03.054. [DOI] [PubMed] [Google Scholar]
- Zeb A.; Nisar P. Effects of high temperature frying of spinach leaves in sunflower oil on carotenoids, chlorophylls, and tocopherol composition. Front Chem. 2017, 5, 250923 10.3389/fchem.2017.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.; Kim M.; Choe E. Study on the changes of tocopherols and lignans and the oxidative properties of roasted sesame oil during manufacturing and storage. Kor. J. Food Sci. Technol. 2008, 40, 15–20. [Google Scholar]
- Hemalatha S.; Ghafoorunissa Sesame lignans enhance the thermal stability of edible vegetable oils. Food Chem. 2007, 105, 1076–85. 10.1016/j.foodchem.2007.05.023. [DOI] [Google Scholar]
- Čukelj N.; Novotni D.; Sarajlija H.; Drakula S.; Voučko B.; Ćurić D. Flaxseed and multigrain mixtures in the development of functional biscuits. LWT - Food Science and Technology 2017, 86, 85–92. 10.1016/j.lwt.2017.07.048. [DOI] [Google Scholar]
- Daskalaki D.; Kefi G. Evaluation of phenolic compounds degradation in virgin olive oil during storage and heating. J. Food Nutrition Res. 2009, 48, 31–41. [Google Scholar]
- Leonard W.; Zhang P.; Ying D.; Adhikari B.; Fang Z. Fermentation transforms the phenolic profiles and bioactivities of plant-based foods. Biotechnol Adv. 2021, 49, 107763 10.1016/j.biotechadv.2021.107763. [DOI] [PubMed] [Google Scholar]
- Simbalista R. L.; Frota K de MG; Soares R. A. M.; Arêas J. A. G. Effect of storage and processing of Brazilian flaxseed on lipid and lignan contents. Food Science and Technology 2012, 32, 374–80. 10.1590/S0101-20612012005000037. [DOI] [Google Scholar]
- Makowska A.; Waśkiewicz A.; Chudy S. Lignans in triticale grain and triticale products. J. Cereal Sci. 2020, 93, 102939 10.1016/j.jcs.2020.102939. [DOI] [Google Scholar]
- Hyvärinen H. K.; Pihlava J. M.; Hiidenhovi J. A.; Hietaniemi V.; Korhonen H. J. T.; Ryhänen E. L. Effect of processing and storage on the stability of flaxseed lignan added to dairy products. J. Agric. Food Chem. 2006, 54, 8788–8792. 10.1021/jf061285n. [DOI] [PubMed] [Google Scholar]
- Park W. S.; Koo K. A.; Bae J.-Y.; Kim H.-J.; Kang D.-M.; Kwon J.-M.; et al. Dibenzocyclooctadiene Lignans in Plant Parts and Fermented Beverages of Schisandra chinensis. Plants 2021, 10, 361. 10.3390/plants10020361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain Zaki U. K.; Fryganas C.; Trijsburg L.; Feskens E. J. M.; Capuano E. Influence of different processing method on lignan content of selected Malaysian plant-based foods. Food Chem. 2023, 404, 134607. 10.1016/j.foodchem.2022.134607. [DOI] [PubMed] [Google Scholar]
- Hyvärinen H. K.; Pihlava J. M.; Hiidenhovi J. A.; Hietaniemi V.; Korhonen H. J. T.; Ryhänen E. L. Effect of processing and storage on the stability of flaxseed lignan added to bakery products. J. Agric. Food Chem. 2006, 54, 48–53. 10.1021/jf0507590. [DOI] [PubMed] [Google Scholar]
- Shahidi F, Ho C. T., Van Chuyen N.. Process-Induced Chemical Changes in Food; Springer: New York, 2013. [Google Scholar]
- Özdemir E. E.; Görgüç A.; Gençdağ E.; Yılmaz F. M. Physicochemical, functional and emulsifying properties of plant protein powder from industrial sesame processing waste as affected by spray and freeze drying. LWT 2022, 154, 112646 10.1016/j.lwt.2021.112646. [DOI] [Google Scholar]
- Li X.; Li J.; Dong S.; Li Y.; Wei L.; Zhao C.; et al. Effects of germination on tocopherol, secoisolarlciresinol diglucoside, cyanogenic glycosides and antioxidant activities in flaxseed (Linum usitatissimum L.). Int. J. Food Sci. Technol. 2019, 54, 2346–54. 10.1111/ijfs.14098. [DOI] [Google Scholar]
- Fuentealba C.; Figuerola F.; Estévez A. M.; Bastías J. M.; Muñoz O. Bioaccessibility of lignans from flaxseed (Linum usitatissimum L.) determined by single-batch in vitro simulation of the digestive process. J. Sci. Food Agric 2014, 94, 1729–38. 10.1002/jsfa.6482. [DOI] [PubMed] [Google Scholar]
- Reboredo-Rodríguez P.; Olmo-García L.; Figueiredo-González M.; González-Barreiro C.; Carrasco-Pancorbo A.; Cancho-Grande B. Application of the INFOGEST Standardized Method to Assess the Digestive Stability and Bioaccessibility of Phenolic Compounds from Galician Extra-Virgin Olive Oil. J. Agric. Food Chem. 2021, 69, 11592–11605. 10.1021/acs.jafc.1c04592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintero-Florez A.; Pereira-Caro G.; Sanchez-Quezada C.; Moreno-Rojas J. M.; Gaforio J. J.; Jimenez A.; Beltran G.; et al. Effect of olive cultivar on bioaccessibility and antioxidant activity of phenolic fraction of virgin olive oil. Eur. J. Nutr 2018, 57, 1925–1946. 10.1007/s00394-017-1475-2. [DOI] [PubMed] [Google Scholar]
- Eran Nagar E.; Okun Z.; Shpigelman A. In vitro bioaccessibility of polyphenolic compounds: The effect of dissolved oxygen and bile. Food Chem. 2023, 404, 134490 10.1016/j.foodchem.2022.134490. [DOI] [PubMed] [Google Scholar]
- Hussain Zaki U. K.; Fryganas C.; Trijsburg L.; Feskens E. J. M.; Capuano E. In vitro gastrointestinal bioaccessibility and colonic fermentation of lignans from fresh, fermented, and germinated flaxseed. Food Funct 2022, 13, 10737–47. 10.1039/D2FO02559K. [DOI] [PubMed] [Google Scholar]
- Domínguez-Fernández M.; Ludwig I. A.; De Peña M. P.; Cid C. Bioaccessibility of Tudela artichoke (Cynara scolymus cv. Blanca de Tudela) (poly)phenols: the effects of heat treatment, simulated gastrointestinal digestion and human colonic microbiota. Food Funct 2021, 12, 1996–2011. 10.1039/D0FO03119D. [DOI] [PubMed] [Google Scholar]
- El-Messery T. M.; Aly E.; López-Nicolas R.; Sánchez-Moya T.; Ros G. Bioaccessibility and antioxidant activity of PCL-microencapsulated olive leaves polyphenols and its application in yogurt. J. Food Sci. 2021, 86, 4303–15. 10.1111/1750-3841.15893. [DOI] [PubMed] [Google Scholar]




